Abstract
Injury or inflammation release a range of inflammatory mediators that increase the sensitivity of sensory neurons to noxious thermal or mechanical stimuli. The heat- and capsaicin-gated channel TRPV1, which is an important detector of multiple noxious stimuli, plays a critical role in the development of thermal hyperalgesia induced by a wide range of inflammatory mediators. We review here recent findings on the molecular mechanisms of sensitisation of TRPV1 by inflammatory mediators, including bradykinin, ATP, NGF and prostaglandins. We describe the signalling pathways believed to be involved in the potentiation of TRPV1, and our current understanding of how inflammatory mediators couple to these pathways.
Key Words: Pain, sensory transduction, inflammation, protein kinase, intracellular signalling, capsaicin, heat, TRPV1
INTRODUCTION
Pain-producing stimuli are detected in vivo by the nerve terminals of primary sensory neurons, whose cell bodies are found in sensory ganglia such as the dorsal root ganglia (DRG). The signal, in the form of action potentials, is then transmitted along primary sensory nerve fibres to the dorsal horn of the spinal cord, and from there on to higher brain centres, where it is interpreted as pain [67]. The specialised primary sensory neurons involved in the transduction of painful stimuli into action potentials are called nociceptors, a term coined by Sherrington 100 years ago [104]. Nociceptors can be activated by a wide range of thermal, mechanical and chemical stimuli (reviewed in ref. [30]). A unique feature of nociceptors is that the gain of signal transduction can be enhanced, or sensitised, by inflammatory mediators, such as prostaglandins, bradykinin, ATP, protons and nerve growth factor (NGF), which are released during tissue injury, metabolic stress and inflammation [13,21,106,120,126]. The effects of these inflammatory mediators on nociceptors are commonly produced by activation or sensitisation of membrane ion channels at nociceptor terminals, through the action of receptor-initiated second messenger cascades (reviewed in ref. [68]).
The heat and capsaicin receptor TRPV1 (formerly known as VR1), an important ion channel involved in pain sensation, plays a critical role in the development of inflammatory hyperalgesia. Knockout studies have shown that mice lacking the TRPV1 receptor fail to develop thermal hyperalgesia during inflammation [18,25]. In addition, pharmacological studies using capsazepine and other more potent TRPV1 antagonists have found that mechanical hyperalgesia was also attenuated in a variety of pain models, supporting the hypothesis that activation of TRPV1 causes mechanical hyper-algesia even though TRPV1 is not directly involved in the detection of noxious mechanical stimuli [48,55,89,129]. Thus, the central implication of TRPV1 in hyperalgesia makes it a potential target for novel analgesic and anti-inflammatory drugs. Since the channel was first cloned, intensive work has been carried out to understand the pharmacological properties of TRPV1 and its regulation by inflammatory mediators.
THE TRPV1 RECEPTOR
TRPV1 is a member of the transient receptor potential (TRP) channel family, and was first isolated from a rodent DRG cDNA library by using a calcium-imaging based expression assay [19]. In agreement with an essential role in nociception, the expression of TRPV1 is mainly detected in small to medium size neurons in the trigeminal ganglia (TG) and dorsal root ganglia (DRG) [38]. Within DRG neurons the level of TRPV1 mRNA is highly variable, being high in a group of neurons expressing the TrkA receptor for NGF but low in somatostatin containing cells [70]. TRPV1 is colocalised both with the peptidergic marker substance P, and with the lectin IB4 which labels non-pep-tidergic neurons in the DRG [29,37,43]. TRPV1 is also found in most cells of the nodose ganglia [38,70]. TRPV1 immunopositive fibers are detected in a variety of tissues such as skin [27,37,45], urinary bladder [7,134], gastrointestinal tract [130], tooth pulp [95], lung [53] and prostate [124]. However, the expression of TRPV1 is not restricted to peripheral neuronal tissues. Functional TRPV1 channels are also found in many areas of the central nervous system [69, 97] and in some non-neuronal tissues, such as epidermal keratinocytes of human skin [112], gastric epithelial cells [51], and epithelial cells of the urothelium and smooth muscle [11]. While our knowledge of the in vivo distribution of TRPV1 is expanding, the function of TRPV1 in cells other than primary sensory neurons remains poorly understood.
In common with other TRP channels, functional TRPV1 channels are homo- or heterotetramers of four single subunits [52]. Each single subunit of TRPV1 contains six transmembrane domains and a pore loop between domain five and six (see Fig. 1) [19]. When expressed together with other TRPV members in HEK 293 cells, the TRPV1 subunits preferentially assemble into homomeric complexes [39]. In addition, expression of TRPV1 alone in heterologous systems [19,36, 90] can fulfill the major electrophysiological properties of native receptors in DRG neurons [21]. These observations suggest that TRPV1 is most likely to form a homotetramer in vivo. Although some recent studies suggest that TRPV1 may form heteromultimers with the other TRPV family members, such as TRPV2 [60] and TRPV3 [110], the possible existence and functional roles of TRPV1 heterometers in native neurons are currently unclear.
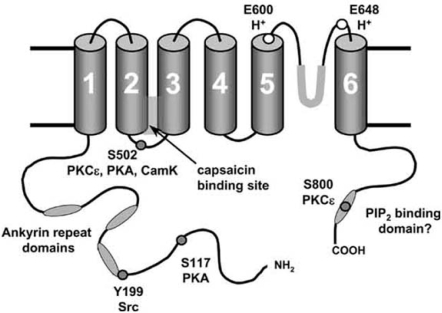
Fig. (1).
Sites on TRPV1 modulating function.
The hypothesised 6-transmembrane domain structure of TRPV1 is shown, with a pore loop between TM5 and TM6. A functional homomeric channel is formed from four such subunits. Sites at which phosphorylation is known to modulate TRPV1 function are shown together with the kinases responsible. Protons can bind to two extracellular glutamates, and protonation of E600 has an apparently similar effect on TRPV1 gating to phosphorylation at S502. Ankyrin domains, the capsaicin-binding domain and the hypothesised C-terminal PIP2 binding domain are also shown.
TRPV1 functions as a polymodal sensor of physical and chemical noxious stimuli in nociceptors. Studies of the ion selectivity of TRPV1 in HEK 293 cells reveal that TRPV1 is a non-selective cation channel with a high relative permeability to calcium [19]. To date, TRPV1 is believed to be the only receptor for vanilloids such as capsaicin, based on the finding that vanilloids evoked no pain related behaviours in TRPV1 knock-out mice [18]. In addition to its response to vanilloids, TRPV1 is also directly gated by noxious heat (>42 °C) and strong acidic conditions (pH<6, ref [118]), by ethanol [121] and by a variety of endogenous lipids such as anandamide [123,138], lipoxygenase products [44] and the potent endovanilloids N-arachidonoyl-dopamine, NADA [42] and N-oleoyldopamine, OLDA [22].
SIGNALLING PATHWAYS MODULATING TRPV1
The sensitivity of TRPV1 to noxious stimuli may be influenced by a number of intracellular signalling pathways (see Fig. 1). There are a number of potential target sites on TRPV1 for phosphorylation by protein kinases. For example, several residues on rat TRPV1 (S502, T704, S744, S800 and S820) have been reported to be phosphorylated by protein kinase C (PKC), although the evidence to date suggests that only S502 and S800 play a significant functional role [8,81]. Protein kinase A (PKA) phosphorylates TRPV1 at six residues (S116, T144, T370, S502, S774 and S800), amongst which S116 appears to be the only important site for PKA modulation [10,72]. The Y199 site (Y200 in human TRPV1), which is located at the start of the first N-terminal ankyrin repeat, plays a critical role in the modulation of TRPV1 trafficking to the neuronal membrane by the tyrosine kinase Src [136]. These kinases may sensitise the channel by increasing the channel open probability, which seems to be the principal effect of phosphorylation by PKCε [126], by reversing desensitisation, in the case of PKA [10], and/or by rapid recruitment of new channels to the cell surface membrane by regulated exocytosis [74,122,136], a process which is mediated by phosphorylation by Src [136]. It has also been suggested that phosphorylation of two sites (S502 and T704) on TRPV1 by CaMKII is required for vanilloid binding to TRP V1, but has no effect on the channel response to acid [50]. Unlike PKC, PKA or Src, however, whose actions have been well studied, little is known about how phosphorylation by CaMKII may alter the function of TRPV1.
Protons are released into the external medium under conditions of anoxia or inflammation, and can interact with TRPV1 in two ways: a low external pH (<6) can directly activate TRPV1 by protonating a glutamate (E648) adjacent to the pore region, but milder acidification protonates E600, at the immediate external face of transmembrane helix 5, leading to an enhancement of the activation of TRPV1 by other stimuli without directly activating the channel [49], a mechanism which is reminiscent of the effects of phosphorylation by PKCε [20,21].
TRPV1 may also be modulated by endogenous PIP2 which has been suggested to bind to the C-terminal of TRPV1 and to inhibit the function of the channel [23,92]. Removal of PIP2 by application of exogenous PLC to isolated membrane patches was found to increase the current activated by capsaicin, heat and protons [23], and removal of a supposed PIP2 binding cassette in the C-terminal domain ablated the sensitising effect of NGF, which activates PLCγ and so metabolises PIP2 [92]. More recently, however, it has been found that the resynthesis of PIP2 by PI4K promotes recovery of TRPV1 from desensitisation [61], an effect apparently contrary to that described above. It has also recently been reported that the removal of the supposed PIP2 binding domain promotes phosphorylation of the Y199 site by Src [136], an action which may explain the effect of removal of this domain without the need to invoke binding of PIP2. Thus the physiological role (if any) of PIP2 binding to TRPV1 is currently unresolved and is a major issue to be clarified in future studies.
SENSITISATION OF TRPV1 BY INFLAMMATORY MEDIATORS
Inflammatory mediators have multiple effects on TRPV1 that lead to nociceptor sensitisation. Some pro-inflammatory mediators, such as protons and certain lipid mediators (e.g. anandamide) are capable of directly activating TRPV1, albeit at rather high and perhaps unphysiological concentrations. Probably a more physiologically relevant mechanism is the modulation of the gating of TRPV1 by the actions of protein kinases, phosphatases, and/or lipid messengers produced by receptor-coupled intracellular signalling pathways. Many inflammatory mediators (e.g. bradykinin, prostaglandins, glutamate, chemokines, prokineticins and ATP) bind to receptors belonging to the G-protein-coupled receptor (GPCR) family, which signal to PKC and PKA to sensitise TRPV1 (see Fig. 2). Furthermore, some inflammatory mediators, such as NGF, insulin and IGF-1, may induce hyperalgesia by increasing the number of TRPV1 channels available to be activated on the nociceptor surface membrane. This process includes both transferring functional TRPV1 from intracellular vesicles to the plasma membrane [74,122,136] and up regulating the expression level of TRPV1 [46,93].
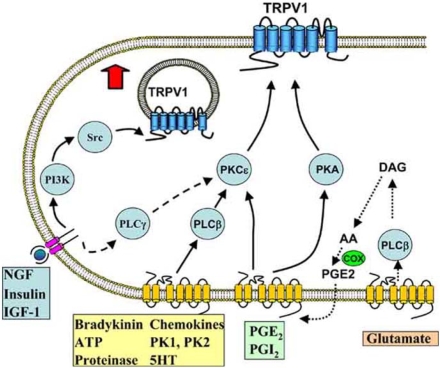
Fig. (2).
Inflammatory mediators and intracellular signalling pathways modulating TRPV1.
NGF and other mediators activating tyrosine kinase-coupled receptors promote the insertion of TRPV1 into the membrane, from a pool located in subcellular vesicles, via a pathway in which PI3K plays an early role while the downstream tyrosine kinase Src activates trafficking to the membrane by phosphorylating Y199 in the N-terminal tail of TRPV1. These receptors also couple to a lesser extent to TRPV1 via PLCγ and PKCε. Seven-transmembrane G-protein coupled receptors activated by a range of inflammatory mediators, including bradykinin and ATP, activate PLCβ and consequently PKCε, which enhances the activity of TRPV1 in the surface membrane by phosphorylating S502 and S800. Prostaglandins PGE2 and PGI2 activate EP and IP receptors, which couple to PKA and PKCε. Glutamate (and probably other agonists such as bradykinin) appears to act at least in part via an indirect route in which the production of arachidonic acid (AA) leads to the synthesis of prostaglandins via the cyclo-oxygenase (COX) pathway and activation of prostaglandin receptors.
Bradykinin
Bradykinin, a nonapeptide which is generated following tissue injury and noxious stimulation, has been known for many years to be a major contributor to the inflammatory response [30]. Injection of bradykinin into human skin produces a dose-dependent pain and a heat hyperalgesia [63], suggesting that bradykinin is capable of exciting nociceptors as well as sensitising their response to heat.
The biological effects of bradykinin in vivo are mediated through two transmembrane G-protein-coupled receptors, the B1 and B2 receptors [65,87]. Functional B2 receptors are widely expressed in the nervous system and in other tissues, but the B1 receptor, on the other hand, is thought to be mainly absent under normal non-inflamed conditions, but to be induced and overexpressed during chronic inflammation [24,88]. Recent data, however, has demonstrated the existence of functional B1 receptors in a small proportion (∼ 2%) of normal DRG neurons [127]. Activation of the B1 receptor sensitises the heat-evoked current in DRG neurons with a more prolonged effect than the B2 receptor, indicating that the B1 receptor may play a more important role in the maintenance of inflammatory pain [127].
B2 receptor activation initiates a number of signal transduction cascades. A major target is PLCβ, which catalyses breakdown of PIP2 into IP3, causing a rise of calcium in the cell, and release of diacylglycerol (DAG), which in turn activates PKC. B2 receptor activation is also known to activate phospholipases A2 and D, and in addition protein tyrosine kinases, phosphatases and MAP kinases [24].
Bradykinin was found to enhance the heat-activated current in isolated DRG neurons [21]. The bradykinin-induced sensitisation of the neuronal response to heat could be mimicked by directly activating PKC, and PKCε was observed to be translocated to the membrane after application of bradykinin [20]. These findings indicate that PKCε plays a vital role in bradykinin-evoked thermal hyperalgesia. Subsequent experiments have confirmed the idea that enhancement of TRPV1 activity through the PLCβ/PKCε pathway is a major molecular mechanism for bradykinin-induced sensitisation of nociceptors [116]. Activation of PKCε by bradykinin via the B2 receptor leads to phosphorylation of TRPV1 at two serine residues, S502 and S800, which potentiates the gating of TRPV1 by noxious stimuli [57,81,91,126]. Another mechanism which may be involved in bradykinin-induced acute senstisation of TRPV1 is hydrolysis of PIP2 by PLC, which has been proposed to sensitise the channel by relieving TRPV1 from PIP2 inhibition [23], though as mentioned above, the current status of this proposal is uncertain.
In addition to sensitising the nociceptor response to heat, bradykinin is able to activate these neurons and so produce pain. TRPV1 may contribute to the excitation of neurons by bradykinin, based on the observation that bradykinin-evoked action potentials were reduced by the TRPV1 antagonist capsazepine [17,56], and that the response of C-fibres to bradykinin was significantly less in TRPV1 knockout mice than that in wild type mice [53]. However, the mechanism by which bradykinin activates TRPV1 seems to be different from that by which it sensitises the channel. The number of bradykinin-induced action potentials in C-fibres was reduced by lipoxygenase inhibitors, suggesting that lipoxygenase products may be involved in this process [17]. One likely hypothesis is that activation of PLA2 induces mobilisation of arachidonic acid (AA) and generation via the lipoxygenase pathway of 12-HPETE, which has been shown to activate TRPV1 directly [105].
ATP
Another important inflammatory mediator released from damaged tissues is ATP, which both produces pain by directly exciting nociceptors and induces hyperalgesia by sensitisation of nociceptors to other noxious stimuli [16,31,102]. The ATP receptors expressed in neurons are divided into two groups: ionotropic P2X receptors and metabotropic P2Y receptors [94]. The current gated by extracellular ATP in sensory neurons is believed to be mediated by P2X receptors, while P2Y receptors play an important role in the ATP-induced sensitisation of nociceptors to noxious stimuli. In TRPV1 knock-out mice, extracellular ATP fails to induce thermal hyperalgesia, suggesting a critical role of TRPV1 in mediating the sensitising effect of extracellular ATP [76, 119]. Patch clamp experiments confirmed that extracellular ATP enhanced the TRPV1-dependent capsaicin and proton-evoked response and reduced the temperature threshold for TRPV1 activation [120]. The potentiating effects of extracellular ATP on TRPV1 activity were almost completely abolished by PKC inhibitors, indicating the activation of PKC via a PLCβ-coupled signalling cascade as a possible mechanism [119,120]. Initial experiments suggested that the P2Y1 receptor for ATP was important [120], but more recent data show that it is P2Y2 which initiates the downstream pathways leading to TRPV1 sensitisation [54,76].
NGF
Nerve growth factor (NGF), a member of the neurotrophin family, is essential for the development and maintenance of the central and peripheral nervous systems [33,58]. The interest in NGF as a mediator of inflammatory pain was originally stimulated by the finding that it was upregulated after inflammation or injury [131]. Injection of NGF into adult rat paw produced a rapid and prolonged hypersensitivity to noxious thermal stimulation [59], confirming an important role for NGF in inflammatory pain. This thermal hyperalgesia became noticeable within a few minutes and was maintained for several days [100].
NGF interacts with two types of cell surface receptors, the tyrosine kinase receptor A (TrkA) and the pan-neurotrophin p75NTR receptor, each of which activates different sets of signalling pathways [26,78,98]. The effect of NGF in promoting inflammatory hyperalgesia is thought to be predominantly mediated via TrkA receptors [66].
NGF-mediated thermal hyperalgesia results principally from sensitisation of TRPV1, because in TRPV1 null mice, the thermal hyperalgesia induced by injection of NGF is largely absent [23]. Short term (∼ 10 minutes) administration of NGF acutely enhances the response of DRG neurons to heat and capsaicin [13,34,107], while long term (4 ∼ 6 days) application of NGF increases the number of capsaicin-sensitive DRG neurons in culture [132,133], indicating that NGF enhances both the activity of existing TRPV1 and the expression level of TRPV1 in DRG neurons.
The short-term sensitisation of TRPV1 by NGF occurs so rapidly that it must be mediated by post-translational processing. Several groups have investigated the mechanisms underlying the acute effect of NGF on TRPV1, but the intracellular signalling involved in this sensitisation process is still controversial. Phosphorylation of TRPV1 via PKA was at first suggested to be involved in the sensitisation of DRG neurons by NGF [107]. Later, experiments using calcium imaging rather than patch clamp, in order to measure sensitisation in a larger number of neurons and so improve the statistics, discounted PKA as a participant but found that partial or total inhibition of the action of NGF was produced by inhibitors of PI3 kinase (PI3K), PKC and CaMKII but not of the Ras-MEK-Erk cascade, at least on a short-term time scale [13]. Later studies, however, have suggested an involvement of the Ras-MEK-Erk cascade [3,137]. A different mechanism was proposed by Chuang and colleagues [23], who suggested that NGF sensitises TRPV1 by activating PLCγ, which hydrolyses PIP2, thereby releasing TRPV1 from PIP2-mediated inhibition. A region was identified in the C-terminal domain of TRPV1 (777-820), which was proposed to be essential for PIP2 –TRPV1 interaction [92]. However, this mechanism was questioned by calcium imaging experiments using isolated DRG neurones [13] and patch clamp and behavioural experiments in adult rats [137] which demonstrated the participation of PI3K and not PLCγ in both the enhancement of TRPV1 function and the NGF-induced hyperalgesia in vivo.
More recent data have shown that sensitisation of TRPV1 by NGF is mediated by two pathways [136]. The major pathway is activated by the Y760 site on TrkA, which stimulates PI3 kinase, with Src kinase being activated downstream of PI3K. Src kinase phosphorylates TRPV1 at a single tyrosine residue, Y199 (Y200 in human TRPV1), leading to trafficking and insertion of the channel into the surface membrane and thus enhancing membrane ionic currents carried by TRPV1 (see Fig. 2). A second and more minor pathway is the PLCγ/PKCε signalling pathway, which causes phosphorylation of TRPV1 at the S502 and S801 sites. Furthermore, deletion of the proposed PIP2 binding cassette [92] increased hTRPV1 tyrosine phosphorylation at the Y200 site and sensitised the basal activity of the channel, suggesting that the change in behaviour of TRPV1 following the 777-820 deletion is a consequence of sensitisation of the channel by Src, and not of removal of a PIP2 binding site per se [136].
In addition to its short-term action, NGF is responsible, at least in part, for the upregulation of TRPV1 expression following inflammation [6,46]. However, in DRG neurons, neither an increase in TRPV1 mRNA levels was detected after the injection of NGF [46], nor was there any change in the TRPV1 mRNA level found in PC12 cells treated with NGF [93]. In contrast, increased axonal transport of TRPV1 mRNA was observed after carrageenan treatment [117], and expression of TRPV1 protein was promoted by NGF in both DRG neurons and PC12 cells [46,93]. Therefore, TRPV1 does not show parallel NGF-induced changes in mRNA and protein levels, indicating that NGF regulates TRPV1 expression by increasing translation and transport of the channel without changing the degree of transcription. The increase in TRPV1 expression induced by NGF was found to be mediated by the TrkA-dependent MAPK pathways [15]. Small GTP binding regulatory proteins, Ras and Rac, are believed to play an important role in initiating the signalling pathway [15]. It has recently been suggested that Rac activates NADPH oxidase, which subsequently generates reactive oxygen species (ROS) leading to stimulation of p38 MAPK activity [93]. Activation of p38 MAPK results in upregulation of TRPV1 expression and increases pain perception [46]. Other studies show that Erk, another member of the MAPK family, and PI3K may also be involved in this process [15].
Lipid Mediators
Prostaglandins are generated in response to noxious stimuli and inflammatory insults [12]. Several prostanoid receptor subtypes are reported to be expressed in DRG neurons, including the PGE2 receptors EP(1-4) [84,114] and the PGI2 receptor IP [64]. Critical actions of EP and IP receptors in inflammation and pain were later demonstrated by behavioural studies of mice lacking IP, EP1 or EP3 receptors, where in all three cases, mice lacking one of these receptors showed a significant reduction in pain perception and thermal hyperalgesia [71,75,77,113]. At the cellular level, when TRPV1 was knocked out, the PGI2-induced thermal hyperalgesia was found to almost completely disappear, indicating that TRPV1 is an essential contributor to prostaglandin-mediated thermal hyperalgesia [75]. Two second-messenger pathways are implicated in potentiation of TRPV1 by PGE2 and PGI2 (see Fig. 2): the PKC-dependent pathway [75] and the cAMP / PKA signalling cascade, both of which sensitise TRPV1 by phosphorylating specific sites on the channel [35,62,111].
Prostaglandins work not only as mediators that directly activate downstream cascades leading to sensitisation of TRPV1, but also function as second messengers in the potentiationof TRPV1 by other inflammatory mediators. One such mediator is glutamate, which contributes to the development of inflammatory hyperalgesia through the group I metabotropic glutamate receptors, of which mGluR1 and mGluR5 are expressed in sensory neurons [9,128]. Application of an mGluR1/5 agonist DHPG enhanced the capsaicin response in DRG neurons, indicating that TRPV1 is involved in the hyperalgesia evoked by mGluR1/5 activation [41]. mGluR1/5 receptors primarily couple to the PLC pathway, which activates PKC downstream [14]. However, the DHPG-induced enhancement of the capsaicin response does not seem to depend on the activation of PKC but instead on the activation of cyclooxygenases (COXs) and PKA, suggesting that mGluR1/5 activation sensitises TRPV1 by a novel mechanism: PLC activation results in generation of lipid messengers (e.g. arachidonic acid), which are metabolised by COX into prostaglandins, ultimately activating the PKA pathway through prostaglandin receptors [41]. Thus, prostaglandins may play a crucial role in glutamate-induced TRPV1 sensitisation (Fig. 2).
Anandamide (N-arachidonoyl-ethanolamide) is another potential lipid mediator in inflammatory hyperalgesia [86, 138]. The role of anandamide in nociception is potentially complex, because two receptors are activated by anandamide in DRG neurones: TRPV1 itself, and the cannabinoid 1 (CB1) receptor, both of which show a high degree of colocalisation [1,2]. The significant structural similarity between anandamide and some of the synthetic vanilloids, such as olvanil, suggested that anandamide may be an endogenous agonist of TRPV1 [28]. However, the potency of anandamide for TRPV1 is 5 ∼ 10 fold lower than capsaicin in various native tissues [99]. For, example, in DRG neurons, the EC50 of anandamide is 6 ∼ 10 μM [44,85,109]. Thus micromolar concentrations of anandamide are required to activate TRPV1 and it is unclear whether endogenous concentrations of anandamide will be sufficient to activate the channel. Some evidence suggests that during inflammation, other inflammatory mediators, such as bradykinin and PGE2, may activate pathways that convert anandamide to a potent TRPV1 activator [108]. However, at much lower concentrations anandamide activates the CB1 receptor, which has a dual effect on the sensitivity of TRPV1 [40]. Firstly, activation of the CB1 receptor was observed to enhance the TRPV1 response to capsaicin, an effect suggested to be mediated by PI3K and PLC pathways [40]. On the other hand, CB1 receptor activation has been found to attenuate rather than enhance TRPV1 activation when PKA is stimulated by forskolin [40]. Such an effect might explain the findings that cannabinoids can reduce hyperalgesia and inflammation, at least in part by inhibition of TRPV1-mediated release of transmitters from terminals of sensory neurones [96]. Thus, anandamide may play a dual role on TRPV1 activity, directly and via the CB1 receptor. However, it has been suggested that during inflammation or other pathological conditions, because of the increase in TRPV1 expression in neurons, an increased anandamide concentration in terminals may lead to an enhancement rather than a decrease in inflammatory heat hyperalgesia [108]. This hypothesis was supported by the earlier findings that anandamide increases release of CGRP in diabetic animals but inhibits it in normal animals [32].
Other Inflammatory Mediators
As the role of different inflammatory mediators in modulating the activity of TRPV1 is more extensively studied, more candidates have been added to the list of mediators that may regulate the TRPV1 response during inflammation (see Fig. 2). For example, following a similar pattern to NGF (discussed above), IGF-1 and insulin have been found to enhance TRPV1-mediated membrane currents both by enhancing the channel gating properties and by translocation of TRPV1 to the plasma membrane [122]. As with NGF, both PI3K and PKC-mediated TRPV1 phosphorylation were found to be essential for the enhancement of the TRPV1 response by IGF-1 / insulin [122].
Phosphorylation of TRPV1 by PKC is involved in the sensitisation of TRPV1 by several other inflammatory mediators. Chemokines, in particular CCL3 (formerly known as macrophage inflammatory protein 1α), play an important role in mediating inflammation [83,101]. In a similar manner to bradykinin or ATP, CCL3 sensitises TRPV1 by activation of its major receptor CCR1, which leads to activation of the PLC / PKC pathway [135]. The protease-activated receptor-2 (PAR-2), whose activation is associated with inflammation in the lung [103], was found to promote TRPV1 function through both the PKC-dependent and the PKA-dependent pathways [4,5]. Serotonin (5HT) also appears to sensitise TRPV1 via both PKC and PKA pathways [115]. Recent data indicate that PKCε is also responsible for the sensitisation of TRPV1 by the prokineticin/prokineticin receptor system [125]. Prokineticins (or their anuran homolog, Bv8) induce hyperalgesia in rats through binding to the prokineticin receptors 1 and 2 (PKR1 and PKR2, refs [73,79,80]; see article by L. Negri in this issue). Activation of PKRs by Bv8 induces translocation of PKCε to the membrane in DRG neurons, indicating that Bv8 potentiates TRPV1 via phosphorylation of the channel by PKCε [125]. Finally, sensitisation of TPRV1 by PKC appears to underlie the IL-1β- induced potentiation of heat-evoked currents in rat DRG neurons, and tyrosine phosphorylation of TRPV1 may also be involved [47,82].
CONCLUSION
We have reviewed here the role of inflammatory mediators in modulating activities of TRPV1 following tissue injury and inflammation. The available evidence suggests that inflammatory mediators alter the sensitivity of TRPV1 by various signalling pathways (including PLC/PKC, PKA, ERK/ MAPK, and PI3K/Src), suggesting a complex network in vivo for modulation of channel function under both physiological and pathological conditions. Sensitisation of TRPV1 via phosphorylation by PKC and/or PKA is by now a well-established mechanism of sensitisation of the gating of TRPV1, which is employed by a number of inflammatory mediators. Insertion of new TRPV1 channels into the nociceptor membrane is a recently-discovered and novel mechanism for the development and maintenance of inflammatory hyperalgesia. Recent work has shown that the trafficking of TRPV1 to the neuronal membrane is upregulated by phosphorylation of TRPV1 by Src. While this pathway was first elucidated for NGF-induced hyperalgesia, it seems likely that other inflammatory mediators activating tyrosine kinase receptors will employ the same route to induce hyperalgesia. Modulation of TRPV1 may be further complicated by crosstalk between the various signalling pathways.
REFERENCES
- 1.Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience. 2002;110:747–753. doi: 10.1016/s0306-4522(01)00601-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 3.Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A- dependent mechanisms in rats and mice. J Physiol. 2006 doi: 10.1113/jphysiol.2006.111534. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 7.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience. 2002;109:787–798. doi: 10.1016/s0306-4522(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 8.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- 10.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 11.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bley KR, Hunter JC, Eglen RM, Smith JA. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci. 1998;19:141–147. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- 13.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. doi: 10.1016/s0301-0082(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 15.Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22:118–132. doi: 10.1016/s1044-7431(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 16.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 17.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 18.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 21.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu CJ, Huang SM, De PL, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 23.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 24.Couture R, Harrisson M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation. Eur J Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- 25.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 26.Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol. 2003;29:211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 27.Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, Matsunaga K. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285:1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- 28.Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, Pertwee R, De Petrocellis L. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- 29.Dinh QT, Groneberg DA, Mingomataj E, Peiser C, Heppt W, Dinh S, Arck PC, Klapp BF, Fischer A. Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides. 2003;37:245–250. doi: 10.1016/s0143-4179(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 30.Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 31.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 32.Ellington HC, Cotter MA, Cameron NE, Ross RA. The effect of cannabinoids on capsaicin-evoked calcitonin generelated peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology. 2002;42:966–975. doi: 10.1016/s0028-3908(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 33.Farinas I. Neurotrophin actions during the development of the peripheral nervous system. Microsc Res Tech. 1999;45:233–242. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<233::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–541. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- 35.Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol. 2003;89:1985–1993. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- 36.Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) J Physiol. 2000;525:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 38.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 39.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 40.Hermann H, De Petrocellis L, Bisogno T, Schiano MA, Lutz B, Di Marzo V. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci. 2003;60:607–616. doi: 10.1007/s000180300052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu HJ, Bhave G, Gereau RW. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang SM, Bisogno T, Trevisani M, Al Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang SJ, Valtschanoff JG. Vanilloid receptor VR1-positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neurosci Lett. 2003;349:41–44. doi: 10.1016/s0304-3940(03)00750-x. [DOI] [PubMed] [Google Scholar]
- 44.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002;291:124–129. doi: 10.1006/bbrc.2002.6393. [DOI] [PubMed] [Google Scholar]
- 46.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalge-sia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 47.Jin X, Morsy N, Winston J, Pasricha PJ, Garrett K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol. 2004;287:C558–C563. doi: 10.1152/ajpcell.00113.2004. [DOI] [PubMed] [Google Scholar]
- 48.Jones RC III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 51.Kato S, Aihara E, Nakamura A, Xin H, Matsui H, Kohama K, Takeuchi K. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol. 2003;66:1115–1121. doi: 10.1016/s0006-2952(03)00461-1. [DOI] [PubMed] [Google Scholar]
- 52.Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 53.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1-/- mice. J Physiol. 2004;555:115–123. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol. 2005;25:819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J, Lee J, Kang M, Shin M, Kim JM, Kang SU, Lim JO, Choi HK, Suh YG, Park HG, Oh U, Kim HD, Park YH, Ha HJ, Kim YH, Toth A, Wang Y, Tran R, Pearce LV, Lundberg DJ, Blumberg PM. N-(3-acyloxy-2-benzylpropyl)-N’-[4-(methylsulfonylamino)benzyl]thiourea analogues: novel potent and high affinity antagonists and partial antagonists of the vanilloid receptor. J Med Chem. 2003;46:3116–3126. doi: 10.1021/jm030089u. [DOI] [PubMed] [Google Scholar]
- 56.Lee MG, Macglashan DW, Jr, Undem BJ. Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol. 2005;566:205–212. doi: 10.1113/jphysiol.2005.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SY, Lee JH, Kang KK, Hwang SY, Choi KD, Oh U. Sensitization of vanilloid receptor involves an increase in the phosphorylated form of the channel. Arch Pharm Res. 2005;28:405–412. doi: 10.1007/BF02977669. [DOI] [PubMed] [Google Scholar]
- 58.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 59.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22:825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopshire JC, Nicol GD. Activation and recovery of the PGE2-mediated sensitization of the capsaicin response in rat sensory neurons. J Neurophysiol. 1997;78:3154–3164. doi: 10.1152/jn.1997.78.6.3154. [DOI] [PubMed] [Google Scholar]
- 63.Manning DC, Raja SN, Meyer RA, Campbell JN. Pain and hyperalgesia after intradermal injection of bradykinin in humans. Clin Pharmacol Ther. 1991;50:721–729. doi: 10.1038/clpt.1991.212. [DOI] [PubMed] [Google Scholar]
- 64.Matsumura K, Watanabe Y, Onoe H, Watanabe Y. Prostacyclin receptor in the brain and central terminals of the primary sensory neurons: an autoradiographic study using a stable prostacyclin analogue [-3H]iloprost. Neuroscience. 1995;65:493–503. doi: 10.1016/0306-4522(94)00505-y. [DOI] [PubMed] [Google Scholar]
- 65.McEachern AE, Shelton ER, Bhakta S, Obernolte R, Bach C, Zuppan P, Fujisaki J, Aldrich RW, Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci USA. 1991;88:7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 67.McMahon SB, Koltzenburg M. Wall and Melzack’s Textbook of Pain. London: Churchill Livingston; 2005. [Google Scholar]
- 68.McNaughton PA. In: Pain Transduction: Modulation and Gating of Ion Channels In: Transduction Channels in Sensory Cells. Frings S, Bradley J, editors. Weinheim: Wiley-VCH; 2004. pp. 251–270. [Google Scholar]
- 69.Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minami T, Nakano H, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Ito S. Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. Br J Pharmacol. 2001;133:438–444. doi: 10.1038/sj.bjp.0704092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 73.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 74.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 75.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugi-moto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 me-tabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, Ichikawa A, Aze Y, Tanaka T, Yoshida N, Ueno A, Oh-ishi S, Narumiya S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 78.Nebreda AR, Martin-Zanca D, Kaplan DR, Parada LF, Santos E. Induction by NGF of meiotic maturation of Xenopus oocytes expressing the trk proto-oncogene product. Science. 1991;252:558–561. doi: 10.1126/science.1850550. [DOI] [PubMed] [Google Scholar]
- 79.Negri L, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, Porreca F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: Focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Negri L, Lattanzi R, Giannini E, Colucci MA, Mignogna G, Barra D, Grohovaz F, Codazzi F, Kaiser A, Kreil G, Melchi-orri P. Biological activities of Bv8 analogues. Br J Pharmacol. 2005;146:625–632. doi: 10.1038/sj.bjp.0706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 82.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 83.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichi-kawa A, Narumiya S. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olah Z, Karai L, Iadarola MJ. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- 86.Park KA, Vasko MR. Lipid mediators of sensitivity in sensory neurons. Trends Pharmacol Sci. 2005;26:571–577. doi: 10.1016/j.tips.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Pesquero JB, Pesquero JL, Oliveira SM, Roscher AA, Metzger R, Ganten D, Bader M. Molecular cloning & functional characterization of a mouse bradykinin B1 receptor gene. Biochem Biophys Res Commun. 1996;224:281. doi: 10.1006/bbrc.1996.1020. [DOI] [PubMed] [Google Scholar]
- 88.Petersen M, Segond vB, Heppelmann B, Koltzenburg M. Nerve growth factor regulates the expression of bradykinin binding sites on adult sensory neurons via the neurotrophin receptor p75. Neuroscience. 1998;83:161–168. doi: 10.1016/s0306-4522(97)00374-6. [DOI] [PubMed] [Google Scholar]
- 89.Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphy-ridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide(BCTC),a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II in vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- 90.Premkumar LS, Agarwal S, Steffen D. Single-channel properties of native and cloned rat vanilloid receptors. J Physiol. 2002;545:107–117. doi: 10.1113/jphysiol.2002.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 92.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 93.Puntambekar P, Mukherjea D, Jajoo S, Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem. 2005;95:1689–1703. doi: 10.1111/j.1471-4159.2005.03518.x. [DOI] [PubMed] [Google Scholar]
- 94.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 95.Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003;17:245–250. [PubMed] [Google Scholar]
- 96.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 97.Roberts JC, Davis JB, Benham CD. [-3H]Resinifera-toxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Tebar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 99.Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rueff A, Dawson AJ, Mendell LM. Characteristics of nerve growth factor induced hyperalgesia in adult rats: dependence on enhanced bradykinin-1 receptor activity but not neurokinin-1 receptor activation. Pain. 1996;66:359–372. doi: 10.1016/0304-3959(96)03060-6. [DOI] [PubMed] [Google Scholar]
- 101.Saeki T, Naya A. CCR1 chemokine receptor antagonists. Curr Pharm Des. 2003;9:1201–1208. doi: 10.2174/1381612033454937. [DOI] [PubMed] [Google Scholar]
- 102.Sawynok J, Sweeney MI. The role of purines in nocicep-tion. Neuroscience. 1989;32:557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- 103.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 104.Sherrington CS. The integrative action of the nervous system. New York: Scribner; 1906. [Google Scholar]
- 105.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reich-ling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalge-sia. Proc Natl Acad Sci USA. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 107.Shu X, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- 108.Singh TA, Santha P, Nagy I. Inflammatory mediators convert anandamide into a potent activator of the vanilloid type 1 transient receptor potential receptor in nociceptive primary sensory neurons. Neuroscience. 2005;136:539–548. doi: 10.1016/j.neuroscience.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 109.Smart D, Jerman JC, Gunthorpe MJ, Brough SJ, Ranson J, Cairns W, Hayes PD, Randall AD, Davis JB. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. Eur J Pharmacol. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- 110.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 111.Smith JA, Davis CL, Burgess GM. Prostaglandin E2-induced sensitization of bradykinin-evoked responses in rat dorsal root ganglion neurons is mediated by cAMP-dependent protein kinase A. Eur J Neurosci. 2000;12:3250–3258. doi: 10.1046/j.1460-9568.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 112.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratino-cytes. J Pharmacol Exp Ther. 2003;304:217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 113.Stock JL, Shinjo K, Burkhardt J, Roach M, Taniguchi K, Ishikawa T, Kim HS, Flannery PJ, Coffman TM, McNeish JD, Audoly LP. The prostaglandin E2 EP1 receptor mediates pain perception and regulates blood pressure. J Clin Invest. 2001;107:325–331. doi: 10.1172/JCI6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sugimoto Y, Shigemoto R, Namba T, Negishi M, Mizuno N, Narumiya S, Ichikawa A. Distribution of the messenger RNA for the prostaglandin E receptor subtype EP3 in the mouse nervous system. Neuroscience. 1994;62:919–928. doi: 10.1016/0306-4522(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 115.Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- 117.Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 118.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 119.Tominaga M, Numazaki M, Iida T, Moriyama T, Togashi K, Higashi T, Murayama N, Tominaga T. Regulation mechanisms of vanilloid receptors. Novartis Found Symp. 2004;261:4–12. [PubMed] [Google Scholar]
- 120.Tominaga M, Wada M, Masu M. Potentiation of cap-saicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates no-ciceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 122.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van der Aa F, Roskams T, Blyweert W, De Ridder D. Interstitial cells in the human prostate: a new therapeutic target? Prostate. 2003;56:250–255. doi: 10.1002/pros.10264. [DOI] [PubMed] [Google Scholar]
- 124.Van der Stelt M, Trevisani M, Vellani V, De Petrocellis L, Schiano MA, Campi B, McNaughton P, Geppetti P, Di Marzo V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J. 2005;24:3026–3037. doi: 10.1038/sj.emboj.7600784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anan-damide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. J Physiol. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini F, Kuhn R, Urban L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 129.Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- 130.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 131.Weskamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- 132.Winter J, Forbes CA, Sternberg J, Lindsay RM. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- 133.Winter J, Walpole CS, Bevan S, James IF. Characterization of resiniferatoxin binding sites on sensory neurons: co-regulation of resiniferatoxin binding and capsaicin sensitivity in adult rat dorsal root ganglia. Neuroscience. 1993;57:747–757. doi: 10.1016/0306-4522(93)90021-7. [DOI] [PubMed] [Google Scholar]
- 134.Yiangou Y, Facer P, Ford A, Brady C, Wiseman O, Fowler CJ, Anand P. Capsaicin receptor VR1 and ATP-gated ion channel P2X3 in human urinary bladder. BJU Int. 2001;87:774–779. doi: 10.1046/j.1464-410x.2001.02190.x. [DOI] [PubMed] [Google Scholar]
- 135.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemo-kine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci USA. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphati-dylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanil-loid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]