Abstract
Schizophrenia has been associated with a dysfunction of brain dopamine (DA). This, so called, DA hypothesis has been refined as new insights into the pathophysiology of schizophrenia have emerged. Currently, dysfunction of prefrontocortical glutamatergic and GABAergic projections and dysfunction of serotonin (5-HT) systems are also thought to play a role in the pathophysiology of schizophrenia. Refinements of the DA hypothesis have lead to the emergence of new pharmacological targets for antipsychotic drug development. It was shown that effective antipsychotic drugs with a low liability for inducing extra-pyramidal side-effects have affinities for a range of neurotransmitter receptors in addition to DA receptors, suggesting that a combination of neurotransmitter receptor affinities may be favorable for treatment outcome.
This review focuses on the interaction between DA and 5-HT, as most antipsychotics display affinity for 5-HT receptors. We will discuss DA/5-HT interactions at the level of receptors and G protein-coupled potassium channels and consequences for induction of depolarization blockade with specific attention to DA neurons in the ventral tegmental area (VTA) and the substantia nigra zona compacta (SN), neurons implicated in treatment efficacy and the side-effects of schizophrenia, respectively. Moreover, it has been reported that electrophysiological interactions between DA and 5-HT show subtle, but important, differences between the SN and the VTA which could explain (in part) the effectiveness and lower propensity to induce side-effects of the newer atypical antipsychotic drugs. In that respect the functional implications of DA/5-HT interactions for schizophrenia will be discussed.
Key Words: Schizophrenia, antipsychotic drug, substantia nigra, ventral tegmental area
DOPAMINE AND SCHIZOPHRENIA
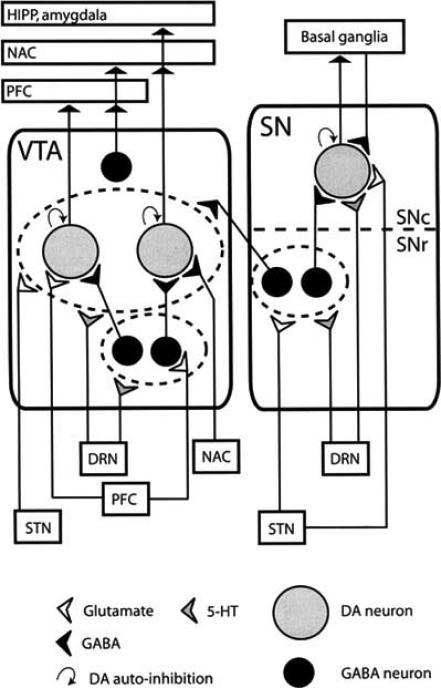
The mesocortical pathway, the mesolimbic pathway, the nigrostriatal pathway and the tuberoinfundibular pathway have all been postulated to be involved in the pathophysiology of schizophrenia and the propensity of antipsychotic drugs to induce side-effects [144]. Hypofunction of the mesocortical pathway and hyperfunction of the mesolimbic pathway [44, 55, 144] are thought to be responsible for the symptoms that can be observed (see also Sesack and Carr, 2002 [138])) and points to one of the many difficulties for effective treatment: increasing dopamine (DA) activity in the mesocortical pathway, while concomitantly decreasing DA activity in the mesolimbic pathway. The nigrostriatal and tuberoinfundibular pathways are involved in side-effects of antipsychotic drug treatment, such as extra-pyramidal side effects and hyperprolactinemia, respectively [10, 30] which are related to changes in firing activity of neurons in these pathways, especially the DA neurons. What lies at the root of this mesocortical mesolimbic dysfunction is unclear but loss of cholinergic interneurons in the striatum, hypoglutamatergia or "miswiring" of glutamatergic and _-amino butyric acid (GABA)-ergic projections from the prefrontal cortex (PFC) have all been proposed [5, 22, 23, 34, 66, 154]. The glutamatergic and GABAergic PFC projections synapse directly and indirectly {via e.g. the nucleus accumbens) to VTA DA neurons (Fig. (1), partly based on [138]). The GABAergic inputs together with local GABAergic interneurons in the VTA and SN (Fig. (1)) reduce DA neuronal firing activity by exerting an inhibitory tone on DA neuronal activity through GABAA and GABAB receptors present on DA neurons [26, 43, 151]. Thus, dysfunction of the PFC pathway could disinhibit VTA DA neurons leading to hyperactivity of VTA neurons.
Fig. (1).
A schematic view of SN and VTA connections. DA neurons receive input from the local GABAergic interneurons and input from other brain areas. Both areas receive serotonergic input from the dorsal raphe nucleus (DEN). The dotted line indicates the subdivision of the SN: the SN pars reticulata (SNr) and the SN pars compacta (SNC). Neurons receiving a collective input are grouped (dotted circles). GABAergic, glutamatergic and serotonergic inputs are indicated by black, white and gray symbols, respectively. HIPP, hippocampus; NAC, nucleus accumbens; PFC, prefrontal cortex; STN, subthalamic nucleus.
THE DA RECEPTOR AND ANTIPSYCHOTIC DRUGS
The discovery of DA in the brain [8] and subsequent discovery in the late 1950s that schizophrenia could be treated with antipsychotic drugs, which antagonize DA D2 receptors, reducing mesolimbic DA neuronal hyperactivity, has led to the DA hypothesis for schizophrenia. This hypothesis has been refined recently to incorporate other neurotransmitters such as glutamate, GABA and serotonin (5-hydroxytryptamine; 5-HT) [11, 23, 24, 136, 155]. However, attempts to develop an effective antipsychotic drug that lacks DA D2 receptor antagonism have been, generally, unsuccessful. For example selective 5-HT2A receptor antagonists [75, 92] and DA D4 receptor antagonists [12, 169] failed to show efficacy in schizophrenia reinforcing a pivotal role for DA D2 receptors in the treatment efficacy of schizophrenia. However, recent studies have suggested that the neurokinin (NK)3 receptor antagonist does have effects in schizophrenia [100].
DA D2 RECEPTORS AND DA NEURON FIRING ACTIVITY
DA D2-like receptors are present as auto-receptors on the DA neurons in SN and VTA and play an important role in the regulation of DA neuronal firing activity by means of auto-inhibition (Fig. (1). These G protein-coupled receptors are activated by somatodendritically released DA [7] and their activation opens G protein-coupled inward rectifying potassium channels (GIRKs) (Fig. (2)). Opening of the GIRK channels leads to hyperpolarization of the cell membrane and consequently a decrease in firing activity [81]. Furthermore, the hyperpolarization mediated via the DA D2 auto-receptor-activated GIRK channels decreases somatodendritical release of DA [141], allowing the DA neuron to depolarize via voltage-dependent calcium current activation and the non-selective cation current Ih [139, 168]. This in turn leads to increased DA release, increased DA D2 auto-receptor-mediated GIRK current and so on, contributing to the maintainance of a spontaneous, pace-maker-like firing pattern.
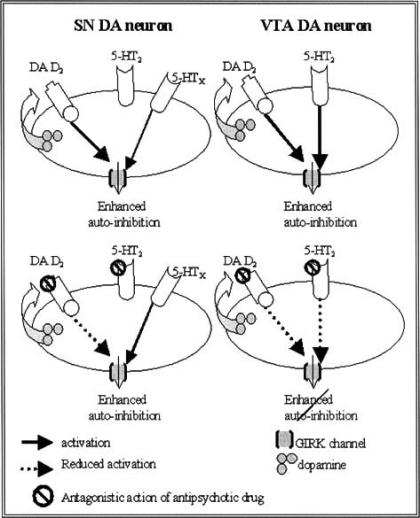
Fig. (2).
Model scheme for atypical antipsychotic drug action. The two schemes above represent the situation under normal conditions, where activation of 5-HT receptors (an unknown (5-HT£) receptor on SN and the 5-HT2 receptor on VTA DA neurons) can enhance the DA D2 receptor-mediated auto-inhibitory process. The two schemes below represent the situation in the presence of an atypical antipsychotic drug. This drug antagonizes a portion of the DA D2 receptors, thereby reducing GIRK channel function, resulting in depolarization and increased firing activity of the DA neurons. Prolonged depolarization could, theoretically, lead to the induction of depolarization blockade and subsequently therapeutic efficacy and extra-pyramidal side-effects (EPS). In VTA DA neurons an atypical antipsychotic drug will, by blocking the 5-HT2 receptors, prevent 5-HT2-mediated enhancement of GIRK channels, and thus auto-inhibition. This would permit a further depolarization, and ultimately depolarization blockade. In SN DA neurons "normal" enhancement of auto-inhibition can occur through the 5-HTx receptor (which is not affected by the antipsychotic drug), a process that will reduce the chance that depolarization blockade will develop.
DEPOLARIZATION BLOCKADE THEORY AND ANTIPSYCHOTIC DRUG EFFICACY
In vitro studies demonstrate that DA neurons usually fire at low pacemaker-like frequencies of 1-8 Hz [61, 162] and this pacemaker-like firing is also observed in vivo, although DA neurons in vivo also display irregular and burst firing activity [18, 47, 140]. In general, it is thought that the tonic release of DA (via regular firing rates) serves to maintain a steady-state level of DA in the brain, while the phasic DA release through bursting activity gives rise to high but transient DA levels which convey discrete signals [42, 46]. Switching the firing pattern from regular to bursting or back is thought to be dependent on coinciding glutamatergic and cholinergic inputs from the subthalamic and pedunculo-pontine nuclei, respectively [80, 87, 130, 159]. In the absence of coinciding glutamate and acetylcholine signaling, switching to a bursting firing pattern is unlikely, although the firing rate can be increased through depolarization of the DA neuron. It has been shown that upon depolarization (e.g. through GIRK channel inhibition) DA neurons cannot sustain increases in the firing rates above a plateau level [170]. At depolarization levels above that producing maximum frequency, DA neuronal firing ceases; such a state is referred to as acute depolarization blockade, a mechanism that could function to curb neuronal excitation [59, 65, 145, 170]. Although difficult to prove in vivo, it is believed that antipsychotic drugs induce a more or less chronic depolarization blockade in VTA DA neurons, thereby effectively reducing mesolimbic hyperactivity [60]. The development of depolarization blockade by antipsychotic drug treatment probably involves the blockade of the DA D2-like autoreceptors on the VTA DA neurons and activation of excitatory feedback systems, a process that develops over time (i.e. during chronic antipsychotic drug treatment) and is often characterized by an initial increase in DA neuronal activity. However, this mechanism of depolarization blockade will also be effective in SN DA neurons. This is reflected by one of the side-effects of the classical antipsychotic drugs: extra-pyramidal side effects (EPS), a collective name for symptoms such as dystonia, akinesia, dyskinesia, and akathisia [19, 91]. Due to the high liability for EPS induction the search for better antipsychotics continued. The introduction of clozapine was a large step-forward in achieving this goal [9, 17, 29, 49, 94, 111, 142] and mesolimbic hyperactivity could be reduced without inducing EPS. Clozapine, and other so-called atypical antipsychotics known as such for their low propensity to induce EPS [31, 67, 150], has a much lower tendency to induce EPS compared to the older, classical antipsychotic drugs [149]. However, it has been demonstrated that a selective depolarization blockade of only VTA DA neurons may be involved in the underlying mechanism [14]. In vivo studies suggest that atypical antipsychotic drugs appear to preferentially modulate VTA DA neuronal firing activity [27,28,58, 167]. Pharmacological studies have demonstrated that most atypical antipsychotic drugs are not only DA D2 antagonists, but also have affinities for a range of other neurotransmitter receptors such as 5-HT2, adrenergic, muscarinic and histamine receptors [20, 21]. These additional receptor affinities could contribute to the selective development of depolarization blockade of only VTA DA neurons and consequently the lower incidence of EPS and improved efficacy compared to the classical antipsychotic drugs.
Besides the development of depolarization blockade of VTA DA neurons, other mechanisms, for instance at the level of the prefrontal cortex, are also likely to improve antipsychotic drug efficacy. It is reported that clozapine facilitates NMDA-mediated neurotransmission in prefrontal cortical neurons [3] and atypical antipsychotic drugs, but not classical antipsychotic drugs, increase acetylcholine release in the forebrain [69]. The release of DA and noradrenaline is also increased by antipsychotic drugs [126, 166]. For a comprehensive review on these mechanisms in the forebrain and their implications for schizophrenia treatment see Moore et al., 1999 ([108]).
Not all atypical antipsychotic drugs combine DA D2 receptor antagonism with affinities for other receptors [107], and it has been suggested that multi-receptor affinity is not the key to atypical antipsychotic effectiveness. It has been proposed that their effectiveness is related to selective affinities for DA receptors in specific areas of the brain or receptors in specific conformational states [107]. Also a fast dissociation theory has been proposed [73, 74, 109]. This theory suggests a "hit-and-run" action of the antipsychotic drug at the DA receptor insofar as the drug rapidly binds and then dissociates from the receptor, thereby not "rigidly" blocking all DA neurotransmission. This mechanism might explain the clinical atypical antipsychotic profile of amisulpride, a pure D2/D3 receptor antagonist, although it has been suggested that the possible selective preference for mesolimbic versus nigrostriatal DA D2-like receptors by amisulpride or its D3 receptor antagonism might account for the atypicality [86, 107, 122]. So far, not all the evidence for the “hit-and-run” theory is conclusive and the hypothesis that multi-receptor affinities underlie atypicality is still very much favored [101]. From the large variety in receptor affinities, besides DA D2 receptors that are displayed by atypical antipsychotic drugs, especially affinity for the 5-HT(2) receptor has received considerable attention for its potential role in atypicality.
5-HT AND DA PATHWAYS
In vivo DA-dependent behaviors can be modulated by 5-HT receptor activation, as demonstrated using 5-HT1 5-HT2 and 5-HT4 receptor agonists. For example synergistic enhancement of the acoustic startle reflex by 5-HT1A receptor agonists [98] or 5-HT2 and 5-HT4 receptor-mediated modulation of cocaine-induced locomotor activity [95–97]. In addition, DA release in the medial prefrontal cortex (mPFC), dorsal and ventral striatum can be increased by (cortical) 5-HT2A receptor activation, while 5-HT2C receptors suppress DA release (likely via effects on DA cell bodies) [33, 56, 57, 119, 120]. Cortical 5-HT1A receptor activation appears to increase DA release in the mPFC, possibly in concert with mesolimbic 5-HT2 receptor antagonism [70]. Others have also extensively demonstrated that 5-HT facilitates DA release and neurotransmission via 5-HT receptors in the forebrain, with specific roles for 5-HT1, 5-HT2 and 5-HT4 receptors [6, 76] and secondary messengers, such as protein kinase A [164] or nitric oxide [165]. Moreover, the modulation of DA synthesis and release is under control of different mechanisms [48, 77, 165], often depending on the projection field [127, 156]. These findings show that 5-HT influences and shapes DA neurotransmission by actions at the somatic and terminal-field level. The findings that DA neurotransmission to the PFC is influenced by 5-HT suggests a role for 5-HT receptors in antipsychotic modulation of mesolimbic hyperactivity (for a comprehensive review see Werkman et al., in press ([161])).
5-HT AND SN AND VTA DA NEURON ACTIVITY
VTA and SN DA neurons (as well as the local GABAergic interneurons) express a range of 5-HT receptor types that can be activated by 5-HT input from the dorsal raphe nucleus (DRN) [62] (Fig. (1)). In Table (1) a summary is provided of the 5-HT receptors and the effect their activation has on DA neuronal firing activity. Some of these receptors have been found on GABAergic interneurons, such as the 5-HT1B and 5-HT2C receptor, although the latter might also be present on the DA neurons [128]. The activation of 5-HT1B receptors inhibits GABA release [71, 171], while 5-HT2C receptor activation excites GABAergic interneurons [36] and stimulation of the 5-HT1A receptor has been reported to excite DA neurons [84]. 5-HT3 receptors are not well studied yet in the SN and VTA, but based on findings in other brain areas they are expected to be located presynaptically [110]. In vivo, i.v. administration of 5-HT3 receptor antagonists is reported to increase DA neuronal firing, although the mechanism underlying this remains unclear [105, 117]. 5-HT4 receptor activation increases nigral DA firing activity, but it is not clear if the receptors are present on the DA neurons or GABAergic interneurons [13, 90, 118]. The 5-HT6 receptor is present on interneurons, and possibly also on the terminals of GABAergic and possibly cholinergic projections to DA neurons [50] but is not thought to be directly involved in modulating DA neurotransmission, but likely indirectly via the regulation of cholinergic neurotransmission [15]. Little is known about the presence or function of the 5-HT7 receptor in SN and VTA areas, although it has been proposed as an interesting candidate for future antipsychotic drugs, as a number of atypical antipsychotics bind to this 5-HT receptor subtype [99, 133].
Table 1.
5-HT Receptor Types in SN and VTA. An Overview of 5-HT Receptor Types with Their Locations in SN and VTA Areas and Reported (Indirect) Effects on DA Neuron Firing Activity. n.d.=not Determined
| Location | DA neuron firing activity | references | |
|---|---|---|---|
| 5-HT<sub>1A</sub> | DA neurons Tresynaptic processes | increase or pattern change | [2,41,85, 106, 123] |
| 5-HT<sub>1B</sub> | GABAergic interneuron | increase | [71, 171] |
| 5-HT<SUB>M</SUB> | DA neurons | increase | [40, 114, 123] |
| 5-HT<sub>2C</sub> | GABAergic interneuron DA neuron? | decrease | [38,57, 157] |
| 5-HTj | n.d. | increase | [104, 110, 117] |
| <bold>5-HT<sub>4</sub></bold> | n.d. | increase | [152] |
| <bold>5-HT<sub>6</sub></bold> | GABAergic interneuron DA neuron? | n.d. | [50, 132] |
These findings, and the reports that 5-HT receptor-induced changes in DA neuronal activity are dependent on the activation state of the DA neurons, i.e. when the DA impulse flow is activated [37, 90, 129, 143], show that 5-HT regulates DA neuronal firing activity both in a tonic and phasic manner. Furthermore, differential roles appear to exist for some 5-HT receptor types, such as the 5-HT2 receptors, i.e. 5-HT2C receptors change DA neuronal firing activity and bursting in VTA, but not in SN DA neurons [35, 39, 116], while 5-HT4 receptors play a more pronounced role in SN DA neurons [90].
The existence of differential roles for a 5-HT receptor type can be related to the diversity of second messenger pathways that 5-HT receptors utilize [4]. Most 5-HT receptors are G protein-coupled receptors (GPCRs) (with the ligand-gated 5-HT3 receptor being the exception) and they can activate second messenger systems such as phospholipase C, and ion channels linked to specific G protein subunits, including GIRK channels [54, 115] (for a comprehensive review see Barnes and Sharp, 1999 ([4])). 5-HT receptor-mediated activation of second messenger pathways and ion channels can directly increase or decrease DA neuronal firing activity as well as influence the neuronal response to activation of other receptors, such as DA D2 receptors [16, 51, 88, 112, 116, 124].
5-HT MODULATION OF DA D2 RECEPTOR-MEDIATED INHIBITION
As mentioned previously, midbrain DA neurons tonically regulate their own firing activity via a feedback auto-inhibition. Extracellular recordings of SN and VTA DA neurons in vitro have shown that concentrations of 5-HT that do not change the firing rate when applied alone enhanced DA D2 receptor-mediated auto-inhibition [16, 116]. It was demonstrated that this enhancement of auto-inhibition was mediated via different 5-HT receptors in the SN and VTA, with 5-HT2 receptors being critically involved in the VTA, in line with in vivo findings that 5-HT2 receptors differentially affect DA neuronal activity in SN and VTA [35, 39]. Various mechanisms underlying this DA/5-HT interaction have been suggested. One proposed mechanism is the inhibition of the Ih current [88]. However, 5-HT concentrations higher than the concentrations used to enhance auto-inhibition were necessary to inhibit Ih [16]. This finding and previous reports that inhibition of Ih current occurs secondary to GIRK channel activation by DA or GABA [25, 102, 160] suggest that additional mechanisms may also involved. The close link between GIRK channel and Ih channel activities [147, 148] is not fully understood, but as GIRK channels play a prominent role in the regulation of DA neuronal firing by translating DA D2 receptor activation into inhibition of firing activity, 5-HT receptor-mediated modulation of GIRK channels may underlie 5-HT-enhanced auto-inhibition (Fig. (2)).
GIRK CHANNEL MODULATION
GIRK channels have up to four binding sites for βγ G protein subunits. Upon activation of a GPCR such as the DA D2 receptor, the attached G protein dissociates and separates into the α and the βγ subunit. Binding of one βγ subunit only partially activates the channel and additional binding (to 3-4 subunits) results in full activation of the channel [32, 113, 134, 135]. It has been established that this graded activation is a form of positive co-operativity [134]. Besides the capability of binding multiple βγ subunits that contribute in a graded manner to channel (in)activation, it has been suggested recently that GIRK channels can be inhibited by binding of the G protein α subunit to the channel [83, 121, 137, 172].
Thus, different GPCRs can influence their respective effects on GIRK channel activation directly at the level of the channel. For instance, GABAB and DA D2 receptors activate the same GIRK channel. Submaximal activation of both receptors results in an additive effect on the GIRK current [81], probably due to the positive co-operativity of the βγ subunits. Such effects at the level of the GIRK channel itself can also lead to inhibitory actions. Neurotensin receptors are coupled to G proteins that inhibit GIRK function; simultaneous activation of neurotensin and DA D2 receptors in midbrain DA neurons results in a decreased GIRK current [45] and consequently an attenuation of DA D2 receptor-mediated auto-inhibition [163]. Furthermore, receptors that activate second messenger pathways (such as 5-HT receptors) can modulate GPCR-activated GIRK channels, as it has been shown that intracellular components such as protein kinases and phosphatidyl inositol diphosphate (PIP2) are able to stabilize the channel in the open state or facilitate βγ subunit binding [63, 64, 68, 82, 89, 125, 135].
Altogether, GPCR stimulation and intracellular mechanisms that influence GPCR and G protein coupling can affect GIRK channel activation, and thus the effect of GPCR activation on DA neuronal firing. Such interactions have been demonstrated for estrogen receptors in hypothalamic neurons, which upon activation, disrupt the coupling of GABAB receptors to GIRK channels through an action on the G protein [78, 79]. As the relative size of the G protein pools that GPCRs have access to differs [115], it is possible to attain a weighted effect on GIRK channel activity upon receptor activation through different input systems.
FUNCTIONAL MECHANISM OF 5-HT MODULATION AND IMPLICATIONS FOR ANTIPSYCHOTIC DRUG ACTIVITY
5-HT2 receptors differentially affect SN and VTA DA neuronal activity, both in vitro and in vivo. In animal models of schizophrenia, limited as they are, it appears that 5-HT2 receptor antagonism indeed plays a role in a differential modulation of DA neuron firing activity by antipsychotic drugs [52, 53, 72, 146, 153] and this may underlie the reduced EPS liability in drugs possessing mixed D2 / 5-HT2A receptor modalities.
How are the affinities of atypical antipsychotic drugs for DA D2 and 5-HT2 receptors translated to functional changes in mesolimbic (and possibly mesocortical) DA neuronal firing? DA D2 receptor-mediated auto-inhibition can be enhanced by 5-HT2 receptors in the VTA, but in the SN probably another 5-HT receptor type is involved [116] (Fig. (2)). Antagonism of part of the DA D2 receptor population increases the firing activity of DA neurons in SN and in VTA [103, 131, 162]. However, in the presence of 5-HT, the inhibitory effect remaining through DA D2 receptor activation will be enhanced, thus (partly) counteracting the effect of the DA D2 receptor antagonism. This can occur in both SN and VTA DA neurons. In the case of treatment of schizophrenia, the preferential effect of the atypical antipsychotic drugs is the induction of depolarization blockade in VTA DA neurons, while leaving the firing activity of SN DA neurons largely unchanged. Classical antipsychotic drugs, as discussed before, will induce depolarization blockade in both areas [28]. In contrast, atypical antipsychotic drugs have an additional feature that could assist in achieving selective depolarization blockade [1, 60]. The atypical antipsychotic drugs also block the DA D2 receptors in SN and VTA DA neurons. However, the 5-HT2 receptor antagonistic action of these drugs [20] results in a blockade of the 5-HT-mediated enhancement of the remaining DA auto-inhibition in VTA DA neurons, allowing a further depolarization and increase in firing rate (Fig (2)), ultimately leading to depolarization blockade. In SN DA neurons however, since another 5-HT receptor subtype may be responsible for the enhancement of auto-inhibition, the remaining DA D2 receptor mediated auto-inhibition can still be enhanced. In general, it is thought that 5-HT2 receptor antagonism increases the likely-hood that DA D2 receptor antagonism induces depolarization blockade of VTA DA neurons and not of SN DA neurons, thereby decreasing the liability for EPS induction. This mechanism is supported by the observations that elevated 5-HT level seems related to clinical efficacy of some atypical antipsychotics, and that atypical antipsychotics can increase 5-HT levels in the brain [93, 158], but this appears very brain area dependent.
In conclusion, 5-HT receptor-mediated modulation of DA neuron physiological function involves direct effects on DA neuron firing activity and indirect effects such as the enhancement of DA D2 receptor-mediated auto-inhibition. Moreover, 5-HT2 receptors have been established to be differentially involved in shaping DA D2 receptor-mediated auto-inhibition, possibly through actions on the GIRK channel via second messenger pathways. This indicates that this channel plays a central role in determining SN and VTA DA neuron activity and possibly the development of depolarization blockade. Differences in control mechanisms of the GIRK channel by DA and 5-HT receptors in both SN and VTA areas allows compounds like atypical antipsychotic drugs to have differential effects on the electrical activity of SN and DA neurons. A pivotal role for the modulation by 5-HT on DA neurotransmission has been established, not only in SN and VTA, but also in areas such as the mPFC. This points to the relevance of 5-HT2 receptor modulation of DA neurotransmission in schizophrenia, either in understanding the pathophysiology of the disease or, perhaps more pronounced, in the treatment of the disease with antipsychotic drugs.
REFERENCES
- 1.Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathe JM, Svensson TH. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:374–385. doi: 10.1007/BF00172774. [DOI] [PubMed] [Google Scholar]
- 2.Arborelius L, Chergui K, Murase S, Nomikos GG, Hook BB, Chouvet G, Hacksell U, Svensson TH. The 5-HT1A receptor selective ligands, (R)-8-OH-DPAT and (S)-UH301, differentially affect the activity of midbrain dopamine neurons. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:353–362. doi: 10.1007/BF00165384. [DOI] [PubMed] [Google Scholar]
- 3.Arvanov VL, Wang RY. Clozapine, but not haloperidol, prevents the functional hyperactivity of N-methyl-D-aspartate receptors in rat cortical neurons induced by subchronic administration of phencyclidine. J Pharmacol Exp Ther. 1999;289:1000–1006. [PubMed] [Google Scholar]
- 4.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 5.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 6.Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther. 1993;265:373–377. [PubMed] [Google Scholar]
- 7.Bernardini GL, Gu X, Viscardi E, German DC. Amphetamine-induced and spontaneous release of dopamine from A9 and A10 cell dendrites: an in vitro electrophysiological study in the mouse. J Neural Transm Gen Section. 1991;84:183–193. doi: 10.1007/BF01244969. [DOI] [PubMed] [Google Scholar]
- 8.Bertler A, Rosengren E. Occurrence and distribution of catechol amines in brain. Acta Physiol Scand. 1959;47:350–361. [PubMed] [Google Scholar]
- 9.Birmaher B, Baker R, Kapur S, Quintana H, Ganguli R. Clozapine for the treatment of adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 1992;31:160–164. doi: 10.1097/00004583-199201000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Blair DT, Dauner A. Extrapyramidal symptoms are serious side-effects of antipsychotic and other drugs. Nurse Pract. 1992;17:56, 62-54–67. doi: 10.1097/00006205-199211000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5:159–179. doi: 10.1017/S1461145702002894. [DOI] [PubMed] [Google Scholar]
- 12.Boeckler F, Russig H, Zhang W, Lober S, Schetz J, Hubner H, Ferger B, Gmeiner P, Feldon J. FAUC 213, a highly selective dopamine D4 receptor full antagonist, exhibits atypical antipsychotic properties in behavioural and neurochemical models of schizophrenia. Psychopharmacology (Berl) 2004;175:7–17. doi: 10.1007/s00213-004-1782-1. [DOI] [PubMed] [Google Scholar]
- 13.Bonhomme N, De Deurwaerdere P, Le Moal M, Spampinato U. Evidence for 5-HT4 receptor subtype involvement in the enhancement of striatal dopamine release induced by serotonin: a microdialysis study in the halothane-anesthetized rat. Neuropharmacology. 1995;34:269–279. doi: 10.1016/0028-3908(94)00145-i. [DOI] [PubMed] [Google Scholar]
- 14.Boye SM, Rompre PP. Behavioral evidence of depolarization block of dopamine neurons after chronic treatment with haloperidol and clozapine. J Neurosci. 2000;20:1229–1239. doi: 10.1523/JNEUROSCI.20-03-01229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branchek TA, Blackburn TP. 5-ht6 receptors as emerging targets for drug discovery. Annu Rev Pharmacol Toxicol. 2000;40:319–334. doi: 10.1146/annurev.pharmtox.40.1.319. [DOI] [PubMed] [Google Scholar]
- 16.Brodie MS, Bunney EB. Serotonin potentiates dopamine inhibition of ventral tegmental area neurons in vitro. J Neurophysiol. 1996;76:2077–2082. doi: 10.1152/jn.1996.76.3.2077. [DOI] [PubMed] [Google Scholar]
- 17.Brunello N, Masotto C, Steardo L, Markstein R, Racagni G. New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharmacology. 1995;13:177–213. doi: 10.1016/0893-133X(95)00068-O. [DOI] [PubMed] [Google Scholar]
- 18.Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- 19.Burki HR. Extrapyramidal side-effects. Pharmacol Ther [B] 1979;5:525–534. doi: 10.1016/0163-7258(79)90127-x. [DOI] [PubMed] [Google Scholar]
- 20.Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- 21.Bymaster FP, Hemrick-Luecke SK, Perry KW, Fuller RW. Neurochemical evidence for antagonism by olanzapine of dopamine, serotonin, alpha 1-adrenergic and muscarinic receptors in vivo in rats. Psychopharmacology (Berl) 1996;124:87–94. doi: 10.1007/BF02245608. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson A, Hansson LO, Waters N, Carlsson ML. Neurotransmitter aberrations in schizophrenia: new perspectives and therapeutic implications. Life Sci. 1997;61:75–94. doi: 10.1016/s0024-3205(97)00228-2. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson ML, Carlsson A, Nilsson M. Schizophrenia: from dopamine to glutamate and back. Curr Med Chem. 2004;11:267–277. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- 25.Cathala L, Paupardin-Tritsch D. Effect of catecholamines on the hyperpolarization-activated cationic Ih and the inwardly rectifying potassium I(Kir) currents in the rat substantia nigra pars compacta. Eur J Neurosci. 1999;11:398–406. doi: 10.1046/j.1460-9568.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 26.Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- 27.Chiodo LA, Bunney BS. Possible mechanisms by which repeated clozapine administration differentially affects the activity of two subpopulations of midbrain dopamine neurons. J Neurosci. 1985;5:2539–2544. doi: 10.1523/JNEUROSCI.05-09-02539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu E, Burrows G, Stevenson J. Double-blind comparison of clozapine with chlorpromazine in acute schizophrenic illness. Aust N Z J Psychiatry. 1976;10:343–347. doi: 10.3109/00048677609159524. [DOI] [PubMed] [Google Scholar]
- 30.Compton MT, Miller AH. Antipsychotic-induced hyperprolactinemia and sexual dysfunction. Psychopharmacol Bull. 2002;36:143–164. [PubMed] [Google Scholar]
- 31.Conley RR, Kelly DL. Current status of antipsychotic treatment. Curr Drug Targets CNS Neurol Disord. 2002;1:123–128. doi: 10.2174/1568007024606221. [DOI] [PubMed] [Google Scholar]
- 32.Corey S, Clapham DE. The Stoichiometry of Gbeta gamma binding to G-protein-regulated inwardly rectifying K+ channels (GIRKs) J Biol Chem. 2001;276:11409–11413. doi: 10.1074/jbc.M100058200. [DOI] [PubMed] [Google Scholar]
- 33.De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deutch AY. Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson’s disease. J Neural Transm Gen Section. 1993;91:197–221. doi: 10.1007/BF01245232. [DOI] [PubMed] [Google Scholar]
- 35.Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. Preferential modulation of mesolimbic vs nigrostriatal dopaminergic function by serotonin(2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse. 2000;35:53–61. doi: 10.1002/(SICI)1098-2396(200001)35:1<53::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Di Giovanni G, Di Matteo V, La Grutta V, Esposito E. m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience. 2001;103:111–116. doi: 10.1016/s0306-4522(00)00561-3. [DOI] [PubMed] [Google Scholar]
- 37.Di Mascio M, Esposito E. The degree of inhibition of dopaminergic neurons in the ventral tegmental area induced by selective serotonin reuptake inhibitors is a function of the density-power-spectrum of the interspike interval. Neuroscience. 1997;79:957–961. doi: 10.1016/s0306-4522(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 38.Di Matteo V, Cacchio M, Di Giulio C, Esposito E. Role of serotonin(2C) receptors in the control of brain dopaminergic function. Pharmacol Biochem Behav. 2002;71:727–734. doi: 10.1016/s0091-3057(01)00705-5. [DOI] [PubMed] [Google Scholar]
- 39.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 40.Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–185. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- 41.Doherty MD, Pickel VM. Targeting of serotonin 1A receptors to dopaminergic neurons within the parabrachial subdivision of the ventral tegmental area in rat brain. J Comp Neurol. 2001;433:390–400. doi: 10.1002/cne.1147. [DOI] [PubMed] [Google Scholar]
- 42.Dreher JC, Burnod Y. An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Netw. 2002;15:583–602. doi: 10.1016/s0893-6080(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 43.Engberg G, Kling-Petersen T, Nissbrandt H. GABAB-receptor activation alters the firing pattern of dopamine neurons in the rat substantia nigra. Synapse. 1993;15:229–238. doi: 10.1002/syn.890150308. [DOI] [PubMed] [Google Scholar]
- 44.Erhardt S, Mathe JM, Chergui K, Engberg G, Svensson TH. GABA(B) receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:173–180. doi: 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- 45.Farkas RH, Chien PY, Nakajima S, Nakajima Y. Neurotensin and dopamine D2 activation oppositely regulate the same K+ conductance in rat midbrain dopaminergic neurons. Neurosci Lett. 1997;231:21–24. doi: 10.1016/s0304-3940(97)00530-2. [DOI] [PubMed] [Google Scholar]
- 46.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 47.Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36:1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- 48.Galloway MP, Wolf ME, Roth RH. Regulation of dopamine synthesis in the medial prefrontal cortex is mediated by release modulating autoreceptors: studies in vivo. J Pharmacol Exp Ther. 1986;236:689–698. [PubMed] [Google Scholar]
- 49.Gelenberg AJ, Doller JC. Clozapine versus chlorpromazine for the treatment of schizophrenia: preliminary results from a double-blind study. J Clin Psychiatry. 1979;40:238–240. [PubMed] [Google Scholar]
- 50.Gerard C, Martres MP, Lefevre K, Miquel MC, Verge D, Lanfumey L, Doucet E, Hamon M, el Mestikawy S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997;746:207–219. doi: 10.1016/s0006-8993(96)01224-3. [DOI] [PubMed] [Google Scholar]
- 51.Gervais J, Rouillard C. Dorsal raphe stimulation differentially modulates dopaminergic neurons in the ventral tegmental area and substantia nigra. Synapse. 2000;35:281–291. doi: 10.1002/(SICI)1098-2396(20000315)35:4<281::AID-SYN6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 52.Geyer MA. Behavioral studies of hallucinogenic drugs in animals: implications for schizophrenia research. Pharmacopsychiatry. 1998;31(2):73–79. doi: 10.1055/s-2007-979350. [DOI] [PubMed] [Google Scholar]
- 53.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 54.Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH. Differential effects of regulator of G protein signaling (RGS) proteins on serotonin 5-HT1A, 5-HT2A, and dopamine D2 receptor-mediated signaling and adenylyl cyclase activity. Cell Signal. 2004;16:711–721. doi: 10.1016/j.cellsig.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Glenthoj B, Mogensen J, Laursen H, Holm S, Hemmingsen R. Electrical sensitization of the meso-limbic dopaminergic system in rats: a pathogenetic model for schizophrenia. Brain Res. 1993;619:39–54. doi: 10.1016/0006-8993(93)91594-i. [DOI] [PubMed] [Google Scholar]
- 56.Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 57.Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein JM, Litwin LC, Sutton EB, Malick JB. Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:293–298. doi: 10.1007/BF02244924. [DOI] [PubMed] [Google Scholar]
- 59.Grace AA, Bunney BS. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther. 1986;238:1092–1100. [PubMed] [Google Scholar]
- 60.Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends Neurosci. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- 61.Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- 63.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 64.Ho IH, Murrell-Lagnado RD. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J Physiol. 1999;520:645–651. doi: 10.1111/j.1469-7793.1999.00645.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollerman JR, Abercrombie ED, Grace AA. Electrophysiological, biochemical, and behavioral studies of acute haloperidol-induced depolarization block of nigral dopamine neurons. Neuroscience. 1992;47:589–601. doi: 10.1016/0306-4522(92)90168-2. [DOI] [PubMed] [Google Scholar]
- 66.Holt DJ, Herman MM, Hyde TM, Kleinman JE, Sinton CM, German DC, Hersh LB, Graybiel AM, Saper CB. Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience. 1999;94:21–31. doi: 10.1016/s0306-4522(99)00279-1. [DOI] [PubMed] [Google Scholar]
- 67.Horacek J. Novel antipsychotics and extrapyramidal side effects. Theory and reality. Pharmacopsychiatry. 2000;33(1):34–42. doi: 10.1055/s-2000-7660. [DOI] [PubMed] [Google Scholar]
- 68.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 69.Ichikawa J, Dai J, O’Laughlin IA, Fowler WL, Meltzer HY. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 70.Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 71.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones-Humble SA, Durcan MJ, Lyerly D, Norton RM, Tang FL, Russell AV, Watson MJ, Gengo PJ, Morgan PF, Wang CM, Cooper BR, Cox RF. Preclinical neurochemical and electrophysiological profile of 1192U90, a potential antipsychotic. Neuropsychopharmacology. 1996;15:217–230. doi: 10.1016/0893-133X(96)00019-X. [DOI] [PubMed] [Google Scholar]
- 73.Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J Psychiatry Neurosci. 2000;25:161–166. [PMC free article] [PubMed] [Google Scholar]
- 74.Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- 75.Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- 76.Kelland MD, Freeman AS, Chiodo LA. Serotonergic afferent regulation of the basic physiology and pharmacological responsiveness of nigrostriatal dopamine neurons. J Pharmacol Exp Ther. 1990;253:803–811. [PubMed] [Google Scholar]
- 77.Kelland MD, Freeman AS, Chiodo LA. SKF 38393 alters the rate-dependent D2-mediated inhibition of nigrostriatal but not mesoaccumbens dopamine neurons. Synapse. 1988;2:416–423. doi: 10.1002/syn.890020409. [DOI] [PubMed] [Google Scholar]
- 78.Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- 80.Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 81.Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lei Q, Jones MB, Talley EM, Garrison JC, Bayliss DA. Molecular mechanisms mediating inhibition of G protein-coupled inwardly- rectifying K+ channels. Mol Cells. 2003;15:1–9. [PubMed] [Google Scholar]
- 83.Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves G(alpha)q family subunits, phospholipase C, and a readily diffusible messenger. J Biol Chem. 2001;276:16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- 84.Lejeune F, Millan MJ. Pindolol excites dopaminergic and adrenergic neurons, and inhibits serotonergic neurons, by activation of 5-HT1A receptors. Eur J Neurosci. 2000;12:3265–3275. doi: 10.1046/j.1460-9568.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 85.Lejeune F, Millan MJ. Induction of burst firing in ventral tegmental area dopaminergic neurons by activation of serotonin (5-HT)1A receptors: WAY 100,635-reversible actions of the highly selective ligands, flesinoxan and S 15535. Synapse. 1998;30:172–180. doi: 10.1002/(SICI)1098-2396(199810)30:2<172::AID-SYN7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 86.Leucht S. Amisulpride a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(1):S15–20. doi: 10.1017/S1461145704004109. [DOI] [PubMed] [Google Scholar]
- 87.Li YX, Bertram R, Rinzel J. Modeling N-methyl-Daspartate-induced bursting in dopamine neurons. Neuroscience. 1996;71:397–410. doi: 10.1016/0306-4522(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–3212. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- 89.Logothetis DE, Zhang H. Gating of G protein-sensitive inwardly rectifying K+ channels through phosphatidylinositol 4,5bisphosphate. J Physiol. 1999;520 doi: 10.1111/j.1469-7793.1999.00630.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lucas G, Di Matteo V, De Deurwaerdere P, Porras G, Martin-Ruiz R, Artigas F, Esposito E, Spampinato U. Neurochemical and electrophysiological evidence that 5-HT4 receptors exert a state-dependent facilitatory control in vivo on nigrostriatal, but not mesoaccumbal, dopaminergic function. Eur J Neurosci. 2001;13:889–898. doi: 10.1046/j.0953-816x.2000.01453.x. [DOI] [PubMed] [Google Scholar]
- 91.Marsden CD, Jenner P. The pathophysiology of extrapyramidal side-effects of neuroleptic drugs. Psychol Med. 1980;10:55–72. doi: 10.1017/s003329170003960x. [DOI] [PubMed] [Google Scholar]
- 92.Martin P, Waters N, Carlsson A, Carlsson ML. The apparent antipsychotic action of the 5-HT2a receptor antagonist M100907 in a mouse model of schizophrenia is counteracted by ritanserin. (Rapid communication) J Neural Transm. 1997;104:561–564. doi: 10.1007/BF01277672. [DOI] [PubMed] [Google Scholar]
- 93.Martin P, Waters N, Schmidt CJ, Carlsson A, Carlsson ML. Rodent data and general hypothesis: antipsychotic action exerted through 5-Ht2A receptor antagonism is dependent on increased serotonergic tone. J Neural Transm. 1998;105:365–396. doi: 10.1007/s007020050064. [DOI] [PubMed] [Google Scholar]
- 94.Matz R, Rick W, Thompson H, Gershon S. Clozapine-a potential antipsychotic agent without extrapyramidal manifestations. Curr Ther Res Clin Exp. 1974;16:687–695. [PubMed] [Google Scholar]
- 95.McCreary AC, Cunningham KA. Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology. 1999;20:556–564. doi: 10.1016/S0893-133X(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 96.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–363. [PubMed] [Google Scholar]
- 97.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(4) receptors attenuates hyperactivity induced by cocaine: putative role for 5-hydroxytryptamine(4) receptors in the nucleus accumbens shell. J Pharmacol Exp Ther. 1999;291:300–307. [PubMed] [Google Scholar]
- 98.Meloni EG, Davis M. Synergistic enhancement of the acoustic startle reflex by dopamine D1 and 5-HT1A agonists and corresponding changes in c-Fos expression in the dorsal raphe of rats. Psychopharmacology (Berl) 2000;151:359–367. doi: 10.1007/s002130000474. [DOI] [PubMed] [Google Scholar]
- 99.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 100.Meltzer HY, Arvanitis L, Bauer D, Rein W. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- 101.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors : their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 102.Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 103.Mereu G, Casu M, Gessa GL. (-)-Sulpiride activates the firing rate and tyrosine hydroxylase activity of dopaminergic neurons in unanesthetized rats. Brain Res. 1983;264:105–110. doi: 10.1016/0006-8993(83)91125-3. [DOI] [PubMed] [Google Scholar]
- 104.Minabe Y, Ashby CR, Jr., Wang RY. Effects produced by acute and chronic treatment with granisetron alone or in combination with haloperidol on midbrain dopamine neurons. Eur Neuropsychopharmacol. 1992;2:127–133. doi: 10.1016/0924-977x(92)90022-z. [DOI] [PubMed] [Google Scholar]
- 105.Minabe Y, Ashby CR, Jr., Wang RY. The effect of acute and chronic LY 277359, a selective 5-HT3 receptor antagonist, on the number of spontaneously active midbrain dopamine neurons. Eur J Pharmacol. 1991;209:151–156. doi: 10.1016/0014-2999(91)90163-k. [DOI] [PubMed] [Google Scholar]
- 106.Minabe Y, Schechter L, Hashimoto K, Shirayama Y, Ashby CR., Jr. Acute and chronic administration of the selective 5-HT1A receptor antagonist WAY-405 significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse. 2003;50:181–190. doi: 10.1002/syn.10255. [DOI] [PubMed] [Google Scholar]
- 107.Moller HJ. Amisulpride: limbic specificity and the mechanism of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1101–1111. doi: 10.1016/j.pnpbp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999;46:40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- 109.Mortimer AM. How do we choose between atypical antipsychotics? The advantages of amisulpride. Int J Neuropsychopharmacol. 2004;7(1):S21–25. doi: 10.1017/S1461145704004134. [DOI] [PubMed] [Google Scholar]
- 110.Mylecharane EJ. Ventral tegmental area 5-HT receptors: mesolimbic dopamine release and behavioural studies. Behav Brain Res. 1996;73:1–5. doi: 10.1016/0166-4328(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 111.Nair NP, Zicherman V, Schwartz G. Dopamine and schizophrenia. A reappraisal in the light of clinical studies with clozapine. Cnd Psychiatr Assoc J. 1977;22:285–293. doi: 10.1177/070674377702200604. [DOI] [PubMed] [Google Scholar]
- 112.Nedergaard S, Flatman JA, Engberg I. Excitation of substantia nigra pars compacta neurones by 5-hydroxy- tryptamine in-vitro. Neuroreport. 1991;2:329–332. doi: 10.1097/00001756-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 113.Nemec J, Wickman K, Clapham DE. Gbetagamma binding increases the open time of IKACh: kinetic evidence for multiple Gbetagamma binding sites. Biophys J. 1999;76:246–252. doi: 10.1016/S0006-3495(99)77193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nocjar C, Roth BL, Pehek EA. Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- 115.Odagaki Y, Fuxe K. 5-HT1A, GABAB, and pirenzepineinsensitive muscarinic receptors are functionally coupled to distinct pools of the same kind of G proteins in rat hippocampus. Brain Res. 1995;689:129–135. doi: 10.1016/0006-8993(95)00576-c. [DOI] [PubMed] [Google Scholar]
- 116.Olijslagers JE, Werkman TR, McCreary AC, Siarey R, Kruse CG, Wadman WJ. 5-HT(2) receptors differentially modulate dopamine-mediated auto-inhibition in A9 and A10 midbrain areas of the rat. Neuropharmacology. 2004;46:504–510. doi: 10.1016/j.neuropharm.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 117.Palfreyman MG, Schmidt CJ, Sorensen SM, Dudley MW, Kehne JH, Moser P, Gittos MW, Carr AA. Electrophysiological, biochemical and behavioral evidence for 5-HT2 and 5-HT3 mediated control of dopaminergic function. Psychopharmacology (Berl) 1993;112:S60–67. doi: 10.1007/BF02245008. [DOI] [PubMed] [Google Scholar]
- 118.Patel S, Roberts J, Moorman J, Reavill C. Localization of serotonin-4 receptors in the striatonigral pathway in rat brain. Neuroscience. 1995;69:1159–1167. doi: 10.1016/0306-4522(95)00314-9. [DOI] [PubMed] [Google Scholar]
- 119.Pehek EA. Local infusion of the serotonin antagonists ritanserin or ICS 205,930 increases in vivo dopamine release in the rat medial prefrontal cortex. Synapse. 1996;24:12–18. doi: 10.1002/(SICI)1098-2396(199609)24:1<12::AID-SYN2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 120.Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- 121.Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 122.Pelissolo A, Krebs MO, Olie JP. Treatment of negative symptoms in schizophrenia by amisulpride. Review of the literature. Encephale. 1996;22:215–219. [PubMed] [Google Scholar]
- 123.Peroutka SJ, Snyder SH. Two distinct serotonin receptors: regional variations in receptor binding in mammalian brain. Brain Res. 1981;208:339–347. doi: 10.1016/0006-8993(81)90562-x. [DOI] [PubMed] [Google Scholar]
- 124.Pessia M, Jiang ZG, North RA, Johnson SW. Actions of 5-hydroxytryptamine on ventral tegmental area neurons of the rat in vitro. Brain Res. 1994;654:324–330. doi: 10.1016/0006-8993(94)90495-2. [DOI] [PubMed] [Google Scholar]
- 125.Petit-Jacques J, Sui JL, Logothetis DE. Synergistic activation of G protein-gated inwardly rectifying potassium channels by the betagamma subunits of G proteins and Na(+) and Mg(2+) ions. J Gen Physiol. 1999;114:673–684. doi: 10.1085/jgp.114.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pira L, Mongeau R, Pani L. The atypical antipsychotic quetiapine increases both noradrenaline and dopamine release in the rat prefrontal cortex. Eur J Pharmacol. 2004;504:61–64. doi: 10.1016/j.ejphar.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 127.Pitts DK, Kelland MD, Freeman AS, Chiodo LA. Repeated amphetamine administration: role of forebrain in reduced responsiveness of nigrostriatal dopamine neurons to dopamine agonists. J Pharmacol Exp Ther. 1993;264:616–621. [PubMed] [Google Scholar]
- 128.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 129.Porras G, Di Matteo V, De Deurwaerdere P, Esposito E, Spampinato U. Central serotonin(4) receptors selectively regulate the impulse- dependent exocytosis of dopamine in the rat striatum: in vivo studies with morphine, amphetamine and cocaine. Neuropharmacology. 2002;43:1099–1109. doi: 10.1016/s0028-3908(02)00212-5. [DOI] [PubMed] [Google Scholar]
- 130.Prisco S, Natoli S, Bernardi G, Mercuri NB. Group I metabotropic glutamate receptors activate burst firing in rat midbrain dopaminergic neurons. Neuropharmacology. 2002;42:289–296. doi: 10.1016/s0028-3908(01)00192-7. [DOI] [PubMed] [Google Scholar]
- 131.Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther. 1994;271:1181–1192. [PubMed] [Google Scholar]
- 132.Roberts JC, Reavill C, East SZ, Harrison PJ, Patel S, Routledge C, Leslie RA. The distribution of 5-HT(6) receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT(6) receptor antagonist [(125)I]SB-258585. Brain Res. 2002;934:49–57. doi: 10.1016/s0006-8993(02)02360-0. [DOI] [PubMed] [Google Scholar]
- 133.Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr., Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- 134.Sadja R, Alagem N, Reuveny E. Graded contribution of the Gbeta gamma binding domains to GIRK channel activation. Proc Natl Acad Sci USA. 2002;99:10783–10788. doi: 10.1073/pnas.162346199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- 136.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 137.Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N. Inhibition of an inwardly rectifying K+ channel by G-protein alpha-subunits. Nature. 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- 138.Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 139.Seutin V, Massotte L, Renette MF, Dresse A. Evidence for a modulatory role of Ih on the firing of a subgroup of midbrain dopamine neurons. Neuroreport. 2001;12:255–258. doi: 10.1097/00001756-200102120-00015. [DOI] [PubMed] [Google Scholar]
- 140.Shepard PD, German DC. Electrophysiological and pharmacological evidence for the existence of distinct subpopulations of nigrostriatal dopaminergic neuron in the rat. Neuroscience. 1988;27:537–546. doi: 10.1016/0306-4522(88)90287-4. [DOI] [PubMed] [Google Scholar]
- 141.Shi WX, Pun CL, Smith PL, Bunney BS. Endogenous DA-mediated feedback inhibition of DA neurons: involvement of both D(1)- and D(2)-like receptors. Synapse. 2000;35:111–119. doi: 10.1002/(SICI)1098-2396(200002)35:2<111::AID-SYN3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 142.Shopsin B, Klein H, Aaronsom M, Collora M. Clozapine, chlorpromazine, and placebo in newly hospitalized, acutely schizophrenic patients: a controlled, double-blind comparison. Arch Gen Psychiatry. 1979;36:657–664. doi: 10.1001/archpsyc.1979.01780060047005. [DOI] [PubMed] [Google Scholar]
- 143.Spampinato U, Esposito E, Samanin R. Serotonin agonists reduce dopamine synthesis in the striatum only when the impulse flow of nigro-striatal neurons is intact. J Neurochem. 1985;45:980–982. doi: 10.1111/j.1471-4159.1985.tb04092.x. [DOI] [PubMed] [Google Scholar]
- 144.Stahl SM. Essential Psychopharmacology. New York: Cambridge University Press; 2000. [Google Scholar]
- 145.Stowe ZN, Nemeroff CB. The electrophysiological actions of neurotensin in the central nervous system. Life Sci. 1991;49:987–1002. doi: 10.1016/0024-3205(91)90300-z. [DOI] [PubMed] [Google Scholar]
- 146.Svensson TH, Mathe JM, Andersson JL, Nomikos GG, Hildebrand BE, Marcus M. Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: role of 5-HT2 receptor and alpha 1-adrenoceptor antagonism [corrected] J Clin Psychopharmacol. 1995;15:11S–18S. doi: 10.1097/00004714-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 147.Svoboda KR, Lupica CR. Opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J Neurosci. 1998;18:7084–7098. doi: 10.1523/JNEUROSCI.18-18-07084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takigawa T, Alzheimer C. G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol. 1999;517:385–390. doi: 10.1111/j.1469-7793.1999.0385t.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann Clin Psychiatry. 2002;14:123–129. doi: 10.1023/a:1016811222688. [DOI] [PubMed] [Google Scholar]
- 150.Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs. 2002;16:23–45. doi: 10.2165/00023210-200216010-00003. [DOI] [PubMed] [Google Scholar]
- 151.Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thorre K, Ebinger G, Michotte Y. 5-HT4 receptor involvement in the serotonin-enhanced dopamine efflux from the substantia nigra of the freely moving rat: a microdialysis study. Brain Res. 1998;796:117–124. doi: 10.1016/s0006-8993(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 153.Tricklebank MD. The antipsychotic potential of subtype-selective 5-HT receptor ligands based on interactions with mesolimbic dopamine systems. Behav Brain Res. 1996;73:15–17. doi: 10.1016/0166-4328(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 154.Trudeau LE. Glutamate co-transmission as an emerging concept in monoamine neuron function. J Psychiatry Neurosci. 2004;29:296–310. [PMC free article] [PubMed] [Google Scholar]
- 155.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 156.Tyler CB, Galloway MP. Acute administration of amphetamine:differential regulation of dopamine synthesis in dopamine projection fields. J Pharmacol Exp Ther. 1992;261:567–573. [PubMed] [Google Scholar]
- 157.Ugedo L, Grenhoff J, Svensson TH. Ritanserin, a 5-HT2 receptor antagonist, activates midbrain dopamine neurons by blocking serotonergic inhibition. Psychopharmacology. 1989;98:45–50. doi: 10.1007/BF00442004. [DOI] [PubMed] [Google Scholar]
- 158.Wadenberg MG, Browning JL, Young KA, Hicks PB. Antagonism at 5-HT(2A) receptors potentiates the effect of haloperidol in a conditioned avoidance response task in rats. Pharmacol Biochem Behav. 2001;68:363–370. doi: 10.1016/s0091-3057(00)00483-4. [DOI] [PubMed] [Google Scholar]
- 159.Wang T, O’Connor WT, Ungerstedt U, French ED. N-methyl-D-aspartic acid biphasically regulates the biochemical and electrophysiological response of A10 dopamine neurons in the ventral tegmental area: in vivo microdialysis and in vitro electrophysiological studies. Brain Res. 1994;666:255–262. doi: 10.1016/0006-8993(94)90780-3. [DOI] [PubMed] [Google Scholar]
- 160.Watts A, Williams J, Henderson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J Neurophysiol. 1996;76:2262–2270. doi: 10.1152/jn.1996.76.4.2262. [DOI] [PubMed] [Google Scholar]
- 161.Werkman TR, Glennon JC, Wadman WJ, McCreary AC. Dopamine receptor pharmacology: interactions with serotonin and significance for the aetiology and treatment of schizophrenia. 2006 doi: 10.2174/187152706784111614. in press. [DOI] [PubMed] [Google Scholar]
- 162.Werkman TR, Kruse CG, Nievelstein H, Long SK, Wadman WJ. In vitro modulation of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area by antipsychotic drugs. Neuropharmacology. 2001;40:927–936. doi: 10.1016/s0028-3908(01)00015-6. [DOI] [PubMed] [Google Scholar]
- 163.Werkman TR, Kruse CG, Nievelstein H, Long SK, Wadman WJ. Neurotensin attenuates the quinpiroleinduced inhibition of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area. Neuroscience. 2000;95:417–423. doi: 10.1016/s0306-4522(99)00449-2. [DOI] [PubMed] [Google Scholar]
- 164.West AR, Galloway MP. Regulation of serotonin-facilitated dopamine release in vivo: the role of protein kinase A activating transduction mechanisms. Synapse. 1996;23:20–27. doi: 10.1002/(SICI)1098-2396(199605)23:1<20::AID-SYN3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 165.West AR, Galloway MP, Grace AA. Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse. 2002;44:227–245. doi: 10.1002/syn.10076. [DOI] [PubMed] [Google Scholar]
- 166.Westerink BH, de Boer P, de Vries JB, Kruse CG, Long SK. Antipsychotic drugs induce similar effects on the release of dopamine and noradrenaline in the medial prefrontal cortex of the rat brain. Eur J Pharmacol. 1998;361:27–33. doi: 10.1016/s0014-2999(98)00711-0. [DOI] [PubMed] [Google Scholar]
- 167.White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- 168.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wong AH, Van Tol HH. The dopamine D4 receptors and mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1091–1099. doi: 10.1016/j.pnpbp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 170.Wu HQ, Schwarcz R, Shepard PD. Excitatory amino acid-induced excitation of dopamine-containing neurons in the rat substantia nigra: modulation by kynurenic acid. Synapse. 1994;16:219–230. doi: 10.1002/syn.890160307. [DOI] [PubMed] [Google Scholar]
- 171.Yan QS, Zheng SZ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis. Brain Res. 2004;1021:82–91. doi: 10.1016/j.brainres.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 172.Zhang Q, Dickson A, Doupnik CA. Gbetagammaactivated inwardly rectifying K(+) (GIRK) channel activation kinetics via Galphai and Galphao-coupled receptors are determined by Galpha-specific interdomain interactions that affect GDP release rates. J Biol Chem. 2004;279:29787–29796. doi: 10.1074/jbc.M403359200. [DOI] [PubMed] [Google Scholar]