Abstract
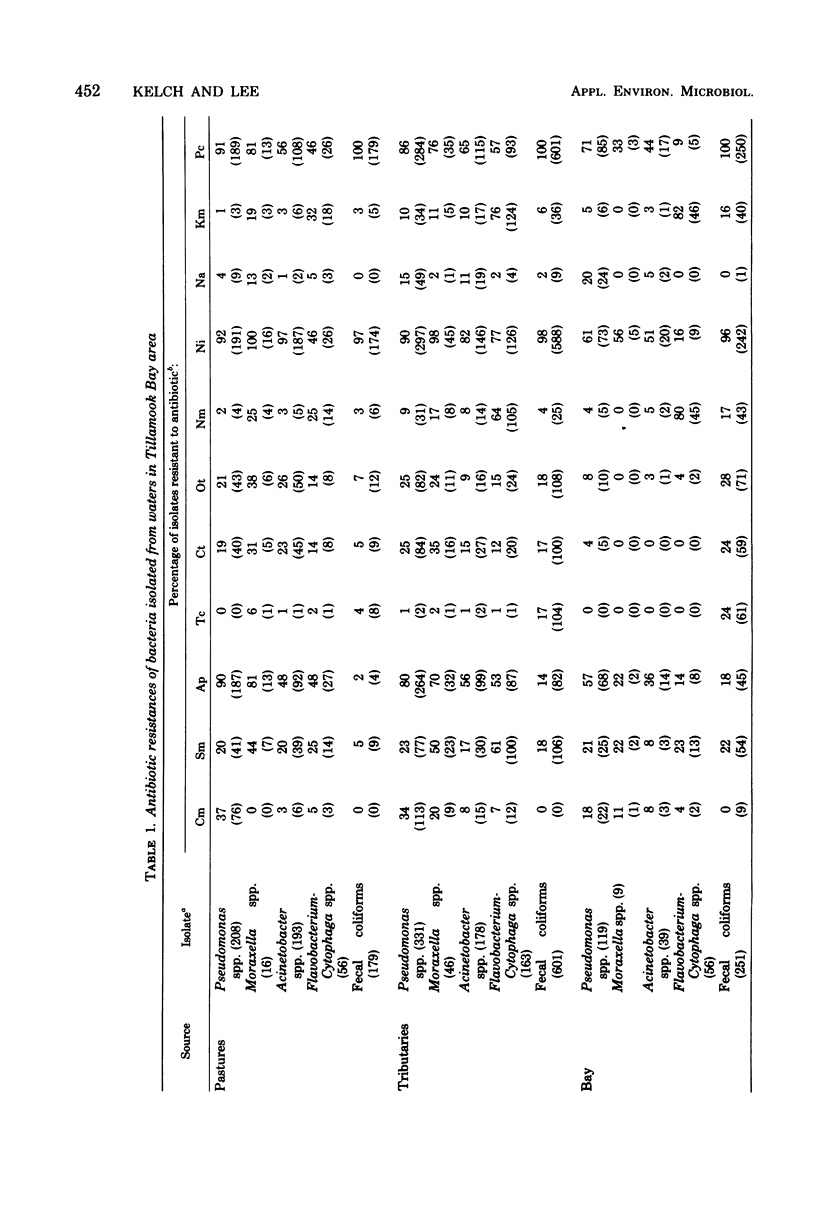
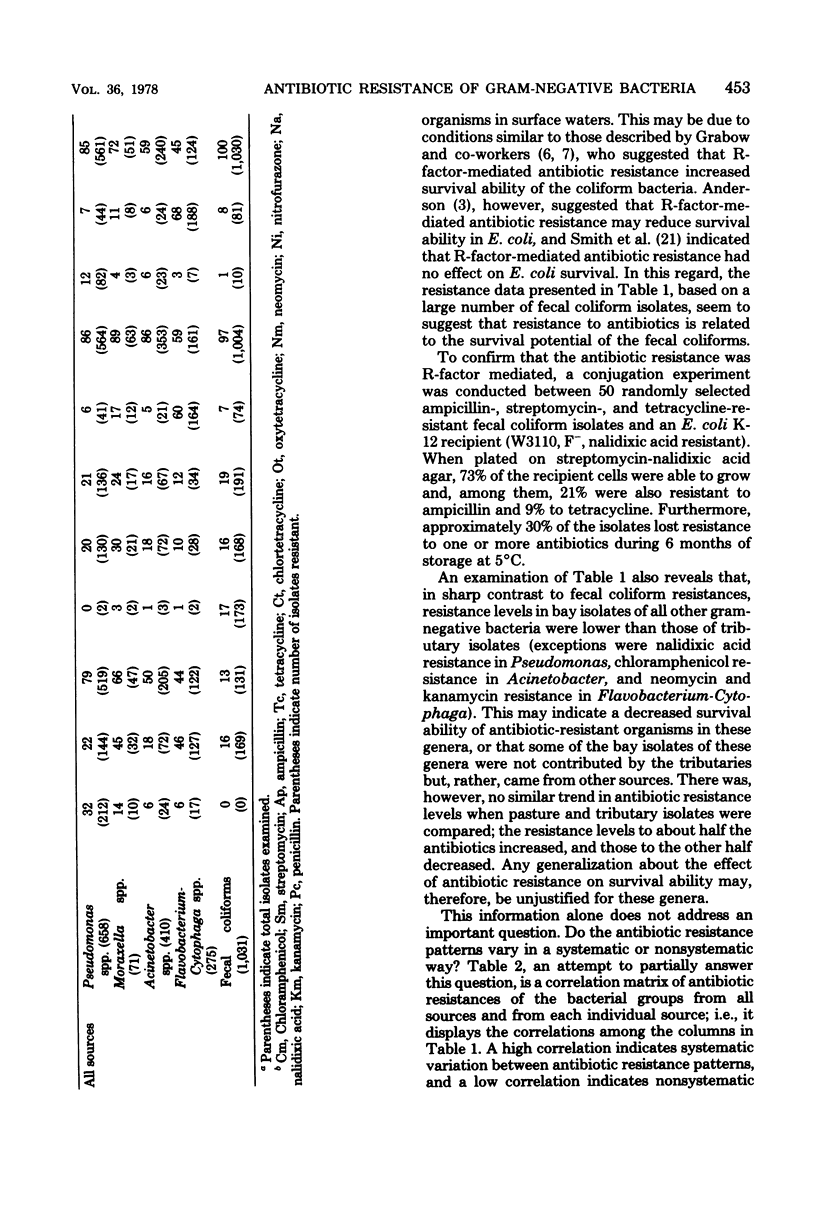
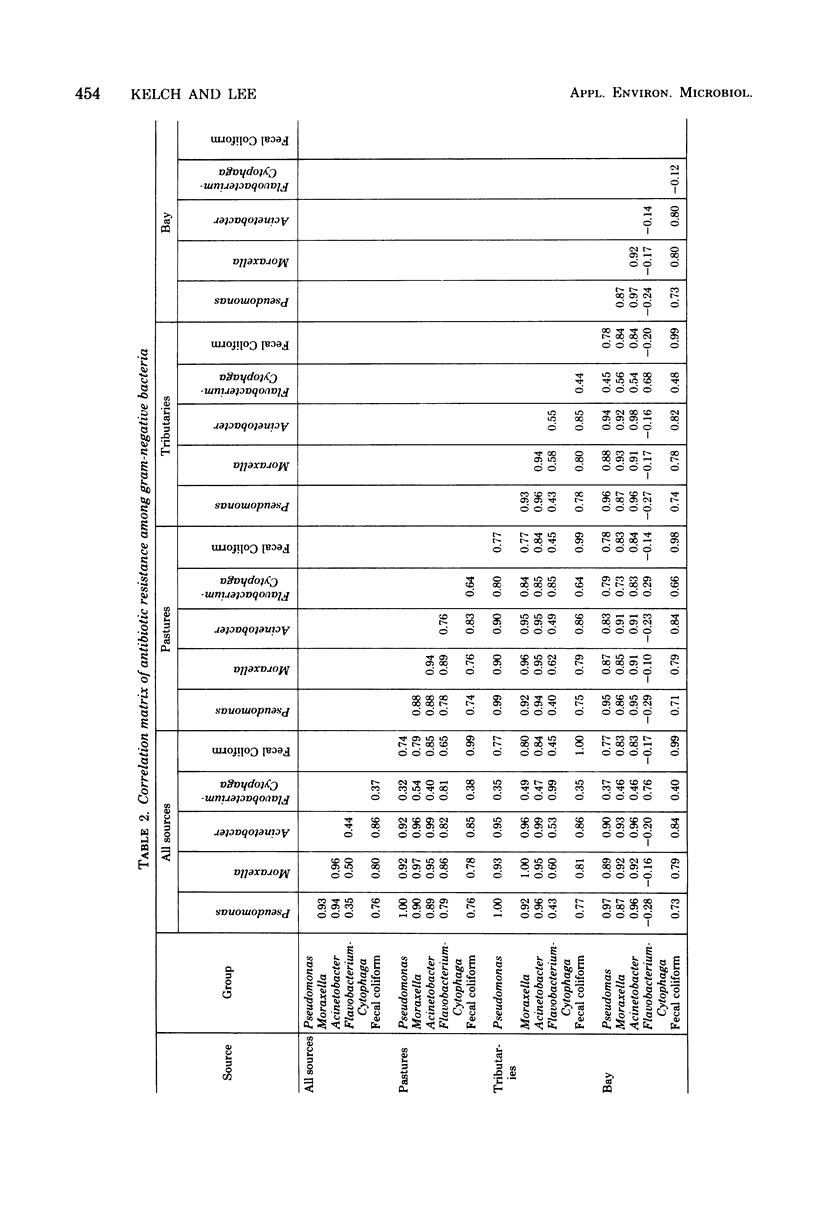
A total of 2,445 gram-negative bacteria belonging to fecal coliform, Pseudomonas, Moraxella, Acinetobacter, and Flavobacterium-Cytophaga groups were isolated from the rivers and bay of Tillamook, Oregon, and their resistances to chloramphenicol (25 microgram/ml), streptomycin (10 microgram/ml), ampicillin (10 microgram/ml), tetracycline (25 microgram/ml), chlortetracycline (25 microgram/ml), oxytetracycline (25 microgram/ml), neomycin (50 microgram/ml), nitrofurazone (12.5 microgram/ml), nalidixic acid (25 microgram/ml), kanamycin (25 microgram/ml), and penicillin G (10 IU/ml) were determined. Among fecal coliforms the bay isolates showed greater resistance to antibiotics than those from tributaries or surface runoff. No such well-defined difference was found among other bacterial groups. The antibiotic resistance patterns of gram-negative bacteria from different sources correlated well, perhaps indicating their common origin. The antibiotic resistance patterns of gram-negative bacteria of different general also correlated well, perhaps indicating that bacteria which share a common environment also share a common mode for developing antibiotic resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G., Bogokovsky B. In-vitro sensitivity of Elavobacterium meningosepticum to antimicrobial agents. J Med Microbiol. 1971 May;4(2):296–299. doi: 10.1099/00222615-4-2-296. [DOI] [PubMed] [Google Scholar]
- Anderson J. D. The effect of R-factor carriage on the survival of Escherichia coli in the human intestine. J Med Microbiol. 1974 Feb;7(1):85–90. doi: 10.1099/00222615-7-1-85. [DOI] [PubMed] [Google Scholar]
- Breuil C., Novitsky T. J., Kushner D. J. Characteristics of a facultatively psychrophilic Acinetobacter species isolated from river sediment. Can J Microbiol. 1975 Dec;21(12):2103–2108. doi: 10.1139/m75-301. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Antimicrobial susceptibility as a diagnostic aid in the identification of nonfermenting gram-negative bacteria. Appl Microbiol. 1971 Nov;22(5):821–823. doi: 10.1128/am.22.5.821-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: identity of laboratory-constructed plasmids and clinical isolates. J Bacteriol. 1977 Jan;129(1):530–533. doi: 10.1128/jb.129.1.530-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFrancois M., Baum J. L. Flavobacterium endophthalmitis following keratoplasty. Use of a tissue culture medium-stored cornea. Arch Ophthalmol. 1976 Nov;94(11):1907–1909. doi: 10.1001/archopht.1976.03910040617007. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Preifer D. K. Microbiological characteristics of Dungeness crab (Cancer magister). Appl Microbiol. 1975 Jul;30(1):72–78. doi: 10.1128/am.30.1.72-78.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowbury E. J., Babb J. R., Roe E. Clearance from a hospital of gram-negative bacilli that transfer carbenicillin-resistance to Pseudomonas aeruginosa. Lancet. 1972 Nov 4;2(7784):941–945. doi: 10.1016/s0140-6736(72)92469-5. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Kreger B. E., Johns M. Type-specific and cross-reactive antibodies in gram-negative bacteremia. N Engl J Med. 1972 Aug 10;287(6):261–267. doi: 10.1056/NEJM197208102870601. [DOI] [PubMed] [Google Scholar]
- Nagy A. E., Csatáry K. N. Bacteriological and pharmacological investigations with Garamycin (gentamicin). Acta Microbiol Acad Sci Hung. 1974;21(3-4):289–291. [PubMed] [Google Scholar]
- Pedersen M. M., Marso E., Pickett M. J. Nonfermentative bacilli associated with man. 3. Pathogenicity and antibiotic susceptibility. Am J Clin Pathol. 1970 Aug;54(2):178–192. doi: 10.1093/ajcp/54.2.178. [DOI] [PubMed] [Google Scholar]
- Pintér M., Kántor M. Quantitative antibiotic sensitivity pattern of Acinetobacter strains. Antonie Van Leeuwenhoek. 1971;37(2):197–200. doi: 10.1007/BF02218481. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. Some environmental consequences of the use of antibiotics: or 'what goes up must come down'. J Appl Bacteriol. 1972 Jun;35(2):155–176. doi: 10.1111/j.1365-2672.1972.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. R., Farrell E., Dunican K. Survival of R+ Escherichia coli in sea water. Appl Microbiol. 1974 May;27(5):983–984. doi: 10.1128/am.27.5.983-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K. J., Vivian A. RP4-mediated conjugation in Acinetobacter calcoaceticus. J Gen Microbiol. 1976 Apr;93(2):355–360. doi: 10.1099/00221287-93-2-355. [DOI] [PubMed] [Google Scholar]
- Tunstall A. M., Gowland G. Susceptibility to antibiotics of marine stains of Pseudomonas. J Appl Bacteriol. 1974 Sep;37(3):455–457. doi: 10.1111/j.1365-2672.1974.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Von Graevenitz A., Redys J. J. Disc sensitivity as an aid in the identification of some gram-negative non-fermentative rods. Health Lab Sci. 1968 Apr;5(2):107–112. [PubMed] [Google Scholar]
- Washington J. A., 2nd Antimicrobial susceptibility of enterobacteriaceae and nonfermenting gram-negative bacilli. Mayo Clin Proc. 1969 Nov;44(11):811–824. [PubMed] [Google Scholar]


