Abstract
In mammals, the cells of the renal medulla are physiologically exposed to interstitial osmolalities several-fold higher that found in any other tissue. Nevertheless, these cells not only have the ability to survive in this harsh environment, but also to function normally, which is critical for maintenance of systemic electrolyte and fluid homeostasis. Over the last two decades, a substantial body of evidence has accumulated, indicating that sequential and well orchestrated genomic responses are required to provide tolerance to osmotic stress. This includes the enhanced expression and action of immediate-early genes, growth arrest and DNA damage inducible genes (GADDs), genes involved in cell cycle control and apoptosis, heat shock proteins, and ultimately that of genes involved in the intracellular accumulation of nonperturbing organic osmolytes. The present review summarizes the sequence of genomic responses conferring resistance against osmotic stress. In addition, the regulatory mechanisms mediating the coordinated genomic response to osmotic stress will be highlighted.
Key Words: Osmotic stress, osmoadaptation, gene expression, renal medullary cells, osmoprotective genes
INTRODUCTION
Osmolalities exceeding the physiological range of 280-300 mosm/kg H2O have severe consequences as they induce cell shrinkage, oxidative stress, DNA damage, cell cycle delay, and ultimately apoptosis in the absence of protective mechanisms. Renal medulla-resident cells provide an excellent paradigm for the adaptation of mammalian cells to osmotic stress, because, depending on the hydration status, these cells are exposed physiologically to wide fluctuations in ambient tonicity. In situations of water shortage (i.e. systemic volume depletion), the urinary concentrating mechanism is activated rapidly, which entails generation of papillary interstitial osmolalities several times higher that of the systemic circulation. In contrast, during prolonged diuresis, papillary interstitial osmolalities may drop progressively to values close to that of the systemic plasma. In the absence of counterregulatory mechanisms, medullary cells would not be able to correct changes in cell volume caused by osmotically obliged movement of water across the cell membrane, driven by the transmembrane osmotic gradient.
Following a rise in extracellular tonicity, renal medullary cells shrink and activate membrane transport pathways mediating the net uptake of NaCl and, in consequence, recovery of cell volume by osmotically driven entry of water [1–3]. Concommitantly, most of the imported sodium ions are replaced rapidly by potassium due to increased activity of the Na+,K+-ATPase [2]. Although these manoeuvres return the cell volume to normal or nearly normal values, they are inevitably associated with severe disturbances of intracellular ion homeostasis.
CELLULAR EFFECTS OF ELEVATED NaCl
Rise in Cellular Ionic Strength
Following an acute rise in ambient tonicity, the intracellular concentration of monovalent inorganic electrolytes (particularly potassium and chloride) increases in renal medullary cells to maintain osmotic equilibrium with the ex-tracellular space [4]. A prolonged rise in the intracellular concentration of inorganic electrolytes, i.e. the cellular ionic strength, however, may negatively affect the function and integrity of cellular macromolecules [5, 6]. Specifically, the activity of several enzymes is reduced and both protein and DNA synthesis are diminished [7, 8]. In the initial phase following the induction of osmotic stress, specific heat shock proteins (HSPs) are assumed to protect medullary cells from the adverse effects of high intracellular electrolyte concentration in view of their protein-stabilizing properties [9, 10]. Subsequently, excess inorganic electrolytes are replaced continuously by compatible organic osmolytes (see below) [11].
In addition to interference with protein structure and function, a persistent rise in cellular ionic strength is associated with increased incidence of DNA damage and apoptotic cell death [12]. Specifically, chromosome alterations, sister chromatid exchanges and DNA single- and double-strand breaks have been detected in cells exposed to osmotic stress [12, 13]. These alterations occur in the initial phase of hyper-tonicity and are specific for impermeant substances that exert osmotic stress, since solutes that equilibrate rapidly across the cell membrane, like glycerol or urea, fail to elicit these effects [12, 13].
Tonicity-Induced Oxidative Stress
Recent data indicate that renal medullary cells are exposed to substantial concentrations of reactive oxygen species, particularly in situations of systemic volume contraction with concomitant activation of the urinary concentrating mechanism and rising medullary interstitial NaCl concentrations. The major sites for intracellular ROS production are the mitochondria, which convert approximately 0.2-2% of the oxygen available to ROS, particularly superoxide anions [14, 15]. To a lesser extent, extra-mitochondrial oxidases, hydroxylases, and cytochrome P450 enzymes contribute to ROS formation [14]. In addition to their role as signalling molecules, excessive production of ROS may cause severe cell damage [14].
The increased abundance of carbonylated proteins is regarded as evidence for extensive oxidative stress within renal medullary cells both in vivo and in vitro [16]. Protein car-bamylation is recognized as a measure for severely oxidatively damaged proteins and is associated with a variety of disorders associated with excessive local oxidative stress [17, 18]. Although the mechanism of protein carbamylation during osmotic stress is incompletely understood, it is likely that ROS rather than NaCl per se mediate this effect, since both NaCl and raffinose increase the carbonyl content of cellular proteins and both solutes induce ROS in renal medullary cells [16]. Accumulation of carbamylated proteins may affect cellular function negatively either by loss of catalytic and/or structural integrity, or by interruption of regulatory pathways [19].
In medullary thick ascending limb cells, generation of ROS is linked to the stimulation of the Na+,K+-ATPase and the Na+/H+ exchanger in response to osmotic stress, whereas inhibition of the Na+,K+-ATPase by ouabain abolishes ROS production [20, 21]. Thus, increased ATP demand to maintain secondary and/or primary active transmembrane transport processes with elevated mitochondrial respiration may represent the primary mechanism for ROS production in renal medullary cells in response to osmotic stress. In addition, angiotensin II promotes the formation of superoxide via NADPH oxidase [22] and a process involving PKC in peri-cytes of descending vasa recta [23].
TONICITY-RESPONSIVE SIGNAL TRANSDUCTION
Although the genetic response to osmotic stress has been studied extensively in eukaryotic cells over the last two decades, to date the precise sequence of intracellular signalling events leading to osmotolerance has been identified only in yeast. In these cells, two membrane-resident osmosensors, SLN1 and sho-1, have been identified. These transduce an increase in ambient tonicity via several steps into activation of the mitogen-activated protein kinase (MAPK) HOG1, a homologue of mammalian p38. Activated HOG1 in turn drives the expression of enzymes involved in the intracellu-lar accumulation of glycerol and trehalose, both of which represent the major organic osmolytes in these cells [24–26]. In mammalian cells a membrane-bound osmosensor has not yet been identified. Nevertheless, osmotic stress entails a series of intracellular events that culminate in transcriptional activation of a trans-acting protein, known as tonicity-responsive enhancer binding protein (TonEBP), that stimulates the expression of various genes conferring osmoresis-tance and ensuring proper function of the renal medulla during antidiuresis (see below). TonEBP has been cloned independently by several groups; the corresponding cis-acting sequence in the 5’-regions of osmoprotective genes was identified some years ago [27–29]. The latter is known as tonicity-responsive enhancer (TonE), which is essential for induction of osmosensitive genes in response to hyperosmolar stress. Evidence that TonE confers osmotic inducibility of these genes has been provided by several experimental approaches, demonstrating that both single-base mutations or deletion of TonE elements blunt the osmotic induction of TonE-driven reporter constructs [24]. The following tonicity-responsive genes have been identified as TonEBP target genes: aldose reductase (AR), the betaine γ-amino-butyrate transporter (BGT-1), the sodium myo-inositol transporter (SMIT), the taurine transporter (TauT), heat shock protein 70 (HSP70), and aquaporin (AQP)-2 [29–33]. Indeed, mice with targeted disruption of TonEBP show severe and progressive atrophy of the renal medulla in parallel with reduced expression of osmoprotective genes [34]. Although the importance of TonEBP for renal medullary cells has been demonstrated convincingly, other tonicity-inducible genes essential for the integrity and function of the renal medulla appear to be regulated independently from TonEBP (e.g Na+,K+-ATPase, Cox-2).
INDUCTION OF OSMOSENSITIVE GENES
Immediate-EarlyGenes
Immediate-early genes (IEGs) are activated rapidly and transiently in response to a broad variety of cellular stimuli, including osmotic stress. The induction of IEGs is far earlier than that of genes involved in osmolyte accumulation, suggesting important functions under conditions in which organic osmolytes have not been accumulated in sufficient quantities.
Well characterized is the transiently increased expression of Fos and Jun proteins in response to osmotic stress in different tissues. Fos and Jun interact with each other in the heterodimeric AP-1 protein complex. AP-1 is a transcription factor that stimulates gene expression by binding to its consensus sequence, the AP-1 binding site, which is localized in the promoter region of target genes. In supraoptic nucleus neurons, Fos and Jun proteins appear within 30 min after the onset of osmotic stress, peak after 90-120 min, and disappear almost completely within 240 min [35]. Also induced by hyperosmolarity in supraoptic nucleus neurons is the early growth response protein-1 (EGR-1), also known as NGFI-A [36]. In renal cells, the transcription rate of EGR-1 is increased by hyperosmotic urea as well as by hypotonic conditions [37]. EGR-1 is a zinc-finger-containing transcription factor, activating the transcription of various downstream genes, encoding growth factors, structural proteins, adhesion molecules or other transcription factors [38].
Expression of IEGs is probably mediated by tonicity-activated MAPK pathways [39] and can be considered as the first phase of the genomic response to exposure to osmotic stress. IEGs often encode transcription factors, which in turn activate the transcription of other genes, also called late-response genes. Therefore, the expression of IEGs is a prerequisite for the activation of late-response genes (such as genes involved in osmolyte accumulation or HSP genes), that protect the cell from deleterious effects of hypertonicity.
Genes Involved in DNA Damage Repair, Growth Arrest and Apoptosis
As mentioned above, an increase in cellular ionic strength is associated with DNA damage. DNA double-strand breaks are detected by the ataxia telangiectasia-mutated (ATM) kinase, which in turn activates other proteins. Accordingly, ATM contributes to full activation of TonEBP, although ATM alone is not sufficient for this process [40–42]. As a consequence of DNA damage due to increased intracellular ionic strength, renal medullary cells activate mechanisms leading to cell cycle delay and growth arrest, thus allowing repair of damaged DNA and preservation of genomic stability [43]. Increased expression and activation of the tumor suppressor gene product p53 by ATM kinase and p38 kinase mediates G1 and G2/M arrest, thereby possibly protecting medullary cells from apoptosis after a rise in ambient tonicity and allowing repair of damaged DNA [40, 44, 45]. Prevention of the increased p53 expression, and therefore prevention of growth arrest under hyper-tonic conditions promotes subsequent cell death during the S-phase [46]. The p53-induced growth arrest observed after the onset of osmotic stress persists for several hours, whereas when adaptation to hypertonicity is complete, cultured cells proliferate indefinitely [47], although at significantly lower rates than under isotonic conditions.
Hypertonicity also increases the copy number of the growth arrest and DNA damage (GADD)-induced proteins 45 and 153 [48]. GADD45 exists in three isoforms, α, β and γ[49]. In mouse inner medullary collecting duct cells, GADD45 is up-regulated by moderate osmotic stress (540 mosm/kg) and contributes to cell cycle arrest at the G2/M cell-cycle checkpoint. During severe osmotic stress (620 mosm/kg), however, GADD45γ enhances apoptosis while GADD45α and β inhibit later stages of apoptosis [50]. These results indicate that GADD45, besides p53, plays a crucial role in the regulation of the balance between cell survival and apoptosis during osmotic stress.
Other DNA-damaging processes also cause transient growth arrest allowing activation of DNA repair mechanisms such as the meiotic recombination (Mre)11 complex [51]. Literature-data are inconsistent with respect to repair of NaCl-induced DNA breaks. Dmitrieva et al. have reported, that under conditions of elevated NaCl concentrations, Mre11 exits the nucleus, thus leaving the DNA unrepaired, even after completion of osmoadaptation of the cell and resume of proliferation [52, 53]. The latter studies suggested that checkpoint kinase (CHK) 1, involved in initiation of cell-cycle arrest, and histone H2AX, which is required for successfull DNA repair, are also inhibited by high NaCl. However, in contrast, Sheen et al. have observed that Mre11 remains in the nucleus following an increase in tonicity and that also H2AX was induced, resulting in an intact cellular response to tonicity-induced DNA damage [54]. The reasons for these inconsistencies are not clear.
Heat Shock Proteins
The induction of heat shock proteins (HSPs) is one of the most conserved mechanisms protecting mammalian cells from various cellular stress conditions, including osmotic stress. HSPs are a group of proteins highly conserved from bacteria to mammalian cells. Cell stress entails the accumulation of misfolded or aggregated proteins, while HSPs act as molecular chaperones, which assist other proteins to fold correct and assemble correctly. HSPs are also involved in protein transport and degradation of misfolded proteins, and interfere with the apoptotic signaling cascade [55]. According to their molecular weight, the HSPs are classified into several families (for a review see [56]). In mammalian cells, particularly members of the small HSP- (sHSP), HSP70- and HSP110-family are up-regulated in response to osmotic stress. It is well established that induction in response to heat stress occurs by binding of the heat shock transcription factor (HSF) to a heat-shock element (HSE) in the promoter region of HSP genes. Although osmotic stress also activates the transcription factor HSF-1, this event seems not to contribute substantially to increased mRNA-levels of HSP genes [57]. Instead, transcriptional activation by TonEBP plays a crucial role in HSP expression during hyperosmolality.
HSP27 belongs to the family of sHSPs. In renal cells, HSP27 expression is increased by osmotic stress and contributes to protection of renal medullary cells from high urea concentrations [58]. The distribution of HSP27 in the kidney follows the corticomedullary osmotic gradient, with low abundance in the cortex and high amounts in the inner medulla, that are elevated even further during antidiuresis [58]. The molecular mechanisms regulating HSP27 expression in response to osmotic stress are largely unknown. However, hyper- and hypoosmolarity induces phosphorylation of HSP27 via the p38 MAPK pathway, and subsequent capping of actin by HSP27, thereby promoting actin polymerization and stabilization of the actin cytoskeleton [59].
Another member of the sHSP family is αB-crystallin, which is also induced during osmotic stress. It shows homology to HSP25, is a molecular chaperone [60], and is one of the major structural proteins of the occular lens of vertebrates. In human retinal epithelial cells and in kidney cells, expression of αB-crystallin increases in response to hyper-tonicity [61–63]. Furthermore, overexpression of αB-crystallin in HEK293 cells enhances cell survival following osmotic stress [63].The increased expression of αB-crystallin apparently does not involve TonEBP [32], while the JNK kinase pathway seems to be involved in the up-regulation during hypertonicity [63].
Proteins of the HSP70 family may be expressed both constitutively and may be inducible by osmotic stress. In mice and human, there are two major HSP70 isoforms, of which only one is induced by osmotic stress [32, 64]. Tonicity-inducible HSP70 genes in different species contain TonE sites in their 5’-flanking region, suggesting that the increased transcription during osmotic stress is mediated by TonEBP [32]. In addition to its function as a molecular chaperone, stress-inducible HSP70 inhibits apoptosis by direct interaction with Apaf-1, thus preventing the recruitment of caspase-9 to the apoptosome complex [65, 66]. In the isoosmotic renal cortex, the stress-inducible HSP70 is barely detectable, while in the hypertonic renal medulla, the protein is highly abundant [67] and is regulated according to the diuretic state [67, 68]. By virtue of its chaperoning functions, HSP70 protects other proteins from destabilization, and counteracts protein aggregation [55]. These properties are consistent with the role of HSP70 in protection of renal cells against high urea concentrations [69].
The osmotic stress protein (OSP) 94 is a member of the HSP110 family [70], and is a molecular chaperone in vitro [71]. Expression of OSP94 in the kidney correlates with the corticomedullary osmotic gradient and the highest abundance is observed in the inner medulla [70]. During antidi-uresis, the already high expression in the inner medulla is elevated even further [72]; indeed, recent data imply that OSP94 is one of the most strongly induced genes following tonicity stress in the proteome of mouse inner medullary collecting duct (mIMCD) cells [73]. In OSP94-deficient mice, 12% of the animals develop hydronephrosis [74] and are preferentially susceptible to osmotic stress. Kojima et al. have detected a TonE-like element in the 5’-flanking region of the gene, to which TonEBP probably binds and upregu-lates the transcription in response to osmotic stress [75].
Genes Involved in Osmolyte Accumulation
As mentioned above, a rise in ambient tonicity results in initially increased cellular ionic strength, accompanied by adverse effects on cellular macromolecules. To overcome these adverse effects, cells have evolved mechanisms to replace excess inorganic ions by a group of organic osmolytes that preserve the osmotic balance but do not interfere with macromolecular structure and function. Consistent with their role in osmoadaptation and their neutral effect on cell metabolism, these compounds are termed compatible organic osmolytes. The best characterized organic osmolytes in mammalian cells are the polyols sorbitol and myo-inositol, the trimethylamines betaine and glycerophosphocholine (GPC), and free amino acids like taurine. Exposure of cells to osmotic stress activates various mechanisms that enable intracellular accumulation of these compounds.
The polyol sorbitol is produced from glucose, catalyzed by the enzyme aldose reductase (AR). AR abundance increases substantially following osmotic stress, reflecting enhanced transcription of the gene [76]. In the 5’-flanking region of the AR gene, several TonE-sites are present, to which TonEBP binds under hyperosmotic conditions and enhaces gene transcription [27, 29, 77]. The tonicity-induced expression of AR is implicated in serious complications in patients with diabetes. The high level of accumulated sorbi-tol is involved in the development of the characteristic diabetic cataract in lens cells and the pathogenesis of diabetic nephropathy and neuropathy. However, clinical trials using AR inhibitors to ameliorate these complications have failed to date (for a review, see [78]).
Under hyperosmotic conditions, myo-inositol is transported from the extracellular space into the cytosol by the sodium-myo-inositol transporter SMIT. The transport of 1 myo-inositol molecule is energized by the symport of 2 sodium ions. SMIT probably posseses 14 transmembrane domains and belongs to the family of the Na+/glucose cotrans-porters. Expression of SMIT is stimulated under hypertonic conditions by TonEBP binding to the promoter region of the gene [29]. In the isoosmotic renal cortex, only low amounts of SMIT are expressed, while in the hyperosmotic renal medulla, expression is several-times higher, particularly in cells of the thick ascending limb of Henle’s loop (TALH) [79] and in collecting duct cells [80]. Expression of SMIT in the renal medulla is also significantly increased during antidiuresis [81]. Pharmacological inhibition of SMIT activity results in severe injury of the tubular cells of the TALH, emphasizing the crucial role of myo-inositol accumulation during osmotic stress [82].
The methylamine betaine is accumulated in the cytosol by two different mechanisms. First, under hyperosmotic conditions, TonEBP enhances transcription of the gene encoding the betaine-GABA-transporter (BGT)-1 [29, 30], a 614-amino acid protein [83]. BGT-1 transports betaine from the extracellular space into the cell along with 3 sodium- and 1 or 2 chloride ions [83]. The normal serum betaine concentration is 20 - 144 μ M, however, in renal medullary cells, it may be concentrated up to 50 mM [84]. A second mechanism by which betaine can be accumulated under hypertonic conditions, is the production of betaine aldehyde from choline, catalyzed by choline dehydrogenase, and the subsequent conversion to betaine by betaine aldehyde dehydrogenase [85]. However, in renal medullary cells the betaine uptake from the extracellular space by BGT-1 is the most important mechanism [86].
Glycerophosphorylcholine (GPC) in the cell is derived from phosphatidylcholine, which is deacylated by phosphol-ipases A1and A2 and lysophospholipase [87]. The degradation of GPC is catalyzed by GPC:choline phosphodiesterase. Hyperosmotic concentrations of NaCl or urea decrease the enzymatic activity of GPC:choline phosphodiesterase, thereby resulting in cellular accumulation of GPC [87]. In addition to this mechanism, mouse inner medullary collecting duct cells can synthesize GPC from phosphatidylcholine via neuropathy target esterase (NTE), a phospholipase B that is up-regulated by osmotic stress, probably via TonEBP [88].
Transport system A is a Na+-dependent transporter for neutral amino acids like glycine or alanine that may serve as compatible osmolytes. Three system-A transporters are known to date, ATA1-3. ATA2 is induced by hypertonicity [89, 90], directly or indirectly dependent on TonEBP [91]. Another free amino acid accumulated during osmotic stress is taurine [92]. Taurine is transported from the extracellular space into the cell via the taurine transporter (TauT) [93]. TauT is upregulated at the functional level by increased activity, and at the transcriptional level during hypertonic stress [93–95]. A TonE-site has been identified in the promoter region of TauT [31], indicating that taurine accumulation also depends on hypertonicity-induced TonEBP activation.
Other Proteins Induced by Osmotic Stress
The Na+,K+-ATPase exchanges 3 sodium ions from the intracellular space for 2 potassium ions at the cost of 1 ATP. Functional Na+,K+-ATPase is essential for maintenance of potassium and sodium gradients across the cell membrane, the latter being essential for sodium-coupled solute and os-molyte transport in renal medullary cells [96]. The Na+,K+-ATPase is composed of an α-subunit which contains the catalytic activity and an additional β-subunit without enzymatic properties, but which is required for the expression of the functionally active enzyme [97, 98]. Recent evidence indicates that in the kidney in vivo, an additional γ-subunit, expressed exclusively in the outer and inner medulla, modulates Na+,K+-ATPase affinity for ATP, sodium, and potassium [99–102], thereby possibly adjusting Na+,K+-ATPase activity to metabolic demands in cells exposed to osmotic stress. Although increased abundance of all three subunits has been reported in response to tonicity stress [103, 104], the γ-subunit appears to contribute decisively to osmotoler-ance of renal medullary cells. Specifically, the γ-subunit is induced at both the transcriptional and translational level, and is subsequently incorporated into the basolateral membrane in medullary collecting duct cells exposed to acute or chronic hypertonic stress [105]. This process is apparently not dependent on TonEBP activation, but requires activation of JNK for induction of the γ-subunit at the transcriptional level, while increased expression at the translational level involves PI3K [106].
Cyclooxygenase (Cox) is a key regulatory enzyme in the synthesis of prostaglandins from arachidonic acid. There are at least two Cox isoforms. While Cox-1 is expressed constitutively in various mammalian tissues, Cox-2 is induced by various cellular stresses, including osmotic stress [107]. Cox-2 is up-regulated by osmotic stress in cells of the mammalian liver, lung, kidney [107–109]. Several studies have suggested that the majority of renal medullary PGE2 originates from Cox-2 [109], and contributes to protection of medullary cells from hypoxia by vasodilatory effects on descending vasa recta [110], and by stimulating the expression of osmoprotective genes like HSP70, SMIT and AR [111]. The importance of tonicity-induced Cox-2 expression in the kidney is illustrated by the well-known adverse effects of non-steroidal anti-inflammatory drugs, used in the therapy of joint pain and fever by virtue of their potent inhibition of prostaglandin synthesis. Their long-term use is limited by renal side effects, probably due to the reduced cytoprotection in the absence of PGE2. The tonicity-induced up-regulation of Cox-2 is probably not mediated by TonEBP [112]. Instead, as described above, hypertonicity induces the generation of ROS [113], leading to transactivation of the EGF receptor [114] and activation of MAP kinase pathways [115], which subsequently stimulate Cox-2 expression.
AQPs are a family of water channels, ubiquitous from bacteria to mammals. All aquaporins share a common tetrameric structure, with each subunit containing 6 trans-membrane domains. In the mammalian kidney, the hormone vasopressin stimulates the translocation of AQP2 from the cytoplasm to the apical cell membrane, thus increasing water reabsorption in collecting duct cells. In cultured kidney cells, expression of the AQP2 gene is increased by osmotic stress, which is, at least in part, dependent on a TonE in the promoter region of the gene [33, 116]. Possible additional sites upstream from TonE also participate in the hypertonicity-induced up-regulation of AQP2 [117]. AQP1, also induced by hypertonicity in cultured kidney cells [118], lacks a TonE, but another cis-acting element conferring osmosensitivity has been identified in the promoter and termed “hypertonicity-responsive element” (HRE), however, the corresponding trans-acting factors are still unidentified [119, 120]. Hyperosmotic stress also increases expression of AQP3 in human keratinocytes [121] and MDCK cells [122], that of AQP5 in mouse lung epithelial cells [123] and that of AQP4 and AQP9 in rat astrocytes [124]. In all of these studies, elevated AQP expression was induced only by functionally membrane-impermeant solutes like NaCl, which establish an osmotic trans-membrane gradient; in contrast, hy-perosmolality generated by membrane-permeant solutes like glycerol or urea failed to induce AQP expression.
As mentioned above, the major mechanism regulating water reabsorption in collecting duct principle cells in an-tidiuresis is the shuttling of AQP2 between the cytoplasm and the apical cell membrane [125]. The physiological role of enhanced expression of AQP2 by osmotic stress in medullary collecting duct cells may be related to the maintenance of increased levels of AQP2 protein, thereby contributing to urine concentration during prolonged antidiuresis. Accordingly, osmotic stress-induced AQP2 expression in the kidney may be more important for the function of the whole organ rather than for cell protection against the deleterious effects of hyperosmolality. The precise role of elevated AQP expression in response to osmotic stress in other tissues remains to be determined. Hill et al. have proposed a model in which AQPs can function as osmosensors and can initiate signaling pathways within the cell [126].
CONCLUDING REMARKS
Following exposure to osmotic stress, cells establish a timely well-orchestrated network of genetic responses that allow cell survival and function under these potentially harmful conditions. Early events include the activation of MAP kinase pathway(s), resulting in the expression of IEGs like Fos and Jun, which in turn promote expression of other cytoprotective genes (Fig. 1). The rapid water efflux and cell shrinkage due to hypertonic conditions is counteracted by activation of ion transport pathways that mediate the import of particularly sodium and chloride ions, and subsequent exchange of sodium for potassium by the Na+,K+-ATPase, resulting in elevated intracellular electrolyte concentrations and the passive influx of water to restore cell volume. NaCl-induced DNA double-strand breaks activate a p53-mediated cell-cycle arrest, thus allowing DNA repair or, in the case of irreversible damage, induce apoptosis. Later events, often mediated by the transcriptional activator TonEBP, include expression of genes involved in accumulation of organic osmolytes to replace excess electrolytes, the expression of HSP, which assist other proteins to prevent misfolding or aggregation, and enhanced expression of Cox-2 and increased PGE2-synthesis (Fig. 2). These later events are observed particularly in renal medullary cells, which are indeed the only mammalian cells exposed routinely to substantial hypertonic stress. The cooperation of the systems described above confers upon these cells the remarkable ability to tolerate the extreme conditions present in the medulla of the concentrating mammalian kidney well, whilst dysfunction of one or more of these systems may entail renal medullary damage, as observed in various pathological situations.
Fig. (1A).
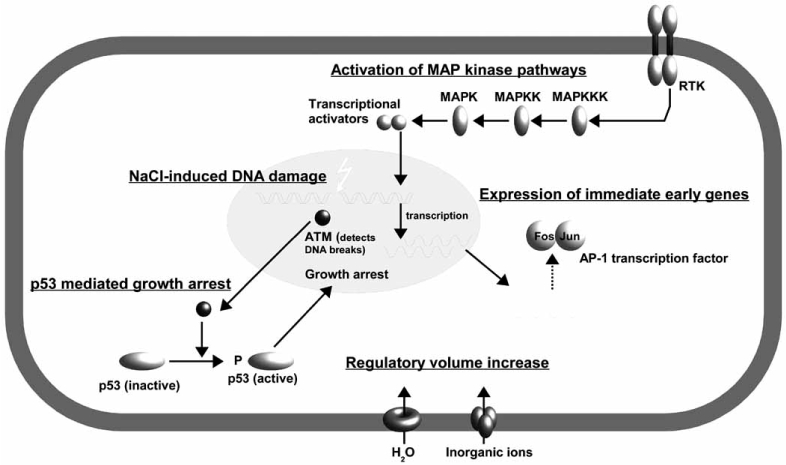
Early events in cells exposed to osmotic stress.
Hypertonic stress causes rapid water efflux and subsequent cell shrinkage. Transport of inorganic ions (Na+, Cl−) into the cell leads to passive water influx and restoration of cell volume, a mechanism termed regulatory volume increase. High [NaCl] cause DNA double-strand breaks, that are detected by the ataxia telangiectasia mutated (ATM) kinase. ATM activates p53, which in turn induces growth arrest by activation of the G1 checkpoint, thus allowing activation of repair mechanisms or induction of apoptosis. Osmotic stress activates MAP kinase pathways, probably mediated by one or more receptor tyrosine kinases, resulting in transcriptional activation of immediate early genes like Fos and Jun. These proteins assemble to the AP-1 transcription factor, which participates in expression of various cytoprotective genes.
Fig. (1B).

Late events in cells exposed to osmotic stress.
Upon phosphorylation, tonicity-enhancer binding protein (TonEBP) enters the nucleus and stimulates the transcription of several osmoprotec-tive genes, amongst them the osmolyte accumulating genes aldose reductase (AR), betaine-GABA transporter (BGT)-1, sodium-myo-inositol transporter (SMIT) and taurine transporter (TauT). The corresponding proteins mediate accumulation of compatible organic osmolytes that can replace excess electrolytes. TonEBP-mediated expression of heat shock proteins contributes to protection of proteins against misfolding and aggregation due to increased intrtacellular ionic strength. Tonicity-induced expression of Cox-2 is probably independent of TonEBP. Synthesis of Prostaglandin E2(PGE2) protects the cell by vasodilatory effects and by stimulating expression of several osmoprotective genes. Recent data suggest, that DNA repair systems like the meiotic recombination (Mre) 11 complex may enter the nucleus and repairs NaCl-induced DNA double-strand breaks.
Fig. (2).

Schematic representation of the time-course of activation of important osmoprotective mechanisms after an increase in ambient tonicity.
ACKNOWLEDGEMENTS
Work in the authors’ laboratory was supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Nierenstiftung, the Münchener Medizinische Wochenschrift, and by the Friedrich Baur Institut, Munich. Critical reading of the manuscript by Dr. John Davis is greatfully acknowledged.
REFERENCES
- 1.Hebert SC. Hypertonic cell volume regulation in mouse thick limbs. II. Na+-H+ and Cl(-)-HCO3- exchange in basolateral membranes. Am J Physiol Cell Physiol. 1986;250:C920–C931. doi: 10.1152/ajpcell.1986.250.6.C920. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- 3.Paillard M. H+ and HCO3- transporters in the medullary thick ascending limb of the kidney: molecular mechanisms, function and regulation. Kidney Int Suppl. 1998;65:S36–41. [PubMed] [Google Scholar]
- 4.Beck FX, Neuhofer W. Cell volume regulation in the renal papilla. Contrib Nephrol. 2006;152:181–197. doi: 10.1159/000096323. [DOI] [PubMed] [Google Scholar]
- 5.Somero GN. Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol. 1986;251:R197–213. doi: 10.1152/ajpregu.1986.251.2.R197. [DOI] [PubMed] [Google Scholar]
- 6.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 7.Petronini PG, Tramacere M, Mazzini A, Kay JE, Borghetti AF. Control of protein synthesis by extracellular Na+ in cultured fibroblasts. J Cell Physiol. 1989;140:202–211. doi: 10.1002/jcp.1041400203. [DOI] [PubMed] [Google Scholar]
- 8.Beck FX, Neuhofer W. Response of renal medullary cells to osmotic stress. Contrib Nephrol. 2005;148:21–34. doi: 10.1159/000086041. [DOI] [PubMed] [Google Scholar]
- 9.Beck FX, Neuhofer W, Müller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279:F203–F215. doi: 10.1152/ajprenal.2000.279.2.F203. [DOI] [PubMed] [Google Scholar]
- 10.Cohen DM, Wasserman JC, Gullans SR. Immediate early gene and HSP70 expression in hyperosmotic stress in MDCK cells. Am J Physiol Cell Physiol. 1991;261:C594–C601. doi: 10.1152/ajpcell.1991.261.4.C594. [DOI] [PubMed] [Google Scholar]
- 11.Neuhofer W, Beck FX. Survival in hostile environments: strategies of renal medullary cells. Physiology (Bethesda) 2006;21:171–180. doi: 10.1152/physiol.00003.2006. [DOI] [PubMed] [Google Scholar]
- 12.Galloway SM, Deasy DA, Bean CL, Kraynak AR, Armstrong MJ, Bradley MO. Effects of high osmotic strength on chromosome aberrations, sister-chromatid exchanges and DNA strand breaks, and the relation to toxicity. Mutat Res. 1987;189:15–25. doi: 10.1016/0165-1218(87)90029-2. [DOI] [PubMed] [Google Scholar]
- 13.Kültz D, Chakravarty D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc Natl Acad Sci USA. 2001;98:1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 15.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Dmitrieva NI, Park JH, Levine RL, Burg MB. High urea and NaCl carbonylate proteins in renal cells in culture and in vivo, and high urea causes 8-oxoguanine lesions in their DNA. Proc Natl Acad Sci USA. 2004;101:9491–9496. doi: 10.1073/pnas.0402961101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 18.Stadtman ER, Levine RL. Why have cells selected reactive oxygen species to regulate cell signaling events? Hum Exp Toxicol. 2002;21:83. doi: 10.1191/0960327102ht215oa. [DOI] [PubMed] [Google Scholar]
- 19.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 20.Abe M, O’Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol. 2006;291:F350–357. doi: 10.1152/ajprenal.00407.2005. [DOI] [PubMed] [Google Scholar]
- 21.Mori T, Cowley AW., Jr Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension. 2004;43:341–346. doi: 10.1161/01.HYP.0000113295.31481.36. [DOI] [PubMed] [Google Scholar]
- 22.Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension. 2003;42:588–593. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Rhinehart K, Kwon W, Weinman E, Pallone TL. ANG II signaling in vasa recta pericytes by PKC and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2004;287:H773–H781. doi: 10.1152/ajpheart.01135.2003. [DOI] [PubMed] [Google Scholar]
- 24.Burg MB, Kwon ED, Kultz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 25.Raitt DC, Posas F, Saito H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000;19:4623–4631. doi: 10.1093/emboj/19.17.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wurgler-Murphy SM, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]
- 27.Ko BC, Ruepp B, Bohren KM, Gabbay KH, Chung SS. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J Biol Chem. 1997;272:16431–16437. doi: 10.1074/jbc.272.26.16431. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko T, Takenaka M, Okabe M, Yoshimura Y, Yamauchi A, Horio M, Kwon HM, Handler JS, Imai E. Osmolarity in renal medulla of transgenic mice regulates transcription via 5’-flanking region of canine BGT1 gene. Am J Physiol. 1997;272:F610–616. doi: 10.1152/ajprenal.1997.272.5.F610. [DOI] [PubMed] [Google Scholar]
- 31.Ito T, Fujio Y, Hirata M, Takatani T, Matsuda T, Muraoka S, Takahashi K, Azuma J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem J. 2004;382:177–182. doi: 10.1042/BJ20031838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Feraille E, Martin PY. Tonicity-Responsive Enhancer Binding Protein Is an Essential Regulator of Aquaporin-2 Expression in Renal Collecting Duct Principal Cells. J Am Soc Nephrol. 2006;17:1521–1531. doi: 10.1681/ASN.2005121317. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA. 2004;101:2392–2397. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K, Guldenaar SE, McCabe JT. Fos and Jun expression in rat supraoptic nucleus neurons after acute vs. repeated osmotic stimulation. Brain Res. 1997;746:117–125. doi: 10.1016/s0006-8993(96)01216-4. [DOI] [PubMed] [Google Scholar]
- 36.Kawasaki M, Yamaguchi K, Saito J, Ozaki Y, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J Neuroendocrinol. 2005;17:227–237. doi: 10.1111/j.1365-2826.2005.01297.x. [DOI] [PubMed] [Google Scholar]
- 37.Cohen DM, Chin WW, Gullans SR. Hyperosmotic urea increases transcription and synthesis of Egr-1 in murine inner medullary collecting duct (mIMCD3) cells. J Biol Chem. 1994;269:25865–25870. [PubMed] [Google Scholar]
- 38.Fu M, Zhu X, Zhang J, Liang J, Lin Y, Zhao L, Ehrengruber MU, Chen YE. Egr-1 target genes in human endothelial cells identified by microarray analysis. Gene. 2003;315:33–41. doi: 10.1016/s0378-1119(03)00730-3. [DOI] [PubMed] [Google Scholar]
- 39.Tian W, Boss GR, Cohen DM. Ras signaling in the inner medullary cell response to urea and NaCl. Am J Physiol Cell Physiol. 2000;278:C372–380. doi: 10.1152/ajpcell.2000.278.2.C372. [DOI] [PubMed] [Google Scholar]
- 40.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 41.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphati-dylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proc Natl Acad Sci USA. 2006;103:8882–8887. doi: 10.1073/pnas.0602911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irarrazabal CE, Liu JC, Burg MB, Ferraris JD. ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA. 2004;101:8809–8814. doi: 10.1073/pnas.0403062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dmitrieva NI, Bulavin DV, Fornace AJ, Jr, Burg MB. Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc Natl Acad Sci USA. 2002;99:184–189. doi: 10.1073/pnas.231623498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dmitrieva N, Kultz D, Michea L, Ferraris J, Burg M. Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J Biol Chem. 2000;275:18243–18247. doi: 10.1074/jbc.M000522200. [DOI] [PubMed] [Google Scholar]
- 45.Kishi H, Nakagawa K, Matsumoto M, Suga M, Ando M, Taya Y, Yamaizumi M. Osmotic shock induces G1 arrest through p53 phosphorylation at Ser33 by activated p38MAPK without phosphorylation at Ser15 and Ser20. J Biol Chem. 2001;276:39115–39122. doi: 10.1074/jbc.M105134200. [DOI] [PubMed] [Google Scholar]
- 46.Dmitrieva NI, Michea LF, Rocha GM, Burg MB. Cell cycle delay and apoptosis in response to osmotic stress. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:411–420. doi: 10.1016/s1095-6433(01)00439-1. [DOI] [PubMed] [Google Scholar]
- 47.Capasso JM, Rivard CJ, Berl T. Long-term adaptation of renal cells to hypertonicity: role of MAP kinases and Na-K-ATPase. Am J Physiol Renal Physiol. 2001;280:F768–776. doi: 10.1152/ajprenal.2001.280.5.F768. [DOI] [PubMed] [Google Scholar]
- 48.Kultz D, Madhany S, Burg MB. Hyperosmolality causes growth arrest of murine kidney cells. Induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J Biol Chem. 1998;273:13645–13651. doi: 10.1074/jbc.273.22.13645. [DOI] [PubMed] [Google Scholar]
- 49.Chakravarty D, Cai Q, Ferraris JD, Michea L, Burg MB, Kultz D. Three GADD45 isoforms contribute to hypertonic stress phenotype of murine renal inner medullary cells. Am J Physiol Renal Physiol. 2002;283:F1020–1029. doi: 10.1152/ajprenal.00118.2002. [DOI] [PubMed] [Google Scholar]
- 50.Mak SK, Kultz D. Gadd45 Proteins Induce G2/M Arrest and Modulate Apoptosis in Kidney Cells Exposed to Hyperosmotic Stress. J Biol Chem. 2004;279:39075–39084. doi: 10.1074/jbc.M406643200. [DOI] [PubMed] [Google Scholar]
- 51.D’Amours D, Jackson SP. The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- 52.Dmitrieva NI, Bulavin DV, Burg MB. High NaCl causes Mre11 to leave the nucleus, disrupting DNA damage signaling and repair. Am J Physiol Renal Physiol. 2003;285:F266–274. doi: 10.1152/ajprenal.00060.2003. [DOI] [PubMed] [Google Scholar]
- 53.Dmitrieva NI, Cai Q, Burg MB. Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci USA. 2004;101:2317–2322. doi: 10.1073/pnas.0308463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheen MR, Kim SW, Jung JY, Ahn JY, Rhee JG, Kwon HM, Woo SK. Mre11-Rad50-Nbs1 complex is activated by hypertonicity. Am J Physiol Renal Physiol. 2006;291:F1014–1020. doi: 10.1152/ajprenal.00153.2006. [DOI] [PubMed] [Google Scholar]
- 55.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 56.Burel C, Mezger V, Pinto M, Rallu M, Trigon S, Morange M. Mammalian heat shock protein families. Expression and functions. Experientia. 1992;48:629–634. doi: 10.1007/BF02118307. [DOI] [PubMed] [Google Scholar]
- 57.Caruccio L, Bae S, Liu AY, Chen KY. The heat-shock transcription factor HSF1 is rapidly activated by either hyper- or hypo-osmotic stress in mammalian cells. Biochem J. 1997;327(Pt 2):341–347. doi: 10.1042/bj3270341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neuhofer W, Fraek ML, Ouyang N, Beck FX. Differential expression of heat shock protein 27 and 70 in renal papillary collecting duct and interstitial cells - implications for urea resistance. J Physiol. 2005;564:715–722. doi: 10.1113/jphysiol.2004.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schafer C, Ross SE, Bragado MJ, Groblewski GE, Ernst SA, Williams JA. A role for the p38 mitogen-activated protein kinase/Hsp 27 pathway in cholecystokinin-induced changes in the actin cytoskeleton in rat pancreatic acini. J Biol Chem. 1998;273:24173–24180. doi: 10.1074/jbc.273.37.24173. [DOI] [PubMed] [Google Scholar]
- 60.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 61.Dasgupta S, Hohman TC, Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- 62.Head MW, Corbin E, Goldman JE. Coordinate and independent regulation of alpha B-crystallin and hsp27 expression in response to physiological stress. J Cell Physiol. 1994;159:41–50. doi: 10.1002/jcp.1041590107. [DOI] [PubMed] [Google Scholar]
- 63.Michl M, Ouyang N, Fraek ML, Beck FX, Neuhofer W. Expression and regulation of alphaB-crystallin in the kidney in vivo and in vitro. Pflugers Arch. 2006;452:387–395. doi: 10.1007/s00424-005-0033-6. [DOI] [PubMed] [Google Scholar]
- 64.Lee JS, Seo JS. Differential expression of two stress-inducible hsp70 genes by various stressors. Exp Mol Med. 2002;34:131–136. doi: 10.1038/emm.2002.19. [DOI] [PubMed] [Google Scholar]
- 65.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 66.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 67.Muller E, Neuhofer W, Ohno A, Rucker S, Thurau K, Beck FX. Heat shock proteins HSP25, HSP60, HSP72, HSP73 in isoos-motic cortex and hyperosmotic medulla of rat kidney. Pflugers Arch. 1996;431:608–617. doi: 10.1007/BF02191910. [DOI] [PubMed] [Google Scholar]
- 68.Muller E, Neuhofer W, Burger-Kentischer A, Ohno A, Thurau K, Beck F. Effects of long-term changes in medullary osmolality on heat shock proteins HSP25, HSP60, HSP72 and HSP73 in the rat kidney. Pflugers Arch. 1998;435:705–712. doi: 10.1007/s004240050572. [DOI] [PubMed] [Google Scholar]
- 69.Neuhofer W, Lugmayr K, Fraek ML, Beck FX. Regulated overexpression of heat shock protein 72 protects Madin-Darby canine kidney cells from the detrimental effects of high urea concentrations. J Am Soc Nephrol. 2001;12:2565–2571. doi: 10.1681/ASN.V12122565. [DOI] [PubMed] [Google Scholar]
- 70.Kojima R, Randall J, Brenner BM, Gullans SR. Osmotic stress protein 94 (Osp94). A new member of the Hsp110/SSE gene subfamily. J Biol Chem. 1996;271:12327–12332. doi: 10.1074/jbc.271.21.12327. [DOI] [PubMed] [Google Scholar]
- 71.Matsumori M, Itoh H, Toyoshima I, Komatsuda A, Sawada K, Fukuda J, Tanaka T, Okubo A, Kinouchi H, Mizoi K, Hama T, Suzuki A, Hamada F, Otaka M, Shoji Y, Takada G. Characterization of the 105-kDa molecular chaperone. Identification, biochemical properties, and localization. Eur J Biochem. 2002;269:5632–5641. doi: 10.1046/j.1432-1033.2002.03272.x. [DOI] [PubMed] [Google Scholar]
- 72.Santos BC, Chevaile A, Kojima R, Gullans SR. Characterization of the Hsp110/SSE gene family response to hyperosmolality and other stresses. Am J Physiol. 1998;274:F1054–1061. doi: 10.1152/ajprenal.1998.274.6.F1054. [DOI] [PubMed] [Google Scholar]
- 73.Valkova N, Kultz D. Constitutive and inducible stress proteins dominate the proteome of the murine inner medullary collecting duct-3 (mIMCD3) cell line. Biochim Biophys Acta. 2006;1764:1007–1020. doi: 10.1016/j.bbapap.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Held T, Paprotta I, Khulan J, Hemmerlein B, Binder L, Wolf S, Schubert S, Meinhardt A, Engel W, Adham IM. Hspa4l-deficient mice display increased incidence of male infertility and hydronephrosis development. Mol Cell Biol. 2006;26:8099–8108. doi: 10.1128/MCB.01332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kojima R, Randall JD, Ito E, Manshio H, Suzuki Y, Gullans SR. Regulation of expression of the stress response gene, Osp94: identification of the tonicity response element and intracellular signalling pathways. Biochem J. 2004;380:783–794. doi: 10.1042/BJ20040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Perez A, Martin B, Murphy HR, Uchida S, Murer H, Cowley BD, Jr, Handler JS, Burg MB. Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem. 1989;264:16815–16821. [PubMed] [Google Scholar]
- 77.McGowan MH, Iwata T, Carper DA. Characterization of the mouse aldose reductase gene and promoter in a lens epithelial cell line. Mol Vis. 1998;4:2. [PubMed] [Google Scholar]
- 78.Chung SS, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6:475–486. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 79.Yamauchi A, Nakanishi T, Takamitsu Y, Sugita M, Imai E, Noguchi T, Fujiwara Y, Kamada T, Ueda N. In vivo osmoregulation of Na/myo-inositol cotransporter mRNA in rat kidney medulla. J Am Soc Nephrol. 1994;5:62–67. doi: 10.1681/ASN.V5162. [DOI] [PubMed] [Google Scholar]
- 80.Burger-Kentischer A, Muller E, Marz J, Fraek ML, Thurau K, Beck FX. Hypertonicity-induced accumulation of organic osmolytes in papillary interstitial cells. Kidney Int. 1999;55:1417–1425. doi: 10.1046/j.1523-1755.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- 81.Burger-Kentischer A, Müller E, Neuhofer W, Marz J, Thurau K, Beck F. Expression of aldose reductase, sorbitol dehydro-genase and Na+/myo-inositol and Na+/Cl-/betaine transporter mRNAs in individual cells of the kidney during changes in the diuretic state. Pflügers Arch. 1999;437:248–254. doi: 10.1007/s004240050776. [DOI] [PubMed] [Google Scholar]
- 82.Kitamura H, Yamauchi A, Sugiura T, Matsuoka Y, Horio M, Tohyama M, Shimada S, Imai E, Hori M. Inhibition of myoinositol transport causes acute renal failure with selective medullary injury in the rat. Kidney Int. 1998;53:146–153. doi: 10.1046/j.1523-1755.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 83.Rasola A, Galietta LJ, Barone V, Romeo G, Bagnasco S. Molecular cloning and functional characterization of a GABA/betaine transporter from human kidney. FEBS Lett. 1995;373:229–233. doi: 10.1016/0014-5793(95)01052-g. [DOI] [PubMed] [Google Scholar]
- 84.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- 85.Grossman EB, Hebert SC. Renal inner medullary choline dehydrogenase activity: characterization and modulation. Am J Physiol. 1989;256:F107–112. doi: 10.1152/ajprenal.1989.256.1.F107. [DOI] [PubMed] [Google Scholar]
- 86.Miller B, Schmid H, Chen TJ, Schmolke M, Guder WG. Determination of choline dehydrogenase activity along the rat nephron. Biol Chem Hoppe Seyler. 1996;377:129–137. doi: 10.1515/bchm3.1996.377.2.129. [DOI] [PubMed] [Google Scholar]
- 87.Bauernschmitt HG, Kinne RK. Metabolism of the ‘organic osmolyte’ glycerophosphorylcholine in isolated rat inner medullary collecting duct cells. I. Pathways for synthesis and degradation. Biochim Biophys Acta. 1993;1148:331–341. doi: 10.1016/0005-2736(93)90147-r. [DOI] [PubMed] [Google Scholar]
- 88.Gallazzini M, Ferraris JD, Kunin M, Morris RG, Burg MB. Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc Natl Acad Sci USA. 2006;103:15260–15265. doi: 10.1073/pnas.0607133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alfieri RR, Petronini PG, Bonelli MA, Caccamo AE, Cavazzoni A, Borghetti AF, Wheeler KP. Osmotic Regulation of ATA2 mRNA Expression and Amino Acid Transport System A Activity. Biochem Biophys Res Commun. 2001;283:174–178. doi: 10.1006/bbrc.2001.4729. [DOI] [PubMed] [Google Scholar]
- 90.Bevilacqua E, Bussolati O, Dall’Asta V, Gaccioli F, Sala R, Gazzola GC, Franchi-Gazzola R. SNAT2 silencing prevents the osmotic induction of transport system A and hinders cell recovery from hypertonic stress. FEBS Lett. 2005;579:3376–3380. doi: 10.1016/j.febslet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol. 2002;169:5477–5488. doi: 10.4049/jimmunol.169.10.5477. [DOI] [PubMed] [Google Scholar]
- 92.Uchida S, Nakanishi T, Kwon HM, Preston AS, Handler JS. Taurine behaves as an osmolyte in Madin-Darby canine kidney cells. Protection by polarized, regulated transport of taurine. J Clin Invest. 1991;88:656–662. doi: 10.1172/JCI115350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uchida S, Kwon HM, Yamauchi A, Preston AS, Marumo F, Handler JS. Molecular cloning of the cDNA for an MDCK cell Na(+)- and Cl(-)-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci USA. 1992;89:8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El-Sherbeny A, Naggar H, Miyauchi S, Ola MS, Maddox DM, Martin PM, Ganapathy V, Smith SB. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and muller cells. Invest Ophthalmol Vis Sci. 2004;45:694–701. doi: 10.1167/iovs.03-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takasaki M, Satsu H, Shimizu M. Physiological significance of the taurine transporter and taurine biosynthetic enzymes in 3T3-L1 adipocytes. Biofactors. 2004;21:419–421. doi: 10.1002/biof.552210183. [DOI] [PubMed] [Google Scholar]
- 96.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol. 2005;67:531–555. doi: 10.1146/annurev.physiol.67.031103.154456. [DOI] [PubMed] [Google Scholar]
- 97.Shull GE, Schwartz A, Lingrel JB. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985;316:691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- 98.Shull GE, Lane LK, Lingrel JB. Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature. 1986;321:429–431. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- 99.Therien AG, Goldshleger R, Karlish SJ, Blostein R. Tissue-specific distribution and modulatory role of the gamma subunit of the Na,K-ATPase. J Biol Chem. 1997;272:32628–32634. doi: 10.1074/jbc.272.51.32628. [DOI] [PubMed] [Google Scholar]
- 100.Therien AG, Karlish SJ, Blostein R. Expression and functional role of the gamma subunit of the Na, K-ATPase in mammalian cells. J Biol Chem. 1999;274:12252–12256. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- 101.Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na(+) and K(+) affinity of the renal Na,K-ATPase. J Biol Chem. 1999;274:33183–33185. doi: 10.1074/jbc.274.47.33183. [DOI] [PubMed] [Google Scholar]
- 102.Wetzel RK, Sweadner KJ. Immunocytochemical localization of Na-K-ATPase alpha- and gamma-subunits in rat kidney. Am J Physiol Renal Physiol. 2001;281:F531–545. doi: 10.1152/ajprenal.2001.281.3.F531. [DOI] [PubMed] [Google Scholar]
- 103.Yordy MR, Bowen JW. Na,K-ATPase expression and cell volume during hypertonic stress in human renal cells. Kidney Int. 1993;43:940–948. doi: 10.1038/ki.1993.132. [DOI] [PubMed] [Google Scholar]
- 104.Capasso JM, Rivard CJ, Enomoto LM, Berl T. Adaptation of murine inner medullary collecting duct (IMCD3) cell cultures to hypertonicity. Ann N Y Acad Sci. 2003;986:410–415. doi: 10.1111/j.1749-6632.2003.tb07222.x. [DOI] [PubMed] [Google Scholar]
- 105.Pihakaski-Maunsbach K, Tokonabe S, Vorum H, Rivard CJ, Capasso JM, Berl T, Maunsbach AB. The gamma-subunit of Na-K-ATPase is incorporated into plasma membranes of mouse IMCD3 cells in response to hypertonicity. Am J Physiol Renal Physiol. 2005;288:F650–657. doi: 10.1152/ajprenal.00162.2004. [DOI] [PubMed] [Google Scholar]
- 106.Capasso JM, Rivard CJ, Berl T. Synthesis of the Na-K-ATPase gamma-subunit is regulated at both the transcriptional and translational levels in IMCD3 cells. Am J Physiol Renal Physiol. 2005;288:F76–81. doi: 10.1152/ajprenal.00026.2004. [DOI] [PubMed] [Google Scholar]
- 107.Zhang F, Warskulat U, Wettstein M, Schreiber R, Henninger HP, Decker K, Haussinger D. Hyperosmolarity stimulates prostaglandin synthesis and cyclooxygenase-2 expression in activated rat liver macrophages. Biochem J. 1995;312(Pt 1):135–143. doi: 10.1042/bj3120135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lim WC, Park M, Bahn JJ, Inoue H, Lee YJ. Hypertonic sodium chloride induction of cyclooxygenase-2 occurs independently of NF-kappaB and is inhibited by the glucocorticoid receptor in A549 cells. FEBS Lett. 2005;579:5430–5436. doi: 10.1016/j.febslet.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 109.Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol. 1999;277:F1–9. doi: 10.1152/ajprenal.1999.277.1.F1. [DOI] [PubMed] [Google Scholar]
- 110.Harris RC. Cyclooxygenase-2 and the kidney: functional and pathophysiological implications. J Hypertens Suppl. 2002;20:S3–9. [PubMed] [Google Scholar]
- 111.Neuhofer W, Fraek ML, Beck FX. Prostaglandin E2 stimulates expression of osmoprotective genes in MDCK cells and promotes survival under hypertonic conditions. J Am Soc Nephrol. 2006;17:95A. doi: 10.1113/jphysiol.2007.135178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 Stimulates Transcription of HSP70 in Response to Hypertonicity. Mol Cell Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang T, Zhang A, Honeggar M, Kohan DE, Mizel D, Sanders K, Hoidal JR, Briggs JP, Schnermann JB. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J Biol Chem. 2005;280:34966–34973. doi: 10.1074/jbc.M502430200. [DOI] [PubMed] [Google Scholar]
- 114.Zhao H, Tian W, Tai C, Cohen DM. Hypertonic induction of COX-2 expression in renal medullary epithelial cells requires transactivation of the EGFR. Am J Physiol Renal Physiol. 2003;285:F281–288. doi: 10.1152/ajprenal.00030.2003. [DOI] [PubMed] [Google Scholar]
- 115.Yang T, Huang Y, Heasley LE, Berl T, Schnermann JB, Briggs JP. MAPK mediation of hypertonicity-stimulated cyclooxygenase-2 expression in renal medullary collecting duct cells. J Biol Chem. 2000;275:23281–23286. doi: 10.1074/jbc.M910237199. [DOI] [PubMed] [Google Scholar]
- 116.Storm R, Klussmann E, Geelhaar A, Rosenthal W, Maric K. Osmolality and solute composition are strong regulators of AQP2 expression in renal principal cells. American J Physiol Renal Physiol. 2003;284:F189–F198. doi: 10.1152/ajprenal.00245.2002. [DOI] [PubMed] [Google Scholar]
- 117.Kasono K, Saito T, Saito T, Tamemoto H, Yanagidate C, Uchida S, Kawakami M, Sasaki S, Ishikawa SE. Hypertonicity regulates the aquaporin-2 promoter independently of arginine vasopressin. Nephrol Dial Transplant. 2005;20:509–515. doi: 10.1093/ndt/gfh677. [DOI] [PubMed] [Google Scholar]
- 118.Jenq W, Mathieson IM, Ihara W, Ramirez G. Aquaporin-1: an osmoinducible water channel in cultured mIMCD-3 cells. Biochem Biophys Res Commun. 1998;245:804–809. doi: 10.1006/bbrc.1998.8518. [DOI] [PubMed] [Google Scholar]
- 119.Umenishi F, Schrier RW. Identification and characterization of a novel hypertonicity-responsive element in the human aquaporin-1 gene. Biochem Biophys Res Commun. 2002;292:771–775. doi: 10.1006/bbrc.2002.6709. [DOI] [PubMed] [Google Scholar]
- 120.Umenishi F, Schrier RW. Hypertonicity-induced Aquaporin-1 (AQP1) Expression Is Mediated by the Activation of MAPK Path-ways and Hypertonicity-responsive Element in the AQP1 Gene. J Biol Chem. 2003;278:15765–15770. doi: 10.1074/jbc.M209980200. [DOI] [PubMed] [Google Scholar]
- 121.Sugiyama Y, Ota Y, Hara M, Inoue S. Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim Biophys Acta. 2001;1522:82–88. doi: 10.1016/s0167-4781(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 122.Matsuzaki T, Suzuki T, Takata K. Hypertonicity-induced expression of aquaporin 3 in MDCK cells. Am J Physiol Cell Physiol. 2001;281:C55–63. doi: 10.1152/ajpcell.2001.281.1.C55. [DOI] [PubMed] [Google Scholar]
- 123.Hoffert JD, Leitch V, Agre P, King LS. Hypertonic Induction of Aquaporin-5 Expression through an ERK-dependent Pathway. J Biol Chem. 2000;275:9070–9077. doi: 10.1074/jbc.275.12.9070. [DOI] [PubMed] [Google Scholar]
- 124.Arima H, Yamamoto N, Sobue K, Umenishi F, Tada T, Katsuya H, Asai K. Hyperosmolar Mannitol Stimulates Expression of Aquaporins 4 and 9 through a p38 Mitogen-activated Protein Kinase-dependent Pathway in Rat Astrocytes. J Biol Chem. 2003;278:44525–44534. doi: 10.1074/jbc.M304368200. [DOI] [PubMed] [Google Scholar]
- 125.Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 126.Hill AE, Shachar-Hill B, Shachar-Hill Y. What are aquaporins for? J Membr Biol. 2004;197:1–32. doi: 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]


