Abstract
Prostaglandins, in particular PGE2 and prostacyclin PGI2 have diverse biological effects. Most importantly, they are involved in inflammation and pain. Prostaglandins in nano- and micromolar concentrations sensitize nerve cells, i.e. make them more sensitive to electrical or chemical stimuli. Sensitization arises from the effect of prostaglandins on ion channels and occurs both at the peripheral terminal of nociceptors at the site of tissue injury (peripheral sensitization) and at the synapses in the spinal cord (central sensitization). The first step is the binding of prostaglandins to receptors in the cell membrane, mainly EP and IP receptors. The receptors couple via G proteins to enzymes such as adenylate cyclase and phospholipase C (PLC). Activation of adenylate cyclase leads to increase of cAMP and subsequent activation of protein kinase A (PKA) or PKA-independent effects of cAMP, e.g. mediated by Epac (=exchange protein activated by cAMP). Activation of PLC causes increase of inositol phosphates and increase of cytosolic calcium. This article summarizes the effects of PGE2, PGE1, PGI2 and its stable analogues on non-selective cation channels and sodium, potassium, calcium and chloride channels. It describes the mechanism responsible for the facilitatory or inhibitory prostaglandin effects on ion channels. Understanding these mechanisms is essential for the development of useful new analgesics.
Key Words: Prostaglandin, cAMP, Epac, protein kinase A and C, dorsal root ganglion cells, neuroblastoma cells, ion channels, sensitization
INTRODUCTION
Prostaglandins are potent and ubiquitous lipid messenger molecules. Arachidonic acid, released from phospholipids by the action of phospholipase A2, is converted by the cyclooxygenase enzymes to prostaglandin H2. Prostaglandin H2 is, in turn, the precursor of multiple prostaglandins.
Prostaglandins have a variety of functions. The present review concentrates on the effects of prostaglandins on ion channels. Such effects have been mainly studied on nerve cells and, more recently, in heterologous expression systems.
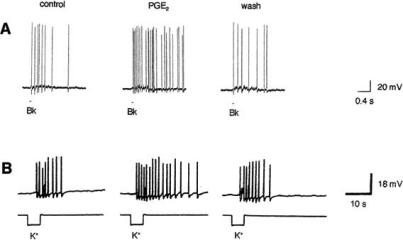
An effect on ion channels underlies the well known sensitizing action of prostaglandins PGE2 and PGI2. They lower the firing threshold of sensory neurons and increase the number of action potentials elicited by a suprathreshold stimulus. Thereby, they “induce a state of hypersensitivity in which normally non-painful stimuli become painful – for example, the rubbing of clothes against sunburnt skin” (quoted from [94]). In recordings from isolated sensory neurons grown in culture, treatment with PGE2 enhances the number of action potentials generated by exposure either to elevated levels of potassium or focally applied bradykinin (Fig. 1). Prostaglandins often lower the resting potential, but sensitization is not simply the consequence of the depolarization. It is also seen when the resting potential changes little [80, 53] or is brought back to its normal value by a hyperpolarizing current (Fig. 2), suggesting a direct effect of prostaglandins on Na+ or K+ channels.
Fig. (1).
Examples for the sensitizing action of PGE2. Treatment with PGE2 (1 μM in A, 0.1 μM in B) enhances the number of action potentials elicited in rat sensory neurons by 100 nM bradykinin (Bk) (A) or 50 mM K+ (B). From [79] and [5].
Fig. (2).
Sensitization of a colonic dorsal root ganglion cell by PGE2. Bath application of 1 μM PGE2 decreases the action potential threshold (top) and increases the number of action potentials elicited by a suprathreshold stimulus (bottom). The resting potential was -57 mV before PGE2. Hyperpolarizing current was used to maintain the membrane potential at -57 mV after PGE2 application. From [34].
The enzyme cyclooxygenase (COX) exists in two isoforms: COX-1 and COX-2 (for review, see [41]). COX-1 is constitutively expressed in most tissues throughout the body. COX-2 has a restrictive pattern of distribution under normal conditions, yet is highly induced at sites of inflammation and cell proliferation. Substances which inhibit the cyclooxygenase enzymes, in particular selective COX-2 inhibitors, play an important role in the treatment of inflammation and arthritis. The effect of NS-398, a selective COX-2 inhibitor, on hippocampal slices is opposite to that of PGE2: It reduces the frequency of action potentials elicited by a depolarizing current [19].
Prostaglandins bind to receptors on the cell surface which couple to G proteins. Various types of prostanoid receptors have been described: DP, EP, FP, IP and TP receptors (for review, see [15, 24, 77, 113]). PGE2 binds to EP receptors and only at high concentrations to IP receptors. PGE1, PGI2 and most of its stable analogues bind to IP and EP receptors. Both EP and IP receptors are important in inflammatory pain [10]. Agonists and antagonists for the various prostanoid receptors have been described, but truly selective ligands with high potency are rare. The affinity of prostanoids and selective agonists for the mouse and rat prostanoid receptors has been studied in detail [12, 58]. Agonists acting on human prostanoid receptors have also been investigated [1, 120]. In addition to the classical agonists, a new generation of specific EP agonists and antagonists has been developed by Ono Pharmaceutical Co. Ltd.. Chemical formulae, EC50 values and Ki values are found in [123] and [113]. For IP receptors, the stable PGI2 analogue cicaprost has long been regarded as the “standard IP receptor agonist” [121] because of its high selectivity for IP receptors. A novel IP agonist is the compound ONO-54918-07 [47, 76].
A). PGE2 ACTING ON ION CHANNELS VIA EP RECEPTORS
Non-Selective Cation Channels
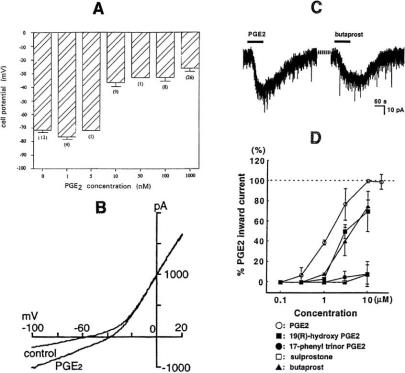
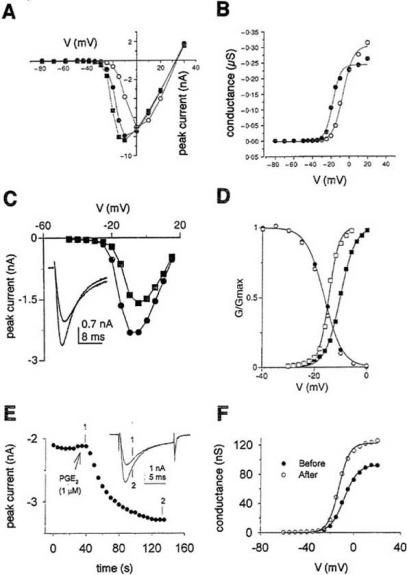
PGE2 can depolarize cells by activating non-selective cation channels. In voltage-clamp experiments, it elicits an inward current. Examples are shown in Fig. 3. At colon crypt cells the depolarization starts at 10 nM and increases only slightly at higher concentrations (Fig. 3A). On voltage-clamped nerve cells of the nucleus tractus solitarius, PGE2 markedly affects the current-voltage curve at voltages more negative than -30 mV (Fig. 3B): It augments the inward current, shifts its reversal potential in the positive direction and increases the slope of the curve. The effect of PGE2 on the membrane current of spinal cord neurons is illustrated in Fig. 3C and D and is mimicked by other EP receptor agonists. The depolarization and the inward current are not affected by changing the internal or external Cl- concentration or by replacing the external Na+ with other monovalent cations like K+, Rb+ or Cs+, but vanish when all cations are replaced by N-methyl-D-glucamine.
Fig. (3).
Effect of PGE2 on non-selective cation channels. A: Membrane potential of rat colon crypt cells at different PGE2 concentrations. From [104]. B: Voltage-clamp recording from neuron of the rat nucleus tractus solitarius with ramp command voltage (-100 to +20 mV in 1.8 s) in control and in 0.1 μM PGE2. From [68]. C and D: Effect of PGE2 and other EP receptor agonists on the membrane current of voltage-clamped neurons in rat spinal cord slices (holding potential -70 mV, agonist concentration 10 μM in C). From [4].
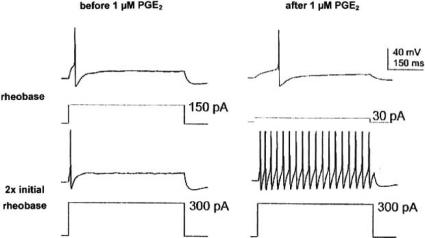
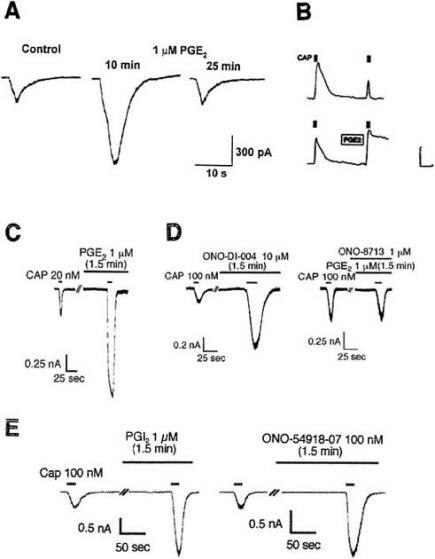
It is tempting to speculate that the PGE2-activated non-selective cation channels in the experiments of Fig. 3 belong to the superfamily of TRP (=transient receptor potential) channels (for review, see [23]). Such channels are present in most cells, e.g. in dorsal root ganglion (DRG) cells (see Table 1 of [117]). TRPV1 channels in DRG cells are receptors for the vanilloid capsaicin. They are opened by capsaicin, resulting in an inward current carried by Na+ and Ca+ and reversing at approximately 0 mV. Capsaicin sensitivity is completely absent in TRPV1 knock-out mice. The capsaicin-induced inward current is markedly enhanced by prostaglandins (Fig. 4). This phenomenon was first observed by Pitchford and Levine [85] and Lopshire and Nicol [64] and recently studied by Narumiya’s group [76]. Fig. 4A shows the transient inward current following focal application of capsaicin. A second application after a 10 min exposure to PGE2 produces a larger current. After 25 min exposure the sensitization by PGE2 has disappeared. The inward current is accompanied by a rise in internal [Ca 2+] (Fig. 4B). Normally, a second application 10 min later has little effect (Fig. 4B top). It becomes, however, highly effective after pretreatment with 0.25 μM PGE2 (Fig. 4B bottom). Likewise, augmentation of the Ca2+ transient by PGE2 has been observed in vagal sensory neurons [36]. Potentiation of the capsaicin-activated current by PGE2 is also seen in human embryonic kidney-derived HEK293 cells expressing rat TRPV1 and mouse EP1 receptor (Fig. 4C), but not with other mouse EP receptors. The specific EP1 agonist ONO-DI-004 mimics the PGE2 effect and the EP1 antagonist ONO-8713 prevents it (Fig. 4D). PGI2 and the specific IP agonist ONO-54918-07 act in a similar way to PGE2 (Fig. 4E). PGE2 (10 nM) enhances also heat-activated non-selective cation current in a subpopulation of cultured rat DRG cells [87]. Furthermore, PGE2 (10 μM) sensitizes mechanosensitive cation channels of cultured DRG neurons by shifting the relation between channel activity (NPO) and pressure to smaller pressures [20].
Table 1.
EP Receptor Subtypes Mediating the PGE2 Effect on Different Preparations
| Receptor | Preparation | Effect | Mimicked by | Reference |
|---|---|---|---|---|
| EP1/3 | melanotrophs | inhibition of ICa | sulprostone | [111] |
| EP1/4 | TRPV1 channel and dorsal root ganglion | enhancement of capsaicin effect | ONO-DI-004 | [76] |
| EP2 | spinal dorsal horn | depolarization | butaprost | [4] |
| EP2 | spinal dorsal horn | block of inhibitory glycin receptors | butaprost | [3] |
| EP2 | nodose and jugular ganglion | action potential firing | butaprost | [59] |
| EP2/4 | nodose ganglion | increase in TTX-R INa | ONO-AE1-259 ONO-AE1-329 |
[67] |
| EP2/4 | osteoclasts | activation of ICl | ONO-AE1-259 ONO-AE1-329 |
[83] |
| EP3 | dorsal raphe nucleus | depolarization | M&B 28767 | [74] |
| EP3 | paratracheal ganglion | inhibition of ICa | sulprostone | [48] |
| EP3 | trigeminal ganglion | inhibition of ICa | ONO-AE-248 | [14] |
| EP3C/4 | dorsal root ganglion | cAMP increase | * | [108] |
| EP4 | supraoptic nucleus | depolarization | ONO-AE1-329 | [100] |
blocked by antisense oligonucleotides.
Fig. (4).
Sensitization of TRPV1 channels by prostaglandins. Records from mouse DRG cells, except record C which is from a HEK293 cell expressing rat TRPV1 and mouse EP1 receptor. A: PGE2 enhances the amplitude of the inward current elicited by capsaicin. From [64]. B: PGE2 augments the Ca2+ signal produced by 20 nM capsaicin (calibration 0.2 ΔF/F and 150 s). From [44]. C: PGE2 enhances the inward current elicited by capsaicin in a HEK293 cell coexpressing TRPV1 with EP1. D: The EP1 agonist ONO-DI-004 mimics the PGE2 effect, the EP1 antagonist ONO-8713 prevents it. E: PGI2 and the IP agonist ONO-54918-07 also enhance the capsaicin effect. C-E from [76].
A special type of non-selective cation current is the hyperpolarization-activated current Ih. In a subpopulation of cultured guinea-pig neurons, 1 μM PGE2 produces a small (18%) increase of its amplitude and a small shift (+4 mV) of its voltage dependence [46].
Sodium Channels
The action of PGE2 on tetrodotoxin-resistant (TTX-R) Na+ currents in DRG cells offers a ready explanation for the sensitizing, hyperalgesic effect of PGE2 and has therefore received much attention (for review, see [61, 122]). In addition to TTX-sensitive Na+ channels and multiple types of Ca+ channels, nociceptive DRG cells express two types of slowly gated TTX-R Na+ channels, Nav1.8 (=PN3, SNS) and Nav1.9 (=PN5,NaN) channels (for details, see [92]). The major transient TTX-R channel isotype is Nav1.8 while persistent TTX-R Na+ currents which inactivate slowly and incompletely are probably flowing through Nav1.9 channels. The distribution of the sensory neuron-specific Nav1.8 channels is restricted mainly to those subpopulations of DRG cells that give rise to unmyelinated C-fibres. As illustrated in Fig. 5, 1 μM PGE2 enhances the TTX-resistant Na+ current in DRG cells by shifting its activation curve to more negative potentials and increasing its slope. This will increase the excitability of sensory neurons. The effect is observed on cutaneous and colonic DRG cells and also on pulmonary DRG neurons [60]. There are, however, several striking differences between cutaneous and colonic neurons with respect to PGE2-induced changes in resting potential and excitability [34]. The importance of Nav1.8 channels is emphasized by the finding [57] that PGE2-induced hyperalgesia is inhibited by antisense oligonucleotides directed against Nav1.8 channels. The PGE2 effect on Nav1.9 channels was studied in both Nav1.8–null and wild-type mice [93]. In these experiments, treatment with 10 μM PGE2 for 1 h increased the persistent TTX-R Na+ current attributed to Nav1.9; the increase will lead to depolarization and augmented firing of spikes. A 5 min exposure to PGE2 has no effect on Nav1.9 [6]. Other inflammatory mediators like adenosine and serotonin have been shown to modulate the activity of TTX-R Na+ current in a manner similar to that seen for PGE2. TTX-R Na+ channels are therefore drug targets for pain therapy [25].
Fig. (5).
PGE2 (1 μM) increases the TTX-resistant Na+ current INa of dorsal root ganglion (DRG) cells and shifts the Na+ conductance vs. voltage curve g(V) to the left. A: INa(V) curves of neonatal rat DRG cells in control (○) and 3 (●) or 5 min (■) after application of PGE2. B: g(V) curves for the experiment in A. A and B from [26]. C: INa(V) curves of rat DRG cells before (■) and after (●) application of PGE2. Inset shows currents at V=0 mV. D: g(V) curves before (■) and after (□) PGE2 and steady-state inactivation curve (circles) from a similar experiment. C and D from [31]. E: Effect of PGE2 on the peak INa of a colonic rat DRG cell recorded at V=0 mV. Inset shows currents at times indicated by 1 and 2. F: g(V) curves from the experiment in E. E and F from [33]. All measurements in the presence of TTX (50 nM – 1 μM).
Potassium Channels
Possibly, the enhancement of excitability by PGE2 results also in part from the suppression of the delayed rectifier current. In DRG cells of embryonic rats, 1 μM PGE2 produces a time-dependent suppression of IK [80]. After a 20 min exposure IK at +60 mV is reduced by 48%. PGE2 (1 μM) inhibits the IK of neonatal rat DRG neurons by only 10% [26] and the IK of primary cultured hippocampal neurons by 22.5% [19]. A 50% inhibition of IK by 10 μM PGE2 has been reported for T-lymphocytes [7]. In vascular smooth muscle cells, a 50% inhibition of IK requires 3.5 μM PGE2 [89]. All these observations can be summarized by saying that PGE2 concentrations between 1 and 10 μM inhibit IK by 50% or less. Much greater PGE2 sensitivity has been reported for the lipopolysacchande-induced K+ outward current in cultured rat microglia: 50% inhibition by 10 nM, 80% inhibition by 100 nM PGE2[17].
PGE2 (100 nM) almost totally blocks the Ca2+-activated K+ current which gives rise to a slow, Ca2+-dependent spike after-hyperpolarization in rabbit nodose neurons [118]. By contrast, 5 nM PGE2 activates large conductance Ca2+-activated K+ channels from otherwise silent patches in human osteoblast bone cells [75]. PGE2 (10 μM) also activates G protein-gated inwardly rectifying K+ (GIRK) channels heterologously expressed in rat superior cervical ganglion [91].
Calcium Channels
Depending on the preparation studied, 1 μM PGE2 either enhances or inhibits the hva (=high-voltage-activated) Ca2+ current ICa. On cultured chicken DRG neurons held at V=-60 mV and pulsed to +10 mV, peak ICa is 1.8fold increased by 1 μM PGE2 [78]. On other preparations a strong inhibition is observed. Fig. 6 illustrates some of these results. PGE2 produces a marked reduction of the sympathetic neuron Ca2+ current accompanied by a pronounced slowing of its rising phase (Fig. 6A and B). In paratracheal ganglion cells, nanomolar concentrations of PGE2 are sufficient for a clear inhibition of ICa (IC50=6.4 nM) and this effect is likewise observed with various prostanoid agonists (Fig. 6C). The inhibitory effect of PGE2 in concentrations ≥10 nM in mouse trigeminal neurons is also mimicked by an EP3 agonist (Fig. 6D). (See below for a full discussion of Fig. 6C and D). The slowing of the Ca2+ current rising phase, conspicuous in Fig. 6A, was less pronounced or absent in the experiments of Fig. 6C and D. Only a weak (11%) inhibition of 1Ca by 1 μM PGE2 has been seen on hippocampal CA1 pyramidal neurons [19]. Inhibition of Ica by PGE2 is not only seen in nerve cells. In rabbit carotid body chemoreceptor cells [35] and rat melanotrophs [111] the inhibition of ICa observed with 0.3 or 1 μM PGE2 is 54 and 35%, respectively.
Fig. (6).
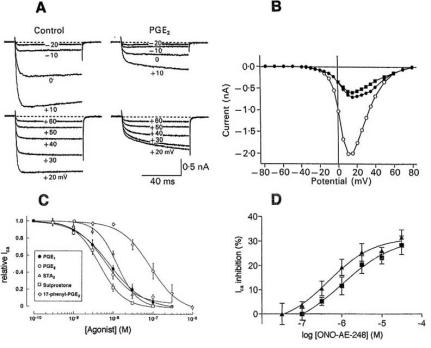
Inhibition of hva (=high-voltage-activated) Ca2+ currents by PGE2 and various EP agonists. A: Leak-subtracted Ca2+ currents of a rat superior cervical ganglion cell in control and in 1 μM PGE2. B: Current-voltage curves from the experiment in A in control (o) and in PGE2 (● , current 10 ms after start of pulse; ■, rapid component of Ca2+ current rising phase determined by double exponential fit). A and B from [45]. C: Concentration-response curves for the inhibition of hva Ca2+ current of rat paratracheal ganglion cells by various prostanoid agonists. Peak Ca2+ currents were measured with pulses to +10 or +20 mV and expressed as fraction of control current. From [48]. D: Inhibition of hva Ca2+ current of Type 1 (■) and Type 2 (▲) mouse trigeminal neurons by the EP3 agonist ONO-AE-248. From [14].
Chloride Channels
Inratosteoclasts, PGE2 (>10 nM) stimulates an outwardly rectifying Cl- current in a concentration-dependent manner and causes a long-lasting depolarization [83]. Likewise, PGE2 (10 μM) activates the Cl- current in pigmented ciliary epithelial cells [29].
Synaptic Channels
In contrast to the activation of Cl- currents just mentioned, PGE2 partially inhibits the glycin-operated Cl- influx and the resulting inhibitory postsynaptic currents at synapses in the rat spinal cord [3]. PGE2 acts via the EP2 receptor and PKA on the α3 glycinreceptor [3, 40, 127]. No effect of PGE2 on other inhibitory or excitatory spinal synapses, operated respectively by GABA, AMPA or NMDA, was seen [3]. Another study on rat spinal cord [4] also failed to observe an effect of PGE2 on glutamatergic excitatory postsynaptic currents (EPSCs) whereas in the mouse spinal cord a long-lasting facilitation of EPCPs by PGE2 was found [72]. PGE2 seems also able to modulate synaptic transmission in the sympathetic nervous system. In cultured chick sympathetic ganglion neurons, PGE2 (25 nM) inhibits significantly the fast inward current flowing through acetylcholine-activated (AChR) channels [110]. The effect results from a decrease in the frequency of channel opening with no change in open time and conductance. PGE2 (0.1-1 μM) also reduces the amplitudes of both fast and slow EPSPs in guinea-pig gallbladder ganglia [49].
Which Types of EP Receptor are Involved?
To answer this question selective agonists for the various EP receptors are required. Until recently, only substances with limited selectivity or low potency were available. Examples of such drugs are sulprostone which binds to both EP1 and EP3 receptors with high affinity and butaprost, the classical EP2 receptor agonist, which suffers from low potency. As mentioned above, recently a new generation of highly selective, potent agonists for the four EP receptors has been developed: ONO-DI-004 (EP1 agonist), ONO-AE1-259 (EP2 agonist), ONO-AE-248 (EP3 agonist) and ONO-AE1-329 (EP4 agonist) [123]. The four new receptor agonists – even at concentrations 1000 times higher than their respective Ki values – have little effect on Chinese hamster ovary (CHO) cells expressing other EP receptors.
Examples of experiments using different EP agonists are illustrated in Fig. 3C and D, Fig. 4D and Fig. 6C and D. As shown in Fig. 3C and D, the effect of PGE2 on the membrane current of spinal cord neurons is mimicked by the EP2 agonist butaprost and the non-selective EP agonist 19(R)-hydroxy PGE2, but not by the EP1/EP3 agonist sulprostone and the EP1 agonist 17-phenyl trinor PGE2. By contrast, sulprostone, the TP ligand STA2 and the highly selective EP3 agonist ONO-AE-248 potently inhibit the Ca2+ current of nerve cells as does PGE2 (Fig. 6C and D). In Fig. 4D, the EP1 agonist ONO-DI-004 mimics the potentiating effect of a short (1.5 min) PGE2 application on TRPV1 channels. Data obtained by such experiments on various preparations are collected in Table 1. A study with antisense oligonucleotides is also included. As can be seen, all four receptor subtypes EP1, EP2, EP3, its splice variant EP3C and EP4 are found.
As shown in Table 1, the potentiating effect of PGE2 on the capsaicin-induced current through TRPV1 channels [76] is mediated by EP1 (see Fig. 4D) as well as EP4 receptors. The pathway downstream of EP1 involves PKC and PLC, the pathway via EP4 causes PKA activation and comes only into play during a fairly long (6.5 min) PGE2 application (see below). Stimulation of EP1 can also cause a robust increase in [Ca2+]i as demonstrated on Chinese hamster ovary (CHO) cells expressing the mouse EP1 receptor [54] (for details, see below). EP2 receptors mediate the PGE2 effects on membrane potential and glycine receptors in rat spinal dorsal horn [3, 4]. Both EP2 and EP4 are known to activate adenylate cyclase via G protein Gs. Thus, the experiments described in [67] support the view that the increase in TTX-R INa by PGE2 is caused by an increase in cAMP level. The same is true for the stimulation of outwardly rectifying Cl- current in osteoclasts observed in [83]. The situation is less clear with regard to EP3 which according to Table 1 seems to be the receptor subtype responsible for inhibition of ICa-EP3 receptors and their isoforms generally inhibit cAMP generation via Gi, but additional signaling mechanisms have also been described. They include stimulation of adenylate cyclase via Gs, Ca2+ mobilization via Gq and signaling through the small G protein Rho (for literature, see [15, 113]). Any of these mechanisms could be responsible for the EP3-mediated PGE2 effects (see below).
B). PGI2 AND PGE1 ACTING ON ION CHANNELS VIA IP RECEPTORS
Whereas EP receptors have equal affinity to PGE1 and PGE2 (Fig. 6C), IP receptors have high binding affinity to PGI2 as well as PGE1, but not to PGE2. Interaction of PGE2 with rat IP receptors occurs only at PGE2 concentrations approximately 300fold higher than its Kd for the rat EP2 and EP4 receptors (quoted from [90]). Comparison between PGE1 and PGE2 sensitivity can be used to distinguish between EP and IP receptors. In addition, two fairly selective IP agonists, cicaprost and the novel ONO-54918-07, are available. However, cicaprost in a concentration of 1 μM is likely to stimulate also EP3 receptors (also quoted from [90]). When studying the cardioprotective action of PGI2 and cicaprost, stimulation of EP3 receptors by 10 μM cicaprost but not by 1 μM was observed [102].
According to [82], prostacyclin receptor mRNA is most abundantly expressed in neurons of the DRG and moderately in arterial smooth muscle cells of a variety of tissues. Likewise, neuroblastoma and neuroblastoma x glioma hybrid cells have a high density of IP receptors.
Arterial Smooth Muscle
PGI2 and its stable analogue iloprost have a potent vasodilatory effect. At concentrations between 1 nM and 1 μM iloprost hyperpolarizes the smooth muscle cells of the canine carotid artery, presumably by opening K+ channels, and relaxes the tension of isolated carotid segments [103]. In smooth muscle cells of the rat tail artery, iloprost (0.5 μM) enhances the outward current through Ca2+-activated and ATP-sensitive K+ channels [96, 97].
DRG Cells and Other Neurons
PGI2 like PGE2 enhances the non-selective inward current elicited by capsaicin in mouse DRG cells [85]. The potentiating effect of PGI2 and ONO-54918-07 on the capsaicin-induced current through TRPV1 channels was recently studied by Narumiya’s group [76] and has been illustrated in Fig. 4E. Earlier work had been concerned with the effect of PGI2 and IP agonists on K+ channels. On embryonic rat DRG cells, a time dependent inhibition of the delayed rectifier current not only by PGE2 but also by the IP agonist carba prostacyclin (1 μM) was found [80], confirming the view that DRG cells possess both EP and IP receptors. In guinea pig nodose ganglion neurons, 0.1 μM prostacyclin abolishes the Ca2+-activated K+ current that gives rise to a slow afterhyperpolarization [114]. PGE2 (0.3 μM) had no effect, but inhibited the slow afterhyperpolarization in rabbit nodose ganglion cells (see above).
At the rat isolated vagus nerve, PGI2 in concentrations as low as 0.1-1 nM causes depolarization [105]. The rank order of agonist potency is PGI2 = PGE1 > PGE2, consistent with IP receptor activation.
Neuroblastoma and Neuroblastoma x Glioma Hybrid Cells
In a comparison of selected activators of adenylate cyclase in the NCB-20 neuronal hybrid cell line, prostacyclin was observed to be 17 times more potent than PGE1 and over 1000 times more potent than PGE2 [8], PGI2 and PGE1 compete for a single receptor in these cells [9]. Likewise, the receptor on mouse neuroblastoma x rat glioma hybrid NG108-15 cells for prostanoid agonists has been demonstrated to have the pharmacological profile of a prostacyclin receptor (quoted from [2]). It binds PGE1 with a KD of 13-20 nM [69]. Cicaprost, the standard IP receptor agonist, potently activates cAMP accumulation in F-11 (embryonic rat DRG x neuroblastoma hybrid), NG108-15 and human neuroblastoma SK-N-SH cells [105, 22].
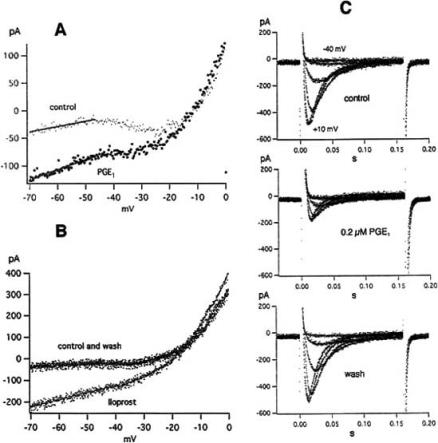
In neuroblastoma cells neither a capsaicin-induced inward current nor a PGE1 effect on the delayed rectifier have been found. PGE1 and iloprost have, however, a pronounced effect on the leakage current and the Ca2+ currents of voltage-clamped NG108-15 cells (Figs 7 and 8). Voltage ramps (Fig. 7A and B) and rectangular pulses (Figs 7C and 8) are used. The current-voltage curves recorded with voltage ramps consist of a linear leakage current (between -70 and -45 mV), an inward current (between -45 and -10 mV) and a strong outward current (between -10 and 0 mV). The latter two currents represent T-type Ca2+ current and delayed rectifier, respectively. PGE1 and iloprost have a clear and reversible effect on the leakage current and the T-type Ca2+ current whereas the delayed rectifier current is not affected. As seen in Fig. 7A, PGE1 (3 μM) increases the conductance (g) of the leakage current (estimated from the slope of the current-voltage curve in its most negative voltage range), shifts the current’s reversal potential Vrev (derived by extrapolating to I=0 the linear fitting of the I-V relationship) to more positive values and increases the inward current Ihold at the holding potential (-70 mV). The effect does not disappear when the NaCl in the bath is replaced by N-methyl-D-glucamine or TrisCl, indicating that PGE1 opens channels with large pore diameters. Iloprost (5 nM) acts in a way similar to PGE1 (Fig. 7B). PGE1 also inhibits the T-type Ca2+ current. The inhibition of T-type Ca2+ current by PGE1 is seen in Fig. 7A, but more clearly seen when rectangular clamp pulses are used (Fig. 7C). In a total of 39 experiments 0.2 μM PGE1 inhibited T-type Ca2+ current (at pulse potentials between -15 and 0 mV) by 37.1 ± 3.6% (mean ± SEM). The inhibition by 50 nM iloprost was 26.2 ± 5.7% (n=9).
Fig. (7).
| g [nS] | Vrev [mV] | |
| A control | 0.99 | -31 |
| PGE1 | 1.77 | 0 |
| B control | 0.70 | -21 |
| iloprost | 3.22 | -1 |
| wash | 0.80 | -21 |
Fig. (8).
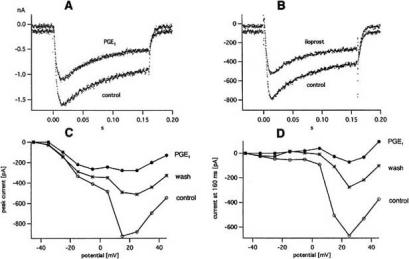
Effect of PGE1 and iloprost on the hva (=high voltage activated) Ca2+ current. A: Hva Ca2+ current at +15 mV in control and 0.2 μM PGE1. B: Hva Ca2+ current at +15 mV in control and 50 nM iloprost. C: Peak current vs pulse potential from a different cell in control, 3 μM PGE1 and wash. D: Same for current at end of 160 ms pulses. Ruptured patch with bath containing 10 mM Ca2+ and K+-free pipette solution. Holding potential -70 mV. Temperature 20-21ºC. From [71].
In further experiments the pulse potential range was extended in order to study the effect of PGE1 and iloprost on hva (=high voltage activated) Ca2+ currents. Such currents are shown in Fig. 8A and B). Their time course and amplitude distinguish them clearly from the fast inactivating, smaller T-type Ca2+ currents. The inhibition of the hva Ca2+ currents by PGE1 (0.2 μM) and iloprost (50 nM), visible in Fig. 8A and B), was stronger than the inhibition of the T-type Ca2+ currents measured on the same cell. Fig. 8C and D illustrate an experiment with 3 μM PGE1. The current-voltage curves for the peak current and the current at pulse end show a conspicuous kink at +5 mV. Upon application of 3 μM PGE1, the peak hva current in the entire potential range from +15 to +45 mV was inhibited by 69-77% while the peak T-type current was only decreased by 36%. The difference is even more pronounced in Fig. 8D because hva current at the end of 160 ms pulses is not contaminated by T-type current.
The smallest PGE1 concentration which in some cells produced an effect was 2.4 nM. The mean values for ΔIhold and gPGE/gcontr measured with 22 nM were similar to the mean values for 0.2 and 3 μM. Likewise, the curve for cAMP production versus [PGE1] rises steeply to its maximum in the nanomolar range (see Fig. 17 of [38]). The same was true for iloprost. Mean values of ΔIhold and giloprost/gcontr were similar for 5, 50 and 200 nM (compare with curve cAMP production versus [iloprost] in Fig. 4 of [56]). The smallest effective iloprost concentration was 1 nM. The sensitivity to PGE1 and iloprost varied greatly from cell to cell. This made it difficult to obtain proper dose-response curves. With increasing passage number cells often ceased to respond at all.
IP receptors couple to Gs and Gq/11 and in some cases to Gi. They activate adenylate cyclase via Gs and phospholipase C (PLC) via Gq/11. Coupling to Gi, leading to inhibition of adenylate cyclase, is cell type-dependent for the mouse IP receptor and absent for the human IP receptor [22]. Thus, either increase of cAMP or [Ca2+], or activation of PKC is responsible for the effects of PGE1, PGI2 and its stable analogues (see below).
C). MECHANISM OF ACTION
EP Receptors Acting via cAMP-Dependent Potein Kinase A or via Protein Kinase C
Exposing sensory neurons to PGE2 at concentrations that sensitize causes an increase in the production of cAMP. This has been first reported by Hingtgen, Waite and Vasko in 1995 [43] and then confirmed in many later publications. Prostaglandin effects are often mediated by cAMP activated protein kinase A (PKA). Such prostaglandin effects are mimicked by forskolin or membrane-permeable cAMP analogues and blocked by compounds that inhibit adenylate cyclase or PKA. The best examples are the effects of PGE2 on TTX-R Na+ channels and on K+ channels. They disappear when a peptide inhibitor of PKA or the powerful cAMP antagonist Rp-cAMPS, a phosphorothioate derivative of cAMP, are included in the pipette solution [26, 32, 27]. The observations on rat DRG cells were confirmed on TTX-R Na+ channels expressed in COS-7 cells, a cell line derived from African green monkey kidney [28]. The effect of forskolin vanished when all five PKA consensus phosphorylation sites within the intracellular I-II loop had been eliminated by site-directed mutagenesis (Fig. 9). Also, the enhancement of the capsaicin-induced inward current by PGE2 (see Fig. 4A) is mimicked by forskolin and completely blocked by intracellular perfusion of the PKA inhibitor PKI14-24 [64]. Sensitization of mechanosensitive channels by PGE2 is blocked by the PKA inhibitor H-89, an isoquinoline sulfonamide [20]. On osteoclasts, forskolin or dibutyryl cAMP reproduce the stimulation of Cl- current by PGE2 whereas the cAMP antagonist Rp-cAMPS and the PKA inhibitor H-89 abolish it [83].
Fig. (9).
Effect of 10 μM forskolin on TTX-R INa expressed in COS-7 cells. A: Wild-type SNS. B: Mutant SNS (SA) with all five PKA consensus sites eliminated. Peak Na+ currents (elicited by stepping from -90 to +15 mV (A) or +20 mV (B)) are plotted against time. Currents recorded at times 1 and 2 are shown in insets. From [28].
So far, the situation seemed straightforward. Further work showed, however, that it is in reality more complicated. First, it turned out that in addition to PKA the protein kinase C (PKC) plays an important role. Second, results were published which seem to suggest that under certain conditions neither PKA nor PKC are involved in the sensitizing effect of PGE2.
It was found that augmentation of TTX-R INa by PGE2 is attenuated not only by inhibitors of PKA (Rp-cAMPS or Wiptide) but also by the PKC inhibitors PKC19-36 or staurosporine, “suggesting that PKC activity is necessary for PGE2-induced modulation of TTX-R INa” [32]. A recent study [76] on adult mouse DRG cells and on HEK293 cells (expressing TRPV1 channels and either EP1 or EP4 receptors) has shown that potentiation of capsaic-activated currents by PGE2 (see Fig. 4C-E) involves both PKC-dependent and PKA-dependent pathways. A short PGE2 application (1.5 min) activates the pathway EP1 — PKC, a longer application (6.5 min) is required to stimulate the pathway EP4 — PKA. The effect of a short PGE2 application is almost completely prevented by the specific PKC inhibitor calphostin C, blocked by the EP1 antagonist ONO-8713, significantly diminished by the PLC inhibitor U73122, mimicked by the EP1 agonist ONO-DI-004 and by the PKC activator PMA (phorbol 12-myristate 13-acetate). A mixture of forskolin, IBMX and dbcAMP, applied for 6.5 min, reproduces the effect of a long PGE2 application.
PKA and PKC phosphorylate serine or threonine residues on ion channels, thereby modulating their function. Na+ channels have five sites of cAMP dependent phosphorylation (see Fig. 9), all situated on the large intracellular loop that connects domains I and II of the α subunit. The effects of phosphorylation on Na+ channels are fully discussed in a recent review [18]. Two target serine residues are found in the capsaicin receptor TRPV1 expressed in HEK293 cells [81]. They are phosphorylated by PKCε and responsible for the potentiation of capsaicin-evoked currents by the phorbolester PMA.
Two studies did not confirm the view that the potentiating PGE2 effect on capsaicin-induced current and TTX-R Na+ current is mediated by PKA and PKC. In the experiments of Lee, Lee, Jung, Oh and Kaang [62], activation of PKA by 8-Br-cAMP or forskolin with IBMX failed to enhance capsaicin-evoked inward currents in Xenopus oocytes expressing TRPV1 channels. A recent study on neonatal rat nodose ganglion neurons suggested that the increase in TTX-R INa induced by PGE2 application may not involve the activation of either PKA or PKC activity [67]. In these experiments, bath application of 1 μM PGE2 produced the well known increase of TTX-R INa together with a leftward shift of the Na+ conductance curve gNa(V). A subsequent intracellular application of the PKA inhibitor PKI (in the continuing presence of 1 μM PGE2) decreased INa again, but did not reverse the shift of the gNa(V) curve. Likewise, staurosporine reversed the PGE2 effect on the peak amplitude of INa, but had no significant effect on the hyperpolarizing shift of gNa(V). Obviously, it depends very much on the experimental conditions whether evidence for the involvement of PKA and PKC is obtained. Further work must clarify the problems.
EP Receptors Acting via Increase of Intracellular Calcium
Clearly, some of the PGE2 effects are caused by an increase of intracellular calcium. As mentioned above, PGE2 acting on EP1 and EP3 receptors can increase [Ca2+]i. On CHO cells stably expressing the mouse EP1 receptor the increase in [Ca2+]i elicited by the EP1 agonist sulprostone consists of two parts [54]. Most of it is caused by Ca2+ influx from the bath, a small part by Ca2+ release from an internal store; the latter is sensitive to the PLC inhibitor U73122 and mediated via Gq/11 [109]. The increases in intracellular calcium elicited by PGE2 and various other agonists in HEK293 cells expressing apo-aequorin and the rat EP1 receptor have been measured [12]. The elevation of cytosolic calcium by PGE2 in neonatal rat DRG neurons has been studied [106]. PGE2 at 1 μM – in addition to increasing cAMP – evoked an increase of [Ca2+]i in 50% of the small DRG neurons. Pretreatment with H-89 reduced the number of cells that responded to 24%. The Ca2+ signal disappeared in Ca2+-free bath containing 0.2 mM EGTA and was not accompanied by an increase of IP3 levels, suggesting that it is caused by Ca2+ influx from the bath. This may occur either through voltage-gated calcium channels (as suggested by the authors) or through non-selective cation channels. Also on rat DRG neurons, the PGE2-induced Ca2+-influx is blocked by the non-selective calcium channel blocker Cd2+ [63]. The membrane permeant cAMP analogue dibutyryl cAMP acted similarly to PGE2. An increase of [Ca2+]i due to Ca2+ influx is most likely the reason for the activation of Ca2+-activated K+ channels in human osteoblast bone cells by PGE2 [75]. The effect is mimicked by cAMP + forskolin and disappears in Ca2+-free bath. An increase of [Ca2+]i could also be responsible for the inhibition of Ca2+ currents by 1 μM PGE2 in rat sympathetic neurons [45] illustrated in Fig. 6A and B. Inhibition became smaller when the pipette solution contained 10 mM BAPTA instead of EGTA.
cAMP Acting Directly on Ih Channels
As mentioned above, PGE2 increases the hyperpolarization-activated current Ih in some guinea-pig neurons [46]. The effect consists of a shift of the Ih activation curve to more positive potentials and is mimicked by forskolin. The PKA inhibitor PKI does not affect the activation curve and does not block the effect of forskolin, suggesting modulation of Ih through direct action of cAMP on the channel proteins. An enhancement of Ih by a PKA-independent, direct action of cAMP was also demonstrated in Ih channels of hippocampal pyramidal cells [84] and in heterologously expressed hyperpolarization-activated channels [65]. By contrast, a PKA-dependent regulation by cAMP was found in bullfrog sympathetic neurons [112] and more recently in rat olfactory receptor neurons [115]. Experiments on mouse DRG cells [86] suggest two separate processes responsible for the enhancement of Ih, a direct effect and, in addition, phosphorylation through PKA. Conflicting results were also obtained for the hyperpolarization-activated current in cardiac tissue. A direct cAMP effect was observed by DiFrancesco’s group (see [13]) whereas another study [125] reported an important role of phosphorylation via cAMP-dependent PKA. Obviously, phosphorylation-dependent modulation of Ih varies from cell type to cell type, a situation similar to that described above for the PGE2 effect on the capsaicin-elicited current and on TTX-R INa.
IP Receptors Coupling to Adenylate Cyclase and Phospholipase C
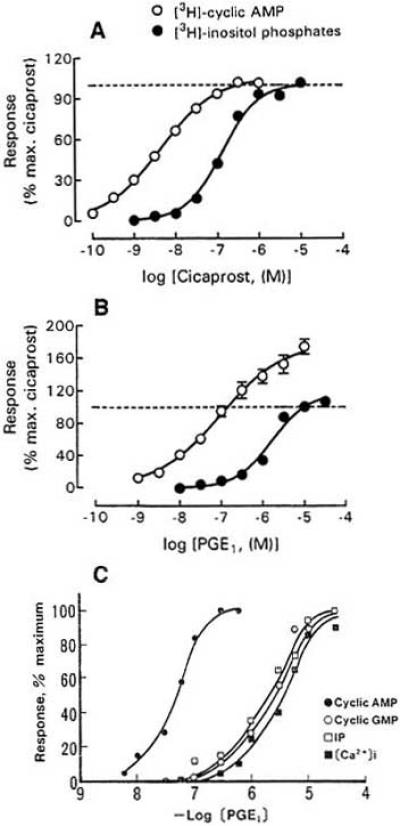
In the simplest case an IP agonist acts solely through elevation of intracellular cAMP. This is true for the iloprost-induced inward Cl- current observed in Xenopus oocytes coexpressing the human IP receptor and the cystic fibrosis transmembrane conductance regulator [11]. In general, however, the situation is more complicated because IP receptors not only couple to adenylate cyclase but also to PLC, i.e. produce inositol phosphates and [Ca2+]i increase in addition to cAMP (for review of the older literature, see [121]). The EC50 values for coupling to PLC are higher than those for cAMP production, especially in transfected cells. For the human prostacyclin receptor expressed in HEK293 cells and activated by iloprost EC50 = 0.1 and 43.1 nM for cAMP and inositol phosphate production, respectively, were obtained [107]. On CHO cells expressing the mouse prostacyclin receptor, PLC is stimulated with 10-40 fold lower potency than adenylate cyclase [21] (Fig. 10A and B). Accumulation of cAMP and [3H]-inositol phosphates in adult rat DRG cells treated with PGI2, cicaprost or iloprost has been described [105]. In these cells, cicaprost and iloprost stimulate adenylate cyclase activity with EC50 values of 22 and 28 nM, respectively [90]. Detailed quantitative studies were carried out on neuroblastoma cells (N1E-115) and neuroblastoma x glioma hybrid cells (NG108-15). As described above, these cells endogenously express IP receptors. Application of PGE1 causes a marked increase of cAMP, reaching a plateau after 10-15 min [39, 99]. The half maximal effect is seen at 20 nM in Fig. 17 of [38] and at 70 nM in Fig. 10C. Iloprost is an even more potent agonist. According to [119] the EC50 for iloprost is 24 nM, much lower than the average of ca. 100 nM for PGE1 estimated from the results of different authors. PGE2 is less effective than PGI2 and PGE1 (see Fig. 2B of [101], Fig. 3 of [42] and Table 1 of [50]). As illustrated in Fig. 10C, PGE1 also causes an increase of inositol phosphates, cGMP and cytosolic Ca2+ in N1E-115 cells, but the EC50 values (1-5 μM) are more than 10 times higher than the EC50 for cAMP production (70 nM). More recently, Chow, Jones and Wise [22] studied the effect of cicaprost on [3H]-cAMP and Gq/11-mediated [3H]-inositol phosphate production in NG108-15 cells and human neuroblastoma SK-N-SH cells as well as CHO and HEK293 cells expressing human or mouse IP receptors. Cicaprost activated PLC in all cells except the SK-N-SH cells, but the EC50 (49-457 nM) was much higher than the EC50 for cAMP production (1.5-22 nM).
Fig. (10).
Coupling of IP receptors to adenylate cyclase and phospholipase C. A: Production of [3H]-cAMP and [3H]-inositol phosphates in CHO cells transfected with mIP plotted against cicaprost concentration. B: Same for PGE1. C: Production of cAMP, cGMP, inositol phosphates and cytosolic calcium in neuroblastoma cells N1E-115 plotted against PGE1 concentration. The levels of cAMP, cGMP and inositol phosphates were measured after 20, 15 and 45 min incubation with the indicated PGE1 concentrations. The values for [Ca2+]i give the maximum reached at 10-20 s. A and B from [21], C from [50].
In the case of adipose cells, the PGI2-induced transient Ca2+ accumulation does not appear to depend on extracellular calcium, i.e. is due to release from an internal store [116]. In erythroleukemia cells, cicaprost and iloprost increase [Ca2+]i by mobilization from internal stores and influx from the extracellular space [98]. In other preparations Ca2+ entry from the bath prevails. Thus, the PGI2-induced increase of [Ca2+]i in rat DRG cells disappears in Ca2+-free bath [106]. According to [50] and [73] the increase of [Ca2+]i produced by PGE1 and other prostaglandins in neuroblastoma cells also depends on the presence of Ca2+ in the extracellular solution, i.e. reflects Ca2+ entry. It is important to notice that the increase of [Ca2+]i is only transient, reaching a peak after 10-20 s and returning to the base line within 2 min (see Fig. 3 of [73]), whereas the cAMP level is sustained for minutes. Fig. 10C gives the peak values for [Ca2+]i, but values for cAMP, cGMP and inositol phosphates determined after 15 – 45 min incubation (see figure legend).
The PGI2 effects on arterial smooth muscle and TRPV1 channels are mediated by PKA and PKC. The enhancement of outward currents through Ca2+-activated K+ channels by 0.5 μM iloprost in arterial smooth muscle (see above) appears to be caused by PKA phosphorylating the channels. The iloprost effect is mimicked by the catalytic subunit of PKA, applied via pipette together with 0.1 mM MgATP [96]. By contrast, the potentiation of the capsaicin-elicited inward current by PGI2 (see Fig. 4E) in mouse DRG neurons and HEK293 cells expressing TRPV1 channels and IP receptors involves both PKC and PKA [76]. The situation is similar to that described above for the PGE2 effect. A short application (1.5 min) of a fairly high PGI2 concentration (1 μM) activates predominantly the pathway IP → Gq → PKC, a long (6.5 min) treatment with 0.1 μM PGI2 stimulates the pathway IP → Gs → PKA. Blockers of PKC and PLC prevent only the effect of a short (1.5 min) PGI2 application.
Effects of PGE1 on Ionic Currents of Neuroblastoma Cells
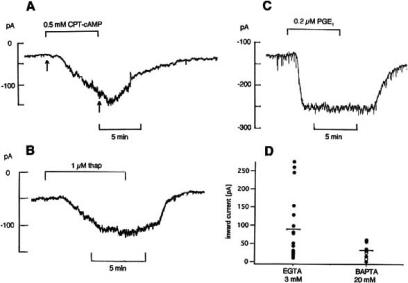
The effects of PGE1 and iloprost on ionic currents of NG108-15 cells (Fig. 7 and 8) are caused by an increase of both cAMP and [Ca2+]i. Experiments that support this view are shown in Fig. 11. An increase of holding current like that seen with PGE1 and iloprost is produced by the membrane permeable cAMP analogue CPT-cAMP (Fig. 11A), the adenylate cyclase activator forskolin and thapsigargin (Fig. 11B). The latter substance releases Ca2+ from internal stores and produces in NG108-15 and NlE-115 cells an immediate increase of [Ca2+]i by 150-200 nM [88, 66]. On average, the change in holding current was -151.5 ± 27.3 pA (n=5) in 0.5 mM CPT-cAMP [70] and -67.5 ± 17.9 pA (n=5) in 1 μM thapsigargin [71]. Together with the increase in holding current the current-voltage curves measured with voltage ramps become steeper between -70 and -50 mV, indicating an increase in conductance. In Ca2+ - and Mg2+-free bath solution with 1.1 mM EGTA, 0.2 μM PGE1 could still produce a clear change in holding current. It was -125 pA in Fig. 11C and on average -103.1 ± 28.1 pA (n=8) [71], As shown by the scatter plot in Fig. 11D, the PGE1 effect is, however, markedly reduced when the pipette solution contains 20 mM of the fast acting chelating agent BAPTA instead of the usual 3 mM EGTA. Likewise, the inhibition of the T-type Ca2+ current by 0.2 μM PGE1 (see Fig. 7C) becomes smaller; it is 37.1 ± 3.6% (n=39) with 5 mM EGTA in the pipette solution and only 16.0 ± 2.5% (n=5) with 5 mM BAPTA. In conclusion, the experiments of Fig. 11 suggest that the PGE1 effect is caused by an increase of internal cAMP and by release of Ca2+ from intracellular stores. In addition, the transient Ca2+ entry from the bath may play a role. It may be responsible for the large increase of holding current seen in many cells (e.g. effect of 50 nM iloprost in Fig. 1C of [71]).
Fig. (11).
Experiments supporting the view that PGE1 acts on NG108-15 cells via increase of intracellular cAMP and Ca2+. A-C: Pen records of holding current recorded at -70 mV. A: Effect of 0.5 mM CPT-cAMP. B: Effect of 1 μM thapsigargin. C: Effect of 0.2 μM PGE1 in Ca2+- and Mg2+-free bath with 1.1 mM EGTA. D: Scatter plot showing the effect of 3 μM PGE1 on holding current with 3 mM EGTA (n=18) or 20 mM BAPTA (n=7) in pipette solution; mean values indicated by horizontal bars. Perforated patch in A-C, ruptured patch in D. Room temperature. From [70, 71] and Meves (unpublished).
The change in holding current was significantly reduced by fiufenamic acid (FFA), an inhibitor of Ca2+-activated non-selective cation current [30, 37]. With 0.1-0.9 mM FFA in the bath, the increase in holding current produced by 3 μM PGE1 was only -32.2 ±7.7 pA (n=9) compared with -106.7 ± 23.3 pA (n=16) in control cells of the same batch.
In rat DRG cells, the membrane permeant dibutyryl cAMP causes Ca2+ entry and increase of [Ca2+]i [106]. In N1E-115 cells, the adenylate cyclase activator forskolin increases [Ca2+]i by activation of PLC-ε [95]. Thus, it may be thought that the first step in the PGE1 effect on neuroblastoma cells is a rise in cAMP which then increases [Ca2+]i by a dual mechanism, opening of non-selective cation channels and release from internal store. Increase of intracellular Ca2+ would cause a further opening of non-selective cation channels (as described in [124]), thus permitting further Ca2+ to enter. This regenerative process would explain the strong conductance increase and the concomitant blockage of Ca2+ currents observed upon application of PGE1 or iloprost. A synergistic action of intracellular Ca2+ and cAMP is also thought to occur in pancreatic β cells where it is responsible for the initiation of Ca2+-induced Ca2+ release [52].
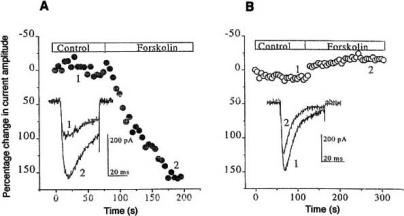
Some support for the assumed key role of cAMP comes from the observation that cAMP production and effect on holding current both start at the same PGE1 concentration, namely at about 10 nM [70], Rp-cAMPS almost totally abolishes the effect of 0.2 and 3 μM PGE1 on holding current, when applied via patch pipette [71]. By contrast, the PKA inhibitor H-89 which was applied via bath in the fairly high concentration of 10-30 μM or via pipette, was ineffective. It did not prevent the PGE1 effect on holding current and T-type Ca2+ current. Fig. 12A shows nearly total blockage of the T-type ICa by 0.2 μM PGE1 in a cell pretreated with bath containing 10μM H-89 for 88 min. Likewise, PKC19-31, a peptide inhibitor of protein kinase C, did not inhibit the PGE1 effect on T-type ICa.
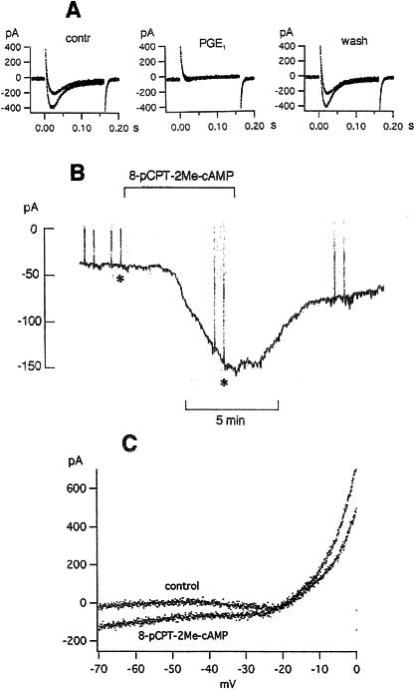
Fig. (12).
The PKA inhibitor H-89 does not prevent blockage of T-type Ca2+ current by PGE1 in NG108-15 cells, but the Epac agonist 8-pCPT-2Me-cAMP mimics the PGE1 effect on holding current and current-voltage curve. A: T-type Ca2+ currents at -25 and -15 mV in control, in 0.2 μM PGE1 (applied for 3 min) and after 4 min wash. When PGE1 was applied, the cell had been in bath with 10 mM CaCl2 and 10 μM H-89 for 88 min. Temperature 26.5ºC. B and C: Effect of 300 μM 8-pCPT-2Me-cAMp on the holding current (B) and on the current-voltage curve (C). Asterisks in B indicate current-voltage curves shown in C. Temperature 22.8ºC. From [71] and Meves (unpublished).
The mechanism by which PGE1 and forskolin produce an increase of [Ca2+]i in N1E-115 cells has been analyzed in detail [95, 55]. According to this study, cAMP acts via Epac (=exchange protein activated by cAMP) on PLC-ε which in turn produces inositol phosphates and an increase of [Ca2+]i PKA is not involved in the signaling path. Experiments illustrated in Fig. 12B and C show that the Epac agonist 8-pCPT-2Me-cAMP can indeed mimic the PGE1 effect on holding current and current-voltage curve of NG108-15 cells. However, signaling via Epac and PLC-ε cannot be the only mechanism responsible for the PGE1 effect on NG108-15 cells. As shown in [71], the PGE1 effect on NG108-15 cells is not significantly reduced by the aminosteroid U73122, a powerful PLC inhibitor, but totally inhibited by the cAMP antagonist Rp-cAMPS (for which Epac is said to have a low affinity, see [51]). Very likely, there are cAMP-dependent events independent of either PKA or Epac.
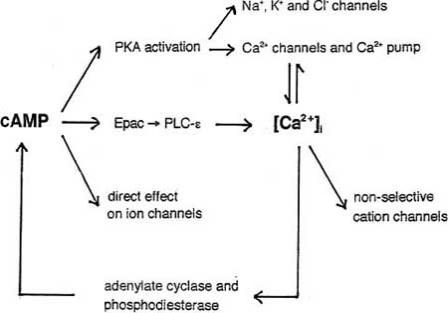
The diagram in Fig. 13 depicts the various pathways so far known to be involved in the effects of cAMP. It emphasizes the fact that cAMP alters the intracellular Ca2+ concentration which in turn affects [cAMP]i.
Fig. (13).
Diagram summarizing the known effects of cAMP and illustrating the interplay of cAMP and Ca2+ signaling. For details of cAMP – Ca2+ interplay see [16, 126].
The mechanism of action is, in general, similar for EP-and IP-mediated prostaglandin effects. In both cases it includes a rise of the intracellular level of cAMP and Ca2+ and, in some preparations, activation of protein kinases A and C. The effects are also similar: Sensitization of nerve cells, potentiation of the capsaicin-elicited inward current, inhibition of K+ and Ca2+ currents. Kwong & Lee [60] have drawn attention to one remaining open question: Whether PGI2 and PGE2, acting via IP receptors, enhance the TTX-resistant Na+ current, has yet to be determined.
REFERENCES
- 1.Abramovitz M, Adam M, Boie Y, Carriàre MC, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta – Molec Cell Biol Lipids. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 2.Adie EJ, Mullaney I, McKenzie FR, Milligan G. Concurrent down-regulation of IP prostanoid receptors and the αsubunit of the stimulatory guanine-nucleotide-binding protein (GS) during prolonged exposure of neuroblastoma x glioma cells to prostanoid agonists. Quantification and functional implications. Biochem J. 1992;285:529–536. doi: 10.1042/bj2850529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2001;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- 4.Baba H, Kohno T, Moore KA, Woolf CJ. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J Neurosci. 2001;21:1750–1756. doi: 10.1523/JNEUROSCI.21-05-01750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baccaglini PI, Hogan PG. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proc Nat Acad Sci USA. 1983;80:594–598. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–867. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastin B, Payet MD, Dupuis G. Effects of modulators of adenylyl cyclase on interleukin-2 production, cytosolic Ca2+ elevation, and K+ channel activity in Jurkat T cells. Cell Immunol. 1990;128:385–399. doi: 10.1016/0008-8749(90)90035-p. [DOI] [PubMed] [Google Scholar]
- 8.Blair IA, Hensby CN, MacDermot J. Prostacyclindependent activation of adenylate cyclase in a neuronal somatic cell hybrid: prostanoid structure-activity relationships. Br J Pharmacol. 1980;69:519–525. doi: 10.1111/j.1476-5381.1980.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair IA, MacDermot J. The binding of [3H]-prostacyclin to membranes of a neuronal somatic hybrid. Br J Pharmacol. 1981;72:435–441. doi: 10.1111/j.1476-5381.1981.tb10994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bley KR, Hunter JC, Eglen RM, Smith JAM. The role of IP prostanoid receptors in inflammatory pain. TiPS. 1998;19:141–147. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- 11.Boie Y, Rushmore TH, Darmon-Goodwin A, Grygorczyk R, Slipetz DM, Metters KM, Abramovitz M. Cloning and expression of a cDNA for the human prostanoid IP receptor. J Biol Chem. 1994;269:12173–12178. [PubMed] [Google Scholar]
- 12.Boie Y, Stocco R, Sawyer N, Slipetz DM, Ungrin MD, Neuschäfer-Rube F, Püschel GP, Metters KM, Abramovitz M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- 13.Bois P, Renaudon B, Baruscotti M, Lenfant J, DiFrancesco D. Activation of f-channels by cAMP analogues in macropatches from rabbit sino-atrial node myocytes. J Physiol. 1997;501:565–571. doi: 10.1111/j.1469-7793.1997.565bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgland SL, Connor M, Ryan RM, Ball HJ, Christie MJ. Prostaglandin E2 inhibits calcium current in two subpopulations of acutely isolated mouse trigeminal sensory neurons. J Physiol. 2002;539:433–444. doi: 10.1113/jphysiol.2001.013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signalling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 16.Bruce JIE, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 17.Caggiano AO, Kraig RP. Prostaglandin E2 and 4-aminopyridine prevent the lipopolysacchande-induced outwardly rectifying potassium current and interleukin-1β production in cultured rat microglia. J Neurochem. 1998;70:2357–2368. doi: 10.1046/j.1471-4159.1998.70062357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;93:929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- 20.Cho H, Shin J, Shin CY, Lee SY, Oh U. Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci. 2002;22:1238–1247. doi: 10.1523/JNEUROSCI.22-04-01238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow KBS, Wong YH, Wise H. Prostacyclin receptor-independent inhibition of phospholipase C activity by nonprostanoid prostacyclin mimetics. Br J Pharmacol. 2001;134:1375–1384. doi: 10.1038/sj.bjp.0704388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow KBS, Jones RL, Wise H. Protein kinase A-dependent coupling of mouse prostacyclin receptors to Gi is cell-type dependent. Eur J Pharmacol. 2003;474:7–13. doi: 10.1016/s0014-2999(03)02006-5. [DOI] [PubMed] [Google Scholar]
- 23.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 24.Coleman RA, Smith WL, Narumiya S. VIII. International Union of Pharmacology. Classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 25.Coward K, Baker M, editors. Sodium Channels, Pain, and Analgesia. Heidelberg: Springer Verlag; 2005. [Google Scholar]
- 26.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J Physiol. 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J Physiol. 1999;516:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleischhauer JC, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. PGE2, Ca2+, and cAMP mediate ATP activation of Cl- channels in pigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2001;281:C1614–C1623. doi: 10.1152/ajpcell.2001.281.5.C1614. [DOI] [PubMed] [Google Scholar]
- 30.Gögelein H, Dahlem D, Englert HC, Lang HJ. Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett. 1990;268:79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- 31.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Nat Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold MS, Levine JD, Correa AM. Modulation of TTXR INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E2 modulates TTX-R INa in rat colonic sensory neurons. J Neurophysiol. 2002;88:1512–1522. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- 34.Gold MS, Traub RJ. Cutaneous and colonic rat DRG neurons differ with respect to both baseline and PGE2-induced changes in passive and active electrophysiological properties. J Neurophysiol. 2004;91:2524–2531. doi: 10.1152/jn.00866.2003. [DOI] [PubMed] [Google Scholar]
- 35.Gómez-Nino A, López-López JR, Almaraz L, González C. Inhibition of [3H] catecholamine release and Ca2+ currents by prostaglandin E2 in rabbit carotid body chemoreceptor cells. J Physiol. 1994;476:269–277. doi: 10.1113/jphysiol.1994.sp020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu Q, Kwong K, Lee LY. Ca transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: Role of cAMP/PKA signalling pathway. J Neurophysiol. 2003;89:1985–1993. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- 37.Guinamard R, Rahmati M, Lenfant J, Bois P. Characterization of a Ca2+-activated nonselective cation channel during dedifferentiation of cultured ventricular cardiomyocytes. J Membrane Biol. 2002;188:127–135. doi: 10.1007/s00232-001-0180-4. [DOI] [PubMed] [Google Scholar]
- 38.Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma – glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- 39.Hamprecht B, Schultz J. Influence of noradrenalin, prostaglandin E1 and inhibitors of phosphodiesterase activity on levels of the cyclic adenosine 3’:5’-monophosphate in somatic cell hybrids. Hoppe-Seyler’s Z Physiol Chem. 1973;354:1633–1641. doi: 10.1515/bchm2.1973.354.2.1633. [DOI] [PubMed] [Google Scholar]
- 40.Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Müller U. GlyR α3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 41.Hersh EV, Lally ET, Moore PA. Update on cyclooxygenase inhibitors: has a third COX isoform entered the fray? Curr Med Res Opin. 2005;21:1217–1226. doi: 10.1185/030079905X56367. [DOI] [PubMed] [Google Scholar]
- 42.Higashida H, Nakagawa Y, Miki N. Facilitation of synaptic transmission by prostaglandin D2 at synapses between NG108-15 hybrid and muscle cells. Brain Res. 1984;295:113–119. doi: 10.1016/0006-8993(84)90821-7. [DOI] [PubMed] [Google Scholar]
- 43.Hingtgen CM, Waite KJ, Vasko MR. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3’,5’-cyclic monophosphate transduction cascade. J Neurosci. 1995;15:5411–5419. doi: 10.1523/JNEUROSCI.15-07-05411.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu HJ, Bhave G, Gereau RW. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda SR. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol. 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue M, Rashid MH, Kawashima T, Matsumoto M, Maeda T, Kishioka S, Ueda H. The algogenic-induced nociceptive flexion test in mice: studies on sensitivity of the test and stress on animals. Brain Res Bull. 2003;60:275–281. doi: 10.1016/s0361-9230(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 48.Ito Y, Murai Y, Ishibashi H, Onoue H, Akaike N. The prostaglandin E series modulates high-voltage activated calcium channels probably through the EP3 receptor in rat paratracheal ganglia. Neuropharmacol. 2000;39:181–190. doi: 10.1016/s0028-3908(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 49.Jennings LJ, Mawe GM. PGE2 hyperpolarizes gallbladder neurons and inhibits synaptic potentials in gallbladder ganglia. Am J Physiol Gastrointestinal and Liver Physiol. 1998;274:G493–G502. doi: 10.1152/ajpgi.1998.274.3.G493. [DOI] [PubMed] [Google Scholar]
- 50.Kanba S, Sasakawa N, Nakaki T, Kanba KS, Yagi G, Kato R, Richelson E. Two possibly distinct prostaglandin E1 receptors in N1E-115 clone: one mediating inositol trisphosphate formation, cyclic GMP formation, and intracellular calcium mobilization and the other mediating cyclic AMP formation. J Neurochem. 1991;57:2011–2015. doi: 10.1111/j.1471-4159.1991.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 51.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2’-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li Wh, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release (CICR) in pancreatic β-cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasai M, Mizumura K. Effects of PGE(2) on neurons from rat dorsal root ganglia in intact and adjuvant-inflamed rats: role of NGF on PGE(2)-induced depolarization. Neurosci Res. 2001;41:345–353. doi: 10.1016/s0168-0102(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 54.Katoh H, Watabe A, Sugimoto Y, Ichikawa A, Negishi M. Characterization of the signal transduction of prostaglandin E receptor EP1 subtype in cDNA-transfected Chinese hamster ovary cells. Biochim Biophys Acta. 1995;1244:41–48. doi: 10.1016/0304-4165(94)00182-w. [DOI] [PubMed] [Google Scholar]
- 55.Keiper M, Stope MB, Szatkowski D, Böhm A, Tysack K, vom Dorp F, Saur O, Oude Weernink PA, Evellin S, Jakobs KH, Schmidt M. Epac- and Ca2+-controlled activation of Ras and extracellular signal-regulated kinases by GS-coupled receptors. J Biol Chem. 2004;279:46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 56.Kelly E, Keen M, Nobbs P, MacDermot J. NaF and guanine nucleotides modulate adenylate cyclase activity in NG10815 cells by interacting with both GS and Gi. Br J Pharmacol. 1990;100:223–230. doi: 10.1111/j.1476-5381.1990.tb15786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- 58.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specifities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwong K, Lee LY. PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol. 2002;93:1419–1428. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- 60.Kwong K, Lee LY. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol. 2005;564:437–450. doi: 10.1113/jphysiol.2004.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- 62.Lee YS, Lee JA, Jung J, Oh U, Kaang BK. The cAMP-dependent kinase pathway does not sensitize the cloned vanilloid receptor type 1 expressed in xenopus oocytes or Aplysia neurons. Neurosci Lett. 2000;288:57–60. doi: 10.1016/s0304-3940(00)01208-8. [DOI] [PubMed] [Google Scholar]
- 63.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118:69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 64.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: Whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 66.Mathes C, Thompson SH. The relationship between depletion of intracellular Ca2+ stores and activation of Ca2+ current by muscarinic receptors in neuroblastoma cells. J Gen Physiol. 1995;106:975–993. doi: 10.1085/jgp.106.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsumoto S, Ikeda M, Yoshida S, Tanimoto T, Takeda M, Nasu M. Prostaglandin E2-induced modification of tetrodotoxin-resistant Na+ currents involves activation of both EP2 and EP4 receptors in neonatal rat nodose ganglion neurones. Br J Pharmacol. 2005;145:503–513. doi: 10.1038/sj.bjp.0706212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumura K, Watanabe Y, Onoe H, Watanabe Y, Tanaka S, Shiraki T, Kobayashi S. Prostaglandin E2 excites neurons of the nucleus tractus solitarius by activating cation channels. Brain Res. 1993;626:343–346. doi: 10.1016/0006-8993(93)90600-r. [DOI] [PubMed] [Google Scholar]
- 69.McGee R, Kenimer JG. The effects of exposure to unsaturated fatty acids on opiate receptors, prostaglandin E1 receptors, and adenylate cyclase activity of neuroblastoma x glioma hybrid cells. Mol Pharmacol. 1982;22:360–368. [PubMed] [Google Scholar]
- 70.Meves H. Prostaglandin E1 induces an inward current in voltage-clamped NG108-15 cells. Prostaglandins Other Lipid Mediat. 2003;71:265–276. doi: 10.1016/s1098-8823(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 71.Meves H. The effect of prostaglandin E1 on ion currents of NG108-15 cells. Prostaglandins Other Lipid Mediat. 2005;76:117–132. doi: 10.1016/j.prostaglandins.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Minami T, Okuda-Ashitaka E, Hori Y, Sakuma S, Sugimoto T, Sakimura K, Mishina M, Ito S. Involvement of primary afferent C-fibres in touch-evoked pain (allodynia) induced by prostaglandin E2. Eur J Neurosci. 1999;11:1849–1856. doi: 10.1046/j.1460-9568.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- 73.Miwa N, Sugino H, Ueno R, Hayaishi O. Prostaglandin induces Ca2+ influx and cyclic GMP formation in mouse neuroblastoma x rat glioma hybrid NG108-15 cells in culture. J Neurochem. 1988;50:1418–1424. doi: 10.1111/j.1471-4159.1988.tb03025.x. [DOI] [PubMed] [Google Scholar]
- 74.Momiyama T, Todo N, Sugimoto Y, Ichikawa A, Narumiya S. Membrane depolarization by activation of prostaglandin E receptor EP3 subtype of putative serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn-Schmiedeberg’s Arch Pharmacol. 1996;353:377–381. doi: 10.1007/BF00261433. [DOI] [PubMed] [Google Scholar]
- 75.Moreau R, Hurst AM, Lapointe JY, Lajeunesse D. Activation of maxi-K+ channels by parathyroid hormone and prostaglandin E2 in human osteoblast bone cells. J Membrane Biol. 1996;150:175–184. doi: 10.1007/s002329900042. [DOI] [PubMed] [Google Scholar]
- 76.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1 doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 78.Nicol GD, Klingberg DK, Vasko MR. Prostaglandin E2 increases calcium conductance and stimulates release of substance P in avian sensory neurons. J Neurosci. 1992;12:1917–1927. doi: 10.1523/JNEUROSCI.12-05-01917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicol GD, Cui M. Enhancement by prostaglandin E2 of bradykinin activation of embryonic rat sensory neurones. J Physiol. 1994;480:485–492. doi: 10.1113/jphysiol.1994.sp020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- 81.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 82.Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, Narumiya S. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamoto F, Kajiya H, Fukushima H, μMi E, Okabe K. Prostaglandin E2 activates outwardly rectifying Cl-channels via a cAMP-dependent pathway and reduces cell motility in rat osteoclasts. Am J Physiol Cell Physiol. 2004;287:C114–C124. doi: 10.1152/ajpcell.00551.2003. [DOI] [PubMed] [Google Scholar]
- 84.Pedarzani P, Storm JF. Protein kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proc Nat Acad Sci USA. 1995;92:11716–11720. doi: 10.1073/pnas.92.25.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pitchford S, Levine JD. Prostaglandins sensitize nociceptors in cell culture. Neurosci Lett. 1991;132:105–108. doi: 10.1016/0304-3940(91)90444-x. [DOI] [PubMed] [Google Scholar]
- 86.Raes A, Wang Z, van den Berg RJ, Goethals M, van de Vijver G, van Bogaert PP. Effect of cAMP and ATP on the hyperpolarization-activated current in mouse dorsal root ganglion neurons. Pflügers Arch. 1997;434:543–550. doi: 10.1007/s004240050434. [DOI] [PubMed] [Google Scholar]
- 87.Reichling DB, Levine JD. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proc Nat Acad Sci USA. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reiser G, Cesar M, Binmöller FJ. Bradykinin and muscarine induce Ca2+-dependent oscillations of membrane potential in rat glioma cells indicating a rhythmic Ca2+-release from internal stores: thapsigargin and 2,5-Di(tert-butyl)-1,4benzohydroquinone deplete InsP3-sensitive Ca2+ stores in glioma and in neuroblastoma –glioma hybrid cells. Exp Cell Res. 1992;202:440–449. doi: 10.1016/0014-4827(92)90097-r. [DOI] [PubMed] [Google Scholar]
- 89.Ren J, Karpinski E, Benishin CG. Prostaglandin E2 contracts vascular smooth muscle and inhibits potassium currents in vascular smooth muscle cells of rat tail artery. J Pharmacol Exp Therap. 1995;275:710–719. [PubMed] [Google Scholar]
- 90.Rowlands DK, Kao Cl, Wise H. Regulation of prostacyclin and prostaglandin E2 receptor mediated responses in adult rat dorsal root ganglion cells, in vitro. Br J Pharmacol. 2001;133:13–22. doi: 10.1038/sj.bjp.0704028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruiz-Velasco V, Ikeda SR. Heterologous expression and coupling of G protein –gated inwardly rectifying K+ channels in adult rat sympathetic neurons. J Physiol. 1998;513:761–773. doi: 10.1111/j.1469-7793.1998.761ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rush AM, Bräu ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol. 1998;511:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant NaV1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023:264–271. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 94.Salmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43:285–296. doi: 10.1093/oxfordjournals.bmb.a072183. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt M, Evellin S, Oude Weernink PA, vom Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 96.Schubert R, Serebryakov VN, Engel H, Hopp HH. Iloprost activates KCa channels of vascular smooth muscle cells: role of cAMP-dependent protein kinase. Am J Physiol Cell Physiol. 1996;271:C1203–C1211. doi: 10.1152/ajpcell.1996.271.4.C1203. [DOI] [PubMed] [Google Scholar]
- 97.Schubert R, Serebryakov VN, Mewes H, Hopp HH. Iloprost dilates rat small arteries: role of KATP- and KCa-channel activation by cAMP-dependent protein kinase. Am J Physiol Heart Circ Physiol. 1997;272:H1147–H1156. doi: 10.1152/ajpheart.1997.272.3.H1147. [DOI] [PubMed] [Google Scholar]
- 98.Schwaner I, Seifert R, Schultz G. Receptor-mediated increases in cytosolic Ca2+ in the human erythroleukaemia cell line involve pertussis toxin-sensitive and –insensitive pathways. Biochem J. 1992;281:301–307. doi: 10.1042/bj2810301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Nat Acad Sci USA. 1975;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shibuya I, Setiadji SV, Ibrahim N, Harayama N, Maruyama T, Ueta Y, Yamashita H. Involvement of postsynaptic EP4 and presynaptic EP3 receptors in actions of prostaglandin E2 in rat supraoptic neurones. J Neuroendocrinol. 2002;14:64–72. doi: 10.1046/j.1365-2826.2002.00741.x. [DOI] [PubMed] [Google Scholar]
- 101.Shimizu T, Mizuno N, Amano T, Hayaishi O. Prostaglandin D2, a neuromodulator. Proc Nat Acad Sci USA. 1979;76:6231–6234. doi: 10.1073/pnas.76.12.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am J Physiol Heart Circ Physiol. 2005;288:H2093–H2101. doi: 10.1152/ajpheart.01003.2004. [DOI] [PubMed] [Google Scholar]
- 103.Siegel G, Carl A, Adler A, Stock G. Effect of the prostacyclin analogue iloprost on K+ permeability in the smooth muscle cells of the canine carotid artery. Eicosanoids. 1989;2:213–222. [PubMed] [Google Scholar]
- 104.Siemer C, Gögelein H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflügers Arch. 1992;420:319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- 105.Smith JAM, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br J Pharmacol. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith JAM, Davis CL, Burgess GM. Prostaglandin E2-induced sensitization of bradykinin-evoked responses in rat dorsal root ganglion neurons is mediated by cAMP-dependent protein kinase A. Eur J Neurosci. 2000;12:3250–3258. doi: 10.1046/j.1460-9568.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 107.Smyth EM, Nestor PV, FitzGerald GA. Agonist-dependent phosphorylation of an epitope-tagged human prostacyclin receptor. J Biol Chem. 1996;271:33698–33704. doi: 10.1074/jbc.271.52.33698. [DOI] [PubMed] [Google Scholar]
- 108.Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem. 2001;276:16083–16091. doi: 10.1074/jbc.M011408200. [DOI] [PubMed] [Google Scholar]
- 109.Tabata H, Tanaka S, Sugimoto Y, Kanki H, Kaneko S, Ichikawa A. Possible coupling of prostaglandin E receptor EP1 to TRP5 expressed in Xenopus laevis oocytes. Biochem Biophys Res Com. 2002;298:398–402. doi: 10.1016/s0006-291x(02)02455-5. [DOI] [PubMed] [Google Scholar]
- 110.Tan W, Du C, Siegelbaum SA, Role LW. Modulation of nicotinic AChR channels by prostaglandin E2 in chick sympathetic ganglion neurons. J Neurophysiol. 1998;79:870–878. doi: 10.1152/jn.1998.79.2.870. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka K, Shibuya I, Kabashima N, Ueta Y, Yamashita H. Inhibition of voltage-dependent calcium channels by prostaglandin E2 in rat melanotrophs. Endocrinology. 1998;139:4801–4810. doi: 10.1210/endo.139.12.6383. [DOI] [PubMed] [Google Scholar]
- 112.Tokimasa T, Akasu T. Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol. 1990;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsuboi K, Sugimoto Y, Ichikawa A. Prostanoid receptor subtypes. Prostaglandins Other Lipid Mediat. 2002;68-69:535–556. doi: 10.1016/s0090-6980(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 114.Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. J Autonomic Nervous System. 1993;44:17–34. doi: 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- 115.Vargas G, Lucero MT. Modulation by PKA of the hyperpolarization-activated current (Ih) in cultured rat olfactory receptor neurons. J Membrane Biol. 2002;188:115–125. doi: 10.1007/s00232-001-0178-y. [DOI] [PubMed] [Google Scholar]
- 116.Vassaux G, Gaillard D, Ailhaud G, Négrel R. Prostacyclin is a specific effector of adipose cell differentiation. Its dual role as a cAMP- and Ca2+-elevating agent. J Biol Chem. 1992;267:11092–11097. [PubMed] [Google Scholar]
- 117.Wang H, Woolf CJ. Pain TRPs. Neuron. 2005;46:9–12. doi: 10.1016/j.neuron.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 118.Weinreich D, Wonderlin WF. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol. 1987;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams RJ, Kelly E. GSα-dependent and –independent desensitization of prostanoid IP receptor-activated adenylyl cyclase in NG108-15 cells. Eur J Pharmacol. 1994;268:177–186. doi: 10.1016/0922-4106(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 120.Wilson RJ, Rhodes SA, Wood RL, Shield VJ, Noel LS, Gray DW, Giles H. Functional pharmacology of human prostanoid EP2 and EP4 receptors. Eur J Pharmacol. 2004;501:49–58. doi: 10.1016/j.ejphar.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 121.Wise H, Jones RL. Focus on prostacyclin and its novel mimetics. TiPS. 1996;17:17–21. doi: 10.1016/0165-6147(96)81565-3. [DOI] [PubMed] [Google Scholar]
- 122.Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol. 2004;61:55–71. doi: 10.1002/neu.20094. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto H, Maruyama T, Sakata K, Koketsu M, Kobayashi M, Yoshida H, Seki A, Tani K, Maruyama T, Kondo K, Ohuchida S. Novel four selective agonists for prostaglandin E receptor subtypes. Prostaglandins Other Lipid Mediators. 1999;59 [Google Scholar]
- 124.Yellen G. Single Ca2+-activated non-selective cation channels in neuroblastoma. Nature. 1982;296:357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- 125.Yu H, Chang F, Cohen IS. Pacemaker current i(f) in adult canine cardiac ventricular myocytes. J Physiol. 1995;485:469–483. doi: 10.1113/jphysiol.1995.sp020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaccolo M, Pozzan T. cAMP and Ca2+ interplay: a matter of oscillation patterns. Trends Neurosci. 2003;26:53–55. doi: 10.1016/s0166-2236(02)00017-6. [DOI] [PubMed] [Google Scholar]
- 127.Zeilhofer HU. The glycinergic control of spinal pain processing. CMLS Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]