Abstract
Using laser microsurgery and cell fusion we have explored how additional centrosomes and/or chromosomes influence the duration of mitosis in human cells. We find that doubling the chromosome number adds ∼10 minutes to a 20 minute division while doubling the number of centrosomes adds ∼30 minutes more, and extra centrosomes and/or chromosomes prolong mitosis by delaying satisfaction of the spindle assembly checkpoint. Thus mitosis can be prolonged by non genetic means and extra chromosomes and centrosomes likely contribute to the elevated mitotic index seen in many tumors.
Keywords: mitosis, centrosomes, chromosomes, cancer, spindle assembly checkpoint
The spindle assembly checkpoint (SAC) prolongs mitosis until all kinetochores are stably attached to spindle microtubules (MTs). In organisms with low chromosome numbers, like flies1 or fission yeast, the SAC is not essential because spindles form rapidly. However, in higher eukaryotes where the attachment of all chromosomes can take hours2 the SAC is essential.
In animals kinetochore attachment to the spindle is envisioned to occur via a stochastic exploration of space by dynamic MTs nucleated from the centrosomes3. This “search-and-capture” mechanism predicts that spindle assembly would be delayed in cells with extra chromosomes and accelerated in cells with extra centrosomes. Surprisingly, the little data existing on this topic is counterintuitive suggesting that extra chromosomes4,5 and/or centrosomes6 have little influence on the duration of mitosis.
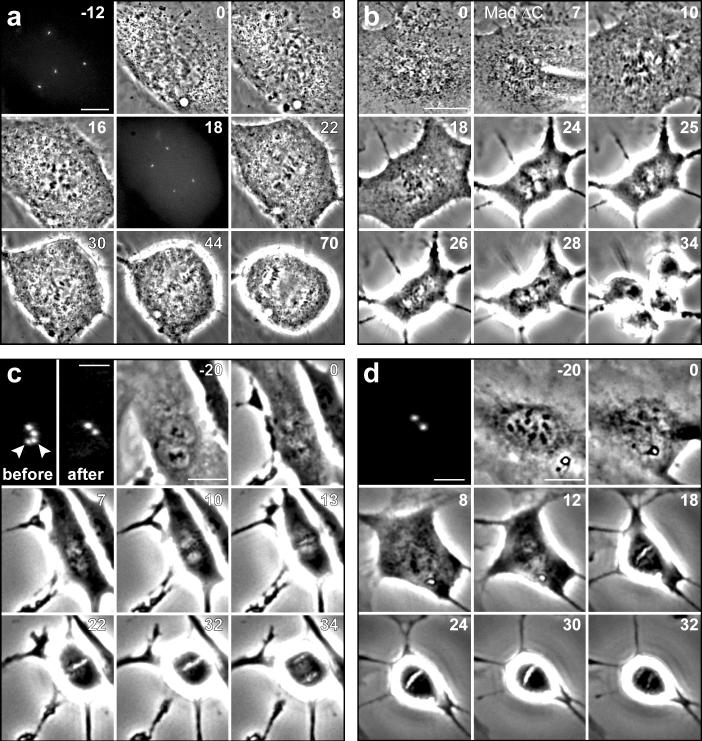
To systematically explore this issue we followed cultures of diploid human cells at 37°C by time-lapse video LM. These records revealed that the duration of mitosis, defined as the interval between nuclear envelope breakdown (NEB) and anaphase onset, is 19 ± 3 minutes in telomerase-immortalized retinal pigment epithelial (RPE-1) cells (mean ± SD; n = 200; throughout the text n represents the number of cells pooled from ≥3 independent experiments). Next we inhibited cytokinesis in RPE-1 cultures with 0.2 μM cytochalasin D. After 16 hrs these cultures contained binucleated cells that after a thorough washing7 entered the next mitosis within 24 hours. Since binucleated cells contain twice the normal number of centrosomes (and chromosomes) they formed multipolar spindles during mitosis. Relative to 2N controls in these cultures, which averaged 20 ± 4 (n = 130) minutes in mitosis, binucleated cells averaged 49 ± 17 (n = 90) minutes (Fig. 1a). Thus, doubling the chromosome number, or doubling the centrosome number, or both, prolongs mitosis >2X. Although binucleated RPE-1 cells initially form tetrapolar spindles, these subsequently transform into bipolar or tripolar spindles so that 52% of the cells ultimately divided into two daughters (Fig. 1a, Supplementary Information, Fig. S1 online) while 48% divided into three. This prolongation of mitosis in binucleated RPE-1 cells is due to a delay in satisfying the SAC because binucleated cells entered anaphase 17−22 minutes (Fig. 1b, n = 6) after microinjection with Mad2-ΔC, a dominant-negative form of Mad2 that abrogates the SAC8.
Figure 1.
Doubling the chromosome number in human RPE-1 cells prolongs mitosis by delaying satisfaction of the spindle assembly checkpoint. (a) The duration of mitosis in binucleated (4N) RPE-1 cells expressing human centrin-1/GFP (a centriolar tag) averaged 49 ± 17 minutes, compared to 20 ± 3 minutes in neighboring mononucleated (2N) controls, and binucleated cells initially formed tetrapolar spindles that reorganize into bipolar or tripolar spindles before anaphase. (b) Injecting binucleated cells shortly after nuclear envelope breakdown (7’) with purified Mad2-ΔC induces anaphase (25’) within 17∼22 min. (c) Destroying one centrosome in a binucleated G1 cell by laser microsurgery produces cells that form normal bipolar spindles that average 33 ± 5 minutes in mitosis. (d) The 4N mononucleated G1 cells produced from the division in (c) contained a single centrosome, and averaged 29±5 min in the next mitosis. The fluorescence images in (c) and (d) were acquired in G1 and scale bars = 2 μm. Scale bars = 10 μm in (a) and 20 μm in the phase images (b, c, d).
Since cancer cells often contain extra chromosomes and centrosomes one would expect their mitosis to be prolonged. Indeed, we found that HeLa (modal chromosome number 80−85, ATCC catalog) average 46 ± 19 (n = 200) minutes in mitosis (see also Supplementary Table 1 online). As in RPE-1, this prolongation is due to the SAC because depleting Mad2 with siRNA induces HeLa to exit mitosis ∼15 minutes after NEB 9. We also found that mitosis in binucleated HeLa containing 2X more chromosomes and centrosomes averages 88 ± 35 (n = 104) minutes. However, unlike RPE-1 cells, 97% of binucleated HeLa cells formed stable tri- or tetra-polar spindles that produce three or four daughter cells.
The duration of mitosis in binucleated rat kangaroo (PtK1) cells is reported to be similar to that of mononucleated cells (57 versus 50 minutes6). This contrasts sharply with our finding that mitosis in binucleated RPE-1 cells is prolonged >2X relative to mononucleated controls. The reasons for this discrepancy are unknown. However, since the 2N chromosome number in PtK1 is 12, binucleated cells enter mitosis with 24 chromosomes which is 4 times fewer than in binucleated RPE-1 cells (and the earlier data do show that, relative to mononucleated PtK1 cells, mitosis takes 7 minutes longer in binucleated cells).
Is it the extra centrosomes, extra chromosomes, or both, that prolongs mitosis in binucleated RPE-1cells? To answer this question we inhibited cytokinesis in RPE-1 expressing human centrin-1/GFP to generate binucleated G1 cells containing two labeled centrosomes10. We then removed one centriole pair (i.e., a centrosome) by laser microsurgery11. To eliminate the possibility that the cytochalasin used to induce bi-nucleation triggered the p38 stress checkpoint 10,7 in our experimental cells, we prophylactically treated the cultures continuously, starting 30 minutes before adding cytochalasin D, with a p38 inhibitor (20-μM SB203580)12. With time these binucleated cells entered mitosis in the presence of two centrosomes and formed bipolar spindles (Fig. 1c) that took 33 ± 5 minutes (n = 10) to enter anaphase. Each division produced two daughter G1 cells containing one centrosome and a 4N nucleus that cycled into the next mitosis which lasted 29 ± 5 minutes (n = 6; Fig. 1d). By contrast, surrounding mononucleated 2N cells with 2 centrosomes, and binucleated cells with 4 centrosomes averaged, respectively, 22 ± 3 minutes (n = 100) and 56 ± 20 minutes (n = 80) in mitosis. Thus, doubling the chromosome number increases the duration of mitosis by ∼50%.
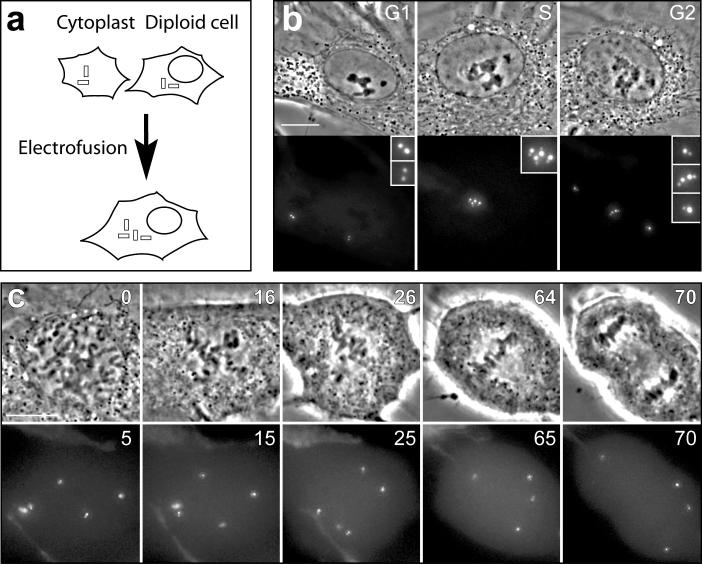
Mitosis in RPE-1 cells with twice the normal chromosome number requires ∼30 min in the presence of 2 centrosomes but ∼50 min in the presence of 4 centrosomes. This implies that doubling the centrosome number in diploid cells will prolong mitosis by ∼100%. To confirm this we fused diploid G1 RPE-1 cells with G1 cytoplasts to create normal 2N G1 cells with an extra centrosome (Fig. 2). After DNA and centrosome replication these cells entered a mitosis that lasted 53 ± 21 min (n = 11), similar to binucleated cells that enter mitosis with 4 centrosomes (49 ± 17 min). Of the eleven cells followed for this experiment, ten formed bipolar spindles and divided into two progeny after first forming tetrapolar spindles. Clearly, doubling the centrosome number in human cells prolongs mitosis ∼3X more than doubling the chromosome number.
Figure 2.
Extra centrosomes prolong mitosis in mononucleated (2N) cells. (a) Enucleated G1 RPE-1 cytoplasts containing one centrin-1/GFP labeled centrosome were electrofused with surrounding G1 cells. (b) This produced G1 cells containing a 2N nucleus and 2 centrosomes, which subsequently replicated into a 4N nucleus and 4 centrosomes. (c) As this cell entered mitosis it formed a tetrapolar spindle (16’) that became bipolar (26’, 64’) so that the chromosomes were segregated into two cells. In this example the duration of mitosis was 64 minutes (the average from 11 cells was 53 ± 21 minutes). Time is in minutes. Scale bars = 10 μm.
Intuitively extra chromosomes are expected to prolong mitosis because each contains sister kinetochores that must become stably attached to the spindle. It is unknown why extra centrosomes delay satisfaction of the SAC. In RPE-1 this delay could result from the progressive reorganization of a tetrapolar spindle into a tri- or bipolar spindle (Fig. 1a, Supplementary Information Fig. S1). However, as in RPE1, mitosis is also prolonged ∼2X in HeLa cells containing 4 centrosomes that do not undergo this spindle reorganization. Possibly the presence of multiple MT asters negatively affects the stability of kinetochore attachments on a transient basis.
Our findings partly explain why tumors exhibit an enhanced mitotic index relative to surrounding tissues. Usually this phenomenon is ascribed to an abnormally accelerated cell cycle13. However, our data suggest that mitosis is prolonged in cancer cells because many are hyper-diploid (polyploid) and/or contain extra centrosomes14,15. Indeed, a strong positive correlation exists between the average duration of mitosis in cancer cell lines and the incidence of multipolar chromosome distribution (Supplementary Information, Table 1). The same is true for transformed lines: on average fully transformed BJ-ELR cells spend ∼ 50% longer in mitosis than their parental BJ cells (33 versus 20 min), and BJ-ELR form multipolar spindles 12X more frequently than BJ cells (∼70% of BJ-ELR cells are also hyper-diploid). The occurrence of bipolar and multipolar mitotic populations also explains the high cell-to-cell variability in the duration of mitosis in cancer cell lines (Supplementary Online Fig. S2). In contrast, cancer cells having normal chromosome and centrosome complements (HT1080) can divide in less than 20 minutes (Supplementary Information Fig. S2 and Table 1 online). Thus, mitosis can be prolonged by non-genetic means.
Acknowledgments
This work was supported by National Institutes of General Medical Sciences grants 40198 (to C.L.R.) and 59363 (to A.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of General Medical Sciences or the National Institutes of Health.
Footnotes
Competing financial interests
The authors declare that they have no competing financial interests.
Supplementary Material
References
- 1.Buffin E, Emre D, Karess RE. Nature Cell Biol. 2007;9:565–572. doi: 10.1038/ncb1570. [DOI] [PubMed] [Google Scholar]
- 2.Rieder CL, Schultz A, Cole R, Sluder G. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirschner M, Mitchison T. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 4.Sisken JE, Bonner SV, Grasch SD. J. Cell. Physiol. 1982;113:219–223. doi: 10.1002/jcp.1041130206. [DOI] [PubMed] [Google Scholar]
- 5.Sisken JE, Bonner SV, Grasch SD, Powell DE, Donaldson ES. Cell Tissue Kinet. 1985;18:137–146. doi: 10.1111/j.1365-2184.1985.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 6.Sluder G, Thompson EA, Miller FJ, Hayes J, Rieder CL. J. Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- 7.Uetake Y, Sluder G. J. Cell Biol. 2004;165:609–615. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canman JC, Salmon ED, Fang G. Cell Motil. Cytoskel. 2002;52:61–65. doi: 10.1002/cm.10032. [DOI] [PubMed] [Google Scholar]
- 9.Meraldi P, Draviam VM, Sorger PK. Devel. Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Uetake Y, et al. J. Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Terra S, et al. J. Cell Biol. 2005;168:713–722. doi: 10.1083/jcb.200411126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuenda A, et al. FEBS Letters. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 13.van diest PJ, Baak JPA. J. Clin. Pathol. 1998;51:716–724. doi: 10.1136/jcp.51.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therman E, Kuhn EM. Crit. Rev. Oncogene. 1989;1:293–305. [PubMed] [Google Scholar]
- 15.Saunders W. Sem. Cancer Biol. 2005;15:25–32. doi: 10.1016/j.semcancer.2004.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.