Abstract
Bv8 is a small protein secreted by frog skin. Mammalian homologues of Bv8, the prokineticins PK1 and PK2, and their G-protein coupled receptors PKR1 and PKR2 have been identified and linked to several biological effects. Bv8 elicits a dose-dependent reduction in nociceptive threshold to thermal and mechanical stimuli applied to the skin of tail and paw of rats and mice and increases the sensitivity to nociceptive mediators as capsaicin and prostaglandins. The receptors for Bv8/PKs are present in a fraction of peptidergic population of C-fibre neurons, and in a fraction of A myelinated-fibre neurons. In mouse and rat dorsal root ganglia, PKR-expressing neurons also express TRPV1 and the activation of PKRs sensitises TPRV1 to the action of capsaicin. Mice lacking PKR1 gene exhibit impaired Bv8-induced hyperalgesia, develop deficient responses to noxious heat, capsaicin and protons and show reduced thermal and mechanical hypersensitivity to paw inflammation, indicating a requirement for PKR1 signalling associated with activation and sensitisation of primary afferent fibres. PKs are highly expressed by neutrophils and other inflammatory cells and must be considered as new pronociceptive mediators in inflammatory tissues. Bv8-like hyperalgesic activity was demonstrated in extracts of rat inflammatory granulocytes. Bv8 stimulate macrophage and T lymphocyte to differentiate between an inflammatory and Th1 profile indicating that Bv8/PK proteins play a role in immuno-inflammatory responses. Blockade of PKRs may represent a novel therapeutic strategy in acute and inflammatory pain conditions.
Key Words: Bv8, prokineticins, prokineticin receptors, nociception, inflammatory pain
INTRODUCTION
Pain is a sensory modality in which specialised primary afferent neurons, called nociceptors, detect noxious stimuli. Nociceptors are remarkable and unusual sensory cells because they respond to a broad range of physical (e.g. heat, cold, and pressure) and chemical (e.g. acid, irritants, and inflammatory mediators) stimuli, but do so only at stimulus intensities capable of causing tissue damage. A fundamental goal in pain biology is to provide a molecular understanding of how physical and chemical stimuli are detected by the nociceptor, how intensity thresholds are specified, and how these thresholds are reset in the setting of tissue injury or disease. Moreover, an important property of pain signalling is that of sensitisation. Many extracellular pain mediators such as prostaglandins, bradykinin, cytokines, neurotrophins, adenosine and protons have been shown to induce nociceptor sensitisation through the activation of their receptors at nociceptor terminals. The number of signalling molecules involved in pain is continuously growing. Pharmacological methods offer alternative strategies for initiating molecular studies, especially when a drug or agent that has robust and selective effects on the cellular or physiological system of interest is identified.
We recently isolated, from the skin secretion of a frog, a small protein that potently induces hyperalgesia in rodents [30, 34]. This peptide was named Bv8, to indicate its origin (Bombina variegata) and its molecular weight (8 kDa). The secretions of the holocrine glands of amphibian skin contain a wide variety of bioactive peptides, nearly all of which have a counterpart in mammalian hormones and neurotransmitters indicating that they are involved in basic cellular or developmental processes conserved throughout evolution [9]. A ‘core’ of gene clusters seems to code for secretory peptides and proteins with homologues found across the zoological scale.
The mammalian homologues of Bv8, the prokineticins (PK1 and PK2), have been successively identified together with their receptors (PKR1 and PKR2). The number of biological activities associated with Bv8 and its mammalian homologues is rapidly increasing. Originally identified as potent agents that contract smooth muscles of the gastrointestinal tract [24, 30], they have also been shown to modulate complex behaviours, such as feeding and drinking [33] and circadian rhythms [6] and are involved in hypothalamic hormone secretions [20], in neuronal survival [29] and angiogenesis [11]. Luckily, the availability of adequate amounts of the amphibian Bv8 protein made it possible to study the pharmacology of Bv8 by topical and systemic administration. In this review we summarise the biological function of Bv8/PKs and focus on the role of these proteins and their receptors in setting the nociception threshold in normal and pathological conditions.
Bv8/PROKINETICIN FAMILY
Homologues of Bv8 have been demonstrated and predicted in skin secretions of other amphibians (Bombina bombina, Bombina orientalis, Bombina maxima, Rana temporaria and Rana esculenta) [17]. A Bv8 homologues, Mamba Intestinal Toxin (MIT-1 or VRPA), was isolated from the venom of the black mamba Dendroaspis polylepsis [16, 37]. The high degree of identity between Bv8 and MIT-1 (58%) suggested that similar peptides could also be present in other species, including mammals. In mice and humans, cDNA cloning identified two orthologues of Bv8, murine Bv8 (mBv8) and human Bv8 (hBv8) [45]. Striking characteristics of these proteins are their identical amino terminal sequence, AVITG, and the presence of ten cysteines with identical spacing that define a five disulphide-bridged motif called a colipase fold [17]. Two forms of mBv8 and hBv8 have been characterised in mouse and man testis. These forms differ in an exon coding for 21 amino acids, the majority of which are basic [45]. Li and colleagues [24] identified sequences encoding two human proteins similar to Bv8, named prokineticin 1 (PK1, a Bv8-like protein) and prokineticin 2 (PK2, or hBv8). The name prokineticin refers to the ability of these peptides to contract guinea pig ileum, a property shared with amphibian Bv8. Screening a library of human secreted proteins for the ability to induce proliferation in capillary endothelial cells, Ferrara and colleagues [21, 22] identified a protein which induced proliferation, migration and fenestration in the endothelial cells of steroid synthesising glands (ovary, testis, adrenals) and named it endocrinegland-derived vascular endothelial growth factor (EG-VEGF). EG-VEGF and PK1 are the same proteins and have an overall identity of 58% and homology of 76% with human PK2 and murine Bv8 and 43% identity with amphibian Bv8. A later study showed that PK2 also possesses a similar angiogenic effect [21]. This angiogenic effect is probably mediated through PK receptors because both PKR1 and PKR2 are expressed in the vascular endothelial cells of the adrenal gland and testis. Because angiogenesis is crucial for many processes including reproductive functions, several groups have further examined the role of Bv8/PKs in the angiogenesis of reproductive organs [1, 12, 18]. Delivery of PK1/EG-VEGF to the ovary elicited potent angiogenesis and cyst formation. PK1/EG-VEGF has been isolated and sequenced also from bovine milk [28].
Rat and human mRNAs for PK1 and PK2 have been cloned and their expression patterns reported in peripheral tissues (gastrointestinal tract, endocrine glands, spleen, human and murine leucocytes) and the central nervous system.
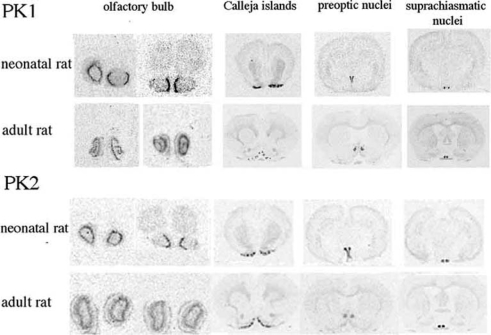
In neonatal and adult rat brain (Fig. 1), both PK1 and PK2 are clearly expressed in the olfactory bulb: olfactory bulb neurogenesis may depend on PK2 signalling because PK2-KO mice display a marked reduction in the size of the olfactory bulb, a loss of normal olfactory bulb architecture, and an accumulation of neuronal progenitors in the rostral migratory stream [35]. PK1 and PK2 are clearly expressed also in Calleja islands and in suprachiasmatic nucleus (SCN).
Fig. (1).
Autoradiograms of PK1 and PK2 mRNA expression in the brain of neonatal and adult rat. PK2 is clearly expressed in neonatal and adult rat brain. PK1 expression tends to diminish during postnatal development to remain clearly expressed in olfactory bulb and in SCN in the adult rat.
The PK2 mRNA expression pattern in the SCN of mice [6] and of rats [33] is rhythmic (being lowest in the dark phase) and is severely blunted in mutant mice deficient in clock or cryptochrome genes. Intracerebroventricular delivery of PK2 [6] or Bv8 [33] during subjective night, when endogenous PKs are low, inhibited the nocturnal wheel running activity and the nocturnal feeding of rats. Collectively, these results indicate that PKs are candidate output molecules that transmit circadian behavioural rhythms from the SCN clock.
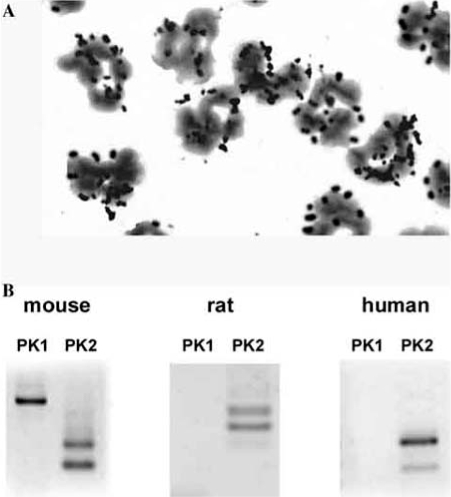
Outside the brain, the mammalian Bv8 (or PK2) is higly expressed in testis, lymphoid organs and in peripheral blood cells, in dendritic cells and in bone marrow [8, 19, 23, 24, 27, 45]. We demonstrated that in murine macrophages [27], in inflammatory granulocytes (Fig. 2) and in HL60 cells (data in preparation), PK2 mRNA is always present as a tissue-specific double-splice variant: the short form encodes for a protein (PK2) similar to amphibian Bv8, whereas the long form (PK2L) encodes for an additional 21 aa domain inserted between residue 47 and residue 48, which had been already described only in mouse and human testis [45] (Fig. 2). The 21-aa insert is rich in arginine and lysine residues, and contains several potential cleavage sites for prohormone convertases. Bullock and colleagues [2] produced a recombinant protein of this splice variant that demonstrates potency about 150 fold lower than PK2, by an aequorin-based assay for [Ca++]i mobilisation in PKR-transfected CHO cells. The secreted PK2L is processed, by proteolytic clevage, into a smaller peptide (PK2Lβ) that loses affinity for PKR2 [5].
Fig. (2).
A: PK2 mRNA in elicited peritoneal granulocytes of rats. Granulocytes were collected 6 h after induction of peritonitis with oyster glycogen. B: RT-PCR amplification of mRNAs for PK1, PK2 and PK2L in elicited purified peritoneal granulocytes from mice and rats, and in human circulating leucocytes.
PROKINETICIN RECEPTORS
Three independent groups identified two closely related G-protein-coupled receptors for Bv8/PKs, prokineticin receptor 1 (PKR1) and prokineticin receptor 2 (PKR2) [25, 28, 39]. PKR1 and PKR2 have an overall identity in their amino acid sequences of 85%, with most differences at the N-terminal and are about 80% identical to the previously described mouse orphan receptor gpr73 [25]. In specific endothelial cells, neurons, and transfected cell lines [25, 29, 34], receptor activation stimulates calcium mobilisation, phosphoinositol turnover, and mitogen-activated protein kinase and Akt pathway activation.
Receptor binding studies [28, 32, 34] showed that PKR2 is an MIT selective receptor, indeed, affinity of MIT for PKR2 (in the range of pM) is about 10 times higher than that of PK2 and 50 times higher than that of PK1. PKR1 is an MIT and PK2 preferring receptor: affinity of MIT for PKR1 (about 5-10 times lower than for PKR2) is comparable to that of PK2 and 60 times higher than that of PK1. Affinity of Bv8 for the receptors is comparable to that of PK2 and is about 40 times higher than that of PK1. IC50 of Bv8 to inhibit [125I]Bv8 binding to rat DRG and dorsal spinal cord homogenates was 4.1 and 7.3 nM, i.e. 20-40 times lower than that of PK1/EG-VEGF (76.4 and 330 nM). The inhibitory potency of Bv8 on the specific binding of 4 pM 125I-MIT at PKR1 and PKR2 receptors in CHO cell membranes was 0.69 and 0.71 nM. Ki of 125I-MIT derived from self-competition binding curves on PKR2-containing membranes was 3.03 pM (PerkinElmer, Biosignal Inc).
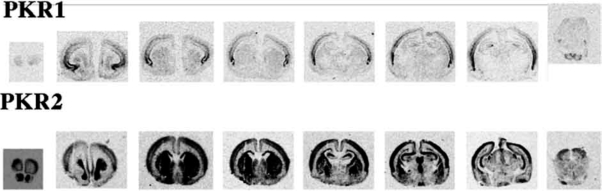
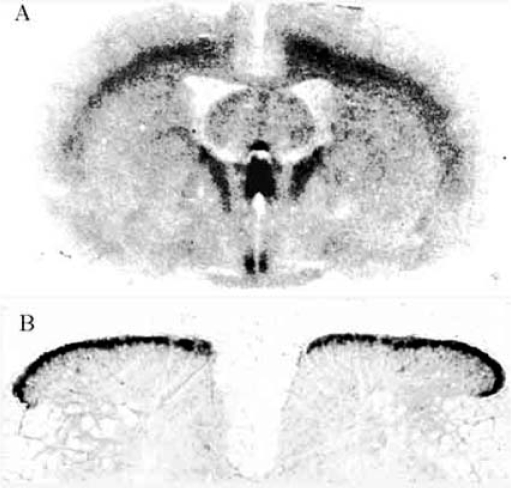
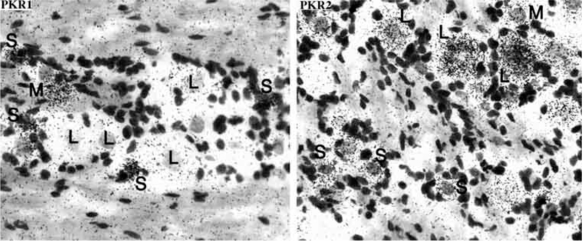
We mapped a comprehensive mRNA distribution of PKR1 and PKR2 by in situ hybridisation with riboprobes and mapped the distribution of the receptor proteins by in situ binding of 125I-MIT, in rat brain, at different ages. In rat embryos from day 12 p.c., both receptors are highly expressed in the neuroepithelium lining ventricles, and in the Gasser-ganglion and dorsal root ganglia (DRG) (Fig. 3). One day after birth, PKR2 is still expressed at high levels in the olfactory bulb, in the neuroepithelium lining the ventricles, in the striatum, hippocampus, thalamic and hypothalamic paraventricular nuclei, suprachiasmatic nucleus, amygdala and cortex, whereas PKR1 is only found in the cortex (Fig. 4). In adult rats, only PKR2 is abundantly or moderately expressed in several discrete brain regions. The presence in the nucleus arcuatus of PKR2-mRNA (demonstrated by in situ hybridisation with ribo-probes [33]) and PKR2-protein (demonstrated by binding of labelled MIT -Fig. 5) explains the anorexogenic effect of Bv8. Whereas, the dipsogenic effect of Bv8 depends on its binding to PKR2 in the subfornical organ (SFO) (Fig. 5A) [33]. Central and peripheral administration of Bv8, in rats, induces oxytocin and vasopressin release and vasopressin-dependent antidiuresis probably by acting on its receptors in paraventricular hypothalamic nuclei [20].
Fig. (3).
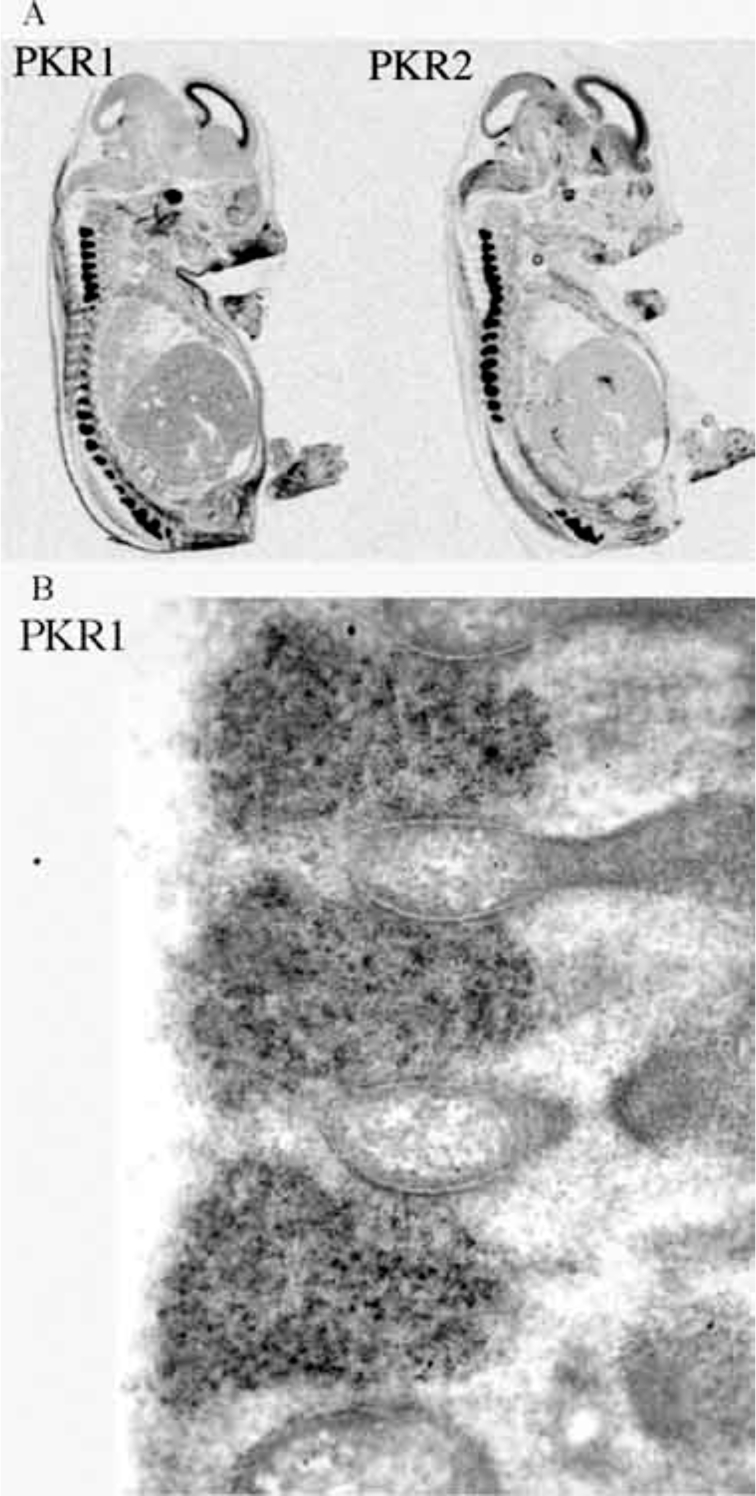
A: autoradiograms of PKR1 and PKR2 mRNAs in a sagittal section of a rat embryo of 18 pc. Both the receptors are highly expressed in the neuroepithelium lining ventricles, in the Gasser-ganglion and in the DRG. B: high magnification of a histological section of DRGs showing a very strong hybridisation signal for PKR2-mRNA.
Fig. (4).
One day after birth, PKR2 is still expressed at high levels in olfactory bulb in the neuroepithelium lining the ventricles, in the striatum, in the hippocampus, in thalamic and hypothalamic paraventricular nuclei, in suprachiasmatic nucleus, in amygdala and in the cortex, while PKR1 is still present only in the cortex.
Fig. (5).
Autoradiograms of coronal section of adult rat brain (A) and transversae section of adult rat lumbar spinal cord (B) showing binding of 125I MIT to PKRs. (A) major areas of binding are identified as cortex, sub fornical organ (SFO), paraventricular thalamic and hypothalamic nuclei, parathenial nuclei and suprachiasmatic nucleus. (B) Intense binding area is present in the outer layers of the dorsal horns of the spinal cord.
PKR1 and PKR2 are both expressed in DRG of neonatal and adult rats: PKR1 is mainly expressed in small and medium size neurons and PKR2 is mainly expressed in large neurons (Fig. 6). PKR mRNA hybridisation is not evident in spinal cord. Conversely, the receptor proteins, revealed by binding with labelled MIT, are present in DRG and in the outer layers of the dorsal horns of the spinal cord (Fig. 5B).
Fig. (6).
In situ hybridization of adult rat DRG sections with riboprobes for PKR1 and PKR2. PKR1 is mainly expressed in small and medium size neurons and PKR2 is mainly expressed in medium and large size neurons.
BV8-INDUCED NOCICEPTIVE SENSITISATION
The major effect of Bv8 and the one we have studied most, is its hyperalgesic effect. Bv8 injected in rats and mice produces sensitisation to thermal and mechanical stimuli, without inducing any spontaneous, overt nocifensive behaviour or local inflammation. An intravenous or subcutaneous injection of Bv8 (from 0.06 to 500 pmol/kg) induces a biphasic hyperalgesia to tactile and thermal stimuli evaluated with paw-pressure test, hot-plate, plantar test and tail-flick test [34]. Allodynia, evaluated with the vonFrey test, displays a comparable biphasic pattern. An initial rapid phase of hyperalgesia peaks after 1 hour, and is followed by a secondary phase peaking at 4 - 5h. The initial phase of hyperalgesia is due at least in part to a local action on nociceptors, because very small amounts of Bv8 (50 fmol/rat) injected intraplantarly cause a strong and localised hyperalgesia with a similar time course to that of the initial phase of hyperalgesia seen with systemic injections. By the intrathecal (i.t.) route, Bv8 (from 6 fmol to 250 pmol) decreased the nociceptive threshold to mechanical or thermal stimuli with the characteristic biphasic time-course. An i.t. injection of 60 fmol of Bv8 halved the nociceptive threshold: hyperalgesia was evident within 2 min, peaked at 30 min, lasted 90-120 min and was followed by a 1h-recovery. The second decrease in the threshold peaked at the 4th h and lasted till the 6th h.
An action of Bv8 through binding to PKR1 and PKR2 on nociceptive neurons is supported by the observation that (i) these receptors are expressed in rat and mouse DRG; (ii) [125I]Bv8 binds to membrane preparations of DRG and dorsal horns of spinal cord; (iii) Bv8 added to rat DRG cultures dose-dependently increases [Ca++]i [34]; and (iv) mice lacking the PKR1 are about 100 times less sensitive to Bv8-induced hyperalgesia (hot-plate test) than wild type mice.
In collaboration with Peter McNaughton and Vittorio Vellani, we thoroughly investigated the cellular basis of hyperalgesia caused by Bv8 in rat DRG neuron cultures [43]. We demonstrated that receptors for Bv8 are present in a fraction of the peptidergic population of C-fibre neurons, and in a fraction of A myelinated-fibre neurons. Of the neurons responding to Bv8, 90% also responded to capsaicin showing a very high degree of co-localisation of functional Bv8 receptors with the heat and capsaicin-activated ion channel TRPV1 [3]. Half of the Bv8-responding neurons expressed calcitonin-gene related peptide (CGRP). Patch clamp experiments showed that a brief exposure to Bv8 tremendously potentiated the inward current activated by capsaicin. Bv8 by itself did not depolarise the plasma membranes. This result agrees with behavioural experiments showing that intrapaw injection of Bv8 does not produce pain per se but produces strong sensitisation to painful stimuli. Moreover, Bv8 caused translocation of PKCε to the neuronal membrane. As already demonstrated for bradykinin and ATP [4], we hypothesise that Bv8-activated PKCε enhances the gating of TRPV1 by phosphorylating two serine residues [36]. Indeed, the Bv8-induced capsaicin potentiation is partially inhibited by the PKC inhibitors staurosporine and RO 31-8220. The observation that part of the sensitisation was not blocked by either inhibitor, however, suggests that other signalling intermediates are also involved.
The data on DRG neuron cultures have been confirmed by “in vivo” experiments. In mice, intraplantar injection of a near-threshold dose of 0.01 nmol of capsaicin induced licking for about 8 s, but the same dose of capsaicin injected 90 min after 50 fmol Bv8, induced marked licking of the paw that lasted more than ten times longer. The effect disappeared within 6 hours after Bv8 administration.
Several in vivo experimental paradigms, in rats and mice, sustain a co-operativity between PKRs and TRPV1 in Bv8 induced hyperalgesia. Pretreatment of rats with capsazepine (0.4 mg, i.pl.) strongly reduced (about 100 times) the mechanical hyperalgesia (Randall-Selitto test) induced by intraplantar injection of Bv8: 5 nmol of Bv8 were needed to obtain the same hyperalgesia produced by 50 fmol of Bv8 in control rats. The capsaicin-sensitive primary afferents (CSPA) were ablated by injecting capsaicin into neonatal rats [15] and rats were tested at the age of 52 days. The basal mechanical nociceptive threshold remained unchanged in CSPA-ablated rats compared with non-ablated rats. But ablated rats were about 100 times less sensitive to Bv8-induced hyperalgesia than non-ablated rats: 2.5 nmol/kg, s.c. were needed to obtain the same hyperalgesia produced by 25 pmol/kg, s.c. in non-ablated rats. TRPV1-KO mice are 20 times less sensitive than wild type mice to Bv8-induced thermal hyperalgesia (hot-plate test). Conversely, PKR1-KO mice are less sensitive than WT-mice to capsaicin. They show impaired nociceptive responses to intraplantar and oral capsaicin and a strong reduction of capsaicin-evoked hypothermia (see later). The experiments with TRPV1-KO and and PKR1-KO mice clearly demonstrate a bidirectional cooperativity between the prokineticin and the vanilloid receptors; moreover, rat and mouse experiments also demonstrate that blocking the TRPV1 pathway, strongly reduces but does not abolish Bv8-induced hyperalgesia. Thus we conclude that Bv8-induced pain sensitisation must also involve other pain mediators.
We demonstrated that hyperalgesia produced by Bv8 injections was inhibited by pretreatment of the Bv8-injected paw with the PLA2 inhibitors AACOF3 and HELSS, the COX-1/2 inhibitor indomethacin, the COX-1 inhibitor SC560, the prostaglandin receptor (EP1) antagonist SC51322, the PKA inhibitors H89 and WIPTIDE. The COX2 inhibitor, NS392 was ineffective.
To check further the involvement of prostaglandins in Bv8-induced hyperalgesia, we studied the heat nociceptive response to Bv8 in COX1- and COX2-KO mice. COX1-KO mice were 20 times less sensitive than WT mice to Bv8, whereas COX2-KO mice showed the same sensitivity as WT mice1. Our data confirm that COX-1 is important for nociceptor function. In DRGs, COX-1 immunoreactivity is observed in the cytoplasm, nuclear membrane and axonal processes of small- and medium-sized neuronal cell bodies and is extensively co-localised with CGRP and isolectin B4 (IB4). Conversely, COX-2 labelling was absent in peripheral somatosensory neurons from normal or arthritic rats [7]. Because about 50% of Bv8 responding neurons are CGRP-positive, we conjecture the existence of a peptidergic nociceptor population containing both COX-1 and PKRs where PG may contribute to the excitatory effect of Bv8. Prostaglandin-induced sensitisation of nociceptors is mediated by the PKA signalling pathway [41] and inhibitors of PKA activity at- tenuate PGE2-induced hyperalgesia. Accordingly, PKA inhibitors and PG receptor antagonists impair the hyperalgesic action of Bv8. Other observations further support an interaction between Bv8 and the eicosanoid system. As already demonstrated for capsaicin-induced licking, the heat nociceptive response (hot-plate test) of mice to intraplantar injection of the threshold dose of PGE2 (10 ng) increased tremendously when mice were preinjected with 50 fmol Bv8. Again, the sensitising effect disappeared within 6 hours after Bv8 administration. Experiments are in progress to detect the mechanisms of Bv8-induced PGE2 sensitisation.
Many in vitro data from our laboratory [32, 34] and others [21, 25] demonstrated that PKR1 and PKR2 are Gq-coupled receptors and their signal are amplified by PLC and PKC. Supporting this molecular mechanism, pretreatment of rats with the PLC inhibitor, U73122, and with the PKC-inhibitors, GF1109203X and Ro-37-8220 completely blocked the thermal (plantar test) and mechanical (paw-pressure test) hyperalgesia induced by intrathecal or intraplantar injection of 50 fmol Bv8.
while the specific molecular mechanism by which Bv8 increases the nociceptive potency of capsaicin and PGE2 remains to be elucidated, we tentatively suggest that Bv8 binds to Gq-coupled-receptors and activates PLC and PKC, thus leading to TRPV1 phosphorylation with an ensuing increase in Ca++ influx. Bv8 could also promote eicosanoid pathway activation via PLC-DAG or more directly by activation of PLA2.
Nitric oxide might also be involved [46] because the decrease in the nociceptive threshold induced by intrapaw and intrathecal injection of Bv8 (50 fmol) is abolished by intrapaw and intrathecal pre-injection of non-analgesic doses of the i-NOS inhibitor 7-NI and the NOS inhibitor L-NAME.
Another intriguing property of Bv8 is the characteristic biphasic time-course of the hyperalgesia induced by its systemic and intrathecal administration. This biphasic pattern might be a hallmark of Bv8-induced nociceptive sensitisation. Why the nociceptive response to Bv8 has two phases is unclear. Nevertheless, because the second phase of hyperalgesia develops only after intrathecal or systemic but not after plantar administration of the protein, it may reflect the well-known process of central sensitisation. The strong stimulation of a large number of nociceptive primary afferent terminals, induced by systemically delivered Bv8 or the direct activation of dorsal horn neurons evoked by intrathecal injection of the protein, may trigger a delayed transient state of increased excitability of nociceptive dorsal horn neurons through the mechanism of central sensitisation. Conversely, local unilateral hyperalgesia produced by the low intraplantar dose of Bv8 probably failed to induce sufficient afferent activity to trigger central sensitisation.
To evaluate Bv8 involvement in nociceptive transmission in spinal cord, we monitored the expression of c-fos, a protooncogene widely used as a morphological marker of pain-activated neurons [14], in dorsal horn of lumbar spinal cord after intrapaw injection of Bv8 by itself or followed by a non-nociceptive stimulation. Compared with saline, intraplantar injection of a dose of Bv8 that causes only local hyperalgesia left Fos staining unchanged. Nevertheless, after a non-noxious stimulation, Fos-immunopositive neurons significantly increased in laminae I-II, which correspond to the projection sites of peptidergic nociceptive fibers expressing CGRP and trkA receptors. A Bv8 dose that produces systemic hyperalgesia significanly increased the the number of Fos-positive neurons, in respect to control rats, both in laminae I-II and in laminae III-VI. This trend was amplified three times after a non-noxious stimulation2.
CGRP appears to be a component of Bv8-induced nociceptive sensitisation. CGRP is expressed by small and medium sized cells in the DRG, and CGRP release in the spinal dorsal horn is a critical component of nociceptive transmission. As previously described, about 50% of Bv8-responding neurons contain CGRP. We have demonstrated that Bv8 elicited a significant and concentration-related release of CGRP in minced dorsal lumbar spinal cord of mice. In rats, intrathecal injection of Bv8 (50 fmol/rat, i.t.) resulted in an upregulation of CGRP and substance P immunoreactivity in lumbar dorsal horn and in DRG. Pretreatment with anisomycin (protein-synthesis inhibitor) significantly blocked the Bv8-induced second phase of hyperalgesia, and also blocked Bv8-induced upregulation of immunoreactivity for CGRP and SP3. These data indicate that Bv8-induced hyperalgesia results, at least in part, from increased expression and release of excitatory transmitters in the spinal dorsal horn.
PHYSIOLOGICAL ROLE OF THE PK/PKR SYSTEM IN THE NEUROBIOLOGY OF PAIN: PKR1-KO MICE
To evaluate the role of the newly identified Bv8/PK and their receptor system in the neurobiology of pain, we studied the nociceptive behaviour of mice lacking prokineticin receptors [31]. Unfortunately PKR2-KO mice do not survive beyond weaning. PKR1-KO mice appeared as healthy as wild type mice, with sensory ganglion development being apparently unaltered, functionally, however, disruption of the PKR1 gene produced an array of specific defects related to nociception.
When compared with wild-type litter mates, mice lacking the PKR1 gene showed impaired responsiveness to noxious heat, capsaicin and protons. Nociceptive deficits in PKR1 null mice were significant within the noxious temperature range from 46° to 50°C, the operating range of C-polymodal nociceptors and the vanilloid channel TRPV1 [42]. In the hot-plate and tail-flick test at temperatures of 46° and 48°C, the baseline latency for response was significantly longer (1.5- to 2.3-fold) than the latencies seen in wild-type mice. No differences were found in the baseline latencies of the two genotypes at temperatures of 50° and 52°C.
Disruption of the PKR1 gene significantly impaired nociceptive responses to intraplantar and oral capsaicin. KO mice were less sensitive than WT mice to capsaicin injected into foot pads, as revealed by the licking test, and less sensitive to capsaicin added in scalar doses to sweetened drinking water. Indeed, PKR1-KO mice drank higher volumes of solutions than WT mice. Moreover, in comparison with WT mice, PKR1-null mice also showed a strong reduction of capsaicinevoked hypothermia. Injection of dilute acetic acid into the peritoneum of the two mouse genotypes induced significantly less intense and shorter-lasting writhing episodes in PKR1-null mice than in wild-type mice, supporting an essential expression of PKR1 for TRPV1 activation.
PKR1-null mice also exhibited impaired development of hyperalgesia after tissue injury: the inflammatory agents CFA and mustard oil produced comparable paw oedema in WT and PKR1-null mice, but the decrease in nociceptive threshold to heat (hot-plate and paw immersion test) and to pressure (incapacitance test) was significantly lower in KO than in WT mice demonstrating lower nociceptive sensitisation in KO than in WT mice.
As expected, PKR1 null mice exhibited impaired hyperalgesic responses to Bv8. Moreover, they did not show the increase in the capsaicin-induced licking after intraplantar injection of Bv8 already described in WT mice.
In DRG cultures from PKR1-null mice, Bv8-responsive neurons were rare and showed a reduced [Ca++]i response to capsaicin. Accordingly, in situ hybridisation of DRG sections from PKR1-KO mice confirmed the lack of PKR1, but PKR2 were still expressed in some large neurons. This finding provides an anatomical basis for the residual hyperalgesic effect of Bv8, which in KO-mice can still produce the classic biphasic hyperalgesia, but only at doses a hundred times higher than in WT mice.
These findings indicate a requirement for PKR1 signalling associated with activation and sensitisation of primary afferent fibres. Moreover, activation of TRPV1 seems to depend in large part upon the presence of PKR1. This positive co-operative interaction between PKR1 and TRPV1, together with the Bv8-induced increase in sensitivity to PGE2, suggests that blockade of PKR1 may represent a novel strategy which can diminish the activation and sensitisation of primary afferent nociceptors, thus bringing therapeutic benefit in acute and inflammatory pain conditions.
PROKINETICINS AND PAIN
Where do the endogenous agonists of the algogenic PKR1 and PKR2 come from?
Lymphoid organs, circulating leucocytes and hematopoietic cells express high levels of Bv8-like proteins [23]. By RT-PCR amplification we demonstrated that mouse inflammatory neutrophils (obtained from animals with oysterglycogen-induced peritonitis) express high levels of PK1, PK2, PK2L (the long form of PK2 already described) and both receptors. Rat inflammatory granulocytes express the two forms of PK2 (PK2 and PK2L), but neither PK1 and only one receptor: PKR2. In human circulating neutrophils and in a human promyelocytic cell line (HL60) we detected the two forms of PK2 (PK2 and PK2L), but not PK1 nor any receptor (Fig. 2B). To check whether the Bv8-like proteins of granulocytes maintain the same biological activity as amphibian Bv8, we injected in rats the granulocyte extract preincubated overnight with normal rabbit serum or with an anti-Bv8 antibody raised in rabbits. The granulocyte-extract produced intense hyperalgesia with a time course identical to that of amphibian Bv8. This hyperalgesic effect was abolished by preincubation with the antiBv8-Ab. The granulocyte extract was fractionated using ionic exchange chromatography (Mini S column), gel filtration (Superdex column) and reverse phase chromatography (RP C8 column). A Bv8-like activity eluted from the RP column with a slightly longer retention time than Bv8, accordingly with a higer hydrophobicity of PK2 than Bv8 (9 KDa, pI = 8.85, 81aa vs 8 KDa, pI = 8.4, 77aa). This fraction displaced 125I-MIT binding on PKR1-transfected CHO cell membranes, and produced the Bv8-characteristic biphasic hyperalgesia when injected i.t. in rats. Starting with ~ 1x109, the estimated yield in Bv8 was ~160 ng (manuscript in preparation). Next targets will be to determine the amino acid sequence of this Bv8-like compound.
In situ hybridisation experiments were performed with samples from inflamed tonsil or appendix [23] or from rat CFA inflamed hind paw. The Bv8/PK signal was associated with infiltrating cells, predominantly neutrophils. RT-PCR evaluation of the expression of PK2, PK2L and PKR1 in the rat inflamed paw, 24 h after CFA injection, compared with the contralateral saline-treated paw showed that inflammation induces a strong increase in both isoforms of PK2 and in PKR1. Hence we hypothesise that the PKs released by inflammatory cells, bind the PKRs on the primary sensitive neurons thus contributing to inflammatory pain.
Bv8/PK proteins can promote survival and differentiation of the granulocytic and monocytic lineages. They potentially modulate growth, survival and function of cells of the innate and adaptive immune system [23]. Murine macrophages and splenocytes express PKR1 in large amounts. We demonstrated [27] that Bv8 is able to induce the macrophage to migrate and to acquire a pro-inflammatory phenotype: Bv8 is a potent and efficaceous chemoattractant, since it stimulates macrophage migration at a concentration as low as 10-12 M. Bv8 significantly increases the lipopolysaccharide (LPS)-induced production of proinflammatory cytokines (IL-1 and IL-12) and decreases that of anti-inflammatory cytokines (IL-10) leaving TNF unaffected. Moreover, Bv8 stimulates T lymphocyte differentiation towards a Th1 profile. The effects start at the very low concentration of 10-11M. Studies conducted using PKR1 knock-out mice clearly indicate that all the activities exerted by Bv8 on macrophages and splenocytes are mediated by PKR1. Considering that the foregoing immune cells produce Bv8/PKs and possess specific PKRs, these molecules could act as a paracrine/autocrine factor that modulates immune and inflammatory processes.
Evidence now suggests that the cytokines Interleukin (IL)1 and Tumor Necrosis Factorα (TNFα) are deeply involved in peripheral nerve and neural excitability leading to the development of persistent pain. Conversely, the anti-inflammatory cytokines have been reported to limit the hyperalgesic response induced by inflammatory stimuli and by the administration of IL-1 and of TNF [40, 44]. A possible outcome of the Bv8/PK-induced modulation of immune and inflammatory processes could be a transition from acute to chronic inflammation. Hence, Bv8/PK-induced modulation of cytokines could be yet another element contributing to its hyperalgesic activity.
CONCLUSION
Bv8 sensitises peripheral nociceptors and activates neutrophils and macrophages through binding to the prokineticin receptor-1 and -2. Bv8 stimulates cytokine release from immunocompetent cells. Bv8-like peptides isolated from inflammatory granulocytes induce hyperalgesia comparable to that induced by Bv8 injections. The potent action of Bv8, both in vivo and in vitro, together with the specific expression of receptors for Bv8/PKs in sub-populations of nociceptive neurons, strongly suggests that PK1 and PK2, the mammalian homologues of Bv8, play a role in physiological hyperalgesia following injury, infection and inflammation.
The signalling molecules expressed by peripheral nociceptors and inflammatory cells are potential targets for drugs, which block the nociceptive information before it reaches the brain. Identifying of the structural determinants required for receptor binding and hyperalgesic activity of Bv8 was the first step in a search for PKR-antagonist molecules. The highly conserved amino terminal sequence (AVITGA) of all members of the Bv8/PK family is important for their biological activity: deletions and substitutions in the conserved N-terminal sequence of Bv8 and of PK1 yielded inactive and, sometimes, antagonist molecules [2, 32]. We have demonstrated that a N-terminally shortened form of Bv8, desAlaVal-Bv8, succeeded in antagonising Bv8-induced hyperalgesia [32].
ACKNOWLEDGEMENTS
This work was supported by grants from the Italian Ministry of University and Scientific Research (PRIN 2004057339, PRIN 2004037781) and from the University of Rome, “La Sapienza”.
Footnotes
Negri L, Lattanzi R, Giannini E and Melchiorri P. “Nociceptor sensitization by the secretory protein Bv8” XIV World Congress of Pharmacology. San Francisco (CA) July 7-12 2002
Rodella LF, Borsani E, Negri L, Melchiorri P. “Fos and pain: a role of Bv8 modulation” National Congress of Italian Society for Neuroscience and Joint Italian-Sweedish Neuroscience Meeting. Ischia (Napoli), October 1-4, 2005.
DeFelice M Porreca F, Melchiorri P and Negri L. “Mechanisms of Bv8-induced hyperalgesia: increased evoked excitatory transmitter release” XXXII Congress of Italian Society of Pharmacology, Napoli, June 1-4, 2005.
REFERENCES
- 1.Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 2.Bullock CM, Li JD, Zhou QY. Structural determinants required for bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 4.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol Pharmacol. 2005;67:2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 6.Cheng MJ, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 7.Chopra B, Giblett S, Little JG, Donaldson LF, Tate S, Evans RJ, Grubb BD. Cyclooxygenase-1 is a marker for a subpopulation of putative nociceptive neurons in rat dorsal root ganglia. Eur J Neurosci. 2000;12:911–920. doi: 10.1046/j.1460-9568.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- 8.Dorsch M, Qiu Y, Soler D, Frank N, Duong T, Goodearl A, O’neil S, Lora J, Fraser C. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–34. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 9.Erspamer V. Bioactive secretions of the Amphibian integument. In: Heatwole H, editor. Amphibian Biology. Surrey Beatty & Sons Publ; 1994. pp. l78–350. [Google Scholar]
- 10.Farkas-Szallasi T, Lundberg JM, Wiesenfeld-Hallin Z, Hokfelt T, Szallasi A. Increased levels of GMAP, VIP and nitric oxide synthase, and their mRNAs, in lumbar dorsal root ganglia of the rat following systemic resiniferatoxin treatment. Neuroreport. 1995;6:2230–2234. doi: 10.1097/00001756-199511000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Le Coutre J, Lin R, Peale F. EG-VEGF AND Bv8: a novel family of tissue-restricted angiogenic factors. Biochem Biophys Acta. 2003;1654:69–78. doi: 10.1016/j.bbcan.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- 13.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 15.Jancso G, Karcsu S, Kiraly E, Szebeni A, Toth L, Bacsy E, Joo F, Parducz A. Neurotoxin induced nerve cell degeneration: possible involvement of calcium. Brain Res. 1984;295:211–216. doi: 10.1016/0006-8993(84)90969-7. [DOI] [PubMed] [Google Scholar]
- 16.Joubert FJ, Strydom DJ. Hoppe Seylers Z Physiol Chem. Vol. 361. 1980. Snake venom. The amino acid sequence of Protein A from Dendroaspis polylepsis (black mamba) venom; pp. 1787–1794. [DOI] [PubMed] [Google Scholar]
- 17.Kaser A, Winklmayr M, Lepperdinger G, Kreil G. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep. 2003;4:469–473. doi: 10.1038/sj.embor.embor830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisliouk T, Podlovni H, Meidan R. Unique expression and regulatory mechanisms of EG-VEGF/prokineticin-1 and its receptors in the corpus luteum. Ann Anat. 2005;187:529–537. doi: 10.1016/j.aanat.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Lattanzi R, Giannini E, Melchiorri P, Negri L. Pharmacology of Bv8: a new peptide from amphibian skin. Br J Pharmacol. 2001;133:45P. [Google Scholar]
- 20.Lattanzi R, Giannini E, Negri L. Bv8, a small protein from frog skin induces antidiuresis in rats. Pharmacol Res. 2001;43(A):30. [Google Scholar]
- 21.LeCouter J, Ferrara N. EG-VEGF and Bv8: a novel family of tissue-selective mediators of angiogenesis, endothelial phenotype and function. TMC. 2003;13:276–282. doi: 10.1016/s1050-1738(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 22.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, Deguzman L, Keller G, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:876–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 23.Lecouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. PNAS. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharm. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 25.Lin Dch, Bullock CM, Ehlert FJ, Chen Jl, Thian H, Zhou QY. Identification and molecular characterization of two closely related G-protein coupled receptors activated by prokineticins/EG-VEGF. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 26.Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- 27.Martucci C, Franchi S, Giannini E, Tian H, Melchiorri P, Negri L, Sacerdote P. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol. 2006;147:225–234. doi: 10.1038/sj.bjp.0706467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Comm. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 29.Melchiorri D, Bruno V, Besong G, Ngomba R, Cuomo L, Deblasi A, Copani A, Moschella C, Storto M, Nicoletti F, Lepperdinger G, Passarelli F. The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur J Neurosci. 2001;13:1694–1702. doi: 10.1046/j.1460-9568.2001.01549.x. [DOI] [PubMed] [Google Scholar]
- 30.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homolog from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 31.Negri L, Lattanzi R, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, De Felice M, Porreca F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negri L, Lattanzi R, Giannini E, Colucci A, Mignogna G, Donatella Barra D, Grohovaz F, Codazzi F, Kaiser A, Kreil G, Melchiorri P. Biological activities of Bv8 analogues. Br J Pharmacol. 2005;146:625–632. doi: 10.1038/sj.bjp.0706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol. 2004;142:141–151. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P. Nociceptive sensitisation by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 36.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase C-epsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 37.Robbins RA, Grisham MB. Nitric oxide. Int J Biochem Cell Biol. 1997;29:857–860. doi: 10.1016/s1357-2725(96)00167-7. [DOI] [PubMed] [Google Scholar]
- 38.Schweitz H, Bidard JN, Lazdunski M. Purification and pharmacological characterization of peptide toxins from the black mamba (Dendroaspis polylepsis) venom. Toxicon. 1999;28:847–856. doi: 10.1016/s0041-0101(09)80007-x. [DOI] [PubMed] [Google Scholar]
- 39.Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Molecular cloning and characterization of prokineticin receptors. Biocem Biophis Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 40.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- 42.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 43.Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 45.Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 1999;462:177–181. doi: 10.1016/s0014-5793(99)01473-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Verge V, Wiesenfeld-Hallin Z, Ju G, Bredt D, Synder SH, Hokfelt T. Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol. 1993;335:563–575. doi: 10.1002/cne.903350408. [DOI] [PubMed] [Google Scholar]