Abstract
Cannabis extracts and synthetic cannabinoids are still widely considered illegal substances. Preclinical and clinical studies have suggested that they may result useful to treat diverse diseases, including those related with acute or chronic pain. The discovery of cannabinoid receptors, their endogenous ligands, and the machinery for the synthesis, transport, and degradation of these retrograde messengers, has equipped us with neurochemical tools for novel drug design. Agonist-activated cannabinoid receptors, modulate nociceptive thresholds, inhibit release of pro-inflammatory molecules, and display synergistic effects with other systems that influence analgesia, especially the endogenous opioid system. Cannabinoid receptor agonists have shown therapeutic value against inflammatory and neuropathic pains, conditions that are often refractory to therapy. Although the psychoactive effects of these substances have limited clinical progress to study cannabinoid actions in pain mechanisms, preclinical research is progressing rapidly. For example, CB1mediated suppression of mast cell activation responses, CB2-mediated indirect stimulation of opioid receptors located in primary afferent pathways, and the discovery of inhibitors for either the transporters or the enzymes degrading endocannabinoids, are recent findings that suggest new therapeutic approaches to avoid central nervous system side effects. In this review, we will examine promising indications of cannabinoid receptor agonists to alleviate acute and chronic pain episodes. Recently, Cannabis sativa extracts, containing known doses of tetrahydrocannabinol and cannabidiol, have granted approval in Canada for the relief of neuropathic pain in multiple sclerosis. Further double-blind placebo-controlled clinical trials are needed to evaluate the potential therapeutic effectiveness of various cannabinoid agonists-based medications for controlling different types of pain.
Key Words: Analgesia, cannabidiol, cannabinoid receptor, cannabis, endocannabinoid, inflammatory pain, neuropathic pain, tetrahydrocannabinol
INTRODUCTION
Hemp, Cannabis sativa, is a coarse bushy annual plant with palmate leaves and clusters of small green flowers that grows wild in regions of mild or tropical weather and can attain a height of 3 metres. The genus name Cannabis is complemented by sativa (which means useful). Cannabis has indeed been used throughout history for a variety of purposes, including the production of fibre for paper and textile manufacture. However, its current popularity lies in its use as a recreational drug with psychoactive properties. The plant contains many chemical compounds that have different pharmacological properties, varying in quantity and quality depending on the strain and culture and storage conditions. Extracts of the dried flowers, buds, or leaves are known as either cannabis (British term) or marijuana (North American term, probably originating from Mexican slang). Hashish is made from a resin secreted by the flowers of female plants. Consumption of cannabis derivatives (by smoking, eating, or drinking) produces euphoria, relaxation, a general sense of well being, and time distortion. Heavy consumption may precipitate hallucinations, anxiety, depression, and psychoses.
Cannabis has been utilised for centuries throughout the world to alleviate disease. Its derivatives were named “panacea”, or “cure-all”, and were sold as a legal medicine, mainly for pain [12, 135]. In the United States, cannabis has been illegal since 1937. In 1961, cannabis was added to the United Nations Single Convention on Narcotic Drugs (amended in 1972) and to Article 33 of the United Nations Convention on the Rights of the Child. One hundred fifty countries ratified theseConventions. Nonetheless, drug policies have varied. For example, during the late 1990s, 13 states of the United States made laws that permit cannabis for medical necessity, requiring to be prescribed by physicians; and being especially used to relieve AIDS patients treatment side effects. Regarding recreational use in Europe, some countries are being permissive, like Switzerland, Portugal, Spain, Italy, and Belgium, and others strict, such as Sweden, where it is illegal to consume cannabis. In the Netherlands, cannabis has long been decriminalised. The widely held view of cannabisrelated products as drugs of abuse has slowed progress in the development of studies designed to take advantage of the properties of cannabinoid compounds for therapeutic purposes. Nonetheless, the discovery of the endogenous cannabinoid system in the early 1990s renewed interest in this field and strongly stimulated research. In 1964, Mechoulam and colleagues [42] found that delta-9-tetrahydrocannabinol (THC) was the major psychoactive ingredient of cannabis. THC causes a variety of effects in different animal species, such as antinociception, hypoactivity, catalepsy, hypothermia, and cardiovascular changes. In the late 1980s, Howlett and colleagues [29] identified and characterised a receptor in rat brain that met criteria for a high-affinity, stereoselective, pharmacologically distinct cannabinoid receptor, by means of radiolabelled agonist ligand binding and functional assays for G-protein coupled receptors. Moreover, they found a correlation between the potency of cannabinoid compounds in producing analgesia in vivo and the inhibition of adenylcyclase in vitro, indicating the presence of a G-proteincoupled “cannabinoid analgesic receptor” in brain [64]. A few years later, in 1990, the cannabinoid type 1 (CB1) receptor was cloned from brain tissue [100], followed by cloning of the type 2 (CB2) cannabinoid receptor in 1993 [108]. Meanwhile, a torrent of new discoveries has yielded insight into the components and mechanisms of action of the endogenous cannabinoid system. Consequently, some endogenous ligands of cannabinoid receptors have been investigated, as well as their synthesis, transport, and degradation [for reviews see: 39, 120]. A number of synthetic cannabinoid receptor agonists and antagonists have been formulated and genetically modified mice have been produced [13, 27, 86]. Altogether, research has provided valuable tools for developing new pharmacologic cannabinoid products. One of the more promising applications appears to be the use of cannabisbased medicines to relieve pain, given the antinociceptive and anti-hyperalgesic effects of cannabinoid receptor agonists observed in animal models. Unfortunately, the underlying potential for abuse and dependence after heavy cannabis consumption is still a drawback in the search for effective pharmacologic preparations, which ideally would have minimal or no psychoactive side effects.
In this review, we will describe the different components of the endogenous cannabinoid system and their mechanisms of action, with especial emphasis on those implicated in analgesic effects. We will also discuss agents that may interfere pharmacologically with cannabinoid functions to enhance their antinociceptive effects and clinical conditions in which cannabinoids hold promise for effective therapeutic applications.
ENDOGENOUS CANNABINOID SYSTEM
Endogenous Cannabinoid Receptors
The biological effects of cannabinoid compounds are mediated by their binding to and further activation of cannabinoid receptors. Four subtypes of these receptors have been identified. Two have been cloned, type 1 (CB1) and type 2 (CB2) cannabinoid receptors [100, 108], while the other two, WIN and abnormal-cannabidiol (abn-CBD) receptors (the latter also known as anandamide receptor), have been characterised pharmacologically [8, 31, 51, 71, 107]. In addition, some truncated forms of the CB1 receptor, like the CB1A, have been found, resulting from alternative splicing [144]; and there may be more subtypes of cannabinoid receptors yet undiscovered [8, 51].
Cannabinoid receptors are Gi/o-protein coupled receptors anchored in the cell membrane. Structurally they consist of seven folded transmembrane helices with intra-and extracellular loops, functionally involved in signal transduction. The CB2 receptor is located mainly in the immune system, but has been found in others sites, as in keratinocytes [67]. On the other hand, the CB1 receptor, which is the cannabinoid receptor that has been most studied, has high levels in brain but also lower levels in spinal and peripheral nervous tissue (including areas important for pain perception, as will be discussed below). CB1 receptors are also disseminated in several other non-nervous tissues like endothelial cells, uterus, and others.
Neuroanatomic Distribution of CB1 Receptors in the Nervous System
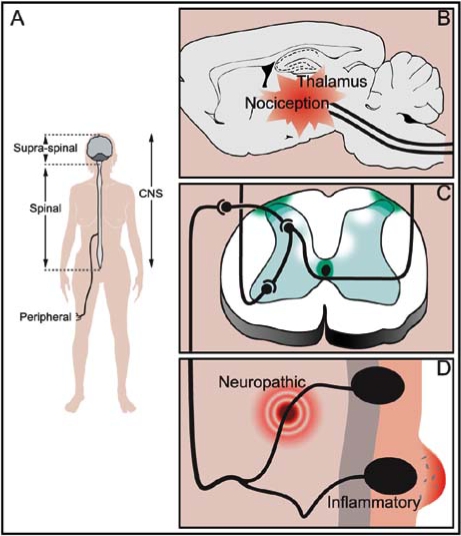
CB1 receptors are abundant and widely dispersed throughout the brain. Their distribution has been mapped by autoradiographic studies, immunohistochemical techniques, in situ histochemistry, and electrophysiological studies [53, 78, 140]. CB1 receptors have shown particularly high levels of expression in cortex, basal ganglia, hippocampus, and cerebellum and low levels of expression in brainstem nuclei. They are present in brain areas involved in nociceptive perception, such as the thalamus and amygdala (Fig. 1B) [93, 99]. CB1 receptors are also expressed in cells of the midbrain periaqueductal grey matter (PAG), and in the substantia gelatinosa of the spinal cord (receiving nociceptive input from primary afferent neurons), which are key sites for modulating nociceptive information [87, 92, 106]. In the medulla oblongata and spinal cord, structures involved in processing pain signals, more dense concentrations of CB1 receptors are detected in the superficial dorsal horn, and in the dorsolateral funiculus of the spinal cord (Fig. 1C) [35, 53, 59, 140, 159]. CB1 receptors of the spinal cord dorsal horn are predominantly found in interneurons, particularly in a double band of CB1 immunoreactivity in laminae I, II, and inner/III transition, and in lamina X [35]. In the superficial dorsal horn of rats, CB1 receptors are located primarily on the axons of intrinsic interneurons [35, 159], indicating a presynaptic site of action that is consistent with modulation of neurotransmitter release by endocannabinoids. Furthermore, CB1 receptors are synthesised in neurons of the rat dorsal root ganglia (that express neuropeptide markers found in nociceptive primary afferents) [59], and these receptors are transported both centrally, reaching superficial dorsal horn terminals [59] and peripherally towards peripheral nerve terminals of sensory nerves [58]. Interestingly, these sensory nerves are engaged in the ascent of nociceptive stimuli to the spinal cord (Fig. 1A, C, D). On the other hand, although CB1 receptor mRNA expression has been described in the trigeminal ganglia in medium and large diameter neurons, the majority of these CB1-expressing neurons do not seem to be involved in nociceptive neurotransmission in the noninjured animal [122]. Finally, CB1 receptors are found on only a small percentage of C-fibres, while the majority are on axons of larger diameter neurons with myelinated Afibres [11]. The described anatomical distribution of CB1 receptors is consistent with their function of modulating pain perception at both peripheral and central (spinal and supraspinal) levels (Fig. 1).
Fig. (1).
Schematic representation of cannabinoid receptors distribution. A: Cannabinoid receptors are present in the pain pathway at the peripheral and central (spinal and supraspinal) levels. B: Supraspinal CB1 receptors are distributed in areas of the brain and brainstem nuclei involved in nociceptive perception as thalamus, amygdala, and periaqueductal grey matter. C: The highest abundance of spinal CB1 receptors are found in the dorsolateral funiculus, in the surroundings of the central canal and in the superficial dorsal horn. D: CB1 receptors are also present in the peripheral sensory nerve endings, and both CB1 and CB2 receptors have been detected in non-neuronal cells participating in immune and inflammatory processes in the proximity of the primary afferent neurons nerve terminals.
Endocannabinoids
The endocannabinoids, or endogenous cannabinoids, are a family of bioactive lipids that activate cannabinoid receptors to exercise their effects, modulating neural transmission. They are present in only small amounts in brain and other tissues and participate in the regulation of various cerebral functions, including pain perception, mood, appetite, and memory. Exogenously administered cannabinoid compounds of natural or synthetic origin mimic their effects. Even though we still have much to learn about the relative roles of different endocannabinoids, they appear to be promising potential targets for manipulation, for instance, to slow their degradation for analgesic proposes. Endocannabinoids possess submicromolar affinity for cannabinoid receptors and act as retrograde signal molecules in synapses. Despite the similarity of their chemical structures, endocannabinoids are produced by their own biochemical pathways. They are synthesised locally on demand in postsynaptic terminals, which requires Ca2+ influx, and released in selected regions to activate presynaptic cannabinoid receptors situated in specific small areas (Fig. 2). Thereafter, they are rapidly inactivated by a combination of a transporter mechanism and further enzyme degradation [32, 44, 118]. Noxious stimuli increase endocannabinoid release [168], which supports that they intervene in pain modulation.
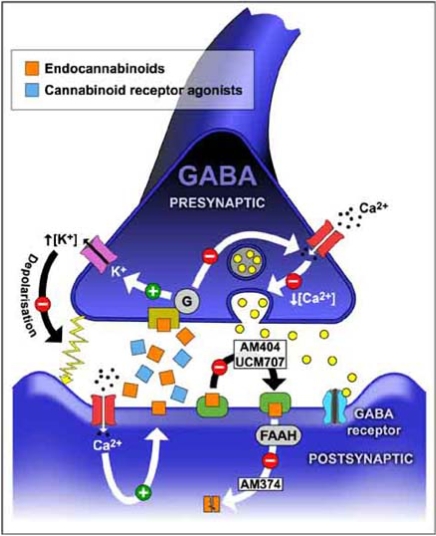
Fig. (2).
Schematic representation of a GABAergic synapsis, containing CB1 receptors, to show potential targets for therapeutic intervention. Endocannabinoids are synthesised in membranes of neurons and other nervous cells and released to the synaptic space to activate presynaptic CB1 receptors. Enhancement of cannabinoid receptors activity can be obtained by different pharmacological manipulations as, for example, administering exogenous cannabinoid receptor agonists or inhibiting either the reuptake or the degradation of the endocannabinoids.
The first endocannabinoid isolated (from porcine brain) and structurally characterised was arachidonylethanolamide (AEA), commonly designated anandamide [30]. The name comes from the Sanskrit word ananda, which means “bliss”, and amide. Bliss means happiness that invokes physiologic and psychologic harmony and, in Buddhism, indicates an elevated consciousness since Ananda was one of the principal disciples of the Buddha. Anandamide acts in pain, depression, appetite, memory, and fertility (due to its uterine synthesis). Anandamide is synthesised enzymatically in brain areas that are important in memory and higher thought processes, and in areas that control movement. Anandamide, or arachidonylethanolamide, is an amide derivative of arachidonic acid and ethanolamine. It is synthesised by hydrolysis of the precursor N-arachidonoyl phophatidylethanolamine, which is catalysed by the enzyme phosphodiesterase phospholipase D [16, 32]. After release from the postsynaptic terminal, anandamide interacts with presynaptic cannabinoid receptors. It is rapidly removed from the synaptic space by a high-affinity transport system present in neurons and astrocytes. Once internalised, anandamide is hydrolysed by the enzyme fatty-acid amide hydrolase (FAAH), an intracellular membrane-bound enzyme. In cerebellum, hippocampus, and neocortex, FAAH is expressed at high levels in the somatodendritic regions of neurons postsynaptic to CB1-positive axon terminals. Thus CB1 receptors and FAAH have a close and complementary anatomical distribution [34].
Other endogenous ligands for cannabinoid receptors are 2arachidonoylglycerol (2-AG), noladin ether, virodhamine, and N-arachidonoyldopamine (NADA) [103, 121, 168]; although anandamide is the endocannabinoid most exten-sively studied.
The endogenous cannabinoid 2-AG is produced by neurons and other nervous cells in a stimulus-dependent manner, binds to cannabinoid receptors and undergoes rapid biological inactivation through transport into cells and catalytic hydrolysis [103, 151]. It is synthesised by cleavage of an inositol-1,2-diacylglycerol, which is catalysed by phospholipase C [16, 32]. The levels of 2-AG found in some regions of the brain are much higher than anandamide levels, and the biosynthesis of thesemolecules is controlled separately [120].
Noladin ether, or 2-arachidonylglyceryl-ether, has been isolated from porcine brain and is an endocannabinoid that binds to CB1 cannabinoid receptor to signal food consumption and weight control [52].
Virodhamine is a partial agonist with in vivo antagonistic activity at the CB1 receptor, but acts as a full agonist on the CB2 receptor. Its concentrations are higher than those of anandamide, but its ability to alter pain sensitivity has not been tested. [168].
NADA was identified in rat and bovine brain [65], activates CB1 (and VR1 receptors) and elicits cannabimimetic effects, including analgesia, following systemic administration. It interacts with FAAH and the anandamide transporter. The highest levels are found in the striatum and hippocampus, and at low levels in the dorsal root ganglia [65, 168].
CANNABINOID PHARMACOLOGY
In this section we will describe the mechanisms of action and the effects that occur after endocannabinoids bind to their specific receptors, while highlighting their role in pain modulation.
Activated CB1 receptors couple to Gi/o-protein to inhibit adenylate cyclase, decrease Ca2+ conductance, increase K+ conductance, and increase mitogen-activated protein kinase activity [for review see: 63]. The presynaptic localisation of CB1 receptors indicates that cannabinoids modulate neurotransmitter release from axon terminals. The effect of cannabinoids on synaptic function consists of inhibition of the release of a variety of neurotransmitters and also the inhibition of electrical activity by a depolarisation phenomenon (Fig. 2) [79, 141]. The neurotransmitters whose release is inhibited by activation of cannabinoid receptors include L-glutamate, GABA, noradrenaline, dopamine, serotonin, and acetylcholine. Therefore, depending on the nature of the presynaptic terminal, endocannabinoids induce either suppression of inhibition or suppression of excitation, namely depolarisation-induced suppression of inhibition (DSI) or of excitation (DSE) [84, 85]. CB1 receptor antagonists block DSI and DSE. However, if the CB1 receptor agonist remains present, the depolarisation phenomenon is blocked by occlusion [115, 175] and inhibitory inputs are transient. This is why cannabinoid receptor agonists cannot mimic the same physiologic effects of locally released endocannabinoids. Since they cause long-lasting activation of CB1 receptors in all brain regions, their overall effect is persistent inhibition of neurotransmitter release from nerve endings that express CB1 receptors and, as a consequence, they temporarily occlude and prevent DSI and DSE phenomena. On the contrary, endocannabinoids are involved in the rapid modulation of synaptic transmission in the central nervous system by a retrograde signalling pathway that can influence synapses in a local region, with inhibitory effects on both excitatory and inhibitory neurotransmitter release that persist for tens of seconds. This may be important in the control of neural circuits, such as nociceptive signalling.
Activation of cannabinoid receptors inhibits GABAergic synaptic transmission in a number of central nervous system regions, including areas participating in nociceptive signalling like the amygdala [93], periaqueductal grey matter [162], rostral ventromedial medulla (RVM) [163], and superficial dorsal horn [72]. Depolarisation of the postsynaptic neurons leads to DSI by inhibition of GABA release. However, even though cannabinoid receptor-mediated inhibition of glutamatergic transmission occurs in some brain regions including PAG neurons [162], it has not been detected in the medullary dorsal horn [143, 154, 162].
ANALGESIC EFFICACY OF CANNABINOIDS
Activation of Cannabinoid Receptors
Cannabinoid receptor agonists effects in the central nervous system (CNS) include disruption of psychomotor behaviour, short-term memory impairment, intoxication, stimulation of appetite, antiemetic effects, and antinociceptive actions [68]. Administration of natural or synthetic cannabinoid receptor agonists has shown therapeutic value for a number of important medical conditions, including pain (particularly against pain of neuropathic origin), anxiety, glaucoma, nausea, emesis, muscle spasms, and wasting diseases. Insofar as pain is concerned, it is well known that cannabinoid receptor agonists have antinociceptive and anti-hyperalgesic effects at the peripheral and central (spinal and supraspinal) levels, as has been demonstrated in acute and chronic pain models [69, 116]. Cannabinoid receptors and endocannabinoids are present in pain circuits from the peripheral sensory nerve endings up to the brain (Fig. 1). Cannabinoid receptor agonists modulate nociceptive thresholds by regulating neuronal activity [4], but they also relieve pain by acting on non-nervous tissues. CB1 receptor is involved in the attenuation of synaptic transmission, and a proportion of the peripheral analgesic effect of endocannabinoids can be attributed to a neuronal mechanism acting through CB1 receptors expressed by primary afferent neurons. However, recent findings suggest that CB1 receptors are also present in mast cells and may participate in some anti-inflammatory effects. Thus, activated CB1 receptors present in mast cells induce sustained cAMP elevation, which, in turn, suppresses degranulation [146]. On the other hand, although CB2 receptors have been related traditionally to the peripheral effects of cannabinoids (mainly modulation of the immunologic responses), they also contribute to antinociception by inhibiting the release of proinflammatory factors by non-neuronal cells located near nociceptive neuron terminals. CB2 receptors are expressed in several types of inflammatory cells and immunocompetent cells. For that reason, activation of peripheral CB2 receptors generates an antinociceptive response in situations of inflammatory hyperalgesia and neuropathic pain [66, 160], while selective CB2 receptor agonists are not antihyperalgesic against chronic inflammatory pain in CB2 knockout mice [160]. Possible mechanisms of this CB2-mediated effect include the attenuation of NGF-induced mast cell degranulation and of neutrophil accumulation, both of which are processes known to contribute to the generation of inflammatory hyperalgesia [131]. Therefore, since activation of CB1 receptors is associated with central side effects, including ataxia and catalepsy, selective CB2 receptor agonists have the potential to treat pain without eliciting the centrallymediated side effects. Furthermore, CB2 receptors have novel pain control actions. A CB2-mediated effect exists, consisting in the indirect stimulation of opioid receptors located in primary afferent pathways [67], as will be described in more detail in the next section. Thus, cannabinoid compounds can modulate hyperalgesia of various origins and they are effective even in inflammatory and neuropathic pain [10, 133], which are conditions often refractory to treatment. In the CNS, although CB2 receptor mRNA has not been detected in the neuronal tissue of human or rat brain, a role in antinociception in inflammatory processes of the nervous system cannot be excluded due to its presence in activated microglia [166].
Animal Models of Pain
Different validated animal models are used to explore the analgesic effects of cannabinoid compounds. However, since most responses are quantified by behavioural tests, it is important to remember that cannabinoids may inhibit or enhance motor activity [139], depending on the dose and structure of the compound administered, which may influence behavioural reactions and mask the results of analgesic tests. In order to counteract these effects, complementary analyses are used to demonstrate the antinociceptive effects of cannabinoids. In this context, cannabinoids block spinal c-fos expression in response to noxious stimulation and suppress the electrophysiologic responses of spinal cord neurons [69, 116, 166]. In the spinal cord lamina receiving primary afferent fibres, noxious stimuli enhance c-fos expression, making it a good marker for spinal nociceptive activity. Following noxious heat stimulation, cannabinoid receptor agonists diminish stimulation in deep dorsal horn neurons, while the CB1-specific antagonist SR141716A facilitates nociceptive responses [18, 60]. Temporary inactivation of neural activity in the RVM in rat brainstem circumvents the analgesic effects of systemically administered cannabinoids, while leaving motor activity effects untouched [104]. This reflects cannabinoid receptor agonists actions that specifically target sensory pathways passing through the RVM. Noxious stimulation evokes enhanced release of the anandamide, as observed in the PAG of brainstem [167], which is evidence that endocannabinoids modulate nociceptive information. Further confirmation of the role of the endocannabinoid system in the control of pain is that the blockade of cannabinoid receptors, whether by antagonists, antibodies, or genetic deletion, inhibits or attenuates pain perception [22, 33, 86]. Thus, the antinociceptive potency of a series of cannabinoid receptor agonists correlates strongly with their capacity to displace radioligands from the cannabinoid receptor and to inhibit adenylate cyclase. Also, cannabinoid-induced antinociception can be attenuated by pertussis toxin and other substances that interfere with the signal transduction of CB1 receptors connected to protein G [128]. Finally, cannabinoid receptors, both CB1 and CB2, are upregulated in models of chronic pain. Therefore, one response of the body to chronic pain is to increase the number of these receptors, suggesting that their function in such situations may be important. For example, animal models of neuropathic pain stimulate upregulation of CB1 receptors expression in nervous structures involved in the processing of pain, like the ipsilateral superficial spinal cord dorsal horn [90] or contralateral thalamus [145], enhancing the analgesic effects of cannabinoid receptor agonists. This upregulation of central CB1 receptors following peripheral nerve injury indicates a role for them in these pathologies and also explain the therapeutic effects of cannabinoid receptor agonists on chronic pain conditions as neuropathic pain. Chronic pain models associated with peripheral nerve injury, but not peripheral inflammation, induce CB2 receptor expression in a highly restricted and specific manner within the lumbar spinal cord. Moreover, the appearance of CB2 expression coincides with the appearance of activated microglia [178].
Sites of Pain Modulation
When investigating cannabinoid actions in the control of pain, special consideration should be given to the level at which such actions take place to determine whether the mechanisms are central or peripheral. Pain perception of a hot-plate is thought to be mediated by a central mechanism (eliciting supraspinally controlled behavioural responses to the noxious heat stimulus), whereas the second late phase of response to an intraplantar injection of formalin reflects peripheral inflammatory pain mechanisms [156]. The tailflick assay consists of induction of a spinal tail flick response to noxious thermal stimuli (spinal reflex). Therefore, the analgesic response recorded in these different acute pain models allows identification of their location.
Another crucial factor in the approach to cannabinoid analgesia would be the route of administration, which not only provides information about the site of action but also helps to improve therapeutic results through selection of the most effective route for dispensation. Systemic and intracerebroventricular administration of cannabinoid receptor agonists both produce analgesia [56, 89], implying that they exercise antinociception centrally. Cannabinoid receptor agonists produce antinociception in spinal and supraspinal sites of action, as investigated in spinally transected and intact rats. In spinally intact animals, cannabinoid receptor agonists injected intravenously produced a potent, longlasting elevation of tail-flick latencies, but spinal transection significantly attenuated antinociception [89].
The cellular actions of cannabinoids on supraspinal and spinal descending antinociception pathways have also been studied [56, 162, 163]. CB1 receptors present in the PGA and dorsolateral funiculus intervene in the important descending controls in cannabinoid-mediated analgesia [35, 167]. Cannabinoid receptor-mediated processing of nociceptive stimuli at higher (supraspinal) levels, as the thalamus and sensory cerebral cortex, may indicate the existence of more specific, finer-tuned, descending responses, which may be more effective in combating the origin of pain. Messages from the brain back to the periphery modulate the received nociceptive information by, for example, ordering release of chemicals with analgesic effects. Evidence that the cannabinoid system modulate the activity of neurons projecting from the brain to the spinal cord comes from an investigation in which CB1 receptor agonist injected in the cerebral ventricle suppressed activity evoked, by application of noxious heat to the hind paw, in wide dynamic range neurones of the lumbar dorsal horn of urethane-anaesthetized rats [56]. Moreover, when the CB1 cannabinoid agonist was given intravenously, the noxious heat-evoked activity of these neurons was not suppressed in animals with spinal transection or after administration of CB1 receptor antagonist. The existence of supraspinal antinociceptive cannabinoid actions is also confirmed by cannabinoid administration directly injected on specific brain structures, as well as by the blockade of their analgesic effect when administered simultaneously with a cannabinoid receptor antagonist. Microinjection of cannabinoids into several brain regions, including the posterolateral ventral thalamus (an area with many nociceptive neurons receiving spinothalamic pathway inputs), amygdala, RVM, and PAG, produces antinociception [87, 98, 104].
Cannabinoid antinociceptive activity at the peripheral level has been investigated by intradermal administration of agonists selective for both CB1 and CB2 receptors, as well as by the lack of analgesic effects with simultaneous administration of specific CB1 and CB2 receptor antagonists. Peripheral cannabinoid receptors are less abundant than in central nervous system areas and non-neuronal cells located near nociceptive neuronal nerve endings are involved in the modulation of pro-inflammatory factors released [66, 146, 160].
Wind-up Phenomenon
Wind-up is an exaggerated neuronal response that occurs upon repeated noxious stimulation, residing at the synapse between the primary afferent and the spinothalamic neuron. Sensitisation causes the release of more neurotransmitters at this synapse enabling better transmittance of pain signals to the spinothalamic neuron and brain. There are no reliably satisfactory treatments for pain diseases showing no evidence of peripheral abnormalities (such as myofascial pain, irritable bowl syndrome, and fibromyalgia), and these diseases may be manifestations of an exaggerated central sensitisation mechanism or wind-up. Interestingly, CB1 receptor agonists administered systemically exert analgesic effects on wind-up (elicited by transcutaneous stimulation) in the dorsal horn [56, 152]. The inhibition of wind-up is probably mediated by supraspinal actions. Intrathecal administration of cannabinoid receptor agonists also produces analgesia [89, 148], indicating spinal antinociceptive action.
Neurochemical Signalling
Superficial dorsal horn CB1 receptors are mainly present in GABAergic neurons [157]. Since these presynaptic CB1 receptors located in GABAergic neurons inhibit the GABAergic inhibitory effect, this disinhibition would result in activation of postsynaptic communication. For this reason, it has been suggested that cannabinoids are paradoxically hyperalgesic at the level of the medullary dorsal horn because of their selective inhibition of GABAergic and glycinergic transmission [56, 89], an effect that appears to be mediated by CB1 receptors located presynaptically in interneurons. However, a disinhibitory action on lamina II neurons, which emit local branches, may be necessary for modulating nociceptive information before it is transmitted to deeper laminae of the spinal cord or to higher centres. On the other hand, there is a controversy as to whether cannabinoid receptor-mediated antinociception maintains a baseline tone and, therefore, whether blockade of cannabinoid transmission will originate hyperalgesia. It has been suggested that although CB1 receptor antagonists block the antinociceptive effects of cannabinoid receptor agonists, the antagonists by themselves do not alter baseline pain thresholds [22]. In opposition, other authors suggest that there is baseline endogenous cannabinoid activity at the spinal level, as intrathecal administration of the CB1 receptor antagonist SR141716A induces hyperalgesic effects [132, 134]. Moreover, the CB1 receptor agonist WIN 55, 212-2 has no effect on either primary afferent-evoked excitatory glutamatergic transmission or postsynaptic K+ conductance from primary afferents in lamina II neurons in the medullary dorsal horn, further supporting a lack of cannabinoid influence on excitatory synapses at this spinal level [72].
CB1 receptor agonists presynaptically inhibit both GABAergic and glycinergic neurotransmission in lamina II neurons in the medullary dorsal horn, having no effect on either primary afferent-evoked excitatory glutamatergic transmission or postsynaptic K+ conductance [72]. Therefore, since the pathways used for cannabinoid receptors-mediated analgesia at the level of the superficial dorsal horn are different from those characterised for μ-opioid receptormediated analgesia, it may explain why cannabinoid receptor agonists retain their efficacy, in contrast with morphine, in animal models of neuropathic pain [95].
Vanilloid Receptor Type One (VR1)
Not all antinociceptive effects of cannabinoid compounds are mediated by cannabinoid receptors. For instance, antagonists of the CB1 receptor do not block antinociception induced by systemic administration of anandamide. This has been noted in CB1 receptor knockout mice as well. In these mice, lacking functional CB1 receptors, certain cannabinoid receptor agonists have antinociceptive effects in the hot-plate or formalin tests [179]. It has been proposed that some cannabinoid effects may be mediated by type one vanilloid receptors (VR1). These receptors have been cloned [17]. They are calcium-permeable, non-selective cation channel present in primary afferent neurons and play an important role in nociceptive responses. While low concentration of anandamide results in inhibition of neurotransmitter release from nociceptive primary sensory neurons by a CB1 receptor-mediated mechanism, high concentrations of anandamide increase the frequency of miniature excitatory postsynaptic currents recorded from substantia gelatinosa neurons by a VR1 receptor-mediated mechanism [106]. Therefore, depending on the concentrations of anandamide it would activate different receptors and produce opposite effects. This may be an important presynaptic mechanism modulating pain perception at the spinal level. Indeed, nociceptive primary sensory neurons co-express CB1 and VR1 receptors to a high degree, giving further support to a complementary role for these receptors [1]. However, the existence of undiscovered cannabinoid receptors has not been ruled out and some cannabinoid analgesic effects may be mediated in part by such receptors [8, 51].
SYNERGISM OF ANTINOCICEPTION BY CANNABINOIDS AND OTHER ANALGESIC SUBSTANCES
Cannabinoids and Opioids Analgesic Synergism
The combination of two antinociceptive drugs acting through specific receptor systems yields major benefits. When given in combination with synergistic substances, the required dose of each agent can be reduced to less than would be explained by a simple additive effect. The clinical benefit of this property is important in analgesic treatments because effective pain relief can be achieved with fewer or no side effects.
The opioid system is one of the systems interacting with the cannabinoids that has been most explored [for reviews see: 26, 41, 94]. Electrophysiologic analysis of the effects of cannabinoids on RVM has revealed that cannabinoids have effects similar to those elicited by morphine [104]. Cannabinoid and opioid receptors both exist at various levels in the pain circuits and these two systems may operate synergistically. THC and morphine have been shown to act synergistically, mutually potentiating their antinociceptive actions. Interestingly, this action is inhibited by either cannabinoid or opioid receptors antagonists separately, as demonstrated in acute [41] and chronic pain models [147, 174]. However, although cannabinoids and opioids both produce analgesia within the dorsal horn, their pharmacologic mechanisms of action differ. For instance, μ-opioid receptor agonists inhibit release of glutamate from primary afferent terminals at the level of the spinal and medullary dorsal horn, while CB1 receptors do not have any inhibitory effect on those excitatory neurons [49, 81]. Moreover, μ-opioid receptors presynaptically inhibit both glycinergic and GABAergic synaptic transmission in the medullary dorsal horn [48], but not in the spinal cord [81], as occurs with CB1 receptors. There are other differences at the supraspinal level, where both cannabinoid and opioid recep-tors presynaptically inhibit GABAergic synaptic transmission, disinhibiting nociceptive descending projection neurons, but only μ-opioid receptors directly inhibit putative GABAergic neurons in the PAG and RVM [19, 162, 163]. Depending on the cannabinoid receptor agonist, morphine effects are potentiated differently. Indeed, in the spinal cord, THC interacts synergistically with morphine and, in contrast, CP-55,940 and CP56,667 do not interact if they are administered epidurally, but do interact if they are administered to the cerebral ventricles (the dose-response curve shows that the dose of morphine diminishes 10-fold) [173, 174]. This may be due to the physical and chemical characteristics of the cannabinoid receptor agonists and to variations in the release of endogenous opioids depending on the cannabinoid receptor agonist used. As for anandamide, it has not been possible to demonstrate a synergistic interaction with the opioids, probably because this substance degrades quickly. The biochemical mechanisms involved in the interaction between cannabinoid and opioid receptors relate to the transduction and release of diverse mediators involved in the modulation of nociception and inflammation. On the other hand, cannabinoid receptor agonists induce the synthesis and/or release of endogenous opioid peptides [25, 94]. Subchronic treatment with THC produces an increase in opioids gene expression in the spinal cord, sustaining the hypothesis of an interaction between the cannabinoid and opioid systems in this region [25]. This relation is confirmed by observations, as a reduced cannabinoid and opioid receptor agonist synergistic analgesia in prodynorphin knockout animals or after administering opioid receptor antagonists like, nor-binaltor-phimine (a kappa antagonist) or naltrindole (a delta antagonist), or antibodies against dynorphin, implies that cannabinoid receptor agonists act indirectly on opioid receptors [125, 126]. Interestingly, therefore, although the antinociception of morphine is mediated predominately by μ-opioid receptors, it may be enhanced by THC through the activation of kappa and delta opioid receptors.
As mentioned earlier, new CB2 locations and mechanisms of action, which are important to pain modulation, have been recently identified. CB2 immunolabeling has been detected on β-endorphin-containing keratinocytes in stratum granulosum throughout the epidermis of the rat hindpaw. These CB2 receptors, when activated, stimulate release of the endogenous opioid β-endorphin, which then acts at μ-opioid receptors on local primary afferent neurons to inhibit nociception. The antinociceptive effects of the CB2 receptorselective agonist AM1241 were prevented in rats when naloxone or antiserum to β-endorphin was injected in the hindpaw where the noxious thermal stimulus was applied, or was not observed in skin from CB2 cannabinoid receptor-deficient mice and β-opioid receptordeficient mice, suggesting that β-endorphin is necessary for CB2 receptor-mediated antinociception [67].
Cannabinoid and α 2-Adrenergic Receptors Interactions
Interactions between CB1 and α2-adrenergic receptors have also been postulated. Both receptors presynaptically modulate primary afferent neurons [62, 176] and occur in areas that process nociceptive information at spinal and supraspinal levels [5, 87, 97]. However, some studies suggest that the antinociceptive actions of α2-adrenergic receptors are mediated purely at the spinal level, mainly via A-subtype α2-adrenergic receptors present in the substantia gelatinosa [76, 155].
CB1, α2-adrenergic, and μ-opioid receptors are all Gprotein coupled receptors sharing signal transduction pathways, such as those inhibiting adenylate cyclase and modulating K+ and Ca2+ channels activity [24, 62, 80, 94, 124]. These receptors are distributed similarly in areas of the central nervous system that participate in antinociception, such as the PAG or substantia gelatinosa [5, 80, 87, 97, 116, 176]. Both α2-adrenergic or A-opioid receptors agonists, when combined with a cannabinoid receptor agonist, show significant synergism in antinociception. Interesting findings have been obtained with combinations of these drugs. A cannabinoid receptor agonist combined with a μ-opioid receptor agonist displayed synergism in both the tail-flick and hot-plate assays, whereas a cannabinoid receptor agonist combined with an α2-adrenergic receptor agonist showed simple additivity in the tail-flick assay and synergism in the hot-plate assay [88]. In both assays, combined α2-adrenergic and μ-opioid receptors activation was simply additive.
Interactions Between Cannabinoids and Prostaglandin Inhibitors and COX-2
An interaction between cannabinoids and inhibitors of prostaglandin biosynthesis (like NSAIDs) has been reported, and it appears to be due to the similarity in chemical structure of endogenous cannabinoid ligands and prostaglandins (arachidonic acid derivatives), and to the convergence of prostaglandin and endocannabinoids transduction signals [36]. In addition, there is evidence that the addition of cannabinoid compounds to brain tissue sections originates an accumulation of arachidonic acid [130]. The enhancement of CB1 receptors activity by some NSAIDs (indomethacin, fluribuprofen) has been confirmed [37]. Moreover, the CB1receptor antagonist AM251 can block the antinociceptive effect of these NSAIDs administered intrathecally in a model of inflammatory pain (formalin test) [3]. In the same way, indomethacin loses efficacy in this model of pain in CB1 knockout mice [50]. The explanation for this involves NSAIDs capacity to inhibit the FAAH [61]. However, this may not be the only mechanism because intraperitoneal administration of a nonselective FAAH inhibitor (phenylmethylsulfonyl fluoride) does not affect the response to the formalin test, while AM251 still antagonises its analgesic effect [3]. An alternative hypothesis suggests that the COX-2 enzyme can metabolise the endocannabinoids (like anandamide and 2-AG) and that epidural administration of NSAIDs prevents anandamide destruction by inhibiting the action of COX-2 [82]. Therefore, the administration of NSAIDs increases the amount of anandamide by impeding its metabolisation through inhibition of the effect of COX-2 and/or FAAH.
Cyclooxygenase-2 (COX-2) is an enzyme associated with secondary damage after brain injury, as it facilitates the inflammatory response and delayed neuronal death. COX-2 exerts a negative influence on endocannabinoids because it catabolises them (as anandamide and 2-AG, that have shown neuroprotective properties in the injured brain) [83]. In a traumatic brain injury model, COX-2 inhibitor treatment protected 2-AG levels, enhanced functional recovery, and reduced cell death and inflammation [45], confirming an interaction between the endocannabinoid 2-AG and COX-2 enzyme. This also suggests that COX-2 inhibitors treatment may produce an indirect enhancement of cannabinoid receptors activity, by increasing endocannabinoid levels.
THERAPEUTIC USES OF CANNABINOIDS IN PAIN EPISODES
Together with morphine, cannabinoid receptor agonists have probably been one of the most common medicinal remedies since time immemorial. In recent years, especially since the decade of the 1990s when the endogenous cannabinoid system was discovered, basic knowledge of this system has advanced spectacularly. Now that there is no doubt that they are involved in the regulation of nociception, interest in using cannabinoid receptor agonists to manage pain has renewed. Therapeutic management using an antinociceptive mechanism different from the usual mechanisms opens a new line of therapy for cases in which no other pharmacologic treatments are available. As mentioned previously, combinations of cannabinoid receptor agonists with other analgesic substances to achieve a synergistic effect and improve the efficacy and safety of treatment are also interesting.
Studies in experimental models of acute and chronic pain have demonstrated the efficacy of cannabinoid receptor agonists, even in neuropathic or inflammatory pain. Preclinical data obtained with cannabinoid receptor agonists in pain models have made it possible to assess their efficacy in humans. The results to date suggest that cannabinoid receptor agonists are analgesic substances per se whose potency is similar to that of opioids like codeine and their dosedependent effect is limited by the appearance of adverse effects. Unfortunately, few clinical trials have been made and they have been carried out with major methodologic limitations. It is noteworthy that whereas cannabinoid receptor agonists have shown to be up to 10 times more potent than morphine in animal models of acute and neuropathic pain [38], analgesic efficacy has not been as good in some clinical trials. The main reason for this discrepancy is that doses are greatly reduced to avoid adverse effects, in particular psychotropic. In addition, there are other deficiencies in the scant clinical trials performed to date, such as important variations in doses, schedules, routes of administration, type of cannabinoid receptor agonists compounds, methods of testing analgesia, the selection of nonhomogeneous study groups (patients with diseases of diverse origin), and the extrapolation of results from models of experimental pain in healthy patients (pain circuits activity differ between healthy volunteers and patients). In Tables 1234-5 are summarised some data collected from humans in situations of acute (either in patients suffering pain or in healthy volunteers submitted to noxious stimuli) and chronic pain treated with cannabinoid compounds, as well as from questionnaires from self-medicated patients.
Table 1.
Effects of Cannabinoid Receptor Agonists in Healthy Volunteers Submitted to Acute Noxious Stimuli
| References | Pain Origin and Treatment | Results and Remarks |
|---|---|---|
| Zeidenberg et al., 1973 [177] | Healthy volunteers n=4 THC 5 mg p.o. Thermal stimuli | Antinociceptive effects which remained after memory and psycholinguistic effects were returning to normal levels (i.e. longer time course for effect on pain). |
| Hill et al., 1974 [55] | Healthy volunteers THC smoked 12 mg Electrical stimulation | THC increases pain sensitivity (hyperalgesia). The reason could be a biphasic effect, with initial stimulation followed by sedation. |
| Milstein et al., 1975 [105] | Healthy volunteers - cannabis-experienced - naive subjects • cannabis smoked • placebo Pressure stimuli | Smoked cannabis increased antinociceptive effects. No statistical difference between drug effect and cannabis experience, but there was a definite trend towards a greater increase for the experienced (16%) compared to the naive group (8%). |
| Raft et al., 1977 [129] | Healthy volunteers - THC 0.022 mg/kg i.v. - THC 0.044 mg/kg i.v. Electrical and pressure stimuli | No analgesic effect of THC, but methodological problems: pain assessment better performed by ratings intensity, as most pain is experienced in an intermediate range; while this study measured only the extremes of pain sensation, threshold (lowest intensity perceived as painful) and tolerance (maximum intensity of pain that a subject can withstand). They did not include a positive control; like an established analgesic (as opiate or narcotic). |
| Clark et al., 1981 [20] | Healthy volunteers being regular cannabis users n=16 THC smoked 20mg/cigarette 3-12/day Thermal stimuli | One month (before starting experiment) drug free, second month smoking 3-12 cigarettes/day, third month drug free again. Antinociceptive effects during the first two weeks of smoking, then returning to presmoking pain level maintained during the postsmoking period. Heavy smoking caused increase in pain reports. (i.e. THC originates tolerance in cannabis regular users) |
| Greenwald and Stitzer 2000 [46] | Healthy volunteers being regular cannabis users n=5 THC smoked Sessions: placebo (9 puffs), THC 3, 6 and 9 puffs Combined with naltrexone 0, 50 or 200 mg p.o. Thermal stimuli | Cannabis dose-dependent antinociceptive effects. Naltrexone (randomised, double-blind) did not influence cannabis effects, suggesting no role of endogenous opiates in cannabis-induced antinociception under these conditions. |
| Naef et al., 2003 [109] | Healthy volunteers n=12 - THC 20 mg - morphine 30 mg - THC + morphine - placebo Single dose, p.o. | Randomised, double-blinded, placebo-controlled, crossover. Pain tests (order randomised): heat, cold, pressure, single and repeated transcutaneous electrical stimulation. THC did not significantly reduce pain. In the cold and heat tests it even produced hyperalgesia, which was completely neutralized by THC-morphine. Psychotropic and somatic side effects (sleepiness, euphoria, anxiety, confusion, nausea, dizziness, etc.) were common, but usually mild. |
Table 2.
Effects of Cannabinoid Receptor Agonists on Acute Postoperative Pain
| References | Pain Origin and Treatment | Results and Remarks |
|---|---|---|
| Raft et al., 1977 [129] | Premedication for dental extraction n=10 - THC 0.022 mg/kg i.v. - THC 0.044 mg/kg i.v. - diazepam 0.157 mg/kg i.v. - placebo | Pain thresholds and psychiatric interviews were assessed, supplemented by tests of personality, depression and anxiety. THC analgesic effects less than that after diazepam and placebo, while pain detection thresholds were altered unpredictably with high THC doses. Three subjects at low-dose THC had a better analgesic effect than placebo but not diazepam. Six subjects preferred placebo to low-dose THC as an analgesic. |
| Jain et al., 1981 [70] | Postoperative or trauma pain n=56 Levonantradol 1.5, 2.0, 2.5, or 3.0 mg or placebo Single dose, i.m. | Double-blind. Significant analgesic effects of each dose of levonantradol (a synthetic cannabinoid) as compared to placebo. No dose-response differences. Side effects in 57% levonantradol, especially drowsiness and, less frequently, dry mouth, dizziness, “weird dreams,” mild hallucinations, nervousness, apprehension and confusion. |
| Buggy et al., 2003 [14] | Postoperative pain n=40 Elective abdominal hysterectomy THC 5 mg p.o. single dose 48 h after surgery | Randomised double-blind, placebo-controlled. No analgesic effect of THC in this paradigm. Randomisation took place when postoperative patient-controlled analgesia was discontinued on the second postoperative day. |
Table 3.
Data from Questionnaires / Surveys of Humans, Taking Cannabis as a Medicine to Relief Chronic Non-Cancer Pain of Different Types, Especially as a Symptom of Multiple Sclerosis
| References | Pain Origin and Treatment | Results and Remarks |
|---|---|---|
| Consroe et al., 1997 [23] | Questionnaire to 112 patients with MS taking cannabis for therapeutic reasons (in a self-medication basis), 2-3 times per day, 5-6 days per week, and usually smoked. | Approximately 95% reported cannabis improved spasticity and chronic pain of extremities; and other symptoms (as acute paroxysmal phenomenon, tremor, emotional dysfunction, anorexia/weight loss, fatigue states, double vision, sexual dysfunction, bowel and bladder dysfunctions, vision dimness, dysfunctions of walking and balance, and memory loss). |
| Ware et al., 2002 [171] | Interviewed patients with chronic pain using smoked herbal cannabis therapeutically n=15 (median duration of use six years) | Twelve improved in pain and mood, while 11 improved in sleep. Eight reported a “high” (state of euphoric intoxication); six denied a “high”. Tolerance to cannabis was not reported. |
| Ware et al., 2003 [170] | Anonymous cross-sectional survey to determine the prevalence of medicinal cannabis use among patients with chronic non-cancerous pain n=209 | 35% ever had used cannabis; 15% had used cannabis for pain relief (pain users), and 10% were currently using cannabis for pain relief; 18% denied using cannabis for pain relief (recreational users). Largest group using cannabis had pain by trauma and/or surgery (51%), and predominantly in neck/upper body (68%) and myofascial (65%). Pain, sleep and mood were most frequently reported as improving with cannabis use, and “high” and dry mouth were the most commonly reported side effects. |
| Clark et al., 2004 [21] | Survey to estimate the patterns and prevalence of cannabis use among patients with multiple sclerosis (MS) n=220 | 36% ever had used cannabis for any purpose; 14% use cannabis for symptom treatment. Medical cannabis use associated with male gender, tobacco use, and recreational cannabis use. Symptoms most effectively relieved: stress, sleep, mood, stiffness/spasm, and pain. |
Table 4.
Data from Clinical Trials of Humans Treated with Cannabinoid Receptor Agonists to Relief Chronic Non-Cancer Pain of Different Types, Especially as a Symptom of Multiple Sclerosis or Other Neuropathic Pain
| References | Pain origin and Treatment | Results and Remarks |
|---|---|---|
| Maurer et al., 1990 [101] | Spinal cord injury n=1 - THC 5 mg p.o. - Codeine 50 mg p.o. - placebo | Single case double-blind trial. THC and codeine both had an analgesic effect as compared with placebo. Only THC showed a significant beneficial effect on spasticity. THC did no alter consciousness. |
| Notcutt et al., 1997 [110] | Neuropathic pain n=50 Nabilone 0.25 to 3 mg/day | Observational study. Analgesic effect in 30% of patients. A significant number of patients abandon the drug because dysphoria and drowsiness. Benefits: analgesia, pain distancing, compressing the pain, sleep relief muscle spam, anxiolysis, relief constipation, etc. Patients who had used cannabis for chronic pain prior to trying nabilone (a synthetic analogue of THC) preferred the former |
| Karst et al., 2003 [77] | Neuropathic pain n=21 (all with hyperalgesia and 7 with allodynia). - Placebo - CT-3 40 mg first 4 days and 80 mg following three days Two 7-day treatment (plus 1 week washout) | Randomised, placebo-controlled, double-blind crossover trial (preliminary). Ajulemic acid (AJA or CT-3 or IP-751), a THC analogue, more analgesic effect than placebo, mainly 3 h after intake of CT-3; while 8 h after intake, the pain scale differences between groups were less marked. No dose response. Adverse effects, mainly transient: dry mouth and tiredness, significantly more often during CT-3 treatment. No major adverse effects were observed. |
| Wade et al., 2003 [165] | Neurogenic symptoms unresponsive to standard treatment n=24 Sublingual sprays. - Placebo - THC (cann. extract) - CBD (cann. extract) - THC+CBD (1:1) 2.5-120 mg each/day Two-week treatment periods | Double-blind, randomised, placebo-controlled single-patient cross-over trials. Pathologies were: MS (18), spinal cord injury (4), brachial plexus damage (1), and limb amputation (1). Pain relief by both THC and CBD significantly superior to placebo. Three patients had transient hypotension and intoxication with rapid initial dosing of THC. Cannabis medicinal extracts improved neurogenic symptoms unresponsive to standard treatments. Unwanted effects are predictable and generally well tolerated. |
| Wade et al., 2004 [164] | Multiple sclerosis outpatients n=160; Sublingual sprays. - placebo - THC+CBD (1:1), cann. extract: 2.5-120 mg of each daily, in divided doses. | Parallel group, double-blind, randomised, placebo-controlled study, undertaken in three centres. MS patients experiencing significant problems: spasticity, spasms, bladder problems, tremor or pain. The primary symptom score reduced more with CBME, but not significantly different than placebo. Spasticity was significantly reduced by CBME compared with placebo. No significant adverse effects on cognition or mood, and intoxication was generally mild. |
| Notcutt et al., 2004 [111] | Chronic pain (mainly neuropathic) n=34 Sublingual sprays 12-week period - Placebo - THC 2.5 mg (cann. extract) - CBD 2.5 mg (cann. extract) - THC+CBD (1:1) Randomly change of one of these treatments each week | Randomised, double-blind, placebo controlled, crossover trial. Extracts containing THC proved most effective in symptom control. Wide range of dosing requirements was observed. Side effects were generally acceptable and little different to those seen when other psychoactive agents used for chronic pain. The authors suggests that these initial experiences with Cannabis Based Medicinal Extracts (CBME) open the way to more detailed and extensive studies. |
| Berman et al., 2004 [6] | Central neuropathic pain from brachial plexus avulsion, with intractable symptoms n=48 Sublingual sprays -Placebo - THC (cannabis extract) - THC+CBD (cann. extract) Each patient each treatment for 2 weeks. | Randomised, double-blind, placebo-controlled, three period crossover study. Significant improvement on pain severity and on sleep disturbances. Generally well tolerated, majority of adverse events being mild to moderate in severity and resolving spontaneously. Studies of longer duration in neuropathic pain are required to confirm a clinically relevant, improvement in the treatment of this condition. |
THC is the substance with the greatest psychoactive potency of the natural cannabinoids, and exhibits the greatest analgesic activity [96]. In fact, THC administered epidurally (intrathecal, intraventricular) produces antinociception similar to that obtained with opioid compounds [173]. In addition, the epidural route of administration allows the use of much smaller doses to obtain a more lasting effect than with nonepidural routes. THC is lipophilic and relatively nonselective for CB1 and CB2 receptor subtypes, and has been widely used in experimental studies. Clinical trials have shown that nonselective cannabinoid receptor agonists are relatively safe and therapeutically efficacious, but they also induce psychotropic side effects [120]. However, if the THC is administered in small doses, it is well tolerated and efficient for neuropathic pain, without causing altered states of consciousness. Cardiovascular effects are generally moderate and well tolerated: hypotension can appear in comparison with placebo [158, 165], but no more than with codeine. However, since it may lead to significant increases in heart rate and a lowering of the blood pressure, patients with cardiovascular disease should probably not be treated with cannabinoid drugs. On the other hand, desired therapeutic effects and adverse effects both vary with the cannabinoid substance used. Thus, diverse THC analogues have been used for experimental or preclinical studies, some of which are being marketed in some countries for certain applications. The THC analogues most often used for preclinical or therapeutic purposes in humans are dronabinol, nabilone, levonantradol, CT-3 (or ajulemic acid), and HU211 [15, 117]. Another derivative, benzopyranoperidine, produces a degree of sedation similar to codeine but is ineffective as an analgesic [73].
Levonantradol is a synthetic analogue of THC that is administered intramuscularly. It has been used in clinical trials to palliate postoperative pain or pain due to trauma and has shown more analgesic efficacy than placebo [70]. Levonantradol causes adverse effects more often than THC in many patients, but none of them is considered serious.
Cannabidiol (CBD) is another major constituent of the Cannabis sativa plant, having the same therapeutic effects than THC (analgesic, anti-inflammatory, and others), but with a different pharmacologic profile. Studies have been made with cannabidiol derivatives developed to inhibit peripheral pain responses and inflammation after binding to cannabinoid receptors. Interestingly, some of these cannabidiol derivatives did not have central nervous system effects, but maintained their antinociceptive and anti-inflammatory properties. This means that centrally inactive synthetic cannabidiol analogues may be good candidates for the development of analgesic and anti-inflammatory drugs for peripheral conditions [40].
Medications have also been developed from extracts of the Cannabis sativa plant containing known amounts of THC and CBD [6, 111, 163, 164]. These medications are being used in clinical trials, and a form dispensed as sublingual spray has just been approved to be marketed in Canada.
Other possible therapeutic targets are CB2 receptors by means of specific agonists. As there are no CB2 receptors in neurons, the actions evoked by cannabinoid receptor agonists on the central nervous system seem to depend mainly on the activation of CB1 receptors. Thus CB2 receptor agonists merit special consideration for use as agents with absence of cognitive and psychotropic properties. Therefore one of them, HU-308 [102], does not produce hypothermia, catalepsy, or behavioural changes, while the role of CB2 receptors is fundamental in other cannabimimetic actions, such as immunomodulatory and antiproliferative effects. On the other hand, as mentioned earlier, new CB2 receptor properties are being discovered, as it has been confirmed that they indirectly stimulate opioid receptors located in primary afferent pathways [67].
Some interesting products are the anandamide reuptake inhibitors which inhibit its transport and potentiate its analgesic effects; as N-(4-hydroxyphenyl)-arachidonamide (AM 404), an anandamide analogue [119], and N-(3-furylmethyl) eicosa-5,8,11,14-tetraenamide (UCM707) (Fig. 2) [91]. Another promising target for therapeutic intervention is the fatty acid amide hydrolase (FAAH) enzyme, which is responsible for intracellular anandamide degradation [43]. AM374 (palmitylsulfonyl fluoride) is a potent FAAH inhibitor [28], preventing the hydrolysis of endocannabinoids and, therefore, increasing their synaptic levels and elevating cannabinoid receptors activity (Fig. 2). Indeed, reversible FAAH inhibitors produce analgesia in animal models [7]. In addition, other compounds like the N-acylethanolamines block anandamide degradation [118]. Knockout mice lacking FAAH display elevated concentrations of anandamide in brain and are more sensitive to the biological actions of anandamide [27].
An important detail to consider in cannabinoid treatment is the route of administration. Although oral dispensation is the route of choice for the clinical management of chronic pain, due to its prolonged action and ease of use, many patients prefer inhalation (smoking cannabis) and sublingual sprays have also been prepared for commercial preparations. Nonetheless, there are other possibilities, particularly when analgesia is deficient, oral intolerance exists, and/or the state of consciousness is altered, such as the epidural or intravenous routes. Other alternatives are delayed-release skin patches and, to a lesser extent, rectal suppositories [9].
Smoking cannabis remains the most efficient means of drug delivery since absorption is much more rapid and experienced users dose themselves by adjusting the frequency and depth of inhalation [68]. However, this route has the disadvantage of respiratory and other complications associated with the tobacco mixed with the product for smoking. On the other hand, the effects of natural cannabinoids (plant extracts) seems to be better than those of synthetic cannabinoids. The effects of cannabis, whether smoked or administered intravenously, appear 30 minutes to one hour after administration and last for 2 or 3 hours. Therefore, one disadvantage is a brief analgesic effect.
As with any other therapy, adverse effects must be taken into account. Some patients will suffer side effects, although most of them will appear only in the first days of treatment and disappear as the body adjusts to the drug. Short-term effects, such as unsteadiness, dizziness, difficulty concentrating, drowsiness, dryness of the mouth, and/or headache, are related to depression of the central nervous system. Chronic cannabis use does not produce serious cognitive disorders, as occurs with other substances like alcohol, but it can aggravate pre-existing mental disease. Therefore, treatment with cannabinoid receptor agonist with central actions may be contraindicated, or either rigorously controlled, in individuals predisposed to psychiatric disorders. No human deaths associated to cannabis use have been reported, and the lethal dose of THC in rodents is very high compared to other substances.
With respect to potentially addictive effects, therapeutic doses are smaller than those used for recreational purposes, and derivatives that have little or no central effects are the most valuable candidates formedicinal purposes. Controversy exists regarding the appearance of tolerance and dependence with the use of cannabinoid compounds. It has been claimed that tolerance develops after repeated administration of THC in humans, that 9-20% of regular cannabis users become dependent, and that a significant withdrawal syndrome occurs in human cannabis users [2, 136, 153]. However, it has also been suggested that many clinical studies are poorly controlled and the development of a cannabinoid withdrawal syndrome is debated [for reviews see: 74, 75].
As for clinical applications, one of the best documented therapeutic uses of cannabinoid derivatives, and the one most widely approved today, is as an antiemetic drug [12, 158, 172]. Indeed, cannabinoid-based prescription medicines are now marketed for this use in some countries. They have proven to be extremely effective for relieving nausea and emesis due to gastrointestinal distress caused by acquired immunodeficiency syndrome (AIDS) medications or cancer chemotherapy. These patients either smoke cannabis or take a THC synthetic analogue like dronabinol or nabilone, although they are prescribed only when other medications for nausea and vomiting do not work.
Acute Pain
Opioids are powerful analgesics widely utilised in clinical pain management, but they yield a poor analgesic response in conditions of certain pathologic pain, such as neuropathy. THC induces antinociception in rats with pathologic pain after nerve injury. Moreover, THC antinociception is independent of opioid receptors in rats with some pathologic pain, as the antinociceptive effect after nerve injury is blocked by the CB1 receptor antagonist SR141716A, but not by the opioid receptor antagonist naloxone, and there is no cross-tolerance between the antinociceptive effects of morphine and THC [95]. Therefore, THC and other cannabinoids may be superior to opioids in alleviating intractable pathologic pain syndromes.
What clinical trials are available on the efficacy of cannabinoid receptor agonists in the treatment of acute pain are scant and inconclusive with respect to their analgesic efficacy (Tables 1-2).
Postoperative Pain
One potential indication of cannabinoids would be as analgesic for postoperative pain. Morphine has been used historically to relieve postoperative pain, but it renders unwanted side effects. Postoperative pain interfere with functioning and healing, and it can grow to intolerable levels. However, there is no drug for this condition which produces adequate analgesia with no side effects. Some clinical trials, using cannabinoid derivatives in postoperative pain, have been undertaken or are in progress (Table 2).
Chronic Pain
Multiple Sclerosis
Multiple sclerosis (MS) is a life-long chronic disease in which nerve cells are attacked by the immune system, originating painful muscle spasms and many other problems, including neuropathic pain. There are about 1.1 million worldwide sufferers of MS. Clinical trials have tested the potential medical applications of cannabis for the treatment of MS symptoms, although some of them present a small number of patients ; and there are also data from responses to questionnaires (Tables 3-4) [68, 117]. Smoking cannabis not only has helped to stop spasms, but has halted the progression of multiple sclerosis. Although smoking cannabis is illegal in some countries, estimates suggest that 10% to 30% of MS patients in Europe smoke cannabis to ease the painful and disabling symptoms of the disease. Medications prepared from whole plant cannabis extract, containing known amounts of THC and CBD as the principal components have been prepared to be administered by oral spray to relieve MS symptoms, as well as for the treatment of other disorders with severe neuropathic pain. This product has undergone phase III placebo-controlled trials, which show that it reduces neuropathic pain, spasticity, and sleep disturbances. Its use has been approved only in Canada so far. Furthermore, animal model of multiple sclerosis, have found other advantage of cannabinoid receptor agonists, since they appear to exert CB1 receptor-mediated neuroprotective effects that would be benefitial for the neurodegeneration occurring in MS [123].
Neuropathic Pain
Neuropathic pain, which is frequently chronic, arises when neurons in the brain or peripheral nervous system become hypersensitised and generate abnormal or prolonged impulses. In Europe, about 4 million patients suffer neuropathic pain. There are many causes of neuropathic pain, including diabetic neuropathy, post-herpetic neuralgia, brachial plexus lesion, fibromyalgia, multiple sclerosis, and cancer.
Severe neuropathic pain has proved difficult to treat and evidence suggests that none of the available drugs, mainly opioids, is effective in more than 50% of patients. This is thus an area of significant unmet clinical need. It is important to emphasise that cannabinoid receptor agonists are more effective than opioids in the management of neuropathic pain. Different hypotheses have been proposed to explain this phenomenon [95]. One of them is based on the presence of cannabinoid receptors in primary afferent myelinated Afibres, since this pain is partly due to spontaneous discharge of these fibres [59]. The A-fibres contain fewer μ-opioid receptors than cannabinoid receptors. There is evidence confirming this hypothesis. Thus, WIN 55,212-2, a cannabinoid receptor agonist, which is less potent than morphine in the inhibition of the acute pain response conducted by C-fibres, is a more effective inhibitor of the wind-up phenomenon (a phenomenon that contributes to the development of hyperalgesia and allodynia) [152]. Another hypothesis is based on the fact that, unlike opioids, cannabinoids do not lose effectiveness in the management of neuropathic pain because fewer receptors disappear in this situation (the destruction of primary afferent fibres by rhizotomy [10] or the administration of neonatal capsaicin [57] reduce CB1-receptor expression less than opioid expression). In contrast, there is less expression of the opioid receptors in the posterior horn [10].
This means that cannabinoid receptor agonists have more capacity for suppressing pathophysiologic mechanisms like the wind-up phenomenon linked to this type of pain [152].
Behavioural studies have shown that cannabinoids reduce thermal and mechanical allodynia in rat models of neuropathic pain [38, 54, 69]. Intrathecal administration of CP55, 940 in models of neuropathic pain (chronic L5/6 spinal nerve ligation) or acute pain (tail flick) attenuated tactile allodynia and induced thermal antinociception. These antinociceptive effects appears to be mediated chiefly by the CB1 receptor, but some data also suggest a role for CB2 receptor-mediated antinociception in both acute and neuropathic pain [142].
Cancer Pain
Pain is one of the most frequent symptoms in patients with cancer and the World Health Organisation recommends that they receive adequate pain relief. Cannabinoids are among the compounds under development for the treatment of these patients, and they seem to have analgesic activity (Table 5) [127]. Clinical trials are also under way to assess the effectiveness of cannabis extract preparations (containing THC and CBD) for the relief of cancer pain (neuropathicrelated cancer pain). Around 40% of cancer patients suffer some degree of neuropathic pain.
Table 5.
Data from Clinical Trials of Humans Treated with Cannabinoid Receptor Agonists to Relief Chronic Cancer Pain
| References | Treatment | Results and Remarks |
|---|---|---|
| Noyes et al., 1975 [114] | n=10 - placebo and THC 5, 10, 15, and 20 mg p.o. Each patients all treatments | Double blind placebo-controlled trial (preliminary). Analgesic effect of THC at high doses (15 and 20 mg), significantly superior than placebo. At these doses, substantial sedation and mental clouding. No nausea or emesis. Increased appetite in some patients. |
| Noyes et al., 1975 [113] | n=36 - placebo - THC 10 or 20 mg p.o. - codeine 60 or 120 mg p.o. | Mild analgesic effect of THC. At 20 mg THC (similar to 120 mg codeine) induced side effects that would prohibit its therapeutic use, including somnolence, dizziness, ataxia, and blurred vision; and even some alarming adverse reactions. At 10 mg THC (similar to 60 mg codeine): analgesic potential; well tolerated, sedative effect. |
| Jochimsen et al., 1978 [73] | n=35 - placebo - benzopyranoperidine (2 or 4 mg) p.o. - codeine sulfate (60 or 120 mg) p.o. | Double-blind, 5-way crossover designed study. Significant analgesic relief with 120 mg of codeine, but no differences between placebo and benzopyranopyridine (analogue of THC). Pain perception even appeared to be augmented by both doses of benzopyranopyridine. |
| Staquet et al., 1978 [150] | NIB (nitrogen analogue of THC) 1 mg p.o. - Trial I: NIB vs. codeine - Trial II: NIB vs. secobarbital - Placebo (trials I and II) | Two consecutive, randomised, double-blind trials. Evaluation of mild, moderate, and severe pain. Trial I: NIB superior to placebo and equivalent to 50 mg of codeine. Trial II: NIB superior to placebo and to 50 mg secobarbital (a short-acting barbiturate). However, NIB is not useful clinically because of the frequency of side effects. |
Fibromyalgia
This disease is characterised by the presence of generalised pain throughout the body, confirmed by the presence of tender and painful points on digital palpation in at least 11 of the 18 points established for diagnosis. Pharmacologic treatment usually consists of tricyclic antidepressants combined with NSAIDs. Although there are no specific clinical studies of the use of cannabinoid receptor agonists for symp- tomatic relief of this disease, data that support their therapeutic potential thanks to their anti-inflammatory and sedative properties [138].
Migraine
Migraine is defined as vasomotor headache characterised by its pulsatile nature, the presentation of crises, and its periodic occurrence. It is usually hemicraneal and accompanied by generalised sensorial hyperestesia, sensitivity to light and noise, and nausea and/or vomiting. Ergot alkaloids and 5-HT1D serotonin receptor agonists are used for its treatment. NSAIDs, codeine, and caffeine are also generally used. Historically, cannabis was prescribed for its management in the early 20th century. At present, the antimigraine properties of cannabis have been recognised [137] and there are studies that affirm that the pain relief it produces is comparable, or better than, that achieved with ergotamine and aspirin [112]. The most effective route of administration is by inhalation because of its rapid action. The antiemetic and vasodilator properties of cannabinoid compounds are additional benefits that support its use as an alternative medication in migraine refractory to conventional therapy.
Spasticity
Spasticity entails increased resistance to passive movement. Among other disadvantages, it causes pain per se and also secondary to joint stiffness. Among the therapeutic measures proposed are the elimination or reduction of nociceptive stimuli, rehabilitation (occupational physical therapy), and the use of antispasmodic medication. Clinical trials have evaluated the efficacy of cannabinoids in diverse musculoskeletal entities accompanied by severe spasticity [149, 161, 164].
Phantom Limb Syndrome
Patients who suffer amputation of an extremity can experience a type of referred neuropathic pain in the amputated zone. Pharmacologic management of this condition is complex and it is based mainly on anticonvulsants. The use of cannabinoids is supported by their efficacy in alleviating neuropathic pain. Promising results have been obtained in clinical practice [47].
CONCLUDING REMARKS
Pain severely impairs quality of life. Currently available treatments, generally opioids and anti-inflammatory drugs, are not always effective for certain painful conditions. The discovery of the cannabinoid receptors in the 1990s led to the characterisation of the endogenous cannabinoid system in terms of its components and numerous basic physiologic functions. CB1 receptors are present in nervous system areas involved in modulating nociception and evidence supports a role of the endocannabinoids in pain modulation. Basic research on how cannabinoid receptors and endocannabinoids intervene in pain mechanisms is progressing rapidly. Clinical progress, however, is advancing slowly. Cannabinoids have antinociceptive mechanisms different from that of other drugs currently in use, which thus opens a new line of promising treatment to mitigate pain that fails to respond to the pharmacologic treatments available, especially for neuropathic and inflammatory pains. The combination of cannabinoids with synergistic analgesic substances is interesting because it may improve the efficacy and safety of treatment. One of the drawbacks of investigating cannabinoids is their typification as substances of abuse. However, compounds blunting severe pain allow patients to perform daily activities more easily, so the potential benefits should be weighed against possible adverse effects. Our current understanding of the physiology and pharmacology of the endogenous cannabinoid system has motivated cannabis-based therapeutic drug design, in which attempts are being made to synthesise compounds with the desired therapeutic actions but without psychoactive adverse effects. Medications prepared with cannabinoid receptor agonists or with drugs that enhance endocannabinoid function (by either increasing release or diminishing reuptake of endocannabinoids) may afford the novel therapeutic approaches demanded by disorders in which pain is a prominent symptom. Clinical trials seem to indicate that either extracts of the Cannabis sativa plant containing known amounts of the active compounds (mainly THC and CBD) or diverse synthetic derivatives of THC are promising treatments for painful conditions that do not respond to available treatments, such as neuropathic, inflammatory and oncologic pain. Specifically, cannabis extracts have shown effectiveness to relief some symptoms of the patients with multiple sclerosis, mainly for pain and spasticity. Pharmacologic manipulation directed to elevate endocannabinoids levels like, for example, with anandamide reuptake inhibitors, or by inhibiting the enzyme fatty acid amide hydrolase (FAAH), which is responsible for intracellular anandamide degradation, may well become a valuable therapeutic tool. CB2 receptor selective agonists with no central effects are other promising pain treatment under investigation. Adequately sized and designed, doubleblind placebo-controlled clinical trials are needed to evaluate the potential applications of cannabis-based medications as novel and effective therapeutic drugs for controlling different types of pain.
ACKNOWLEDGEMENTS
We wish to thank Mr. Stuart Ingham for making the illustrations presented in this review; and to the “Fundacion Neurociencias y Envejecimiento” for financial support.
ABBREVIATIONS
- 2-AG
2-arachidonoylglycerol
- AEA
Arachidonylethanolamide or anandamide
- cann. Extract
Extract of Cannabis sativa
- CB1 receptor
Cannabinoid receptor type 1
- CB2 receptor
Cannabinoid receptor type 2
- CBD
Cannabidiol
- CBME
Cannabis Based Medicinal Extracts
- CNS
Central nervous system
- COX-2
Cyclooxygenase-2
- CT-3 or acid AJA or IP-751
Ajulemic
- DSE
Depolarisation-induced suppression of excitation
- DSI
Depolarisation-induced suppression of inhibition
- FAAH
Fatty-acid amide hydrolase
- GABA
Gamma-aminobutyric acid
- MS
Multiple sclerosis
- NADA
N-arachidonoyldopamine
- NIB
Synthetic nitrogen analogue of tetrahy-drocannabinol
- NSAIDs
Non steroidal anti-inflammatory drugs
- PAG
Periaqueductal grey matter
- RVM
Rostral ventromedial medulla
- THC
Delta-9-tetrahydrocannabinol
- VR1
Vanilloid receptor type 1
REFERENCES
- 1.Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 2.Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substance and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- 3.Ates M, Hamza M, Seidel K, Kotalla CE, Ledent C, Guhring H. Intrathecally applied flurbiprofen produces an endocan-nabinoid-dependent antinociception in the rat formalin test. Eur J Neurosci. 2003;17:597–604. doi: 10.1046/j.1460-9568.2003.02470.x. [DOI] [PubMed] [Google Scholar]
- 4.Barinaga M. How cannabinoids work in the brain. Science. 2001;291:2530–2531. doi: 10.1126/science.291.5513.2530. [DOI] [PubMed] [Google Scholar]
- 5.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 6.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, Hwang I, Hedrick MP, Leung D, Acevedo O, Guimaraes CR, Jorgensen WL, Cravatt BF. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. J Med Chem. 2005;48:1849–1856. doi: 10.1021/jm049614v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G-protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- 9.Brenneisen R, Egli A, ElSohly MA, Henn V, Spiess Y. The effect of orally and rectally administered δ9-tetrahydrocannabinol on spasticity: a pilot study with 2 patients. Int J Clin Pharmacol Ther. 1996;34:446–452. [PubMed] [Google Scholar]
- 10.Bridges D, Ahmad K, Rice AS. The synthetic cannabi-noid WIN 55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;113:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohisto-chemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 12.British Medical Association . London: Harwood Academic Publishers; 1997. Therapeutic Uses of Cannabis. [Google Scholar]
- 13.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 14.Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106:169–172. doi: 10.1016/s0304-3959(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 15.Burstein SH, Karst M, Schneider U, Zurier RB. Ajulemic acid: A novel cannabinoid produces analgesia without a “high”. Life Sci. 2004;75:1513–1522. doi: 10.1016/j.lfs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor, a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 18.Chapman V. The cannabinoid CB1 receptor antagonist, SR141716A, selectively facilitates nociceptive responses of dorsal horn neurones in the rat. Br J Pharmacol. 1999;127:1765–1767. doi: 10.1038/sj.bjp.0702758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chieng B, Christie MJ. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark WC, Janal MN, Zeidenberg P, Nahas GG. Effects of moderate and high doses of marihuana on thermal pain: A sensory decision theory analysis. J Clin Pharmacol. 1981;21((8-9 Suppl.)):299S–310S. doi: 10.1002/j.1552-4604.1981.tb02608.x. [DOI] [PubMed] [Google Scholar]
- 21.Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62:2098–2100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- 22.Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A), inhibition of delta 9-tetrahydocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- 23.Consroe P, Musty R, Rein J, Tillery W, Pertwee R. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol. 1997;38:44–48. doi: 10.1159/000112901. [DOI] [PubMed] [Google Scholar]
- 24.Cook SA, Welch SP, Lichtman AH, Martin BR. Evaluation of cAMP involvement in cannabinoid-induced antino-ciception. Life Sci. 1995;56:2049–2056. doi: 10.1016/0024-3205(95)00188-c. [DOI] [PubMed] [Google Scholar]
- 25.Corchero J, Avila MA, Fuentes JA, Manzanares J. Delta 9-tetrahydrocannabinol increases prodynorphin and proenkephalin gene expression in the spinal cord of the rat. Life Sci. 1997;61:L39–L43. doi: 10.1016/s0024-3205(97)00405-0. [DOI] [PubMed] [Google Scholar]
- 26.Corchero J, Manzanares J, Fuentes JA. Cannabi-noid/opioid crosstalk in the central nervous system. Crit Rev Neurobiol. 2004;16:159–172. doi: 10.1615/critrevneurobiol.v16.i12.170. [DOI] [PubMed] [Google Scholar]
- 27.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutsch DG, Lin S, Hill WA, Morse KL, Salehani D, Ar-reaza G, Omeir RL, Makriyannis A. Fatty acid sulfonyl fluorides inhibit anandamide metabolism and bind to the cannabi-noid receptor. Biochem Biophys Res Commun. 1997;231:217–221. doi: 10.1006/bbrc.1997.6072. [DOI] [PubMed] [Google Scholar]
- 29.Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a can-nabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 30.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechou-lam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 31.Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB1 can-nabinoid receptor knockout mice, evidence for non-CB1, non-CB2 receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 33.Edsall SA, Knapp RJ, Vanderah TW, Roeske WR, Consroe P, Yamamura HI. Antisense oligodeoxynucleotide treatment to the brain cannabinoid receptor inhibits antinociception. Neuroreport. 1996;7:593–596. doi: 10.1097/00001756-199601310-00052. [DOI] [PubMed] [Google Scholar]
- 34.Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farquhar-Smith WP, Egertova M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- 36.Fimiani C, Liberty T, Aquirre AJ, Amin I, Ali N, Stefano GB. Opiate, cannabinoid, and eicosanoid signaling converges on common intracellular pathways nitric oxide coupling. Prostaglandins Other Lipid Mediat. 1999;57:23–34. doi: 10.1016/s0090-6980(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 37.Fowler CJ. Possible involvement of the endocannabinoid system in the actions of three clinically used drugs. Trends Pharmacol Sci. 2004;25:59–61. doi: 10.1016/j.tips.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Fox A, Kesingland A, Gentrym C, McNair K, Patel S, Urban L, James I. The role of central and peripheral Cannabinoid 1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 39.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 40.Fride E, Feigin C, Ponde DE, Breuer A, Hanus L, Ar-shavsky N, Mechoulam R. (+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only. Eur J Pharmacol. 2004;506:179–188. doi: 10.1016/j.ejphar.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 41.Fuentes JA, Ruiz-Gayo M, Manzanares J, Vela G, Reche I, Corchero J. Cannabinoids as potential new analgesics. Life Sciences. 1999;65:675–685. doi: 10.1016/s0024-3205(99)00290-8. [DOI] [PubMed] [Google Scholar]
- 42.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1467. [Google Scholar]
- 43.Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci USA. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giuffrida A, Beltramo. M, Piomelli D. Mechanisms of endocannabinoid inactivation, biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- 45.Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Nara-yan RK, Strauss KI. Functional outcomes, provides neu-roprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 47.Grinspoon L, Bakalar JB. Marihuana the forbidden medicine. New Haven: Yale University Press; 1997. [Google Scholar]
- 48.Grudt TJ, Henderson G. Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa, inhibition by mu-opioid and GABAB agonists. J Physiol. 1998;507:473–483. doi: 10.1111/j.1469-7793.1998.473bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grudt TJ, Williams JT. mu-Opioid agonists inhibit spinal trigeminal substantia gelatinosa neurons in guinea pig and rat. J Neurosci. 1994;14:1646–1654. doi: 10.1523/JNEUROSCI.14-03-01646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guhring H, Hamza M, Sergejeva M, Ates M, Kotalla CE, Ledent C, Brune K. A role for endocannabinoids in indo-methacin-induced spinal antinociception. Eur J Pharmacol. 2002;454:153–163. doi: 10.1016/s0014-2999(02)02485-8. [DOI] [PubMed] [Google Scholar]
- 51.Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 52.Hanus L, Abu-Lafi S, Fride E, Breuer A, Shalev DE, Kusta-novich I, Vogel Z, Mechoulam R. 2-Arachidonyl glyc-eryl ether, a novel endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain, a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity can-nabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- 55.Hill SY, Schwin R, Goodwin DW, Powell BJ. Marihuana and pain. J Pharmacol Exp Ther. 1974;188:415–418. [PubMed] [Google Scholar]
- 56.Hohman AG, Walker JM. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J. Neurophysiol. 1999;81:575–585. doi: 10.1152/jn.1999.81.2.575. [DOI] [PubMed] [Google Scholar]
- 57.Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin. Neurosci Lett. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- 58.Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- 59.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopula-tions of rat dorsal root ganglia, a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 60.Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci Lett. 1998;257:119–122. doi: 10.1016/s0304-3940(98)00802-7. [DOI] [PubMed] [Google Scholar]
- 61.Holt S, Fowler CJ. Anandamide metabolism by fatty acidamide hydrolase in intact C6 glioma cells. Increased sensitivity to inhibition by ibuprofen and flurbiprofen upon reduction of extra- but not intracellular pH. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:237–244. doi: 10.1007/s00210-002-0686-z. [DOI] [PubMed] [Google Scholar]
- 62.Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 63.Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142:1209–1218. doi: 10.1038/sj.bjp.0705881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howlett AC, Johnson MR, Melvin LS, Milne GM. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol. 1988;33:297–302. [PubMed] [Google Scholar]
- 65.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petro-cellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Bandera TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- 69.Iversen LL, Chapman V. Cannabinoids, a real prospect for pain relief? Curr Opin Pharmacol. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 70.Jain AK, Ryan JR, McMahon FG, Smith G. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J Clin Pharmacol. 1981;21(8-9 Suppl.):320S–326S. doi: 10.1002/j.1552-4604.1981.tb02610.x. [DOI] [PubMed] [Google Scholar]
- 71.Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct fromCB1or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jennings EA, Vaughan CW, Christie MJ. Cannabinoid actions on rat superficial medullary dorsal horn neurons in vitro. J. Physiol. 2001;534:805–812. doi: 10.1111/j.1469-7793.2001.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jochimsen PR, Lawton RL, VerSteeg K, Noyes R., Jr Effect of benzopyranoperidine, a delta-9-THC congener, on pain. Clin Pharmacol Ther. 1978;24:223–227. doi: 10.1002/cpt1978242223. [DOI] [PubMed] [Google Scholar]
- 74.Jones RT. Behavioral tolerance: lessons learned from cannabis research. NIDA Res Monogr. 1978;18:118–126. [PubMed] [Google Scholar]
- 75.Jones RT. Drug of abuse profile: cannabis. Clin Chem. 1987;33:72B–81B. [PubMed] [Google Scholar]
- 76.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 77.Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on the chronic neuropathic pain. JAMA. 2003;290:1757–1762. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- 78.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 80.Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imi-dazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 81.Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999;518:803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kozak KR, Prusakiewicz JJ, Rowlinson SW, Prudhomme DR, Marnett LJ. Amino acid determinants in cyclooxy-genase-2 oxygenation of the endocannabinoid anandamide. Biochemistry. 2003;42:9041–9049. doi: 10.1021/bi034471k. [DOI] [PubMed] [Google Scholar]
- 83.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prosta-glandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 84.Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous can-nabinoids. J Neurosci. 2001;21(RC174):1–5. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreitzer AC, Regehr WG. Retrograde inhibition of pre-synaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 86.Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabi-noids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 87.Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats, evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- 88.Lichtman AH, Martin BR. Cannabinoid-induced antino-ciception is mediated by a spinal a2-noradrenergic mechanism. Brain Res. 1991;559:309–314. doi: 10.1016/0006-8993(91)90017-p. [DOI] [PubMed] [Google Scholar]
- 89.Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- 90.Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–283. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 91.Lopez-Rodriguez ML, Viso A, Ortega-Gutierrez S, Lastres-Becker I, Gonzalez S, Fernandez-Ruiz JJ, Ramos JA. Design, synthesis and biological evaluation of novel arachidonic acid derivatives as highly potent and selective endocannabinoid transporter inhibitors. J Med Chem. 2001;44:4505–4508. doi: 10.1021/jm015545y. [DOI] [PubMed] [Google Scholar]
- 92.Luo C, Kumamoto E, Furue H, Chen J, Yoshimura M. Anandamide inhibits excitatory transmission to rat substantia ge-latinosa neurones in a manner different from that of capsaicin. Neurosci Lett. 2002;321:17–20. doi: 10.1016/s0304-3940(01)02471-5. [DOI] [PubMed] [Google Scholar]
- 93.Manning BH, Martin WJ, Meng ID. The rodent amygdala contributes to the production of cannabinoid-induced an-tinociception. Neuroscience. 2003;120:1157–1170. doi: 10.1016/s0306-4522(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 94.Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- 95.Mao J, Price DD, Lu J, Keniston L, Mayer DJ. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- 96.Martin BR. Structural requirements for cannabinoid-induced antinociceptive activity in mice. Life Sci. 1985;36:1523–1530. doi: 10.1016/0024-3205(85)90376-5. [DOI] [PubMed] [Google Scholar]
- 97.Martin BR, Lichtman AH. Cannabinoid transmission and pain perception. Neurobiol Dis. 1998;5:447–461. doi: 10.1006/nbdi.1998.0218. [DOI] [PubMed] [Google Scholar]
- 98.Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced an-tinociception as revealed by intracerebral microinjections. Brain Res. 1999;822:237–242. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- 99.Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist, correlation between electrophysiological and antinociceptive effects. J Neurosci. 1996;16:6601–6611. doi: 10.1523/JNEUROSCI.16-20-06601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bon-ner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 101.Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur Arch Psychiatry Clin Neurosci. 1990;240:1–4. doi: 10.1007/BF02190083. [DOI] [PubMed] [Google Scholar]
- 102.Mechoulam R. Looking back at Cannabis research. Curr Pharm Des. 2000;6:1313–1322. doi: 10.2174/1381612003399509. [DOI] [PubMed] [Google Scholar]
- 103.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 104.Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- 105.Milstein SL, MacCannell K, Karr G, Clark S. Marijuana-produced changes in pain tolerance. Experienced and non-experienced subjects. Int Pharmacopsychiatry. 1975;10:177–182. doi: 10.1159/000468188. [DOI] [PubMed] [Google Scholar]
- 106.Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substancia gelatinosa neurons of the spinal cord. J Neurophysiol. 2001;86:40–48. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- 107.Mukhopadhyay S, Chapnick BM, Howlett AC. Anan-damide-induced vasorelaxation in rabbit aortic rings has two components, G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- 108.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 109.Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/s0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 110.Notcutt WG, Price M, Chapman G. Clinical experience with nabilone for chronic pain. Pharm Sci. 1997;3:551–555. [Google Scholar]
- 111.Notcutt W, Price M, Miller R, Newport S, Phillips C, Sim-mons S, Sansom C. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia. 2004;59:440–452. doi: 10.1111/j.1365-2044.2004.03674.x. [DOI] [PubMed] [Google Scholar]
- 112.Noyes R, Jr, Baram DA. Cannabis analgesia. Compr. Psychiatry. 1974;15:531–535. doi: 10.1016/0010-440x(74)90008-x. [DOI] [PubMed] [Google Scholar]
- 113.Noyes R, Jr, Brunk SF, Avery DH, Canter A. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18:84–89. doi: 10.1002/cpt197518184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noyes R, Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9 tetrahydrocannabinol. J Clin Pharmacol. 1975;15:139–143. doi: 10.1002/j.1552-4604.1975.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 115.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsyn-aptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 116.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 117.Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Ther. 2002;95:165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- 118.Piomelli D, Beltramo M, Giuffrida A, Stella N. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5:462–473. doi: 10.1006/nbdi.1998.0221. [DOI] [PubMed] [Google Scholar]
- 119.Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci USA. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Porter AC, Felder CC. The endocannabinoid nervous system, unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 121.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocan-nabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 122.Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM, Ledent C, Petzold A, Thompson AJ, Giovan-noni G, Cuzner ML, Baker D. Cannabinoids inhibit neu-rodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- 124.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 125.Pugh G, Jr, Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–616. [PubMed] [Google Scholar]
- 126.Pugh G, Abood ME, Welch SP. Antisense oligode-oxynucleotides to the k-1 receptor block the antinociceptive effects of D9-THC in the spinal cord. Brain Res. 1995;689:157–158. doi: 10.1016/0006-8993(95)00560-d. [DOI] [PubMed] [Google Scholar]
- 127.Radbruch L, Elsner F. Emerging analgesics in cancer pain management. Expert Opin Emerg Drugs. 2005;10:151–171. doi: 10.1517/14728214.10.1.151. [DOI] [PubMed] [Google Scholar]
- 128.Raffa RB, Stone DJ, Hipp SJ. Differential cholera-toxin sensitivity of supraspinal antinociception induced by the can-nabinoid agonists D9-THC, WIN 55,212-2 and anandamide in mice. Neurosci Lett. 1999;263:29–32. doi: 10.1016/s0304-3940(99)00096-8. [DOI] [PubMed] [Google Scholar]
- 129.Raft D, Gregg J, Ghia J, Harris L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin Pharmacol Ther. 1977;21:26–33. doi: 10.1002/cpt197721126. [DOI] [PubMed] [Google Scholar]
- 130.Reichman M, Nen W, Hokin LE. Delta-9-tetrahydro-cannabinol increases arachidonic acid levels in guinea pig cerebral cortex slices. Mol Pharmacol. 1988;34:823–828. [PubMed] [Google Scholar]
- 131.Rice AS, Farquhar-Smith WP, Nagy I. Endocannabi-noids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- 132.Richardson JD, Aanonsen L, Hargreaves KM. SR 141716A, a cannabinoid receptor antagonist, produces hyperalge-sia in untreated mice. Eur J Pharmacol. 1997;319:R3–R4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- 133.Richardson JD, Aanonsen L, Hargreaves KM. Anti-hyperalgesic effects of spinal cannabinoids. Eur J Pharmacol. 1998;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- 134.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduces hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 135.Robson P. Therapeutic aspects of cannabis and cannabi-noids. Br J Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- 136.Rosenberg MF, Anthony JC. Early clinical manifestations of cannabis dependence in a community sample. Drug Alcohol Depend. 2001;64:123–131. doi: 10.1016/s0376-8716(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 137.Russo E. Cannabis for migraine treatment: the once and future prescription? An historical and scientific review. Pain. 1998;76:3–8. doi: 10.1016/s0304-3959(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 138.Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett. 2004;25:31–39. [PubMed] [Google Scholar]
- 139.Sanudo-Pena MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 140.Sanudo-Pena MC, Strangman NM, Mackie K, Walker JM, Tsou K. CB1 receptor localization in rat spinal cord and roots, dorsal root ganglion, and peripheral nerve. Zhongguo Yao Li Xue Bao. 1999;20:1115–1120. [PubMed] [Google Scholar]
- 141.Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- 142.Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 143.Shen M, Piser TM, Seybold VS, Thayer SA. Cannabi-noid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4324. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, Caput D, Ferrara P. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- 145.Siegling A, Hofmann HA, Denzer D, Mauler F, De Vry J. Cannabinoid CB(1) receptor upregulation in a rat model of chronic neuropathic pain. Eur J Pharmacol. 2001;415:R5–R7. doi: 10.1016/s0014-2999(01)00798-1. [DOI] [PubMed] [Google Scholar]
- 146.Small-Howard AL, Shimoda LM, Adra CN, Turner H. Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells. Biochem J. 2005;388((pt 2)):465–73. doi: 10.1042/BJ20041682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of D9-tetrahydrocannabinol and anandamide antinoci-ception in non-arthritic and arthritic rats. Pharmacol Biochem Behav. 1998;60:183–191. doi: 10.1016/s0091-3057(97)00583-2. [DOI] [PubMed] [Google Scholar]
- 148.Smith PB, Martin BR. Spinal mechanisms of delta 9-tetrahydrocannabinol-induced analgesia. Brain Res. 1992;578:8–12. doi: 10.1016/0006-8993(92)90222-u. [DOI] [PubMed] [Google Scholar]
- 149.Smith PF. Medicinal cannabis extracts for the treatment of multiple sclerosis. Curr Opin Investig Drugs. 2004;5:727–730. [PubMed] [Google Scholar]
- 150.Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clin Pharmacol Ther. 1978;23:397–401. doi: 10.1002/cpt1978234397. [DOI] [PubMed] [Google Scholar]
- 151.Stella N, Schweitzer P, Piomelli DA. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 152.Strangman NM, Walker JM. The cannabinoid WIN 55,212-2 inhibits the activity-dependent facilitation of spinal noci-ceptive responses. J Neurophysiol. 1999;81:472–477. doi: 10.1152/jn.1999.82.1.472. [DOI] [PubMed] [Google Scholar]
- 153.Swift W, Hall W, Tesson M. Cannabis use and dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Addiction. 2001;96:737–748. doi: 10.1046/j.1360-0443.2001.9657379.x. [DOI] [PubMed] [Google Scholar]
- 154.Szabo B, Wallmichrath I, Mathonia P, Pfreundtner C. Cannabinoids inhibit excitatory neurotransmission in the substantia nigra pars reticulata. Neuroscience. 2000;97:89–97. doi: 10.1016/s0306-4522(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 155.Takano Y, Yaksh TL. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J Pharmacol Exp Ther. 1992;261:764–772. [PubMed] [Google Scholar]
- 156.Tjolsen A, Hole K. In: Animal models of analgesia In: The Pharmacology of Pain. Dick-enson L, Bessing J, editors. Heidelberg: Springer; 1997. pp. 1–20. [Google Scholar]
- 157.Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- 158.Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323:16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsou K, Brown MC, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distrubution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 160.Valenzano KJ, Tafessem L, Lee G, Harrison JE, Bouletm JM, Gottshallm SL, Mark L, Pearson MS, Miller W, Shan S, Rabadi L, Rotshteyn Y, Chafferm SM, Turchinm PI, El-semore DA, Toth M, Koetznerm L, Whiteside GT. Pharmacological and pharmacokinetic characterization of the can-nabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 161.Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gat-tlen B, Hagen U, Schnelle M, Reif M. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10:417–424. doi: 10.1191/1352458504ms1048oa. [DOI] [PubMed] [Google Scholar]
- 162.Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- 163.Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- 165.Wade DT, Robson P, House H, Makela P, Ara J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–29. doi: 10.1191/0269215503cr581oa. [DOI] [PubMed] [Google Scholar]
- 166.Walker JM, Hohmann G, Martin WJ, Strangman NM. The neurobiology of cannabinoid analgesia. Life Sci. 1999;65:665–673. doi: 10.1016/s0024-3205(99)00289-1. [DOI] [PubMed] [Google Scholar]
- 167.Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walker JM, Krey JF, Chu CJ, Huang SM. Endocan-nabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids. 2002;121:159–172. doi: 10.1016/s0009-3084(02)00152-4. [DOI] [PubMed] [Google Scholar]
- 169.Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ware MA, Doyle CR, Woods R, Lynch ME, Clarck AJ. Cannabis use for chronic non-cancer pain: results of a prospective survey. Pain. 2003;102:211–216. doi: 10.1016/s0304-3959(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 171.Ware MA, Gamsa A, Persson J, Fitzcharles MA. Cannabis for chronic pain: case series and implications for clinicians. Pain Res Manag. 2002;7:95–99. doi: 10.1155/2002/380509. [DOI] [PubMed] [Google Scholar]
- 172.Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- 173.Welch SP, Dunlow LD, Patrick GS, Razdan RK. Characterisation of anandamide-induced antinociception and cross-tolerance to delta 9-THC after intrathecal administration to mice: blockade of delta 9-THC-induced antinociception. J Pharmacol Exp Ther. 1995;273:1235–1244. [PubMed] [Google Scholar]
- 174.Welch SP, Stevens DL. Antinociceptive activity of in-trathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther. 1992;262:10–18. [PubMed] [Google Scholar]
- 175.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 176.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 177.Zeidenberg P, Clark WC, Jaffe J, Anderson SW, Chin S, Malitz S. Effect of oral administration of delta-9-tetrahydrocannabinol on memory, speech, and perception of thermal stimulation: results with four normal human volunteer subjects. Preliminary report. Comp Psychiatry. 1973;14:549–556. doi: 10.1016/0010-440x(73)90040-0. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Don-nell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 179.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoal-gesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]