Abstract
Pain is universal and vital to survival. It is an essential component of our sense of touch; together, touch and pain have evolved to enable our awareness of the intricacies of our environment and to warn us of danger and possible injury. There is a clear link between temperature sensation and pain—painful temperature sensations occur acutely and are a hallmark of inflammatory and chronic pain disorders of the nervous system. Mounting evidence suggests a subset of Transient Receptor Potential (TRP) ion channels activated by temperature (thermoTRPs) are important molecular players in acute, inflammatory and chronic pain states. Varying degrees of heat activate four of these channels (TRPV1-4), while cooling temperatures ranging from pleasant to painful activate two distantly related thermoTRP channels (TRPM8 and TRPA1). ThermoTRP channels are also chemosensitive, being activated and or modulated by plant-derived small molecules and endogenous inflammatory mediators. All thermoTRPs are expressed in tissues essential to cutaneous thermal and pain sensation. This review examines the contribution of thermoTRP channels to our understanding of temperature and pain transduction at the molecular level.
Key Words: ThermoTRP, pain, DRG, skin, inflammation, TRPA1, TRPV
TRP CHANNELS: A PRIMER
The detection of temperature and pain stimuli is initiated at the level of primary afferent neurons that terminate as free nerve endings embedded in target tissues such as the dermal and epidermal layers of the skin, the oral and nasal mucosa and joints. The cell bodies of these neurons originate from cranial nerve ganglia and dorsal root ganglia (DRG). They relay information regarding environmental stimuli to the central nervous system via central processes projecting to the dorsal horn of the spinal cord. The conduction properties of neurons that respond to stimuli such as heat, cold and mechanical pressure are characteristic of C- and Aδ fibers [91]. These neurons express the receptor tyrosine kinase trkA, are dependent on nerve growth factor (NGF) during development and are peptidergic, expressing nociceptive markers such as calcitonin gene-related peptide (CGRP) and substance P (SP) [121]. A portion of these neurons express c-ret postnatally, are dependent on glial-derived neurotrophic factor (GDNF) and are non-peptidergic. Instead they are distinguished by their ability to bind the plant lectin, isolectin-B4 (IB4) [95,133,164]. Until relatively recently, the mechanisms of how these sensory neurons detect thermal and pain stimuli were poorly understood.
Caterina et al. achieved a breakthrough in our molecular understanding of thermal and pain sensation by identifying the “capsaicin receptor” [24]. Hot chili peppers produce this deterrent vanilloid molecule, which induces a burning sensation when coming in contact with mucous membranes of the mouth and eyes. This biological effect results from the excitation of a sub-population of nociceptive neurons [136]. The subsequent long-lasting desensitization of these nociceptive fibers accounts for the analgesic effect of capsaicin and explain its utility in treating pain associated with chronic diseases such as arthritis. Capsaicin has also been long-recognized as an excellent pharmacological tool for the study of nociceptive neurons [155]. By utilizing capsaicin sensitivity as a readout in an expression cloning strategy, the search by Caterina et al. identified TRPV1 (Transient Receptor Potential ion channel Vanilloid 1, formerly VR1). Heterologous expression of this cloned capsaicin receptor in naïve cells (Xenopus oocytes and Human Embryonic Kidney cells, HEK293) remarkably rendered them capsaicin-sensitive. In addition to activation by capsaicin, the cloned TRPV1 channel exhibited activation by noxious heat, with a thermal threshold of ≥42°C similar to native heat responses of cultured DRG neurons [24].
TRPV1 is the founding mammalian member of the superfamily of outwardly rectifying cation channels related to Drosophila TRP, first identified in fly photoreceptor mutants. The TRP superfamily, of which there are invertebrate and vertebrate members, is divided into seven subfamilies: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPN (Nomp), TRPA (Ankyrin), and the more distantly related TRPP (Polycystin) and TRPML (Mucolipin) [96, 113]. Members of the TRP superfamily have an extensive diversity of physiological functions, with a striking contribution to sensory biology. In a wide variety of organisms including Drosophila, the nematode C. elegans, zebrafish (Danio rerio), rodents and humans they mediate sensory transduction in modalities such as vision, taste, olfaction, mechanosensation and thermosensation [2,33,51,85,131,143, 148,150,160].
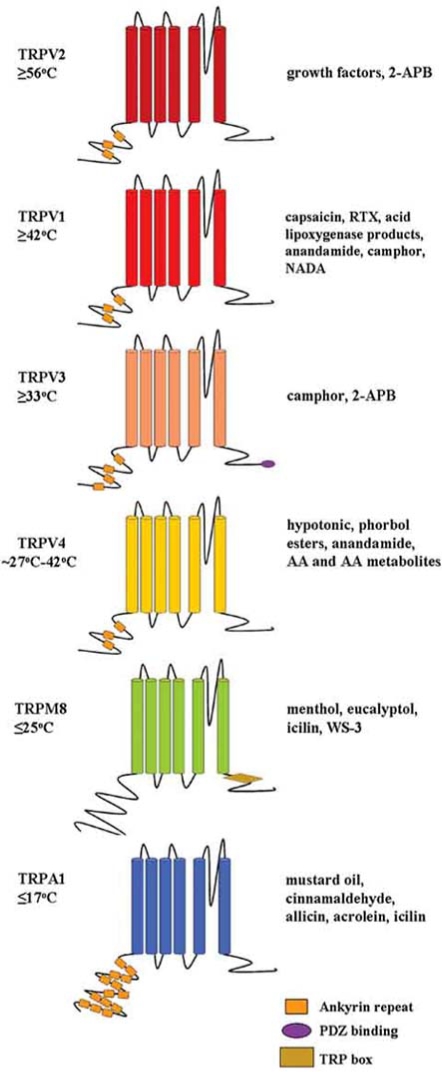
Since the discovery of TRPV1, five additional mammalian TRP channels that are activated by specific temperature thresholds over the perceptible range have been identified (dubbed thermoTRPs) [39,107]. Similar to TRPV1, thermoTRP channels are comprised of six predicted transmembrane domain segments and may form homo- or heterotetrameric complexes (Fig. 1). They are Ca2+-permeable cation channels with a putative pore region between transmembrane segments five and six. The N- and C-termini are thought to be intracellular, with some thermoTRPs possessing a variable number of ankyrin repeat domains in the N-terminus (Fig. 1) [113]. The six mammalian thermoTRP ion channels derive from three diverse TRP subfamilies. In addition to TRPV1, three other TRPV subfamily members are activated by heat or warm stimuli of distinct thermal thresholds; TRPV2 is activated by very high heat stimuli (≥ 52°C), while TRPV3 and TRPV4 are activated by warmth (33°C and ∼27-42°C respectively) [23,49,109,126,153,158]. Two distantly related to thermoTRPs are activated by relatively cool to cold stimuli: TRPM8 exhibits activation at or below room temperature (≤ 25°C) and TRPA1 at cold temperatures that approach the threshold of cold pain reported in human psychophysical tests (∼15°C) [90,108,130]. Not only does each thermoTRP channel display distinct thermal activation thresholds, they are chemosensitive, displaying specific activation by plant-derived and synthetic compounds, many of which evoke thermal and pain sensations (Fig. 1). The thermoTRP channels are regulated by nerve injury and undergo direct activation (and/or modulation) by components of the “inflammatory soup”. Altered thermoTRP activity via these mechanisms is thought to account for phenomena such as hyperalgesia (heightened response to a normally noxious thermal stimulus) and allodynia (pain response to a normally innocuous thermal stimulus). These channels also feature distinguishing biophysical properties and modulation by intracellular signaling events. The sections that follow will examine each thermoTRP channel with respect to these properties, with an emphasis on what is known about their specific roles in pain mechanisms including acute, inflammatory and chronic pain.
Fig. (1).
Schematic depiction of the six mammalian thermoTRP channels. Each channel subunit is made up of six putative transmembrane domains with a pore region between segments five and six. The N- and C-termini are thought to be intracellular. All thermoTRPs, except TRPM8, have a variable number of ankyrin repeat domains in the N-terminus. The C-termini of thermoTRPs also contain special features such as a TRP box or PDZ binding domain. All of the thermoTRPs display distinct thermal thresholds from very hot (TRPV2) to cold (TRPA1). Each thermoTRP is also activated by specific botanicals, endogenous and synthetic substances, some of which induce cutaneous thermal and pain sensations.
TRPV1 AND NOXIOUS HEAT DETECTION
The most comprehensively characterized thermoTRP is TRPV1. As mentioned above, Caterina et al. first cloned and characterized TRPV1 as a cation channel directly gated by capsaicin and noxious heat (≥42°C). TRPV1 currents are blocked by capsazepine and ruthenium red [24]. Within the DRG and trigeminal ganglia, TRPV1 is most abundantly expressed in small diameter C-fibers that express CGRP, SP and IB4. It is also found within sensory fibers that innervate the viscera, urinary bladder and airways [17,42,43,76,93, 139]. Consistent with the central projections of these neurons, TRPV1 immunoreactivity is localized to CNS regions such as the superficial laminae (I and II) of the dorsal horn of the spinal cord and nucleus of the solitary tract [50,139]. Several studies also report low expression of TRPV1 in regions of the brain. For example, TRPV1 is detected in thermoregulatory hypothalamic nuclei, which could explain the effect of TRPV1 agonists in lowering body temperature [92]. TRPV1 expression has also been detected in epithelial cells of both skin and urinary bladder, where TRPV1 appears to be required for proper bladder reflexes [16,17]. The function of TRPV1 in skin cells is unclear, but is discussed below [38,129].
Two groups initially carried out targeted deletion of TRPV1 and behavioral studies of knockout (TRPV1-/-) mice [22,35]. Capsaicin-sensitivity of cultured sensory neurons as well as capsaicin-induced nociceptive behaviors are absent in mice lacking TRPV1, suggesting the channel is the sole receptor for this compound. However, responses to noxious heat stimuli are largely intact in TRPV1-/- mice, with blunted sensitivity becoming apparent only at temperatures >50°C. Moreover, a small proportion of heat-sensitive fibers (peak temperature of activation 47°C) and noxious-heat induced c-Fos staining in spinal cord persist in TRPV1-/- mice [21]. Subsequent recordings from heat-sensitive fibers in skin-nerve and ex vivo preparations from TRPV1 mice-/- have been performed by other investigators [156,162]. Overall, these investigators have failed to detect differences in C-fiber heat responses in TRPV1-/- mice compared to wild-type. The conclusion from these studies is that TRPV1 activation alone does not account for all heat sensation. However, a highly significant result ascertained by the initial knockout studies is that TRPV1 is absolutely required for the development of inflammation-induced thermal hyperalgesia [22, 35]. Consistent with the sensitizing effects of the inflammatory agents mentioned below, mice lacking TRPV1 do not develop heightened sensitivity to heat following administration of Complete Freund’s Adjuvant (CFA), Tumor Necrosis Factor-alpha, mustard oil or carrageenan [22,35, 68].
ROLE OF TRPV1 IN INFLAMMATION AND INJURY
In addition to noxious heat and capsaicin, TRPV1 is activated and/or modulated by a variety of stimuli implicated in nociception and inflammation. The channel is directly gated by resiniferatoxin (RTX, ultra-potent capsaicin analog) and acidic pH (≤5.9) [24,139]. Numerous components of the inflammatory soup sensitize TRPV1 so that it is active at body temperature, providing a plausible mechanism for heat hyperalgesia. In vitro studies show that TRPV1 is sensitized by a number of factors generated during inflammation including mild acidification (pH 6.5), bradykinin (BK), prostaglandin E2 (PGE2), NGF and ATP (Fig. 1) [140,141]. Sensitization of the channel by these factors occurs through PKA- and PKC-dependent phosphorylation [14,15,37,60,111,114, 142]. For example PGE2, which increases the excitability of nociceptive neurons in response to several types of noxious stimuli, sensitizes TRPV1 via specific PKA-dependent phos-phorylation [25,60,94]. In the naïve state, TRPV1 is inhibited by endogenous phosphatidylinositol 4,5-bisphos-phate (PIP2) via interaction with the C-terminal domain of the channel. Activation of phospholipase C through NGF or BK signaling, for example, depletes cellular PIP2 and sensitizes the channel [28,112]. It was also recently reported that high concentrations of camphor, thought to be a specific activator of TRPV3, activates and strongly desensitizes TRPV1 [157]. Therefore camphor, similar to capsaicin, could be analgesic owing to its strong desensitization of TRPV1. Desensitization is dependent on calmodulin binding to the C-terminus of TRPV1 and Ca2+-calmodulin-dependent kinase II releases this inhibition via phosphorylation [72,104]. There was also early speculation that TRPV1 is activated by endogenous ligands, contributing to hyperalgesia during inflammation. Several have been identified including lipoxygenase products, anandamide and N-arachidonoyl dopamine [27,40,63, 125,165].
In addition to the potentiating effects of inflammatory mediators via post-translational modification of TRPV1, pain sensitization may occur through augmented expression of TRPV1 in sensory neurons. The number of DRG neurons and peripheral terminals expressing TRPV1 is increased in inflamed tissues [7,62,120,161]. Also, increased TRPV1 protein is transported specifically to peripheral terminals via NGF-induced activation of p38 MAPK in response to CFA injection [67]. In nerve injury models, upregulated expression of TRPV1 is observed in uninjured ganglia and the antagonist capsazapine alleviates resulting mechanical allodynia and hyperalgesia. In humans, increased TRPV1 in visceral sensory afferents is associated with inflammatory bowel disease [57-59]. An underlying cause of abdominal pain associated with bowel disease is increased mechanical sensitivity induced by inflammation. A recent study of TRPV1-/- mice showed that TRPV1 is in part required for mechanical sensitivity and inflammation-induced hypersensitivity in response to colon distention [69]. A complete summary of the many studies of TRPV1 is outside the scope of this review, but the topic has been thoroughly considered elsewhere [58,101,134,140,141]. Vigorous studies of TRPV1 in rodent and human models of pain clearly demonstrate that this channel is a relevant therapeutic target. The channel is implicated in a variety of human diseases including osteoarthritis, gastrointestinal reflex disease, vulvodynia, and inflammatory disorders of the airways and urinary bladder [48,89,103,146].
TRPV2
A bioinformatics approach to identify sequences related to TRPV1 identified TRPV2 (formerly VRL-1). Similar to TRPV1 (and TRPV3) TRPV2 is activated by 2-aminoethoxydiphenyl borate (2-APB) [61]. It is unresponsive to vanil-loid-compounds, moderate heating or protons [23]. However, very high heat stimuli (≥52°C) evoke TRPV2 currents in heterologous systems, which similar to TRPV1 are blocked by ruthenium red (Fig. 1) [23]. Heat-evoked TRPV2 currents are sensitizing, repeated subthreshold 40°C heat pulses shift the thermal threshold of TRPV2. Immunohisto-chemical analyses of DRG neurons show that TRPV2 is expressed in myelinated medium- to large-diameter CGRP-positive Aδ-fibers and non-nociceptive Aβ -fibers, distinct from cells expressing TRPV1 [1,24,87]. These latter Aβ-fibers are thought to contribute to neuropathic pain through sensitization to low-threshold mechanical stimuli [106]. In addition to expression in DRG sensory neurons, TRPV2 immunoreactivity is observed in neuronal tissues including brain, cranial nerve ganglia and spinal cord [23,82]. In the spinal cord, TRPV2 staining is localized to laminae I and II, consistent with central projections of nociceptive Aδ-fibers, and in the deeper laminae III/IV, consistent with targeting of Aβ-fibers. The heat-response property and expression profile of TRPV2 correlate with a class of myelinated Aδ-mechano- and heat-sensitive (AMH) fiber. Within the DRG there are two types of AMH fibers: type I AMHs respond to high heat of 53°C and type II to a threshold of 43°C. Therefore, in primary afferent nociceptors, TRPV2 is hypothesized to mediate high heat-evoked currents in type I AMH fibers, while TRPV1 mediates responses in capsaicin-sensitive C-fibers, which exhibit heat thresholds of approximately 43°C [91, 144]
Despite the correlation between the heat threshold of the cloned TRPV2 channel and that of noxious heat detection by type I AHMs, a direct role of TRPV2 in pain sensation remains elusive. Acute behavioral responses to noxious heat have not been examined in the absence of this protein, as studies of TRPV2 knockout mice have not been reported. Also, TRPV2 likely has roles outside of nociception, which could confound knockout or knockdown studies. For instance, TRPV2 immunoreactivity has been observed in numerous non-neuronal tissues such as smooth and cardiac muscle cells. TRPV2 is reported to be stretch activated; mediating intracellular calcium influx in vascular smooth muscle cells in response to swelling and stretch [65,66,78,99]. TRPV2 is also activated by insulin growth factor 1 (IGF-1), and through an IGF-1 dependent pathway, may contribute to cardiac muscle degeneration (Fig. 1) [73]. There is some evidence that TRPV2 could contribute to inflammatory pain. Previous studies have shown that NGF regulates the expression of TRPV1, leading to increased heat-sensitivity following intraplantar injection of CFA in mice. A recent study identified a possible role of TRPV2 in contributing to inflammation-induced heat hyperalgesia independent of that mediated by NGF or TRPV1 [122]. First, intraplantar injection of CFA, but not NGF, leads to an increase in the number of TRPV2-expressing neurons suggesting that NGF drives up-regulation of TRPV1 but not TRPV2. Furthermore, while 50°C hot plate responses are attenuated by RTX-mediated killing of TRPV1-expressing neurons, the latency of noci-ceptive responses to 56°C hot plate after inflammation is not affected. Therefore, TRPV2 could mediate heat hyperalgesia at higher temperatures of 56°C, while TRPV1 is required for hyperalgesia at ≤ 50°C.
WARMTH-ACTIVATED THERMOTRPS
Detection of temperatures in the innocuous warmth range is essential to homeostatic regulation of body temperature and maintaining comfort. In addition, otherwise pleasant warmth stimuli can become painful during inflammation or tissue injury (as in preferred bathing temperature with sunburn). A class of primary afferent neuron, distinct from those activated by noxious heat, detects warm temperature. Recordings from single nerve fibers in the skin of several species demonstrate two types of warmth-sensitive fibers; both types of fiber discharge regularly at normal skin temperature (∼30-34°C) and continue firing as temperature rises to 45-47°C. However, one type of fiber displays maximal firing rates at temperatures approaching 41°C, while the second type reaches peak discharge at 47°C [54,55]. Intriguingly, warmth responses are most readily observed in recordings from intact skin-nerve preparations, but rarely in dissociated DRG cultures. This seems to suggest warmth sensation requires some relationship (anatomical and/or biochemical) be maintained between skin cells and peripheral nerve fibers. Remarkably, the cloned channel properties of two additional TRPV subfamily members (TRPV3 and TRPV4) that are enriched in skin cells (keratinocytes) mimic the responses of these warmth-sensitive fibers. Both channels have been targeted and novel behavior assays employed to measure warmth-sensation in mice. The prominent expression of TRPV3 and TRPV4 in skin raises the interesting possibility that the skin itself, classically regarded as a protective barrier, plays a role in thermosensation and pain.
TRPV3
Peier et al. first identified mouse TRPV3 by utilizing a bioinfomatics approach [109]. The channel was identified and characterized in primates (monkey and human) a short time later by two other groups [126,158]. TRPV3 is activated by innocuous warming temperatures with a threshold of activation of ∼33°C and is blocked by ruthenium red (Fig. 1). In addition, TRPV3 exhibits sensitization to repeated heat stimuli and increasing responses to temperatures >33°C. With prolonged stimulation TRPV3 currents are biphasic; sensitization occurs during the initial phase followed by a second phase of high current amplitude where outward rectification is lost [29]. Similar to TRPV1, TRPV3 is chemosensitive, activated by camphor and 2-APB [31,61,97,157]. In contrast to TRPV1, TRPV3 sensitizes to these chemical stimuli and cross-sensitization occurs when repeated heat and chemical pulses are sequentially applied. Therefore, in addition to transducing pure warmth sensation, the sensitizing property of TRPV3 could contribute to heightened sensitivity to repeated stimuli. In the study by Peier et al., expression analyses using northern blot, in situ hybridization and immunohistochemical staining with an antibody raised against TRPV3 demonstrated that in mouse, TRPV3 is not detectable in DRG neurons; rather it is prominently expressed by keratinocytes within the skin [109]. The two subsequent studies of primate TRPV3 reported that TRPV3 is expressed in DRG and trigeminal sensory neurons in addition to skin, hypothalamus and several non-neuronal tissues [126,158]. In human DRG, TRPV3 was co-expressed with TRPV1 and interaction of the proteins in heterologous systems suggested TRPV1 and TRPV3 subunits could form heteromeric channels [126]. However, a more recent study using fluorescence resonance energy transfer and immunoprecipitation approaches failed to detect heteromerization of TRPV1 and TRPV3 [53]. Finally, another study detected TRPV3 immunoreactivity localized to human breast epithelium but not in sensory neurons innervating this tissue [45].
TRPV4
TRPV4 was originally characterized by two groups as a TRP channel highly sensitive to changes in extracellular osmolarity (activated by hypotonicity and inhibited by hypertonicity)[83,132]. TRPV4 is also activated by warmth (∼27-35°C) and inhibited by ruthenium red (Fig. 1) [49,153]. TRPV4 responds to heat-stimuli with increasing amplitude as temperatures rise to 42°C and then declines as temperature is increased further. These characteristics approximate the electrophysiological nature of the type of warmth-sensitive fiber that reaches peak discharge around this temperature in skin-nerve recordings. Heat and osmolarity interact to modulate the activity of TRPV4: heat-evoked responses in hypotonic conditions are larger than isotonic and are inhibited by hypertonicity. In addition, TRPV4 responses evoked by cell swelling are modest at 22°C and increased at 37°C [49]. Similar to the thermoTRPs discussed thus far, TRPV4 is activated by synthetic and endogenous substances. The synthetic phorbol ester, 4 α PDD robustly activates TRPV4. TRPV4 is also activated by the endocanabanoid anandamide, arachadonic acid (AA) and cytochrome P-450 metabolites of AA (Fig. 1) [151,152]. Therefore a diverse array of stimuli can activate TRPV4 and studies demonstrate that although thermal, osmotic and chemical stimuli can act in a convergent manner on TRPV4, they are also mechanistically separable, acting through distinct signaling pathways and protein domains [149]. This appears to be a theme in thermoTRP activation and is discussed further below (see conclusions). Similar to TRPV3, whether TRPV4 is expressed in DRG sensory neurons is somewhat controversial. In situ hybridization analyses have shown that TRPV4 mRNA is localized within sensory neurons, but immunhistochemical and immunoblot studies have not consistently shown expression at the protein level [49,83,84,135]. Consistent with a role in osmo- and thermoregulation, TRPV4 is expressed in several homeostatic regulatory centers of the brain including the vascular organ of the lamina terminalis and in the anterior and median preoptic areas of the hypothalamus [49,83]. It is also expressed in a diversity of peripheral tissues such as vascular endothelial cells, kidney and hair cells of the inner ear [83,159]. Finally, TRPV4 is prominently expressed in the epidermis where it is localized primarily within suprabasal keratinocytes [49].
THERMOTRPVS: SKIN-NERVE CONNECTION
Recent in vitro expression and electrophysiological analyses of cultured cells demonstrate that both TRPV3 and TRPV4 proteins are present in primary mouse keratinocytes and the mouse 308 keratinocyte cell line [30,97]. The proportion of functional channels observed in these cells varies between studies, but demonstrate that skin cells exhibit a rise in intracellular Ca2+ in response to warmth and chemical stimuli via activation of TRPV3 and TRPV4. Two independent studies that performed electrophysiological recordings from mouse primary keratinocytes reveal distinct types of warmth-evoked currents characteristic of TRPV3 and TRPV4 channels. In the first study, most keratinocytes exhibited desensitizing TRPV4-like currents with a thermal activation threshold of 32°C [30]. Currents attributed to TRPV4 activation were absent in cultured keratinocytes derived from TRPV4-/- mice. In the same study, a more rare type of response was attributed to TRPV3-activation: currents sensitized to repeated heat stimuli, were potentiated by 2-APB and persisted in TRPV4-/- mice. A more recent study carried out similar analyses in cultured primary keratinocytes from wildtype and TRPV3-/- mice [97]. In this study, sensitizing TRPV3-like currents were recorded in the majority of keratinocytes (80%), while TRPV4-like currents were present in a minority (30%) of cells. In addition, the TRPV3 agonist camphor activated keratinocyte cells and potentiated warmth responses. Camphor- and warmth-induced sensitizing responses, but not desensitizing TRPV4-like currents, were absent in keratinocytes derived from TRPV3-/- mice. Collectively these in vitro studies confirm that keratinocyte cells respond to warmth and chemical stimuli via specific activation of TRPV3 and TRPV4. Discrepancies in the proportion of TRPV3- and TRPV4-like currents observed may arise from differences in culture preparations.
The functional characterization of the roles of these channels in mediating thermal responsiveness of keratinocytes raises new questions of how neuronal and nonneuronal elements within skin participate in temperature and pain sensation. For example, what are the mechanisms of how skin cells transmit stimuli to cutaneous sensory neurons via activation of these channels? An attractive area of investigation focuses on thermoTRP-mediated release of keratinocyte-derived diffusible factors that could affect the function of sensory neurons. Such a relationship has been demonstrated for TRPV1 [110,128]. Although best characterized in sensory neurons, subsequent RT-PCR and immunostaining analyses showed that TRPV1 is detectable in human (but not mouse) primary keratinocytes and in the HaCaT (human keratinocyte-derived) cell line. Specific capsaicin-mediated activation of TRPV1 in these cells causes a rise in intracellular Ca2+, which is specifically blocked by the antagonist capsazepine. Activation of TRPV1 also induces Ca2+-dependent release of the inflammatory mediators PGE2 and interleukin-8, providing a possible mechanism for hyperalgesia induced by heat injury [128]. In an analogous fashion, TRPV1 appears to be required for mechanically-evoked release of ATP from urothelial and epidermal cells [17,32]. Could ATP be the communicating molecule in warmth sensation? A subset of DRG neurons express ATP-gated ion channel P2X3 and mice deficient in this channel exhibit subtle deficits in their ability to detect warmth. Whether TRPV3- and TRPV4-expressing skin cells release ATP upon warmth-activation is an intriguing possibility that has been considered by several investigators [80,109]. Keratinocytes also release antinociceptive molecules. For example, upon specific activation of the endothelin-B receptor, β-endorphin is released from cultured keratinocyte cells, which could then affect pain signaling via binding to μ-opioid receptors on sensory neurons [64,75]. Therefore, there is precedence that a molecule or molecules could be released upon thermoTRPV-mediated keratinocyte stimulation. Whether such molecules differ depending on which thermoTRPV is activated and then specifically act downstream on warmth- vs. noxious heatencoding units is an intriguing possibility in terms of sensory coding.
WHAT KNOCKOUTS TELL US OF WARMTH SENSATION
Behavioral assays of noxious heat detection utilize high threshold stimuli to score nocifensive behaviors like shaking and licking of the paws. How do investigators measure responses to innocuous thermal stimuli where such types of behaviors would not be thought to occur? In terms of innocuous thermal sensation, one must glean from behavioral tests what an animal “prefers”. Ambient and surface temperature gradients have been used to test thermal preference in rodents [20,41,115,154]. Rodents display a strong preference for a relatively warm surface temperature of ∼35°C. Factors such as circadian rhythm, drug and toxin injection and obesity can shift the thermal preference to warmer or cooler temperatures, suggesting that rodents are highly equipped to detect discrete changes in innocuous thermal stimuli. This ability is connected physiologically to a variety of homeostatic and metabolic processes and adapts depending both on the external environment and other factors that impact physiology such as febrile state. Two recent studies utilized preference tests to examine the physiological roles of TRPV3 and TRPV4 in warmth and heat sensation in knockout mice [81,97]. Knockout animals of both channels are deficient in their ability to detect warmth, but intriguing differences in warm-sensing behavior reveal subtle variations in the physiological roles of TRPV3 and TRPV4.
Moqrich et al. recently reported that TRPV3-/- mice are deficient in their ability to “choose” a warm surface in a two-temperature choice test [97]. Similar to previous reports, this test reveals that wildtype mice prefer a warm surface (35°C) over one kept at room temperature (25°C) as indicated by the amount of time spent there. TRPV3 -/- mice do not discriminate between the two temperatures, spending a nearly equivalent time on both surfaces. The authors also used a thermal gradient to measure preference over a wider range of temperatures (15-55°C). During the two-hour gradient trial, wildtype mice display exploratory behavior during the first 30-minute period. Consistent with thermal preference exhibited in the two choice test, wildtype mice then spend the greater part of the next 30 minutes within the 30-38°C zone. TRPV3-/- mice on the other hand, explore as wildtype mice but then display a marked delay of 30 minutes in “choosing” the 30-38°C zone, not spending the majority of time there until the 60-90 minute-segment of the assay.
Another study has examined innocuous warmth sensation in TRPV4-/- mice using similar surface temperature tests [81]. In addition, they extended their studies to include analyses of physiological responses to changes in ambient temperature. It has been hypothesized that because TRPV4 is expressed in the anterior hypothalamus where central thermoregulatory centers reside, it could serve as a warmth-sensitive channel in specialized neurons in this region that respond to changes in body temperature. However, diurnal fluctuations in body temperature are normal in TRPV4-/- mice and they are able to regulate body temperature normally in response to rapid ambient temperature change, suggesting TRPV4 is dispensable in central thermosensation. Compatible with a role in peripheral warmth sensation however, TRPV4-/- mice differ from wildtype in their preference for surface temperature and in ways distinct from those observed in TRPV3-/- mice. In a 2-hour thermal gradient assay, TRPV4-/- mice demonstrate a strong shift to warmer preferred surface temperature compared to wildtype littermates during the second 60-minute phase of the trial. To rule out the possibility that this shift occurrs due to increased avoidance of noxious temperature at the extremes of the gradient and to test whether TRPV4-/- mice could discriminate between modestly warm surfaces, Lee et al. also performed a two temperature test in which mice were allowed to choose between either 30°C or 34°C surfaces. While wildtype mice are impartial to the two temperatures, TRPV4-/- mice favor the 34°C surface. Taken together, the data from these two assays demonstrate a drive in TRPV4-/- mice toward warmer surface temperatures compared to wildtype mice. Furthermore, although innocuous warmth behavioral assays show that knockout mice of either channel are impaired in their ability to discriminate among temperatures in the innocuous warm range, they are not completely deficient in their ability to sense warmth. This could be explained by compensatory and/or redundant contributions in warmth and heat sensation.
ROLE OF WARMTH-ACTIVATED THERMOTRPVS IN ACUTE AND INFLAMMATORY PAIN
Responses to noxious heat are also impaired in TRPV3 and TRPV4 knockout mice, suggesting these channels play a physiological role in the detection of high heat in addition to warmth [81,97]. Similar to the subtle heat phenotype observed in TRPV1-/- mice, TRPV3-/- mice are deficient in thermal nociceptive behavioral responses to acute heat stimuli ≥50°C in hot plate and tail immersion assays [97]. However, unlike the severe deficit in thermal hyperalgesia caused by the absence of TRPV1, TRPV3-/- mice display normal nociceptive responses to radiant heat following CFA or bradykinin injection of the hindpaw. Two groups have studied the role of TRPV4 in mediating acute noxious heat detection and inflammatory thermal hyperalgesia by analyzing TRPV4-/- mice [81,138]. In the first study, acute noxious heat sensation of TRPV4-/- mice appears intact, with no significant difference compared to wildtype in escape latencies in hot plate (35-50°C) or radiant heat tests [138]. In this same study, TRPV4-/- mice display longer escape latencies from hot plate compared to wildtype following hindpaw injection of carrageenan, indicating that TRPV4 plays a role in mediating inflammation induced thermal hyperalgesia. However, a more recent study by Lee et al. reported that noxious heat sensation is impaired in TRPV4-/- mice, observing longer withdrawal latencies in tail immersion tests (∼46°C) compared to wildtype mice [81]. In addition, Lee et al. observed no differences in temperature selection behavior on the thermal gradient following CFA injection in TRPV4-/- vs. wildtype mice, suggesting that TRPV4 is not required for thermal hyperalgesia. The discrepancies between these two studies could be due to differences between the hot plate escape, tail immersion and gradient assays and may depend on factors including rate of temperature change, genetic background and thermal sensitivity of anatomically distinct tissues. Taken together, the data from these studies are suggestive of partially redundant functions of TRPV1, TRPV3 and TRPV4 in acute noxious heat sensation.
Additional studies implicate TRPV4 channel in inflammatory and neuropathic pain [3-6]. TRPV4-/- mice show subtle impairment of osmotic regulation. TRPV4-/- mice are also reported to be deficient in behavioral responses to intense tail pressure, suggesting that in addition to osmosensing, the channel might function to transduce noxious mechanical stimuli [135]. In human pain models, subcutaneous injection of hyper- or hypotonic solutions produce pain sensations described as diffuse and aching or sharp and pricking, respectively [46,47]. Similarly in rodents, hindpaw injection of these solutions induces nociceptive behavior and activates nociceptive C-fibers. These effects are enhanced by the inflammatory mediator PGE2. One group has utilized both antisense knockdown strategies and analysis of TRPV4-/- mice to show that pain-related behaviors induced by injection of hypo- and hypertonic solutions are mediated in part by TRPV4 [5,6]. The same group has demonstrated a role of TRPV4 in a rat model of chemotherapy pain [4]. In cancer patients, tolerable doses of taxol (a tumor reducing drug) are limited due to its induction of small fiber peripheral neuropathy and resulting pain in the extremities. In rodents, chronic taxol treatment induces hyperalgesia in response to mechanical stimuli and hypotonic solutions through enhancement of nociceptor transduction by the drug. Spinal administration of antisense oligonucleotides against TRPV4 reduce its expression in sensory nerves and attenuates hyperalgesia in response to these stimuli, suggesting the channel plays a role in mechanical hyperalgesia induced in this model of neuropathic pain. The authors further show that mechanical hyperalgesia is not due to upregulation of TRPV4 in nerves but rather via interaction of the channel with integrin/Src tyro-sine kinase signaling pathways [5].
OPEN QUESTIONS: MULTIPLE PLAYERS, MULTIPLE MECHANISMS
Collectively,results of TRPV1,TRPV3 and TRPV4 knockout studies raise important questions regarding the roles of thermoTRPVs in detection of temperatures above 25°C. The biophysical properties of each cloned channel are unique, exhibiting different thermal and chemical sensitivity and current responses to repeated stimuli. However, the thermal sensitivities of these channels are somewhat overlapping and they appear to mediate heat detection in vivo in both redundant and distinct ways. How activation of thermoTRPVs lead to the perception of graded or continuous temperature increases from relatively warm to painfully hot remains unclear. Can these channels functionally compensate for one another? Do they function through convergent signaling pathways when activated over a broad range of warm to hot temperatures acutely and during inflammation? Partial thermosensation in thermoTRPV knockout mice is intact suggesting that combinatorial knockout approaches are warranted. A recent study showed that thermoTRPVs might act in concert to increase neuropathic breast pain [45]. Breast pain is influenced in healthy women by estrogens, which regulate NGF mRNA levels in sensory neurons [127]. The authors hypothesized that estrogens and NGF could contribute to clinical breast pain by regulating the expression of ion channels such as TRPV1, a channel that in part mediates thermal hypersensitivity during inflammation. They performed immunohistological analyses using antibodies against TRPV1, TRPV3, TRPV4 and NGF on breast tissues taken from healthy and diseased subjects. All three thermoTRP channels were significantly up-regulated in the pain group, either in nerve fibers (TRPV1 and TRPV4) or epidermal cells (TRPV3 and TRPV4). The intensity of expression correlated with the graded assessment of pain. Taken together these results suggest that altered expression of these channels, in both skin and nerve cells, could contribute to breast pain. In healthy women, variation in breast pain, as well as overall thermal sensitivity, occurs due to the cycling of estrogens. It is tempting to speculate that estrogens themselves could influence the signaling and/or expression of these channels and alter heat (or cold) tolerance across the menstrual cycle.
THERMOTRPS AND COLD SENSATION
Similar to innocuous warmth, sensations elicited by moderately cool stimuli are pleasant (like those from the breeze of a fan on a hot day). Very cold temperature however can cause pain and these pain sensations are qualitatively different from those we experience from very high temperature stimuli (consider reflexive withdraw from a hot surface vs. immersing the hand in very cold water). Cold pain is complex, described as burning, pricking or aching in quality, with acute pain components and pain which develops over time [36]. The activation of cold-receptive C- and Aδ -fibers allows for the perception of decreases in skin temperature by as little as 1°C across a broad range of temperatures in the innocuous (cool) and noxious (painfully cold) range [19,26,123,124]. In terms of acute cold detection, healthy human subjects generally report pain sensations when skin temperature is locally cooled below 15°C. In neuropathic conditions producing hypersensitivity to cooling stimuli (cold allodynia), cooling temperatures in the innocuous range (15-30°C) become painful [163]. Two distantly related thermoTRP channels, TRPM8 and TRPA1 appear to contribute to our ability to detect cooling temperatures both in the innocuous and painful range (Fig. 1).
TRPM8
Several decades of work encompassing early electro-physiological recordings and subsequent calcium imaging studies resulted in the hypothesis that menthol (the cooling, mint-derived compound) and cold activate the same Ca2+-permeable ion channel expressed by sensory neurons [56,117,119,147]. A search to identify this channel led to the discovery of TRPM8 (CMR1, cold and menthol receptor) by two independent groups [90,108]. One group utilized a genomics approach reasoning that sequence homologies to TRP channels might encode novel temperature-activated ion channels [108]. The other group (the same that identified TRPV1) utilized menthol-sensitivity in an expressioncloning screen of a sensory neuron cDNA library [90]. When heterologously expressed, TRPM8 is activated by both menthol and cooling temperatures with a thermal activation threshold of ∼25-28°C. Many biophysical features of the cloned TRPM8 channel closely resemble those of the native channel including adaptation to prolonged cold stimuli and a rise in the thermal activation threshold in the presence of menthol. In addition to menthol, TRPM8 is activated by a variety of naturally occurring and synthetic cooling agents including eucalyptol, spearmint, WS-3 and the ultra-cooling compound icilin (Fig.1) [10,13,90]. Therefore, similar to the “hot” sensations evoked by capsaicin, which activate heat-sensing TRPV1, and the “warm” sensations of camphor induced by TRPV3-activation, menthol is cooling by virtue of its activation of TRPM8. Consistent with the proportion of cool/menthol sensitive DRG and trigeminal neurons observed in culture, in situ hybridization analysis shows that TRPM8 is expressed in approximately 10% of small-diameter C-fiber neurons. Consistent with its expression in a subset of pain- and temperature-sensing neurons, TRPM8 is not present in trkA knockout mice [108]. However, TRPM8 mRNA does not co-localize with a panel of nociceptive markers includingCGRP, SP, IB4 or TRPV1 [108]. Therefore, TRPM8 appears to mark a small subset of temperature-sensing neurons that are poorly characterized.
The in vivo expression pattern of TRPM8 does not appear to be maintained in culture as numerous investigators have observed menthol/capsaicin co-responsive DRG and trigeminal ganglion neurons [8,90,116,147]. Initially, these observations led to the hypothesis that neurons expressing cold-activated TRPM8 and heat-activated TRPV1 transduce painful cold as well as heat and could contribute to phenomena such as paradoxical cold (resulting from the discharge of cold fibers in response to high heat stimuli) [42]. However, the majority of subsequent studies that have used TRPM8 antibodies do not detect co-localization of this channel with TRPV1.Therefore,capsaicin/menthol co-responsiveness observed in culture could result from dissociation and plating procedures. Alternatively Story et al. proposed that DRG neurons respond to both menthol and capsaicin only in the presence of NGF, but subsequent studies have observed this independent of growth factor content [8,130]. Additional discrepancies among laboratories studying thermoTRP response profiles in DRG cultures have arisen and are discussed further below. Although culture studies provide an excellent tool to confirm that thermoTRP agonists are relevant to sensory transduction, there may be an upper limit in the amount of information they provide.
It remains unclear whether TRPM8 functions in transducing noxious cold stimuli. Menthol evokes a non-painful cooling sensation, but a recent study reports that high concentrations of this compound (30%) decrease the cold pain threshold and augment pain in response to suprathreshold stimuli [52]. It is difficult to say whether these high concentrations of menthol are relevant strictly to TRPM8 activation, but menthol might be useful in the study of the severity and dermatomal localization of cold allodynia in neuropathic pain patients. Moreover, although TRPM8 is first activated at relatively cool temperatures, current is sustained at lower, more noxious temperature [90,108]. It is therefore possible that TRPM8 could transmit sensations of cold pain. In addition to sensory neurons, TRPM8 is expressed in bladder urothelium and prostate and in these tissues heightened expression of the channel appears to correlate with cancer pain and progression, respectively [44,98,145]. Several stimuli appear to affect TRPV1 and TRPM8 in an opposite manner. Whereas TRPV1 is sensitized/activated by acidic pH and inhibited by PIP2, TRPM8 is conversely inhibited by acidic pH and activated by PIP2 [86,118]. The implications of the opposing modulatory effects on these two channels are interesting in terms of pain transduction. It has also been proposed that inflammation or nerve injury could induce upregulation of TRPM8 and/or ectopic expression in TRPV1 neurons and contribute to hyperalgesia or cold allodynia. However recent studies failed to detect a change in TRPM8 expression after inflammation or nerve injury [74,105]. Rather cold allodynia appeared to result from increased TRPA1 expression (discussed below). Ultimately whether TRPM8 plays a role in transducing signals of cold throughout the pleasant to painful range will not be clear until knockout animals become available.
FUNCTION OF TRPA1 IN COLD SENSATION
Some disagreement surrounds the physiological role(s) of TRPA1 (ANKTM1), a distantly related TRP channel [10, 70,100,130]. This thermoTRP channel is characterized by a large number of N-terminal ankyrin repeats (predicted 16) and is the only mammalian member of the TRPA subfamily (Fig. 1). Story et al. initially reported that in heterologous expression systems, TRPA1 is activated over a broad range of temperatures with an average activation threshold approaching that of cold pain (≤17°C). TRPA1 is insensitive to menthol, but similar to TRPM8 is activated by the ultra-cooling compound icilin [130]. Activation of TRPA1 by cold is inhibited by ruthenium red and camphor appears to inhibit basal currents of the channel [157]. In vivo expression analyses of DRG show that TRPA1 mRNA is localized within a small subset of nociceptive C-fibers (and possibly thinly myelinated Aδ-fibers) marked by CGRP and SP [130]. Accordingly, the TRPA1 population of DRG neurons does not overlap with TRPM8. Instead TRPA1-expressing neurons strikingly occur as a subset of TRPV1 neurons, suggesting first that TRPM8 and TRPA1 serve different roles in transducing cold stimuli and second, neurons that co-express TRPA1 and TRPV1 might function as polymodal nociceptors (responding to both noxious cold and heat) [130].
While the in vivo expression pattern of TRPA1 in nociceptive neurons is widely accepted, its activation by noxious cold is not without controversy (discussed further below). Two studies subsequent to that of Story et al. failed to reproduce noxious cold activation of TRPA1 in heterologous systems [70,100]. This could result from differences in cell types chosen for over-expression and also in the manner in which cold temperatures are applied (length of stimulus, rate of temperature change). Despite these discrepancies, multiple recent studies demonstrate that TRPA1 comprises a molecular site of integration of multiple pain producing stimuli. It is specifically activated by multiple pungent derivatives from mustard oil (allyl isothiocyanates), cinnamon oil (cinnamaldehyde) and garlic (allicin), all of which induce a sensation of pain that is burning in quality [10,12,70,88]. Recently, the highly toxic irritant acrolein, formed from the breakdown of air pollutants such as fumes from burning gasoline, was also reported to activate TRPA1 [11]. Therefore, TRPA1 is acutely activated by a variety of chemicals all of which induce pain and irritation. Providing a potential mechanism whereby TRPA1 could contribute to inflammatory pain, it robustly couples to BK signaling via the G-protein-coupled BK receptor B2R in vitro [10].
Although a role of TRPA1 in acute noxious cold detection in vivo remains unclear (see below) two recent studies from the same group have shown that TRPA1 contributes to cold hyperalgesia (heightened sensitivity to painful cold temperature) induced by inflammation and injury [74,105]. They examined the contribution of TRPA1 to this condition in models of inflammation (CFA injection) and nerve injury (spinal nerve ligation). Under these conditions, rodents display increased nociceptive behavior (number of paw lifts) in response to exposure to a 5°C cold plate. In both models, TRPA1 mRNA (but not TRPM8) is up-regulated. The studies also demonstrate that increased TRPA1 expression occurs via NGF-induced p38 MAPK activation in noxious-cold activated neurons. Blocking this pathway attenuates cold hyperalgesia. Spinal administration of anti-NGF antibody, a p38 MAPK inhibitor or antisense TRPA1 all alleviate cold hyperalgesia. These studies clearly establish that TRPA1 contributes to the pathogenesis of cold-hyperalgesia in vivo, a condition common in patients suffering from neuropathic pain.
Two groups have now independently generated lines of TRPA1 knockout mice and carried out cold-related behavioral analyses, but whether TRPA1 contributes to acute noxious cold detection in vivo remains unclear [11,79]. Both groups utilized acetone and cold plate tests as a measure of acute cold-sensing in wildtype and TRPA1-/- mice. While one study detected no behavioral deficits in response to cold stimuli, TRPA1-/- mice in the other study showed significantly fewer paw lifts on a 0°C cold plate and reduced sensitivity to evaporative cooling compared to wiltype mice. The studies differed in the way that behavioral tests were scored. That is, while TRPA1-/- mice do not differ from wildtype mice in paw withdrawal response latencies to the cold challenges as measured by Bautista et al., Kwan et al. observe that TRPA1-/- mice respond fewer times compared to wildtype over the course of the assays. Which measure is most relevant to the detection of cold pain should be given careful consideration. Also, Kwan et al. included female TRPA1-/- mice in their analyses, which show a much more significant deficit in both tests compared to a modest trend toward diminished sensitivity in male TRPA1-/- mice. Gender differences in rodent and human pain sensitivity have been documented and may explain why cold pain phenotypes are more evident in female mice [77].
ROLE OF TRPA1 IN CHEMICAL AND MECHANICAL PAIN
Both studies of TRPA1-/- mice demonstrate that TRPA1 is the major (if not sole) site of action of mustard oil. Mustard oil (found in food garnishes such as wasabi and horseradish) causes acute nociceptive behavior as well as neuro-genic inflammation. In a drinking test, Kwan et al. showed that TRPA1 deficient mice are less averse to water containing mustard oil, still consuming water (albeit less than normal) containing such high mustard oil concentrations that wildtype mice completely cease drinking [79]. In tests where mustard oil is applied to the hindpaw, both studies find that TRPA1 deficient mice do not respond with shaking and licking of the treated paw as in wildtype mice. Although significant attenuation of pain behaviors is observed by both groups, one subtle difference is that Kwan et al. observe a low residual sensitivity to mustard oil in TRPA1-/- mice [11,79]. In addition to whether TRPA1 is activated by cold, there is some discrepancy regarding whether mustard oil is specific only to TRPA1 [10,70]. Several studies (including those of TRPA1-/- mice) have identified a population of cultured DRG neurons activated by both mustard oil and capsaicin (presumed TRPA1/TRPV1 neurons). There are two conflicting reports on whether these neurons are also coldsensitive. Nevertheless, this population of mustard oilresponsive neurons is absent in TRPA1-/- mice, while overall capsaicin sensitivity is intact. However, no mustard oil responses were present in cultures derived from TRPA1-/- mice in the study by Bautista et al., while a remaining subset of mustard oil-sensitive/capsaicin-insensitive neurons was present in the second study. This result resembles the observations reported by Bandell et al. who previously hypothesized another receptor for mustard oil is present in DRG neurons [10]. These inconsistencies are difficult to resolve, but differences in observations among laboratories could result from the caveats of DRG cultures previously discussed.
TRPA1 is expressed in hair cells within the inner ear and has been proposed as the mechanosensory transduction channel that mediates hearing [34]. However, TRPA1-/- mice are not deaf, suggesting another transduction channel in this somatosensory modality. It has also been hypothesized that TRPA1 possesses electrophysiological properties peripheral mechanonociceptors [100]. One study of TRPA1-/- mice (the same that reported a cold phenotype), found that mice deficient in TRPA1 exhibited reduced sensitivity to punctuate suprathreshold stimuli applied to the hindpaw (von Frey filaments) and to blunt pressure (Randall-Selitto test) [79]. Studies of TRPA1-/- mice also confirm an essential role of TRPA1 in the development of BK-induced mechanical and thermal hyperalgesia. First, the threshold of mechanically induced pain after BK-mediated inflammation is significantly higher in TRPA1-/- mice compared to wildtype. Also, TRPA1 appears to act in cooperation with TRPV1 to excite nociceptors in response to BK and induce thermal hyperalgesia [11]. Intriguingly similar to TRPV1-/- mice, BK-induced thermal hyperalgesia is attenuated in TRPA1-/- mice. This could be due to functional coupling between TRPA1 and TRPV1 in neurons expressing both channels. Unlike TRPV1-/- mice, thermal hyperalgesia in response to CFA injection is preserved TRPA1-/- mice. Therefore it appears that TRPA1 is modulated by specific components of the inflammatory soup such as bradykinin. Taken together, the results of these studies demonstrate that TRPA1 is a key component of the pain pathway. Further study is necessary to understand its exact role in the detection of noxious cold stimuli. Perhaps more detailed analyses of thermal selection behavior (choice and gradient tests) will reveal more subtle roles of the channel. In addition, when TRPM8 knockout mice become available, side-by-side analysis of cold-related behavior in both strains and the generation of double knockout will surely be informative.
CONCLUDING REMARKS
Intense work on the thermoTRP channels has demonstrated their significant contribution to temperature and pain transduction. The thermal activation thresholds of these channels account for almost the entire range of perceptible temperatures and in many cases correlate well with the properties of native responses. However, other mechanisms of temperature sensation might be in play [39]. For example, Talavera et al. recently showed that warming temperatures (increasing temperatures from 15-35°C) activate two TRP channels from the Melastatin subfamily, TRPM4 and TRPM5 [137]. TRPM5 was previously characterized as a calciumimpermeable cation channel required to generate depolarization in taste receptor cells and mediate responses to sweet, umami and bitter taste. In this latest study, the authors show that TRPM5 is required for thermal modulation of activation of sensory neurons innervating the taste papillae in response to sweet stimuli. They hypothesize that this relationship could provide a mechanism for warmth enhancement of sweet taste reported by human subjects. Whether TRPM4 or TRPM5 function in thermosensation in sensory neurons in addition to gustatory neurons requires further investigation.
Recent studies have begun to further elucidate the mechanisms of modulation and activation of these channels. For instance, what are the mechanisms of how varied stimuli activate (or modulate) these channels? Previous studies demonstrated that a specific domain confers vanilloidsensitivity to TRPV1 [71]. Recent work using novel high-throughput mutagenesis has shed light on TRPM8 channel gating, suggesting that the mechanisms of activation by menthol and cold are separable [9]. By using a chimeric protein approach, another recent study has demonstrated that the C-terminal domains of TRPV1 and TRPM8 are necessary and sufficient to confer thermal sensitivity, PIP2 modulation and gating kinetics characteristic of either channel [18]. Adding further complexity to these multi-functional channels, Nilius and colleagues have proposed a “voltage connection” in the gating of TRP channels [102]. Recent work reveals that gating of both TRPM8 and TRPV1 is weakly voltage-dependent and that ligand activation shifts the activation curve of these channels to more physiological voltages. Whereas heat and capsaicin induced a leftward shift to more negative voltages, cold and menthol induce this effect on TRPM8. Thus, the open probability of TRPV1 channels is greater at high temperature, while cold temperature has this effect on channel gating of TRPM8. Alternatively cold and voltage could gate TRPM8 via separate domains [18]. These studies have revealed an intriguing thermodynamic property of TRPV1 and TRPM8 and whether similar mechanisms function to gate other thermoTRPs warrants further investigation.
In conclusion, we have only reached the threshold in our understanding of the ways in which modulation of these channels can exacerbate pain perhaps by altering nociceptor plasticity. A crucial next step is to move from the level of the primary afferent neuron and examine how thermoTRP channels affect processing at higher levels of the pain pathway.
REFERENCES
- 1.Ahluwalia J, Rang H, Nagy I. The putative role of vanil-loid receptor-like protein-1 in mediating high threshold noxious heat-sensitivity in rat cultured primary sensory neurons. Eur J Neurosci. 2002;16:1483–1489. doi: 10.1046/j.1460-9568.2002.02231.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Anzi B, Tracey WD, Jr., Benzer S. Response of Drosophila to Wasabi Is Mediated by painless, the Fly Homolog of Mammalian TRPA1/ANKTM1. Curr Biol. 2006;16(10):1034–40. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential va-nilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JDN. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 7.Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimo-sato G, Tominaga MY, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- 8.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- 9.Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 10.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 11.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Bautista DM, Movahed P, Hinman A, Axelsson HEO, Sterner OED, Hogestatt ED, Julius D, S Jordt E, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and va-nilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, G S Oxford GS, Gereau RWt. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 16.Birder LA, Kanai AJ, de Groat, Kiss WCS, Nealen ML, Burke NE, Dineley KE, Watkins IJ, Reynolds S, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Wat-kins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 18.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensa-tion in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlisle HJ, Frost TS, Stock MJ. Thermal preference behavior following clonidine, norepinephrine, isoproterenol, and ephedrine. Physiol Behav. 1999;66:585–589. doi: 10.1016/s0031-9384(98)00328-x. [DOI] [PubMed] [Google Scholar]
- 21.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 22.Caterina D, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 23.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 24.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 25.Cesare P, McNaughton P. Peripheral pain mechanisms. Curr Opin Neurobiol. 1997;7:493–499. doi: 10.1016/s0959-4388(97)80028-1. [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Rainville P, Bushnell MC. Noxious and innocuous cold discrimination in humans: evidence for separate afferent channels. Pain. 1996;68:33–43. doi: 10.1016/S0304-3959(96)03180-6. [DOI] [PubMed] [Google Scholar]
- 27.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous cap-saicin-likelipidthat produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 28.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4, 5) P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 29.Chung MK, Guler AD, Caterina MJ. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem. 2005;280:15928–15941. doi: 10.1074/jbc.M500596200. [DOI] [PubMed] [Google Scholar]
- 30.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- 31.Chung MK, Lee A, Mizuno M Suzuki, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockayne DA, Hamilton SG, Zhu, Dunn PM, Zhong Y, No-vakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 33.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath A, Amalfitano EL, Cheung BH, Derfler A, Duggan GS, Geleoc PA, Gray MP, Hoffman MA, Rehm HL, Tama-sauskas D, Zhang DS. TRPA1 is a candidate for the mecha-nosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 35.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 36.Davis KD. Cold-induced pain and prickle in the glabrous and hairy skin. Pain. 1998;75:47–57. doi: 10.1016/S0304-3959(97)00203-0. [DOI] [PubMed] [Google Scholar]
- 37.Petrocellis LDe, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V. The vanilloid receptor (VR1) -mediated effects of anan-damide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem. 2001;77:1660–1663. doi: 10.1046/j.1471-4159.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 38.Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, Matsunaga K. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285:1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- 39.Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 40.Marzo VDi, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr Opin Neurobiol. 2002;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 41.Dickinson SD, Cunningham CL. Altered ambient temperature and ethanol-induced conditioned place preference in mice. Alcohol. 1998;16:13–18. doi: 10.1016/s0741-8329(97)00168-7. [DOI] [PubMed] [Google Scholar]
- 42.Dodt E, Zoterman Y. The discharge of specific cold fibers at high temperatures (the paradoxical cold) Acta Physio Scand. 1952;26:358–365. doi: 10.1111/j.1748-1716.1952.tb00917.x. [DOI] [PubMed] [Google Scholar]
- 43.Funakoshi K, Nakano M, Atobe Y, Goris RC, Kadota T, Yazama F. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 2006;323:27–41. doi: 10.1007/s00441-005-0013-3. [DOI] [PubMed] [Google Scholar]
- 44.Geirsson G. Evidence of cold receptors in the human bladder: effect of menthol on the bladder cooling reflex. J Urol. 1993;150:427–430. doi: 10.1016/s0022-5347(17)35501-5. [DOI] [PubMed] [Google Scholar]
- 45.Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graven-Nielsen T, Arendt-Nielsen L, Svensson P, Jensen TS. Stimulus-response functions in areas with experimentally induced referred muscle pain--a psychophysical study. Brain Res. 1997;744:121–128. doi: 10.1016/s0006-8993(96)01077-3. [DOI] [PubMed] [Google Scholar]
- 47.Graven-Nielsen T, McArdle A, Phoenix J, Arendt-Nielsen L, Jensen TS, Jackson MJ, Edwards RH. In vivo model of muscle pain: quantification of intramuscular chemical, electrical, and pressure changes associated with saline-induced muscle pain in humans. Pain. 1997;69:137–143. doi: 10.1016/s0304-3959(96)03270-8. [DOI] [PubMed] [Google Scholar]
- 48.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170:1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 49.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immu-nocytochemical localization of the vanilloid receptor 1 (VR1) : relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 51.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 52.Hatem S, Attal N, Willer JC, Bouhassira D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196. doi: 10.1016/j.pain.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 53.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 54.Hensel H. Thermoreception and temperature regulation. Monogr Physiol Soc. 1981;38:1–321. [PubMed] [Google Scholar]
- 55.Hensel H, Iggo A. Analysis of cutaneous warm and cold fibres in primates. P flugers Arch. 1971;329:1–8. doi: 10.1007/BF00586896. [DOI] [PubMed] [Google Scholar]
- 56.Hensel H, Zotterman Y. The effect of menthol on the thermoreceptors. Acta Physiol Scandinavica. 1951:27–34. doi: 10.1111/j.1748-1716.1951.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 57.Holzer P. Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin Ther Targets. 2004;8:107–123. doi: 10.1517/14728222.8.2.107. [DOI] [PubMed] [Google Scholar]
- 58.Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 59.Holzer P. Vanilloid receptor TRPV1: hot on the tongue and inflaming the colon. Neurogastroenterol Motil. 2004;16:697–699. doi: 10.1111/j.1365-2982.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 60.Hu HJ, Bhave G, Gereau RWt. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu M. X. 2-Aminoethoxydiphe-nyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279(36):37423–30. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 62.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 63.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue R, Morita H, Ito Y. Newly emerging Ca2+ entry channel molecules that regulate the vascular tone. Expert Opin Ther Targets. 2004;8:321–334. doi: 10.1517/14728222.8.4.321. [DOI] [PubMed] [Google Scholar]
- 66.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa MA. novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 68.Jin X, Gereau RWT. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensi-tivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zyg-munt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 71.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 72.Jung J, Shin JS, Lee wang SYH, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca+2/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2003;279:7048–54. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 73.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Ko-jima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 74.Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.01.031. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 75.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 76.Kollarik M, Undem BJ. Activation of Bronchopulmon-ary Vagal Afferent Nerves with Bradykinin, Acid and Vanilloid Receptor Agonists in Wildtype and TRPV1-/- Mice. J Physiol. 2003;555(pt1):115–23. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–160. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Kowase T, Nakazato Y, Yoko OH, Morikawa A, Kojima I. Immunohistochemical localization of growth factor-regulated channel (GRC) in human tissues. Endocr J. 2002;49:349–355. doi: 10.1507/endocrj.49.349. [DOI] [PubMed] [Google Scholar]
- 79.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 80.Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 2005;451:160–167. doi: 10.1007/s00424-005-1438-y. [DOI] [PubMed] [Google Scholar]
- 81.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ewinter RD, Skinner LK, Julius D, Basbaum AI. Immunoreactive TRPV-2 (VRL-1), a capsaicin receptor homolog, in the spinal cord of the rat. J Comp Neurol. 2004;470:400–408. doi: 10.1002/cne.20024. [DOI] [PubMed] [Google Scholar]
- 83.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Va-nilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4, 5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma QP. Vanilloid receptor homologue, VRL1, is expressed by both A- and C-fiber sensory neurons. Neuroreport. 2001;12:3693–3695. doi: 10.1097/00001756-200112040-00018. [DOI] [PubMed] [Google Scholar]
- 88.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepa-tol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 90.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 91.Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. Phila-delphia: Elsevier; 2006. pp. 3–34. [Google Scholar]
- 92.Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1- like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mizumura K. Peripheral mechanism of hyperalgesia--sensitization of nociceptors. Nagoya J Med Sci. 1997;60:69–87. [PubMed] [Google Scholar]
- 95.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen QYan, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 96.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005 doi: 10.1126/stke.2722005re3. 2005, re3. [DOI] [PubMed] [Google Scholar]
- 97.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GMA. Patapou-tian, Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 98.Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK, Anand P. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006;6:6. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 100.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Noci-ceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagy I, Santha P, Jancso G, Urban L. The role of the vanil-loid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 102.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nilius B, Voets T, Peters J. TRP channels in disease. Sci STKE. 2005 doi: 10.1126/stke.2952005re8. re8. [DOI] [PubMed] [Google Scholar]
- 104.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyper-algesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- 107.Patapoutian A, Peier AP, Story GM, Viswanath V. ThermoTRPs and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 108.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 109.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan SA. Patapoutian, A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 110.Petho G, Izydorczyk I, Reeh PW. Effects of TRPV1 receptor antagonists on stimulated iCGRP release from isolated skin of rats and TRPV1 mutant mice. Pain. 2004;109:284–290. doi: 10.1016/j.pain.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 111.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 112.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 113.Ramsey IS, Delling M, Clapham DE. An introduction to trp channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 114.Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ray B, Mallick HN, Kumar VM. Changes in thermal preference, sleep-wakefulness, body temperature and locomotor activity of rats during continuous recording for 24 hours. Behav Brain Res. 2004;154:519–526. doi: 10.1016/j.bbr.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 116.Reid G, Flonta ML. Ion channels activated by cold and menthol in cultured rat dorsal root ganglion neurons. Neurosci Lett. 2002;324:164–168. doi: 10.1016/s0304-3940(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 117.Reid G, Flonta ML. Physiology Cold current in ther-moreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- 118.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4, 5) P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 119.Schafer K, Braun HA, Isenberg C. Effect of menthol on cold receptor activity Analysis of receptor processes. J Gen Physiol. 1986;88:757–776. doi: 10.1085/jgp.88.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schicho R, Florian W, Liebmann I, Holzer PI. T f TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 121.Scott SA. Sensory neurons: diversity, development, and plasticity. New York: Oxford University Press; 1992. [Google Scholar]
- 122.Shimosato G, Amaya F, Ueda M, Tanaka Y, Decosterd I, Tanaka M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain. 2005;119:225–232. doi: 10.1016/j.pain.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Simone DA, Kajander KC. Excitation of rat cutaneous nociceptors by noxious cold. Neurosci Lett. 1996;213:53–56. doi: 10.1016/0304-3940(96)12838-x. [DOI] [PubMed] [Google Scholar]
- 124.Simone DA, Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol. 1997;77:2049–2060. doi: 10.1152/jn.1997.77.4.2049. [DOI] [PubMed] [Google Scholar]
- 125.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanil-loid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 127.Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratino-cytes. J Pharmacol Exp Ther. 2003;304:217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 129.Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch T, Brzoska U, Lippert BM, Henz TA, Luger D, Metze M, Steinhoff V. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 130.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 131.Stowers L, Holy TE, Meister Dulac MC, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 132.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 133.Stucky CL, Lewin G, Isolectin RB. (4) -positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11:2687–2698. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- 135.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 136.Szolcsanyi J, ancso-Gabor A. Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency of pungent agents. Arzneimittelforschung. 1975;25:1877–1881. [PubMed] [Google Scholar]
- 137.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 138.Todaka H, Taniguchi J, Satoh JI, Mizuno A, Suzuki M. Warm temperature-sensitive TRPV4 plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279(34):35133–8. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- 139.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 140.Tominaga M, Numazaki M, Iida T, Moriyama T, Togashi K, Higashi T, Murayama N, Tominaga T. Regulation mechanisms of vanilloid receptors. Novartis Found Symp. 2004;261:4–12. discussion 12-18, 47-54. [PubMed] [Google Scholar]
- 141.Tominaga M, Tominaga T. Structure and function of TRPV1. P flugers Arch. 2005;45:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 142.Tominaga M, Wada M, Masu M. Potentiation of cap-saicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila Gene Essential for Nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 144.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in noci-ceptive primary afferents innervating monkey skin. J Physiol. 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 146.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain. 2004;8:129–133. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 147.Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- 148.Viswanath V, Story GM, Peier AP, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite ther-mosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 149.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;10:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 151.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens W, Cairns U, Wissenbach J, Prenen V, Flock-erzi G, Droogmans CD, Benham J, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 152.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 153.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 154.Wilson LM, Sinha HL. Thermal preference behavior of genetically obese (ob/ob) and genetically lean (+/?) mice. Physiol Behav. 1985;35:545–548. doi: 10.1016/0031-9384(85)90138-6. [DOI] [PubMed] [Google Scholar]
- 155.Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- 156.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 159.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and va-nilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 161.Zhou Y, Li GD, Zhao ZQ. State-dependent phosphoryla-tion of epsilon-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J Neurochem. 2003;85:571–80. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 162.Zimmermann K, Leffler A, Fischer MM, Messlinger K, Nau C, Reeh PW. The TRPV1/2/3 activator 2-aminoethoxydiphenyl borate sensitizes native nociceptive neurons to heat in wildtype but not TRPV1 deficient mice. Neuroscience. 2005;135:1277–1284. doi: 10.1016/j.neuroscience.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 163.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 164.Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neuro-sci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanil-loid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]