Abstract
The identification and cloning of the vanilloid receptor 1 (TRPV1) represented a significant step for the understanding of the molecular mechanisms underlying the transduction of noxious chemical and thermal stimuli by peripheral nociceptors. TRPV1 is a non-selective cation channel gated by noxious heat, vanilloids and extracellular protons. TRPV1 channel activity is remarkably potentiated by pro-inflammatory agents, a phenomenon that is thought to underlie the peripheral sensitisation of nociceptors that leads to thermal hyperalgesia. Cumulative evidence is building a strong case for the involvement of this receptor in the etiology of both peripheral and visceral inflammatory pain, such as inflammatory bowel disease, bladder inflammation and cancer pain. The validation of TRPV1 receptor as a key therapeutic target for pain management has thrust intensive drug discovery programs aimed at developing orally active antagonists of the receptor protein. Nonetheless, the real challenge of these drug discovery platforms is to develop antagonists that preserve the physiological activity of TRPV1 receptors while correcting over-active channels. This is a condition to ensure normal pro-prioceptive and nociceptive responses that represent a safety mechanism to prevent tissue injury. Recent and exciting advances in the function, dysfunction and modulation of this receptor will be the focus of this review.
THE TRP RECEPTOR SUPERFAMILY
The Transient Receptor Potential (TRP) mammalian gene superfamily consists of 28 different gene products encoding non-selective cation channels that play a wide diversity of physiological functions [24,79]. These channels are considered molecular gateways in sensory systems, since several of these channels transduce chemical and physical stimuli into neuronal activity, i.e. action potentials. Thus, TRP channels have emerged as major transducers of chemically and physically evoked sensations.
Based upon their sequence homology, mammalian TRP channels are divided into six subfamilies, namely TRPC, TRPV, TRPP, TRPM, TRPA and TRPML [24,79,82]. An additional family, TRPN, has been described in Drosophila. These proteins have a common topology of six transmembrane segments (S1-S6) with a pore region between the fifth and sixth segment, and cytoplasmic N- and C-termini. TRPV and TRPC contain two-to-four ankyrin domains that are thought to interact with the cytoskeleton, as well as with other cytosolic proteins [24]. TRP proteins are remarkable channels because of the diversity of their activation mechanisms, cation selectivity and biological function. Some members are activated through the phospholipase C-inositol trisphosphate pathway, although the specific mechanism of activation is still elusive [24,77]. Others, such as some TRPV members, are ionotropic receptors activated by chemical and physical stimuli [24,61]. The molecular diversity of TRP proteins correlates with their wide number of biological functions, ranging from fertility to vision, taste, olfaction, osmo/mechanosensation, and nociception [61,77,79]. Therefore, TRP channels constitute a family of sensory receptors.
THE TRPV1 CHANNEL
Among the TRP channel superfamily, TRPV1, TRPV2, TRPV3, TRPV4, TRPM8 and TRPA1 are thermoreceptors [80]. These receptors are designed to detect a wide range of temperatures from hot (TRPV1 and TRPV2) and cold (TRPA1) noxious temperatures to innocuous thermal stimuli (TRPV3, TRPV4 and TRPM8) [24,80]. In addition, these channels are activated by chemical agents such as capsaicin (TRPV1), menthol (TRPM8), camphor (TRPV3) and mustard (TRPA1) [19,60,74,81,80,100]. Furthermore, TRPV1 is also gated by protons and endocannabinoids [19,61]. Notably, the physiological properties of the receptor have signalled it out as a key thermosensory transducer in the nervous system [19,80]. In support of this tenet, analysis of vanilloid receptor null mice has substantiated the involvement of this channel in pain sensation [18,30].
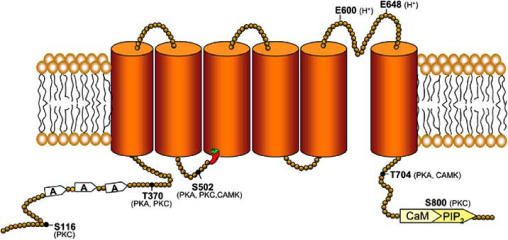
At the molecular level, TRPV1 is a calcium permeable non-selective cation channel. The functional receptor is a tetrameric membrane protein with four identical subunits assembled around a central aqueous pore, although heteromeric association with TRPV3 has been reported [98]. Structurally, each TRPV1 subunit protein shows a membrane domain composed of six transmembrane segments (S1-S6), with an amphipathic region between the fifth and sixth segment that forms the channel conductive pore. This region contains glutamic acids that are involved in the pH-dependent gating of the receptor [19]. The protein also has a cytoplasmic N- and C-termini (Fig. 1). In the N-terminus, TRPV1 channels exhibit three ankyrin domains that mediate protein-protein interactions with cytosolic proteins, and show consensus sequences for protein kinases. The protein displays a cytosolic C-terminus domain containing phosphoinositide, and calmodulin binding (CAM) domains, as well as phosphorylation sites [24]. In addition, the C-end has a TRP-like motif that functions as an association domain of receptor subunits [39].
Fig. (1).
Proposed model of the topology of TRPV1 subunits. Each subunit consists of six putative transmembrane segments and intracellular N- and C- domains. Functional regulatory domains of the receptors and phosphorylation sites are indicated. The capsaicin binding site is labelled withe the chili icon.
TRPV1 is an integrator of noxious stimuli that gates in response to heat (≥ 42°C), vanilloids, protons, and proalgesic substances [19,61]. Temperature sensing seems related to voltage dependent gating, as evidenced by the shift in TRPV1 voltage-dependent activation in response to changes in temperature [111]. Chemical activators of TRPV1 channels can be grouped into those agents that directly gate the channel, and those that allosterically modulate the protein by post-translational modifications. Furthermore, agonists of the receptor act as gating modifiers reducing the temperature threshold of the channel gating [111]. Although TRPV1 is a temperature-gated channel with a temperature threshold of activation of 42°C, it was recently shown that TRPV1 may not be required for the detection of noxious heat in intact C-fiber afferents [117]. However, the channel becomes a pivotal heat sensor under pathological/ inflammatory states as concluded from genetic and pharmacological TRPV1 knock-out studies [18,30,38]. The receptor has two abundant single nucleotide polymorphisms that produce amino acid substitutions, one in codon 315 (Met315 Ile) at the N-terminus domain, and the other at amino acid 585 (Ile585 Val) located in the fifth transmembrane segment. Interestingly, gender, ethnicity and temperament seem to contribute to individual variation in thermal and cold pain sensitivity by interactions in part with these TRPV1 single nucleotide polymorphisms [66].
A large body of evidence indicates that post-translational modification of TRPV1 increases the channel activity of the receptor [11,32]. Indeed, TRPV1 has several consensus sequences for protein kinases A (PKA), C (PKC), calmodulin kinase II (CAMKII) and the tyrosine specific kinase Src (Fig. 1). PKA phosphorylation potentiates TRPV1 responses by reducing agonist-induced desensitisation. Desensitisation of the receptor depends on the presence of extracellular Ca2+, and it is characterised by a profound run-down of its channel activity upon repetitive agonist stimulation. PKA exerts its action through partial rescue of desensitised receptors by direct phosphorylation of amino acids S116 and T370 at the N-terminus domain. Similarly, CaMKII-mediated phosphorylation, along with calcineurin-induced dephosphorylation of the receptor, notably contributes to determine its activation and desensitisation state [63,78]. Likewise, in colonic dorsal root ganglion neurons, the channel activity of TRPV1 is enhanced by tyrosine phosphorylation with the nonreceptor tyrosine kinase Src [58]. Tyrosine phosphorylation of the protein may underlie in part to the IL-1β-induced potentiation of heat-activated currents in rat sensory neurons [86].
PKC-dependent phosphorylation of TRPV1 can be primarily induced either by extracellular ATP released from injured cells, IL-1β, proteases, neurotrophins and bradykinin [86,102,110]. In vitro studies have identified S502, T704 and S800 as PKC phosphorylation sites (Fig. 1). Different PKC isoforms have been involved in TRPV1 sensitisation. For instance, phorbol esters primarily activate PKCα [87], although they can also act as a direct ligands of TRPV1 [10,83]. In contrast, stimulation of bradykinin B1 and B2 receptors leads to PKCε activation [20,102]. More recently, PKCμ, a kinase related to, but distinct of the PKC family has been shown to phosphorylate and sensitise both heterologously expressed and native TRPV1 channels [115]. PKC phosphorylation of the vanilloid receptor notably enhances its responsiveness by augmenting the channel open probability [93]. Akin to vanilloids and pH, TRPV1 phosphorylation by PKC decreases the receptor heat threshold, thus leading to activation of the channel at body temperature. In addition, PKC activation promotes the rapid recruitment of vesicular receptors to the cell surface [83]. The occurrence of both events provides a molecular mechanism for the pain experienced in response to a warm stimulus, such as showering sunburnt skin.
EXPRESSION OF TRPV1 CHANNELS
In situ hybridisation, immunocytochemical analysis, and drug binding assays have shown TRPV1 expression in ≈50% of dorsal root and trigeminal ganglion neurons, in dorsal horn of spinal cord and caudal nucleus of spinal trigeminal complex [19,107]. The majority of TRPV1 positive neurons also colocalise with the nerve growth factor (NGF) receptor trkA, the lectin IB4, and the neuropeptides involved in nociceptive transmission such as substance P (SP) and calcitonin gene related peptide (CGRP) [19,61]. Vanilloid sensitive nociceptors are peptidergic, small diameter neurons that give rise to unmyelinated C fibers, although some Aδ fibers are responsive to vanilloid derivatives [49]. Somatic and visceral primary afferents express TRPV1 at both the spinal and peripheral terminals. Furthermore, TRPV1 expression has been reported in vagal afferents in jugular and nodose ganglion neurons [54,84].
In addition to a subset of nociceptors, TRPV1 is present in neurons of the central nervous system and non-neuronal cells. For instance, TRPV1 mRNA or protein is widely expressed in brain regions such as the olfactory nuclei, cerebral cortex, dentate gyrus, central amygdala, striatum, centromedian and paraventricular thalamic nuclei, hypothalamus, substantia nigra, reticular formation, locus coeruleus, inferior olive and cerebellar cortex [see 84 and references therein]. The role of TRPV1 in the central nervous system is still elusive,although it may mediate endovanilloid signalling promoting the release of excitatory neurotransmitter such as L-glutamate, noradrenaline and dopamine [71,72].
In the skin, TRPV1 positive cells have been found in the dermis and epidermis [46], primarily in Meissner corpuscles and keratinocytes [56,88]. Moreover, this receptor is also expressed in mast cells, where its activation could release mast cell pro-algesic mediators that bind to histamine and proteinase-activated receptors on sensory terminals [99]. More recently, functional TRPV1 channels were found in the human hair follicle [15]. Activation of this receptor in cultured keratinocytes inhibited cellular proliferation, induced apoptosis, up regulated known endogenous hair growth inhibitors, and down regulated hair growth promoters. Thus, these results suggest an important role of TRPV1 in epithelial growth disorders [15].
It is also worth mentioning that TRPV1 receptors appear to be highly expressed in visceral afferents [53], and are present in the gastrointestinal (GI) tract within the myenteric and submucous plexus and some enteric intrinsic neurons [116]. Although capsaicin does not desensitise gastric epithelial cells, activation of TRPV1 expressing primary afferents has been shown to thicken the protective barrier in the stomach and duodenum [84], and sensitisation of this receptor plays a role in GI inflammation and function [41]. In pathological conditions, TRPV1 has been implicated in the hypermotility of the GI tract in abdominal pain associated to functional bowel disorders, and in the neurogenic component of pancreatitis [1,49].
TRPV1 expression is also prominent in other tissues. In the urinary bladder, functional channels are expressed in the uroepithelium in both the superficial and basal layers [4], where they are implicated in the development of the micturition reflex in both normal and pathological conditions [12]. In the lungs, capsaicin stimulates airway specific C-fibers and may play a role in the generation of non-productive cough [65]. Neurogenic inflammation of mucosal nociceptors also seems to be a key factor in the pathogenesis of asthma [44]. Under normal conditions, these fibers do not express SP but begin to produce it after allergic inflammation and viral infection [17,51]. In the vascular system, TRPV1-expressing fibers accompany blood vessels in all layers of the viscera. These receptors appear involved in Bayliss myogenic constriction which leads to hypertension [97]. Furthermore, TRPV1 seems to contribute to myocardial protection by releasing CGRP and nitric oxide from capsaicin-sensitive afferents in the ischemic heart [101]. Another tissue that expresses TRPV1 channels is the inner ear, where the protein is present in inner and outer hair cells, inner and outer pillar cells, Hensen cells and satellite cells [8,121]. These channels appear to be involved in hearing, as evidenced by the vanilloid-induced alteration of cochlear sensitivity [104].
Taken together, all these findings illustrate that TRPV1 channels are widely expressed in neuronal and non-neuronal cells of both endodermal and mesodermal origin, and suggest that the receptor is involved in diverse physiological functions. These include thermosensory transduction, as well as chemical signalling presumably mediated by endovanilloid compounds. In addition, they hint that dysfunction of the channel may underlie the etiology of pathological sensory transduction such as that occurring in inflammation. Taken together, all these observations underscore the notion that TRPV1 is a widely expressed protein whose function may be critical for diverse physiological conditions.
TRPV1 A THERAPEUTIC TARGET FOR PAIN MANAGEMENT
The pivotal role of TRPV1 in physiology has suggested a contribution of the channel to the mechanism of diverse human diseases. In particular, cumulative evidence is substantiating the tenet that nociceptor sensitisation by inflammatory agents is primarily achieved by TRPV1. This receptor is the endpoint target of intracellular signalling pathways triggered by inflammatory mediators that lead to potentiation of its channel activity which, in turn, promotes the hyperexcitability of nociceptors. Enhancement of TRPV1 function by pro-algesic agents may be accomplished either by direct activation of the channel or by its post-translational modification mediated by intracellular metabolic cascades [24,80]. Direct activation of TRPV1 responses has been reported for lipid mediators such as arachidonic acid metabolites including anandamide, N-arachidonyl-dopamine (NADA), N-oleyldopamine and, 12-(hyperoxy)eicosatet-renoic acid (12-HPETE). These compounds act as weak agonists, but notably increase TRPV1-mediated [Ca2+]i in both heterologous expression systems, and in primary sensory neurons [22,50,52]. In addition, the acidosis that develops in inflamed tissues is also a direct activator of the TRPV1 channel activity [19]. The potency and efficacy of each singular mediator is quite low but in inflammatory conditions, several of these modulators are simultaneously released and act synergistically. Noteworthy, most of these TRPV1 ligands act by reducing the heat threshold of channel activation from 42°C to body temperature (≈35°C) [11]. Therefore, direct gating of TRPV1 responses by inflammatory agents acting as channel agonists notably increases the excitability of nociceptors resulting in a hyperalgesic condition. In addition, the TRPV1-mediated [Ca2+]i rise, in turn, triggers the release of pro-inflammatory agents at peripheral terminals, thus further increasing the excitability of the nociceptors. This feedback circuit notably contributes to enhance the hypersensitivity of inflamed tissue.
Activation of intracellular protein networks during inflammation results in TRPV1 phosphorylation [11], release of tonically-inhibited receptors [23], and an increment of the surface expression of functional channels [83], all being major events underlying the nociceptor activation and sensitisation that leads to hyperalgesia. Indeed, TRPV1 expression is upregulated in tissue samples from patients with inflammatory bowel disease and Crohn’s disease [118], and and also in patients with rectal hypersensitivity [21], as well as those affected of vulvodynia [108]. Thus, TRPV1 receptors are strong candidates for an important role in the sensitisation of primary afferents after injury or inflammation. The involvement of TRPV1 in heat hyperalgesia is underscored by the reduced thermal hypersensitivity of mice lacking TRPV1 [18,30]. Similarly, non-competitive antagonists of the TRPV1 channel notably attenuate the heat hyperalgesia triggered by inflammation in vivo [38]. The important contribution of TRPV1 receptor to the onset and maintenance of neurogenic inflammation has validated it as a therapeutic target for inflammatory pain management.
In addition to the contribution of the vanilloid receptor as a target of the neurogenic inflammation underlying different diseases, TRPV1 is gaining interest for the treatment of neuropathic, postoperative and chronic pain and, recently, for the therapy of epithelial disorders. Thus, for instance, topical capsaicin or resiniferotoxin have been used in postherpetic neuralgia, diabetic neuropathy, postmastectomy pain and arthritis [64,103]. Recently, TRPV1 has been clearly validated as a key target for management of chronic pain in bone cancer [42]. As a result, the development of specific TRPV1 antagonists is a central focus of current drug discovery programs.
PHARMACOLOGY OF TRPV1 RECEPTORS
The TRPV1 receptor is a molecular entity with diverse drug binding sites. Structure-function studies have unmasked the molecular determinants of the capsaicin binding site in an intracellular domain of the receptor (Fig. 1) [40,59], and those involved in the interaction with the non-competitive antagonist ruthenium red at the extracellular vestibule of the pore domain [37]. The existence of these binding sites has prompted the discovery of novel vanilloid-like agonists, as well as competitive and non-competitive antagonists with the hope that they will be of clinical use to treat human TRPV1 receptor dysfunction. Further summarized are the efforts undertaken to develop these three classes of TRPV1 modulators.
TRPV1 AGONISTS
Agonists are molecules that directly gate the channel by a mechanism that involves the reduction of the heat threshold of activation. Prolonged exposure of the receptor to the agonist in the presence of Ca2+ induces channel closure by desensitisation and tachyphylaxia [103], by a mechanism that involves phosphorylation of a key residue at the C-terminus of the protein [11]. Agonist-induced desensitisation is thought to underlie the analgesic activity of capsaicin. However, capsaicin analgesia may also arise from selective deletion of TRPV1-expressing nociceptors due to the Ca2+ overload that may induce the ligand [64]. Thus, high-affinity agonists that promote receptor tachyphylaxia and/or nociceptor ablation could be used as efficacious pain relievers.
The identification of several natural products that act as TRPV1 ligands prompted the use of these molecules as pharmacological tools to study this receptor even before its molecular identity was known. In addition, these studies opened different medicinal chemistry programs based on structure-activity relationships (SAR) directed to improve disease [118], the therapeutic profile of the initial hits.
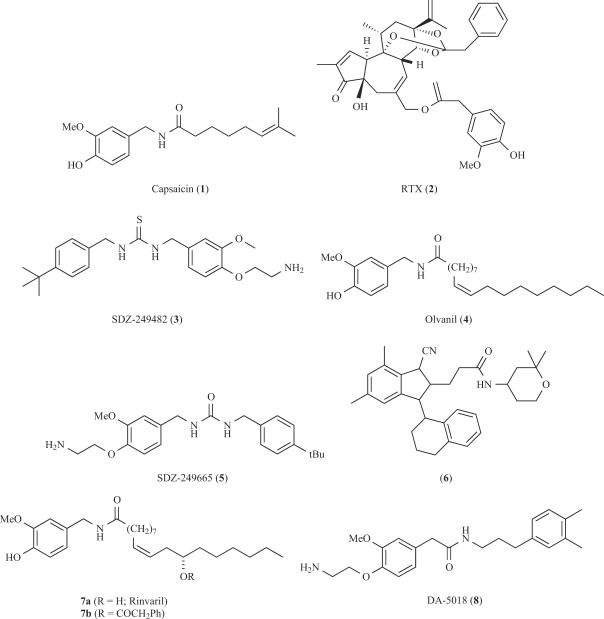
Vanilloids are the best known kind of TRPV1 agonists. Capsaicin (1, Fig. 2) is the representative example of this family. This molecule bears the 4-hydroxy-3-methoxybenzyl moiety characteristic for homovanillin derivatives, an amide linker and a lipophilic region. The homovanillyl motif and the amide linker contain polar groups capable of forming hydrogen bonds. The SAR studies performed on capsaicin analoges suggest that these polar moieties are essential for exciting sensory neurons. In contrast, the hydrophobic region, with an optimal chain length of 8-15 carbon atoms, would interact with a hydrophobic region in the receptor. Unlike other ligand-gated channels that produce fast synaptic transmission, vanilloids show a slow activation kinetics, in part because their binding site is located in the intracellular portion of the receptor (Fig. 1) [31,40,59,62]. Indeed, a cytosolic region spanning the third transmembrane domain in TRPV1 appears to be essential for capsaicin binding, presumably through hydrophobic interactions with the vanilloid carbon chain [40,59]. The structural determinants involved in capsaicin binding are being unravelled and molecular models for the vanilloid site have been proposed which, upon refinement, may facilitate the design of agonists with higher therapeutic index [40].
Fig. (2).
Structures of a selection of natural and synthetic TRPV1 agonists.
There are other known classes of naturally occurring vanilloids, such as Resiniferatoxin (RTX, 2), a compound isolated from Euphorbia resinifera, that exhibits a TRPV1 agonistic activity much more potently than capsaicin (IC50=10 pM). As shown in Fig. 2, RTX is a rather complex molecule and the pharmacophoric groups have not been clearly defined yet. SAR studies suggest that the homovanillic moiety of RTX, the C3-keto group and the orthoester phenyl moiety in ring C are essential structural elements for eliciting its extremely high potency desensitising TRPV1 channel activity. As a consequence, RTX is being developed as a sensory neuron desensitising agent for the treatment of urinary urge incontinence and pain associated with diabetic neuropathy [105], two human pathologies mediated by TRPV1 dysfunction. In spite of these results, the limited availability of RTX from natural sources and its difficult chemical synthesis hamper its therapeutic use.
The efficacy of both capsaicin and RTX for treating overactive bladder and neuropathic pain has stimulated the efforts for identifying orally active TRPV1 agonists. A major shortcoming for the therapeutic use of capsaicin and related vanilloids is the burning sensation and irritation that they provoke. In addition, the phorboid group of RTX has raised concerns of tumorigenicity. The development of more potent, orally active vanilloid derivatives (See Fig. 2) such as SDZ-249482 (3, Fig. 2), olvanil (4, Procter and Gamble), SDZ-249665 (5, Novartis) or the complex capsaicin analogue reported by Takeda (6) did not fully circumvent the discomfort of the side effects derived from irritation. Recently, homoallylic hydroxylation and further acylation of the fatty acyl chain of olvanil has led to the identification of phenylacetylrinvanil (7b, Fig. 2), an ultra-potent capsaicinoid (EC50=11 pM) that reduces bladder detrusor overactivity in vivo in a rat model of urinary incontinence with a potency similar to resiniferatoxin [3].
In parallel to the identification of vanilloid-like agonists, the development of new formulations for capsaicin such as dermal patches (NGX-4010 from NeurogesX) or localised injections (ALGRX-4975 by AlgoRx) are currently evaluated as analgesic strategies. The vanilloid analog DA-5018 (8, Dong A Pharmaceuticals) is also under development as a topical analgesic. Thus far, clinical data gathered indicate that the use of TRPV1 agonists as analgesics has clear cut advantages such as long lasting effects, broad mechanism of action, and high potency for the case of RTX. However, these molecules exhibit side effects such as pungency and pain that limit their clinical application. Therefore, the identification of non-pungent vanilloid-like drugs that induce a long-lasting desensitisation of the receptor will be of great clinical value.
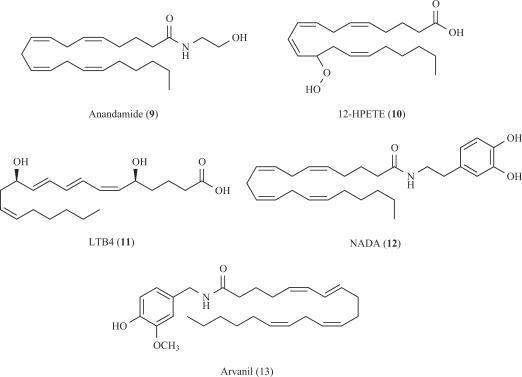
There is good evidence on the occurrence of endogenous vanilloid receptor agonists, coined with the term endovanilloids, and that these agents modulate the sensitivity of TRPV1 channels to thermal stimuli [109]. For instance, it has been described that a polyunsaturated compound derived from arachidonic acid, anandamide (9, Fig. 3) activates both native and recombinant TRPV1 receptors [122]. The structure of the molecule does not give evidence that it is a vanilloid analog. This fact caused some confusion about the term “vanilloid” depending on whether it is used chemically or pharmacologically. Moreover, anandamide is also considered as an endocannabinoid, since it activates cannabinoid (CB) receptors CB1 and CB2 at concentrations lower than those required to gate TRPV1 [122]. Similarly, several eicosanoids, particularly those derived from the enzymatic action of 5-lipoxygenase or 12-lipoxygenase, are capable of activating rat TRPV1. In particular, 12-HPETE (10, Fig. 3) and leukotriene B4 (11, LTB4, Fig. 3) have exhibited the most potent agonistic activity [50]. It is believed that eicosanoids function as intracellular vanilloids of TRPV1 receptors in the cells where they are synthesised. Recently, NADA (12, Fig. 3) has been identified as a brain endovanilloid [52]. NADA is an endogenous anandamide analog several times more potent than anandamide on TRPV1, although it still activates CB1 receptors.
Fig. (3).
Structures of TRPV1 endogenous ligands and hybrid canabinoid-TRPV1 modulators.
Another related approach to modulate TRPV1 signalling is the identification of the so-called hybrid cannabinoids/ TRPV1 ligands. Since TRPV1 colocalises with CB1 receptors in brain, and both proteins have overlapping ligand recognition properties, it is possible to conceive compounds capable of activating both receptor types simultaneously even by different mechanisms. Arvanil (N-[3’-methoxy-4’-hydroxybenzyl]arachidonamide, 13, Fig. 3) is one of these agents. Arvanil has affinity for CB1 receptors and activates TRPV1 receptors more potently than capsaicin or anandamide [75]. This compound is an antiproliferative agent for human breast cancer cells sensitive to both CB1 and TRPV1 receptor antagonists, as well as a spinal analgesic and, a relaxant of mouse vas deferens. Similarly, arachidonyl ethanolamine, an endogenous cannabinoid that also acts on the TRPV1 channel, induced strong apoptosis of uterine cancer cells that aberrantly express the vanilloid receptor [24]. Accordingly, it is of great interest for the future development of improved hybrid CB1/TRPV1 agonists.
TRPV1 ANTAGONISTS
Intense efforts have been carried out to design TRPV1 competitive and non-competitive antagonists [91,106]. The competitive antagonists bind to the agonist binding site, and lock the channel in the closed, nonconductive state. In contrast, non-competitive antagonists interact with additional binding sites on the receptor structure preventing receptor opening by the agonist or blocking its aqueous pore [91]. Non-competitive antagonists acting as open channel blockers are therapeutically attractive because they preferentially recognise the population of over-activated TRPV1 channels with a marginal recognition of physiologically working receptors. Thus, this kind of drugs target pathologically-activated receptors, which can reduce the potential unwanted side effects.
TRPV1 COMPETITIVE ANTAGONISTS
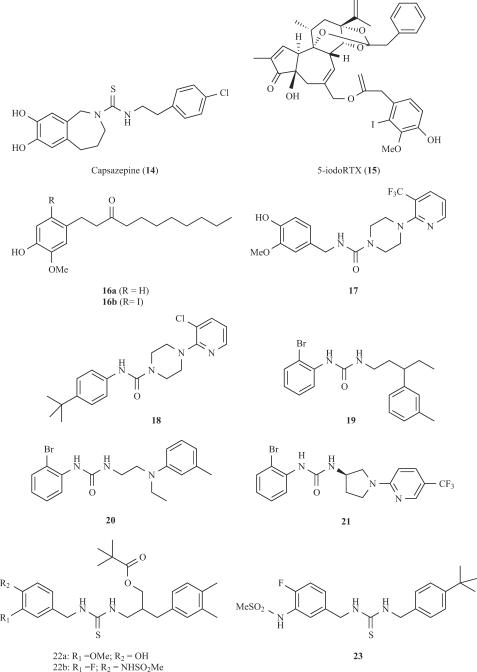
The first competitive TRPV1 antagonist, capsazepine (14, Fig. 4), was identified by Sandoz (now Novartis) and was used as a valuable pharmacological tool to understand the capsaicin-induced effects on nociceptors in vivo before the TRPV1 channel was cloned. This compound can be considered as a conformationally-restricted capsaicin analog bearing a thiourea moiety instead of the urea present in capsaicin. The propylidene linker of the seven-membered ring forces the dihydroxylated aromatic moiety to adopt an orthogonal orientation with respect to the thiourea bond. This steric constraint was considered essential for eliciting the receptor antagonistic activity, although development of acyclic vanilloid-based derivatives lacking this spatial disposition and eliciting higher inhibitory potency than capsazepine questioned the relevance of this SAR statement. Pharmacological studies showed that capsazepine exhibited low metabolic stability and poor pharmacokinetic properties in rodents, thus preventing its clinical development. Furthermore, pre-clinical studies on animal models of pain have provided conflicting results. For instance, the compound shows poor anti-inflammatory and analgesic activity in rats, although it remarkably attenuates thermal and mechanical hyperalgesia in guinea pigs [113].
Fig. (4).
A selection of TRPV1 competitive antagonists.
The discovery that the introduction of an iodine atom on the vanillyl moiety of RTX agonist modulates its pharmacological activity depending on the position of the halogen ended up with the identification of 5-iodoRTX (15, Fig. 4) as a potent antagonist of TRPV1 (IC50 = 3.9 nM) [73,112]. This compound produced analgesic activity in vivo and it is currently in clinical studies. Similar to RTX, a major drawback of compound 15 is its complex chemical structure and, the cost of its synthesis from RTX itself. In addition, 5-iodoRTX exhibits low oral bioavailability probably due to its poor pharmacokinetic properties. Nevertheless, the discovery of compound 15 stimulated the SAR studies on the halogenation of the vanillyl moiety present in simple capsaicin derivatives. For instance, taking nonivamide as a model (16a, Fig. 4), the substitution at C-6 afforded more potent inhibitors than at C-5 and the inhibitory potency correlated with the size of the halogen (I > Br > Cl) [2]. The most potent derivative identified was that bearing the iodine atom at C-6 (16b). Similarly, iodination of phenylacetylrinvanil (7b) produced a potent TRPV1 antagonist (IC50 = 0.8 nM) [3]. These results showed that halogenation could be an appropriate strategy for reversing the activity of vanilloids. This observation, together with the aim of synthesising simpler derivatives of RTX that preserve the inhibitory potency of the parent compound, would constitute an attractive field for developing orally active, competitive TRPV1 antagonists with improved pharmaceutical profiles for clinical use.
A related family of capsaicin analogues was developed by Neurogen through the formal amidation of the homovanillylamino moiety with different 4-(α-pyridyl)piperidine-1-acyl residues. The urea 17 (Fig. 4) is a representative example of this family [6]. The presence of an amino moiety at the hydrophobic region of the capsacinoid appeared to be crucial for obtaining a potent inhibitor that was, at the same time, a highly improved drug lead in terms of hydrophilicity. This observation was also important to recognise that the C-region of capsaicin should be considered as a domain that plays a key role in the interaction with the receptor. Another piperazinyl urea (BCTC, 18) was reported by Purdue-Pharma to be more selective than capsazepine against a wide variety of enzymes and channels. This compound exhibited in vivo oral activity in animal models of inflammatory and neuropathic pain [92]. Less conformationally-restricted analogs of BCTC such as compounds 19 and 20, along with the recently reported pyrrolidine urea 21 (Fig. 4), also elicit a good antagonistic TRPV1 potency maintaining the receptor selectivity. The ongoing pre-clinical characterization of these compounds will uncover whether they have a higher therapeutic index than BCTC [94]. It is worth noting that most of these molecules were identified from the screening of chemical combinatorial libraries, thus showing the usefulness of this strategy in drug discovery programs.
Structurally connected to capsaicin related compounds, thiourea 22a (Fig. 4) elicited a potent agonist activity (Ki < 10 nM). Interestingly, the presence of fluorine at C-3’ and the methanesulfonamido moiety at C-4’, replacing the methoxy and hydroxy groups, respectively, afforded compound 22b, a molecule exhibiting an antagonistic TRPV1 activity [68]. Further modification of these derivatives, now at the lipophilic region, led AmorePacific to obtain optimized compound 23, bearing a tert-butyl group at the aromatic ring [114].
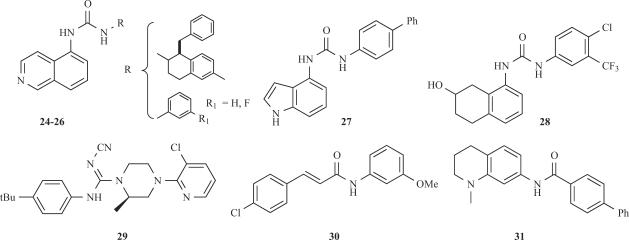
The continuous efforts to improve the pharmacological properties of TRPV1 antagonists have resulted in the development of novel templates (See Fig. 5). In this regard, several non-symmetric ureas have been reported by Janssen [25], Abbott [69] and Merck [16], where the 5-aminoisoquinoline moiety appears as a common feature (cf. 24-26). Alternatively, Bayer has reported the 4-aminoindole 27 [119] and the hydroxylated tetrahydronaphthalene 28 [119] urea derivatives. All these compounds show an inhibitory potency ≤ 10 nM. Taken together, these results emphasise the relevance for antagonistic activity of a functionalised lipophilic moiety, preferentially with a basic group, for interacting with the capsaicin binding site on TRPV1.
Fig. (5).
TRPV1 antagonists based on novel templates.
The replacement of the urea moiety by other isosteric groups has also been explored. Euroceltique reported compound 29 (Fig. 5), based on the piperidine templates reported earlier (cf. 17 and 18), where the cyanoguanidine group substituted the urea [67]. A further alternative to the ureas are the amides explored by GlaxoSmithKline. Compound SB-366791 (29) exhibits a potent competitive antagonistic activity (IC50≤ 5 nM) in both the human and rat TRPV1 receptors and it shows a better selectivity profile than capsazepine [45]. Further studies led to the identification of compound 30, which also exhibits a good in vitro inhibitory activity. In addition, this molecule is readily amenable for structural optimisation, as shown by the synthesis of the tetrahydroquinoline 31.
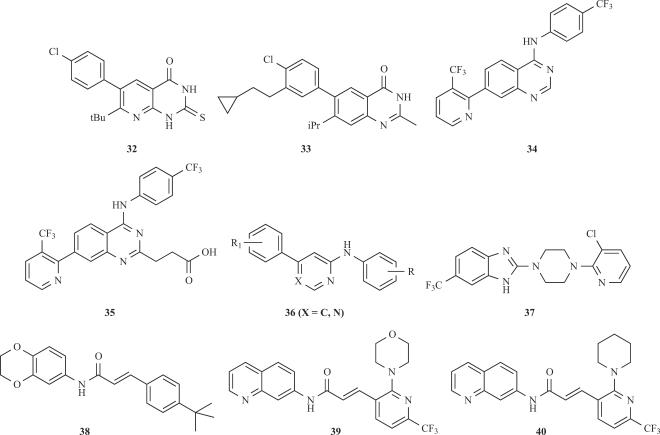
A further approach for the development of TRPV1 efficient antagonists is characterised by those derivatives lacking the urea, thiourea or amide groups (see Fig. 6). Compounds 32-37 are examples of this approach that have been reported from different companies. The pyridine derivative 32 [28], the quinazolin-4-one 33 [29] from Novartis and the quinazoline-4-ylamine 34 [7] from Neurogen were the first compounds disclosed. A second derivative from Neurogen 35 [8] containing a residue of propionic acid (or phosphate or phosphonate) attached to the quinazoline moiety followed. In addition, two more derivatives, compounds 36 [14] and 37 [9], from Amgen containing heterocyclic moieties at the central regions have been also reported. More recently, the group of Abbott has described a collection of urea derivatives from which an analogue of compound 24 displaying a trifluoromethyl substituent at the meta position of the phenyl residue exhibited an IC50 = 4 nM, a 46% of oral bioavailability and, in vivo analgesic activity in animal models of visceral and inflammatory pain [43]. Overall, the strength of the heterocyclic pharmacophores studied was 5-isoquinoline > 8-quinoline = 8-quinazoline > 8-isoquinoline ≥ cinnoline ≈ phtalazine ≈ quinoxaline ≈ 5-quinoline. Lastly, a group from Amgen has published a study on different analogs based on its acrylamide antagonist 38. From the N-aryl cinnamides synthesised, optimised compounds 39 and 40 exhibited high antagonist potency and good oral bioavailability in rats, as well as a favourable pharmacokinetic profile [33]. Although much of these compounds display in vivo anti-inflammatory and analgesic activity in animal models, further studies are required to assess the efficacy in the clinic. These exciting results are eagerly awaited.
Fig. (6).
Novel TRPV1 antagonists lacking the urea or thiourea groups (31-36), or based on the N-arylcinnamide moiety (37-39).
Collectively, all these findings illustrate that development of potent competitive vanilloid antagonists of TRPV1 activity is a hot field of current neuropharmacology. Analysis of the structures shows the use of scaffolds that preserve little resemblance to the original vanilloid group or even to the capsacinoid family. On the other hand, even in the absence of detailed knowledge of the topology of the TRPV1 receptor, the wide variety of active structures reported thus far provide a significant database for initiating molecular modelling and SAR approaches aimed at the identification of the pharmacophoric features required for orally active, potent antagonistic activity. These studies are also necessary to clarify whether the above structural variety is due to the interaction with sames of different drug binding sites on the receptor.
TRPV1 NON-COMPETITIVE ANTAGONISTS
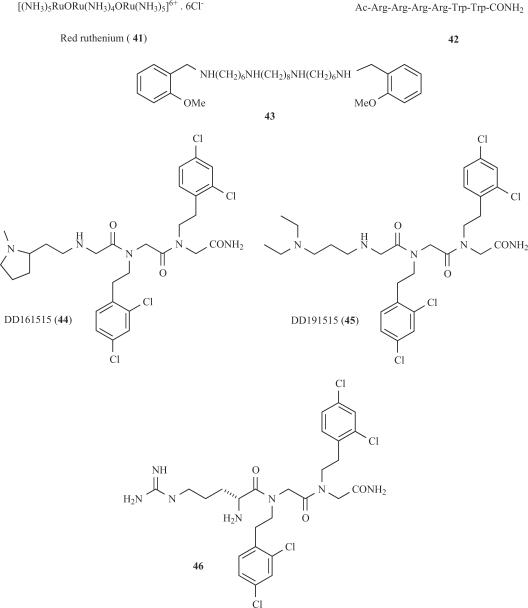
The first non-competitive TRPV1 antagonist, and the only molecule available for several years as vanilloid receptor blocker was the trinuclear polyamine complex, Ruthenium red (41, Fig. 7). This compound binds to the pore region of the channel with high potency (IC50, 100 nM) and weak voltage dependency by a mechanism that involves interaction with negatively-charged residues present at the outer vestibule of the ionic pore [37]. The poor receptor selectivity of this compound seems to underlie its proconvulsive activity in animal models that preclude the clinical development. Arginine-rich hexapeptides such as RRRRWW-NH2 block recombinant TRPV1 channels expressed in Xenopus oocytes in a non-competitive manner with submicromolar potency (42, Fig. 7) [90]. However, it was later suggested that these derivatives might partially act as competitive antagonists [48]. Nonetheless, similar to ruthenium red, their lack of receptor selectivity resulted in severe side effects and toxicity, thus preventing further their development as analgesics.
Fig. (7).
TRPV1 non-competitive antagonists.
A parallel effort was carried out to evaluate polymethylene tetraamines as TRPV1 channel blockers [76]. These studies identified methoctramine (43) as a non-competitive capsaicin antagonist with an IC50 of 2 μM, and notable voltage-dependent blockade (Fig. 7). This molecule antagonised native TRPV1 receptors in dorsal root ganglion neurons activated by either capsaicin or protons. Therefore, the inhibitory activity of methoctramine analogs has provided a new pharmacophoric scaffold for non-competitive antagonists of TRPV1. Thus far, the lack of receptor selectivity has restrained the use of these compounds in vivo.
The first small organic molecules identified as noncompetitive TRPV1 emerged from the screening of a peptidomimetic combinatorial library. N-Alkylglycines (also known as peptoids), constitute a class of compounds of synthetic origin that exhibit interesting biological properties derived from their structural features, i.e, the absence of CO-NH bonds and then of the hydrogen bond interactions that they induce, an improved stability against proteases and a high degree of conformational freedom. In addition, the structural simplicity of peptoids makes these peptidomimetic compounds amenable to structural manipulation, thus facilitating the optimisation of identified hits for improving their drug-like properties [47]. This is important if it is considered that the high conformational mobility of these molecules can generate selectivity problems due to undesired interactions with non-target receptors.
The screening of the library of trimers of N-alkylglycines led to the identification of two compounds referred to as DD161515 (44) and DD191515 (45) that preferentially block TRPV1 channel activity with micromolar potency and moderate voltage-dependency (Fig 7) [38]. These peptoids appear to recognise a receptor site different from that of capsaicin. Intraperitoneal administration of both trialkylglycines into mice significantly attenuated pain and neurogenic inflammation induced by injection of capsaicin into the hindpaws and, reduced thermal hyperlalgesia due to mustard oil-evoked tissue irritation. In contrast, these peptoids did not affect capsaicin triggered-mechanical hypersensitivity [38]. These findings suggested that TRPV1 active trialkylglycines may be developed into analgesics to treat inflammatory pain. However, the in vivo doses required for analgesic and anti-inflammatory activity were too high (≥25 mg/kg), thus preventing their development into useful drugs. Therefore, the design of more potent, non-competitive antagonists of TRPV1 is a yet unmet goal of drug discovery platforms. Recent progress in this arena, along with molecular modelling of the TRPV1 pore domain [35], is providing this kind of compounds. For instance, by combining the chemical features of arginine-rich peptides and peptoid DD161515, a potent non-competitive antagonist (H-Arg-15-15C, 46) of TRPV1 was discovered (Fig. 7) [34]. Compound H-Arg-15-15C blocked capsaicin-induced TRPV1 activity with 10-fold higher potency than the original peptoid DD161515, without compromising the receptor selectivity. Noteworthy, at variance with the parental peptoids, compound H-Arg-15-15C potently abrogated pH-activated TRPV1 responses. As for DD161515, the blockade mechanism was non-competitive acting as a channel blocker. Thus, introduction of a strong positive charge in the pharmacophore provided by peptoid DD161515 results in a significant increase in the binding efficacy of the molecule to the receptor. Noteworthy, compound 46 notably attenuated the inflammatory discomfort exerted by intraplantar injection of capsaicin. The i.p. dose required to produce analgesia was 10-fold lower than that of peptoid DD161515. In addition, the compound showed activity on the Phase II of the formalin model, although at doses relatively high, suggesting a modest activity of the antagonist in neuropathic pain models [96]. Indeed, H-Arg-1515C did not mitigate mechanical allodynia in the partially ligated sciatic nerve model of neuropathic pain. In contrast, 46 completely reversed the complete Freund Adjuvant (CFA)-induced thermal hyperalgesia at therapeutic doses (5 mg/kg, i.p.). Collectively, these findings show that compound H-Arg-15-15C is a potent non-competitive antagonist of the receptor that exhibits notable anti-inflammatory and analgesic activity in pre-clinical models of acute and chronic pain. However, compound 46 displays limited oral and i.p. bioavailability in vivo, presumably because of its lack of compliance with the Lipinski rule of 5 [70]. Thus, chemical modification on the H-Arg-15-15C structure will be necessary to increase its therapeutic potential. All these results demonstrate that TRPV1 antagonists that act on a site different from the vanilloid binding site are also promising candidates for analgesic/anti-inflammatory drug development.
THE TRPV1 RECEPTOR COMPLEX AS A THERAPEUTIC TARGET
Similar to other TRP channels, TRPV1 is arranged in major molecular complexes establishing high order signalling networks that notably determine the response to external stimuli. These protein networks are in turn the target of intracellular signaling pathways that modulate their composition, structure and function. Thus, an emerging notion is that ion channels are not isolated entities in cell membranes but pivotal components of these macromolecular assemblies. These complexes are composed of a plethora of diverse polypeptides including scaffolding proteins, receptors, enzymes and cytoskeletal proteins. The molecular components of the TRPV1 complex identified thus far include the high affinity neurotrophic receptor (TrkA), phospholipase C (PLC), and calmodulin [85,95]. The composition of these assemblies appears important for the regulation of the receptor activity. Activation of trkA and PLC, along with translocation of PKC, decrease the tonic inhibition that phosphatidylinositol bisphosphate exerts on TRPV1, thus promoting channel activity [23]. Similarly, activation of PKA and CAMKII gives rise to a decrease in Ca2+-induced receptor desensitisation and tachyphylaxia. Cumulative evidence indicates that modulation of receptor complexes underlie the TRPV1 potentiation by inflammatory mediators.
A yeast-two hybrid screen of a rat brain library using the N-terminus of TRPV1 as a bait identified two synaptic vesicle proteins, Snapin and Synaptotagmin IX, as interacting partners of TRPV1 [83]. Noteworthy, these proteins bind to SNARE proteins and participate in neuronal exocytosis [36,55], suggesting that surface delivery of TRPV1 channels is a highly regulated, Ca2+-dependent exocytotic process. Indeed, the interaction of both vesicular proteins with TRPV1 appears temporal and seems not involved in the formation of the molecular complexes at the cell surface. However, these interactions are pivotal for the trafficking and surface expression of TRPV1 channels. In support of this tenet, PKC-induced TRPV1 trafficking to the plasma membrane was blocked by botulinum neurotoxins, thus indicating that PKC- sensitisation of TRPV1 receptors is due at least in part to the regulated exocytosis of channels located in a reserve pool of cytosolic vesicles [83]. A recent observation that botulinum neurotoxin attenuates heat hyperalgesia substantiates this hypothesis [27], although additional in vivo experiments are needed to determine the precise role of receptor exocytosis to the onset and maintenance of neurogenic inflammation. Therefore, regulation of TRPV1 surface density in nociceptor peripheral terminals appears to be an important mechanism for both the development and preservation of inflammatory hyperalgesia. CFA-induced inflammation triggers an increased translation and trafficking of TRPV1 channels to the peripheral terminals [57]. These findings imply that modulation of TRPV1 trafficking may be a therapeutic approach for pain management. Accordingly, botulinomimetic compounds such as small peptides that inhibit SNARE-dependent exocytosis could be developed as anti-inflammatory and analgesic compounds [13].
CONCLUSION
The recognition of the important role the TRPV1 in the etiology of neurogenic inflammation and pain transduction has thrust the development of down regulators of its channel activity as analgesic drugs. Several of these compounds are initiating clinical studies in humans and their results are awaited. However, a note of caution must be sound because of the pivotal and diverse physiological functions played by the TRPV1 channel. Its widespread distribution in different tissues should be taken into account since undiscriminate pharmacological block of the receptor may lead to severe side effects that may complicate the use of antagonists of TRPV1 activity. Thus, the challenge of molecular neuropharmacology would be to develop receptor-selective drugs that preserve the physiological activity of TRPV1 while down regulating the function of overactive receptors. This kind of compound would not compromise the normal sensory signaling that is essential for survival.
ACKNOWLEDGMENTS
We thank all members of our group and colleagues of collaboration groups for their fundamental contribution to the results herein presented. We are indebted to the Financial support from the MEC, FIS, GVA, and Fundació La Caixa.
ABBREVIATIONS
- TRPV1
transient receptor potential vanilloid subunit 1
- PKA
protein kinase A
- PKC
protein kinase C
- NGF
nerve growth factor
- RTX
resiniferatoxin
- SP
substance P
- CGRP
calcitonin gene related peptide
- NADA
N-arachidonyl-dopamine
- SAR
structure-activity relationships
- CFA
complete Freund adjuvant
- CAMK
calmodulin kinase
- PLC
phospholipase C
- GI
gastrointestinal
- TrkA
high affinity neurotrophic receptor
REFERENCES
- 1.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Sensory pathways and cyclooxygenase regulate mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G470–474. doi: 10.1152/ajpgi.2001.280.3.G470. [DOI] [PubMed] [Google Scholar]
- 2.Appendino G, Harrison S, De Petrocellis L, Daddario N, Bianchi F, Schiano F, Moriello A, Trevisani M, Benvenuti F, Geppetti P, Di Marzo V. Halogenation of a capsaicin analogue leads to novel vanilloid TRPV1 receptor antagonists. Br J Pharmacol. 2003;139:1417–1424. doi: 10.1038/sj.bjp.0705387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, Gazzieri D, Ligresti A, Campi B, Fontana G, Pinna C, Geppetti P, Di Marzo V. Development of the first ultra-potent "capsaicinoid" agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther. 2005;312:561–570. doi: 10.1124/jpet.104.074864. [DOI] [PubMed] [Google Scholar]
- 4.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 5.Bakthavatchalam R, Hutchison A, Desimone RW, Hodgetts KJ, Krause JE, White GG. WO 0208221-A. Capsaicin receptor ligands. 2002
- 6.Bakthavatchatam R, Blum CL, Brielmann HL, Caldwell TM, Delombaert S, Hodgetis KJ, Yoon T, Zheng X. WO 03/062209. Substituted quinazolin-4-ylamine analogues as modulators of capsaicin. 2003
- 7.Bakthavatachalam R, Blum CA, Brielmann H, Caldwell TM, De Lombaert S, Hodgetts KJ, Zhen X. WO 04/055004. Carboxylic acid, phosphate or phosphonate substituted quinazolin-4-ylamine analogues as capsaicin receptor modulators. 2003
- 8.Balaban CD, Zhou J, Li HS. Type I vanilloid receptor expression by mammalian inner ear glanglion cells. Hear Res. 2003;175:165–170. doi: 10.1016/s0378-5955(02)00734-7. [DOI] [PubMed] [Google Scholar]
- 9.Balan C, Bo Y, Dominguez C, Christopher H, Gore UK, Ma VV, Norman MH, Ognyanov VY, Quian YX, Wang X, Xi N, Xu S. WO 04/035549. Benzimidazole derivatives and their use as vanilloid receptor ligands. 2003
- 10.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhave G, Gereau RW., 4th Posttranslational mechanisms of peripheral sensitization. Int J Neurobiol. 2004;61:88–106. doi: 10.1002/neu.20083. [DOI] [PubMed] [Google Scholar]
- 12.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 13.Blanes-Mira C, Merino JM, Valera E, Fernández-Ballester G, Gutiérrez LM, Viniegra S, Pérez-Payá E, Ferrer-Montiel A. Small peptides patterned after the N-terminus domain of SNAP25 inhibit SNARE complex assembly and regulated exocytosis. J Neurochemistry. 2004;88:124–135. doi: 10.1046/j.1471-4159.2003.02133.x. [DOI] [PubMed] [Google Scholar]
- 14.Bo YY, Chakrabarti PP, Chen N, Doherty EM, Fotsch CH, Han N, Kelly MG, Liu Q, Norman MH, Wang X, Zhu J, Ornyanov VI. WO 03/049702. Vanilloid receptor ligands and their use in treatments. 2003
- 15.Bodó E, Biró T, Telek A, Czifra G, Griger Z, Tóth BI, Mescalchin A, Ito T, Bettermann A, Kovács L, Paus R. Involvement of vanilloid receptor -1 (VR1/TRPV1) signalling in human hair growth control. Am J Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RE, Doughty VA, Hollingworth GJ, Brian JA, Lindon MJ, Moyes CR, Rogers L. WO 03/080578. Heteroaromatic urea derivatives as VR-1 receptor modulators for treating pain. 2003
- 17.Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir. 2002;165:1071–1075. doi: 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- 18.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 20.Cesare P, McNaughton P. Peripheral pain mechanisms. Curr Opin Neurobiol. 1997;7:457–482. doi: 10.1016/s0959-4388(97)80028-1. [DOI] [PubMed] [Google Scholar]
- 21.Chan CL, Facer P, Davis JB, Smith GA, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- 22.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JA, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 23.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 24.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 25.Codd L, Dax S, Jetter M, Mcdonell M, Mcnally J, Youngman M. WO 03/097586. Aminotetralin-derived urea modulators of vanilloid vr1 receptor. 2003
- 26.Contassot E, Tenan M, Schnüriger V, Pelte MF, Dietrich PY. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol Oncol. 2004;93:182–188. doi: 10.1016/j.ygyno.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 27.Cui M, Khanijou S, Rubino J, Aoki K. Subcutaneous administration of botulinum Toxin A reduces formalin-induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Culshaw AJ, Gull P, Hallet A, Kim HY, Seiler MP, Zimmermann K, Liu Y, Prashad M. WO 02/076946. Pyridine derivatives. 2002
- 29.Culshaw AJ, Dziadulewicz ED, Hallet A, Hart TW. WO 04/033435. Quinazolinone derivatives useful as anti-hyperalgesic agents. 2003
- 30.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 31.De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and in limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- 32.De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppeti P, Di Marzo V. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem. 2001;77:1660–1663. doi: 10.1046/j.1471-4159.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 33.Doherty EM, Fotsch C, Bo Y, Chakrabarti PP, Chen N, Gavva N, Han N, Kelly MG, Kincaid J, Klionsky L, Liu Q, Ognyanov VI, Tamir R, Wang X, Zhu J, Norman MH, Treanor JJ. Discovery of potent, orally available vanilloid receptor-1 antagonists. Structure-activity relationship of N-aryl cinnamides. J Med Chem. 2005;48:71–90. doi: 10.1021/jm049485i. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer-Montiel A, Fernández-Carvajal A, García-Martínez C, Belmonte-Martínez C, Van Den Nest W, Carreño C. WO 03/097670. Compounds which can block the responses to chemical substances or thermal stimuli or mediators of inflammation of nociceptors, producing method thereof and composition containing them. 2003
- 35.Ferrer-Montiel A, Garcia-Martinez C, Morenilla-Palao C, Garcia-Sanz N, Fernandez-Carvajal A, Fernandez-Ballester G, Planells-Cases R. Molecular architecture of the vanilloid receptor. Insights for drug design. Eur J Biochem. 2004;271:1820–1826. doi: 10.1111/j.1432-1033.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda M. Vesicle-associated membrane protein2/synaptobrevin binding to synaptotagmin I promotes Oglycosylation of synaptotagmin I. J Biol Chem. 2002;277:30351–30358. doi: 10.1074/jbc.M204056200. [DOI] [PubMed] [Google Scholar]
- 37.García-Martínez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel AV. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- 38.García-Martínez C, Humet M, Planells-Cases R, Gomis A, Caprini M, Viana F, De La Pena E, Sanchez-Baeza F, Carbonell T, De Felipe C, Perez-Paya E, Belmonte C, Messeguer A, Ferrer-Montiel A. Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc Natl Acad Sci USA. 2002;99:2374–2379. doi: 10.1073/pnas.022285899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Sanz N, Ferández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernández-Ballester G, Ferrer-Montiel A. Identification of tetramerization domain in the C-terminus of the vanilloid receptor. J Neurosci. 2004;24:5306–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ. Molecular determinants of vanilloid receptor sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 41.Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor, role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomtsyan A, Bayburt EK, Schmidt RG, Zeng GZ, Perner RJ, Didomenico S, Koenig JR, Turner S, Jinkerson T, Drizin I, Hannick SM, Macri BS, McDonald HA, Honore P, Wismer CT, Marsh KC, Wetter J, Stewart KD, Oie T, Jarvis MF, Surowy CS, Faltynek CR, Lee CH. Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain, Structure-activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline and cinnoline moieties. J Med Chem. 2005;48:744–752. doi: 10.1021/jm0492958. [DOI] [PubMed] [Google Scholar]
- 44.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2004;59:1139–1152. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 45.Gunthorpe MJ, Rami HK, Jeman JC, Smart D, Gill CH, Soffin EM, Hannan SL, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterization of SB-366791, a potent and a selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacol. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- 46.Guo A, Vluchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor I (VR1), relationship to neuropeptides, the P2X3 purinoreceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 47.Hann MM, Leach AR, Harper G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J Chem Inf Comput Sci. 2001;41:856–864. doi: 10.1021/ci000403i. [DOI] [PubMed] [Google Scholar]
- 48.Himmel HM, Kiss T, Borvendeg SJ, Gillen C, Ille P. The arginine rich hexapeptide R4W2 is a stereoselective antagonist of the vanilloid receptor 1, A Ca2+imaging study in adult rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2002;301:981–986. doi: 10.1124/jpet.301.3.981. [DOI] [PubMed] [Google Scholar]
- 49.Holzer P. TRPV1 and the gut, from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CI, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter DD, Myers AC, Undem BJ. Nerve-growth factor induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- 52.Hwang SW, Cho J, Kwak J, Lee SY, Kang J, Jung S, Cho KH, Min YG, Suh D, Kim U, Oh U. Direct activation of capsaicin receptors by products of lipooxygenases, endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6056. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang SJ, Valtschanoff JG. Vanilloid receptor VR1positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neuorsci Lett. 2003;349:41–44. doi: 10.1016/s0304-3940(03)00750-x. [DOI] [PubMed] [Google Scholar]
- 54.Ichikawa H, Sugimoto T. The co-expression of VR1 and VRL-1 in the rat vagal sensory ganglia. Brain Res. 2003;980:293–296. doi: 10.1016/s0006-8993(03)02998-6. [DOI] [PubMed] [Google Scholar]
- 55.Ilardi JM, Mochida S, Sheng ZH. Snapin, a SNARE-associated protein implicated in synaptic transmission. Nat Neurosci. 1999;2:119–124. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- 56.Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002;291:124–129. doi: 10.1006/bbrc.2002.6393. [DOI] [PubMed] [Google Scholar]
- 57.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf C. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 58.Jin X, Morsy N, Winston J, Pasricha PJ, Garret K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol. 2004;287:C558–C563. doi: 10.1152/ajpcell.00113.2004. [DOI] [PubMed] [Google Scholar]
- 59.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to hot chilli peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 60.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 61.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 62.Jung J, Hwang SW, Kwak J, Lee SY, Kang JC, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 64.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karlsson JA. A role for capsaicin sensitive, tachykinin containing nerves in chronic coughing and sneezing but not in asthma, a hypothesis. Thorax. 1993;48:396–400. doi: 10.1136/thx.48.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Kyle DJ, Sun Q, Fatesse L, Zhang C, Zhou X. WO 04/002983. Therapeutic agents useful for treating pain. 2003
- 68.Lee J, Kang M, Shin M, Kim JM, Kang SU, Lim JO, Choi HK, Suh YG, Park HG, Oh U, Kim HD, Park YH, Ha HJ, Kim YH, Toth A, Wang Y, Tran R, Pearce LV, Lundberg DJ, Blumberg PM. N-(3-Acyloxy-2-benzylpropyl)-N[4-(methylsulfonylamino) benzyl]thiourea analogues, novel potent and high affinity antagonists and partial antagonists of the vanilloid receptor. J Med Chem. 2003;46:3116–3126. doi: 10.1021/jm030089u. [DOI] [PubMed] [Google Scholar]
- 69.Lee CH, Bayburt EK, Didomenico DS, Drizin I, Gomtsyan AR, Koenig JR, Perner RJ, Schmidt RG, Turner SC, White TK, Zheng X. WO 03/070247. Fused azabicyclic compounds that inhibit VR-1. 2003
- 70.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 71.Marinelli S, Vaughan CW, MacDonald JC, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol. 2002;5432:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marinelli S, Pascucci T, Bernardi G, Puglisi-Allegra S, Mercuri NB. Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology. 2005;30:864–870. doi: 10.1038/sj.npp.1300615. [DOI] [PubMed] [Google Scholar]
- 73.McDonnel ME, Zhang SP, Dubin AE, Dax SL. Synthesis and in vitro evaluation of a novel iodinated resiniferatoxin derivative that is an agonist at the human vanilloid VR1 receptor. Bioorg Med Chem Lett. 2002;12:11189–11192. doi: 10.1016/s0960-894x(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 74.McKemy DD, Neuhasser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 75.Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Unsaturated long chain N-acyl-vanillyl-amides (N-AVAMS), vanilloid receptor ligands that inhibit anandamide-facilitated transpor and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- 76.Mellor IR, Ogilvie J, Pluteanu F, Clothier RH, Parker TL, Rosini M, Minarini A, Tumiatti V, Melchiorre C. Methoctramine analogues inhibit responses to capsaicin and protons in rat dorsal root ganglion neurons. Eur J Pharmacol. 2004;505:37–50. doi: 10.1016/j.ejphar.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 78.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 79.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 80.Montell C. Thermosensation, Hot findings make TRPNs very cool. Curr Biol. 2003;13:R476–R478. doi: 10.1016/s0960-9822(03)00406-8. [DOI] [PubMed] [Google Scholar]
- 81.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 82.Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Morenilla-Palao C, Planells-Cases R, García-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 84.Nagy I, Sántha P, Jancsó G, Urbán L. The role of vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 85.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obreja O, Rathee KP, Lips KS, Distler C, Kress M. IL-1_ potentiates heat-activated currents in rat sensory neurons, involvement of IL-1R1, tyrosine kinase and protein kinase C. FASEB J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 87.Olah Z, Karai L, Iadarola MJ. Protein kinase C(alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signalling pathways. J Biol Chem. 2002;277:35752–35759. doi: 10.1074/jbc.M201551200. [DOI] [PubMed] [Google Scholar]
- 88.Pare M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL. The Meissner corpuscule revised, a multiafferent mechanoreceptor with nociceptor immunological properties. J Neurosci. 2001;21:7236–7246. doi: 10.1523/JNEUROSCI.21-18-07236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Anderson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 90.Planells-Cases R, Aracil A, Merino J, Gallar J, Pérez-Payá E, Belmonte C, Ferrer-Montiel AV. Arginine rich peptides are blockers of VR-1 channels with analgesic activity. FEBS Lett. 2000;481:131–36. doi: 10.1016/s0014-5793(00)01982-7. [DOI] [PubMed] [Google Scholar]
- 91.Planells-Cases R, Garcia-Martinez C, Royo M, Pérez-Payá E, Carreño C, Albericio F, Messeguer A, Ferrer-Montiel A. Small molecules targeting the vanilloid receptor complex as drugs for inflammatory pain. Drugs Future. 2003;28:787–795. [Google Scholar]
- 92.Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carboxi-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties, II. in vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- 93.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 94.Rami HK, Thompson M, Wyman P, Jerman JC, Egerton J, Brough S, Stevens AJ, Randall AD, Smart D, Gunthorpe MJ, Davis JB. Discovery of small molecule antagonists of TRPV1. Bioorg Med Chem Lett. 2004;14:3631–3634. doi: 10.1016/j.bmcl.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 95.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. Brain Res. 2004;996:176–183. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schaible HG, Richter F. Pathophysiology of pain. Langenbecks Arch Sur. 2004;389:237–243. doi: 10.1007/s00423-004-0468-9. [DOI] [PubMed] [Google Scholar]
- 97.Scotland RS, Chauhan S, Davis C, DeFelipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C fibers, and vascular autoregulation, a novel mechanism involved in myogenic constriction. Circ Res. 2004;95:1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- 98.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 99.Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitc V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 100.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Anderesson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 101.Strecker T, Messlinger K, Weyand M, Reeh PW. Role of different proton sensitive channels in releasing calcitonin gen related peptide from isolated hearts and mutant mice. Cardiovascular Res. 2005;5:405–410. doi: 10.1016/j.cardiores.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 102.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- 103.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 104.Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- 105.Szallasi A. Vanilloid receptor ligands, hopes and realities for the future. Drugs Aging. 2001;18:561–573. doi: 10.2165/00002512-200118080-00001. [DOI] [PubMed] [Google Scholar]
- 106.Szallasi A, Appendino G. Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. Are we putting the cart before the horse. J Med Chem. 2004;47:2717–2723. doi: 10.1021/jm030560j. [DOI] [PubMed] [Google Scholar]
- 107.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 108.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain. 2004;8:129–133. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 109.Van der Stelt M, Di Marzo V. Endovanilloids, Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 110.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature–dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 112.Wahl P, Foged C, Tullin S, Thomsen C. Iodo-Resiniferatoxin, a New Potent Vanilloid Receptor Antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- 113.Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Szabo T, Welter JD, Toth A, Tran R, Lee J, Kang SU, Suh YG, Blumberg PM, Lee J. High affinity antagonists of the vanilloid receptor. Mol Pharmacol. 2002;62:947–956. doi: 10.1124/mol.62.4.947. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y, Kedei N, Wang M, Wang QJ, Huppler AR, Toth A, Tran R, Blumberg PM. Interaction between protein kinase C-mu and the vanilloid receptor type 1. J Biol Chem. 2004;279:53674–53682. doi: 10.1074/jbc.M410331200. [DOI] [PubMed] [Google Scholar]
- 116.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal track. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 117.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreativity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- 119.Yura T, Mogi M, Ikegami Y, Masuda T, Kokubo T, Urbahns K, Yoshida N, Marumo N, Shiroo M, Masaomi T, Takeshita K, Moriwaki T, Tusikimi Y. WO 03/055484. Urea derivatives. 2003
- 120.Yura T, Mogi M, Ikegami Y, Masuda T, Kokubo T, Urbahns K, Yoshida N, Marumo N, Shiroo M, Masaomi T, Takeshita K, Moriwaki T, Tusikimi Y. WO 03/095420. Hydroxytetrahydronaphtalenylurea derivatives. 2003
- 121.Zheng J, Dai C, Steyger PS, Kim Y, Vass Z, Ren T, Nuttal AL. Vanilloid receptors in hearing, altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti. J Neurophysiol. 2003;90:444–455. doi: 10.1152/jn.00919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zymunt PM, Peterson J, Anderson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediates the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]