Abstract
A new process for ortho-prenylation of phenols is presented within the context of known methods. All of the processes are briefly assessed with regards to the substitution patterns and accompanying functional groups tolerated by each strategy. The conclusion reached is that a new procedure using ortho-quinone methides, for which an experimental protocol is provided, offers the greatest generality and flexibility in the preparation of ortho-prenylated phenol derivatives.
Keywords: prenylation, phenols, ortho-alkylation, ortho-methide
1 Introduction
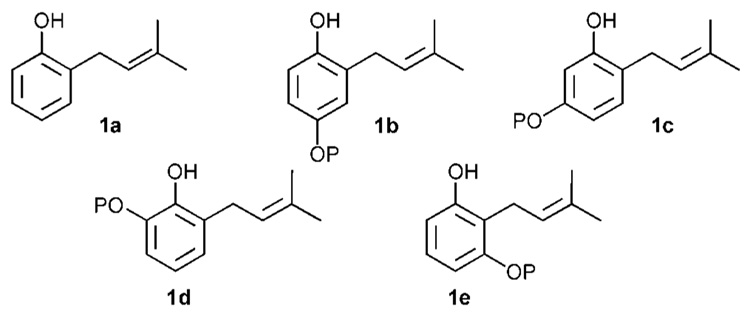
ortho-Prenylated phenols play an important role in mediating many biological processes. Prenylated ubiquinones are essential in cellular respiration.1 Prenylated napthaquinones, such as shikonin, are potent anti-bacterial agents.2 Arnebinol, an ansa ortho-prenylated phenol, inhibits the biosynthesis of prostaglandin.3 Other prenylated phenols exhibit anti-fungal,4 anti-tumor,5 anti-HIV6 and anti-Alzheimer activity.7 Derivatives of ortho-prenylated phenols, such as the corresponding dimethyl benzopyrans, exhibit an even broader range of interesting physiological effects.8 Clearly, such an important structural motif needs a general strategy for its preparation, particularly for systems in which other aromatic hydroxyl residues are differentiated, as is often the case with therapeutic natural products containing this pharmacophore. However, until recently no single method readily encompassed preparations of differentially protected dihydroxy aromatics such as 1b–e (Figure 1).9 In this document, attributes and admonitions are presented regarding the procedures for synthesizing ortho-prenylated phenols. In addition, a new strategy involving ortho-quinone methides is presented which increases access to these systems.
Figure 1.
All of the analogs of differentially protected ortho-prenylated dihydroxyphenol nuclei (PH).
2 Desymmetrization of Dihydroxy Aromatics
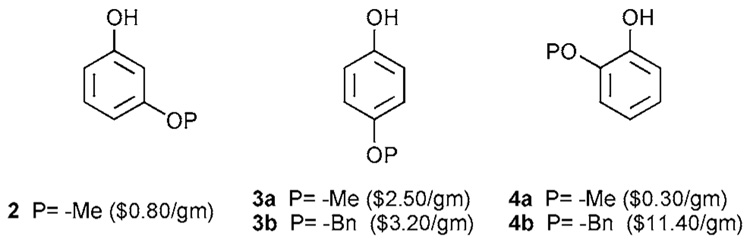
Distinguishing functional groups in the same chemical, electronic, and structural environments can be a difficult task. In chiral systems such a challenge is referred to as meso-desymmetrization often requiring the use of enzymes or chiral catalysts. If a desymmetrized starting material is unavailable, substantial material is lost in order to distinguish the groups. This problem must be solved in order to construct derivatives of 1b–e. Commercially available desymmetrized dihydroxyphenols are shown in Figure 2 to inform the readers of the approximate cost and availability of potential starting materials.
Figure 2.
Differentially protected dihydroxyphenols that are commercially available.
3 Directed ortho-Metalation (DoM) and Alkylation
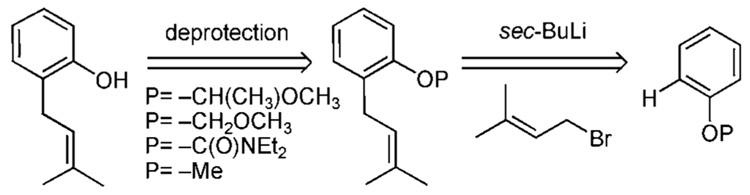
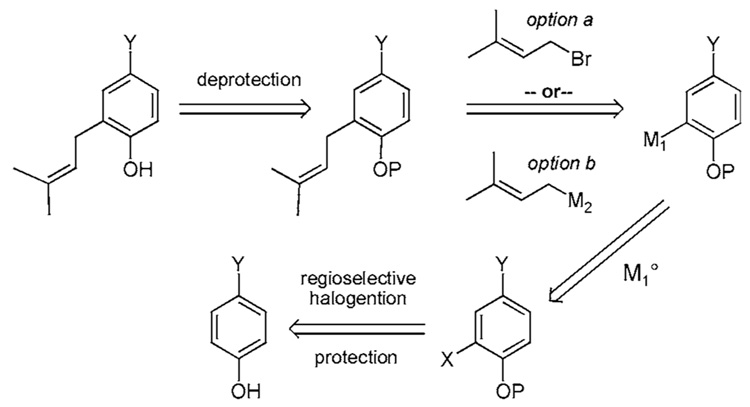
Directed ortho-metalation (DoM) is thought by most to be the best strategy for site-specific functionalization of aromatic compounds (Scheme 1).10 The procedure works well for the ortho-prenylation of symmetric phenol derivatives as well as derivatives that have already been desymmetrized and contain two or fewer oxygen substituents. Aromatic compounds displaying two or more oxygen substituents, however, can prove difficult to deprotonate. Often times, a proton transfer from a neighboring group such as −OBn or −OMOM quenches the aryl anion. Acetal [P=−CH(R)OMe] and carbamate [P=−C(O)NR2] protecting groups avoid this potential complication. However, these ortho directing groups are not without their own limitations. For example, a carbamate might migrate to the ortho-lithiated aryl carbon atom at temperatures above −60 °C affording the corresponding salicylicamide.10b Furthermore, carbamate deprotection requires harsh conditions such as reduction with LiAlH4. Snieckus reports that an N-cumyl N-methyl benzene-Ocarbamate undergoes cleavage under milder conditions.10c Substituted acetals display their own peculiarities. The 1-ethoxylethyl ether (OEE) often complicates 1H NMR data interpretation by virtue of its chirality and these types of ethers are surprisingly unstable towards acidic conditions, often hydrolyzing upon chromatography. On the other hand, MOM protected phenols are robust and often require very harsh conditions for deprotection. But, as previously mentioned, a proton exchange can decrease the effectiveness of MOM ethers in DoM processes.10d Some methyl ethers have proven effective in DoM protocols, however, cleavage of an aryl methyl ether requires extremely harsh conditions, such as BBr3, or refluxing MeMgI.11 Specific examples of DoM processes in regards to prenylation are shown in Scheme 1a–Scheme 1d.
Scheme 1.
The general DoM strategy
Scheme 1a.
Scheme 1d.
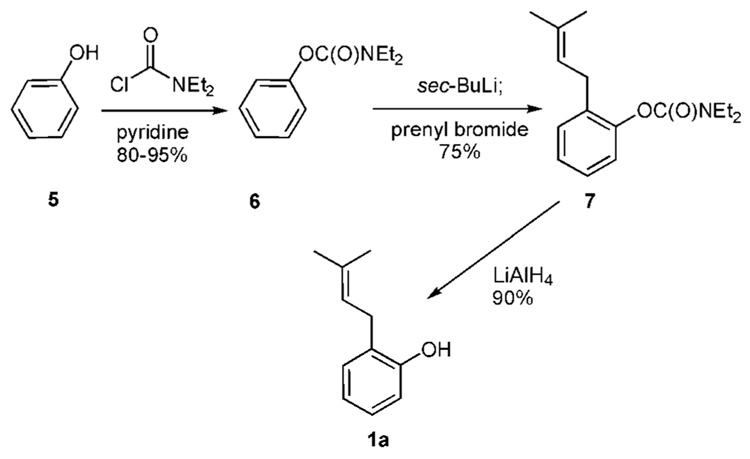
Phenol (5) smoothly couples with the carbamyl chloride to afford the carbamate 6 (Scheme 1a). Deprotonation of 6 is carried out at −78 °C with sec-butyllithium. Subsequent addition of prenyl bromide to the aryl anion affords the prenylated material 7 in 75% yield. Reduction of the carbamate moiety with LiAlH4 produces the o-prenylated phenol 1a in yields ranging from 54–64% from 5.10
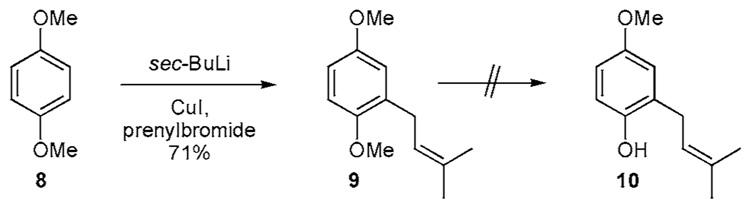
Despite the availability of the differentially protected hydroquinones 3a and 3b, application of these materials in the construction of 1b has not been reported. However, 1,4-dimethoxybenzene (8) has been prenylated utilizing the DoM protocol to produce 9 in 69% yield (Scheme 1b).12 The addition of catalytic CuI improves the yield of 9 to 71%. Cleavage of a single methyl ether residue in 9 to produce 10 would be a difficult transformation and to our knowledge is unknown.
Scheme 1b.
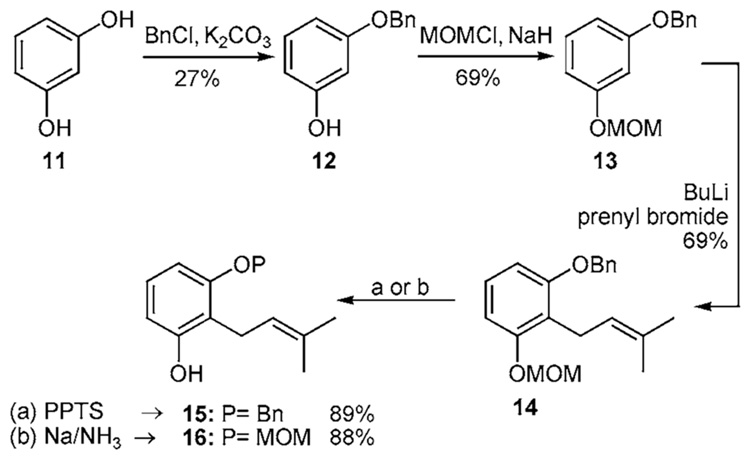
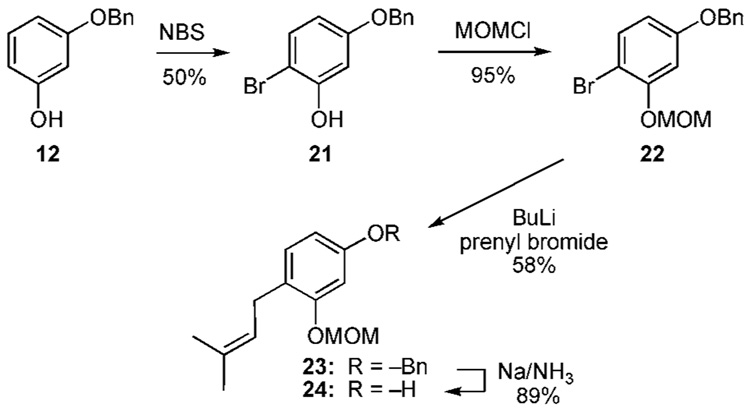
Application of the DoM sequence becomes problematic (Scheme 1c) with the resorcinol nucleus.13 Desymmetrization of resorcinol (11) is a non-trivial affair. Mono-protection affords 12 in 27% yield. The subsequent etherification with MOMCl proceeds without difficulty and the oxygen substituents cooperate in the deprotonation of 13 with butyllithium. Subsequent addition of prenyl bromide occurs exclusively at the 2-position affording 14 in 69% yield. Selective cleavage of the MOM and benzyl moieties is achieved with PPTS and Na/NH3, respectively, affording 15 and 16 in 89% and 88% yield. However, because desymmetrization of 11 proves so inefficient the overall yield in either of these sequences is only 11%.
Scheme 1c.
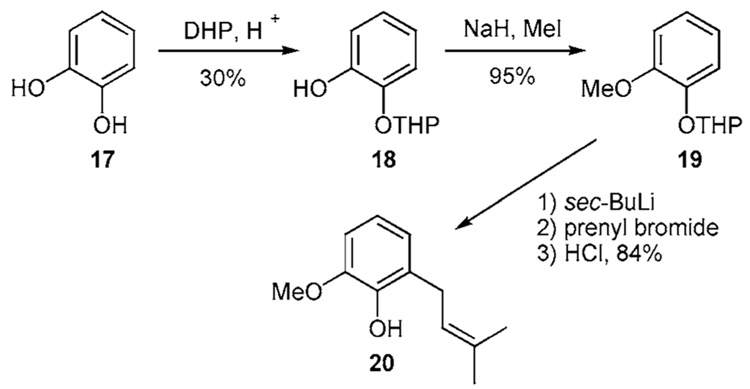
Desymmetrization of 17 has been accomplished with DHP in 30% yield to produce the mono-ether 18 (Scheme 1d).14 Methylation of the remaining hydroxyl residue affords 19. The DoM protocol proceeds smoothly resulting in an ortho-prenylated THP ether. Subsequent deprotection of the THP residue affords the differentially protected catechol 20 in 84% yield, a 23% overall yield from 17.
4 Metal-Halogen Exchange and Metal-Mediated Coupling
In metal-halogen exchange (MHE) mediated transformations, the issue of regiocontrol is reassigned to the preparation of the ortho-halogenated material. Regioselective halogenation remains challenging and follows different trends than the DoM alkylation strategy. If a halogenated phenol is readily available, two processes can be used (options a and b shown in Scheme 2). In option a, an electrophile is added after oxidative insertion with lithium, magnesium or zinc. In option b, a nucleophilic prenylated metal species is added after oxidative insertion with transition metals such as Ni(0) or Pd(0). The former strategy suffers from many of the earlier discussed problems; namely, the complications arising with attendant functional groups under highly basic conditions. The transition metal mediated process tolerates a wider variety of functional groups, including alcohols. However, these couplings proceed better with protected hydroxyl groups. In addition, transition metal mediated couplings can prove challenging with electron rich aryl halides. Furthermore, most prenyl metal species favor reverse prenylation rather than direct prenylation. Examples of these two related strategies are shown in Scheme 2a–Scheme 2e.
Scheme 2.
General metal exchange strategies for prenylation
Scheme 2a.
Scheme 2e.
As previously explained in regards to the DoM strategy, 1,3-oxygen substituents direct deprotonation at the 2-position. Therefore, the DoM protocol is not applicable to the construction of the 6-prenylated resorcinol 1c. However, option a of the MHE strategy allows access to these types of materials (Scheme 2a).13a The mono-O-benzylated resorcinol 12, available from resorcinol as shown in Scheme 1c, undergoes bromination with NBS to produce 21 in 50% yield. Etherification of the free phenol with MOMCl affords 22. At this juncture, lithium-halogen exchange and subsequent addition to prenyl bromide produces 23 in 58% yield. This material can be selectively deprotected as shown earlier (Scheme 1c) yielding 24 in 6.3% overall yield from resorcinol.
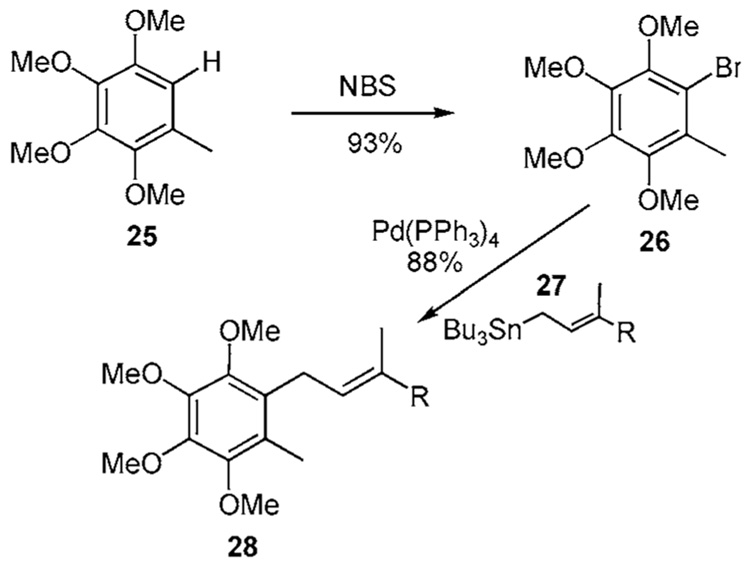
Option b of the MHE process is shown in Scheme 2b. Bromination of 25 with NBS in CH2Cl2 proceeds at the vinyl site to afford the aryl bromide 26 in 93% yield. A palladium mediated Stille coupling between aryl bromide 26 and stannane 27 affords 28 in 88% yield.15 Some have speculated that ortho substituent(s) aid(s) this coupling process by facilitating reductive elimination. Interestingly, reverse prenylation is not observed with the stannane species 27. However, this metal species is difficult to procure. 15b Considering the challenge of procuring 27 and poor selective cleavage of a methyl ether in 33, this process loses its utility.
Scheme 2b.
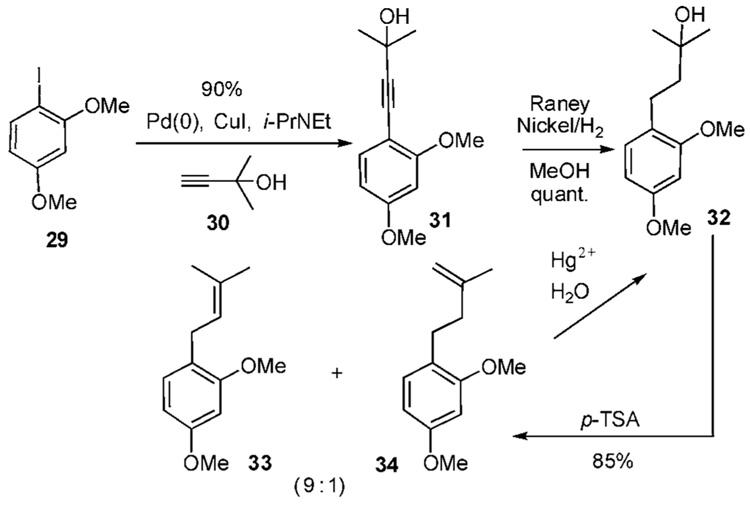
Because metal species, such as 27, are relatively inaccessible and in most cases favor reverse prenylation, several fragments have been developed to serve as masked prenyl derivatives. For example, the Sonogashira coupling of iodophenol 29 and alkyne 30 mediated by Pd(0) produces alkyne 31 in 90% yield (Scheme 2c).16 Subsequent Raney nickel reduction of 31 in methanol affords the tertiary alcohol 32 in nearly quantitative yield. para-Toluenesulfonic acid prompts elimination of the tertiary alcohol and affords a 9:1 mixture of 33 and 34. The undesired isomer 34 can be recycled to the tertiary alcohol 32 by addition of Hg(II) and H2O.
Scheme 2c.
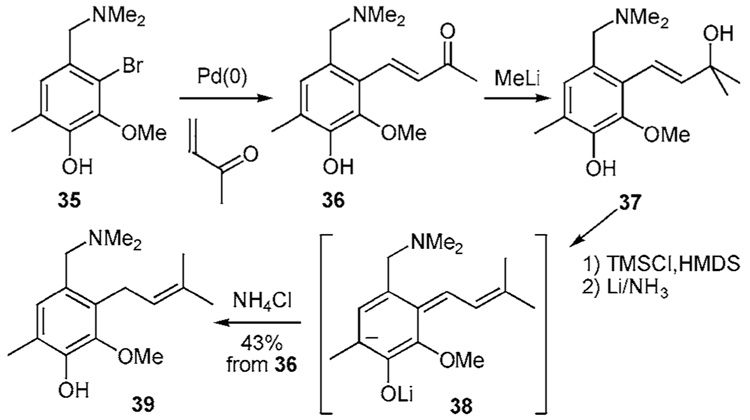
The Heck-type coupling reaction in Scheme 2d also uses a masked prenyl group.17 Coupling of the aryl bromide 35 and methyl vinyl ketone (MVK) affords enone 36. Subsequent 1,2-addition of methyl lithium produces the tertiary alcohol 37. After per-silylation, treatment with Li/NH3 affords anion 38 by sequential single electron transfers and elimination. This intermediate undergoes protonation to produce 39 in 43%.
Scheme 2d.
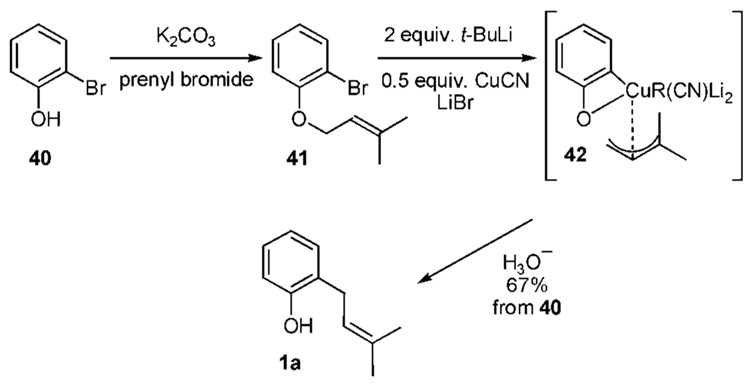
An example of reverse prenylation using the MHE sequence involving copper and resulting in ortho-prenylation is shown in Scheme 2e.18 O-Prenylation of the commercially available ortho-bromophenol (40) affords ether 41. It has been postulated that treatment with 2 equivalents of t-BuLi and 0.5 equivalents of CuCN results in a π-allyl complex 42 followed by migration to afford product 1a.
5 Phenoxide ortho-C-Alkylation
A favored strategy for preparing ortho-prenylated phenols under mild basic conditions is shown in Scheme 3. After phenols are deprotonated, the resulting phenoxide resembles an enolate. These phenoxides react at the adjacent carbon with prenyl bromide to yield the corresponding ortho-prenylated phenols.19 Because of competing side reactions such as para-prenylation, bis-prenylation, and oxygen-prenylation, the yields for this procedure are moderate (30–50%).19e When the para position is blocked (Y H) the reaction is synthetically useful. Electron rich phenols, which work poorly in previously discussed metalation procedures, are the best substrates in these types of processes. The [Na+] favors C-alkylation; whereas [K+] and [Li+] favor O-prenylation and bis-prenylation.19c A modified process using BaO/Al2O3 has been shown to work well.19h Applications of ortho-C-alkylations are shown in Scheme 3a,Scheme 3b.
Scheme 3.
General phenoxide C-prenylation strategy
Scheme 3a.
Scheme 3b.
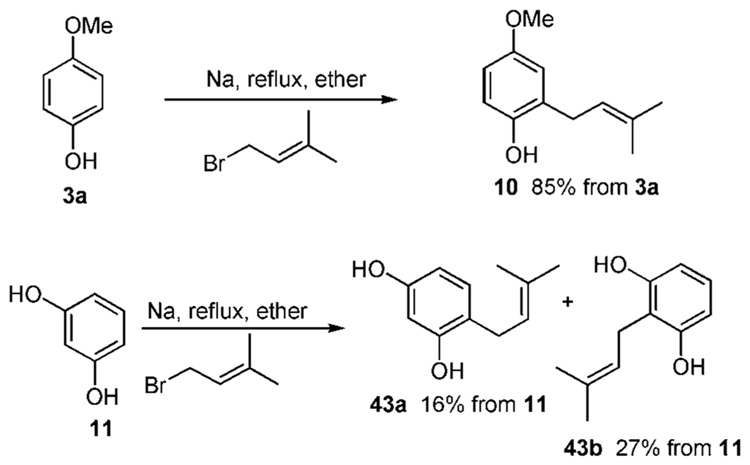
An example of this strategy is illustrated in Scheme 3a. Sodium metal is used to form the phenoxide in the differentially protected hydroquinone 3a and resorcinol (11). The phenoxide of 3a refluxed with prenyl bromide affords the prenylated phenol 10 smoothly in 85% yield.12 This reaction proves less successful for resorcinol (11). A mixture of 43a and 43b emerges in 16% and 27% yields, respectively, along with several other byproducts.19g.
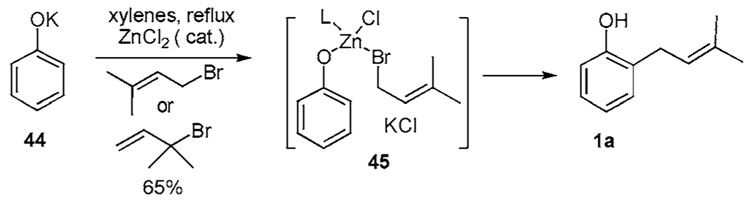
Addition of ZnCl2 in MHE reactions improves ortho control even when the para site remains unsubstituted. It is quite remarkable that phenoxide 44 undergoes addition to either prenyl bromide or 3-bromo-3-methyl-1-butene in the presence of catalytic ZnCl2 to provide only the ortho-functionalized isomer 1a in 65% yield (Scheme 3b).19i In the absence of ZnCl2, 1a emerges from 44 in less than 25% yield. This technique is reminiscent boron mediated ortho-alkylations of phenols previously reported by Sugasawa. 19j,k
6 Claisen Rearrangement
Another ortho-prenylation strategy involves the Claisen rearrangement of a reverse prenyl aryl ether (Scheme 4).20 Although the process is mild and tolerates various substitution patterns, the formation of a reverse prenyl aryl ether requires several manipulations. Moreover, in the case of dihydroxylated aromatic compounds the hydroxy residues must be distinguished prior to etherification. Examples of this strategy are shown in Scheme 4a,Scheme 4b.
Scheme 4.
The general Claisen rearrangement strategy
Scheme 4a.
Scheme 4b.
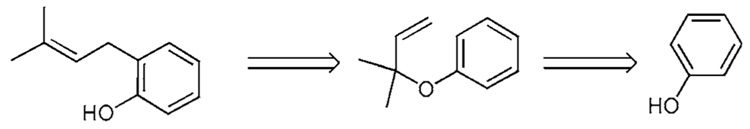
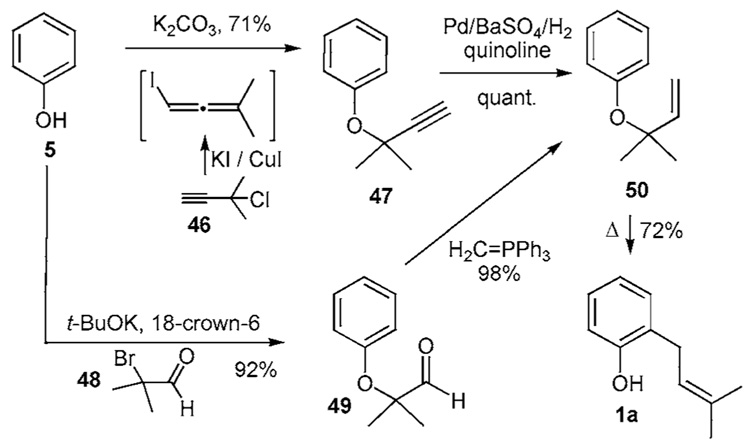
Reverse prenyl aryl ethers have been constructed using one of two processes (Scheme 4a). In one method, phenol (5) undergoes coupling as its corresponding potassium salt with 3-chloro-3-methylbutyne (46) mediated by potassium iodide and copper iodide to produce the alkynyl ether 47.20b,c The allenyl intermediate is presumed to be formed from 46 and undergoes addition-elimination to produce alkyne 47. Subsequent partial reduction of the alkyne using Lindlar’s conditions affords the reverse prenyl ether 50. In the alternative method, the phenoxide of 5 is coupled with the ortho-bromoaldehyde (48) to generate aldehyde 49 in good yield. This material undergoes a subsequent Wittig olefination to afford the identical reverse prenyl aryl ether 50.20f Compound 50 smoothly undergoes a [3,3]-sigmatropic rearrangement upon heating (130 °C) to produce 1a, delivering the prenyl residue to the most accessible ortho site thereby producing phenol 1a.
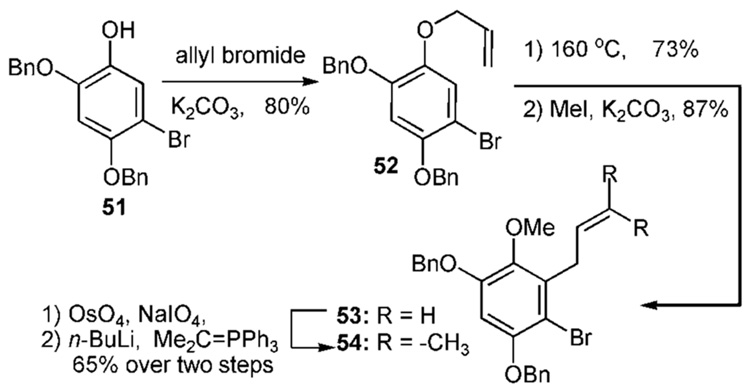
A related strategy avoids the rather cumbersome formation of the reverse prenyl aryl ether (Scheme 4b).21 O-Allylation readily proceeds by combination of allyl bromide and the potassium phenoxide of 51 to produce the allyl ether 52. This material undergoes rearrangement at temperatures >180 °C and affords 53 upon subsequent methylation of the phenol. Oxidative cleavage of the olefin side-chain in 53 affords an aldehyde followed by Wittig reaction of the dimethyl phosphonium ylide to yield 54. In the case of 1b–e, application of this strategy would require differentially protected phenols such as 2–4.
7 Friedel–Crafts-like Prenylations
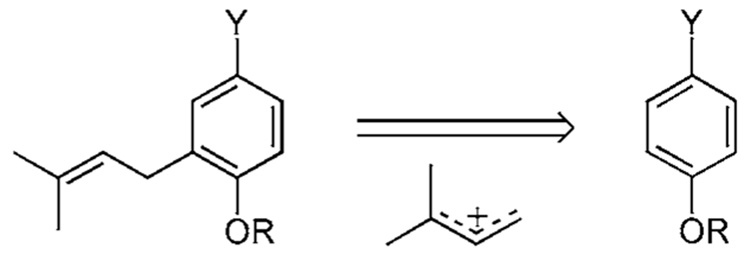
Another method to produce ortho-prenylated phenols utilizes a Friedel–Crafts (FC) type reaction shown in Scheme 5. The reaction generally occurs at the less substituted carbon of the prenyl cation and the most nucleophilic site of the aromatic compound.22 However, success mandates that the phenol substrate bear multiple electron donating substituents and the process proceeds with only moderate regiocontrol. Furthermore, these types of processes are difficult to stop after a single substitution since the addition of a prenyl residue makes the aromatic system more electron rich and hence more inclined to undergo a second addition. For these reasons, the reaction is unsuccessful with resorcinol and catechol nuclei, but works well in other instances. It is most useful when prenylating a symmetric electron rich phenol in which the para position is substituted. Examples are shown in Scheme 5a–Scheme 5e.
Scheme 5.
General Friedel–Crafts prenylation strategy
Scheme 5a.
Scheme 5e.
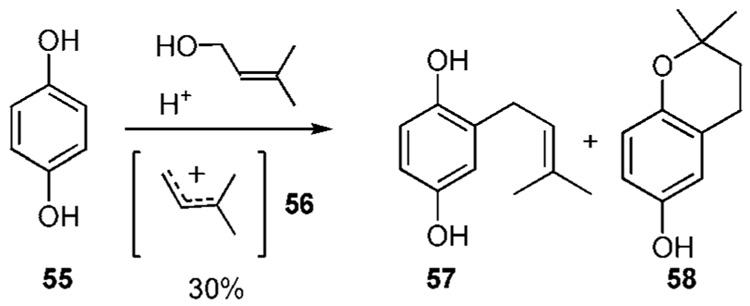
In weakly acidic media, simple phenols prove unreactive. However, more electron rich phenols such as hydroquinone (55) undergo prenylation with cation 56, produced by the addition of acid to the prenyl alcohol shown, to produce 57 in 30% yield (Scheme 5a).22a,b Significant amounts of 55 are recovered along with the chroman 58 that results by cyclization between the phenol residue in 57 and the prenyl residue. To discourage the formation of 58 the transformation can also be carried out with nonprotic Lewis acids such as BF3•OEt2.22c,d
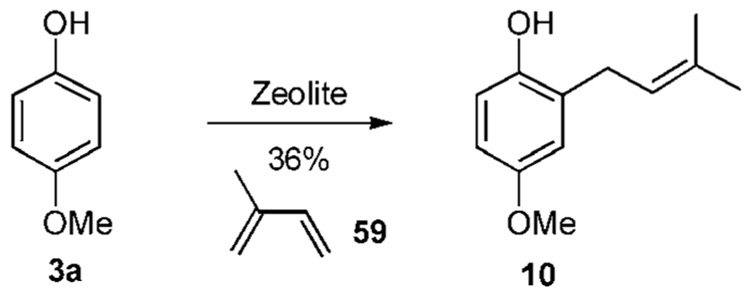
Acidic clays are thought to work in the same manner. For example, refluxing phenol 3a with butadiene 59 in the presence of clay zeolites affords the hydroquinone derivative 10 in 36% yield (Scheme 5b).22e A similar reaction with anisole leads to a mixture of para- and ortho-prenylation products.
Scheme 5b.
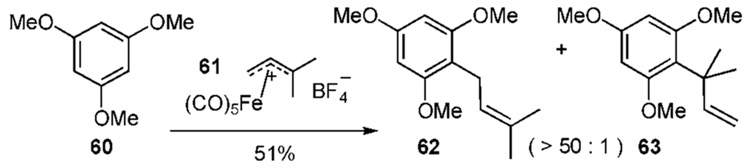
The (η3-allyl)Fe(CO)4 cation metal complex 61 is significantly more stable than cation 56 and often proves better behaved. Complex 61, however, is unreactive towards phenol and hydroquinone. Electron rich ring systems, such as 60, undergo mono-prenylation in modest yields (Scheme 5c).22f
Scheme 5c.
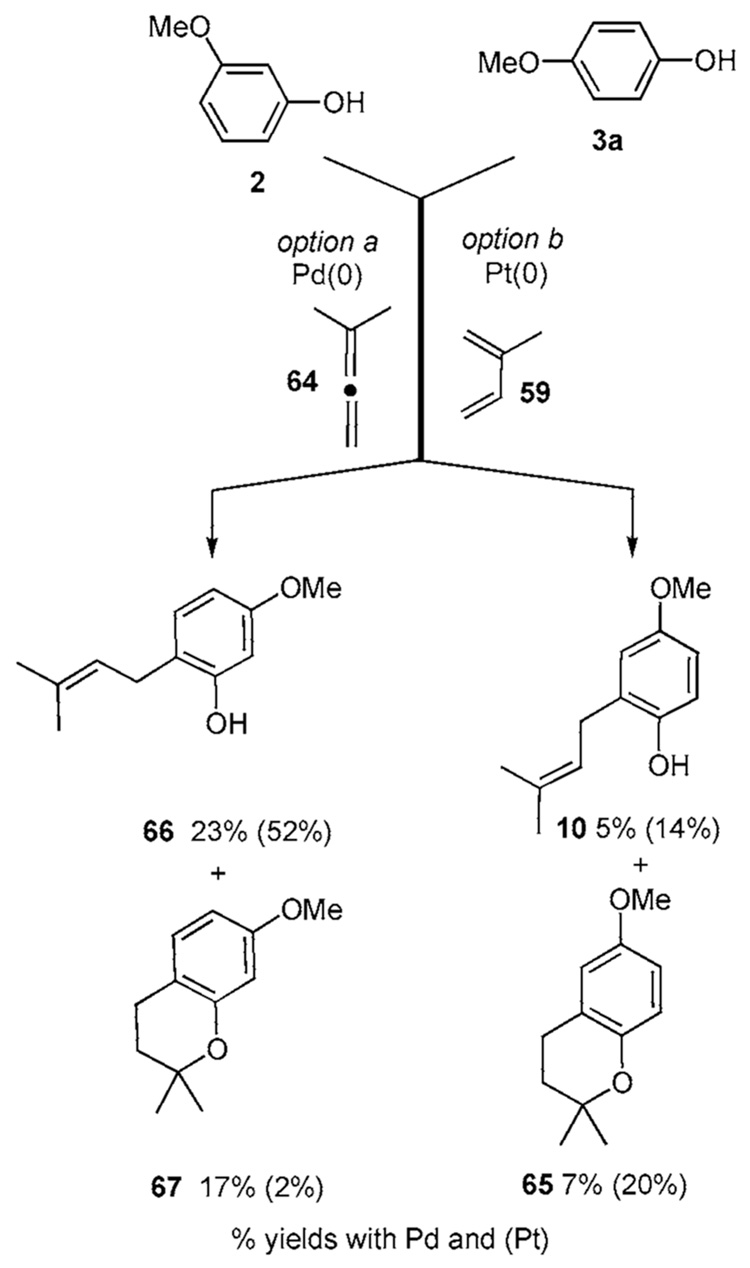
Another strategy that can be loosely classified as a FC reaction involves Pt(II) and butadiene 59 or Pd(II) and allene 64 (Scheme 5d).22g,h Hydroquinone 3a undergoes reaction under the Pd(II) protocol to afford 10 and 65 in 5% and 7% yields, respectively. The Pt(II) protocol applied to 3a affords 10 and 65 in 14% and 20% yields, respectively. The resorcinol derivative 2 on the other hand undergoes reaction in the palladium protocol to produce 66 and 67 in 23% and 17% yields, respectively, while the platinum procedure affords 66 and 67 in 52% and 2% yields, respectively.
Scheme 5d.
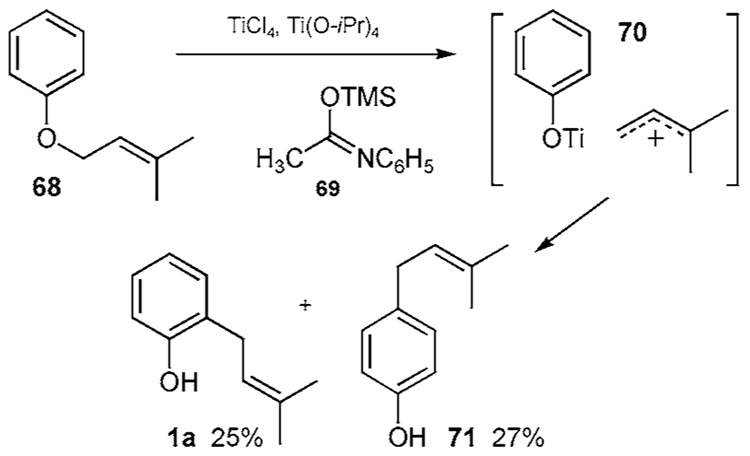
Another FC related strategy starts with prenyl ether 68, which upon treatment with 3 equivalents of a 1:1 mixture of TiCl4/Ti(O-iPr)4 proceeds to the ion pair 70 and ultimately phenols 1a and 71 in 25% and 27% yields, respectively (Scheme 5e).22i The imine 69 serves as a HCl scavenger and prevents formation of the chroman adduct from 1a. Montmorillonite clay is reported to catalyze a similar transformation in O-prenylated ethers in yields ranging from 35–53%.22j,k
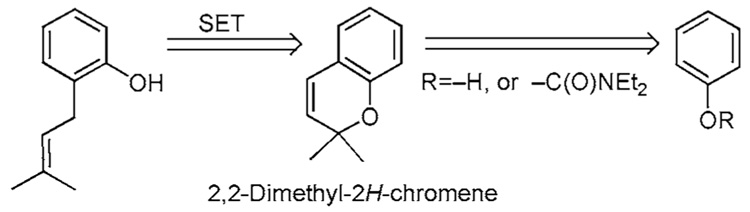
8 Birch Reduction of 2,2-Dimethyl-2Hchromenes
The Birch strategy for the preparation of ortho-prenylated phenols entails reductively opening 2,2-dimethyl-2Hchromenes by a series of single electron transfers (Scheme 6).23,24 Examples are shown in Scheme 6a.
Scheme 6.
General Birch reductions strategy
Scheme 6a.
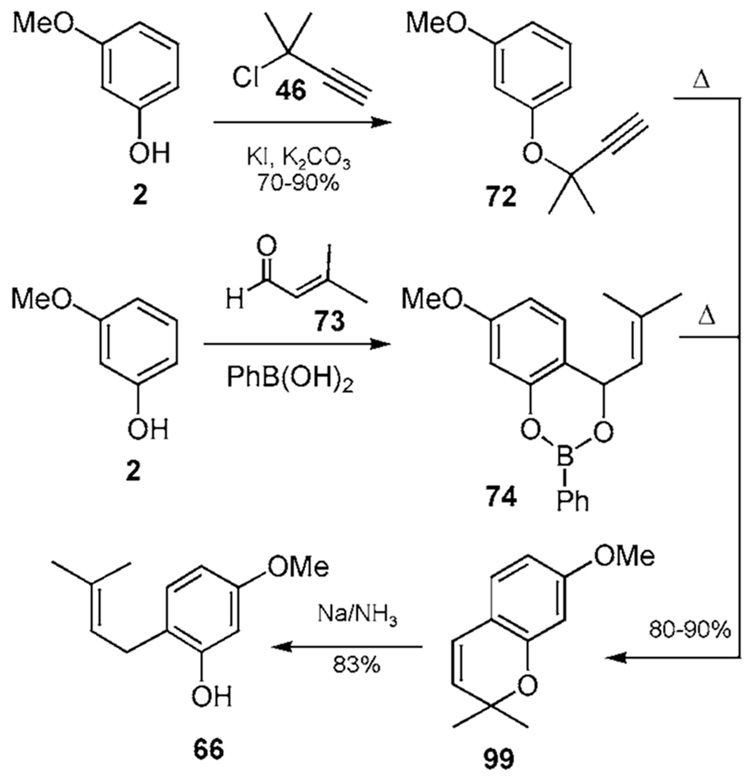
The chromene 99 can be constructed in a regioselective manner by several processes (Scheme 6a). In one, propargylation of phenol 2 is effected by conditions described earlier in Scheme 4a. Subsequent thermal treatment of propargyl ether 72 affords chromene 99 by cyclization. In another procedure, an aldol-like reaction is employed, followed by heat. Treatment of phenol 2 with PhB(OH)2 and 3,3-dimethylacrylaldehyde (73) affords the cyclic borate 74.23a,b Warming this material leads to elimination, resulting in an ortho-quinone methide which undergoes subsequent electrocyclization to produce chromene 99. In yet another protocol, a −C(O)NEt2 protected phenol is ortho-lithiated and added to 3,3-dimethyl acrolein. Subsequent treament of the benzyl alcohol with acid is speculated to afford an allyl cation that undergoes cyclization with the freed phenol to afford the corresponding chromene.24c All of these methods confront the questions of ortho/para regioselectivity and multiple additions, provided that the starting phenol can be obtained with differentially protected hydroxy groups. Chromene 99 can then be opened via a series of single electron transfers using Birch reduction conditions to afford the ortho-prenylated phenol 66. One or more of these methods could be applied in the synthesis of ortho-prenylated materials 1a,b,d,e.
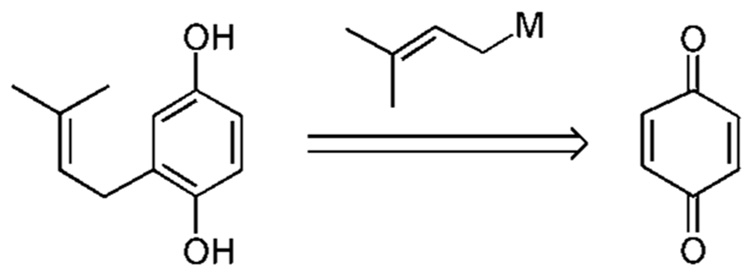
9 Net Conjugate Addition to para-Quinone
Although applicable only to the hydroquinone nucleus, a net 1,4-conjugate addition of a prenyl organometallic reagent to para-quinone would in principle provide a straightforward route to a prenylated hydroquinone (Scheme 7). Specific examples of this strategy are shown in Scheme 7a.
Scheme 7.
1,4-Conjugate addition strategy
Scheme 7a.
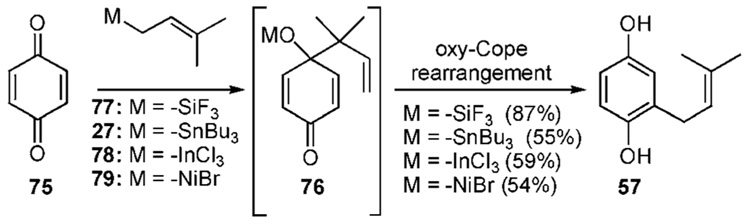
The propensity of most prenyl metal species to favor reverse prenylation is utilized in the following method. A 1,2-prenylation of the carbonyl followed by an oxy-Cope- [3,3]-rearrangement constitutes a net conjugate addition of a prenyl residue to quinone. Several metal species have been found to undergo this process (Scheme 7a).25–28 For example, treatment of 1,4-benzoquinone (75) with trifluoro-(3-methyl-2-butenyl)-silane (77) and TBAF affords the intermediate cyclohexadienone alcohol 76, which undergoes a subsequent rearrangement to restore aromaticity and generate the prenylated hydroquinone 57 in a respectable 87% yield.25 The corresponding species of stannane (27),26a–d indium (78),27 and nickel (79)28 have all been employed in similar processes.
10 Benzylic Couplings
An alternative disconnection strategy avoids the issue of regioselectivity by coupling a vinyl metal species at the benzylic position of the appropriately protected phenol (Scheme 8). Because many of these benzylic derivatives are procured from the corresponding salicylaldehyde, access to differentially protected systems are easily accommodated. Examples of these processes are shown in Scheme 8a,b.
Scheme 8.
General benzylic coupling strategy
Scheme 8a.
Scheme 8b.
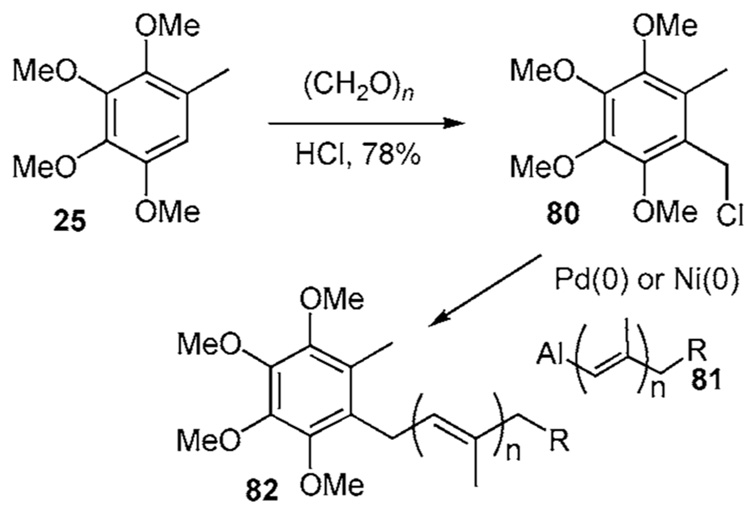
The highly reactive benzyl chloride 80, available in 78% yield from 25, undergoes Pd(0) or Ni(0) mediated coupling with the aluminum species 81 to afford CoQ10 (82) in 88% yield (Scheme 8a).29 While only the application of the vinyl aluminum reagent has been reported, the coupling is amendable to zirconium, stannyl, and magnesium vinyl species.
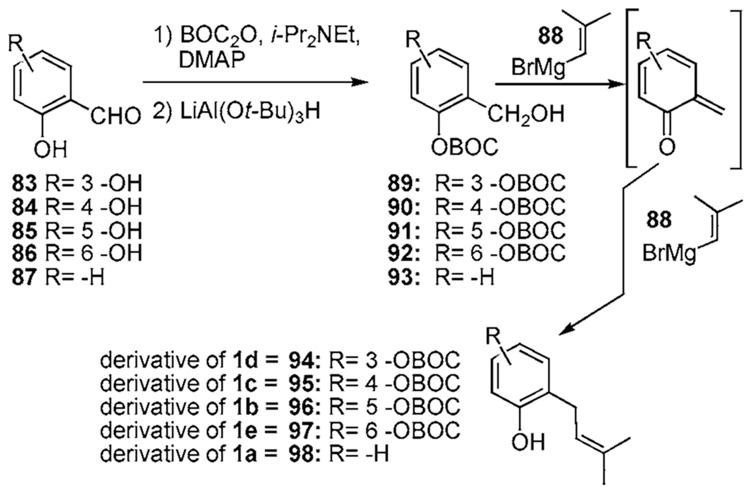
A milder variant of this strategy, which avoids preparation and purification of the highly reactive benzyl chloride intermediate 80, is shown in Scheme 8b.30 The appropriate salicylaldehydes 83–8731 are converted by protection and reduction into the corresponding o-OBOC alcohols 89– 93. Addition of a benzyl alcohol to an excess of Grignard compound 88 results in deprotonation of the alcohol, migration of the BOC group, and elimination yielding a ortho-quinone methide intermediate. Subsequent 1,4-addition of 88 to this intermediate affords the desired ortho-prenylated phenol in respectable yields (94–98, 25–67% from the starting aldehydes 83–87).32 Inverse addition was found to be crucial in order to prevent formation of a chroman cycloadduct side product that arises by a [4+2] reaction of the hydrido form of 88 with the ortho-quinone methide.
11 Conclusions
We hope this discussion has illuminated the difficulties associated with the preparation of ortho-prenylated phenols. In dihydroxylated aromatic derivatives the problem is far more challenging than it would first seem. The only strategy examined that provides application to all of the differentially protected dihydroxylated nuclei (1b–e) in a mild manner is shown in Scheme 8b. The ortho-quinone methide strategy is exceptionally diverse considering it is such a synthetic challenge. We hope synthetic groups find the discussion useful and the ortho-quinone process applicable in their future preparations of natural products that contain an ortho-prenylated phenol.30c
General Protocol for Construction of 1a–e
Procedure for Acylation and Reduction of Salicylaldehydes 83–87
To a dry, nitrogen flushed, 10 mL round-bottom flask, equipped with a magnetic stir bar, charged with salicylaldehyde (93 mg, 0.673 mmol) in anhyd THF (3.4 mL, 0.2 M) was added di-tert-butyl dicarbonate (297 mg, 1.36 mmol). N,N-Diisopropylethylamine (59 μL, 0.337 mmol) and DMAP (2.5 mg, 0.02 mmol) were added to the reaction mixture which was stirred for 10 h at r.t. The reaction mixture was concentrated in vacuo and purified by flash silica gel chromatography (EtOAc–petroleum ether, 95:5).
To a dry, nitrogen flushed, 10 mL round-bottom flask, charged with di-BOC protected salicylaldehyde (16.2 mg, 0.4788 mmol) in anhyd THF (0.5 mL, 0.1 M) was added lithium tri-tert-butoxyaluminohydride until completion (5 min, monitored by TLC analysis). The reaction mixture was quenched with 0.1 M HCl (0.5 mL) and extracted with Et2O (3 × 2 mL). The combined organic fractions were washed with brine (2 mL), dried over Na2SO4, and concentrated in vacuo. The crude product was purified by flash silica gel chromatography (EtOAc–petroleum ether, 85:15) to afford the corresponding benzyl alcohol.
Special Notes (1–5) associated with the preparation of alcohols 89–93, distinguishable because of hydrogen bonding:
1) Preparation of 89 required the addition of 5 equiv of lithium tri-tert- butoxyaluminohydride as solid at a reaction temperature of 0 °C, then the reaction was allowed to warm up to r.t. for 15 min before quenching with 0.1 M citric acid.
2) Preparation of 90 required 1 equiv of lithium tri-tert-butoxyaluminohydride for reaction completion.
3) Preparation of 91 is done in dry Et2O at 0 °C and required the solution to warm up to r.t. for 20 min after addition of 1.5 equiv of lithium tri-tert-butoxyaluminohydride for reaction completion.
4) Preparation of 92 required a reaction temperature of 0 °C and 5 equiv of lithium tri-tert-butoxyaluminohydride for reaction completion. The migration of the BOC group to the benzylic alcohol was observed and purification through flash chromatography was avoided because of the resulting cleavage of the BOC group.32
5) Preparation 93 required 1.2 equiv of lithium tri-tert-butoxyaluminohydride for reaction completion.
Preparation of [(CH3)2C=CHMgBr]
Commercially available 88 contains significant amounts of Mg(OH)2 which greatly impedes the efficiency of the reaction. For this reason, 88 was freshly prepared before each use. To a dry, nitrogen flushed, 10 mL round-bottom flask, charged with magnesium powder (356 mg, 14.6 mmol) and covered with anhyd THF (4 mL), 2-methyl-1-propenylbromide was added neat (1 mL, 9.8 mmol) at r.t. Dibromoethane (0.168 mL, 2.0 mmol) was added and the reaction mixture was heated to 40 °C. After completion (1 h) the reaction was cooled to r.t. and diluted to a 0.2 M solution.
General Procedure for Addition of the Organomagnesium Compound to ortho-OBOC Alcohol
A dry, nitrogen flushed, 10 mL round-bottom flask, equipped with a magnetic stir bar was charged with 88 (3.8 mL, 0.2 M in THF, 0.8 mmol) and cooled to 0 °C. The benzylic alcohol (51.6 mg, 0.2 M in THF, 0.15 mmol) was added dropwise by a cannula. The reaction was allowed to warm to r.t. over 10 min. The reaction was quenched with 0.1 M HCl (1.5 mL) and extracted with EtOAc (3 × 5 mL). The combined organic fractions were washed with brine (5 mL), dried over Na2SO4, and concentrated in vacuo. The crude product was purified by flash chromatography (EtOAc–petroleum ether) to yield the prenylated phenols 94–98.
Special Notes (1–6) associated with the preparation of 94–98 resulting from the addition of RMgX to alcohols 89–93:
1) DA adduct arises upon non-inverse addition of 88 to 90
1H NMR (400 MHz, CDCl3): δ = 6.92 (dd, J = 2.8, 8.6 Hz, 1 H), 6.86 (d, J = 2.6 Hz, 1 H), 6.80 (d, J = 8.8 Hz, 1 H), 5.04 (d, J = 8.0 Hz, 1 H), 2.95 (d, J = 3.8 Hz, 1 H), 2.83 (d, J = 16.3 Hz, 1 H), 2.40 (d, J = 16.3 Hz, 1 H), 1.56 (s, 9 H), 1.08 (s, 3 H), 0.97 (s, 3 H); 13C NMR (100 MHz, CDCl3): δ = 152.6, 148.5, 144.9, 122.6, 122.1, 120.3, 117.4, 98.6, 83.5, 35.1, 32.4, 32.9, 24.3, 24.0.
2) Compound 94
(petroleum ether–EtOAc, 80:20), 69% isolated yield: The benzylic alcohol (0.2 M in Et2O) was added dropwise via syringe. The reaction was kept at 0 °C for 20 min before quenching with NH4Cl (0.1 M) to reduce the BOC cleavage observed under acidic conditions. 1H NMR (400 MHz, CDCl3): δ = 7.06–6.77 (m, 3 H), 5.60 (s, OH), 5.25 (t, J = 7.22 Hz, 1 H), 3.27 (d, J = 7.22 Hz, 2 H), 1.74 (s, 3 H), 1.71 (s, 3 H), 1.57 (s, 9 H); 13C NMR (100 MHz, CDCl3): δ = 144.3, 142.2, 135.3, 127.6, 122.0, 121.7, 120.8, 113.5, 94.6, 27.9, 26.0, 18.1.
Compound 95
(petroleum ether–EtOAc, 95:5), 68% isolated yield: 1H NMR (400 MHz, CDCl3): δ = 7.07 (d, J = 2.4 Hz, 1 H), 6.68 (dd, J = 8.3, 2.4, 1 H), 6.64 (d, J = 2.4, 1 H), 5.33 (s, OH), 5.29 (m, 1 H), 3.32 (d, J = 6.9 Hz, 2 H), 1.77 (s, 6 H), 1.56 (s, 9 H); 13C NMR (100 MHz, CDCl3): δ = 155.0, 152.2, 150.4, 135.3, 130.4, 124.7, 121.7, 113.5, 109.3, 83.7, 29.5, 27.9, 26.0, 18.1.
Compound 96
(petroleum ether–EtOAc, 95:5), 65% isolated yield: 1H NMR (400 MHz, CDCl3): δ = 6.92–6.89 (m, 2 H), 6.78–6.76 (m, 1 H), 5.32–5.30 (m, 1 H), 5.08 (s, OH), 3.33 (d, J = 7.3 Hz, 1 H), 1.78–1.77(m, 6 H), 1.56 (s, 9 H), 3.32 (d, J = 6.9 Hz, 2 H), 1.77 (s, 6 H), 1.56 (s, 9 H); 13C NMR (100 MHz, CDCl3): δ = 152.1, 144.7, 135.6, 128.0, 122.6, 121.3, 120.2, 116.3, 94.6, 83.5, 27.9, 26.0, 18.1.
Compound 97:32
(petroleum ether–EtOAc, 80:20), 25% isolated yield: The benzylic alcohol (0.05 M in Et2O) was added dropwise via a syringe. The reaction was kept at 0 °C for 20 min before quenching with 0.1 M HCl. 1H NMR (400 MHz, CDCl3): δ = 7.10 (t, J = 8.1 Hz, 1 H), 6.73 (d, J = 8.3 Hz, 2 H), 5.36 (s, OH), 5.22 (t, J = 7.1 Hz, 1 H), 3.34 (d, J = 7.1 Hz, 2 H), 1.81 (s, 3 H), 1.74 (s, 3 H), 1.55 (s, 9 H); 13C NMR (100 MHz, CDCl3): δ = 155.8, 152.2, 149.9, 135.3, 127.4, 121.1, 120.0, 114.6, 113.9, 83.6, 29.9, 27.9, 26.0, 23.5, 18.1.
Compound 98
(petroleum ether–EtOAc, 97:3), 63% isolated yield: 1H NMR (400 MHz, CDCl3): δ = 7.15–7.11 (m, 2 H), 6.89 (td, J = 1.2, 2.4, 8.6 Hz, 1 H), 6.82 (dd, J = 1.1, 8.3 Hz, 1 H), 5.36–5.34 (m, 1 H), 5.17 (s, OH), 3.38 (d, J = 7.22, 1 H), 1.80 (s, 6 H); 13C NMR (100 MHz, CDCl3): δ = 154.48, 134.99, 130.15, 127.72, 127.01, 121.98, 120.94, 115.88, 30.00, 26.00, 18.07.
Acknowledgment
Research Grants from Research Corporation (R10296), the UC Committee on Cancer Research for two awards (19990641) and (SB010064), the National Science Foundation for two awards (CHE-9971211) and (0135031), the Petroleum Research Fund (PRF34986-G1), NIH (GM64831), and UC-AIDS (K00-SB-039) are greatly appreciated. Thanks to Christopher Lindsey, Ryan Jones, and Ryan Van De Water for the development and application of the ortho-quinone methide strategy for prenylation of phenols. Thanks to Carolyn Selenski and Lupe Mejorado for editorial insight.
Biographies

Christophe Hoarau was born in Marseille France in 1972. In 1995 he graduated from the Université de Luminy with a B.S. in biochemistry. In 1995 he moved to America and obtained a M.S. from San Francisco State University under the direction of Dr. Ihsan Erden. In 1999 he began working towards his PhD in the research group of Dr. Pettus.

Thomas R. R. Pettus was born in Richmond Virginia in 1967. After a false start at his father’s alma mater, he eventually graduated from Longwood College summa cume laude with honors in Chemistry. After research with Professors Tomás Hudlicky, Richard Schlessinger and Samuel Danishefsky as an undergraduate, graduate and Postdoc, respectively, he began work at the University of California at Santa Barbara in 1998.
References
- 1.Brandt U. Biofactors. 1999;9:95. doi: 10.1002/biof.5520090203. [DOI] [PubMed] [Google Scholar]
- 2.Fukui H, Feroj Hassan AFM, Ueoka T, Kyo M. Phytochemistry. 1998;47:1037. [Google Scholar]
- 3.Mori K, Waku M, Sakakibara M. Tetrahedron. 1985;41:2825. [Google Scholar]
- 4.(a) Basset C, Sherwood RT, Kepler JA, Hamilton PB. Phytopathology. 1967;57:1046. [Google Scholar]; (b) Wächter GA, Hoffmann JJ, Furbacher T, Blake ME, Timmermann BN. Phytochemistry. 1999;52:1469. doi: 10.1016/s0031-9422(99)00221-6. [DOI] [PubMed] [Google Scholar]
- 5.Ghirtis K, Pouli N, Marakos P, Skaltsounis A-L, Leonce S, Caignard DH, Atassi G. Heterocycles. 2000;53:93. doi: 10.1016/s0014-827x(00)00068-9. [DOI] [PubMed] [Google Scholar]
- 6.Meragelman KM, McKee TC, Boyd MR. J. Nat. Prod. 2001;64:546. doi: 10.1021/np0005457. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kang SY, Lee KY, Sung SH, Park MJ, Kim YC. J. Nat. Prod. 2001;64:683. doi: 10.1021/np000441w. [DOI] [PubMed] [Google Scholar]; (b) Carney JR, Krenisky JM, Williamson RT, Luo J. J. Nat. Prod. 2001;64:203. doi: 10.1021/np010374l. [DOI] [PubMed] [Google Scholar]; (c) Verotta L, Appendino G, Beelloro E, Bianchi F, Sterner O, Lovati M, Bombardelli E. J. Nat. Prod. 2002;65:433. doi: 10.1021/np0105681. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao G-Q, Barluenga S, Mitchell HJ. J. Am. Chem. Soc. 2000;122:9939. [Google Scholar]
- 9.Wang Y, Tan W, Li WZ, Li Y. J. Nat. Prod. 2001;64:196. doi: 10.1021/np0001124. [DOI] [PubMed] [Google Scholar]
- 10.(a) Snieckus V. Chem. Rev. 1990;90:879. [Google Scholar]; (b) Snieckus V, Sibi MP. J. Org. Chem. 1983;48:1937. [Google Scholar]; (c) Metallinos C, Nerdinger S, Snieckus V. Org. Lett. 1999;1:1183. [Google Scholar]; (d) Ronald RC, Winkle MR. J. Org. Chem. 1982;47:2101. [Google Scholar]
- 11.Mechoulam R, Gaoni Y. J. Am. Chem. Soc. 1965;87:3273. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 12.Kirschleger B, Bouzbouz S. Synthesis. 1994:714. [Google Scholar]
- 13.(a) Simas ABC, Coelho AL, Costa PRR. Synthesis. 1999:1017. [Google Scholar]; (b) Fitton AO, Ramage GR. J. Chem. Soc. 1962:4870. [Google Scholar]
- 14.(a) Bieber LW, Chiappeta ADA, De Moraes E, Souza MA, Generino RM, Rolim Neto P. J. Nat. Prod. 1990;53:706. [Google Scholar]; (b) Anderson EL, Parham WE. J. Am. Chem. Soc. 1948;70:4187. doi: 10.1021/ja01192a062. [DOI] [PubMed] [Google Scholar]
- 15.(a) Jung Y-S, Joe B-Y, Seong C-M, Park N-S. Bull. Korean Chem. Soc. 2000;21:463. [Google Scholar]; (b) Depew KM, Danishefsky SJ, Rosen N, Sepp-Lorenzino L. J. Am. Chem. Soc. 1996;118:12463. [Google Scholar]
- 16.(a) Tsukayama M, Kikuchi M, Kawamura Y. Heterocycles. 1994;38:1487. [Google Scholar]; (b) Tsukayama M, Kikuchi M, Kawamura Y. Chem. Lett. 1994:1203. [Google Scholar]; (c) Kim YH, Yang SG. Tetrahedron Lett. 1999;40:6051. [Google Scholar]
- 17.(a) Beckwith ALJ, Gara WB. J. Chem. Soc., Perkin Trans. 2. 1975:795. [Google Scholar]; (b) Ballester P, Capo M, Saa JM. Tetrahedron Lett. 1990;31:1339. [Google Scholar]; (c) Garcias X, Ballester P, Capo M, Saa JM. J. Org. Chem. 1994;59:5093. [Google Scholar]; (d) Weinstock J, Ladd DL, Wilson JM, Brush CK, Yim NCF, Gallagher G, Jr, McCarthy ME, Silvestry J, Sarau HM, Flaim KE, Ackerman DM, Setler PE, Tobia AJ, Hahn RA. J. Med. Chem. 1986;29:2315. doi: 10.1021/jm00161a029. [DOI] [PubMed] [Google Scholar]
- 18.Barluenga J, Sanz R, Fañanás FJ. Tetrahedron Lett. 1997:6103. [Google Scholar]
- 19.For examples of this procedure, see:Kuhnke J, Bohlmann F. Tetrahedron Lett. 1985;26:3955.Chen KM, Semple JE, Joullie MM. J. Org. Chem. 1985;50:3997.Le Noble WJ. Synthesis. 1970:1.De Bernardi M, Vidari G, Vita Finzi P, Fronza G. Tetrahedron. 1992;48:7331.; observes 31% of C-prenylated product of 4-methoxyphenol. Note, if O-prenylation occurs, the subsequent Claisen rearrangement leads to a reverse prenyl residueYamada S, Ono F, Katagiri T, Tanaka J. Synth. Comm. 1975;5:181.Fürstner A, Gastner T. Org. Lett. 2000:2467. doi: 10.1021/ol0061236.Yamada S, Futara O, Katagiri T, Tanaka J. Bull. Chem. Soc. Jpn. 1977;50:750.Glüsenkamp K-H, Büchi G. J. Org. Chem. 1986;51:4481.Casiraghi G, Bigi F, Sartori G. Synthesis. 1981:310.Toyoda T, Sasakura K, Sugasawa T. J. Org. Chem. 1981;46:189.Piccolo O, Filippini L, Tinucci L, Valoti E, Cittero A. Tetrahedron. 1986;42:885.
- 20.(a) Hlubucek J, Ritche E, Taylor WC. Tetrahedron Lett. 1969:1369. [Google Scholar]; (b) Pettus TRR, Inoue M, Chen X-T, Danishefsky SJ. J. Am. Chem. Soc. 2000;122:6160. [Google Scholar]; (c) Pernin R, Muyard F, Bevalot F, Tillequin F, Vaquette J. J. Nat. Prod. 2000;63:245. doi: 10.1021/np9902845. [DOI] [PubMed] [Google Scholar]; (d) Monteiro N, Arnold A, Balme G. Synlett. 1998:1111. [Google Scholar]; (e) Bell D, Davies MR, Geen GR, Mann IS. Synthesis. 1995:707. [Google Scholar]; (f) Li J, Nicolaou KC. Angew. Chem. Int. Ed. 2001;40:4264. doi: 10.1002/1521-3773(20011119)40:22<4264::AID-ANIE4264>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Iikubo K, Ishikawa Y, Ando N, Umezawa K, Nishiyama S. Tetrahedron Lett. 2002;43:291. [Google Scholar]
- 22.(a) Pochini A, Marchelli R, Bocchi V. Gazz. Chim. Ital. 1975;105:1245. [Google Scholar]; (b) Pochini A, Marchelli R, Bocchi V. Gazz. Chim. Ital. 1975;105:1253. [Google Scholar]; (c) Jain AC, Prasad AK. Indian J. Chem., Sect. B. 1990;29:525. [Google Scholar]; (d) Sudalai A, Rao GSK. Indian J. Chem., Sect. B. 1989;28:760. [Google Scholar]; (e) Bigi F, Carloni S, Maggi R, Muchetti C, Rastelli M, Sartori G. Synthesis. 1998:301. [Google Scholar]; (f) Dieter JW, Li Z, Nicholas KM. Tetrahedron Lett. 1987;28:5415. [Google Scholar]; (g) De Renzi A, Panunzi A, Saporito A, Vitagliano A. J. Chem. Soc., Perkin Trans. 2. 1983:993. [Google Scholar]; (h) De Felice V, De Renzi A, Funicello M, Panuzi A, Saporito A. Gazz. Chim. Ital. 1985;115:13. [Google Scholar]; (i) Narasaka K, Bald E, Mukaiyama T. Chem. Lett. 1975:1041. [Google Scholar]; (j) Dauben WG, Cogen JM, Behar V. Tetrahedron Lett. 1990;31:3241. [Google Scholar]; (k) Corey EJ, Wu LI. J. Am. Chem. Soc. 1993;115:9327. [Google Scholar]
- 23.(a) Bissada S, Lau CK, Bernstein MA, Dufresne C. Can. J. Chem. 1994;72:1866. [Google Scholar]; (b) Chauder BA, Lopes CC, Lopes RSC, Da Silva AJM, Snieckus V. Synthesis. 1998:279. [Google Scholar]
- 24.For the reduction of chromenes, see:Aniol M, Wawrzenczyk C. Heterocycles. 1994;38:2655.Birch AJ, Maung M, Pelter AJ. Aust. J. Chem. 1969;22:1923.Chauder BA, Kalinin AV, Snieckus V. Synthesis. 2001:140.
- 25.Silane:Hagiwara E, Hatanaka Y, Gohda K-I, Hiyama T. Tetrahedron Lett. 1995;36:2773.
- 26.Tin:Maruyama K, Naruta Y. J. Org. Chem. 1978;43:3796.Naruta Y. J. Org. Chem. 1980;45:4097.Takuwa A, Soga O, Mishima T. J. Org. Chem. 1987;52:1261.Naruta Y, Maruyama K. Org. Synth. 1992;71:125.
- 27.Indium:Akari S, Katsumura N, Butsugan Y. J. Organomet. Chem. 1991;415:7.
- 28.π-Allylnickel complex:Hegedus LS, Waterman EL, Catlin J. J. Am. Chem. Soc. 1972;94:7155. doi: 10.1021/ja00775a051.Hegedus LS, Evans BR. J. Am. Chem. Soc. 1978;100:3461.
- 29.(a) Negishi E-T, Matsushita H, Okukado N. Tetrahedron Lett. 1981;22:2715. [Google Scholar]; (b) Lipshutz BH, Bulow G, Fernandez-Lazaro F, Kim S-K, Lowe R, Mollard P, Stevens KL. J. Am. Chem. Soc. 1999;121:11664. [Google Scholar]; (c) Lipshutz BH, Bulow G, Lowe R, Stevens KL. J. Am. Chem. Soc. 1996;118:5512. [Google Scholar]; (d) Srogl J, Allred GD, Liebeskind LS. J. Am. Chem. Soc. 1997;119:12376. [Google Scholar]; (e) Zhang S, Marshall D, Liebeskind LS. J. Org. Chem. 1999;64:2796. doi: 10.1021/jo982250s. [DOI] [PubMed] [Google Scholar]
- 30.(a) Van De Water RW, Magdziak DJ, Chau JN, Pettus TRR. J. Am. Chem. Soc. 2000;122:6502. [Google Scholar]; (b) Jones RM, Van De Water RW, Lindsey CC, Hoarau C, Ung T, Pettus TRR. J. Org. Chem. 2001;66:3435. doi: 10.1021/jo001752e. [DOI] [PubMed] [Google Scholar]; (c) Lindsey CC, Gómez-Días C, Villalba JM, Pettus TRR. Tetrahedron. 2002;58:4559. [Google Scholar]
- 31.The 2,6-dihydroxybenzaldehyde 88 was prepared from the demethylation of 2,6-dimethoxybenzaldehyde in 58% following reported literature procedureBoulos Z, Attardo G, Barriault N, Penney C. J. Chem. Soc., Perkin Trans. 1. 1997:2925.
- 32.The lability of the remaining OBOC residue in 92 and our inability to purify this substance is believed to be responsible for the reduced yield of 97 when compared with the overall yields of 95, 96 and 98