Abstract
Duchenne muscular dystrophy (DMD; dystrophin-deficiency) causes dilated cardiomyopathy in the second decade of life in affected males. We studied the dystrophin-deficient mouse heart (mdx) using high frequency echocardiography, histomorphometry, and gene expression profiling. Heart dysfunction was prominent at 9-10 months of age and showed significantly increased LV internal diameter (end systole) and decreased posterior wall thickness. This cardiomyopathy was associated with a 30% decrease in shortening fraction. Histologically, there was a 10-fold increase in connective tissue volume (fibrosis). mRNA profiling with RT-PCR validation showed activation of key pro-fibrotic genes, including Nox4 and Lox. The Nox gene family expression differed in mdx heart and skeletal muscle, where Nox2 was specifically induced in skeletal muscle while Nox4 was specifically induced in heart. This is the first report of an altered profibrotic gene expression profile in cardiac tissue of dystrophic mice showing echocardiographic evidence of cardiomyopathy.
Keywords: Duchenne muscular dystrophy, cardiomyopathy, echocardiography, mouse, Nox4, Lox
Introduction
Duchenne muscular dystrophy (DMD) is an inherited X-linked disorder with an incidence of 1 in 3,500 male births that is due to the absence of dystrophin, a large protein linking the intracellular cytoskeleton to the extracellular matrix. [1] DMD is typically diagnosed at about 3 years of age when patients present with weakening of skeletal muscles. Patients lose their ability to ambulate around age 10 and develop multiple medical problems related to skeletal and respiratory muscle weakness. Cardiomyopathy is a common feature developing in the second decade. [2], [3], [4] Death usually occurs in the second to third decades of life from respiratory or cardiac failure. As the treatment of skeletal and respiratory systems improves and patients live longer, an estimated 10-20% of DMD patients are dying of complications from cardiomyopathy. [5], [6]
Becker muscular dystrophy (BMD) occurs in approximately 1 in 30,000 males and results from reduced and/or abnormal dystrophin proteins. [7] BMD is caused by partial loss of function in the dystrophin protein and shows a more variable clinical course. [8] Although the skeletal muscle function is more mildly affected, BMD patients show a high prevalence of cardiac disease and can require cardiac transplantation. [9], [10]
The precise sequence of events connecting the initial cellular insult of dystrophin deficiency to tissue pathology and dysfunction remains relatively poorly understood and the mechanistic pathways are likely distinct for the heart and skeletal muscle. For example, skeletal muscle undergoes degeneration and regeneration cycles, with the regenerative potential decreasing with age, resulting in expected muscle wasting and weakness over time. The heart, on the other hand, shows little regenerative capacity, and the progressive pathology may be driven instead by a combination of fibrosis and/or cardiomyocyte loss. [11], [12] A better understanding of the molecular and cellular pathogenesis of the heart involvement in DMD would help to identify suitable therapeutic targets that may eventually improve the quality of life and prolong survival in DMD patients.
The animal model of DMD, the mdx mouse, is genetically similar to the human deletion in the Xp21.1 locus. [1], [13], [14] Although the underlying gene defect is the same in human and the mdx mouse, the clinical picture is quite different. The mdx skeletal muscle undergoes an early acute phase of degeneration at about 3-4 weeks of age followed by a successful regeneration phase. The mdx mouse also develops a cardiomyopathy with decreased cardiac function and cardiac fibrosis. [15]
We studied the mdx mouse using high frequency echocardiography to better characterize the cardiac dysfunction. We utilized an increased frequency (30 MHz) compared to clinical systems (15 MHz) and this enabled better two dimensional (2D) visualization of the cardiac structures and more precise measurements of the blood flow Doppler velocities in the mdx mouse. We also correlated functional and histological parameters with molecular networks induced in the mdx heart using mRNA expression profiling and quantitative real time-PCR validations. These molecular and physiologic correlates may provide new therapeutic insights into the treatment of DMD and other forms of cardiomyopathy.
Materials and methods
Animal Care
All mice were handled according to guidelines from the Institutional Animal Care and Use Committee. One, three and nine month old C57Bl/6J (wild type) and C57BL/10ScSn-Dmdmdx/J (mdx) mice weighing 20-30 grams were purchased from The Jackson Laboratory (Bar Harbor, Ma). All mice were housed in an individually vented cage system with a 12 hour light-dark cycle and received standard mouse chow and water ad libitum.
Echocardiography
Wild type (n=10) and mdx (n=14) mice were scanned at 1, 3 and 9 months. Mice were placed in an induction chamber with constant inflow of 5% isoflurane mixed with 100% oxygen. Once the mouse was asleep, it was removed from the induction chamber, weighed and placed on a heating platform with electrocardiogram contact pads (THM 100, Indus Instruments, Houston, TX) and the nose was placed into a nose cone with 1-2% isoflurane in 100% oxygen. Excess gases were evacuated passively using an activated charcoal absorption filter (VaporGuard, VetEquip, Pleasanton, Ca). The eyes were covered with a petroleum based ophthalmic ointment. Electrode gel was placed on the paws and the paws were taped over the electrocardiogram contact pads on the heating platform. A rectal probe was lubricated with gel, placed in the rectum and taped to the platform. The temperature was maintained at 36.5 to 37.5 °C. A blood pressure (BP) cuff was placed around the tail and the tail was then placed in a sensor assembly for non-invasive blood pressure monitoring (SC1000, Hatteras Instruments, Cary, NC). Depilatory cream was applied to the chest of the mouse and removed after two minutes. Ultrasound gel was placed on the chest of the anesthetized mouse. The ultrasound probe (RMZ 702a, Vevo 660, VisualSonics, Toronto, Canada) was placed in contact with the ultrasound gel and scanning was performed over 20 minutes. B-mode, M-mode and spectral Doppler images were obtained. The temperature, heart rate (HR) and BP were constantly monitored during the scanning. Once completed, all probes and monitors were removed from the mouse. The mouse was cleaned with water and allowed to recover on the heated platform. Once awake, the mouse was returned to its cage.
Qualitative and quantitative measurements were made offline using analytic software (VisualSonics, Toronto, Canada). Electrocardiogram kilohertz-based visualization (EKV™) software analysis produced offline reconstruction for simulated 250 to 1,000 Hz static and Cine loop images. Echocardiographic measurements included vessel diameters, ventricular wall thickness and chamber size, and blood flow velocities across the atrioventricular and semilunar valves. EKV™ images in the modified parasternal short and long axes were used to measure left ventricular mass (LVM) via the area-length method (LVM (g) = 1.05 {[5/6 * LV epicardial area (LV long axis in diastole + myocardial thickness)] − [5/6* LV endocardial area *LV long axis in diastole)]}. [16] Modified parasternal long axis EKV loops were also used to measure ejection fraction (EF) via Simpson’s method (Simp) (Figure 1A). M-mode images (Figure 1B) were used to measure left ventricular (LV) chamber sizes and wall thicknesses. Percent shortening fraction was calculated from M-mode measurements using the leading edge to leading edge method via the formula %Shortening Fraction (%SF) = left ventricular internal diameter (diastole) [LVID(d)] − left ventricular internal diameter (systole) [LVID(s)] / LVID(d).
Figure 1. Dystrophin-deficient mdx mice shows decreased cardiac function using high frequency echocardiography.

(A) Long axis view of the left ventricle with the end-diastolic volume measured using Simpson’s method. (B) Enlarged view of M-mode tracing of left ventricle showing measurements used to calculate shortening fraction.
Other calculations were performed using echocardiographic derived values and blood pressure measurements. Strove volume [SV (calc)] was derived using the formula SV= {[π (Aortic diameter)2/4]*[Aortic velocity time integral]. Cardiac output [CO (calc)] derived using formula CO = SV(calc)*heart rate (HR). The velocity of circumferential fiber shortening corrected for heart rate (VCFc) was calculated using {[LVID(d)] − [LVID(s)] / LVID(d)}/ Ejection time/square root of the preceding RR interval. The left ventricular meridional wall stress (WSm) was derived using formula 0.334[systolic blood pressure (SBP) * LVID(s)] / {left ventricular posterior wall thickness in systole (LVPWs)[1+(LVPWs/ LVIDs)]}. The myocardial performance index (MPI) was calculated using the formula (isovolumic contraction time + isovolumic relaxation time/ejection time).
Quantitation of fibrosis
Paraffin embedded cardiac tissue sections were stained with hematoxylin and eosin (H&E) (Sigma, St. Louis, Mo) and Gomori’s Tri-Chrome stain containing: fast green FCF, chromotrope 2R, and phosphotungstic acid (Sigma, St. Louis, Mo). The tissue was imaged under a light microscope and a digital image was obtained using computer software (Olympus C.A.S.T. Stereology System, Olympus America Inc., Center Valley, PA). The digital images were copied into PowerPoint, adjusted to the same size, and printed. The image was placed into an overlay containing 50 randomly spaced dots. Using the tri-chrome stained slides, counts were made for all dots which overlaid areas of blue-stained connective tissue. Connective tissue area was expressed as a ratio of the number of counted dots divided by the total number of dots on the overlay. [17]
RNA isolation
Left ventricular tissue was frozen in isopentane cooled with liquid nitrogen. The tissue was placed into a tube with 1 ml of Trizol and homogenized. The total RNA was isolated and then cleaned using the Qiagen RNeasy Mini kit according to the manufacturer’s instructions (Qiagen, Valencia, Ca). The resulting total RNA was checked on a gel for RNA integrity and quantified using the Nanodrop ND-100 Spectrophotometer (Nanodrop Technologies, Wilmington, De).
Gene expression profiling
To determine candidate molecular networks, gene expression profiling was studied in the left ventricles of mdx (n=3) and wild type (n=3) mice at 9-10 months of age. Total RNA extracted from the left ventricles was amplified (one-cycle) and hybridized to Affymetrix GeneChip® Mouse Expression Set 430 2.0 per the Affymetrix protocol (Affymetrix USA).
Gene expression data analysis was performed using different probe set algorithms (MAS5.0, dCHIP, RMA), and data visualization and statistical analyses using GeneSpring Software (Stratagene, La Jolla, Ca). [18] Candidate probe sets showing pathology-related changes were selected based upon those concordant in RMA and dChip algorithms with at least one present call and a Welch ANOVA t-test of p<0.05 compared to wild type and a two-fold difference in gene expression. We also used the Ingenuity Pathway Analysis program (Ingenuity Systems, Red Wood City, Ca) to define known protein-protein and gene-protein networks that involved the selected transcripts.
All microarray data are publicly available on the Public Expression Profiling Resource web site (http://pepr.cnmcresearch.org/home.do). This website contains .DAT, .CEL, and .TXT files for every microarray as well as online time query and visualization tools to assist with analysis of the database.
Real time Polymerase Chain Reaction (RT-PCR)
mRNA transcripts relevant to the pathophysiology were selected and confirmed by RT-PCR. Samples independent from gene profiling experiments of dystrophin deficient heart (n=4) and gastrocnemius (n=5) and wild type heart (n=3) and gastrocnemius (n=5) were used for validation studies. Total RNA was isolated as above and 300 ng of total RNA was used with the ABI high capacity cDNA kit (Applied Biosystems, Foster City, Ca) to generate cDNA. The cDNA was added to TaqMan 2x Universal PCR mix and the desired TaqMan Gene Expression Assay primer (Applied Biosystems, Foster City, Ca). Real time PCR was performed using the 7900 HT Fast Real Time PCR system (Applied Biosystems, Foster City, Ca) in the relative quantification ΔΔct mode. Data was analyzed using manufacturer supplied SDS2.2 software.
Immunofluorochemistry
Heart tissue was quickly frozen in isopentane and then stored at -80°C. Tissue slides of 6 micron thick sections were made using a cryostat and fixed in 4% paraformaldehyde. The slides were placed in 0.1% triton-X. Blocking was performed with 1% goat serum. Slide were incubated with anti-mouse Nox4 antibodies, a gift from Dr. J. David Lambeth, Department of Pathology and Laboratory Medicine, Emory University, overnight at 4°C at a dilution of 1:200. Slides were incubated with the secondary antibody AlexaFlour 594 (Invitrogen, Inc., Carlsbad, CA) at a dilution of 1:1000 for 1 hour at room temperature. Coverslips were applied with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and sealed with clear nail polish. [19] Slides were viewed using a Zeiss Axiovert 200M ApoTome microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY).
Statistics
Echocardiographic values were compared using unpaired t-test analysis for each measured or calculated variable at 1, 3, and 9 months of age. Significance in amount of fibrosis was calculated using an unpaired t-test. Gene expression data used a Welch ANOVA t-test with a p<0.05 to determine significance. RT-PCR utilized unpaired t-test analysis comparing 2-ΔΔCt±Std. Dev. (ΔCt) compared to wild type and normalized to HPRT1 control probe.
Results
High Frequency echocardiography shows cardiac dysfunction in mdx heart
We did not detect changes in cardiac structure or function at 1 and 3 months of age (data not shown). However, significant differences in a number of functional parameters were present at 9-10 months of age. Table 1 shows anatomical measurement results from M-mode imaging of the left ventricle in mdx and C57Bl/6J at 9-10 months of age. Significant differences include an increase in the left ventricular internal dimension at the end of systole [LVID(s)] (p=0.0023) and a decrease in the thickness of the left ventricular posterior wall at the end of systole [LVPW(s)] (p=0.0019). However, there were no significant differences seen in Doppler velocity measurements of both the inflow and outflow tracts (Table 2).
Table 1. Mdx mice show significant changes in left ventricular M-mode measurements compared to wild type at 9-10 months of age.
| M-mode
Measurement (mm) |
9-10 months | |
|---|---|---|
| wild type | mdx | |
| LV anterior wall (d) | 0.89±0.14 | 0.90±0.14 |
| LV internal diameter (d) | 3.61±0.33 | 3.85±0.39 |
| LV posterior wall (d) | 0.82±0.12 | 0.78±0.12 |
| LV anterior wall (s) | 1.31±0.22 | 1.14±0.22 |
| LV internal diameter (s) | 2.40±0.36 | 2.94±0.39* |
| LV posterior wall (s) | 1.12±0.15 | 0.94±0.10* |
p value < 0.05.
All measurements are presented as the average ± standard deviation. LV – left ventricle, (d) – diastole, (s) – systole, mm- millimeters.
Table 2. No significant differences in spectral Doppler measurements in 9-10 month old wild type and mdx mice.
| Spectral Doppler
measurement |
9-10 months old | |
|---|---|---|
| wild type | mdx | |
| Aortic artery Vmax (mm/s) | 880±101 | 882±165 |
| Aortic artery VTI (mm) | 2.31±0.34 | 2.71±0.64 |
| Pulmonary artery Vmax (mm/s) | 667±59 | 619±78 |
| Pulmonary artery VTI (mm) | 2.3±0.4 | 2.3±0.5 |
| Mitral valve E wave peak velocity (mm/s) | 629±46 | 610±97 |
| Tricuspid valve A wave velocity (mm/s) | 464±104 | 426±68 |
No significant differences were found using unpaired t-test. All measurements are presented as average ± standard deviation. Vmax – maximum velocity, VTI – velocity time integral, mm – millimeters, s- seconds.
Significant functional differences in several parameters were observed between mdx and wild type mice (Table 3). Diastolic (p<0.0001) and mean blood pressures (p<0.0001) were significantly decreased in mdx mice. The systolic blood pressure showed a trend towards a significant decrease (p=0.05). Most importantly, mdx mice showed a striking decrease in shortening fraction (p<0.0001), the gold standard for evaluation of cardiac systolic function. Ejection fraction was also calculated and significantly decreased in mdx mice versus in wild type mice (p<0.05). The calculation of meridional wall stress (WSm) was also significantly increased in mdx mice (p<0.01). The VCFc was significantly decreased in mdx mice (p<0.0001). The left ventricular mass (derived by the area-length formula) at 9-10 months of age trended toward an increase in mdx mice, but this did not reach statistical significance. [20], [21] Finally, the myocardial performance index (MPI), a global assessment of systolic and diastolic function, was significantly increased in mdx mice (p<0.05) and indicative of decreased cardiac diastolic and systolic function. Overall, the echocardiographic data was consistent with the presence of cardiomyopathy in 9-10 month old mdx mice compared to wild type controls.
Table 3. Significant differences in functional assessments of 9-10 month old mdx mice compared to wild type.
| Functional Measurement | wild type | mdx |
|---|---|---|
| Heart rate (bpm) | 506±82 | 460±83 |
| Systolic Blood Pressure (mmHg) | 90±13 | 80±10 |
| Diastolic Blood Pressure (mmHg) | 75±7 | 45±6*** |
| Mean Blood Pressure (mmHg) | 81±6 | 56±6*** |
| Shortening Fraction % | 33.6±4.8 | 23.5±5.1*** |
| Ejection Fraction % (Simp) | 54±11 | 40±14* |
| Stroke Volume (calc) ml | 0.037±0.01 | 0.043±0.01 |
| Cardiac Output (calc)ml/min | 18.62±3.5 | 19.21±5.1 |
| Wall Stressm (g/cm2) | 43±15 | 63±12** |
| VCFc (circ/sec) | 0.29±0.04 | 0.20±0.04*** |
| Left ventricular mass (mg) | 75±11 | 90±23 |
| Myocardial Performance Index | 0.78±0.12 | 0.88±0.06* |
p value < 0.05,
p value < 0.01,
p value < 0.0001.
mmHg – millimeters mercury, bpm – beats per minute, VCFc – velocity of circumferential fiber shortening corrected for heart rate, Wall Stressm – left ventricular meridional wall stress, Simp – value derived using Simpson’s method, calc – value based on calculation described in Methods section.
Cardiac fibrosis is significantly elevated in mdx heart
An increased connective tissue ratio was seen in the left ventricles of mdx hearts compared to wild type mice (p<0.0001) indicating increased fibrosis in the 9-10 month old mice (Figure 2A). Staining showed that the fibrosis was patchy and found throughout the left ventricle, including the lateral free wall and the interventricular septum (Figure 2B-I).
Figure 2. Left ventricle of mdx mice shows increased fibrosis area by histomorphometry.

(A) Graphic depiction of ratio of connective tissue area to normal myocardium in 9-10 month old mdx mice and wild type mice. Significantly elevated tissue area of fibrosis was seen in the mdx left ventricle (p=0.0001). (B) H&E stained left ventricle in wild type mice (10X). (C) H&E stained left ventricle in mdx mice showing patchy areas of fibrosis (10X). (D) H&E stained left ventricle myocardium in wild type mice showing normal cellular morphology (40X). (E) H&E stained left ventricle myocardium in mdx mice showing focal cardiomyocyte degeneration and increased fibroblast infiltration (40X). (F) Corresponding tri-chrome stained tissue to plate B (10X). (G) Corresponding tri-chrome stained tissue to plate C showing increased areas of collagen (10X). (H) Corresponding tri-chrome stained tissue to plate D showing normal cellular morphology (40X). (I) Corresponding tri-chrome stained tissue to plate E showing extensive collagen infiltration of myocardium.
Gene expression profiling shows pro-fibrotic gene expression
Gene expression analysis of mdx and control (wild-type) left ventricles was done at 10 months of age (n=3 for each group) and microarrays analyzed using two probe set algorithms (dCHIP and RMA). We and others have shown approximately 30-40% concordance between different methods of interpreting the same microarray images (probe set algorithms). [18] One approach to reducing false positive and false negative gene selections in microarray studies is to study those transcripts showing statistical significance for two probe set algorithms. Using this approach with a p<0.05 threshold for both probe set algorithms, and two additional data filters (‘present call’ filter, and >2-fold change filter), we found mdx and wild-type hearts to show significant differences in several genes that participate in profibrotic pathways (Table 4). Many of the up-regulated genes are related to TGF-β signaling and include cyclin dependent kinase inhibitor (Cdkn1A/p21), NADPH oxidase 4 (Nox4), Lysyl oxidase (Lox), vascular endothelial growth factor C (Vegfc), fibroblast growth factor 12 (Fgf12), connective tissue growth factor (Ctgf) and matrix metalloproteinase 14 (Mmp14/MT1-MMP). Other genes of interest included natriuretic peptide precursor type B (Nppb), the precursor to the protein BNP which is secreted during heart failure and cardiac volume overload, and sarcolipin (sln), a sarcoplasmic reticulum calcium handling protein.
Table 4. Gene profiling data showing up-regulated genes found in the left ventricles of 9-10 month old mdx mice and the fold increase (FI) compared to wild type mice.
All transcripts shown were significant (p<0.05) for two probe set algorithms (dCHIP, RMA).
| Genbank ID | Gene name | Brief Description | FI |
|---|---|---|---|
| BC005446 | Cap1 | CAP, adenylate cyclase-associated protein 1 (yeast) | 12.14 |
| NM_007669 | Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) | 9.82 |
| NM_007598 | Cap1 | CAP, adenylate cyclase-associated protein 1 (yeast) | 7.97 |
| BM233251 | Wdfy1 | WD40 and FYVE domain containing 1 | 6.44 |
| BC021831 | Erdr1 | erythroid differentiation regulator 1 | 6.05 |
| AK013278 | Btbd5 | BTB (POZ) domain containing 5 | 5.82 |
| AK008863 | Sln | Sarcolipin | 4.99 |
| BQ031098 | Wdfy1 | WD40 and FYVE domain containing 1 | 4.30 |
| BC021378 | Nox4 | NADPH oxidase 4 | 3.47 |
| NM_010728 | Lox | Lysyl oxidase | 3.44 |
| AK007630 | Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) | 3.35 |
| NM_010858 | Myl4 | High mobility group nucleosomal binding domain 1 | 3.21 |
| AK004853 | Dkk3 | Dickkopf homolog 3 (Xenopus laevis) | 3.11 |
| NM_022879 | Myl7 | Myosin, light polypeptide 7, regulatory | 2.86 |
| NM_013749 | Tnfrsf12a | Tumor necrosis factor receptor, member 12a | 2.86 |
| NM_013749 | Tnfrsf12a | Tumor necrosis factor receptor, member 12a | 2.83 |
| NM_009003 | Rab4a | RAB4A, member RAS oncogene family | 2.64 |
| NM_015814 | Dkk3 | Dickkopf homolog 3 (Xenopus laevis) | 2.64 |
| NM_009994 | Cyp1b1 | Cytochrome P450, family 1, subfamily b, polypeptide 1 | 2.59 |
| AW228853 | Vegfc | vascular endothelial growth factor C | 2.57 |
| BC019757 | Hist1h4i | histone 1, H4i | 2.49 |
| AK011892 | Plcd3 | Phospholipase C, delta 3 | 2.49 |
| NM_008966 | Ptgfr | Prostaglandin F receptor | 2.48 |
| BB774399 | Mlana | melan-A | 2.40 |
| BB559501 | Rora | RAR-related orphan receptor alpha | 2.38 |
| AF020738 | Fgf12 | Fibroblast growth factor 12 | 2.37 |
| AB013592 | Myoc | Myocilin | 2.37 |
| AK017926 | Ddit4 | DNA-damage-inducible transcript 4 | 2.31 |
| NM_008726 | Nppb | Natriuretic peptide precursor type B | 2.28 |
| BB353211 | Inhbb | inhibin beta-B | 2.26 |
| BG966751 | Etv5 | ets variant gene 5 | 2.23 |
| NM_022814 | Polydom | Von Willebrand factor type A, EGF and pentraxin | 2.19 |
| X16834 | Lgals3 | Lectin, galactose binding, soluble 3 | 2.16 |
| AV343573 | Cbln4 | cerebellin 4 precursor protein | 2.14 |
| NM_010217 | Ctgf | Connective tissue growth factor | 2.13 |
| AV273591 | Nt5e | 5’ nucleotidase, ecto | 2.09 |
| NM_008608 | Mmp14 | Matrix metalloproteinase 14 (membrane-inserted) | 2.08 |
| BC018236 | Alad | Aminolevulinate, delta-, dehydratase | 2.04 |
| BC014731 | Parp16 | Poly (ADP-ribose) polymerase family, member 16 | 2.01 |
RT-PCR shows Nox4 up-regulation is specific to cardiac muscle
RT-PCR was performed in 9-10 month old mdx and wild type left ventricular tissue independent from samples used in expression profiling. Transcripts selected for validation included those known to be involved in cardiac cell survival and cardiac fibrosis. Nox4 showed a 5 fold increase in mdx mice compared to wild type mice (p=0.001). No significant differences in NADPH oxidase 1 (Nox1) or Nox2 (Cybb) mRNA levels were seen compared to wild type. Lox mRNA levels were significantly increased compared to wild type (p=0.0016) (Figure 3). Nox4 and Lox mRNA levels were also studied in 3 month old mdx and wild type mice without evidence of a cardiomyopathy. Nox4 mRNA was not significantly altered (1.25±0.56 in mdx vs. 1.0±0.38 in wild type, p= 0.26). However, Lox was significantly increased at this early age (1.96±0.29 fold increased in mdx vs. 1.0±0.38 in wild type, p< 0.0001). Therefore, cardiac muscle showed early increased Lox mRNA levels followed by increased Nox4 and Lox mRNA levels at 9-10 months of age.
Figure 3. Increased Nox4 mRNA expression in left ventricle of mdx mice with cardiomyopathy.
Real time PCR results for Nox1, Nox2, Nox4 and Lox in cardiac (A) and skeletal muscle (B) tissue of 9-10 month old mdx and wild type mice. Significant increases were seen in Nox4 and Lox in mdx mice in cardiac muscle and in Nox2 and Lox in skeletal muscle. P values are for mdx vs. wild type for each respective gene.
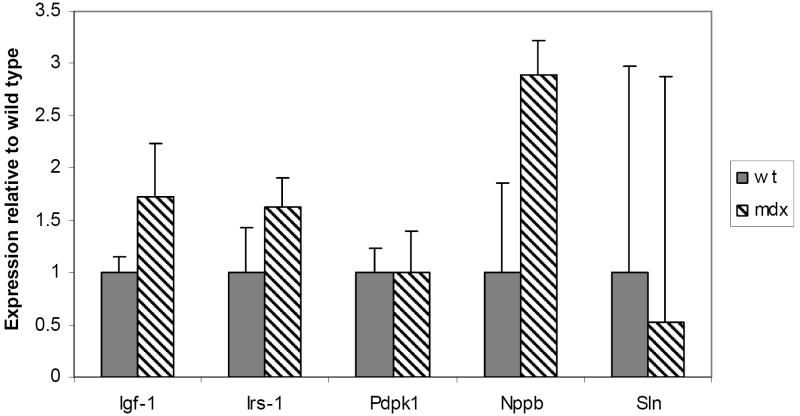
In skeletal muscle samples, no significant differences were seen in Nox4 and Nox1. However, Nox2 (p=0.008) and Lox (p=0.0001) were both significantly increased in mdx mouse skeletal muscle compared to wild type (Figure 3). Nppb mRNA levels also validated the increased gene expression profiling data (p= 0.009). Other genes involved in cardiac cell survival, IGF-1, IRS-1, trended towards significance (p=0.06 and 0.07, respectively). Sarcolipin was actually decreased in mdx hearts, contrary to expression profiling data (Figure 4).
Figure 4. Increased Nppb mRNA expression in left ventricle of mdx mice showing cardiomyopathy.
Real time PCR results for Igf-1, Irs-1, Pdpk1, Nppb and Sln in cardiac tissue of 9-10 month old mdx and wild type mice. A significant increase in Nppb was seen in mdx mice (p=0.009).
Immunofluorescence shows increased Nox4 protein levels in left ventricular tissue
Immunofluorescence was performed on ventricular tissue from 9 month old mdx and normal mice. Anti-Nox4 antibody showed increased binding in the nuclei of the cardiomyocytes (Figure 5). This is consistent with studies in other tissue types, i.e. vascular smooth muscle cells in culture. [22] These results support the gene expression and RT-PCR data showing increased expression of Nox4 in ventricular tissue.
Figure 5. Increased Nox4 protein found in the nuclei of left ventricular cardiomyocytes in 9-10 month old mdx mice.

Immunofluorescence using primary Nox4 antibodies demonstrates increased binding in cardiomyocytes found in the left ventricles of mdx (top panel) mice compared to wild type (wt) mice (bottom panel). Counterstaining with DAPI confirms localization to the nuclei.
Discussion
As life prolonging treatments for skeletal and respiratory muscle dysfunction improve, cardiomyopathy is becoming the cause of death in an increasing number of DMD patients. [5], [6] A better understanding of the pathogenesis of this unique cardiomyopathy is needed to improve patient outcomes and quality of life. This study utilized high resolution echocardiography for non-invasive assessment of cardiac function and identified molecular changes that occur in the cardiomyopathy of the mouse model for DMD, the mdx mouse. Decreased cardiac function was evident at 9-10 months of age and increased Nox4 and Lox gene expression was present in left ventricular tissue. This is the first report of global gene expression changes seen in mdx cardiomyopathy.
A previous study using conventional M-mode imaging documented a dilated cardiomyopathy in mdx mice. [15] In that study, conscious mdx mice and aged matched C57BL10ScSn controls were studied at 8, 29 and 42 weeks of age. No differences were seen in 8 and 29 week old mice. In the 42 week old mdx mice, the left ventricular end diastolic dimension increased compared to wild-type mice. Also, the percent shortening fraction decreased in mdx mice compared to controls. This is evidence of a dilated cardiomyopathy in 42 week old mdx mice.
Our study also did not find any significant differences in 6 and 18-20 week old mice. In older mice, our study concurred with Quinlan et al. (2004) and found a significantly decreased percent shortening fraction in mdx mice using high frequency echocardiography. [15] The values are different since the previous study used conscious mice and our study was performed using inhaled isoflurane anesthesia. Isoflurane anesthesia is known to decrease hearts rates and contractility in mice, so our study showed lower heart rates and decreased overall percent shortening fraction. [23] Although the variability in heart rates seen with anesthesia can affect calculated values such as cardiac output, the effects on shortening fraction are consistent between groups. The decrease in cardiac ventricular function corresponded with a significant increase in the left ventricular end systolic dimension. However, we did not find a significantly increased left ventricular end diastolic dimension, indicative of a dilated cardiomyopathy, as was seen by Quinlan et al. (2004).
We expanded upon the previous report by utilizing increased frequency (30 MHz) ultrasound and an echocardiography system using EKV™ imaging, ECG kilohertz-based visualization. This 2D reconstruction of a single heart beat using M-mode image data acquired at a resolution of 1000 Hz provides improved visualization of the endocardial and epicardial borders. By using EKV™ imaging, we were able to trace the endocardial border of the heart in systole and diastole to derive an ejection fraction. This echocardiographic view (Figure 1A) is not the same as the one used clinically, so there is concern that the left ventricle is slightly foreshortened and may lead to smaller measured ventricular volumes. However, using this technique, we consistently showed a decreased ejection fraction in mdx mice compared to wild type controls. While the ejection fraction is a common measurement of function in the human clinical echocardiography lab, previous technology has not allowed this measurement in mice, so this is a novel finding for the assessment of cardiac function in small animal models.
The improved resolution of the high frequency system also provides more capabilities to perform functional assessments. Many of these assessments are derived calculations that require data including heart rate and blood pressure. Significant decreases in blood pressure were found in 9-10 month old mdx mice. The exact mechanism for this decrease in blood pressure is not entirely clear, but decreased cardiac function and anesthesia may be contributors. Also, in mdx mice, there is known dysregulation of vascular endothelial function involving nitric oxide synthase. An inappropriate response of nitric oxide could affect vascular resistance and blood pressure. [24], [25]
Other significant functional assessments include wall stress, an estimate of how much stress the cardiac muscle fibers are experiencing during systole. This value was significantly increased in mdx mice and indicates that the thinning, poorly functioning myocardium is actually under more stress than the normal heart, possibly contributing to increased myocardial cell death over time. Another functional assessment, the VCFc, is a calculation of the speed at which cardiac muscle fibers are shortening. This was significantly decreased in mdx mice and consistent with worsening cardiac function. These are novel measurements of cardiac function derived non-invasively from mdx mice and improve our assessment and understanding of the cardiomyopathy seen in the mdx mouse model.
Recent studies have confirmed that high frequency echocardiography is more feasible and reproducible for studying cardiac function in mouse models compared to conventional clinical platforms. [26] Our results using high frequency echocardiography for the measurement of shortening fraction are also consistent with other studies using similar techniques in different mouse models. [27], [28], [29], [30] The calculation of ejection fraction is also consistent with a recent study. [31] The additional capability of directly measuring percent ejection fraction in the 2D parasternal long axis allows high frequency echocardiography to be used alongside other newer imaging modalities such as 3D echo and MRI. [32], [33], [34]
Our histological results are in agreement with previous studies demonstrating patchy fibrosis in the ventricles of mdx mice. [15], [35], [36], [37], [38] The loss of functional muscle tissue and resulting fibrosis can lead to decreased cardiac systolic and diastolic function. Since the fibrosis is patchy and does not localize specifically to areas with the greatest muscle involvement, i.e. the left ventricular free wall, there are likely mechanisms involved that are not solely related to membrane instability and abnormal transmission of shear forces. However, these pathways and the extent of their contribution to cardiac cell death are still not known.
The cardiomyopathy seen in mdx mice can also have a variable clinical presentation. In our study, we feel the observed variation was not significant. From a functional standpoint, the standard deviation seen in the measurement of shortening fraction was ±4.8 % for wild type and ±5.1% for mdx . This is indicative of a similar variation found within control mice and mdx mice. When using p values as a filtering method in gene expression profiling, the use of n=3 replicates may not prove sensitive for more subtle changes, but should still retain adequate specificity. Given that we did use relatively low numbers of animals, we focused on validation of key pathway members in independent samples using alternative methods, namely quantitative RT-PCR and immunofluorescence. By confirmation of these results in independent samples, we show further evidence for significant differences not affected by individual variations.
As with our study, gene expression profiling has been used extensively to interrogate genetic variations in many other mouse and human cardiomyopathy models. In a mouse viral myocarditis model, increases in BNP and Lox were seen 3 and 9 days after infection. There were also up-regulated genes affecting the extracellular matrix, i.e. collagen type IV, and stress responses, i.e. heat shock proteins 27, 60, 86. [39] In mice lacking muscle LIM protein, cardiomyopathy was associated with increased BNP, Ctgf and heat shock proteins 25 and 27. Ctgf also showed significantly increased expression during progression from early to late heart failure. [40] BNP and Ctgf were also shown to be markers for human idiopathic dilated cardiomyopathies. [41], [42] Other studies in humans show up-regulation of many other extracellular matrix related genes. [43], [44]
We found that Nox4 and Lox mRNA’s were increased in left ventricular tissue from 9-10 month old mdx mice, whereas oxidases Nox1 and Nox2 (Cybb) were not up-regulated (Figure 3). There is no mention of increased Nox4 expression in any of the previous cardiomyopathy models mentioned. Lox mRNA was also increased in left ventricular tissue from 3 month old mdx mice. Thus, Nox4 and Lox may specifically play an important role in the increased cardiac fibrosis seen in mdx mice.
Lox is an extracellular, copper dependent enzyme that initiates the cross-linking of collagen and elastin. [45] Lox has been shown to be quite important in cardiovascular development and function, especially related to the elastin layer in the aorta. [46], [47] This gene family is also important in cardiac remodeling. Up-regulation of Lox mRNA was also found in fibrotic myocardium distant from an infarct site in a mouse coronary artery ligation model. [48] In a study using cultured rat cardiac fibroblasts, collagen production was decreased when lysyl oxidase was inhibited by arphamenine A, an aminopeptidase B inhibitor. [49] Another lysyl oxidase inhibitor, aminoproprionitrile, abolished collagen contraction in adult male cardiac fibroblasts when stimulated with angiotensin I, II and III. [50] These studies show that Lox also has an important role in cardiac fibrosis. Notably, we also observed that Lox was increased in the skeletal muscle of the mdx mouse. Therefore, Lox is likely involved in a common pathway of fibrosis seen in both skeletal and cardiac muscle.
Nox2 is expressed in neutrophils and macrophages and is mainly involved in host defense and inflammatory responses. [51] Therefore, increases in Nox2 levels in mdx skeletal muscle are likely related to inflammatory responses associated with muscle cell death and regeneration. In a study using Nox2 (gp91phox) deficient mice, a 90% reduction in the number of muscle fibers showing extensive membrane injury was found compared to controls. [52] However, increased Nox2 activity was not seen in cardiac tissue and there were not extensive inflammatory infiltrates seen in mdx myocardial tissue (Figure 3). Therefore, the fibrosis seen in the mdx heart does not appear related to significant inflammatory cellular infiltrates and Nox2 is likely more involved with skeletal muscle pathology than cardiac muscle.
This study demonstrates that increased Nox4 is unique to cardiac tissue and located in the nuclei of cells. There was no increase of Nox4 in mdx skeletal muscle (Figure 6) and extensive human skeletal muscle data available from our lab does not show an increase in Nox4 mRNA in multiple muscular dystrophies (http://sas.cnmcresearch.org). In vitro studies are beginning to look at cardiac fibroblasts and the secondary messengers related to fibrosis and scarring. Cucoranu et al. (2006) showed in tissue culture that TGF-β1 stimulation causes primary human cardiac fibroblasts to differentiate into cardiac myofibroblasts which express increased amounts of smooth muscle α-actin mRNA. [53] They also showed that cardiac fibroblasts displayed an increased expression of Nox4 mRNA when stimulated by TGF-β. Inhibiting Nox4 expression with siRNA’s reduced the expression of smooth muscle α-actin mRNA by 95%. Lastly, TGF-β signaling is known to use Smad 2/3 phosphorylation and depletion of Nox4 decreased Smad 2/3 phosphorylation by 75%. Colston et al. (2005) found that primary human cardiac fibroblasts produced reactive oxygen species with the addition of hydrogen peroxide to the culture. They also showed that adult cardiac fibroblasts express Nox4 quite abundantly compared to Nox2. [54] These studies, along with our findings of increased Nox4 in mdx mice, show that Nox4 likely plays an important role on the fibrotic response of cardiac tissue.
The interactions and effects of Lox and Nox4 are not completely understood at this time in dystrophin-deficient cardiomyopathy. Both are involved in cardiac fibrosis in response to other signaling mechanisms, potentially TGF-β or reactive oxygen species. As we continue to look for a cure for DMD, potential treatments at this time may be effective if directed at decreasing the amount of cardiac fibrosis. By modulating Nox4 and/or Lox, there may be preservation of cardiac function, improved quality of life and decreased cardiac mortality in DMD patients. Thus, the Nox4 pathway appears to be specific to cardiac tissue, a critical factor in cardiac fibrosis, and may provide a novel therapeutic window for DMD cardiomyopathy.
Acknowledgments
This work was supported by grant NIH/ NICHD K12 CHRCDA K12HD001399-04, Department of Defense USAMRAA grant W81XWH-05-1-0616 (Mouse Drug Screening Core to K. Nagaraju), and NIH grant 1U54HD053177-01A1 (Wellstone Muscular Dystrophy Center to EP Hoffman). Support for mRNA profiling was provided by the MRDDRC (1P30HD40677-01), and the National Center for Medical Rehabilitation Research (5R24HD050846-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman EP, Brown RH, Kunkel LM. Dystrophin, the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Gilroy J, Cahalan JL, Berman R, Newman M. Cardiac and pulmonary complications in Duchenne’s progressive muscular dystrophy. Circulation. 1963;27:484–493. doi: 10.1161/01.cir.27.4.484. [DOI] [PubMed] [Google Scholar]
- 3.De Kermadec JM, Becane HM, Chenard A, Tertrain F, Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J. 1994;127:618–23. doi: 10.1016/0002-8703(94)90672-6. [DOI] [PubMed] [Google Scholar]
- 4.Kirchmann C, Kececioglu D, Korinthenberg R, Dittrich S. Echocardiographic and electrocardiographic findings of cardiomyopathy in Duchenne and Becker-Kiener muscular dystrophies. Pediatr Cardiol. 2005;26:66–72. doi: 10.1007/s00246-004-0689-2. [DOI] [PubMed] [Google Scholar]
- 5.Gulati S, Saxena A, Kumar V, Kalra V. Duchenne Muscular Dystrophy: Prevalence and Patterns of Cardiac Involvement. Indian J Pediatr. 2005;72:389–93. doi: 10.1007/BF02731732. [DOI] [PubMed] [Google Scholar]
- 6.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in left expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 7.Emery AE. Population frequencies of inherited neuromuscular diseases: a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman EP, Kunkel LM, Angelini C, Clarke A, Johnson M, Harris JB. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology. 1989;39:1011–1017. doi: 10.1212/wnl.39.8.1011. [DOI] [PubMed] [Google Scholar]
- 9.Melacini P, Fanin M, Danieli GA, et al. Cardiac involvement in Becker muscular dystrophy. J Am Coll Cardiol. 1993;22(7):1927–34. doi: 10.1016/0735-1097(93)90781-u. [DOI] [PubMed] [Google Scholar]
- 10.Melacini P, Fanin M, Danieli GA, et al. Myocardial involvement is very frequent among patients affected with subclinical Becker’s muscular dystrophy. Circulation. 1996;94:3168–3175. doi: 10.1161/01.cir.94.12.3168. [DOI] [PubMed] [Google Scholar]
- 11.Leferovich JM, Bedelbaeva K, Samulewicz S, et al. Heart regeneration in adult MRL mice. Proc Natl Acad Sci USA. 2001;98(17):9830–5. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 13.Ryder-Cook AS, Sicinski P, Thomas K, et al. Localization of the mdx mutation within the mouse dystrophin gene. EMBO. 1988;7(10):3017–3021. doi: 10.1002/j.1460-2075.1988.tb03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 15.Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantification if the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 17.Curtis AS. Area and volume measurements by random sampling methods. Med Biol Illus. 1960;10:261. [PubMed] [Google Scholar]
- 18.Seo J, Hoffman EP. Prove set algorithms: is there a rational best bet? BMC Bioinformatics. 2006;7:395–407. doi: 10.1186/1471-2105-7-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–69. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 20.Collins KA, Korcarz CE, Shroff SG, et al. Accuracy of echocardiographic estimates of left ventricular mass in mice. Am J Physiol Heart Circ Physiol. 2001;280:H1954–62. doi: 10.1152/ajpheart.2001.280.5.H1954. [DOI] [PubMed] [Google Scholar]
- 21.Youn HJ, Rokosh G, Lester SJ, Simpson P, Schiller NB, Foster E. Two-dimensional echocardiography with a 15-MHz transducer is a promising alternative for in vivo measurement of left ventricular mass in mice. J Am Soc Echocardiogr. 1999;12:70–5. doi: 10.1016/s0894-7317(99)70175-6. [DOI] [PubMed] [Google Scholar]
- 22.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–83. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 23.Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–40. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- 24.Loufrani L, Matrougui K, Gorny D, et al. Flow (Shear stress)-Induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin. Circulation. 2001;103(6):864–70. doi: 10.1161/01.cir.103.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loufrani L, Levy BI, Henrion D. Defect in microvascular adaptation to chronic changes in blood flow in mice lacking the gene encoding for dystrophin. Circ Res. 2002;91(12):1183–9. doi: 10.1161/01.res.0000047505.11002.81. [DOI] [PubMed] [Google Scholar]
- 26.Okajima K, Abe Y, Fujimoto K, et al. Comparative study of high-resolution microimaging with 30-Mhz scanner for evaluating cardiac function in mice. J Am Soc Echocardiogr. 2007;20:1203–1210. doi: 10.1016/j.echo.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YQ, Foster FS, Nieman BJ, et al. Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics. 2004;18:232–244. doi: 10.1152/physiolgenomics.00026.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Fedak PW, Dai X, et al. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006;114:2271–2279. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 29.Radke MH, Peng J, Wu Y, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci. 2007;104:3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Yong W, Ren S, et al. Over expression of bone morphogenetic protein 10 in myocardium disrupts cardiac postnatal hypertrophic growth. J Biol Chem. 2006;281:27481–27491. doi: 10.1074/jbc.M604818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Takagawa J, Sievers RE, et al. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H1187–1192. doi: 10.1152/ajpheart.00895.2006. [DOI] [PubMed] [Google Scholar]
- 32.Dawson D, Lygate CA, Saunders J, et al. Quantitative 3-dimensional echocardiography for accurate and rapid cardiac phenotype characterization in mice. Circulation. 2004;110:1632–1637. doi: 10.1161/01.CIR.0000142049.14227.AD. [DOI] [PubMed] [Google Scholar]
- 33.Wilding JR, Schneider JE, Sang AE, Davies KE, Neubauer S, Clarke K. Dystrophin- and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 2005;19:79–81. doi: 10.1096/fj.04-1731fje. [DOI] [PubMed] [Google Scholar]
- 34.Kober F, Iltis I, Cozzone PJ, Bernard M. Cine-MRI assessment of cardiac function in mice anesthetized with ketamine/xylazine and isoflurane. MAGMA. 2004;17:157–61. doi: 10.1007/s10334-004-0086-0. [DOI] [PubMed] [Google Scholar]
- 35.Bridges LR. The association of cardiac muscle necrosis and inflammation with the degenerative and persistent myopathy of MDX mice. J Neurol Sci. 1986;72(23):147–57. doi: 10.1016/0022-510x(86)90003-1. [DOI] [PubMed] [Google Scholar]
- 36.Lefaucher JP, Pastoret C, Sebille A. Phenotype of dystrinopathy in old mdx mice. Anat Rec. 1995;242(1):70–6. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- 37.Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest. 2001;107(2):R1–R7. doi: 10.1172/JCI11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura A, Yoshida K, Takeda S, Dohi N, Ikeda S. Progression of dystrophic features and activation of mitogen-activated protein kinases and calcineurin by physical exercise , in hearts of mdx mice. FEBS Lett. 2002;520(13):18–24. doi: 10.1016/s0014-5793(02)02739-4. [DOI] [PubMed] [Google Scholar]
- 39.Taylor LA, Carthy CM, Yang D, et al. Host gene regulation during coxsackievirus B3 infection in mice; assessment by microarrays. Circ Res. 2000;87:328–334. doi: 10.1161/01.res.87.4.328. [DOI] [PubMed] [Google Scholar]
- 40.Blaxall BC, Spang R, Rockman HA, Koch WJ. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiol Genomics. 2003;15:105–114. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- 41.Tan FL, Moravec CS, Li J, et al. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci USA. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barth AS, Kuner R, Buness A, et al. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J Am Coll Cardiol. 2006;48:1610–17. doi: 10.1016/j.jacc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Sanoudou D, Vafiadaki E, Arvanitis DA, Kranias E, Kontrogianni-Konstantopoulos A. Array lessons from the heart: focus on the genome and transcriptome of cardiomyopathies. Physiol Genomics. 2005;21:131–143. doi: 10.1152/physiolgenomics.00259.2004. [DOI] [PubMed] [Google Scholar]
- 44.Hwang JJ, Allen PD, Tseng GC, et al. Microarray gene expression profiles in dilated and hypertrophic cardiomyopathic end-stage heart failure. Physiol Genomics. 2002;10:31–44. doi: 10.1152/physiolgenomics.00122.2001. [DOI] [PubMed] [Google Scholar]
- 45.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(1920):2304–16. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maki JM, Rasanen J, Tikkanen H, et al. Inactivation of the Lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi K, Fong KS, Mercier F, Boyd CD, Csiszar K, Hayashi M. Comparative immunocytochemical localization of Lysyl oxidase (LOX) and the Lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol. 2004;35(89):845–55. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 48.Tsuda T, Gao E, Evangelist L, Markova D, Ma X, Chu ML. Post-ischemic myocardial fibrosis occurs independent of hemodynamic changes. Cardiovasc Res. 2003;59(4):926–33. doi: 10.1016/s0008-6363(03)00519-4. [DOI] [PubMed] [Google Scholar]
- 49.Lijnen PJ, Petrov VV, Turner M, Fagard RH. Collagen production in cardiac fibroblasts during inhibition of aminopeptidase B. J Renin Angiotensin Aldosterone Syst. 2005;6(2):69–77. doi: 10.3317/jraas.2005.012. [DOI] [PubMed] [Google Scholar]
- 50.Lijnen P, Petrov V, Diaz-Araya G, Fagard R. Effect of bestatin on angiotensin I-, II-, III-induced collagen gel contraction in cardiac fibroblasts. J Renin Angiotensin Aldosterone Syst. 2004;5(4):183–8. doi: 10.3317/jraas.2004.038. [DOI] [PubMed] [Google Scholar]
- 51.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochem Biophys Acta. 2004;1657(1):1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen HX, Tidball JG. Null mutation of gpphox91 reduces muscle membrane lysis during muscle inflammation in mice. J Physiol. 2003;553(pt 3):833–41. doi: 10.1113/jphysiol.2003.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 54.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox4 through PLA2 dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005;579:2533–2540. doi: 10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]