Abstract
Dopamine (DA) and noradrenaline (NA) in the prefrontal cortex (PFC) modulate superior cognitive functions, and are involved in the aetiology of depressive and psychotic symptoms. Moreover, microdialysis studies in rats have shown how pharmacological treatments that induce modifications of extracellular NA in the medial PFC (mPFC), also produce parallel changes in extracellular DA.
To explain the coupling of NA and DA changes, this article reviews the evidence supporting the hypothesis that extracellular DA in the cerebral cortex originates not only from dopaminergic terminals but also from noradrenergic ones, where it acts both as precursor for NA and as a co-transmitter.
Accordingly, extracellular DA concentration in the occipital, parietal and cerebellar cortex was found to be much higher than expected in view of the scarce dopaminergic innervation in these areas.
Systemic administration or intra-cortical perfusion of α2-adrenoceptor agonists and antagonists, consistent with their action on noradrenergic neuronal activity, produced concomitant changes not only in extracellular NA but also in DA in the mPFC, occipital and parietal cortex.
Chemical modulation of the locus coeruleus by locally applied carbachol, kainate, NMDA or clonidine modified both NA and DA in the mPFC.
Electrical stimulation of the locus coeruleus led to an increased efflux of both NA and DA in mPFC, parietal and occipital cortex, while in the striatum, NA efflux alone was enhanced.
Atypical antipsychotics, such as clozapine and olanzapine, or antidepressants, including mirtazapine and mianserine, have been found to increase both NA and DA throughout the cerebral cortex, likely through blockade of α2-adrenoceptors. On the other hand, drugs selectively acting on dopaminergic transmission produced modest changes in extracellular DA in mPFC, and had no effect on the occipital or parietal cortex.
Acute administration of morphine did not increase DA levels in the PFC (where NA is diminished), in contrast with augmented dopaminergic neuronal activity; moreover, during morphine withdrawal both DA and NA levels increased, in spite of a diminished dopaminergic activity, both increases being antagonised by clonidine but not quinpirole administration.
Extensive 6-hydroxy dopamine lesion of the ventral tegmental area (VTA) decreases below 95% of control both intra- and extracellular DA and DOPAC in the nucleus accumbens, but only partially or not significantly in the mPFC and parietal cortex.
The above evidence points to a common origin for NA and DA in the cerebral cortex and suggests the possible utility of noradrenergic system modulation as a target for drugs with potential clinical efficacy on cognitive functions.
INTRODUCTION
Cognitive functions such as working memory, attention and executive tasks are mainly dependent on frontal cortex activity, the performance of which is modulated by dopamine (DA) and noradrenaline (NA). These catecholamines exert a biphasic effect on cognitive functioning, determining an inverted-U shaped dose-curve, normal cognitive operation occurring only within a limited range of dopaminergic and noradrenergic activity [7, 8, 47, 73, 82, 125]. Consequently, imbalances in dopaminergic and noradrenergic system functioning are involved in the aetiology of different psychiatric diseases such as schizophrenia, ADHD and depressive illness [13, 39, 56, 85, 126, 128].
The cerebral cortex receives dense and widespread noradrenergic innervation, whereas dopaminergic terminals are concentrated in the medial prefrontal cortex (mPFC), anterior cingulate, rhinal and entorhinal cortices [23, 69, 102]. However, DA receptors are not confined to the innervated regions, but instead are widely spread throughout the cerebral cortex [45, 67, 71, 97, 121]. Moreover, cortical tissue NA concentrations exceed those of DA even in regions with overlapping dopaminergic and noradrenergic innervations, such as the mPFC [35].
Experimental evidence indicates a coupling between NA and DA release in the PFC [62], and different conditions including stress [36, 37, 61, 83], antidepressants [29, 76, 114], psychostimulants [22, 98] antipsychotic drugs [27, 66, 91, 123], α2 adrenoceptor agonists and antagonists [24, 28, 50, 62] have been shown to produce concomitant changes in extracellular DA and NA in the PFC. To explain these parallel changes in extracellular NA and DA concentration, the “heterologous uptake” hypothesis has been formulated: indeed, plasma membrane monoamine transporters lack selectivity for their substrates, and the uptake transporters DAT, NET and SERT, in spite of their exclusive expression in neurons that, respectively, synthesise and release DA, NA or serotonin, also accumulate monoamines released from different neuronal systems [15, 80, 115, 119]. Moreover, NET displays higher affinity for DA than for NA in vitro [58, 95], and DAT is scarcely expressed in the mPFC [21, 107], while NET and SERT are well represented [77, 103]. Even though NET expressing fibres are generally separated from dopa-minergic neurons and they never contact the same dendritic structures [78], DA and NA can interact through extra-synaptic process, being diffused by volume transmission [81, 132]. As a consequence of these observations, it is likely that in the cerebral cortex, a consistent fraction of extracellular DA is recaptured by NET into noradrenergic terminals. Thus, it has been postulated that in the mPFC, parallel increases of DA and NA are due to competition for the same transporter; accordingly in some circumstances, DA increase might be the passive consequence of increased extracellular NA [15, 80, 93, 127]. However, data from our laboratory, as well as from other groups, indicate that DA, in addition to being recaptured, is also released with NA from noradrenergic terminals, being not only a precursor but also a co-transmitter of NA in the cerebral cortex.
EXTRACELLULAR CATECHOLAMINE CONCENTRATIONS
Our studies are conducted by means of cerebral microdialysis in freely moving animals. This technique provides for an implant in specific cerebral areas of probes constructed using semi-permeable membrane, through which an artificial cerebral spinal fluid is constantly circulated, allowing exchange of substances with extracellular fluid along concentration gradients. This method facilitates the collection of neurotransmitters, or the administration of drugs, over a well circumscribed area of the brain.
In cerebral areas such as the parietal and occipital cortex and the cerebellum, we observed that extracellular DA levels are higher than expected from their scarce or absent dopaminergic innervation, and are fairly similar to the level found in the prefrontal cortex, which receives a dense dopaminergic innervation [24]. We confirmed our early data, obtained by transversal microdialysis, by means of experiments performed by vertical probes [31, 32]. However, others [118] have reported DA levels in occipital and parietal cortex lower than those they found in the prefrontal cortex. Because the main difference between these experiments is in the anaesthetic used, perhaps the anaesthesia with ketamine [118] or Equithesin (our conditions) could differentially affect basal DA levels in the cortex, even hours after administration. Indeed, it has been demonstrated that ketamine anaesthesia causes a significant decrease in cortical extracellular level of glutamate and other excitatory aminoacids, while Equithesin has no significant effect [101].
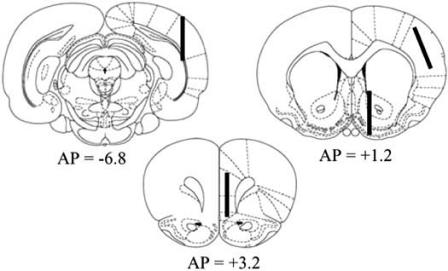
Table 1 shows extracellular concentrations of NA, DA and its main metabolite DOPAC, determined in the same conditions in the mPFC, parietal and occipital cortex and in the nucleus accumbens, which receives a preponderant dopaminergic innervation. Correspondent probe positions are schematically depicted in (Fig. (1)).
Table 1.
Extracellular NA, DA and DOPAC Concentrations in Different Rat Brain Areas
| Cerebral area | NA | DA | DOPAC |
|---|---|---|---|
| Medial prefrontal cortex (163) | 3.1 ± 0.2 | 2.8 ± 0.2 | 188.2 ± 11.1 |
| Parietal cortex (64) | 3.0 ± 0.2 | 2.3 ± 0.1 | 122.7 ± 17.7 |
| Occipital cortex (11) | 3.8 ± 0.5 | 2.6 ± 0.3 | 12.9 ± 1.7 |
| Nucleus Accumbens (19) | 9.0 ± 1.0 | 59.1 ± 10.4 | 2494.2 ± 221.5 |
Values are the means ± SEM of data obtained in the number of rats indicated in parenthesis, and are expressed as pg/sample injected on column.
Fig. (1).
Schematic representation of probe positioning in the mPFC (A=+3.0), the parietal cortex and the nucleus accumbens (A=+1.2), and the occipital cortex (A=−6.8), according to [89].
Consistent with the homogeneous noradrenergic innervation, NA levels are very similar in the three cerebral cortices but, quite surprisingly, no significant difference exists among cortical DA values. On the other hand, DOPAC concentrations decrease from mPFC to occipital cortex, each concentration differing significantly from the others.
Extracellular DOPAC concentration mostly originates from intracellular catabolism of newly synthesised DA, thus it could be considered as an index of dopaminergic neuronal activity [110, 130]. In this regard, it should be underlined how the highest DOPAC value was found in the prevalently dopaminergic nucleus accumbens, where DA level is more than 20 times higher than in the cortices, according to a concentration gradient strictly resembling the intensity of dopaminergic innervation. Even though DOPAC must be taken into account just as an indirect measure of dopaminergic activity, the extracellular concentration of DOPAC, rather than DA, seems to reflect the presence of dopaminergic innervation.
Since dopaminergic innervation of the cerebral cortex is maximal in the mPFC and minimal or absent in the parietal and occipital cortex [23, 69], the equal and relatively high levels of extracellular DA in these cortical areas are intriguing and suggest that DA might originate from non dopaminergic neurons. Indeed, this DA is neuronal in origin, as its release is increased following membrane depolarisation by local perfusion of high concentration of K+, and decreased by Na-channel blocking, by means of tetrodotoxin local perfusion [24, 30, 31].
It could be hypothesised that DA found in scarcely innervated cortical areas originates from areas where DA neurons are present and diffuse throughout the cortex. Indeed, DAT expression is very scarce in the cerebral cortex, and DA diffusion is strictly correlated to DAT activity [107]. Thus, the relatively high extracellular DA levels could be due to its diffusion, secondary to low DAT presence. However, even though DA volume transmission is likely to occur [132], long-distance diffusion of DA should be limited by NA transport system which, as already stated (see above), actively re-uptakes DA and is well represented throughout the cerebral cortex.
NORADRENERGIC DRUGS
Being the precursor of NA, DA is synthesised by tyrosine hydroxylase not only in dopaminergic but also in noradrenergic neurons, in whose synaptic vesicles it is converted into NA by dopamine-β-hydroxylase. Kinetic studies suggest that the rate of NA production could be much slower than the uptake of DA into vesicles [5]. Thus, we hypothesise that DA is not totally turned into NA, and is coreleased with NA from noradrenergic terminals. If this hypothesis is true, then drugs affecting noradrenergic function must also influence extracellular DA levels in the cerebral cortex.
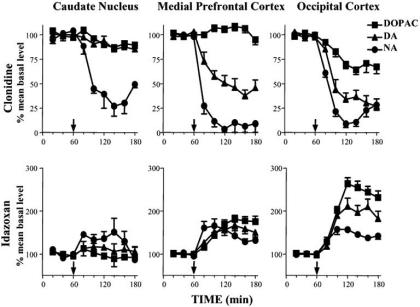
The α2-agonist clonidine, through activation of auto-receptors, inhibits noradrenergic neuronal activity and NA release [3]. Clonidine produces a dramatic decrease in extracellular levels not only of NA but also of DA throughout the cerebral cortex, after systemic administration [24, 27] (Fig. (2)) and after local perfusion into terminal areas [24] or into the locus coeruleus [26, 62], from which cortical noradrenergic innervation originates. Furthermore, after local inhibition of uptake by desipramine perfusion, clonidine administered into the locus coeruleus maintains its ability to reduce cortical DA: thus, DA decrease is not the consequence of augmented re-uptake, but of reduced release [26]. Clonidine perfusion into the locus coeruleus does not affect extracellular DA in the ventral striatum, decreasing only NA concentration in this area [26]. This observation is consistent with the prominent dopaminergic innervation of the ventral striatum and argues against a tonic stimulation of VTA dopaminergic cells by NA. Locus coeruleus noradrenergic neurons project on dopaminergic perikaria, where through postsynaptic α1-adrenoceptors, NA might influence the firing rate of dopaminergic neurons [6, 48] leading to an increased response to other excitatory afferents [3, 4]. It has been shown that clonidine, systemically administered, elicits a regularising action on VTA dopaminergic neurons, while α2-antagonists produce deregularisation and increase of firing [49], thus generating a decrease and an increase, respectively, of DA release in terminal areas [46]. Midbrain dopaminergic neurons do not express α2-adrenoceptor mRNA [104], thus these drugs should act through α2-adrenoceptor located on non-dopaminergic neurons in the midbrain [52, 104, 117].
Fig. (2).
Effect of systemic administration of clonidine (0.15 mg/kg i.p., upper panel) or idazoxan (15 mg/kg i.p., lower panel) on extracellular levels of NA, DA and DOPAC in the caudate nucleus, mPFC and occipital cortex. Arrows indicate time point of drug administration.
Indeed, noradrenergic effects on DA neuron electrical activity have been hypothesised to be mediated by an indirect effect requiring catecholamine release [49, 88].
There is no unequivocal in vivo evidence for the existence of a tonically active noradrenergic input to midbrain DA neurons [49], and recently, in vitro evidence of a direct inhibition, rather than excitation, of the activity of dopaminergic cells by NA has been provided [87].
Alpha2-adrenergic antagonists, such as idazoxan and RS 79948, increase not only NA, but also DA and DOPAC, after systemic administration or local perfusion into medial prefrontal, parietal and occipital cortex [24, 28, 62] (Fig. 2), while in the caudate and accumbens nuclei only NA, and not DA, is affected by idazoxan [57, 112] (Fig (2)). Moreover, perfusion of the ventral tegmental area with tetrodotoxin, which reduces cortical output of DA, does not prevent idazoxan ability to enhance DA efflux in the PFC [57], and idazoxan is still active in increasing cortical DA level in rats lesioned with 6-hydroxy dopamine (6-OHDA) into the medial forebrain bundle [112]. On the other hand, when perfused into the VTA, idazoxan fails to increase cortical DA level, thus arguing against a tonic inhibition of dopaminergic cells by NA in vivo [57]. These findings indicate that α2-adrenergic effects on cortical DA efflux are independent from neuronal activity of VTA dopaminergic cells.
Changes in extracellular DA produced by α2-adrenoceptor ligands might be mediated by α2-heteroreceptors located on dopaminergic terminals [59, 129]. However, several considerations argue against this interpretation:
α2-adrenoceptor agonists and antagonists affect DA efflux not only in the mPFC but also, and to a greater extent in the occipital cortex, where DA terminals are scarce [24, 28, 29, 118] (Fig. (2)).
α2-agonists and antagonists have no or only a scarce effect on DA levels in two brain regions with dense dopaminergic innervation, the nucleus accumbens and the caudate nucleus, in spite of the presence of α2-adrenoceptors [57, 112] (Fig. (2)).
The effect of α2-adrenoceptor antagonists on DA release in the mPFC is suppressed by lesion of the dorsal noradrenergic bundle [118] and not of the medial forebrain bundle [112].
No α2-adrenoceptor mRNA has been detected in midbrain dopaminergic neurons [104].
STIMULATION OF LOCUS COERULEUS
Noradrenergic neuronal activity can be modulated by perfusion of locus coeruleus with specific drugs, as described previously for clonidine, the inhibitory action of which is manifested by a profound decrease in cortical levels of both NA and DA [26, 62]. Stimulation of locus coeruleus neuronal activity by local perfusion with the glutamate agonists NMDA and kainate, or the muscarinic receptor agonist carbachol, or the GABA antagonist bicuculline, elicits parallel increases of both NA and DA in the PFC, while in the nucleus accumbens, extracellular DA remains unchanged [62]. Furthermore, systemic administration of α1-adrenoceptor antagonist prazosin does not modify the effect of carbachol stimulation, thus excluding an indirect effect mediated by tonic NA activity on VTA [62].
Electrical stimulation of the locus coeruleus enhances NA release in the PFC in a frequency-dependent manner [38]. By simultaneous monitoring of NA and DA, we demonstrated that burst stimuli administration into the locus coeruleus increases both catecholamine levels in the mPFC, occipital and parietal cortex, but only NA in the caudate nucleus. These increases are frequency-dependent, are abolished by TTX local perfusion and occur both in freely moving and anaesthetised rats [30, 31]. These findings indicate that in the cerebral cortex, extracellular DA is correlated with noradrenergic neuronal activity.
However, the co-release hypothesis does not exclude that in the mPFC, DA output also originates from dopaminergic neurons. Indeed, cortical DA release can be modulated by intra-VTA drug infusion, and direct stimulation of the VTA by means of infusion of the muscarinic cholinergic agonist oxotremorine elicits an increase in extracellular DA, but not NA, in the PFC [51, 122]. Moreover, selective activation of ascending dopaminergic neurons increases the voltammetric signal identified as DA by fast-scan cyclic voltammetry in the mPFC [43].
ANTIPSYCHOTICS
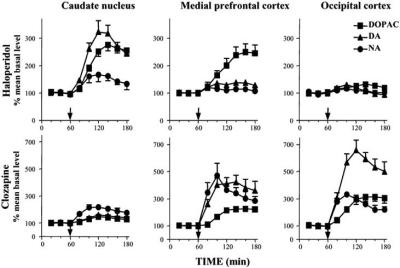
Antipsychotic drugs are known to stimulate dopaminergic neuron activity, through blockade of both pre- and postsynaptic D2 receptors [94]. Thus, haloperidol, classical neuroleptic endowed with D2 receptor antagonist selectivity, is highly effective in increasing nigro-striatal dopaminergic neuronal activity and striatal DA and DOPAC levels [60, 94]. However, despite its efficacy in increasing the activity of antidromically identified meso-cortical DA cells [44], haloperidol produces no effect [24, 29, 44, 55, 63, 90, 127] or only scarce modifications [28, 79, 122] on extracellular DA concentration in the PFC, where it is active in increasing DOPAC concentration only, while in the occipital cortex, neither NA and DA nor DOPAC levels are affected (Fig. (3)), [28]. Anyway, this difference could be ascribed to differences in autoreceptor functioning between mesocortical and mesostriatal DA neurons [9, 10, 20, 40].
Fig. (3).
Effect of systemic administration of haloperidol (0.1 mg/kg i.p., upper panel) or clozapine (10 mg/kg i.p., lower panel) on extracellular levels of NA, DA and DOPAC in the caudate nucleus, mPFC and occipital cortex. Arrows indicate time point of drug administration.
On the other hand, the new generation antipsychotics, which possess antagonistic activity towards different classes of receptors, affect cortical DA to a greater degree than subcortical levels, this property being claimed to subserve their superior efficacy against negative symptoms of schizophrenia [63, 68, 79, 84, 90, 120, 124]. Atypical antipsychotics are also effective in increasing NA level, in line with their affinity for α-adrenergic receptors [24, 27, 66, 91, 123].
Clozapine, the prototype for atypical antipsychotics, has been the object of extensive investigation. We demonstrated that clozapine augments extracellular NA and DA concentrations not only in the mPFC, which receives consistent dopaminergic innervation, but also, and to a greater extent in the prevalently noradrenergic occipital cortex [27] (Fig. (3)). Moreover, both NA and DA increases are completely antagonised by clonidine, while they are not affected by the selective D2 receptor agonist quinpirole [27]. Clozapine interaction with noradrenergic system has been clearly demonstrated. In fact, it has considerable affinity for a2-adrenoceptors [14], it increases the firing rate of NA neurons in the locus coeruleus [96] and prevents d-amphetamineinduced suppression of the firing of NA neurons [111]. Nevertheless, clonidine might reverse the effect of clozapine on DA and NA release by an indirect mechanism, or by physiological antagonism. Moreover, due to clozapine interaction with a wide range of receptors [75], induction of NA and DA co-release through different receptor modulation cannot be excluded.
MORPHINE EFFECTS
Morphine elicits opposite actions on VTA dopaminergic- and locus coeruleus noradrenergic-cell activities. Indeed, acute morphine administration inhibits locus coeruleus NA neurons [2] and activates dopaminergic neurons in the VTA [74]. On the contrary, abstinence from chronic morphine is associated with activation of noradrenergic cells [2] and inhibition of VTA dopaminergic neurons [33]. Consistently, extracellular NA levels are decreased by acute morphine and increased by its withdrawal [100, 109], while DA is increased and decreased, respectively, in the nucleus accumbens, one of the projection areas of VTA DA neurons [1, 92]. Quite surprisingly, in the mPFC, which also receives VTA DA projections, extracellular DA levels are not modified by acute morphine administration, and are markedly increased during withdrawal, in spite of the inhibition of VTA DA neuronal activity [12, 25]. We explain this apparent contradiction with the co-release hypothesis [25], basing our conclusion on the observation that in the parietal cortex, which is not innervated by DA, acute morphine administration elicits a parallel decrease in both NA and DA levels, and that when morphine is coadministered with quinpirole to inhibit VTA DA neuronal activity, a DA decrease is evidenced also in the mPFC. Furthermore, the dramatic increase in NA and DA levels elicited in the mPFC of morphine-dependent rats by naloxone, is completely reverted by clonidine, but not quinpirole administration. Again, these findings indicate that in the cerebral cortex, extracellular DA variations better correlate with noradrenergic than with dopaminergic neuronal activity. The apparent lack of acute morphine effect on mPFC DA might be due to the algebraic sum of increase in DA originating from dopaminergic cells and decrease in DA co-released with NA. Alternatively, it might be due to differences in regulation between meso-cortical and mesolimbic DA neurons. Indeed, mesocortical DA system exhibits different functional characteristics with respect to other DA systems [17, 19, 42]. As for the above-mentioned lack of haloperidol effect, differences in autoreceptor [9, 10, 20, 40] or in glutamatergic afferent input [16], might originate different responses to the same stimulus.
VTA LESION
Destruction of striatal dopaminergic innervation by means of 6-OH-DA injection into the substantia nigra/ventral tegmental area has been extensively investigated, being a useful model of Parkinson’s disease. DA system has been demonstrated to react to the lesion with numerous compensatory neuroadaptations [131]. It is well known that in the nigro-striatal system, at least 80% depletion of tissue DA is required to observe a small reduction in extracellular DA, while after more than 95% reduction of tissue DA content, extracellular DA also falls below 10% of control values. Unlike DA, decreases of extracellular DA metabolites are highly correlated with lesion extent and residual tissue content [18, 99].
The comparison of tissue and extracellular DA and DOPAC in the nucleus accumbens and in two cortical areas, the mPFC and the parietal cortex, after monolateral 6-OHDA injection into the VTA [32] may be useful to better elucidate DA origin in the cerebral cortex. To the best of our knowledge, no other similar studies on meso-cortical dopaminergic system have been published.
Table 2 shows that the lesion produces a clear-cut reduction in tissue DA and DOPAC content in all cerebral areas tested, but in the nucleus accumbens, the decrease is more pronounced than in the cortical areas (95% or more versus 50-70%).
Table 2.
Tissue Content of DA and DOPAC in Intact and 6-OHDA Lesioned Cerebral Areas
| Cerebral Area | DA | DOPAC | ||
|---|---|---|---|---|
| Intact | Lesioned | Intact | Lesioned | |
| Medial prefrontal cortex | 62.1 ± 3.7 | 17.7 ± 2.0 28% |
38.2 ± 4.0 | 20.4 ± 4.0 53% |
| Parietal cortex | 15.5 ± 1.5 | 5.9 ± 0.4 38% |
21.6 ± 2.8 | 12.8 ± 2.4 59% |
| Nucleus accumbens | 5387.2 ± 298.2 | 173.5 ± 27.5 3% |
1652.7 ±120.6 | 95.8 ± 11.3 5% |
Values are expressed as pg/ mg tissue wet weight, and are the mean ± SEM of 50 rats. Rats received monolateral 6-OHDA infusion into the VTA, and intact (controlateral to the lesion) vs. lesioned (ipsilateral to the lesion) side are compared.
Following extensive 6-OHDA lesion of VTA, extracellular DA values are only slightly and not significantly decreased in the mPFC and parietal cortex, but are dramatically reduced in the nucleus accumbens, while extracellular DOPAC decrease corresponds to respective tissue content diminution in all cerebral areas (Table 3).
Table 3.
Extracellular Values of DA and DOPAC in Intact and 6-OHDA Lesioned Cerebral Areas
| Cerebral Area | DA | DOPAC | ||
|---|---|---|---|---|
| Intact | Lesioned | Intact | Lesioned | |
| Medial prefrontal cortex (20) | 2.4 ± 0.3 | 1.9 ± 0.2 80% |
211.8 ± 48.6 | 23.9 ± 3.7 11% |
| Parietal cortex (11) | 1.9 ± 0.3 | 1.6 ± 0.2 84% |
158.6 ± 37.8 | 19.0 ± 3.7 11% |
| Nucleus accumbens (19) | 42.5 ± 7.4 | 3.5 ± 0.6 8% |
2636 ± 371 | 57.3 ± 13.8 2% |
Values are expressed as pg/sample and are the mean ± SEM of the number of rats indicated in parenthesis. Following monolateral 6-OHDA infusion into the VTA, rats were implanted with two probes, one for each cerebral hemisphere, and intact (controlateral to the lesion) vs. lesioned (ipsilateral to the lesion) side values are compared.
Thus, even though dopaminergic innervation of both mPFC and nucleus accumbens originates from the VTA, it appears that cortical areas are less severely affected by 6-OHDA-induced denervation. The relatively high cortical levels remaining in the lesioned side might be due to the presence of DA in noradrenergic neurons. Alternatively, it should be hypothesised that cortical DA originates in part from dopaminergic neurons not arising from VTA, or that meso-cortical dopaminergic neurons may be less sensitive than meso-limbic ones to the effect of 6-OHDA. Even though dopaminergic innervation partly arising from the mediolateral substantia nigra is present in the supragenual cingulate cortex [69], this cortical area has not been included in our study. Indeed, the great majority of dopaminergic terminals in the infralimbic-prelimbic mPFC originate from the VTA [113].
6-OHDA exerts its toxic action after being taken up into neurons by catecholamine transport systems [102], thus neurons devoid of catecholamine re-uptake sites should be protected against lesion. Dopaminergic terminals in the mPFC are known to express fewer DAT with respect to nucleus accumbens innervation [21, 107], however, it is not clear whether this difference is also present in their cellular bodies [108]. If this is the case, then they should be relatively resistant to 6-OH-DA effect.
To date, no experimental evidence has been provided to support either of the aforementioned alternative hypotheses. The only certainty is the presence of DA in noradrenergic neurons innervating the cerebral cortex. Thus, we propose that extensive VTA lesion does not affect extracellular DA in the mPFC and parietal cortex due to being originated prevalently from noradrenergic neurons.
On the other hand, noradrenergic neuron lesion does not alter basal dialysate DA in the mPFC [15, 93, 118, 127] but affects its response to pharmacological challenges, underscoring an increase in DA following previously ineffective dopaminergic drugs, such as haloperidol [127] or the selective DAT inhibitor GBR 12909 [93], and preventing the increase in DA elicited by clozapine [118]. These results could be attributed to a decreased DA clearance by NA transporter, diminished by the lesion, but they could be also due to the compensatory proliferation of dopaminergic terminals that follows noradrenergic degeneration, as suggested by enhanced dopaminergic mechanisms [53, 54, 70, 116]. In this context, the lack of effect of clozapine on DA, in the mPFC and parietal cortex of NA denervated rats, is particularly indicative [118], suggesting that the increase of DA elicited by clozapine in the cerebral cortex is totally due to clozapine effect on noradrenergic system.
CONCLUSION
The evidence presented in this paper converge towards the possibility that in the cerebral cortex, most of DA originates from noradrenergic neurons, from which DA is co-released together with NA.
In the mPFC, DA acts as a neuromodulator [65, 105], and dopaminergic system is responsible for the fine tuning of the neurons it impinges upon [34, 41, 86]. An optimal dopaminergic tone is required to obtain an appropriate reaction to stimuli, or to cope with challenging situations. DA neuro-modulatory role is particularly relevant for the cognitive control exerted by mPFC. In fact, dopaminergic imbalance in the mPFC has been implicated in virtually all psychopathologies, and mainly in schizophrenia related syndromes. A similar regulatory role has also been attributed to NA, particularly in relation with superior cognitive activities and connected psychopathologies [7, 8, 39, 56, 85, 128]. According to our hypothesis, NA and DA neuromodulation in the cerebral cortex is at least in part supported by a common neuronal origin. In fact, even though dopaminergic cortical targets display a well circumscribed localisation in specific areas, namely the mPFC, anterior cingulate, rhinal and entorhinal cortices [23, 69, 106], noradrenergic innervation is uniformly distributed, supplying a dopaminergic tone throughout the entire cerebral cortex and overlapping with proper dopaminergic innervation. This noradrenergic pool of DA might be massively mobilised during conditions of stress (such as morphine withdrawal), and might be utilised by atypical antipsychotics or antidepressants to buffer insufficient dopaminergic tone in the mPFC. Also in case of loss of dopaminergic neurons (such as in Parkinson’s disease), the higher cortical functions might be preserved, thanks to DA co-released with NA. This mechanism may be added to the well known compensations that intervene following dopaminergic neuron loss, such as vigorous sprouting and increase of tyrosine hydroxylase activity in remaining axons, and up-regulation of postsynaptic DA receptors.
In other words, prefrontal cortical function plays such a key role in the organism homeostasis that its neuromodulation is committed to a double control. The noradrenergic system might be involved in DA support in the case of insufficient input, the neuro-modulatory action exerted by DA being reinforced by NA in DA innervated cortical areas, such as the mPFC. At the same time, heterologous uptake of DA by NET might contribute to buffer excessive spread of released DA.
This hypothesis might be related to the actions of drugs endowed with noradrenergic activity, such as atomoxetine, guanfacine or atipamezole [8, 11, 64, 72], as a therapeutic tool to modulate cognitive functions [39].
ACKNOWLEDGEMENTS
The authors wish to convey their sincere thanks and gratitude to Prof G.L. Gessa for his inestimable guidance throughout many fruitful years of study.
REFERENCES
- 1.Acquas E, Carboni E, Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol. 1991;193:133–134. doi: 10.1016/0014-2999(91)90214-b. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK. Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine. Nature. 1978;267:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian GK. In: Catecholamines: neuropharmacology and central nervous system - theoretical aspects. Usdin E, Carlsson A, Dahlstrom A, Engel J, editors. New York: Alan R. Liss; 1984. pp. 85–92. [Google Scholar]
- 4.Aghajanian GK. Modulation of a transient outward current in serotonergic neurons by α1-adrenoceptors. Nature. 1985;315:501–513. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- 5.Ahn NG, Klinman JP. Nature of rate-limiting steps in a compartmentalized enzyme system. Quantitation of dopamine transport and hydroxylation rates in resealed chromaffin granule ghosts. J Biol Chem. 1989;264:12259–12265. [PubMed] [Google Scholar]
- 6.Andén NE, Grabowska M. Pharmacological evidence for a stimulation of dopamine neurons by noradrenaline neurons in the brain. Eur J Pharmacol. 1976;39:275–282. doi: 10.1016/0014-2999(76)90136-9. [DOI] [PubMed] [Google Scholar]
- 7.Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharm. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten AFT. Adrenergic targets for the treatment of cognitive deficit in schizophrenia. Psychopharmacology. 2004;174:25–31. doi: 10.1007/s00213-003-1724-3. [DOI] [PubMed] [Google Scholar]
- 9.Bannon MJ, Michaud RL, Roth RH. Mesocortical dopamine neurons. Lack of autoreceptors modulating dopamine synthesis. Mol Pharmacol. 1981;19:270–275. [PubMed] [Google Scholar]
- 10.Bannon MJ, Reinhard JF, Jr., Bunney EB, Roth RH. Unique response to antipsychotic drugs is due to absence of terminal autoreceptors in mesocortical dopamine neurones. Nature. 1982;296:444–446. doi: 10.1038/296444a0. [DOI] [PubMed] [Google Scholar]
- 11.Barton J. Atomoxetine: a new pharmacotherapeutic approach in the management of attention deficit/hyperactivity disorder. Arch Dis Child. 2005;1:i26–i29. doi: 10.1136/adc.2004.059386. suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G. Non-psychostimulants drugs of abuse activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology. 1996;124:293–299. doi: 10.1007/BF02247433. [DOI] [PubMed] [Google Scholar]
- 13.Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL. The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles. Brain Res Rev. 2005;48:57–73. doi: 10.1016/j.brainresrev.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- 15.Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- 16.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cass WA, Gerhardt GA. In Vivo Assessment of Dopamine Uptake in Rat Medial Prefrontal Cortex: Comparison with Dorsal Striatum and Nucleus Accumbens. J Neurochem. 1995;65:201–207. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- 18.Castaneda E, Whishaw IQ, Robinson TE. Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci. 1990;10:1847–1854. doi: 10.1523/JNEUROSCI.10-06-01847.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cenci MA, Kalen P, Mandel RJ, Bjorklund A. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. doi: 10.1016/0006-8993(92)90711-h. [DOI] [PubMed] [Google Scholar]
- 20.Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- 21.Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- 24.Devoto P, Flore G, Pani L, Gessa GL. Evidence for corelease of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- 25.Devoto P, Flore G, Pira L, Diana M, Gessa GL. Corelease of noradrenaline and dopamine in the prefrontal cortex after acute morphine and during morphine withdrawal. Psychopharmacology. 2002;160:220–224. doi: 10.1007/s00213-001-0985-y. [DOI] [PubMed] [Google Scholar]
- 26.Devoto P, Flore G, Longu G, Pira L, Gessa GL. Origin of extracellular dopamine from dopamine and noradrenaline neurons in the medial prefrontal and occipital cortex. Synapse. 2003;50:200–205. doi: 10.1002/syn.10264. [DOI] [PubMed] [Google Scholar]
- 27.Devoto P, Flore G, Vacca G, Pira L, Arca A, Casu MA, Pani L, Gessa GL. Co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex induced by clozapine, the prototype atypical antipsychotic. Psychopharmacol. 2003;167:79–84. doi: 10.1007/s00213-002-1381-y. [DOI] [PubMed] [Google Scholar]
- 28.Devoto P, Flore G, Longu G, Pira L, Gessa GL. Alpha2-adrenoceptor mediated co-release of dopamine and noradrenaline from noradrenergic neurons in the cerebral cortex. J Neurochem. 2004;88:1003–1009. doi: 10.1046/j.1471-4159.2003.02239.x. [DOI] [PubMed] [Google Scholar]
- 29.Devoto P, Flore G, Pira L, Longu G, Gessa GL. Mirtazapine-induced co-release of dopamine and noradrenaline from noradrenergic neurons in the medial prefrontal and occipital cortex. Eur J Pharmacol. 2004;487:105–111. doi: 10.1016/j.ejphar.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Devoto P, Flore G, Saba P, Fà M, Gessa GL. Corelease of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neuroscience. 2005;6 doi: 10.1186/1471-2202-6-31. http://www.biomedcentral.com/1471-2202/6/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devoto P, Flore G, Saba P, Fà M, Gessa GL. Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J Neurochem. 2005;92:368–374. doi: 10.1111/j.1471-4159.2004.02866.x. [DOI] [PubMed] [Google Scholar]
- 32.Devoto P, Flore G, Saba P, Gessa GL. Co-release of dopamine and noradrenaline in the rat cerebral cortex: evidence from 6-hydroxy-dopamine VTA lesion. Washington DC: Society for Neuroscience, Abstract Viewer/Itinerary Planner; 2005. Program Nº 605.3. [Google Scholar]
- 33.Diana M, Pistis M, Muntoni AL, Gessa GL. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharm Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- 34.Dong Y, Cooper D, Nasif F, Hu XT, White FJ. Dopamine modulates inwardly rectifying potassium currents in medial prefrontal cortex pyramidal neurons. J Neurosci. 2004;24:3077–3085. doi: 10.1523/JNEUROSCI.4715-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fadda F, Gessa GL, Marcou M, Mosca E, Rossetti Z. Evidence for dopamine autoreceptors in mesocortical dopamine neurons. Brain Res. 1984;293:67–72. doi: 10.1016/0006-8993(84)91453-7. [DOI] [PubMed] [Google Scholar]
- 36.Feenstra MGP. Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Progr Brain Res. 2000;126:133–163. doi: 10.1016/S0079-6123(00)26012-3. [DOI] [PubMed] [Google Scholar]
- 37.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 38.Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in the prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- 39.Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer’s disease. Biol Psychiatry. 1999;46:1243–1252. doi: 10.1016/s0006-3223(99)00232-2. [DOI] [PubMed] [Google Scholar]
- 40.Galloway MP, Wolf ME, Roth RH. Regulation of dopamine synthesis in the medial prefrontal cortex is mediated by release modulating autoreceptors: studies in vivo. J Pharmacol Exp Ther. 1986;236:689–698. [PubMed] [Google Scholar]
- 41.Gao WJ, Wang Y, Goldman-Rakic P. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner EL, Ashby CR., Jr. Heterogeneity of the mesotelencephalic dopamine fibers: physiology and pharmacology. Neurosci Biobehav Rev. 2000;24:115–118. doi: 10.1016/s0149-7634(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 43.Garris PA, Collins LB, Jones SR, Wightman RM. Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem. 1993;61:637–647. doi: 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- 44.Gessa GL, Devoto P, Diana M, Flore G, Melis M, Pistis M. Dissociation of haloperidol, clozapine and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacol. 2000;22:642–649. doi: 10.1016/S0893-133X(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 45.Goldsmith SK, Joyce JN. Dopamine D2 receptors in hippocampus and parahippocampal cortex of rat, cat and human in relation to tyrosine hydroxylase-immunoreactive fibers. Hippocampus. 1994;4:354–373. doi: 10.1002/hipo.450040318. [DOI] [PubMed] [Google Scholar]
- 46.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 47.Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- 49.Grenhoff J, Svensson TH. Clonidine modulates dopamine cell firing in the ventral tegmental area. Eur J Pharmacol. 1989;165:11–18. doi: 10.1016/0014-2999(89)90765-6. [DOI] [PubMed] [Google Scholar]
- 50.Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem. 1995;65:111–116. doi: 10.1046/j.1471-4159.1995.65010111.x. [DOI] [PubMed] [Google Scholar]
- 51.Gronier B, Perry KW, Rasmussen K. Activation of the mesocorticolimbic dopaminergic system by stimulation of muscarinic cholinergic receptors in the ventral tegmental area. Psychopharmacol. 2000;147:347–355. doi: 10.1007/s002130050002. [DOI] [PubMed] [Google Scholar]
- 52.Happe HK, Coulter CL, Gerety ME, Sanders JD, O’Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Harik SI, Duckrow RB, LaManna JC, Rosenthal M, Sharma VK, Banerjee SP. Cerebral compensation for chronic noradrenergic denervation induced by locus coeruleus lesion: recovery of receptor binding, isoproterenol-induced adenylate cyclase activity, and oxidative metabolism. J Neurosci. 1981;1:641–649. doi: 10.1523/JNEUROSCI.01-06-00641.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harik SI. Locus coeruleus lesion by local 6-hydroxydopamine infusion causes marked and specific destruction of noradrenergic neurons, long-term depletion of norepinephrine and the enzymes that synthesize it, and enhanced dopaminergic mechanisms in the ipsilateral cerebral cortex. J Neurosci. 1984;4:699–707. doi: 10.1523/JNEUROSCI.04-03-00699.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heidbreder CA, Foxton R, Cilia J, Hughes ZA, Shah AJ, Atkins A, Hunter AJ, Hagan JJ, Jones DNC. Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacology. 2001;156:338–351. doi: 10.1007/s002130100760. [DOI] [PubMed] [Google Scholar]
- 56.Herrmann N, Lanctot KL, Khan LR. The role of norepinephrine in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci. 2004;16:261–276. doi: 10.1176/jnp.16.3.261. [DOI] [PubMed] [Google Scholar]
- 57.Hertel P, Nomikos GG, Svensson TH. Idazoxan preferentially increases dopamine output in the rat medial prefrontal cortex at the nerve terminal level. Eur J Pharmacol. 1999;371:153–158. doi: 10.1016/s0014-2999(99)00175-2. [DOI] [PubMed] [Google Scholar]
- 58.Horn AS. Structure-activity relations for the inhibition of catecholamine uptake into synaptosomes from noradrenergic and dopaminergic neurons in rat brain homogenates. Br J Pharmacol. 1973;47:332–338. doi: 10.1111/j.1476-5381.1973.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ihalainen JA, Tanila H. In vivo regulation of dopamine and noradrenaline release by α2A-adrenoceptors in the mouse prefrontal cortex. Eur J Neurosci. 2002;15:1789–1794. doi: 10.1046/j.1460-9568.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 60.Imperato A, Di Chiara G. Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J Neurosci. 1985;5:297–306. doi: 10.1523/JNEUROSCI.05-02-00297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawahara Y, Kawahara H, Westerink BHC. Comparison of the effects of hypotension and handling stress on the release of noradrenaline and dopamine in the locus coeruleus and medial prefrontal cortex of the rat. Naunyn-Schmiedeberg’s Arch Pharmacol. 1999;360:42–49. doi: 10.1007/s002109900042. [DOI] [PubMed] [Google Scholar]
- 62.Kawahara H, Kawahara Y, Westerink BHC. The noradrenaline-dopamine interaction in the rat medial prefrontal cortex studied by multi-probe microdialysis. Eur J Pharmacol. 2001;418:177–186. doi: 10.1016/s0014-2999(01)00863-9. [DOI] [PubMed] [Google Scholar]
- 63.Kuroki T, Meltzer HY, Ichikawa J. Effect of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharm Exp Ther. 1999;288:774–781. [PubMed] [Google Scholar]
- 64.Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 65.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 66.Li XM, Perry KW, Wong DT, Bymaster FP. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacol. 1998;136:153–161. doi: 10.1007/s002130050551. [DOI] [PubMed] [Google Scholar]
- 67.Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. Dopamine D2 receptors in the cerebral cortex: distribution and pharmacological characterization with [3H]raclopride. Proc Nat Acad Sci USA. 1989;86:6412–6416. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lidow MS, Williams GV, Goldman-Rakic PS. The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci. 1998;19:136–140. doi: 10.1016/s0165-6147(98)01186-9. [DOI] [PubMed] [Google Scholar]
- 69.Lindvall O, Björklund A, Divac I. Organization of the catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- 70.Logan LJ, Harik SI. Specific uptake of norepinephrine and dopamine by homogenates of rat cerebral cortex after locus coeruleus lesion. J Neurosci. 1982;2:394–398. doi: 10.1523/JNEUROSCI.02-03-00394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martres MP, Bouthenet ML, Sales N, Sokoloff P, Schwartz JC. Widespread distribution of brain dopamine receptors evidenced with [125I]iodosulpiride, a highly selective ligand. Science. 1985;228:752–755. doi: 10.1126/science.3838821. [DOI] [PubMed] [Google Scholar]
- 72.Masi G. Pharmacotherapy of pervasive developmental disorders in children and adolescents. CNS Drugs. 2005;18:1031–1052. doi: 10.2165/00023210-200418140-00006. [DOI] [PubMed] [Google Scholar]
- 73.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Nat Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopaminergic neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- 75.Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55:47–52. [PubMed] [Google Scholar]
- 76.Millan MJ, Gobert A, Rivet JM, Adhumeau-Auclair A, Cussac D, Newman-Tancredi A, Dekeyne A, Nicolas JP, Lejeune F. Mirtazapine enhances frontocortical dopaminergic and adrenergic, but not serotonergic, transmission by blockade of α2-adrenergic and serotonin 2C receptors: a comparison with citalopram. Eur J Neurosci. 2000;12:1079–1095. doi: 10.1046/j.1460-9568.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- 77.Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the serotonin transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to dopamine terminals. J Comp Neurol. 2000;427:220–234. doi: 10.1002/1096-9861(20001113)427:2<220::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 78.Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- 79.Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 80.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM. Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem. 2001;79:130–142. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- 82.Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy EK, Sved AF, Finlay JM. Corticotropin-releasing hormone receptor blockade fails to alter stress-evoked catecholamine release in prefrontal cortex of control or chronically stressed rats. Neuroscience. 2003;116:1081–1087. doi: 10.1016/s0306-4522(02)00565-1. [DOI] [PubMed] [Google Scholar]
- 84.Nomikos GG, Iurlo M, Andersson JL, Kimura K, Svensson TH. Systemic administration of amperozide, a new atypical antipsychotic drug, preferentially increases dopamine release in the rat medial prefrontal cortex. Psychopharmacology. 1994;115:147–156. doi: 10.1007/BF02244765. [DOI] [PubMed] [Google Scholar]
- 85.Oades RD, Sadile GA, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russel VA. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Develop Sci. 2005;8:122–131. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- 86.Onn SP, Wang XB. Differential modulation of anterior cingulate cortical activity by afferents from ventral tegmental area and mediodorsal thalamus. Eur J Neurosci. 2005;21:2975–2992. doi: 10.1111/j.1460-9568.2005.04122.x. [DOI] [PubMed] [Google Scholar]
- 87.Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan WH, Sung JC, Fuh SM. Locally application of amphetamine into the ventral tegmental area enhances dopamine release in the nucleus accumbens and the medial prefrontal cortex through noradrenergic neurotransmission. J Pharmacol Exp Ther. 1996;278:725–731. [PubMed] [Google Scholar]
- 89.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd. San Diego, California: Academic Press; 1997. [Google Scholar]
- 90.Pehek EA, Yamamoto BK. Differential effects of locally administered clozapine and haloperidol on dopamine efflux in the rat prefrontal cortex and caudate-putamen. J Neurochem. 1994;63:2118–2124. doi: 10.1046/j.1471-4159.1994.63062118.x. [DOI] [PubMed] [Google Scholar]
- 91.Pira L, Mongeau R, Pani L. The atypical antipsychotic quetiapine increases both noradrenaline and dopamine release in the rat prefrontal cortex. Eur J Pharmacol. 2004;504:61–64. doi: 10.1016/j.ejphar.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 92.Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- 93.Pozzi L, Invernizzi R, Cervo L, Vallebuona F, Samanin R. Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem. 1994;63:195–200. doi: 10.1046/j.1471-4159.1994.63010195.x. [DOI] [PubMed] [Google Scholar]
- 94.Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther. 1994;271:1181–1192. [PubMed] [Google Scholar]
- 95.Raiteri M, Del Carmine R, Bertollini A, Levi G. Effects of sympaticomimetic amines on the synaptosomal transport of noradrenaline, dopamine,5-hydroxytryptamine. Eur J Pharmacol. 1977;41:133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- 96.Ramirez OA, Wang RY. Locus coeruleus norepinephrine-containing neurons : effects produced by acute and subchronic treatment with antipsychotic drugs and amphetamine. Brain Res. 1986;362:165–170. doi: 10.1016/0006-8993(86)91411-3. [DOI] [PubMed] [Google Scholar]
- 97.Richfield EK, Young AB, Penney JB. Comparative distribution of dopamine D1 and D2 receptors in the cerebral cortex of rats, cats and monkeys. J Comp Neurol. 1989;286:409–426. doi: 10.1002/cne.902860402. [DOI] [PubMed] [Google Scholar]
- 98.Roberts DC, Zis AP, Fibiger HC. Ascending catecholamine pathways and amphetamine-induced locomotor activity: importance of dopamine and apparent non-involvement of norepinephrine. Brain Res. 1975;93:441–454. doi: 10.1016/0006-8993(75)90182-1. [DOI] [PubMed] [Google Scholar]
- 99.Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- 100.Rossetti ZL, Longu G, Mercuro G, Gessa GL. Extraneuronal noradrenaline in the prefrontal cortex of morphine-dependent rats: tolerance and withdrawal mechanisms. Brain Res. 1993;609:316–320. doi: 10.1016/0006-8993(93)90889-u. [DOI] [PubMed] [Google Scholar]
- 101.Rozza A, Masoero E, Favalli L, Lanza E, Govoni S, Rizzo V, Montalbetti L. Influence of different anaesthetics on extracellular aminoacids in rat brain. J Neurosci Methods. 2000;101:165–169. doi: 10.1016/s0165-0270(00)00266-1. [DOI] [PubMed] [Google Scholar]
- 102.Sachs C, Johnsson G. Mechanism of action of 6-hydroxydopamine. Biochem Pharmacol. 1975;24:1–8. doi: 10.1016/0006-2952(75)90304-4. [DOI] [PubMed] [Google Scholar]
- 103.Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr. Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 105.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–57. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 106.Seguela P, Watkins KC, Geffard M, Descarries L. Noradrenaline axon terminals in adult rat neocortex: an immunohistochemical analysis in serial thin sections. Neuroscience. 1990;35:249–264. doi: 10.1016/0306-4522(90)90079-j. [DOI] [PubMed] [Google Scholar]
- 107.Sesak SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2696–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shimada S, Kitayama S, Walther D, Uhl G. Dopamine transporter mRNA: Dense expression in ventral midbrain neurons. Mol Brain Res. 1992;13:359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- 109.Silverstone PH, Done C, Sharp T. Clonidine but not nifedipine prevents the release of noradrenaline during naloxone-precipitated opiate withdrawal: an in vivo microdialysis study in the rat. Psychopharmacology. 1992;109:235–238. doi: 10.1007/BF02245506. [DOI] [PubMed] [Google Scholar]
- 110.Soares da Silva P, Garrett MC. A kinetic study of the rate of formation of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the brain of the rat : implications for the origin of DOPAC. Neuropharmacology. 1990;29:869–874. doi: 10.1016/0028-3908(90)90135-e. [DOI] [PubMed] [Google Scholar]
- 111.Souto M, Monti JM, Altier H. Effects of clozapine on the activity of central dopaminergic and noradrenergic neurons. Pharmacol Biochem Behav. 1979;10:5–9. doi: 10.1016/0091-3057(79)90160-6. [DOI] [PubMed] [Google Scholar]
- 112.Srinivasan J, Schmidt WJ. The effect of the α2-adrenoceptor antagonist idazoxan against 6-hydroxy-dopamineinduced Parkinsonism in rats: multiple facets of action? Naunyn-Schmiedeberg’s Arch Pharmacol. 2004;369:629–638. doi: 10.1007/s00210-004-0929-2. [DOI] [PubMed] [Google Scholar]
- 113.Swanson LW. The projection of the ventral tegmental area and adjacent areas: a combined retrograder tracer and immunofluorescence study of the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 114.Tanda G, Bassareo V, Di Chiara G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacol. 1996;123:127–130. doi: 10.1007/BF02246169. [DOI] [PubMed] [Google Scholar]
- 115.Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 116.Tassin JP, Lavielle S, Hervé D, Blanc G, Thierry AM, Alvarez C, Berger B, Glowinski J. Collateral sprouting and reduced activity of the rat mesocortical dopaminergic neurons after selective destruction of the ascending noradrenergic bundle. Neuroscience. 1979;4:1569–1582. doi: 10.1016/0306-4522(79)90020-4. [DOI] [PubMed] [Google Scholar]
- 117.Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984;319:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- 118.Valentini V, Frau R, Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem. 2004;88:917–927. doi: 10.1046/j.1471-4159.2003.02238.x. [DOI] [PubMed] [Google Scholar]
- 119.Vizi ES, Zsilla G, Caron MG, Kiss JP. Uptake and release of norepinephrine by serotonergic terminals in norepinephrine transporter knock-out mice: implications for the action of selective serotonin reuptake inhibitors. J Neurosci. 2004;24:7888–7894. doi: 10.1523/JNEUROSCI.1506-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Volonte M, Monferini E, Cerutti M, Fodritto F, Borsini F. BIMG 80, a novel potential antipsychotic drug: evidence for multireceptor actions and preferential release of dopamine in prefrontal cortex. J Neurochem. 1997;69:182–190. doi: 10.1046/j.1471-4159.1997.69010182.x. [DOI] [PubMed] [Google Scholar]
- 121.Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A. Cortical localization of dopamine D4 recetros in the rat brain - Immunocytochemical study. J Physiol Pharmacol. 2000;51:205–221. [PubMed] [Google Scholar]
- 122.Westerink BHC, Enrico P, Feimann J, De Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- 123.Westerink BHC, de Boer P, de Vries JB, Kruse CG, Long SK. Antipsychotic drugs induce similar effects on the release of dopamine and noradrenaline in the medial prefrontal cortex of the rat brain. Eur J Pharmacol. 1998;361:27–33. doi: 10.1016/s0014-2999(98)00711-0. [DOI] [PubMed] [Google Scholar]
- 124.Westerink BH, Kawahara Y, De Boer P, Geels C, De Vries JB, Wikstrom HV, Van Kalkeren A, Van Vliet B, Kruse CG, Long SK. Antipsychotic drugs classified by their effects on the release of dopamine and noradrenaline in the prefrontal cortex and striatum. Eur J Pharmacol. 2001;412:127–138. doi: 10.1016/s0014-2999(00)00935-3. [DOI] [PubMed] [Google Scholar]
- 125.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 126.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem. 1998;71:274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- 128.Yamamoto K, Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia. Progr Neuro-Psychopharm Biol Psy. 2004;28:913–922. doi: 10.1016/j.pnpbp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 129.Yavich L, Lappalainen R, Sirviö J, Haapalinna A, MacDonald E. α2-adrenergic control of dopamine overflow and metabolism in mouse striatum. Eur J Pharmacol. 1997;339:113–119. doi: 10.1016/s0014-2999(97)01375-7. [DOI] [PubMed] [Google Scholar]
- 130.Zetterstrom T, Sharp T, Collin AK, Ungerstedt U. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]
- 131.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensation after lesion of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- 132.Zoli M, Jansson A, Sykovà E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]