Abstract
The concept of mild cognitive impairment (MCI) identifies persons who are neither cognitively normal nor demented. There is increasing evidence that MCI defines a group of persons who are at near-term risk of developing dementia and particularly Alzheimer's disease (AD). MCI thus constitutes an attractive target population for preventive treatments of AD. MCI is associated with aging and is more prevalent than dementia. There are several clinical and biological markers that are predictive of MCI prognosis, including depressive symptoms, cognitive deficits, brain imaging and neurochemical findings. The clinician needs to be especially alert to depressive and other mood symptoms which are common in MCI and potentially treatable. Trials of current medications for prevention of MCI progression to dementia have been largely negative. There are observational data suggesting that lifestyle modifications including exercise, leisure activities, cognitive stimulation, and social activities may be effective for prevention of MCI progression. There are many novel therapies currently in trials for early AD, and if effective they may prove to be helpful in prevention of MCI progression as well.
Keywords: Mild cognitive impairment, Alzheimer's disease, aging, depressive symptoms, exercise, prevention
Alzheimer's disease (AD) is the most common neurodegenerative disease. It is projected to affect 81 million persons worldwide by 2040 1. It represents a major cause of disability for patients and caregivers, and is associated with huge financial burden to all societies. Clinically, the disease has an insidious onset and slow progression of characteristic cognitive and functional deficits 2,3 and near-universal incidence of neuropsychiatric symptoms 4. Neuropathologically, AD is associated with the deposition of insoluble amyloid-beta in extracellular plaques and phosphorylated tau in intraneuronal neurofibrillary tangles, microglial activation, and neuronal loss 5.
The disease probably affects the brain many years, possibly many decades 6, before its full clinical expression. By the time Alzheimer's dementia becomes clinically apparent, considerable brain damage has occurred, which is likely irreversible. Effective management of AD in the long term will rest on the ability to detect and manage its earliest manifestations in the brain and also clinically. This paper is focused on the latter, namely the earliest clinical manifestations of AD.
Clinicians have long noted that persons who develop AD have cognitive symptoms prior to the onset of dementia. As far back as the 1960s, investigators recognized a group of older persons who were neither cognitively normal nor demented but fit somewhere in between 7. While many of these persons developed dementia, a substantial number did not. This has given rise to the concepts of “cognitive impairment no dementia” (CIND) 8 and “mild cognitive impairment” (MCI) 3,9.
It must be emphasized that MCI represents a risk group and not a widely accepted clinical diagnosis. Even with the use of biomarker profiles and sophisticated clinical evaluations to refine the definition, a substantial number of persons with MCI will not develop dementia.
In this paper we seek to present the current state of knowledge of the MCI concept, as it applies to clinical evaluation and treatment, with particular emphasis on risk and prognostic factors, lifestyle interventions, and the future of treatment in this area.
MCI AND ITS SUBTYPES
Persons with MCI are by definition neither cognitively normal nor demented. The first part of the definition means that they have subjective cognitive complaints and/or objective evidence of abnormal cognitive testing. In addition to the above evidence of a decline in cognitive functioning, the “Petersen criteria” require that to meet criteria for MCI a person must also perform ≥1.5 standard deviations below age-education norms on at least one cognitive test 3. These criteria for MCI are most widely accepted, due to their relatively high specificity.
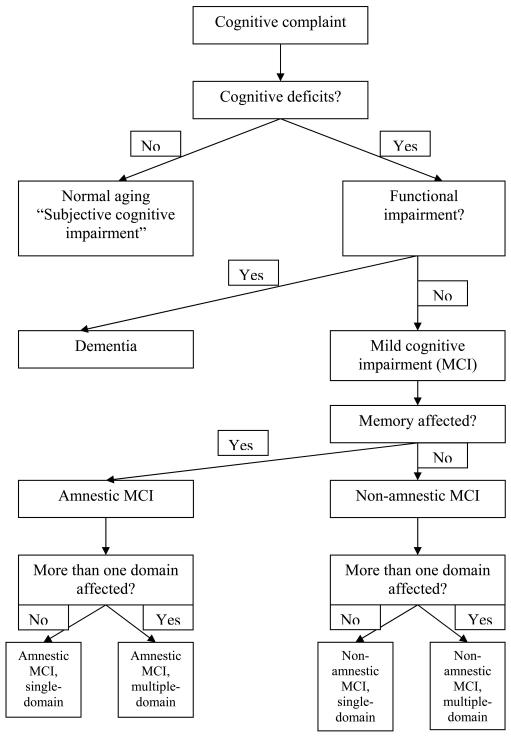
The second part of the MCI definition – that the person not be demented – means that the person has no functional deficits related to cognitive impairment, often defined as no impairment in instrumental activities of daily living (IADLs). In practice this criterion is harder to operationalize, largely because the cognitive demands of functional activities vary greatly by stage of life cycle and by life situation. For example, older persons still in the workforce often have greater day-to-day cognitive demands than persons who are retired, and thus are more likely to be diagnosed with dementia given the same degree of cognitive impairment. The presence of a living spouse often masks minor functional deficits; living in a retirement community likely decreases the cognitive demands of home maintenance, shopping, cooking etc.; while the need to adhere to a complex medical regimen likely heightens cognitive demands in daily life. Perneczky et al 10 found that persons with rigorously defined MCI in fact had mild IADL impairments, particularly in tasks requiring memory or executive function. Thus, while persons with MCI have subtle deficits in IADLs consistent with their cognitive performance, they generally function independently. Only when their functioning declines in several areas, they are said to “cross the border” into dementia (Figure 1).
Figure 1.
Mild cognitive impairment (MCI) and related syndromes (adapted from Rosenberg et al, 65)
MCI has been further subtyped on the basis of cognitive deficits into amnestic vs. non-amnestic and single-domain vs. multiple-domain.
EPIDEMIOLOGY AND PROGNOSIS
The prevalence of MCI in late life varies according to the sample and the definition. Amnestic MCI has a prevalence of 3-6% in population-based samples of older persons, while all MCI subtypes have a prevalence of as high as 16% 11,12. The prevalence of MCI increases with age, ranging in the Cardiovascular Health Study from 15% under age 75 years to 30% over age 85 years 12.
MCI was previously thought to entail an inevitable and relatively rapid progression to dementia. This grim prognosis has been revised recently. In an early study, the rate of progression to dementia was estimated to be 10-15% annually, with >80% of MCI patients developing dementia over 6 years 3,14. Similar rates were reported in the Cache County Study 13. Some investigators concluded that amnestic MCI is simply prodromal AD 15. However, population-based studies or studies with longer follow-up have revised these estimates downward: Devanand et al reported an annual progression rate of 5% 166, Solfrizzi et al of 7.4% 17, and Ganguli et al of 2.7% 18. Clearly the risk of dementia in MCI patients is highly variable, and appears lowest in general population samples.
A substantial number of patients with MCI “revert” to normal (i.e., no longer have subjective or objective cognitive dysfunction). The rate of “reversion” has been reported to range from 17% to 32% 19-21. The rate of progression to AD is highest, while the rate of reversion is lowest, in patients with rigorously defined amnestic MCI 19, particularly if it affects multiple cognitive domains 22, but is still significant in patients with non-amnestic MCI 23. The specificity of MCI subtypes risk for specific dementia subtypes is still unclear. Early reports that amnestic MCI was specific for AD and non-amnestic MCI for other dementias (particularly vascular dementia) have not been replicated 22,23 and are conceptually too simplistic.
Who with MCI is more likely to progress to dementia? In one study, MCI patients who progressed to dementia had worse verbal memory at baseline 24. In MCI patients with a very mild impairment, worse verbal memory and executive function was associated with greater risk of progression 25. Similar findings have been reported from the placebo arm of MCI medication trials 26. Additionally, subtle changes in IADL function predict a worse prognosis 25.
Brain imaging findings clearly can reflect prognosis of MCI. The rate of whole brain or regional volume loss in the hippocampus and entorhinal cortex 27,28, and possibly the rate of increase in ventricular size 29, appear to be good predictors of MCI progression. Decreased glucose uptake in the posterior cingulate and temporo-parietal cortices imaged with fluorodeoxyglucose positron emission tomography (FDG-PET) also predicts MCI conversion to dementia 30,31. Using Pittsburgh Agent B (PIB) (a new PET tracer for imaging amyloid plaques in vivo), binding is higher in MCI patients who progress to dementia than in those who remain functionally stable 32, suggesting that the density of amyloid plaques is higher in MCI patients who develop dementia. Functional magnetic resonance imaging (MRI) may also predict prognosis: for example, Miller et al 33 found that greater hippocampal activation during a visual scene-encoding task was a predictor of future cognitive decline.
Plasma and cerebrospinal fluid (CSF) amyloid and tau levels also hold promise as prognostic markers in MCI. Hansson et al 34 reported that a combination of decreased parietal blood flow and abnormal CSF amyloid-beta and tau levels was a strong predictor of MCI progression. Two studies reported that a decreased Aβ42/Aβ40 ratio is a risk factor for AD in MCI patients 35,36.
The strongest genetic association reported for AD is with the ApoE4 allele 14. This allele may also be a risk factor for progression of MCI to AD 26.
The association of depression and anxiety with MCI prognosis is of particular importance to psychiatrists. Depression, lack of motivation, and anxiety are more prevalent in MCI patients than in cognitively intact elderly 37. Both major depression 38 and anxiety 37 markedly increase risk of MCI progression to dementia; further, depression and apathy were more common in MCI patients who later progressed to AD 21,39. The majority of older adults with major depression also met criteria for MCI, and their cognitive deficits persisted after remission of depression 40. Other studies of late-life depression have noted a particular association with executive dysfunction 41.
DIAGNOSIS
History
To make a diagnosis of MCI, the clinician must determine that the patient has subjective and/or objective cognitive symptoms, but not dementia. The border between MCI and dementia can be subtle, and the initial definition of MCI requiring no deficits in IADLs has been amended to allow for subtle deficits. The clinician needs to determine if the patient is no longer functioning at his/her baseline at work, home, hobbies, social activities, etc.
Patients with MCI are typically aware of their deficits and can provide a valid history, but confirmation with a knowledgeable informant (typically a family member) is important. Patients most commonly complain of deficits in short-term recall, with common examples being: a) cannot remember if they took medications; b) repeat questions; c) difficulty with driving directions in unfamiliar locations; d) difficulty recalling the time sequence of events; and, e) difficulty organizing complex projects, such as doing taxes or writing reports at work. MCI patients may additionally complain of deficits in executive functioning, such as using information to make judgments and decisions, appreciating the consequences of decisions, etc.; these tend to be more evident in the workplace and are harder to assess in retirees.
The importance of mood symptoms
It is clear that depressive and cognitive complaints often co-occur in older persons, and that depression is frequently prodromal to MCI and dementia. Therefore, the clinician must be alert to depressive symptoms in patients with cognitive complaints and must endeavor to distinguish primary mood changes from cognitive changes. Patients with MCI are among the most worried patients seen in a geriatric psychiatry practice; they often are convinced that they are demented and are prone to catastrophizing rather than adapting to their disability. For this reason, it is important that the clinician presents MCI as what it is – a syndrome and a risk group rather than a clearly defined illness.
There are certain mood features that are more common in MCI than in major depression. For example, patients may complain more of lack of motivation rather than sad or depressed feelings 42. Hopelessness is common, but suicidal ideation is not 43. The clinician should be highly attuned to the possibility that cognitive complaints are actually a presentation of an “atypical” depressive disorder and to make treatment decisions accordingly.
Cognitive assessment
Clinical assessment is rarely definitive in MCI, but useful for validating the patient's cognitive complaints. The Mini-Mental State Examination (MMSE) is neither sensitive nor specific enough to confirm or reject an MCI diagnosis, with one study showing 70% sensitivity and specificity using a cutoff of 26 or less for cognitive impairment 44. Instruments such as the Modified Mini-Mental State Examination (3MS or mMMSE) 45,46 or Montreal Cognitive Assessment (MoCA) 47 are more difficult, have less of a “ceiling effect”, and as such are more useful in clinical practice for assessment of MCI. There are normative data for the 3MS derived from population-based samples 46; for example, the mean 3MS for a 75-79 year old person with a high school education is 90, while scores below 80 are below the 5th percentile. Neuropsychological testing adds further depth to the MCI evaluation, and there is growing evidence that sensitive tests of immediate and delayed recall particularly improve the predictive power of the evaluation 48.
Laboratory and physical assessment
While laboratory tests are not always necessary in the workup of MCI, it is important to rule out cognitive effects of medical illnesses other than neurodegenerative disease. For this reason, a thorough physical exam and laboratory assessment should be considered part of the assessment of MCI. Common conditions that either mimic or cause cognitive symptoms, even dementia, include hypothyroidism, vitamin B12 deficiency, neurosyphilis, and hypernatremia. A subacute onset of a delirium can mimic MCI, including in the context of urinary tract infection, pneumonia, congestive heart failure, and the effects of sedating medications (especially anticholinergics, benzodiazepines, and opioid analgesics). A thorough neurologic exam is important to assess for long-tract neurologic signs that might suggest an occult stroke, peripheral neuropathy, a myopathic process, or early Parkinson's disease, which can present with cognitive and motor slowing as a first sign and might mimic MCI.
Brain imaging
In current clinical practice, structural brain imaging is performed largely to rule out uncommon and occult causes of cognitive impairment, such as an occult stroke, subdural hematoma, or brain tumor. As such, it is not of the highest importance in the current diagnosis and management of MCI. But, as reviewed above, new findings from structural MRI, FDG-PET and PIB-PET may greatly improve the clinical utility of these technologies in diagnosing and treating MCI.
MANAGEMENT
The most important aspect of the current management of MCI is making as clear a diagnosis as possible, and supporting patients and their families in the knowledge that they have a risk of dementia but no certainty of outcome. Specific aspects of management include: a) encouraging preventive strategies derived from observational data, and b) treating depression.
Strategies for preventing progression to dementia do not have proven efficacy to date, but there is suggestive evidence for the influence of lifestyle factors. We refer the reader to recent and comprehensive discussions of the biopsychosocial approach to treatment of depression in older persons 49,50 and restrict our comments to lifestyle strategies and medications.
Lifestyle strategies
Patients and families often ask the clinician whether exercise and cognitive activity will improve their memory or prevent dementia. The ideas are attractive and the mechanism of “use it or lose it” is intuitively appealing and widely cited as critical to dementia prevention. Supportive evidence comes from observational studies of community-based samples of older adults. A selection of recent studies is provided in Table 1.
Table 1.
Table 1 Recent studies of lifestyle factors and incident dementia
| Author | Lifestyle factor | Sample (mean follow-up) | Results | Comment |
| Podewils et al (51) | Number of exercise activities | N=3375 (5.4 years) | >3 activities associated with decreased dementia incidence (HR=0.58) | Effect seen in ApoE4 negative |
| Larson et al (66) | Frequency of exercise | N=1740 (6.2 years) | >3 times weekly exercise associated with decreased dementia incidence (HR=0.62) | Greater effect seen in persons with lower exercise performance levels at baseline |
| Wilson et al (67) | Number and frequency of cognitively stimulating activities | N=842 (4.1 years) | More cognitive stimulation associated with decreased dementia incidence (OR=0.36 for one-point increase in composite measure) | No effect seen for physical activity |
| Verghese et al (54) | Number of leisure activities | N=469 (5.1 years) | Greater number of leisure activities was associated with decreased dementia incidence | Activities associated with decreased dementia incidence included reading, playing board games, playing musical instruments, and dancing |
| Wang et al (68) | Performance-based physical function | N=2288 (5.9 years) | Higher levels of baseline physical performance were associated with decreased dementia incidence | Similar association with cognitive decline |
| Scarmeas et al (69) | Number of leisure activities dichotomized at the median | N=1772 (2.9 years) | Greater number of leisure activities was associated with decreased dementia incidence | |
| Rovio et al (69) | Midlife exercise frequency | N=1449 (26 years) | Exercise at least twice weekly in midlife was associated with decreased dementia incidence in late life (OR=0.48) | Note that the association applies to midlife (not late life) exercise frequency |
| Laurin et al (71) | Cognitive activity (compositve measure) | N=801 (4.5 years) | Cognitively stimulating activities were associated with decreased dementia incidence | Similar association with global cognition, working memory, and perceptual speed |
| HR - hazard ratio; OR - odds ratio | ||||
| The samples are selected to lack dementia or significant functional impairment at baseline, but are not chosen in a manner to include or exclude subjects with mild cognitive impairment | ||||
Curiously enough, there is more evidence and stronger results for the protective effect of exercise than for cognitive activity, and moderate exercise (for example, twice weekly in a variety of exercise activities) is sufficient to demonstrate this association 51. The effect of cognitive activity has been less consistently observed and is confounded with education; in other words, education is observed to have a protective effect against dementia and to be associated with cognitive activities in older persons. There may be a similar salutary effect of social activities 52, although recent evidence suggests that reduction of social involvement is more likely to be the result, as opposed to the cause, of impending cognitive decline and dementia 53. In addition, cognitive activities fall into such a variety of categories that it has been difficult to determine the underlying mechanism subsuming different activities such as (for example) crossword puzzles and dancing 54.
The mechanisms of the protective effects of lifestyle factors are not well understood, but exercise and cognitive activity may lead to a greater “cognitive reserve” 55, conceivably through enhanced vascular supply to the brain or more efficient use of cognitive networks 56. “LIFE” is a randomized, controlled trial of an exercise program in physically frail elderly that will examine cognition and dementia risk as secondary outcomes 57.
We recommend that, with an eye toward prevention of cognitive deterioration, persons with MCI: a) pursue a regular but moderate, variable exercise program consisting of at least 30 minutes three times weekly of walking alternating with aerobically challenging exercise, and group sports; b) pursue cognitively stimulating activities according to personal interests, abilities and education; c) keep as socially engaged as practically possible.
Medications
Current FDA-approved medications for AD have been systematically studied for their effects on the symptoms and prognosis of MCI. In preclinical studies, all three acetylcholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and the NMDA antagonist memantine improved cognition in transgenic mouse models of AD 58. Galantamine improved memory symptomatically in MCI patients 59. However, the results of controlled trials of cholinesterase inhibitors on the prognosis of MCI have been largely negative (Table 2). The only positive finding, with donepezil, comes from a secondary analysis in a subgroup of patients, and had limited clinical relevance. There are no reported trials with memantine. Given the current state of knowledge, the clinician should not prescribe these drugs to MCI patients with the hope of preventing progression to AD. However, high-risk patients with amnestic MCI and declining cognitive function may symptomatically benefit from these treatments, since they likely have early AD.
Table 2.
Table 2 Randomized controlled trials of prevention of MCI progressing to dementia or AD
| Author | Treatment | N (duration) | Outcomes | Results | Comments |
| Feldman et al (73) | Rivastigmine | 1018 (48 months) | 1. Progression to AD 2. Change on composite cognitive score | No difference between drug and placebo | No difference in MRI measure (ventricular volume) |
| Salloway et al (74) | Donepezil | 270 (6 months) | 1. Global impression of change | ||
| 2. Change in delayed logical recall | No difference between drug and placebo | ||||
| Petersen et al (75) | Donepezil ± Vitamin E | 769 (36 months) | Incident AD | 1. Donepezil was not protective on primary outcome, but had a limited effect at 12 months in a secondary analysis. | 1. Donepezil effect observed at 36 months in ApoE4 carriers |
| 2. No effect of Vitamin E | 2. No effect on rate of brain atrophy (76) | ||||
| MCI - mild cognitive impairment; AD - Alzheimer's disease; MRI - magnetic resonance imaging | |||||
| Two trials of galantamine in MCI have been reported as negative in a recent systematic review (72) | |||||
CONCLUSIONS
What we know at the present
When patients present with memory deficits, clinicians can evaluate their near-term risk of developing dementia with the clinical tools and diagnostic assessments reviewed here. The most important concept for patients and families is that identifying a patient as having MCI assigns him or her to a risk group and is not a definitive diagnosis of disease, since a substantial proportion of persons with MCI will not develop dementia and will continue to function normally. Mood and anxiety symptoms are very prevalent in MCI and the clinician should pay particular attention to their diagnosis and treatment. Current medications for AD do not appear effective in preventing the progression of MCI to AD, but there is encouraging evidence for the beneficial role of exercise, cognitive stimulation, leisure activities, and socialization.
What the future holds
The rapid pace of innovation in preclinical and translational research in AD has led to an increasing pace of novel AD treatments entering clinical trials, including immunotherapies 60,61, secretase inhibitors 62, inhibition of the receptor for advanced glycation end-products (RAGE) 63, and anti-inflammatory agents 64. Since amnestic MCI includes a large group of patients with prodromal AD, if a new treatment is effective in early AD it may also prevent progression of amnestic MCI to AD. There is much investigation of biomarkers of preclinical AD which will help identify MCI patients at greatest risk of AD, and may allow for identification of patients before they develop MCI, so that treatment becomes possible in a preclinical state. Additionally, the near-future will likely produce an explosion of results on the effectiveness of lifestyle interventions in MCI. The clinician should keep alert for findings in all of these areas, which offer great hope of improving our management of MCI and possibly preventing incident AD and reducing its enormous public health burden.
References
- 1.Ferri CP, Prince M, Brayne C. Alzheimer's Disease International: global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binetti G, Magni E, Padovani A. Executive dysfunction in early Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1996;60:91–93. doi: 10.1136/jnnp.60.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Doody R, Kurz A. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg M, Shao H, Zandi P. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23:170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snowdon DA, Kemper SJ, Mortimer JA. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 7.Kay DW, Beamish P, Roth M. Old age mental disorders in Newcastle Upon Tyne. I. A study of prevalence. Br J Psychiatry. 1964;110:146–158. doi: 10.1192/bjp.110.465.146. [DOI] [PubMed] [Google Scholar]
- 8.Graham JE, Rockwood K, Beattie BL. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Smith GE, Waring SC. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 10.Perneczky R, Pohl C, Sorg C. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21:158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- 11.Feldman HH, Jacova C. Mild cognitive impairment. Am J Geriatr Psychiatry. 2005;13:645–655. doi: 10.1176/appi.ajgp.13.8.645. [DOI] [PubMed] [Google Scholar]
- 12.Lopez OL, Jagust WJ, DeKosky ST. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 13.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67:229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Ivnik RJ. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 15.Morris JC, Storandt M, Miller JP. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 16.Devanand DP, Pradhaban G, Liu X. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 17.Solfrizzi V, D'Introno A, Colacicco AM. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- 18.Ganguli M, Dodge HH, Shen C. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 19.Lopez OL, Kuller LH, Becker JT. Incidence of dementia in mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol. 2007;64:416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 20.Maioli F, Coveri M, Pagni P. Conversion of mild cognitive impairment to dementia in elderly subjects: a preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr. 2007;44(Suppl. 1):233–241. doi: 10.1016/j.archger.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Gabryelewicz T, Styczynska M, Luczywek E. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22:563–567. doi: 10.1002/gps.1716. [DOI] [PubMed] [Google Scholar]
- 22.Busse A, Hensel A, Guhne U. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 23.Fischer P, Jungwirth S, Zehetmayer S. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 24.Albert M, Moss MB, Blacker D. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson BC, Sperling RA, Hyman BT. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleisher AS, Sowell BB, Taylor C. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR Jr, Weigand SD, Shiung MM. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. doi: 10.1212/01.wnl.0000281688.77598.35. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCarli C, Frisoni GB, Clark CM. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 29.Carmichael OT, Kuller LH, Lopez OL. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drzezga A, Grimmer T, Riemenschneider M. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–1632. [PubMed] [Google Scholar]
- 31.Mosconi L, Perani D, Sorbi S. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 32.Forsberg A, Engler H, Almkvist O. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2007.03.029. in press. [DOI] [PubMed] [Google Scholar]
- 33.Miller SL, Fenstermacher E, Bates J. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp.2007.124149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson O, Buchhave P, Zetterberg H. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer's disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2007.06.009. in press. [DOI] [PubMed] [Google Scholar]
- 35.Hansson O, Zetterberg H, Buchhave P. Prediction of Alzheimer's disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 36.Graff-Radford NR, Crook JE, Lucas J. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 37.Palmer K, Berger AK, Monastero R. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 38.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 39.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, Potter GG, Wagner HR. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos GS,/given-names> , Kiosses DN. Heo M et al. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Jorm AF, Parslow RA. Depression in mild cognitive impairment in a community sample of individuals 60-64 years old. Int Psychogeriatr. 2006;18:471–480. doi: 10.1017/S1041610205003005. [DOI] [PubMed] [Google Scholar]
- 43.Lopez OL, Becker JT, Sweet RA. Non-cognitive symptoms in mild cognitive impairment subjects. Neurocase. 2005;11:65–71. doi: 10.1080/13554790490896893. [DOI] [PubMed] [Google Scholar]
- 44.Loewenstein DA, Barker WW, Harwood DG. Utility of a Modified Mini-Mental State Examination with extended delayed recall in screening for mild cognitive impairment and dementia among community dwelling elders. Int J Geriatr Psychiatry. 2000;15:434–440. doi: 10.1002/(sici)1099-1166(200005)15:5<434::aid-gps137>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State Examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- 46.Bravo G, Hebert R. Age- and education-specific reference values for the Mini-Mental and Modified Mini-Mental State Examinations derived from a non-demented elderly population. Int J Geriatr Psychiatry. 1997;12:1008–1018. doi: 10.1002/(sici)1099-1166(199710)12:10<1008::aid-gps676>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 48.Loewenstein DA, Acevedo A, Ownby R. Using different memory cutoffs to assess mild cognitive impairment. Am J Geriatr Psychiatry. 2006;14:911–919. doi: 10.1097/01.JGP.0000229651.62137.e2. [DOI] [PubMed] [Google Scholar]
- 49.Dombrovski AY, Lenzei EJ, Dew MA. Maintenance treatment for old-age depression preserves health-related quality of life: a randomized, controlled trial of paroxetine and interpersonal psychotherapy. J Am Geriatr Soc. 2007;55:1325–1332. doi: 10.1111/j.1532-5415.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 50.Cuijpers P, van Straten A, Smit F. Psychological treatment of late-life depression: a meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry. 2006;21:1139–1149. doi: 10.1002/gps.1620. [DOI] [PubMed] [Google Scholar]
- 51.Podewils LJ, Guallar E, Kuller LH. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 52.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 53.Green RC, Rebok GW, Lyketsos CG. Influence of social network characteristics on cognition and functional status with aging. Int J Geriatr Psychiatry. doi: 10.1002/gps.2023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verghese J, Lipton RB, Katz MJ. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 55.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 56.Scarmeas N, Zarahn E, Anderson KE. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60:359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Espeland MA, Gill TM, Guralnik J. Designing clinical trials of interventions for mobility disability: results from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) trial. J Gerontol A Biol Sci Med Sci. 2007;62:1237–1243. doi: 10.1093/gerona/62.11.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Dam D, Abramowski D, Staufenbiel M. Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology. 2005;180:177–190. doi: 10.1007/s00213-004-2132-z. [DOI] [PubMed] [Google Scholar]
- 59.Koontz J, Baskys A. Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double-blind placebo-controlled study. Am J Alzheimers Dis Other Demen. 2005;20:295–302. doi: 10.1177/153331750502000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arbel M, Solomon B. Immunotherapy for Alzheimer's disease: attacking amyloid-beta from the inside. Trends Immunol. 2007;28:511–513. doi: 10.1016/j.it.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Gardberg AS, Dice LT, Ou S. Molecular basis for passive immunotherapy of Alzheimer's disease. Proc Natl Acad Sci USA. 2007;104:15659–15664. doi: 10.1073/pnas.0705888104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melnikova I. Therapies for Alzheimer's disease. Nat Rev Drug Discov. 2007;6:341–342. doi: 10.1038/nrd2314. [DOI] [PubMed] [Google Scholar]
- 63.Lue LF, Yan SD, Stern DM. Preventing activation of receptor for advanced glycation endproducts in Alzheimer's disease. Curr Drug Targets CNS Neurol Disord. 2005;4:249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- 64.Rosenberg PB. Clinical aspects of inflammation in Alzheimer's disease. Int Rev Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 65.Rosenberg PB, Johnston D, Lyketsos CG. A clinical approach to mild cognitive impairment. Am J Psychiatry. 2006;163:1884–1890. doi: 10.1176/ajp.2006.163.11.1884. [DOI] [PubMed] [Google Scholar]
- 66.Larson EB, Wang L, Bowen JD. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 67.Wilson RS, Bennett DA, Bienias JL. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 68.Wang L, Larson EB, Bowen JD. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 69.Scarmeas N, Levy G, Tang MX. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rovio S, Kareholt I, Helkala EL. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 71.Laurin D, Verreault R, Lindsay J. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(Suppl. 18):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 72.Loy C, Schneider L. Galantamine for Alzheimer's disease and mild cognitive impairment. (CD001747).Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD001747.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman HH, Ferris S, Winblad B. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 74.Salloway S, Ferris S, Kluger A. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63:651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 75.Petersen RC, Thomas RG, Grundman M. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 76.Jack CR Jr, Petersen RC, Grundman M. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2007.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]