Abstract
The role of channel inactivation in the molecular mechanism of calcium (Ca2+) channel block by phenylalkylamines (PAA) was analyzed by designing mutant Ca2+ channels that carry the high affinity determinants of the PAA receptor site [Hockerman, G. H., Johnson, B. D., Scheuer, T., and Catterall, W. A. (1995) J. Biol. Chem. 270, 22119–22122] but inactivate at different rates. Use-dependent block by PAAs was studied after expressing the mutant Ca2+ channels in Xenopus oocytes. Substitution of single putative pore-orientated amino acids in segment IIIS6 by alanine (F-1499-A, F-1500-A, F-1510-A, I-1514-A, and F-1515-A) gradually slowed channel inactivation and simultaneously reduced inhibition of barium currents (IBa) by (−)D600 upon depolarization by 100 ms steps at 0.1 Hz. This apparent reduction in drug sensitivity was only evident if test pulses were applied at a low frequency of 0.1 Hz and almost disappeared at the frequency of 1 Hz. (−)D600 slowed IBa recovery after maintained membrane depolarization (1–3 sec) to a comparable extent in all channel constructs. A drug-induced delay in the onset of IBa recovery from inactivation suggests that PAAs promote the transition to a deep inactivated channel conformation. These findings indicate that apparent PAA sensitivity of Ca2+ channels is not only defined by drug interaction with its receptor site but also crucially dependent on intrinsic gating properties of the channel molecule. A molecular model for PAA-Ca2+ channel interaction that accounts for the relationship between drug induced inactivation and channel block by PAA is proposed.
Keywords: Ca2+ channel, phenylalkylamines, state-dependent block, antiarrhythmic action
Voltage gated L-type calcium (Ca2+) channels are the therapeutic target for phenylalkylamine (PAA) Ca2+ antagonists (1). Channel block by PAA in myocardial (2, 3), smooth muscle (4), and neuronal cells (5) is dependent on the channel conformation (resting, open, inactivated) and accumulates during repetitive depolarization of the membrane. It is widely accepted that this use- (6) or frequency-dependent block plays a significant role in the therapeutic action of PAAs as antiarrhythmics (7). Estimation of use-dependent block of Ca2+ channel currents during trains of test pulses serves, therefore, as a classical protocol to study the interaction of PAA with the pore forming α1 subunit of L-type Ca2+ channels. It has been shown that Ca2+ channels have to pass through an open conformational state to enable efficient channel block by PAA (3, 4, 8). The role of inactivation in Ca2+ channel block by PAA is, however, still not understood. High affinity determinants of the PAA-receptor site of L-type Ca2+ channels have recently been identified on transmembrane segment IVS6 by mutating putative pore-orientated amino acids and subsequent screening of the resulting mutants for PAA-sensitivity (8) or by transferring the responsible L-type amino acids into the α1A subunit of P/Q-type channels thereby introducing PAA sensitivity (10, 11). The resulting highly PAA sensitive triple α1A mutant AL25 (11) displayed significantly faster inactivation kinetics than the wild-type channel. Furthermore, an even more rapid inactivation and higher apparent PAA sensitivity was induced into α1A if an additional phenylalanine in position 1,805 of AL25 was mutated to the corresponding L-type methionine in IVS6 suggesting a close link between inactivation properties of the channel and use-dependent block by PAA.
However, because mutations of the PAA-receptor site itself or of closely located amino acids may not only affect channel inactivation but also the interaction of PAAs with their binding site on transmembrane segment IVS6, no unequivocal conclusions about the role of inactivation in channel block could be drawn (11). To answer this question we designed Ca2+ channels that inactivate at different rates by site directed mutagenesis of segment IIIS6.
We report here that substitutions of putative pore-lining amino acids that are highly conserved between α1A, α1S, and α1C gradually reduce the rate of channel inactivation while also gradually reducing use-dependent block by (−)D600. Our data indicate that changes in Ca2+ channel inactivation induced by site directed mutagenesis modulate use-dependent Ca2+ channel block by PAA. This suggests that channel inactivation is an important determinant of use-dependent Ca2+ channel block by these drugs.
METHODS
Generation of α1 Construct.
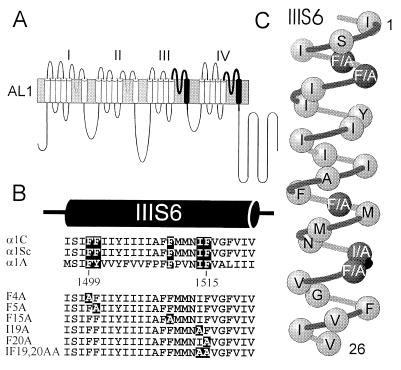
Chimeric α1 cDNAs. Chimeras and mutants were constructed as follows (“silent” restriction sites generated by PCR in previous cloning steps are indicated by asterisks): Chimera AL1 (amino acid composition: α1A1–1406, α1S 965–1104, α1A 1544–1723, α1S 1311–1437, α1A 1856–2424) was as described (12) but with a major portion of the 3′-noncoding region removed. The cDNA of this construct is termed AL1-a. It was constructed by ligating the XhoI (nucleotide position α1A 1698)-ClaI* (nucleotide position α1A 4925) fragment of the previously published AL1 construct into the respective sites of AL14 (12). Single point mutations were introduced into the SalI* (nucleotide position α1S 3317)-ClaI* (nucleotide position α1A 4925) cassette of the AL1-a construct using gene splicing by overlap extension (13). PCR was performed with 35 cycles (1 min at 94°C, 30 sec at 42°C, 1, 5 min at 72°C) using proofreading Pfu polymerase (Stratagene). All PCR-generated mutations were verified by sequence analysis employing the dideoxy chain termination method (14). Single alanine mutations were introduced into the IIIS6 segment at the following positions (amino acid number corresponds to IIIS6 in α1A, (for nomenclature see ref. 15): F1499; F1500, F1510, I1514, and F1515. These residues are located in positions 4, 5, 15, 19, and 20 of IIIS6 from the extracellular side. Therefore the resulting mutants were designated as F4A, F5A, F15A, I19A, F20A, IF19, and 29AA (Fig. 1).
Figure 1.
(A) Schematic representation of chimera AL1 (13) and derived mutants F4A-IF19,20AA. Repeats I and II were from the α1A subunit. L-type sequences that were inserted into the carp skeletal muscle α1S are shown as black transmembrane segments (IIIS6 and IVS6) with bold lines as adjacent S5-S6 connecting loops. (B) Sequence alignment (Lower) of transmembrane segments IIIS6 of chimera AL1 (the carp skeletal muscle α1S, see 33) and segments IIIS6 of the cardiac L-type α1C (34) and the class A α1A subunit (15). Homologous putative pore-orientated phenylalanines in segment IIIS6 of AL1 and alanine mutations are highlighted. (C) Schematic α-helical representation of amino acid sequence of repeat IIIS6 of chimera AL1 (shaded). Substituted putative pore-orientated amino acids of mutants F4A-F20A that are homologous between α1A and α1C are shown (solid).
All constructs were inserted into the polyadenylating transcription plasmids pSPCBI-2 (a kind gift of O. Pongs).
Electrophysiology.
Inward barium currents (IBa) were studied with two microelectrode voltage-clamp of Xenopus oocytes 2–7 days after microinjection of approximately equimolar cRNA mixtures of α1 (0.3 ng/50 nl)/β1a (0.1 ng/50 nl)/α2δ (0.2 ng/50 nl) as previously described (11, 12). All experiments were carried out at room temperature in a bath solution with the following composition: 40 mM Ba(OH)2, 40 mM N-methyl-d-glucamine, 10 mM Hepes, 10 mM glucose (pH adjusted to 7.4 with methanesulfonic acid). Voltage-recording and current-injecting microelectrodes were filled with 2.8 M CsCl, 0.2 M CsOH, 10 mM EGTA, 10 mM Hepes (pH 7.4), and had resistances of 0.3–2 MΩ.
To study the mechanism of Ca2+-channel block by the PAA (−)D600 we applied three different pulse protocols: (i) measurements of use-dependent IBa block by PAA during depolarizing pulse trains at different frequencies, (ii) estimation of the time course of IBa recovery from use-dependent block by (−)D600, and (iii) estimation of IBa recovery from inactivation and block after a single maintained depolarization.
Use-dependent Ca2+ channel block by (−)D600 was measured during 15 consecutive 100-ms pulses applied at a frequency of 0.1 Hz or 1 Hz after a 3-min equilibrium period in drug-containing solution at −80 mV. The same pulse trains were applied in the absence of drug to estimate the accumulation of Ca2+ channels in an inactivated state under control conditions. Resting-state dependent block of IBa (measured as peak current inhibition during the first pulse after a 3-min equilibration in drug containing solution) usually amounted to <10% (see also ref. 11). The holding potential was −80 mV and test potentials were applied to 10 mV that was the peak potential or 10 mV positive to peak of the current-voltage relationships for the channel constructs. The studied point mutations did not affect the current voltage relationship of the resulting mutant channels (data not shown).
Before starting an experiment care was taken to allow the Ca2+ channels to recover from inactivation. The oocytes were held for 15–25 min (depending on the batch of oocytes) at −80 mV until IBa was stable. IBa of AL1 and the derived mutants were then stable under voltage-clamp conditions for 3–4 hr. For the long recovery experiments this was a clear advantage of class A/L-type chimeras compared with IBa of chimera Lh that tends to “run down”.
Recovery of IBa from fast inactivation (defined as IBa recovery within 10 sec) was studied after depolarizing Ca2+channels during a 1 or 3 sec prepulse to 10 mV. The time course of IBa recovery from inactivation was estimated at a holding potential of −80 mV by applying a test pulse to 10 mV at various time intervals after the conditioning prepulse. Peak IBa values were normalized to the peak current measured during the prepulse. IBa recovered between 90–100% during 4 min rest at −100 mV.
IBa of the chimeric channels decays slowly during maintained depolarization and is described by more than a single exponential. Even a 10 sec pulse to 10 mV did not induce complete inactivation in the channel constructs studied (see Fig. 2B). After a 1 sec prepulse (to 10 mV) IBa recovered within 10 sec to 85–90% of its original value mainly reflecting recovery from the fast component of inactivation. A second slow component of IBa recovery became prominent if the prepulse length was extended.
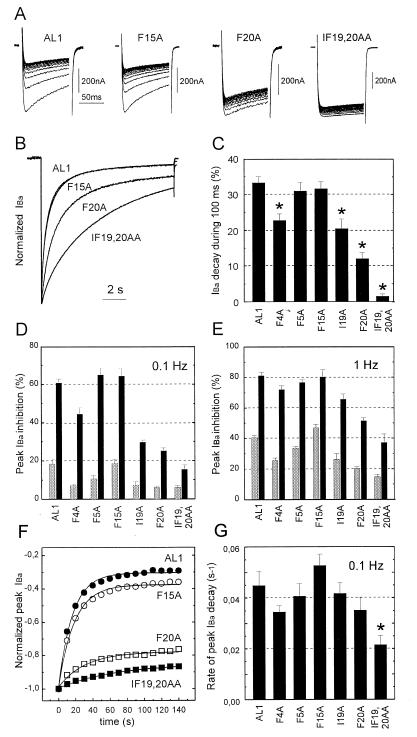
Figure 2.
(A) Use-dependent IBa-inhibition by 100 μM (−) gallopamil of AL1, F15A, F20A, and IF19,20AA. Channel block was estimated as peak IBa inhibition during trains of 15 pulses (100 ms) applied from a holding potential of −80 mV to 10 mV. Note that slowly inactivating mutants are affected less by (−)D600! (B) Normalized control IBa of chimeras AL1, F15A, F20A, and IF19,20AA during 10 sec depolarizing test pulses applied from a holding potential of −80 mV to 10 mV. Traces of AL1 and F15A are superimposed. (C) Comparison of the time course of IBa inactivation of mutants AL1, F4A, F5A, F15A, I19A, F20A, and IF19,20AA (in control) measured as IBa-decay during a 100 ms test pulse from −80 mV to 10 mV (n = 6–9). Oocytes were depolarized to the peak potential of the current voltage relationship (10 mV). Statistically significant acceleration of current inactivation compared with AL1 is indicated by asterisks (*P < 0.05). (D and E) Comparison of the use-dependent IBa block of the AL1 derived mutant α1 subunits as depicted in Fig. 1 B by 100 μM (−)D600. The block of IBa was measured as cumulative current inhibition (in %) during 15 depolarizing pulses (100 ms) after 3 min incubation of the Xenopus oocytes in drug (solid). Peak IBa decay under control conditions (shaded) indicates an incomplete recovery of IBa from inactivation during the train. Test pulses were either applied at a frequency of 0.1 Hz (D) or 1 Hz (E). Bars = mean ± SEM. (n = 3–14). (F) Peak current values of IBa in the presence of 100 μM (−)D600 during a 0.1 Hz pulse train plotted as a function of time. Smooth lines, single-exponential fits to the time courses with time constants of 16.4 sec (AL1), 18.5 sec (F15A), 31.4 sec (F20A), and 65 sec (IF19,20AA). Data are from the experiments shown in A. (G) Mean rate constants of peak current inhibition of the studied chimeras deduced from single exponential fits of the peak IBa decay during trains of 15 test pulses (100 ms) applied at 0.1 Hz. Peak current values were normalized to the first pulse current amplitude. Statistically significant faster rate of peak current decay during a train compared with AL1 is indicated by ∗ (P < 0.05).
Recovery from slow inactivation was measured by applying 10 sec prepulses from −80 to 10 mV and subsequently 20 ms test pulses (to 10 mV) at increasing time intervals starting after a 10 sec interpulse interval to allow the channels to recover from fast inactivation.
IBa-unblock from use-dependent inhibition by (−)D600 was studied at −80 mV by applying a 20 ms test pulse to 10 mV at various time intervals after the last pulse of the train. Because any test pulse applied during the recovery period after a train would induce additional channel block, recovery from use-dependent block was measured only once for a given pulse train. Between subsequent trains and double pulses the membrane voltage was held for 4 min at −100 mV resulting in 90–100% unblock and recovery of the channels from inactivation.
RESULTS AND DISCUSSION
Putative Pore-Orientated Residues in Segment IIIS6 Affect Ca2+ Channel Inactivation and Use-Dependent Ca2+ Channel Block by (−)D600.
We have reported (11) that single amino acid substitutions in transmembrane segments S6 of the α1A subunit affect inactivation of class A Ca2+ channels suggesting that S6 segments participate in inactivation gating. To modify Ca2+ channel inactivation without affecting the proposed high affinity determinants of PAA interaction within IVS6 we mutated single amino acids in segment IIIS6 of the highly PAA-sensitive class A/L-type chimera AL1 (Fig. 1A) (12). Five putative pore-orientated amino acids (four phenylalanines and one isoleucine, see Fig. 1B) that are highly conserved in the segments IIIS6 of all known mammalian α1 subunit isoforms [with exception of F5 (for nomenclature see Methods) that is only conserved in L-type Ca2+ channels] were substituted by alanine. Inactivation properties and use-dependent block of the resulting mutants by (−)D600 were studied after expression in Xenopus oocytes with two microelectrode voltage-clamp.
Alanine substitutions of residues I19 and F20 that are presumably localized closer to the inner mouth of the channel pore, and to an even greater extent the double mutation IF19,20AA (Fig. 1 B and C) slowed the rate of current inactivation (Fig. 2 A–C). Mutation F4A (located presumably closer to the outer channel mouth) also slowed the current inactivation time course and mutants F5A and F15A inactivated with nearly the same rate as AL1 (Fig. 2B). Only the reduced rate in current inactivation of I19A, F20A and IF19,20AA was accompanied by a substantial reduction in use-dependent block of IBa by (−)D600 during 100 ms steps at 0.1 Hz. The least amount of block after 15 pulses was observed for chimeras I19A, F20A and the double mutant IF19,20AA whereas chimeras AL1, F4A, F5A, and F15A were strongly blocked by (−)D600 during a train (see Fig. 2 A–D).
This finding can be explained by mutation-induced alterations in the affinity of the PAA receptor site, changes in channel inactivation properties or both. As the apparent PAA sensitivity was lower in slowly inactivating mutants I19A, F20A, and IF19,20AA compared with faster inactivating AL1, F5A, and F15A it is tempting to speculate that inactivation is an important determinant of PAA block. This was further supported by the finding that an increase in apparent PAA-sensitivity could be induced by depolarizing the membrane at the higher frequency of 1 Hz (Fig. 2E). Furthermore, inactivation properties of the chimeras correlated not only with the extent of use-dependent block but also with the rate of block development during a train (Fig. 2 F and G) suggesting that the rate of IBa inhibition by (−)D600 during a train is substantially determined by the amount of current inactivation during individual pulses.
Evidence for PAA-Induced Inactivation of Ca2+ Channels.
To enable a more detailed analysis of the role of channel inactivation in block development we studied IBa block and channel recovery from block after maintained (1 or 3 sec) depolarizations by means of a two-pulse protocol (Fig. 3A).
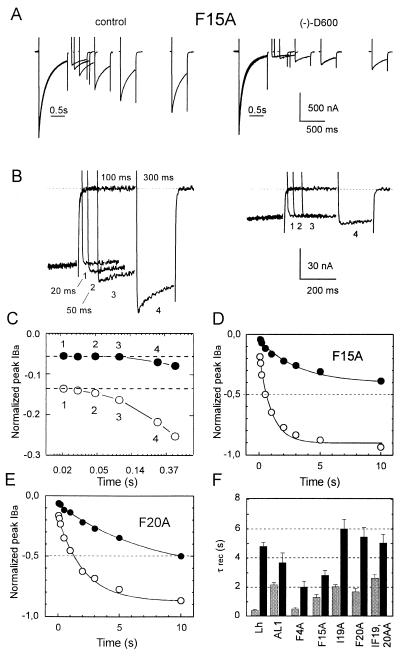
Figure 3.
Delay in recovery of calcium channels in the presence of (−)D600. (A) IBa recovery from inactivation of F15A was measured by a two-pulse protocol in the absence (Left) and presence of 100 μM (−)D600 (Right). The currents were inactivated by 1 sec prepulses to 10 mV. Recovery from inactivation at −80 mV was measured by applying a sequence of test pulses at various times (between 50 ms and 10 sec) after the prepulse (see Methods). After a two-pulse experiment the membrane was hyperpolarized to −100 mV for 4 min to permit complete recovery from inactivation and block. IBa measured during the prepulses are superimposed. Mean time constants of recovery from inactivation and block are shown in F. (B) Initial delay in IBa recovery from inactivation of F15A in (−)D600 (100 μM) at −80 mV after a 1 sec prepulse to 10 mV. Displayed currents were recorded after interpulse intervals of 20, 50, 100, and 300 ms. (C) Semilogarithmic plot of peak IBa during the early phase of recovery (same experiment as shown in B). IBa in the presence of (−)D600 recovered with a initial delay of about 100 ms (•). No delay above 20 ms could be observed in the absence of drug(μ). (D and E) Quantitative analysis of the IBa recovery from fast inactivation of chimeras Lh (C), F15A (D), and F20A (E). IBa recovery was measured at −80 mV with the two-pulse protocol as shown in A. Peak current values measured at different intervals after the prepulse in control (○) and the presence of 100 μM (−)D600 (•) were normalized to peak current values of the prepulse and plotted as a function of time. Data points are fitted by a single exponential function. Continuous lines are single exponential fits to the time courses, with time constants of τcontrol = 0.55 sec, τ(−)D600 = 3.85 sec (Lh, C); τcontrol = 0.93 sec, τ(−)D600 = 2.76 sec (F15A, D) and τcontrol = 1.86 sec, τ(−)D600 = 6.5 sec (F20A, E). (F) Mean time constants of IBa recovery from inactivation in control (shaded) compared with recovery time constants in the presence of 100 μM (−)D600 (solid) of chimeras Lh, AL1, F15A, I19A, F20A, and IF19,20AA. Because of the slower time course of inactivation IBa-recovery of the cardiac construct Lh (12) and IF19,20AA were estimated after 3 sec pulses; recovery of other channel constructs was measured after 1 sec pulses.
IBa recovery of our mutant channels from inactivation was estimated at different intervals after a conditioning prepulse at a holding potential of −80 mV (Fig. 3A, see Methods). Recovery in the absence of drug could be approximated by a single exponential function (Fig. 3 D and E).
In the presence of (−)D600 we consistently observed a small initial delay in the onset of IBa recovery resulting in a sigmoidal rather than exponential time course. This is illustrated for F15A in Fig. 3 B and C, where peak IBa values during a 300-ms recovery interval are shown at a higher time resolution. No significant delay in the onset of IBa recovery from inactivation was found in the absence of PAA for interpulse intervals down to 20 ms.
An initial delay in IBa recovery in the presence of (−)D600 (in the range of 100 ms) was observed for all our mutant Ca2+ channels. This finding was not a peculiarity of our AL1 deduced channel mutants but also observed for the cardiac L-type Ca2+ channel construct Lh (12) (see Fig. 3F for drug-induced slowing of IBa recovery from inactivation).
The delay in Ca2+ channel recovery in the presence of (−)D600 may be explained by recovery of a certain channel fraction from an additional deep or slow inactivated state. Evidence for slow (or ultra slow) inactivation of Ca2+ channels was recently presented by Boyett et al. (16) who described that a second (slow) phase in recovery of current is enhanced with prolonged membrane depolarization and suggested an important role of slow inactivation for the rate dependent shortening of the action potential in ventricular cells as well as for changes in the conduction velocity of the atrioventricular node.
The most striking result was, however, that (−)D600 delayed IBa recovery of the apparently low-sensitivity F20A and IF19,20AA to a comparable or even higher extent (F20A) as of mutants AL1 and F15A that displayed significantly more use-dependent block during low frequency pulse trains (Fig. 2 D and F). Thus, after single maintained depolarizations that allow substantial inactivation also in the slowly inactivating I19A, F20A, and IF19,20AA, apparent differences in PAA sensitivity were no longer observed. This clearly indicates that Ca2+ channel block by PAA is not only determined by the absolute drug affinity for a specific receptor site but also by the intrinsic gating properties of the channel molecule.
Putative Pore-Orientated Amino Acids in Segment IIIS6 Affect Recovery of Ca2+ Channels from Inactivation.
Alanine substitutions of putative pore-orientated residues in IIIS6 of chimera AL1 affected not only the rate of IBa recovery from channel block but also modulated the recovery from inactivation in the absence of drug (Fig. 3F).
Compared with IBa recovery of AL1 (τrec./AL1 = 2.13 ± 0.12 sec, n = 3), chimera F4A, with the alanine substitution located close to the outer mouth of the channel, displayed markedly faster recovery kinetics (τrec./F4A = 0.62 ± 0.04 sec, n = 3, P < 0.05) whereas mutations I19A (τrec./I19A = 2.04 ± 0.15 sec, n = 3, P < 0.05), F20A, and IF19,20FA that are located closer to the inner mouth of the channel pore (Fig. 1C) affect IBa recovery to a lesser extent (τrec./F20A = 1.64 ± 0.23 sec, n = 3 and τrec./IF19,20AA = 2.58 ± 0.29 sec, n = 3, not significantly different to AL1) (Fig. 3F).
These results reveal that in addition to segment IVS6 (11) and IS6 (17) putative pore-orientated amino acids in transmembrane segment IIIS6 determine kinetics of Ca2+ channel inactivation and recovery from inactivation (Fig. 3 and 4) and support our previous hypothesis that pore-lining amino acids of transmembrane segments S6 contribute to Ca2+ channel inactivation (11). Our data obtained with chimeric channel constructs should prompt further studies to investigate the role of highly conserved IIIS6 residues F4, F15, I19, and F20 in inactivation gating of the different Ca2+ channel types.
Recovery of IBa from Slow Inactivation and Block by (−)D600.
If PAAs promote slow inactivation it is likely that recovery from block at rest (−80 mV) occurs with a comparable time course as recovery from a slow inactivated state that is induced by maintained membrane depolarization in the absence of drug. We therefore studied a possible correlation between both processes by investigating IBa recovery after prolonged (10 sec) depolarizations in two fast inactivating (“high sensitive”, AL1 and F15A) and two slowly inactivating (“low sensitive”, F20A and IF19,20AA) chimeras in greater detail. Typical time courses of the slow component of IBa recovery of AL1 are shown in Fig. 4A. The mean time constants of IBa recovery from slow inactivation and the time constant of recovery from use-dependent block by (−)D600 of AL1, F15A, F20A and IF19,20AA are compared in Fig. 4B. Only IBa of chimera IF19,20AA recovered significantly faster from block than from slow inactivation in the absence of drug.
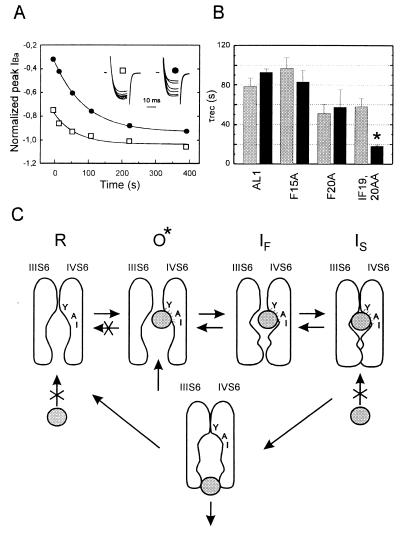
Figure 4.
IBa recovery from slow inactivation and unblock from use-dependent block by 100 μM (−)D600. (A) (□) Time course of the slow component in AL1 IBa recovery from inactivation in control after a 10 sec prepulse to 10 mV (see Methods). (•) Time course of peak IBa recovery from block by 100 μM (−)D600 induced by 15 test pulses (100 ms) applied at 0.1 Hz pulses from −80 mV to 10 mV in oocytes expressing the fast inactivating chimera AL1. The inset shows IBa during 20 ms test pulses to 10 mV applied at different intervals after a single 10 sec prepulse to 10 mV (□) or use-dependent block (•). Peak current values are normalized (see Inset) to IBa of the prepulse (□) or to the first current of the train (•), respectively. Time courses of IBa recovery from use-dependent block and inactivation were fitted to single exponential functions; respective time constants are τunblock = 94 sec and τslow = 68 sec. (B) Mean time constants of IBa recovery from inactivation (shaded) and from use-dependent block (solid) by 100 μM (−)D600 of chimeras AL1, F15A, and F20A. To observe a significant amount of IBa inhibition during a 0.1 Hz train in IF19,20AA we increased the applied (−)D600 concentration to 300 μM. Statistical significant difference in recovery from block and slow inactivation is indicated by asterisks (*P < 0.05). (C) Model for PAA interaction with Ca2+ channels illustrating drug induced slow inactivation. Access of the PAA molecule (symbolized by the grey ball) to the receptor site in the resting state (R) is restricted. After removal of putative pore-orientated guarding amino acids the drug can enter the channel from the cytoplasmic site in the open conformational state (O*) and subsequently interact with its receptor site (24, 25). According to (8) pore-orientated amino acids of transmembrane segment IVS6 contribute high affinity determinants of the PAA receptor site (11, 25). After PAA interaction with its receptor site the channels become non conducting and transitions of Ca2+ channels to the resting state are restricted (see also refs. 27 and 28). If activation and inactivation are coupled processes Ca2+ channels will move to the fast inactivated state (IF) and subsequently accumulate in the slow inactivated state (IS) even if the drug does not directly affect the rate of Ca2+ channel inactivation. Consequently the amount of use-dependent block during a train of pulses will crucially depend on the rate of channel inactivation (see Fig. 2).
Molecular Model for Ca2+ Channel Block by PAA.
To explain the role of inactivation in use-dependent Ca2+ channel block by PAA we propose the model shown in Fig. 4C. It is well established (18–23) that PAAs approach their receptor site in the channel pore from the cytoplasmic site. Furthermore, we have recently unequivocally demonstrated in mammalian cells and mutant Ca2+ channels expressed in Xenopus oocytes that intracellular but not extracellular action of quaternary PAA is strictly use-dependent (24, 25). This finding indicates that PAAs access the channel via a hydrophilic pathway whereas putative pore-orientated amino acids localized in the cytoplasmic direction from the PAA receptor serve as “guarding” structures (26) that prevent channel block at rest. Inactivation per se is insufficient for channel block by PAA (3, 4) and PAAs therefore access their site upon channel opening after removal of these guarding structures.
The PAA-induced delay in the onset of recovery from inactivation (Fig. 3 B and C) suggests that after the drug has entered the channel in the open state transitions to IS occur at a promoted rate (see model in Fig. 4C). In our model we offer an explanation for this finding that is deduced from a model earlier described by Armstrong (ref. 27, also ref. 28, see ref. 9 for review), namely that transitions to the resting state are restricted once the drug is bound to the channel in its open conformation. The primary result of the present study that faster inactivating Ca2+ channels are more efficiently blocked by PAA can therefore be explained without requiring that PAA directly affect the rate of channel inactivation. Such a model predicts that enhanced accumulation of channels in an inactivated state (observed as use-dependent block by PAA) will occur if Ca2+ channels inactivate at a higher rate (AL1, F5A, and F15A) and also explains that slowly inactivating channels (I19A, F20A, IF19,20AA) are blocked more efficiently at a higher frequency of depolarization (Fig. 2 D and E) or prolonged depolarization by single conditioning pulses (Fig. 3F). It has, however, to be emphasized that the indicated changes in channel gating (crossing out of the O → R pathway) are the result of a specific PAA-interaction with its binding site. This is supported by our recent finding that accelerated inactivation per se does not promote use-dependent PAA-block of class A/L-type chimeric channels lacking the high-affinity determinants in IVS6 (25).
Furthermore, the model provides also an explanation for differences observed for PAA sensitivity in previous studies. Faster inactivating Ca2+ currents were found to be more efficiently blocked by PAA than more slowly inactivating Ba2+ currents in cardiomyocytes (2). Heterologously expressed L-type Ca2+ channels are blocked to a larger extent by PAA when β3-subunits, which accelerate current inactivation, are coexpressed with α1C (29).
An unexpected finding was, however, that channel PAA-unblock of IF19,20AA occurred at a significantly faster rate than recovery of IBa from inactivation.
We found that only alanine substitutions close to the cytoplasmic channel mouth affect both inactivation and use-dependent IBa block by PAA. This is illustrated in Figs. 2 and 3 where F4A (close to the outer channel mouth) and I19A (close to the inner channel mouth) inactivate at a comparable rate. However, at low stimulation frequency (0.1 Hz) I19A channels were blocked to a lesser extent than F4A (Fig. 2D). It is therefore tempting to speculate, that bulky amino acids I19 and F20 at the inner mouth of the channel in segment IIIS6 (Figs. 1C and 4C) form part of an inactivation mechanism that prevents drug dissociation from the cytoplasmic side at rest. A single point mutation (I19A or F20A) would weaken its interaction with a putative inactivation particle (Fig. 2 B and C) but not affect PAA dissociation from its trapped state such that recovery from inactivation remains a rate limiting factor (Fig. 4B). Substitution of both residues by alanine removes inactivation to an extent that this process is no longer rate limiting and PAA dissociation at negative voltages occurs at a facilitated rate (Fig. 4B). A key role of the I19,F20-motive at the inner channel mouth is also indirectly supported by our recent finding that mutations of the PAA-receptor site itself (i.e., in the central part of the putative IVS6 alpha helix) have no significant effect on the PAA-dissociation rate (25).
In summary our results are consistent with the idea that PAA access a high affinity receptor site in the open channel conformation where I19 and F20 are likely to participate in forming a receptor site for an inactivation structure determining drug dissociation (Fig. 4C).
Although our data convincingly demonstrate that inactivation plays a key role in Ca2+ channel block by PAA we can, however, not exclude a direct PAA-interaction with I19 and/or F20 in segment IIIS6. We presently favour a model where unblock is substantially determined by removal of inactivation rather than by state independent barrier structures in the pore. Further detailed studies of the voltage dependency of channel unblock are needed for a precise understanding of the molecular pathways of PAA-dissociation from its receptor site in the channel pore. Future research will show if slow inactivation that occurs as a consequence of open channel block by PAA is identical to the ultra slow inactivation of Ca2+ channels described under physiological conditions (16).
A delay in recovery from inactivation (Fig. 3 B and C) was previously observed for sodium channel inactivation and attributed to channel transitions to an additional (slow) inactivated state (30). Accumulation of sodium channels in a slow inactivated state by local anesthetics is induced by long lasting depolarization; channels recover from a slow inactivated state with time constants ranging from several secs to minutes (see ref. 9 for review). By analogy to sodium channel interaction with local anesthetics (9, 31, 32) slow inactivation may consequently have an important role in the antiarrhythmic action of Ca2+ antagonists.
Acknowledgments
We thank Prof. H. Glossmann for continuous support of our work, Dr. M. Grabner for helpful suggestions and Drs. K. Beam and D. Beech for critical comments on the manuscript. We also thank Drs. Y. Mori and K. Imoto for the gift of the α1A cDNA, Dr. A. Schwartz for providing the α1C-a and α2/δ cDNA, B. Kurka and D. Kandler for expert technical assistance, and Dr. Traut (Knoll AG, Ludwigshafen, Germany) for providing the phenylalkylamine (−)D600. This work was supported by grants from the Fonds zur Förderung der Wissenschaftlichen Forschung S6602 (J.S.) and S6603 (S.H.), and the Dr. Legerlotz Stiftung and Hans und Blanca Moser Stiftung (S.A.). This work is part of the thesis of R.L.K. (supported by a grant from the Austrian Academy of Sciences).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PAA, phenylalkylamine; IBa, inhibition of barium currents.
References
- 1.Catterall W A, Striessnig J. Trends Pharmacol Sci. 1992;13:256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- 2.Lee K S, Tsien R W. Nature (London) 1983;302:790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- 3.McDonald T F, Pelzer D, Trautwein W. J Physiol (London) 1984;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hering S, Bolton T B, Beech D J, Lim S P. Circ Res. 1989;64:928–936. doi: 10.1161/01.res.64.5.928. [DOI] [PubMed] [Google Scholar]
- 5.Oyama Y, Hori N, Tokutomi N, Akaike N. Brain Res. 1987;417:143–147. doi: 10.1016/0006-8993(87)90189-2. [DOI] [PubMed] [Google Scholar]
- 6.Courtney K R. J Pharmacol Exp Ther. 1975;195:225–236. [PubMed] [Google Scholar]
- 7.Hondeghem L M, Katzung B G. Annu Rev Pharacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- 8.Hockerman G H, Johnson B D, Scheuer T, Catterall W A. J Biol Chem. 1995;270:22119–22122. doi: 10.1074/jbc.270.38.22119. [DOI] [PubMed] [Google Scholar]
- 9.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 10.Döring F, Degtiar V E, Grabner M, Striessnig J, Hering S, Glossmann H. J Biol Chem. 1996;271:11745–11749. doi: 10.1074/jbc.271.20.11745. [DOI] [PubMed] [Google Scholar]
- 11.Hering S, Aczél S, Grabner M, Döring F, Berjukow S, Mitterdorfer J, Sinnegger M J, Striessnig J, Degtiar D E, Wang Z, Glossmann H. J Biol Chem. 1996;271:24471–24475. doi: 10.1074/jbc.271.40.24471. [DOI] [PubMed] [Google Scholar]
- 12.Grabner M, Wang Z, Hering S, Striessnig J, Glossmann H. Neuron. 1996;16:207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- 13.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 14.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Nature (London) 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 16.Boyett M, R, Honjo H, Harrison S M, Zang W-J, Kirby M S. Pflügers Arch. 1994;428:39–50. doi: 10.1007/BF00374750. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J-F, Ellinor P T, Aldrich R W, Tsien R W. Nature (London) 1994;372:97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]
- 18.Hescheler J, Pelzer D, Trube G, Trautwein W. Pflügers Arch. 1982;393:287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- 19.White E J, Bradford H F. Eur J Pharmacol. 1986;130:243–248. doi: 10.1016/0014-2999(86)90274-8. [DOI] [PubMed] [Google Scholar]
- 20.Kass R S, Arena J P, Chin S. J Gen Physiol. 1991;98:63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klöckner U, Isenberg G. J Physiol Pharmacol. 1991;42:163–179. [PubMed] [Google Scholar]
- 22.Hering S, Savchenko A, Strübing C, Lakitsch M, Striessnig J. Mol Pharmacol. 1993;43:820–826. [PubMed] [Google Scholar]
- 23.Seydl K, Kimball D, Schindler H, Romanin C. Pflügers Arch. 1993;424:552–554. doi: 10.1007/BF00374922. [DOI] [PubMed] [Google Scholar]
- 24.Berjukov S, Aczel S, Beyer B, Kimball D, Dichtl M, Hering S, Striessnig J. Br J Pharmacol. 1996;119:1197–1202. doi: 10.1111/j.1476-5381.1996.tb16022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degtiar V, Aczél S, Döring F, Timin E N, Kimball D, Mitterdorfer J, Hering S. Biophys J. 1997;73:157–167. doi: 10.1016/S0006-3495(97)78056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starmer C F, Grant A O. Mol Pharmacol. 1985;28:348–356. [PubMed] [Google Scholar]
- 27.Armstrong C M. J Gen Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong C M, Bezanilla F. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacinova L, Ludwig A, Bosse E, Flockerzi V, Hofmann F. FEBS Lett. 1995;373:103–107. doi: 10.1016/0014-5793(95)01013-5. [DOI] [PubMed] [Google Scholar]
- 30.Chiu S Y. J Physiol (London) 1977;237:573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khodorov B I, Shishkova L D, Peganov E. Exp Biol Med. 1974;3:10–14. doi: 10.1007/BF00802462. [DOI] [PubMed] [Google Scholar]
- 32.Khodorov B I, Shishkova L D, Peganov E, Revenko S. Biochem Biophys Acta. 1976;433:409–435. [Google Scholar]
- 33.Grabner M, Friedrich K, Knaus H-G, Striessnig J, Scheffauer F, Staudinger R, Koch W J, Schwartz A, Glossmann H. Proc Natl Acad Sci USA. 1991;88:727–731. doi: 10.1073/pnas.88.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Nature (London) 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]