Abstract
Objective
To report the autoantigens of a new category of treatment-responsive paraneoplastic encephalitis.
Methods
Analysis of clinical features, neuropathological findings, tumors, and serum/cerebrospinal fluid antibodies using rat tissue, neuronal cultures, and HEK293 cells expressing subunits of the N-methyl-D-aspartate receptor (NMDAR).
Results
Twelve women (14 – 44 years) developed prominent psychiatric symptoms, amnesia, seizures, frequent dyskinesias, autonomic dysfunction, and decreased level of consciousness often requiring ventilatory support. All had serum/cerebrospinal fluid antibodies that predominantly immunolabeled the neuropil of hippocampus/forebrain, in particular the cell surface of hippocampal neurons, and reacted with NR2B (and to a lesser extent NR2A) subunits of the NMDAR. NR2B binds glutamate and forms heteromers (NR1/NR2B or NR1/NR2A/NR2B) that are preferentially expressed in the adult hippocampus/forebrain. Expression of functional heteromers (not single subunits) was required for antibody binding. Eleven patients had teratoma of the ovary (six mature) and one a mature teratoma in the mediastinum; five of five tumors examined contained nervous tissue that strongly expressed NR2 subunits and reacted with patients’ antibodies. Tumor resection and immunotherapy resulted in improvement or full recovery of eight of nine patients (paralleled by decreased antibody titers); two of three patients without tumor resection died of neurological deterioration. Autopsies showed extensive microgliosis, rare T-cell infiltrates, and neuronal degeneration predominantly involving, but not restricted to, the hippocampus.
Interpretation
Antibodies to NR2B- and NR2A-containing heteromers of the NMDAR associate with a severe but treatment-responsive encephalitis. Our findings provide a diagnostic test and suggest a model of autoimmune NMDAR-related encephalitis with broad implications for other immune-mediated disorders of memory, behavior, and cognition.
Disturbances of memory, behavior, cognition, and seizures can result from immune-mediated encephalitis. One cause of autoimmune encephalitis is the paraneoplastic manifestation of a neoplasm.1 Until now, most paraneoplastic encephalitides have been associated with antibodies to intracellular onconeuronal proteins and cytotoxic T cells presumably against the same proteins.2 These disorders usually associate with malignant tumors and are poorly responsive to immunotherapies or treatment of the cancer.3 In a previous study, we described a disorder that appeared to represent a new category of severe, potentially lethal, but treatment-responsive paraneoplastic encephalitis.4 The affected patients were women who developed prominent psychiatric symptoms, seizures, memory deficits, and decreased level of consciousness often requiring ventilatory support. Three salient features included the young age of the patients, the association with ovarian teratomas, and the detection of antibodies to unknown antigens predominantly expressed in the cell membrane of hippocampal neurons (also referred to as a subgroup of neuropil antigens).5 Since then, we have studied eight additional patients and now report the identification of the target autoantigens, which are heteromers containing NR1 and NR2 subunits of the N-methyl-D-aspartate receptor (NMDAR), also expressed by the associated tumors.
Patients and Methods
Patients include 12 women with paraneoplastic encephalitis associated with teratomas. The six most recently identified patients and neuropathological findings (two cases) are described in detail in the Supplementary materials; the clinical features of the other six patients have been reported previously by us and others.4 – 8 Frozen serum or cerebrospinal fluid (CSF) was available from all 12 patients. Tissues for immunological studies included tumors from five patients (one frozen tissue, four embedded in paraffin), and brain obtained at autopsy of one patient and two neurologically normal individuals. Sera or CSF of 200 individuals, including blood donors and patients with diverse paraneoplastic and nonparaneoplastic encephalitis served as controls. Studies were approved by the University of Pennsylvania Institutional Review Board.
Animal Tissue, Antibodies, and IgG Biotinylation
Wistar rats were killed omitting perfusion with saline or fixatives; the brain was removed, immersed in 4% paraformaldehyde at 4°C for 24 hours, cryoprotected with 40% sucrose, sagittally sectioned, and snap frozen in isopentane chilled with liquid nitrogen. The following antibodies were used at the indicated dilutions: chicken anti-MAP2 (1:20,000; Covance, Princeton, NJ); rabbit anti-NR1 (1:50; amino acids 1–20) and rabbit anti-NR2A (1:50; amino acids 1265–1464) (both from Upstate Biotechnology, Lake Placid, NY); rabbit anti-NR2B (1:50; 251-amino acid sequence from N-terminal portion of NMDAR; Zymed, San Francisco, CA); and CD3, CD19, and CD68 (all 1:100; Dako-Cytomation, Carpinteria, CA).
All immunohistochemical studies with tumor tissue utilized IgG purified from patients’ sera and labeled with biotin to avoid reactivity with endogenous IgG.9
Immunohistochemistry
Paraffin-embedded tissue was deparaffinized and the antigens retrieved, as reported elsewhere.10 Seven-micrometer-thick frozen (or 4μm-thick paraffin) tissue sections were seriallyincubated with 0.3% H2O2 for 20 minutes, 10% goat serum for 1 hour, and patient’s serum (1:250), CSF (1:10) or biotinylated IgG (0.2mg/ml), or the indicated purchased antibodies overnight at 4°C. After using the appropriate secondary antibodies (all 1:2,000), reactivities were developed with the avidin-biotin-peroxidase method. The secondary antibody was omitted when patients’ biotinylated IgG was used. Normal human serum and biotinylated IgG and also normal goat serum served as controls. Double immunolabeling with patients’ IgG, MAP2, and NR2 antibodies was performed using the appropriate Alexa Fluor secondary antibodies diluted 1:2,000 (Molecular Probes, Eugene, OR); results were photographed under a fluorescence microscope using Zeiss Axiovision software (Zeiss, Thornwood, NY).
Immunocytochemistry
Rat hippocampal neuronal cultures were prepared as reported elsewhere.11 Live neurons grown on coverslips were exposed for 1 hour at 37°C to the patients’ or control serum (final dilution 1:400) or CSF (1:10). After removing the media and extensive washing, neurons were fixed with 4% paraformaldehyde, and single or double immunolabeling was performed, as indicated earlier. The reactivity of commercial antibodies to NR1 and NR2 subunits was best seen with cell permeabilization (0.1% Triton X-100) after paraformaldehyde.
HEK293 cells were transfected with plasmids containing rodent NR1, NR2A, or NR2B subunits of the NMDAR (>90% homologous to the human subunits in the extracellular domains) or plasmids without insert (control), as reported previously.12–14 In other experiments, cells were co-transfected with NR1 and NR2A (or NR1 and NR2B) in equimolar ratios.12–14 Cells were grown for 24 hours after transfection before assessment. All cells were routinely grown in the presence of NMDAR antagonists (500μM ketamine) to prevent cell death after transfection.15 Transfected cells were then incubated with patients’ serum (1:400) or CSF (1:10) overnight at 4°C and the appropriate Alexa Fluor secondary antibodies, as described earlier.
Results
Neurological Findings
The median age of the patients was 27 years (range, 14 – 44 years). In 11 patients, the neurological symptoms preceded the diagnosis of the teratoma by 3 weeks to 4 months (median, 2 months), and in one patient occurred after an ovarian cyst had been radiologically detected 1 month earlier. Ten patients had a viral-like prodromic syndrome with hyperthermia (seven cases) and frequent headache (six cases) (Table 1).
Table 1.
Clinical Features
| Case No. | Sex/Age(yr) | Teratoma: Histology, Side, Size | Time to Tumor Diagnosis | Prodrome | Presenting Symptoms | Other Symptoms and Findings during the Course of the Disorder |
|---|---|---|---|---|---|---|
| 1 | F/30 | Immature, right ovary, 10cm | 2 months | Headache, hyperthermia | STMD, decreased level of consciousness, “epilepsia partialis continua” for 2 weeks; sedation, MV, PEG | Restlessness, involuntary movements, hyperthermia |
| 2 | F/35 | Mature, left ovary, 3.5cm | 4 months (autopsy) | Headache, nausea, no fever; received acetaminophen | STMD, generalized tonic-clonic seizure followed by refractory status epilepticus; sedation, MV, and PEG | Partial motor seizures in left lower extremity; dystonic movements; hyperthermia |
| 3 | F/25 | Mature, left ovary, 6cm | 6 weeks | Hyperthermia | STMD, panic attacks, confusion, hallucinations(admitted to psychiatric center); subsequently, partial complex seizures, TPN, PEG | Episodes of staring; minimally reactive to stimuli; catatonic-like episodes; autonomic instability: tachycardia, high blood pressure |
| 4 | F/17 | Immature, left ovary, 7cm | 4 weeks | Hyperthermia | Bizarre behavior, disorganized thinking, restless, wandering aimlessly, hallucinations, catatonic-like episodes (2 admissions in psychiatric centers); subsequently, status epilepticus; sedation, MV | Incoherent speech; episodes of staring, catatonia, oculogyric crisis, choreoathetoid movements; autonomic instability: hyperthermia; mydriasis during episodes of restlessness and agitation |
| 5 | F/32 | Mature, right ovary, 6cm | 2 months | NA | Acute development of personality change, abnormal behavior, confused, agitated, wandering off(admitted to psychiatry); generalized tonic-clonic seizures, partial motor seizures, severe general encephalopathy, PEG | Unable to speak and follow commands for 2 months; episodes of “facial twitches” |
| 6 | F/24 | Mature, right ovary, 1.5cm | 3 months (autopsy) | Headache, hyperthermia, nausea, vomiting, diarrhea | Paranoid thoughts, auditory hallucinations, agitation(admitted to psychiatry); subsequently, generalized seizure; sedation, MV, PEG; remained with decreased level of consciousness and ventilator dependent | Myoclonic and dyskinetic movements in arms and face; autonomic instability: alternating hypothermia/hyperthermia, hypotension/hypertension; during awake periods, tachypnea; during sleep periods, problem triggering ventilator |
| Previously reported patients | ||||||
| 76 | F/19 | Immature, right ovary, 22cm | 4 months | Headache, hyperthermia | Acute personality change, aggressive behavior (seen at a psychiatric center); progressive confusion, decreased level of consciousness; general and partial seizures, refractory status epilepticus; sedation, MV, PEG | Episodes of “chewing, grimacing”; myoclonic and ballistic movements with limbs; rhythmic contractions of abdominal wall; dystonic movements of the trunk and limbs; opisthotonos-like posture; insomnia/hypersomnia |
| 84 | F/26 | Left ovarian dermoid cyst by CT,a1.6cm | 3 weeks | Anorexia and insomnia(3 weeks) | Psychiatric syndrome, generalized seizures, MV, PEG | Incomprehensible speech, decreased of level of consciousness, STMD, hyperthermia |
| 94 | F/40 | Mature, left ovary, 6.0cm | 3 weeks | NA | Secondary generalized seizures, psychiatric syndrome, MV, PEG | Decreased level of con sciousness, STMD |
| 104,8 | F/14 | Immature, left ovary, 1.9cm | 2 months | Hyperthermia, headache, rhinorrhea | Psychiatric syndrome (hallucinations, extreme panic), generalized seizures, MV, PEG | Incomprehensible speech, choreoathetotic movements, hypersomnia, hyperthermia, autonomic instability |
| 114,7 | F/28 | 1st episode: mature, right ovary, 14cm; 2nd episode: “benign,” left, 2cm; 3rd episode: “benign,” left, 1.7cm | 1 month after tumor | Cough, no fever; received antibiotics | 1st episode: psychiatric syndrome (delusional thinking, personality change), auditory hallucinations, STMD, dysphagia, horizontal nystagmus, vertical gaze paresis, MV 2nd episode: dysarthria 3rd episode: diplopia, facial numbness, dysphagia, ataxia | 1st episode: hypersomnia, comatose, flaccid paraplegia |
| 125 | F/44 | Mature, mediastinum, 6.5cm | 3 weeks | Hyperthermia, headache | Admission to psychiatry for acute agitation, personality change, memory loss, generalized tonic-clonic seizures, MV for PE, PEG | Word finding difficulty, mild right hemiparesis, episodes of diaphoresis, hypersomnia, headache |
STMD = short-term memory deficits; MV = mechanical ventilation; PEG = percutaneous endoscopic gastrostomy; PE = pulmonary embolism; TPN = total parenteral nutrition; CT = computed tomography; NA = not available.
Three of the 12 patients presented with short-term memory loss, followed by psychiatric symptoms or confusion and decreased level of consciousness (see Table 1). The other nine patients presented with an acute psychiatric syndrome, including personality and behavioral change, agitation, or paranoid thoughts. Overall, six patients were initially evaluated by psychiatrists and five admitted to psychiatric units. Eleven patients developed generalized or partial complex seizures. After controlling the seizures, 10 patients required mechanical ventilation because of the decreased level of consciousness and hypoventilation in 9 cases and a pulmonary embolism in 1 case; the median time that patients were receiving mechanical ventilation was 12 weeks (2–16 weeks). During that time multiple electroencephalograms showed diffuse general slowing in seven patients and generalized slowing with occasional epileptic activity in the other three.
During the course of the disease, eight patients developed episodes of hyperthermia, alternating in one case with hypothermia (see Table 1). Seven patients developed abnormal movements that included one or more of the following: choreoathetosis, myoclonic and ballistic movements, dystonic movements, dyskinesias in face and arms, rhythmic contractions of the abdominal wall, opisthotonos-like postures, and catatonic-like episodes. Five patients had signs of autonomic instability, including episodes of mydriasis, tachycardia, tachypnea, diaphoresis, or hypertension, which in one case alternated with hypotension. Four patients developed transient sleep dysfunction while recovering from the encephalitis.
Ancillary Tests
All patients underwent several brain magnetic resonance imaging (MRI) at the early stage of the disorder (Table 2): three had bilateral medial temporal lobe fluid-attenuated inversion recovery hyperintensities (one with right frontal cortex involvement; Fig 1), five had small or punctuate areas of fluid-attenuated inversion recovery or T2 hyperintensity in the frontal or parietal cortex (two with cerebellar involvement) and subtle enhancement of overlying meninges, one had transient T2 hyperintensity in the medulla and spinal cord, and three had normal or nonspecific findings.
Table 2.
Diagnostic Tests, Treatment, and Outcome
| Case No. | MRI at Presentation | CSF | Chronologic List of Treatments (immunotherapy and tumor) | Initial Improvement | Outcome (duration follow-up) |
|---|---|---|---|---|---|
| 1 | FLAIR and T2 hyperintensity in medial temporal lobes | 40μl WBC, protein 67mg/dl | Corticosteroids, plasma exchange, IVIg (tumor removal) | Partial clinical and MRI improvement before surgery; further improvement 4 weeks after surgery | Back to work as an internal medicine resident; normal MRI (12 months) |
| 2 | FLAIR and T2 hyperintensity in medial temporal lobes | 189μl WBC, protein 68mg/dl, (+) OGB | Corticosteroids, plasma exchange, IVIg, cyclophosphamide | No surgery (did not improve) | Follow-up MRIs: severe atrophy, mainly in temporal lobes; died 4 months after symptom presentation |
| 3 | Initial MRI normal; subsequent MRI: FLAIR hyperintensity in medial temporal lobes and right frontal cortex | 15μl WBC, protein normal, (−) OGB | Corticosteroids, (tumor removal), plasma exchange | Three days after surgery | Normal examination; normal MRI (12 months) |
| 4 | Single punctuate FLAIR abnormality in the right frontal lobe; two subsequent MRIs: normal | 26μl WBC, protein normal | Plasma exchange, IVIg(tumor removal), corticosteroids and cyclophosphamide | 12 days after surgery and 3 days after corticosteroids and bolus of cyclophosphamide: striking improvement, able to communicate and be extubated | Back to high school with good grades; normal examination; normal MRI (7 months) |
| 5 | Two MRIs normal | 20μl WBC, protein 56mg/dl | Corticosteroids, plasma exchange (2 days) (tumor removal), plasma exchange (3 days) | 2 days after plasma exchange (1 day before surgery); 3 days after surgery: able to sit, talk, eat | Normal examination; normal MRI (7 months) |
| 6 | T2 hyperintensity in sulci of parietal hemispheres; mild enhancement of overlying meninges | 219μl WBC, protein 129mg/dl, (+) OGB | Corticosteroids | No surgery (did not improve) | Died 3 months after symptom presentation |
| Previously reported patients | |||||
| 76 | Small FLAIR abnormality in cerebellum; mild enhancement of meninges of cerebral sulci | 12μl WBC, protein normal | corticosteroids, IVIg(tumor removal), and chemotherapy | 4 weeks after surgery | Alternating insomnia/hypersomnia for 7 months; total recovery of motor function, but MMSE score24/30 (mild STMD); MRI: frontotemporal atrophy (16 months) |
| 84 | FLAIR/T2 hyperintensities in cerebral cortex and cerebellum; mild cortical cerebellar enhancement | 49μl WBC, protein 67mg/dl | corticosteroids | Approximately 7 weeks | Full recovery; normal MRI(24 months) |
| 94 | FLAIR abnormalities involving the cingulum and gray matter of the frontal lobes | 9μl WBC, protein normal | Tumor removal | approximately 16 weeks | Residual cognitive dysfunction and memory problem, MMSE 28/30 (6 years) |
| 104,8 | Three MRIs normal | 115μl WBC, protein 92mg/dl, (3) OGB | Tumor removal, plasma exchange, corticosteroids, IVIg | Transferred to a chronic care facility with MV (no significant improvement) | Unexpected death after mild improvement, about 6 months after symptom presentation |
| 114,7 | T2 hyperintensity in the dorsal aspect of the medulla and 3 similar areas in the spinal cord | 1st episode: 233l WBC, protein61mg/dl 2nd, 3rd episodes: normal, (−) OGB | Each episode: tumor removal, IVIg, corticosteroids | 1st episode: approximately 8 weeks 2nd episode: 6 weeks 3rd episode: 2 weeks | 1st episode: recovered memory and cognitive function; residual mild truncal dysesthesias (6 months) 2nd, 3rd episodes: complete recovery (22 months) |
| 125 | Nonspecific, scattered T2 foci of hyperintensity in frontal lobes; no contrast enhancement | 15μl WBC, protein 18mg/dl, (−) OGB | Tumor removal | Approximately 1 week after surgery | Full recovery; MRI unchanged, 4 years |
Normal cerebrospinal fluid (CSF) values: white blood cell count (WBC): <5μl; total protein concentration: 15–55mg/dl.
MRI = magnetic resonance imaging; FLAIR = fluid-attentuated inversion recovery; OGB = oligoclonal bands; IVIg = intravenous immunoglobulin; MMSE = Mini-Mental State Examination; STMD = short-term memory deficits; MV = mechanical ventilation.
Fig 1.
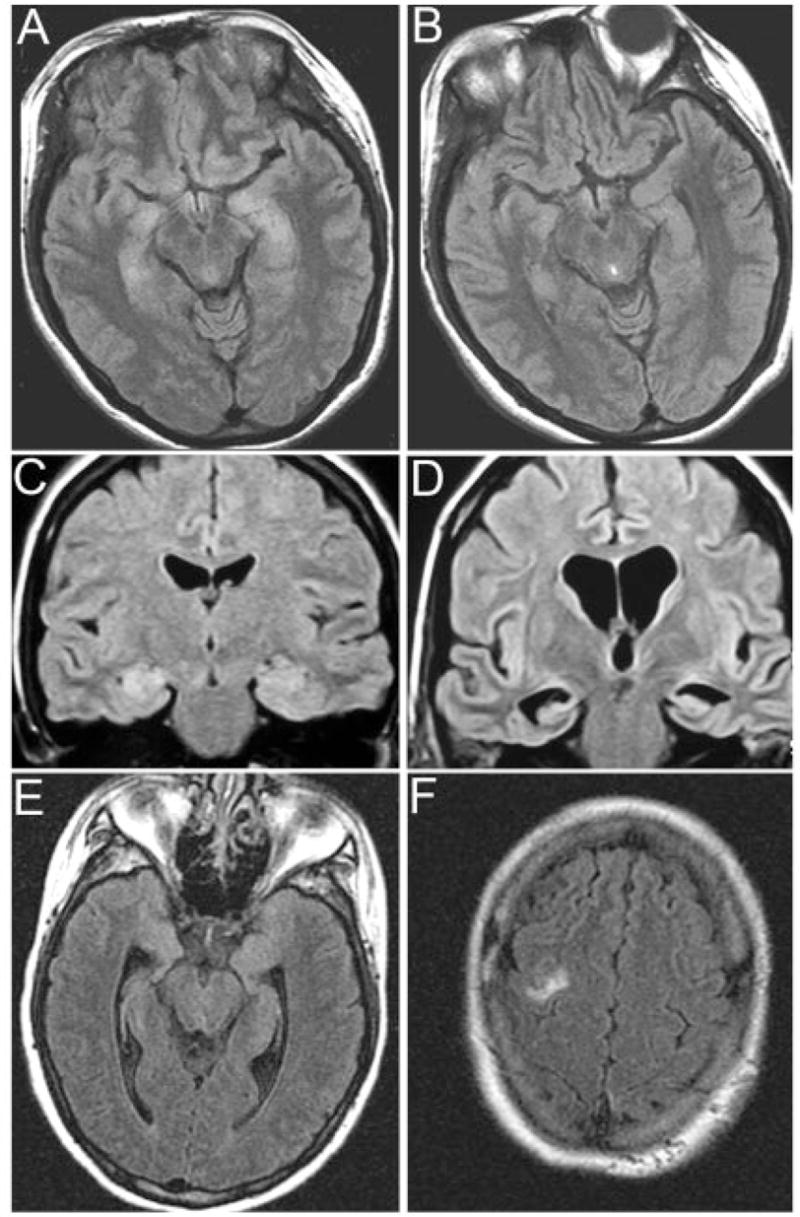
Brain magnetic resonance imaging (MRI) findings in three patients. (A, B) MRI of Patient 1 at symptom presentation (A) and after partial clinical improvement and cerebrospinal fluid normalization with immunotherapy (B); note that the clinical and MRI improvement started to occur before tumor resection. (C, D) MRI of Patient 2 at symptom presentation (C) and 4 months later (D); this patient developed rapidly progressive neurological deterioration that did not respond to immunotherapy. The autopsy demonstrated that the ovarian cyst was a mature teratoma of the ovary. (E, F) MRI of Patient 3 at symptom presentation; note the mild fluid-attenuated inversion recovery hyperintensity in medial temporal lobes and right frontal cortex. After immunotherapy and tumor resection, the MRI was normal (not shown).
CSF lymphocytic pleocytosis was identified in all patients (9 –219 cells/μl; median, 24 cells/μl), and seven also had increased protein concentration (56 –129mg/ dl; median, 67mg/dl); the glucose concentration was normal in all instances (see Table 2). Oligoclonal bands were identified in three of six patients examined. All patients had extensive serum and CSF diagnostic tests with negative or normal results for viral, bacterial, and fungal infections; collagen-vascular autoimmune disorders; thyroid autoimmunity; and comprehensive panels of paraneoplastic and voltage-gated potassium channel (VGKC) antibodies.
Associated Tumors
Computed tomography of the chest, abdomen, and pelvis demonstrated an ovarian mass in 10 patients, a tumor in the anterior mediastinum in 1, and no evidence of tumor in 1 (the tumor was demonstrated at autopsy). The radiological size of the tumors ranged from 1.5 to 22cm (median largest diameter, 6.5cm) (see Table 1). Two patients (Cases 1 and 7) had significant tumor growth during the encephalitic process (Figs 2A, B). Only one patient had increased levels of carcinoembryonic antigen (CEA), CA125, and α-fetoprotein. One patient had a history of a resected contralateral teratoma (without accompanying encephalitis), and another patient had three episodes of neurological symptoms, each heralding a new or recurrent mature teratoma (NR2 antibodies were measured at the last recurrence).
Fig 2.
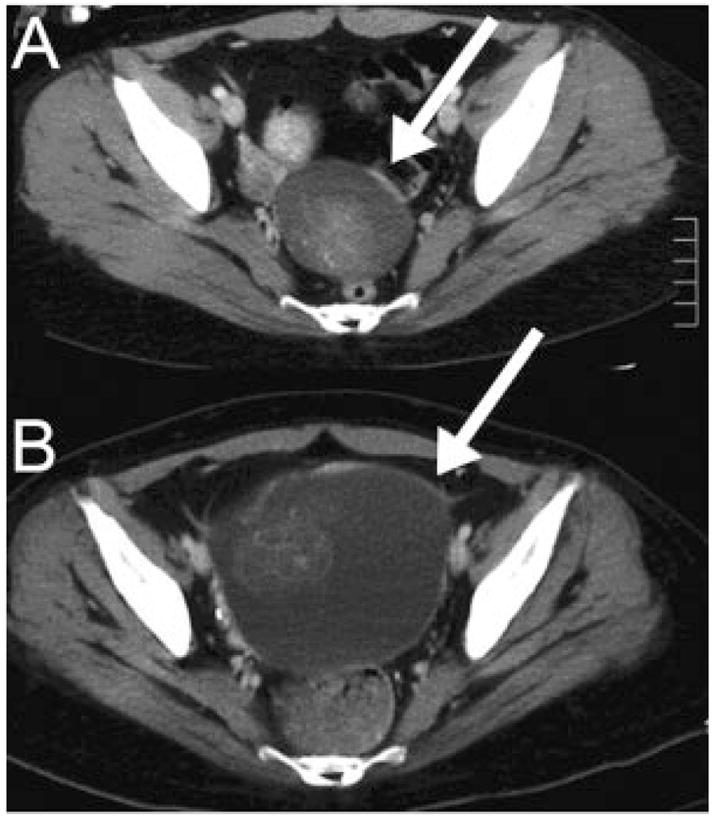
Interval increase of tumor size during encephalitis. (A) Computed tomography of Patient 1 shows a 5cm cystic ovarian lesion (arrow) that doubled in size over 2 months (B). The lesion was not initially removed because of the poor clinical condition of the patient and benign appearance of the ovarian mass. After partial clinical improvement with immunotherapy, the mass was removed (immature teratoma).
Nine patients had complete tumor resection and three did not have surgery (the tumor of two of these cases was studied at autopsy). Overall, pathological studies showed that seven patients had mature teratoma (six ovary, one mediastinum) and four immature teratoma. Review of slides from eight of the ovarian teratomas demonstrated in all instances nervous tissue intermixed with tissues derived from the other germinal layers; tissue was available from five of these tumors for immunological studies (described later).
Treatment and Outcome
Seven patients were treated with tumor resection and immunosuppressants (one with additional chemotherapy); six recovered and one died unexpectedly in a chronic care facility after mild improvement8 (see Table 2). Five of the six patients who recovered returned to work, and the follow-up MRIs were normal; the sixth patient had partial recovery (Mini-Mental State Examination score = 24/30) and developed mild frontotemporal atrophy.
The other five patients were treated with surgery alone (two cases) or immunosuppressants (three cases). Three patients recovered (two returning to work; one partial recovery Mini-Mental State Examination score = 28/30) and the other two died of neurological progression (both without tumor removal). In one of these patients (Case 2), corticosteroids, plasma exchange, and intravenous immunoglobulin (IVIg) had no effect on the symptoms and CSF pleocytosis; multiple MRIs showed progressive atrophy, mainly in the temporal lobes and hippocampi. Seven weeks before her death, she received cyclophosphamide that resulted in normalization of the CSF but no clinical recovery. In the other deceased patient (Case 6), the ovarian teratoma was discovered at autopsy; among many diagnostic tests, this patient had undergone brain biopsy. The neuropathological findings of these two patients are described in the Supplemental information.
Patients’ Antibodies React with Neuronal Cell Membrane Antigens Preferentially Expressed in the Hippocampus
The CSF and serum of all 12 patients, but not control subjects, showed a distinctive pattern of reactivity with the neuropil of rat hippocampus; the reactivity with other forebrain regions was less intense, and hardly visible in the cerebellum (Fig 3A). The immunolabeling predominantly occurred with the cell membrane of neurons and was intense in the molecular layer of the hippocampus (see Fig 3B), as reported in a previous study.4 The addition of CSF or serum of patients, but not control subjects, to cultures of live rat hippocampal neurons produced striking immunolabeling of the cell surface and dendrites (see Fig 3C). Control antibodies to intracellular antigens (ie, HuD) produced no reactivity with live neurons, but showed intracellular reactivity with permeabilized neurons (data not shown). These findings indicate that the patients’ antibodies recognize epitopes exposed on the cell surface.
Fig 3.
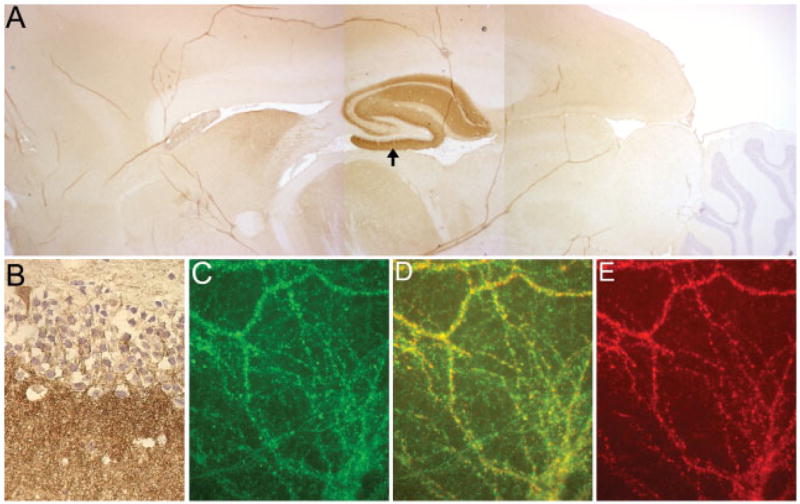
Reactivity of patients’ antibodies with hippocampus and forebrain, and colocalization with the NR2B subunit of the N-methyl-D-aspartate receptor (NMDAR). (A) Sagittal section of rat brain immunolabeled with a patient’s cerebrospinal fluid. Note the robust reactivity with the hippocampus and milder reactivity with forebrain. The cerebellum is largely spared. (B) Higher magnification of the molecular layer of the hippocampus (arrow in A); this pattern of reactivity is identical to that previously reported in patients with paraneoplastic encephalitis and ovarian teratoma.4 (C–E) Double immunolabeling of cultures of rat hippocampal neurons using a patient’s antibodies (C, green) and an antibody against NR2B of the NMDAR (E, red); note the significant colocalization of reactivities (D, yellow). These findings suggested that patients’ antibodies were directed against the NMDAR. Subsequent studies demonstrated that the patient also had antibodies against NR2A (not shown), which explains, in part, the partial colocalization of reactivities. Original magnification ×2.5 (A) and ×400 (B), both counterstained with hematoxylin; ×800 (oil lens), immunofluorescence (C–E).
Using rat brain tissue and serial dilutions of normalized total IgG in paired serum and CSF samples, we noted that 8 of 11 patients had evidence of intrathecal synthesis of antibodies (in 2 patients, the antibodies were barely detectable in serum). Serum antibody titers were followed in eight patients: all seven patients with neurological improvement had a decrease of titers (five became undetectable), and one who died of neurological progression had an increase of titers (from 1:200 to 1:1,600).
The Main Autoantigens Are Functional Heteromers of N-methyl-D-aspartate Receptors
Double immunolabeling of brain and hippocampal rat neurons using patients’ antibodies and diverse markers of candidate autoantigens demonstrated significant co-localization with the NR2B subunit of the NMDAR (see Figs 3C–E). Because this subunit is preferentially expressed in the hippocampus and forebrain (and absent from the cerebellum) forming heteromers with NR1 or with NR1 and NR2A, we subsequently examined the reactivity of patients’ antibodies with HEK293 cells expressing individual subunits (NR1, NR2A or NR2B) or a combination of subunits required for a functional receptor (NR1/NR2B or NR1/ NR2A heteromers). These studies showed that all 12 patients’ CSF and serum reacted with NR1/NR2 heteromers containing NR2B; sera and CSF from eight patients also recognized heteromers containing NR1/ NR2A (Fig 4). Sera did not react with cells transfected with individual subunits (NR1, NR2A, or NR2B). These antibodies were not identified in an extensive group of control sera and CSF, including among others six patients with testicular teratomas and paraneoplastic anti-Ma2 encephalitis.
Fig 4.
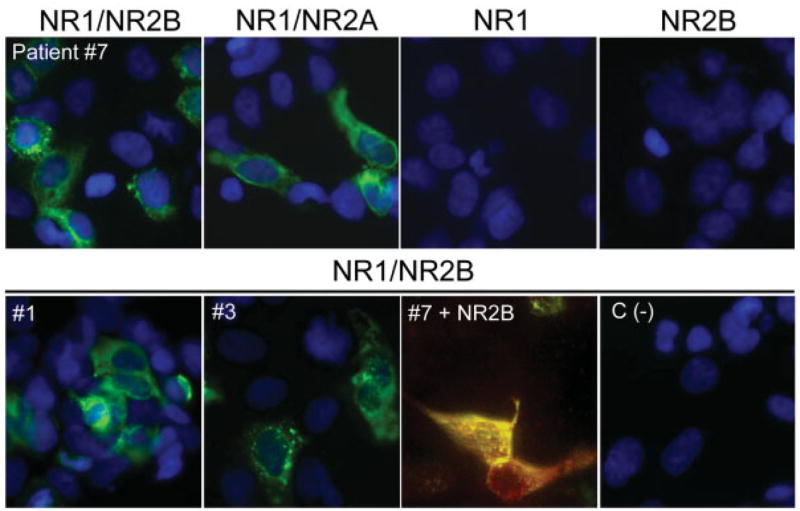
Patients’ antibodies react with heteromers of NR2B and NR2A subunits of the N-methyl-D-aspartate receptor (NMDAR). HEK293 cells expressing heteromers (NR1/NR2B or NR1/NR2A) or transfected with single subunits NR1 or NR2B of the NMDAR were incubated with patients’ serum or cerebrospinal fluid (CSF). (top row) Panels demonstrate that the CSF of Patient 7 reacts with cells expressing heteromers (functional receptors) of NR1/NR2B and NR1/NR2A, but not with cells transfected with single subunits (NR1; NR2B). Cells transfected with single NR2A or plasmid without insert were not reactive with patient’s antibodies (not shown). (bottom row) Panels demonstrate the reactivity of the CSF of Patients 1 and 3 with NR1/NR2B. In the third panel (#7 + NR2B), the cells were coincubated with CSF of Patient 7 and an antibody specific for NR2B, showing colocalization of reactivities. The fourth panel (C (−)) corresponds to the CSF of an individual without paraneoplastic encephalitis (negative control). Original magnification ×800, immunofluorescence; nuclei of cells demonstrated with 4′,6-diamidino-2-phenylindole (DAPI), except “#7 + NR2B,” in which no DAPI was used.
Immunoblots of membrane proteins isolated from hippocampal neurons or HEK293 cells expressing NR1/NR2B or NR1/NR2A heteromers showed reactivity with commercially available antibodies (NR1, NR2A, NR2B) but did not react with patients’ CSF or serum (data not shown). Overall, these findings indicate that patients’ antibodies recognize NR1/NR2 heteromers containing the NR2B (and at a lesser degree NR2A) subunit of the NMDAR and suggest that the epitopes are conformational.
We subsequently examined whether patients’ tumors contained nervous tissue expressing NMDARs and whether these receptors were recognized by patients’ antibodies. Five of five tumors examined showed mature- and immature-appearing neurons together with a variably dense network of fibers expressing MAP2 (a marker of neurons and dendritic processes) (Figs 5A, B). This atypical nervous tissue had intense expression of NR2 subunits of NMDAR; colocalization of reactivities was observed when tumors were incubated with commercially available antibodies to NR2B or NR2A and patients’ antibodies (see Figs 5C–H).
Fig 5.
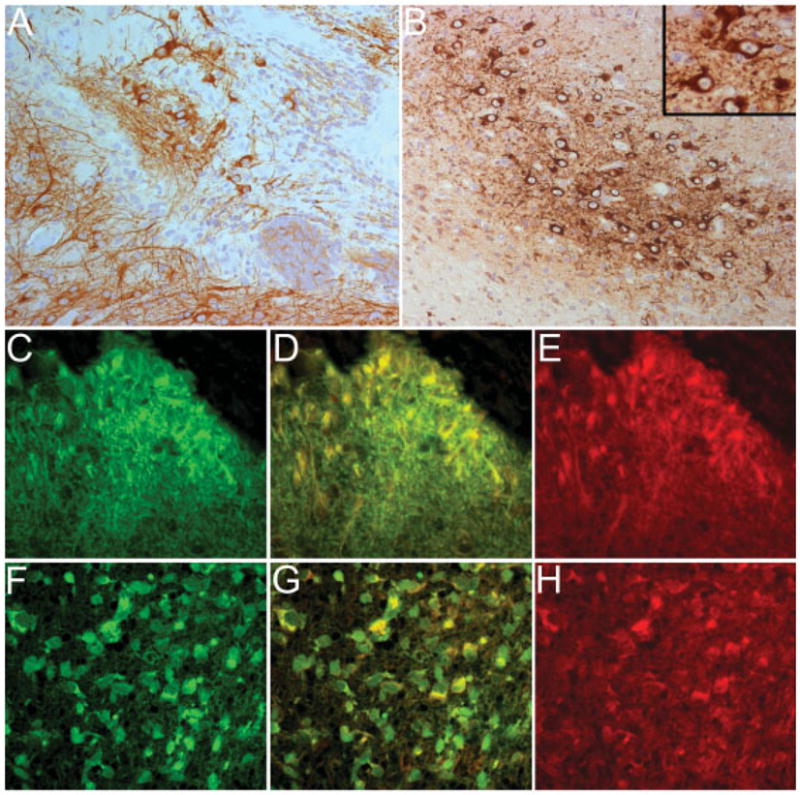
Patients’ antibodies react with NR2 subunits of the N-methyl-D-aspartate receptors (NMDARs) expressed by nervous tissue present in the tumor. (A, B) Panels correspond to the ovarian teratoma of Patients 3 and 5 immunolabeled with MAP2 (brown staining), a marker specific for neurons and dendritic processes. Note the intense reactivity with neuronal-like cells and a network of cell processes that are better developed in A. (B, inset) Some immature neuronal cells at higher magnification. (C–E) Panels correspond to the tumor of Patient 3 immunolabeled with the patient’s antibodies (C, green) and a specific antibody for NR2B (E, red). Note that there is colocalization of reactivities (D, yellow), indicating that the patient’s antibodies react with NR2B expressed in the tumor (similar findings were observed with NR2A, not shown). (F–H) Panels correspond to the tumor of Patient 5 immunolabeled with the patient’s antibodies (F, green) and a specific antibody for NR2B (H, red). There is also colocalization of reactivities (G, yellow), indicating that the patient’s antibodies recognize NR2B expressed in the tumor (similar findings were observed with NR2A, not shown). Original magnification ×200, counterstained with hematoxylin (A, B); ×400, immunofluorescence (C–H).
Discussion
This study facilitates the recognition of a severe form of autoimmune encephalitis that is often responsive to treatment. In addition, the results emphasize the idea that autoimmunity can affect behavior, and particularly that antibodies to heteromers containing the NR2B and NR2A subunits of the NMDAR may alter emotion, memory, and consciousness.16
The frequency of this disorder (which we call paraneoplastic anti-NMDAR encephalitis) is unknown. Before our description of five patients in 2005,4,5 only five other possible clinical cases had been reported in the English literature (reviewed in Vitaliani and colleagues4). Thus, the identification of seven additional patients in the few months after those publications suggests that the disorder is frequently unrecognized. This may be due to several features that make this disorder unique among paraneoplastic encephalitis including: (1) involvement of relatively young women between the second and fifth decades of life; (2) the unusual presentation with prominent psychiatric manifestations; (3) normal or atypical MRI findings, which in 75% of cases consist of mild, transient T2 or fluid-attenuated inversion recovery abnormalities outside the medial temporal lobes, sometimes with cortical enhancement; and (4) the benign appearance of the ovarian tumors.
The clinical picture of all 12 patients shows a recognizable syndrome in most instances. At presentation, a psychiatric disorder is usually considered and patients are often admitted to psychiatric centers. Most patients appear confused, restless, agitated, with frequent paranoid or delusional thoughts sometimes alternating with quiet staring and dystonic or catatonic postures. In addition, most patients develop seizures and a subsequent decrease of level of consciousness, requiring antiepileptic medication, sedation, frequent mechanical ventilation, nutritional support, and management of episodes of autonomic instability and dyskinesias. After controlling the seizures, attempts to decrease the sedation or wean patients from the ventilator demonstrate limited recovery of consciousness and spontaneous breathing, and reappearance or worsening of abnormal movements (often with diffuse slowing of the electroencephalogram). As a result, ventilatory support was required for a median of 12 weeks in 9 patients.
A constant abnormality is the presence of CSF pleocytosis or increased protein concentration that suggests an inflammatory or immune-mediated neurological process. Otherwise, extensive evaluations to identify the cause of the encephalitis are normal or unrevealing, and the associated tumors (usually appearing as “benign” ovarian cysts) are frequently considered unrelated to the disorder.
A remarkable finding is that all patients had antibodies to NMDARs containing the NR2B, and at a lesser degree, the NR2A subunit. NMDARs are usually formed from heteromers of NR1 (which bind glycine) and NR2 subunits (which bind glutamate).17,18 Both subunits are required to create a functional receptor that likely contains two NR1 and two NR2 subunits.19 There are four NR2 subunits (NR2A–D) that have 50 to 70% sequence identity in the extracellular domain (NR2B is 70% identical to NR2A, and 55–58% identical to NR2C and NR2D). These NR2 subunits are coded by four different genes and show developmental and regional variability. NR2B is expressed at high levels prenatally and declines postnatally.20,21 During its decline, expression levels of NR2A and NR2C increase. In adults, NR2A is found in most brain regions, NR2B in the hippocampus and forebrain, NR2C in cerebellum, and NR2D in limited subsets of neurons.20,21 NR1 is ubiquitously distributed in the brain.19 –21 With maturity, many NR1/NR2B receptors become largely extrasynaptic in hippocampal neurons and NR1/NR2A/NR2B becomes the major synaptic receptors in the hippocampus and forebrain. Thus, the predominant reactivity of all patients’ antibodies with hippocampus and forebrain correlates with the distribution of heteromers containing NR2B.22 Furthermore, the antibodies readily access cell-surface epitopes of live neurons and react only with HEK293 cells expressing functional receptors (heteromers of NR1/NR2B or NR1/NR2A). No reactivity is identified when each subunit is expressed individually (even though NR1 in particular assembles in stable but inactive homomeric receptors in HEK293 cells)12 or with immunoblots of cells expressing the functional receptor. These findings suggest that the main epitopes are in the extracellular domain of NR2B and NR2A subunits and are likely conformational. Preliminary studies indicate that some patients also harbor antibodies to functional heteromers of NR2C and NR2D (consistent with the 55–58% homology between these subunits and NR2B); however, no increase of cerebellar immunolabeling was seen in sera or CSF from these patients, suggesting that these antibodies occur at low titers (data not published).
The discovery of NR2B-related antibodies in the serum and CSF of all patients provides a potential diagnostic test for the disorder and suggests a novel immune-mediated mechanism of NMDAR dysfunction. Critical roles of NMDARs include synaptic transmission and remodeling, dendritic sprouting, and hippocampal long-term potentiation, one paradigm of memory formation and learning.19 However, NMDARs are also the major mediator of excitotoxicity, and their dysfunction has been associated with schizophrenia, epilepsy, and several types of dementia. Drugs interacting with NMDARs may result in paranoia, hallucinations, and dyskinesias, all frequent symptoms in our patients.23 Antibodies to NR2 subunits have been identified in patients with neuropsychiatric lupus24,25 and diverse seizure disorders, but the target epitopes (recognized by immunoblot)26,27 and syndromes are different from those of our patients. The antibodies of patients with lupus are anti–double-stranded DNA that cross-react with a single epitope present in NR2A and NR2B28; these antibodies can cause neuronal death by excitotoxicity and apoptosis and result in behavioral abnormalities in an animal model of the disorder.29 None of our 12 patients had symptoms of lupus, and all were negative for anti–double-stranded DNA; furthermore, the target NR2 epitopes in our patients appear to be multiple and are not always present in receptors that contain NR2A.
Although the mechanisms that triggered the immune response are unclear, we postulate the ectopic expression of NR2 subunits by nervous tissue contained in the teratomas contributes to break immune tolerance. All five tumors available for immunological studies showed intense reactivity with patients’ antibodies that colocalized with dramatic expression of NR2 subunits (more robust than that observed in normal hippocampus; data not shown). A combination of factors such as an adjuvant effect of the prodromal viral-like illness30,31 that occurred in most patients, and perhaps a genetic predisposition, could play additional roles in the initiation of the immune response.
The mechanisms of how antibodies breach the blood–brain barrier have been explored in models of lupus. These studies showed that infection or hypertension significantly enhanced antibody entrance to the central nervous system.29,32 Interestingly, the amygdala and hippocampus that have the highest levels of NR2B and NR2A are also the regions where the blood–brain barrier is more vulnerable to these mechanisms. During the course of the disease of our patients, hypertension and symptoms of sympathetic overactivity occurred frequently, possibly enhancing blood–brain barrier leakiness. Furthermore, intrathecal production of antibodies was identified in eight patients.
A possible pathogenic role of NR2 antibodies in paraneoplastic anti-NMDAR encephalitis is suggested by the following factors: (1) the indicated animal models of neuropsychiatric lupus (although the epitopes are different)28,33; (2) the correlation between patients’ symptoms and antibody titers; and (3) the demonstration of deposits of IgG in the hippocampus and amygdala of a patient’s autopsy, in a pattern that showed striking resemblance to the rat brain immunolabeling by patients’ antibodies (see Supplemental data for Case 2 and Supplemental Fig 6). Whereas in other paraneoplastic encephalitis cases (ie, Hu, Ma2) the perivascular and interstitial infiltrates of T cells are prominent,34,35 the encephalitis with NR2 antibodies associates with extensive microglial proliferation, variable neuronal degeneration, and rare inflammatory infiltrates (see Supplemental data for Cases 2 and 6 and Supplemental Fig 7). In these patients and a previously reported case (Case 10),8 the abnormalities predominated in the hippocampi, amygdala, and at a lesser degree, other areas of the neuraxis.
Despite the severity of the symptoms, paraneoplastic anti-NMDAR encephalitis has a better prognosis than most other paraneoplastic encephalitides.3,36 Nine of 12 patients significantly recovered and 7 returned to their jobs. Because most patients had tumor resection and immunotherapy in close temporal association, the relative contribution of these treatments to neurological recovery is difficult to assess in this study. In general, resection of the tumor appeared important to attain final recovery or sustain the improvement that in some cases started soon after immunotherapy (corticosteroids, IVIg, or plasma exchange). Two patients had only tumor resection and both improved, but two of the three patients who did not have tumor resection died of neurological progression. The importance of removing the tumor is also suggested by one patient who developed recurrent neurological symptoms, each heralding a tumor recurrence. We were surprised by the dramatic and rapid recovery of some patients, suggesting a potentially reversible antibody-mediated neuronal dysfunction. The deterioration or partial recovery of a few patients may reflect a more sustained disruption or involvement of other critical epitopes of the NMDAR, or a secondary effect of the prolonged seizures, leading to neuronal degeneration.
While preparing this article, four patients with a similar syndrome, antibodies to NR2B-containing heteromers, and ovarian teratoma (one immature; three mature, in one case bilateral) have been identified (currently under study). These patients, as well as most of those in this study, were initially considered to have a psychiatric illness or viral encephalitis. Because some patients have transient (usually partial) improvement with the empiric use of corticosteroids, IVIg, or plasma exchange, they may be discharged from hospitals without a final diagnosis, and subsequently deteriorate or die if the ovarian mass is not removed. For example, 10 of our patients were seen at several institutions, some with multiple hospital transfers or discharges, before the tumor was diagnosed and removed. This raises the concern that this disorder is more frequent than our current experience suggests. In addition to clinical implications, further studies may provide an important model of immune-mediated dysfunction of NMDARs, with relevance to multiple disciplines.19
Supplementary Material
Acknowledgments
This work was supported by NIH/NCI (RO1CA89054, J.D.; RO1CA107192, J.D.), and NIH (RO1 NS45986, D.R.L.).
We thank Drs F. Lieberman T. Zwerdling for providing clinical information, and M. Maronski for excellent technical assistance.
Footnotes
This work was first presented at the 131st Annual Meeting of the American Neurological Association, Chicago, IL, Oct 10, 2006.
References
- 1.Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 2.Darnell RB, Posner JB. A new cause of limbic encephalopathy. Brain. 2005;128:1745–1746. doi: 10.1093/brain/awh592. [DOI] [PubMed] [Google Scholar]
- 3.Graus F, Keime-Guibert F, Rene R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 4.Vitaliani R, Mason W, Ances B, et al. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koide R, Shimizu T, Koike K, Dalmau J. EFA6A-like antibodies in paraneoplastic encephalitis associated with immature ovarian teratoma: a case report. J Neurooncol. 2006 Jun 29; doi: 10.1007/s11060-006-9200-7. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RB, Mason W, Kong K, Wennberg R. Reversible paraneoplastic encephalomyelitis associated with a benign ovarian teratoma. Can J Neurol Sci. 1999;26:317–320. doi: 10.1017/s0317167100000469. [DOI] [PubMed] [Google Scholar]
- 8.Stein-Wexler R, Wootton-Gorges SL, Greco CM, Brunberg JA. Paraneoplastic limbic encephalitis in a teenage girl with an immature ovarian teratoma. Pediatr Radiol. 2005;35:694–697. doi: 10.1007/s00247-005-1402-1. [DOI] [PubMed] [Google Scholar]
- 9.Furneaux HM, Rosenblum MK, Dalmau J, et al. Selective expression of Purkinje-cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med. 1990;322:1844–1851. doi: 10.1056/NEJM199006283222604. [DOI] [PubMed] [Google Scholar]
- 10.Cattoretti G, Pileri S, Parravicini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 11.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 12.Lynch DR, Anegawa NJ, Verdoorn T, Pritchett DB. N-methyl-D-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol. 1994;45:540–545. [PubMed] [Google Scholar]
- 13.Lynch DR, Lawrence JJ, Lenz S, et al. Pharmacological characterization of heterodimeric NMDA receptors composed of NR 1a and 2B subunits: differences with receptors formed from NR 1a and 2A. J Neurochem. 1995;64:1462–1468. doi: 10.1046/j.1471-4159.1995.64041462.x. [DOI] [PubMed] [Google Scholar]
- 14.Grant ER, Bacskai BJ, Pleasure DE, et al. N-methyl-D-aspartate receptors expressed in a nonneuronal cell line mediate subunit-specific increases in free intracellular calcium. J Biol Chem. 1997;272:647–656. doi: 10.1074/jbc.272.1.647. [DOI] [PubMed] [Google Scholar]
- 15.Anegawa NJ, Lynch DR, Verdoorn TA, Pritchett DB. Trans-fection of N-methyl-D-aspartate receptors in a nonneuronal cell line leads to cell death. J Neurochem. 1995;64:2004–2012. doi: 10.1046/j.1471-4159.1995.64052004.x. [DOI] [PubMed] [Google Scholar]
- 16.Diamond B, Kowal C, Huerta PT, et al. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Adv Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- 17.Kendrick SJ, Lynch DR, Pritchett DB. Characterization of glutamate binding sites in receptors assembled from transfected NMDA receptor subunits. J Neurochem. 1996;67:608–616. doi: 10.1046/j.1471-4159.1996.67020608.x. [DOI] [PubMed] [Google Scholar]
- 18.Laube B, Hirai H, Sturgess M, et al. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 19.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 20.Monyer H, Burnashev N, Laurie DJ, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 21.Standaert DG, Testa CM, Young AB, Penney JB., Jr Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- 23.Lynch DR, Guttmann RP. Excitotoxicity: perspectives based on N-methyl-D-aspartate receptor subtypes. J Pharmacol Exp Ther. 2002;300:717–723. doi: 10.1124/jpet.300.3.717. [DOI] [PubMed] [Google Scholar]
- 24.Lapteva L, Nowak M, Yarboro CH, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 25.Omdal R, Brokstad K, Waterloo K, et al. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12:392–398. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumakura A, Miyajima T, Fujii T, et al. A patient with epilepsia partialis continua with anti-glutamate receptor epsilon 2 antibodies. Pediatr Neurol. 2003;29:160–163. doi: 10.1016/s0887-8994(03)00151-6. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Mori H, Mishina M, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRepsilon2 in patients with Rasmussen’s encephalitis and chronic progressive epilepsia partialis continua. Epilepsia. 2005;46(suppl 5):152–158. doi: 10.1111/j.1528-1167.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 28.DeGiorgio LA, Konstantinov KN, Lee SC, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 29.Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianani R, Sarvetnick N. Viruses, cytokines, antigens, and autoimmunity. Proc Natl Acad Sci U S A. 1996;93:2257–2259. doi: 10.1073/pnas.93.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol. 2005;15:17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- 32.Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Rai G, Ray S, Shaw RE, et al. Models of systemic lupus erythematosus: development of autoimmunity following peptide immunizations of noninbred pedigreed rabbits. J Immunol. 2006;176:660–667. doi: 10.4049/jimmunol.176.1.660. [DOI] [PubMed] [Google Scholar]
- 34.Bernal F, Graus F, Pifarre A, et al. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol (Berl) 2002;103:509–515. doi: 10.1007/s00401-001-0498-0. [DOI] [PubMed] [Google Scholar]
- 35.Blumenthal DT, Salzman KL, Digre KB, et al. Early pathologic findings and long-term improvement in anti-Ma2-associated encephalitis. Neurology. 2006;67:146–149. doi: 10.1212/01.wnl.0000223647.83708.20. [DOI] [PubMed] [Google Scholar]
- 36.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


