Abstract
Herpes simplex virus-type 1 is among the most prevalent and successful humans pathogens. Although infection is largely uncomplicated in the immunocompetent human host, HSV-1 infection can cause blinding corneal disease, and individuals with defects in innate or adaptive immunity are susceptible to herpes simplex encephalitis. Chemokines regulate leukocyte trafficking to inflamed tissues and play a crucial role in orchestrating the immune response to HSV-1 infection. In this review we will focus on the pathways that induce chemokine expression during HSV-1 infection and the implications of chemokine signaling on control of viral replication.
Keywords: HSV-1, Chemokines, Toll-Like Receptors, Chemokine Receptors, Review
2. INTRODUCTION
Herpes simplex virus-type 1 (HSV-1) is a neurotropic member of the alpha herpesvirus family with worldwide seroprevalence rates ranging from between 50-90%. (1-3). Primary infection with HSV-1 typically occurs in childhood or adolescence following inoculation of mucosal epithelial surfaces and is usually mild or asymptomatic in the immunocompetent host. Initial infection of epithelial cells results in a lytic replicative cycle during which the virus infects sensory neurons proximal to the site of primary infection. Virions are then transported via retrograde axonal transport to neuronal cell bodies in the trigeminal ganglia resulting in the establishment of a lifelong latent infection (4,5). Subsequent reactivation of latent HSV-1 leads to renewed lytic infection at epithelial surfaces fed by infected sensory nerve fibers with infectious virus released either through asympomatic viral shedding or the formation of fluid filled vesicles.
Although HSV-1 infection is of low consequence in healthy adults, infection of neonates or immunocompromised individuals may lead to fatal encephalitis, emphasizing the importance of an appropriate immune response. Chemotactic cytokines or chemokines play an important role in the development of immunological resistance to HSV-1 replication acting as a bridge between innate and adaptive immunological recognition of the pathogen and subsequent leukocyte mobilization. This review will focus first on chemokine biology and signaling pathways, and then more specifically on the induction and role of chemokines in the coordination and development of innate and adaptive immune responses to HSV-1.
3. CHEMOKINE SIGNALING
Chemokines are a group of highly structurally, conserved cytokines notable for their crucial role in leukocyte trafficking. The chemokine family is classified into four subfamilies on the basis of the arrangement of conserved Cys amino acids at the N-terminus, being CXC, CC, C, or CX3C. The CXC chemokines are further divided on the basis of the presence or absence of an ELR motif preceding the first N-terminal cysteine, with ELR+ chemokines being broadly associated with the chemoattraction of granulocytes and monocytes through CXCR1/2 signaling while ELR- CXC chemokines induce chemoattraction of lymphocytes through CXCR3/4/5/6 signal transduction (6-11).
Through their interaction with cognate 7-transmembrane heterotrimeric G-protein coupled receptors, chemokines induce the directed movement of leukocyte subsets across endothelial barriers and through tissue microenvironments. By controlling leukocyte influx to foci of infection as well as migration of antigen presenting cells and lymphocytes to secondary lymphoid tissue, chemokines initiate and guide host immune responses (7-10, 12). The directed migration induced by chemokine signaling requires the establishment of chemokine concentration gradients. For all except the transmembrane chemokines CX3CL1 and CXCL16, glycosaminoglycan (GAG) binding through domains along C-terminal alpha helices is required for the establishment of concentration gradients and induction of chemotaxis (13,14). In addition to a role in presentation of chemokines to responding leukocytes, GAG binding may also affect the network of chemokine signaling pathways elicted by receptor ligation (15).
Ligation of chemokine receptors results in the activation of signaling cascades following exchange of GDP for GTP by receptor bound G alpha subunits and subsequent release of beta-gamma subunits. Most chemokine-induced responses are pertussis toxin sensitive indicating coupling of the receptor to G alpha i subunits, though G alpha i signaling is not sufficient to induce chemotaxis (16). G alpha i subunit release results in the inhibition of adenylyl cyclase as well as Src kinase activation, while liberated beta-gamma subunits directly activate PLC-beta and stimulate PI3K gamma activity, with additional PLC and PI3K isoforms contributing to chemokine signaling as well(16,17). Critical downstream effectors of these pathways include Ras superfamily small GTPases, Ras, RhoA, Rac, and Rap1 which mediate actin cytoskeleton rearrangement, integrin activation and microclustering, as well as MAPK pathway activation (18-21).
Both chemokines and their receptors are capable of homo and heterodimerization but considerable debate exists as to the role of oligomerization during in vivo chemokine signaling. In solution chemokines form dimers at millimolar concentrations, orders of magnitude greater than concentrations found in vivo. Monomeric mutants of several chemokines exhibit near wild type chemoattractant function in in vitro assays whereas oligomerization impaired V27F CXCL8 mutant (for example) exhibits near wild type in vivo chemoattraction (22-24). However, GAG binding significantly lowers the concentration threshold required for chemokine oligomerization, and oligomerization is a requirement for the in vivo activity of CCL5 and CXCL10 despite near wild type receptor and GAG affinity of monomeric mutants (25,26). These results suggest that for some members of the chemokine oligomerization is essential for signal transduction events in vivo.
Chemokine and chemokine receptor oligomerization appear to have a role in differential induction of signaling pathways depending on the concentration of chemokines in a given environment. Chemokine receptor dimerization was first explicitly demonstrated for CCR2 and shown to be required for pertussis toxin insensitive induction receptor phosphorylation and JAK/STAT signaling (27, reviewed in greater detail in reference 16). This exciting discovery was the first to suggest a mechanism by which chemokines are capable of inducing both chemoattraction, chemorepulsion, and synergy (28,29). Rather than signaling as discrete biochemical units, chemokine receptors integrate environmental cues and exert distinct biochemical pathways to induce responses beyond leukocyte arrest and transendothelial migration affecting biochemical events as diverse as chemoattraction, T-cell costimulation, and Th polarization (30,31,32).
4. CHEMOKINE PRODUCTION DURING HSV-1 INFECTION
Inflammatory chemokine expression during HSV-1 infection is driven by recognition of viral products by germline encoded receptors of pathogen associated molecular patterns (PAMPs) and specific lymphocyte recognition of viral antigen. Antigen recognition drives chemokine expression as well as production of interferon gamma (IFNg) and tumor necrosis factor-alpha (TNFa) production that further induce chemokine expression (33,34). PAMPs induce signal transduction through ancestral PAMP recognition pathways such as the Toll-like receptor (TLR) family and double stranded RNA inducible protein kinase (PKR). Following PAMP recognition, these pathways either directly induce chemokine production through nuclear factor kappa b (NF-kB) and mitogen-activated protein kinase (MAPK) activation or indirectly through the NF-kB or interferon regulatory factors 3 and 7 driven expression of inflammatory cytokines such as type I IFNs, TNFa, IL-1 and IL-6 (35-40).
To date only four TLRs have been directly implicated in host resistance to HSV-1 infection; TLR3, TLR9, and the TLR2/6 heterodimer which recognize double stranded RNA, CpG DNA motifs, and lipopeptides respectively (41,42). Viral or host lipopeptides responsible for TLR2 agonist activity are currently unknown, and TLR2- mediated recognition of HSV-1 PAMPs only occurs in a relatively small number of laboratory and clinical isolates (43-45). Yet, in TLR2 agonistic isolates, HSV-1 infection was demonstrated to induce TLR2-dependent upregulation of IL-6, IL-12 and CCL2 expression. TLR2 recognition may be particularly important during HSV-1 encephalitis as microglia strongly express TLR2 and are abundant producers of CCL2, -3, -4, -5, -8, and -10 (42,46).
TLR9 signaling has little impact on the course of HSV-1 infection in mouse models. Although early chemokine production during acute HSV-1 infection is reduced in the absence of TLR9, particularly for the monocyte and granulocyte chemoattractants, CCL2 and CXCL1 respectively, neither viral burdens within the nervous system nor survival are affected (47,48).
IRAK-4 is required for normal NF-kB activation and type I IFN (IFNalpha/beta) production following TLR stimulation (49,50) for all TLRs except TLRs 3 and 4 for which IRAK-4 is required for normal NF-kB activation but not IFN production (51). IRAK-4 deficient patients do not exhibit the increased susceptibility to herpes simplex encephalitis (HSE) demonstrating that TLR2/5/6/7/8/9-mediated type I IFN production probably has little impact on the course of primary infection in humans. However, individuals deficient in UNC-93B, which has an as yet unknown role in type I IFN production following TLR3/7/8/9 ligation, are profoundly susceptible to HSE (52). These results suggested that TLR3 is the dominant TLR involved in recognition of HSV-1, and patients with dominant-negative TLR3 mutations were recently found to be highly susceptible to HSE (41). This study did not address chemokine production specifically, and it is likely that any contribution to HSE susceptibility from reduced NF-kB-driven chemokine production in these patients is minor in comparison to the contribution from reduced IFN production, as IRAK-4 is required for optimal NF-kB activation even following TLR3 stimulation (51). However, this result does suggest that dsRNA detection is a critical step in innate recognition of HSV-1 infection implicating TLR3 which is expressed in human neurons, microglia, and astrocytes (53-55). Pattern recognition receptors responsible for dsRNA detection, such as TLR3, PKR, and MDA5 may play key roles in cytokine and chemokine production responsible for immunological control of HSV-1 reactivation from latency.
5. CHEMOKINES AND LEUKOCYTE RECRUITMENT AT SITES OF EARLY VIRAL REPLICATION
Control of HSV-1 during primary infection requires the expression of type I IFNs, recruitment of natural killer (NK) cells, and culminates in the induction of antigen specific responses by T cells which control viral replication through lysis of infected cells and cytokine production. Failure at any step in this process results in uncontrolled viral replication leading to herpes simplex encephalitis (56-59). Conversely, effective innate control of viral replication may lower latent viral burden present in sensory ganglia, and reduce the frequency of subsequent reactivation.
Early chemokine expression is dominated by production of ELR+ CXC chemokines CXCL1/2/8, CCL2, and CXCL10 (48, 60-63). CXCL1/2/8 chemoattracts PMNs through the receptors CXCR1/2, while CCL2 recruits monocytes through CCR2. Recruited PMNs and subsequently monocytes augment local chemokine expression through direct chemokine production including CXCL10 and CCL5 as well as by production of TNFa (64,65,66). The early type I IFN-dependent production of CXCL10 (significant upregulation within <24hrs) presumably plays a role in chemoattraction of monocytes and NK cells (9,62,67,68).
6. CHEMOKINES IN THE SENSORY GANGLIA AND CENTRAL NERVOUS SYSTEM
While PMNs, macrophages, and NK cells effectively suppress local HSV-1 (57), sensory nerve fibers feeding the site of inoculation are infected with the virus which then travels to sensory ganglia via retrograde transport. The role of chemokines in innate control of HSV-1 infection in sensory ganglia and the central nervous system (CNS) mirrors the expression and role of chemokines in the periphery. In the ocular HSV-1 infection of C57BL/6 mice, expression of CCL2, CCL5, CXCL9 and CXCL10 is upregulated in the trigeminal ganglia within 72 hours post infection which is the earliest time point by which HSV-1 replication is detectable in this tissue followed shortly thereafter by upregulation of the potent NK chemoattractant CCL3 (69,70). CCL2 and CXCL10 are believed to be of particular importance in the development of innate immune responses to HSV-1 within the nervous system. CCL2 deficiency significantly reduces monocyte recruitment to the CNS during inflammation. Recruited monocytes/macrophages are the dominant TNFα source during primary HSV-1 infection of the murine trigeminal ganglia, and TNFα neutralization significantly reduces global leukocyte recruitment during HSV-1 infection (71,72).
Currently, the impact of CXCL10 is less clear. CXCL10 is a potent chemoattract for monocytes, NK cells, as well as Th1-polarized CD4+ T-cells and CD8+ T-cells, and is among the first and most highly expressed chemokine that has been detected in murine models in response to HSV-1. Yet, genetic deficiency in the only known receptor for CXCL10, CXCR3, has had a paradoxical impact on the course of HSV-1 in our hands. Our group has observed increased survival in CXCR3-deficient animals compared to wild type C57BL/6 mice (70). Viral titers in the brain stem and trigeminal ganglia of CXCR3 deficient animals are elevated, but only at day 7 post-infection suggestive of an impact on the development of adaptive immune responses.
Regulation of CXCL10 expression is equally perplexing. CXCL10 expression is dramatically upregulated early during the course of HSV-1 by type I and II IFN- dependent mechanisms. Yet, constitutive expression of type I IFN at levels sufficient to nearly completely prevent HSV-1 replication within the trigeminal ganglia and brain stem in murine models, drives only modest CXCL10 expression (73). Still, the potent chemoattractive potential of CXCL10 towards NK cells would suggest that this chemokine contributes to NK cell recruitment along with CCL3 and CCL5 (9, 67,74).
Some controversy exists in the field over the degree of importance of NK cell activity in control of HSV-1 replication as experiments to determine the role of NK cells have typically been confounded by antibody-mediated depletion of non-NK cell populations or effects related to genetic deletion of the common γ chain of the IL-2/15 receptor (75). However, depletion of NK cells in mice using the relatively specific anti-NK1.1 antibody results in elevated HSV-1 viral titers, and indirect evidence taken from studies using CCR5 deficient animals further suggests a prominent role for this population. During corneal infection with HSV-1, CCR5 deficient mice exhibit elevated viral titers in the cornea, trigeminal ganglia and brain stem (69), CCR5 deficient mice exhibit a similarly increased susceptibility during HSV-2 infection which is predominantly attributable to reduced NK cell recruitment (76).
One interesting question that remains with regards to NK biology during HSV-1 infection is what the roles of individual NK cell subsets are. In humans and mice NK cells can be broadly divided into populations with either high cytotoxic activity or abundant cytokine expression that are distinguishable on the basis of CD56 and CD16 expression in humans and CD11b, CD27, and CD127 (IL-7Ralpha) expression in mice. These subsets differentially express various chemokine receptors including CXCR1/3/4 and CCR1/4/5/6/7/9 (77,78). Both subsets express CXCR3, the ligands of which are abundantly expressed during HSV-1 infection at epithelial surfaces as well as within sensory ganglia and the CNS (5). Yet CD56bright NK cells universally express CCR7, while CD56dim cells do not (78). This chemokine receptor is classically associated with lymphoid homing cells and CD56bright NK cells are enriched in secondary lymphoid tissues (79).
However, T cells found in cerebral spinal fluid during CNS inflammation express high levels of CCR7, and the CCR7 ligands CCL19 and CCL21 are expressed proximal at the blood brain barrier during experimental autoimmune encephalitis suggesting that CCR7 signaling may also play a role in differential recruitment of specific NK cell subsets to the CNS under certain circumstances (80,81). Conversely, the chemokine environment present in certain mouse strains or human patients may promote the recruitment of highly cytotoxic NK cells instead. Cytotoxic CD56dim NK cells express high levels of CXCR1 and CX3CR1 (82). The ligand for CX3CR1, CX3CL1 is predominantly expressed within the CNS and is required for NK recruitment to the CNS during experimental autoimmune encephalitis (69). Selective recruitment of highly cytotoxic NK cell subsets by this chemokine could be involved in the paradoxical role of NK cells in control of Toxoplasma gondii. In a study comparing CCR5 deficient versus wild type mice on C57BL/6 backgrounds, mice infected with T. gondii exhibit reduced mortality despite elevated parasite burden following NK cell depletion. However, whether NK cell-mediated neuroinflammation in this model is a result of NK cytotoxicity or cytokine production is still unknown (83).
7. CHEMOKINES AND THE GENERATION OF THE ADAPTIVE IMMUNE RESPONSE
Although innate immune functions such as type I IFN and NK cell activity are required to suppress HSV-1 replication and prevent HSE, the generation of both CD4+ and CD8+ virus-specific T cells are ultimately required to control viral replication within neural tissues, drive HSV-1 into latency, and prevent reactivation (58,59,84-87).
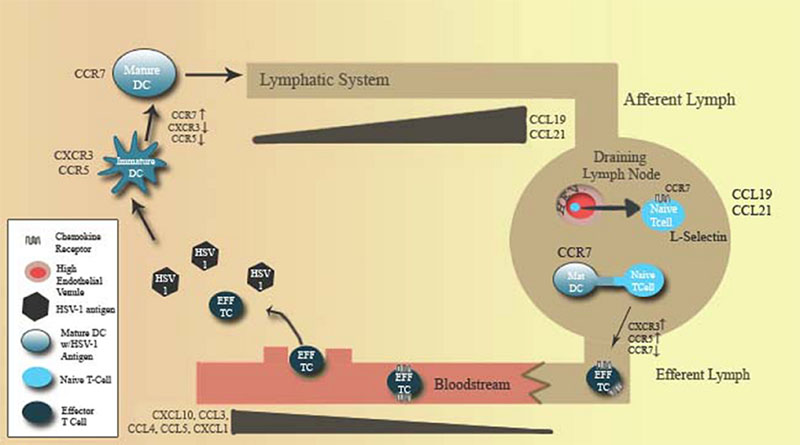
Humans and mice both maintain a number of specialized dendritic cell populations. However, the role of specific dendritic cell populations in HSV-1 antigen presentation is poorly understood. Experiments in mice suggest antigen presentation is driven by two concurrent dendritic cell-mediated processes. Conventional dendritic cells appear to be responsible for trafficking of antigen to draining lymph nodes as antigen carrying Langherhan's cells accumulate during HSV-1 infection (88). Expression of CXCR3 and CCR1/2/5 agonists at foci of infection act to recruit to immature conventional dendritic cells which acquire antigen. Through a cytokine and TLR ligand-dependent maturation process, these cells downregulate inflammatory chemokine receptors and upregulate expression of the lymphoid homing chemokine receptor CCR7. This process drives antigen-loaded DC migration to secondary lymphoid tissues to initiate antigen presentation to naive T cells (89-91). Yet for CD8+ T cells and possibly CD4+ T cells, it appears the final process of antigen presentation and co-stimulation of T-cells occurs through lymph node resident CD8alpha+ dendritic cells possibly through MHC transfer between convential DCs and lymph node resident DCs (88-91) (Fig. 1).
Figure 1.
Chemokine and chemokine receptor expression coordinates the development of the adaptive immune response to HSV-1. The development of T cell responses to HSV-1 requires the transfer of antigen from sites of viral replication to the draining lymph nodes, where antigen is subsequently presented to T cells. Dendritic cell (DC) maturation down regulates expression of inflammatory chemokine receptors including CCR5 and CXCR3. DC maturation also up-regulates expression of lymphoid homing chemokine receptors such as CCR7 driving antigen loaded DCs towards CCL19- and CCL21-expressing lymphoid tissue for presentation of antigen to T cells. Conversely, T cell activation induces the expression of inflammatory chemokine receptors and downregulation of lymphoid homing receptors, thus promoting effector cell migration to sites of viral replication.
Little is known about the impact of specialized dendritic cells of plasmacytoid morphology (pDC) capable of rapid high level expression of IFNa following TLR9-dependent recognition of HSV-1 CpG DNA motifs (47,92). The relatively mild phenotype of TLR9 deficient mice during primary infection as well as direct studies on IFN expression during HSV-1 infection suggest that the majority of type I IFN production during primary HSV-1 infection originates in other cell types including resident cells as well as macrophages and conventional dendritic cells (93,47). However, pDCs have been directly implicated in other facets of control such as activation of NK cells through IL-18 production (94). PDCs also play a role in the generation of anti-HSV CD8+ T cells through type I IFN production following CXCR3/CXCL9- dependent migration to draining lymph nodes rather than directly through antigen presentation (95).
8. T-CELL HOMING TO NEURAL TISSUES
The adaptive immune response to HSV-1 is of a Th1 etiology, HSV-1 specific CD4+ T cells are polarized towards IFNg production and large numbers of virus- specific CD8+ T cells are generated. These cells act to suppress viral replication through IFNg production and possibly cytolytic activity (5,59,91). Th1 polarized CD4+ and virus-specific CD8+ T cells upregulate expression of CCR5 and CXCR3, the ligands highly upregulated at sites of HSV-1 replication as well in latently infected sensory ganglia (5,70,97-99). It is our opinion that during infection the concurrent expression of inflammatory chemokines and selective expression of their receptors on recently activated and therefore, predominantly antigen-specific T cells contributes to the preferential recruitment of antigen-specific T cells to foci of infection. (Figure 2, also 102,103).
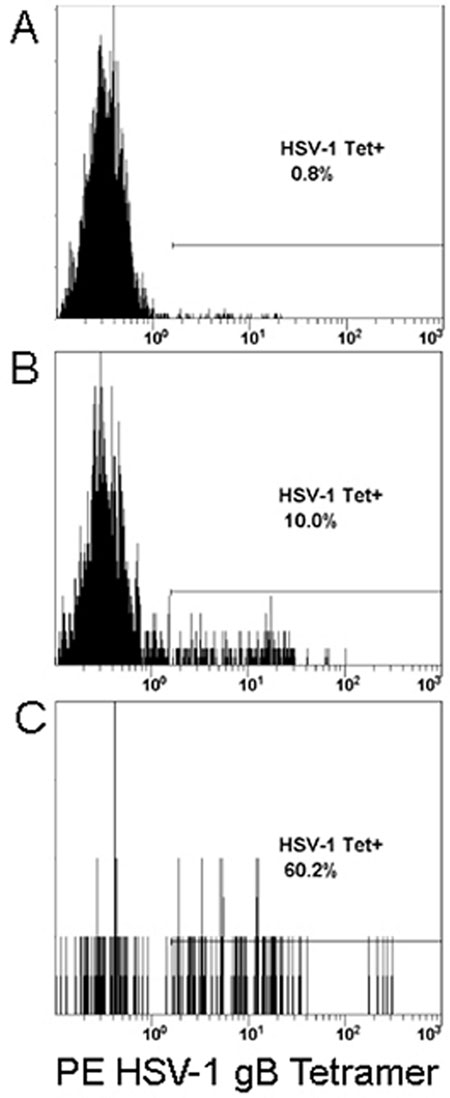
Figure 2.
HSV-1 Specific CD8+ Tcells are preferentially recruited to sites of viral replication. In H-2kB restricted C57BL/6 mice HSV-1 specific CD8+ T cells are almost entirely specific for the HSV-1 glycoprotein B immunodominant epitope SSIEFARL (100,101). Mice were infected with HSV-1 McKrae via corneal inoculation. At day 7 post-infection, leukocytes were taken from the (A) draining lymph nodes, (B) whole blood, or (C) the trigeminal ganglia. Cells were purified and analyzed by flow cytometry for expression CD8, CD45, and staining with phycoerythrin (PE) labeled H-2kB tetramer containing SSIEFARL peptide. Shown are representative histograms of CD45HI and CD8+ gated populations, with PE fluorescence on the x-axis.
Deficiency in either CCR5 or CXCR3 results in elevated viral titers at day 7 post infection suggestive of a defect in adaptive immunity (69,70). However, no quantitative measurements of T cell recruitment to neural tissues in either CCR5 or CXCR3 deficient mice exist in the literature and defective T cell responses in either strain may not be a result of aberrant recruitment to infected tissues. CCR5 accumulates at immunological synapses between APCs and T cells, and CXCR3 utilizes some T cell receptor components for signal transduction (30,32). Thus, deficiency in either receptor may result in T cell functional deficits besides impaired recruitment.
9. CHEMOKINES AND HSV-1 LATENCY
Following resolution and initiation of latency, T cell responses are critical for the control of HSV-1 reactivation. Latently infected trigeminal ganglia exhibit continued upregulation of mRNA for the chemokines CCL3/4/5 and CXCL10 and for their respective chemokine receptors CCR5 and CXCR3 (98,104,105). Chemokine expression is believed to result in the retention of activated CD4+ and CD8+ T cells in latently infected TG which is observed in both humans and mice (106-108).
Virus-specific CD8+ T cells suppress reactivation in ex vivo trigeminal ganglia cultures most likely through IFNg production, and may also suppress reactivation through cytokine production at sensory nerve endings as well (59,84). Furthermore, virus-specific CD8+ T cells are selectively retained at sensory nerve endings in the skin following resolution of viral replication during HSV-2 infection (108). Whether this is the case during HSV-1 infection remains to be formally demonstrated. Multiple subsets of CD8+ T cells have been identified on the basis of both activity and chemokine receptor expression. Central versus effector memory CD8+ T cells are defined partly on the presence or absence of the lymphoid homing chemokine receptor CCR7, and a subset of uniquely cytotoxic CXCR1+ CD8+ T cells has been identified by Luster and colleagues (109-112). However, we are not aware of any studies investigating differential recruitment of CD8+ T cell subsets to the CNS, sensory ganglia, or vesicular lesions during HSV-1 infection.
10. PERSPECTIVE
A number of pathogens are capable of subverting chemokine responses, and members of the herpesvirus family are unusually gifted in this regard. HSV-1 ICP0 blocks transcription of IFN stimulated genes, dampening IFN driven chemokine expression (113). Several gamma herpesviruses encode viral chemokines and chemokine receptors (114-116). Members of the alpha herpesvirus family, of which HSV-1 is a member, also subvert chemokine responses. Secreted virally-encoded glycoprotein Gs from several alpha herpesviruses are potent chemokine binding proteins capable of blocking neutrophil recruitment (117,118). Oddly, HSV-1 gG is an exception; no chemokine binding capability is observed despite homology with other gGs assayed by Bryant et al (117). Alternatively, HSV-1 gG may specifically bind a chemokine(s) not assayed for in this study or use other mechanisms to block chemokine signaling.
Chemokines play a dynamic and powerful role in the development of immune responses that are only beginning to be explored. Through selective recruitment and modulation of specific leukocyte subsets, chemokines initiate and modulate the host immune response. One of the most important unanswered questions in chemokine biology is to what degree chemokine redundancy impacts leukocyte mobilization. The interactions between chemokines and chemokine receptors are notoriously promiscuous. Redundancy and compensatory mechanisms in chemokine signaling probably mask the importance of individual chemokines and receptors analyzed through genetically deficient models. Control of viral infections is associated with production of CXCR3 and CCR5 agonists and recruitment of leukocytes bearing these receptors. Yet, single knockouts of CXCR3 and CCR5 exhibit a mild phenotype when compared to ablation of leukocyte subsets associated with these receptors suggesting functional redundancy between these two and most likely other chemokine receptors. Very few studies have assessed the impact of deficiency of more than one chemokine receptor during viral infection. Consequently, it remains to be seen whether chemokine receptor redundancy is simply a result of chemokine/chemokine receptor variety and promiscuity.
11. ACKNOWLEDGEMENTS
The authors would like to thank Gabby Nguyen for her technical assistance. This work was supported by USPHS grant, AI067309, an unrestricted grant from Research to Prevent Blindness, and NIH/NEI Core grant, EY12190.
12. REFERENCES
- 1.Howard M, Sellors J, Jang D, Robinson N, Fearon M, Kaczorowski J, Chernesky M. Regional of Distribution Antibodies to Herpes Simplex Virus Type 1 (HSV-1) and HSV-2 in Men and Women in Ontario, Canada. J Clin Microbiol. 2003;41(1):84–89. doi: 10.1128/JCM.41.1.84-89.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker K, Garnet G. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81:103–107. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith J, Robinson NJ. Age-Specific Prevalence of Infection with Herpes Simplex Virus Types 2 and 1: A Global Review. The Journal of Infectious Diseases. 2002;186:S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 4.Taylor T, Brockman M, McNamee E, Knipe D. Herpes Simplex Virus. Frontiers in Bioscience. 2002;7:d752–764. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- 5.Carr DJ, Tomanek L. Herpes simplex virus and the chemokines that mediate the inflammation. Curr Top Microbiol Immunol. 2006;303:47–65. doi: 10.1007/978-3-540-33397-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas I, Nicolls M. Chemokine-mediated angiogenesis: an essential link in the evolution of airway fibrosis? J Clin Invest. 2005;115(5):1133–1136. doi: 10.1172/JCI25193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlotnik A, Yoshie O. Chemokines: A New Classification System and Their Role in Immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 9.Luster A. The role of chemokines in linking innate and adaptive immunity. Current Opinion in Immunology. 2002;14(1):129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95(10):3032–3043. [PubMed] [Google Scholar]
- 11.Kim C, Kunkel E, Boisvert J, Johnston B, Campbell J, Genovese M, Greenberg H, Butcher E. Bonzo/CXCR6 expression defines type 1–polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107(5):595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viola A, Contento R, Molon B. T cells and their partners: the chemokine dating agency. Trends in Immunology. 2006;27(9):421–427. doi: 10.1016/j.it.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Koopmann W, Krangel M. Identification of a Glycosaminoglycan-binding Site in Chemokine Macrophage Inflammatory Protein-1alpha. J. Biol. Chem. 1997;272(15):10103–10109. doi: 10.1074/jbc.272.15.10103. [DOI] [PubMed] [Google Scholar]
- 14.Witt D, Lander A. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr. Biol. 1994;4(5):394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 15.Proudfoot A. The biological relevance of chemokine-proteoglycan interactions. Biochem Soc Trans. 2006;34(3):422–426. doi: 10.1042/BST0340422. [DOI] [PubMed] [Google Scholar]
- 16.Mellado M, Rodríguez-Frade J, Mañes S, Martínez-A C. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 17.Curnock A, Logan M, Ward S. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105(2):125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. The Journal of Cell Biology. 161(2):417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katagiri K, Shimonaka M, Kinashi T. Rap1-mediated Lymphocyte Function-associated Antigen-1 Activation by the T Cell Antigen Receptor Is Dependent on Phospholipase C-1 gamma. J. Biol. Chem. 2004;279(12):11875–11881. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 20.Luster A, Alon R, von Andrian U. Immune cell migration in inflammation: present and future therapeutic targets. Nature Immunology. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 21.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nature Reviews Immunology. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 22.Allen S, Crown S, Handel T. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 23.Campanella G, Grimm J, Manice L, Colvin R, Medoff B, Wojtkiewicz G, Weissleder R, Luster A. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177(10):6991–6998. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- 24.Horcher M, Rot A, Aschauer H, Besemer J. IL-8 derivatives with a reduced potential to form homodimers are fully active in vitro and in vivo. Cytokine. 1998;10(1):1–12. doi: 10.1006/cyto.1997.0251. [DOI] [PubMed] [Google Scholar]
- 25.Campanella G, Grimm J, Manice L, Colvin R, Medoff B, Wojtkiewicz G, Weissleder R, Luster A. Oligomerization of CXCL10 Is Necessary for Endothelial Cell Presentation and In Vivo Activity. The Journal of Immunology. 2006;177:6991–6998. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- 26.Baltus T, Weber K, Johnson Z, Proudfoot A, Weber C. Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood. 2003;102(6):1985–1988. doi: 10.1182/blood-2003-04-1175. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Frade J, Vila-Coro A, Martín de Ana A, Albar J, Martínez-A C, Mellado M. The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. PNAS. 1999;96(7):3628–3633. doi: 10.1073/pnas.96.7.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vianello F, Olszak I, Poznansky M. Fugetaxis: active movement of leukocytes away from a chemokinetic agent. J Mol Med. 2005;83(10):752–763. doi: 10.1007/s00109-005-0675-z. [DOI] [PubMed] [Google Scholar]
- 29.Poznansky M, Olszak I, Foxall R, Evans R, Luster A, Scadden D. Active movement of T cells away from a chemokine. Nature Medicine. 2000;6:543–548. doi: 10.1038/75022. [DOI] [PubMed] [Google Scholar]
- 30.Dar W, Knechtle S. CXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signalling through the T-cell receptor. Immunology. 2007;120(4):467–85. doi: 10.1111/j.1365-2567.2006.02534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A, Humphreys T, Kremer K, Bramati P, Bradfield L, Edgar C, Hedin K. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25(2):213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Molon B, Gri G, Bettella M, Gómez-Moutón C, Lanzavecchia A, Martínez-A C, Mañes S, Viola A. T-cell costimulation by chemokine receptors. Nat Immunol. 2005;6(5):465–71. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 33.Huse M, Lillemeier B, Kuhns M, Chen D, Davis M. T cells use two directionally distinct pathways for cytokine secretion. Nature Immunology. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 34.Olson T, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh S, Karin M. Missing Pieces in the NF-кB Puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 36.Zamanian-Daryoush M, Mogensen T, DiDonato J, Williams B. NF-B Activation by Double-Stranded-RNA-Activated Protein Kinase (PKR) Is Mediated through NF-B-Inducing Kinase and IB Kinase. Molecular and Cellular Biology. 2000;20(4):1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnet M, Weil R, Dam E, Hovanessian A, Meurs E. PKR Stimulates NF-B Irrespective of Its Kinase Function by Interacting with the IB Kinase Complex. Molecular and Cellular Biology. 2000;20(13):4532–4542. doi: 10.1128/mcb.20.13.4532-4542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gil J, García M, Gomez-Puertas P, Guerra S, Rullas J, Nakano H, Alcamí J, Esteban M. TRAF Family Proteins Link PKR with NF-B Activation. Molecular and Cellular Biology. 2004;24(10):4502–4512. doi: 10.1128/MCB.24.10.4502-4512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. Innate immune recognition of viral infection. Nature Immunology. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 40.Finberg R, Knipe D, Kurt-Jones E. Herpes Simplex Virus and Toll-like receptors. Viral Immunology. 2005;18(3):457–465. doi: 10.1089/vim.2005.18.457. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku C, Casrouge A, Zhang X, Barreiro L, Leonard J, Hamilton C, Lebon P, Héron B, Vallée L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova J. TLR3 Deficiency in Patients with Herpes Simplex Encephalitis. Science. 2007;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 42.Lokensgard J, Maxim C, Cheeran J, Hu S, Gekker G, Peterson P. Glial Cell Responses to Herpesvirus Infections: Role in Defense and Immunopathogenesis. The Journal of Infectious Diseases. 2002;186:S171–S179. doi: 10.1086/344272. [DOI] [PubMed] [Google Scholar]
- 43.Kurt-Jones E, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold M, Knipe D, Finberg R. Herpes simplex virus 1 interaction with toll-like receptor 2 contributes to lethal encephalitis. PNAS. 2004;101(5):1315–20. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato A, Linehan M, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. PNAS. 2006;103(46):17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mansur D, Kroon E, Nogueira M, Arantes R, Rodrigues S, Akira S, Gazzinelli R, Campos M. Lethal Encephalitis in Myeloid Differentiation Factor 88-Deficient Mice Infected with Herpes Simplex Virus 1. American Journal of Pathology. 2005;166:1419–1426. doi: 10.1016/S0002-9440(10)62359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aravalli R, Hu S, Rowen T, Palmquist J, Lokensgard J. Cutting Edge: TLR2-Mediated Proinflammatory Cytokine and Chemokine Production by Microglial Cells in Response to Herpes Simplex Virus1. The Journal of Immunology. 2005;175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 47.Krug A, Luker G, Barchet W, Leib D, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor. Blood. 2004;103(4):1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 48.Wuest T, Austin B, Uematsu S, Thapa M, Akira S, Carr D. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J. Neuroimmunol. 2006;179(12):46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim W, Staschke K, Bulek K, Yao J, Peters K, Oh K, Vandenburg Y, Xiao H, Qian W, Hamilton T, Min B, Sen G, Gilmour R, Li X. A critical role for IRAK4 kinase activity in Toll-like receptor–mediated innate immunity. The Journal of Experimental Medicine. 2007;204(5):1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang K, Puel A, Zhang S, Eidenschenk C, Ku C, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Maródi L, Davidson D, Speert D, Roifman C, Garty B, Ozinsky A, Barrat F, Coffman R, Miller R, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann H, Jouanguy E, Casanova J. Human TLR-7-, -8-, and -9-Mediated Induction of IFN-α/β and -λ Is IRAK-4 Dependent and Redundant for Protective Immunity to Viruses. Immunity. 2005;23(5):465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzukia N, Saito T. IRAK-4 – a shared NF-кB activator in innate and acquired immunity. Trends in Immunology. 2006;27(12):566–572. doi: 10.1016/j.it.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Casrouge A, Zhang S, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Sénéchal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller R, Héron B, Mignot C, Billette de Villemeur T, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova J. Herpes Simplex Virus Encephalitis in Human UNC-93B Deficiency. Science. 2006;314(5797):308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 53.Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29(3):185–94. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 54.Olson J, Miller S. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 55.Carpentier P, Williams B, Miller S. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55(3):239–252. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- 56.Ghiasi H, Cai S, Perng G, Nesburn A, Wechsler S. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 2000;45(1):33–45. doi: 10.1016/s0166-3542(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 57.Reading P, Whitney P, Barr D, Smyth M, Brooks A. NK cells contribute to the early clearance of HSV-1 from the lung but cannot control replication in the central nervous system following intranasal infection. Eur J Immunol. 2006;36(4):897–905. doi: 10.1002/eji.200535710. [DOI] [PubMed] [Google Scholar]
- 58.Mossman K, Ashkar A. Herpesviruses and the innate immune response. Viral Immunol. 2005;18(2):267–281. doi: 10.1089/vim.2005.18.267. [DOI] [PubMed] [Google Scholar]
- 59.Khanna K, Lepisto A, Decman V, Hendricks R. Immune control of herpes simplex virus during latency. Curr Opin Immunol. 2004;16(4):463–469. doi: 10.1016/j.coi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Tumpey T, Fenton R, Molesworth-Kenyon S, Oakes J, Lausch R. Role for Macrophage Inflammatory Protein 2 (MIP-2), MIP-1α, and Interleukin-1α in the Delayed-Type Hypersensitivity Response to Viral Antigen. J Virol. 2002;76(16):8050–8057. doi: 10.1128/JVI.76.16.8050-8057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan X, Tumpey T, Kunkel S, Oakes J, Lausch R. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39(10):1854–62. [PubMed] [Google Scholar]
- 62.Carr DJ, Tomanek L. Herpes simplex virus and the chemokines that mediate the inflammation. Curr Top Microbiol Immunol. 2006;303:47–65. doi: 10.1007/978-3-540-33397-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanbervliet B, Homey B, Durand I, Massacrier C, Aït-Yahia S, de Bouteiller O, Vicari A, Caux C. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur J Immunol. 2002;32(1):231–242. doi: 10.1002/1521-4141(200201)32:1<231::AID-IMMU231>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 64.Molesworth-Kenyon S, Oakes J, Lausch R. A novel role for neutrophils as a source of T cell-recruiting chemokines IP-10 and Mig during the DTH response to HSV-1 antigen. Journal of Leukocyte Biology. 2005;77:552–559. doi: 10.1189/jlb.0904485. [DOI] [PubMed] [Google Scholar]
- 65.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel S. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 66.Malmgaard L, Melchjorsen J, Bowie A, Mogensen S, Paludan S. Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol. 2004;173(11):6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- 67.Wald O, Weiss I, Wald H, Shoham H, Bar-Shavit Y, Beider K, Galun E, Weiss L, Flaishon L, Shachar I, Nagler A, Lu B, Gerard C, Gao J, Mishani E, Farber J, Peled A. IFN-gamma acts on T cells to induce NK cell mobilization and accumulation in target organs. J Immunol. 2006;176(8):4716–4729. doi: 10.4049/jimmunol.176.8.4716. [DOI] [PubMed] [Google Scholar]
- 68.Taub D, Lloyd A, Conlon K, Wang J, Ortaldo J, Harada A, Matsushima K, Kelvin D, Oppenheim J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. The Journal of Experimental Medicine. 1993;177(6):1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carr D, Ash J, Lane T, Kuziel W. Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1. J Gen Virol. 2006;87(3):489–499. doi: 10.1099/vir.0.81339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wickham S, Lu B, Ash J, Carr D. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. Journal of Neuroimmunology. 2003;162(12):51–59. doi: 10.1016/j.jneuroim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Kodukulal P, Liu T, Van Rooijen N, Jager M, Hendricks R. Macrophage Control of Herpes Simplex Virus Type 1 Replication in the Peripheral Nervous System. The Journal of Immunology. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 72.Rebenko-Molla N, Liua L, Cardonaa A, Ransohoff R. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Current Opinion in Immunology. 2006;18(6):683–689. doi: 10.1016/j.coi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Carr D, Campbell I. Herpes Simplex Virus Type 1 Induction of Chemokine Production is Unrelated to Virus Load in the Cornea but not in the Nervous System. Viral Immunol. 2006;19(4):741–746. doi: 10.1089/vim.2006.19.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taub D, Sayers T, Carter C, Ortaldo J. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. The Journal of Immunology. 1995;155(8):3877–3888. [PubMed] [Google Scholar]
- 75.Halford W, Maender J, Gebhardt B. Re-evaluating the role of natural killer cells in innate resistance to herpes simplex virus type 1. Virol J. 2005;2:56. doi: 10.1186/1743-422X-2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thapa M, Kuziel W, Carr D. Susceptibility of CCR5-Deficient Mice to Genital Herpes Simplex Virus Type 2 Is Linked to NK Cell Mobilization. J Virol. 2007;81(8):3704–3713. doi: 10.1128/JVI.02626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayakawa Y, Huntington N, Nutt S, Smyth M. Functional subsets of mouse natural killer cells. Immunological Reviews. 2006;213(1):47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 78.Berahovich R, Lai N, Wei Z, Lanier L, Schall T. Evidence for NK Cell Subsets Based on Chemokine Receptor Expression. The Journal of Immunology. 2006;177:7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- 79.Ferlazzo G, Thomas D, Lin S, Goodman K, Morandi B, Muller W, Moretta A, Münz C. The Abundant NK Cells in Human Secondary Lymphoid Tissues Require Activation to Express Killer Cell Ig-Like Receptors and Become Cytolytic. The Journal of Immunology. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 80.Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle M, Pedemonte E, Noonan D, Albini A, Bernardi G, Mancardi G, Battistini L, Uccelli A. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. Journal of Leukocyte Biology. 2003;73:584–590. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- 81.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. European Journal of Immunology. 2002;32(8):2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 82.Robertson M. Role of chemokines in the biology of natural killer cells. J. Leukoc Biol. 2002;71(2):173–83. [PubMed] [Google Scholar]
- 83.Khan I, Thomas S, Moretto M, Lee F, Islam S, Combe C, Schwartzman J, Luster A. CCR5 Is Essential for NK Cell Trafficking and Host Survival following Toxoplasma gondii Infection. PLoS Pathog. 2006;2(6):e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liua T, Khannaa K, Chenb X, Fink D, Hendricks R. CD8+ T Cells Can Block Herpes Simplex Virus Type 1 (HSV-1) Reactivation from Latency in Sensory Neurons. The Journal of Experimental Medicine. 2000;191(9):1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simmons A, Tscharke D. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. The Journal of Experimental Medicine. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manickan E, Rouse B. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J. Virol. 1995;69(12):8178–8189. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghiasi H, Cai S, Perng G, Nesburn A, Wechsler S. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol. 84(4):408–12. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosnjak L, Jones C, Abendroth A, Cunningham A. Dendritic cell biology in herpesvirus infections. Viral Immunol. 2005;18(3):419–433. doi: 10.1089/vim.2005.18.419. [DOI] [PubMed] [Google Scholar]
- 89.Allan R, Smith C, Belz G, van Lint A, Wakim L, Heath W, Carbone F. Epidermal Viral Immunity Induced by CD8+ Dendritic Cells But Not by Langerhans Cells. Science. 2003;301(5641):1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 90.de Heusch M, Blocklet D, Egrise D, Hauquier B, Vermeersch M, Goldman S, Moser M. Bidirectional MHC molecule exchange between migratory and resident dendritic cells. Journal of Leukocyte Biol. 2007;82:861–868. doi: 10.1189/jlb.0307167. [DOI] [PubMed] [Google Scholar]
- 91.Allan R, Waithman J, Bedoui S, Jones C, Villadangos J, Zhan Y, Lew A, Shortman K, Heath W, Carbone F. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Megjugorac N, Young H, Amrute S, Olshalsky S, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75(3):504–14. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 93.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. PNAS. 2004;101(31):11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.French A, Yokoyama W. Natural killer cells and viral infections. Current Opinion in Immunology. 2003;15(1):45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 95.Zhu J, Koelle D, Cao J, Vazquez J, Huang M, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. The Journal of Experimental Medicine. 2007;204(3):595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hukkanen V, Broberg E, Salmi A, Erälinna J. Cytokines in experimental herpes simplex virus infection. Int Rev Immunol. 2002;21(45):355–371. doi: 10.1080/08830180213276. [DOI] [PubMed] [Google Scholar]
- 97.Barbi J, Oghumu S, Lezama-Davila C, Satoskar A. IFN-g and STAT1 are required for efficient induction of CXC chemokine receptor 3 (CXCR3) on CD4+ but not CD8+ T cells. Blood. 2007;110(6):2215–2216. doi: 10.1182/blood-2007-03-081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cook W, Kramer M, Walker R, Burwell T, Holman H, Coen D, Knipe D. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol J. 2004;1:5. doi: 10.1186/1743-422X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Garra A, McEvoy L, Zlotnik A. T-cell subsets: chemokine receptors guide the way. Curr Biol. 1998;8(18):R646–649. doi: 10.1016/s0960-9822(07)00413-7. [DOI] [PubMed] [Google Scholar]
- 100.Hanke T, Graham F, Rosenthal K, Johnson D. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 1991;65(3):1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cose S, Kelly J, Carbone F. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytoxic T-cell response showing a preferential V beta bias. J. Virol. 1995;69(9):5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hokeness K, Deweerd E, Munks M, Lewis C, Gladue R, Salazar-Mather T. CXCR3-Dependent Recruitment of Antigen-Specific T Lymphocytes to the Liver during Murine Cytomegalovirus Infection. Journal of Virology. 2007;81(3):1241–1250. doi: 10.1128/JVI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wurbel M, Malissen M, Guy-Grand D, Malissen B, Campbell J. Impaired Accumulation of Antigen-Specific CD8 Lymphocytes in Chemokine CCL25-Deficient Intestinal Epithelium and Lamina Propria. The Journal of Immunology. 2007;178:7598–7606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Halford W, Gebhardt B, Carr D. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157(8):3542–3549. [PubMed] [Google Scholar]
- 105.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent Herpesvirus Infection in Human Trigeminal Ganglia Causes Chronic Immune Response. Am J Pathol. 2003;163(6):2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khanna K, Bonneau R, Kinchington P, Hendricks R. Herpes Simplex Virus-Specific Memory CD8+ T Cells Are Selectively Activated and Retained in Latently Infected Sensory Ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verjans G, Hintzen R, van Dun J, Poot A, Milikan J, Laman J, Langerak A, Kinchington P, Osterhaus A. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. PNAS. 2007;104(9):3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. The Journal of Experimental Medicine. 2005;202(3):425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stock A, Jones C, Heath W, Carbone F. Cutting Edge: Central Memory T Cells Do Not Show Accelerated Proliferation or Tissue Infiltration in Response to Localized Herpes Simplex Virus-1 Infection. J Immunol. 2006;177(3):1411–1415. doi: 10.4049/jimmunol.177.3.1411. [DOI] [PubMed] [Google Scholar]
- 110.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central– and effector–memory CD8 T cells in vivo. The Journal of Experimental Medicine. 2005;201(4):579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 112.Hess C, Means T, Autissier P, Woodberry T, Altfeld M, Addo M, Frahm N, Brander C, Walker B, Luster A. IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood. 2004;104(12):3463–3471. doi: 10.1182/blood-2004-03-1067. [DOI] [PubMed] [Google Scholar]
- 113.Hagglund R, Roizman B. Role of ICP0 in the Strategy of Conquest of the Host Cell by Herpes Simplex Virus 1. J Virol. 2004;78(5):2169–2178. doi: 10.1128/JVI.78.5.2169-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenkilde M, Kledal T. Targeting Herpesvirus Reliance of the Chemokine System. Current Drug Targets. 2006;7(1):103–118. doi: 10.2174/138945006775270259. [DOI] [PubMed] [Google Scholar]
- 115.van Cleef K, Smit M, Bruggeman C, Vink C. Cytomegalovirus-encoded homologs of G protein-coupled receptors and chemokines. J Clin Virol. 2006;35(3):343–348. doi: 10.1016/j.jcv.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 116.Nicholas J. Human gammaherpesvirus cytokines and chemokine receptors. J Interferon Cytokine Res. 2005;25(7):373–383. doi: 10.1089/jir.2005.25.373. [DOI] [PubMed] [Google Scholar]
- 117.Bryant N, Davis-Poynter N, Vanderplasschen A, Alcami A. Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO Jour. 2003;22:833–846. doi: 10.1093/emboj/cdg092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Van de Walle G, May M, Sukhumavasi W, von Einem J, Osterrieder N. Herpesvirus Chemokine-Binding Glycoprotein G (gG) Efficiently Inhibits Neutrophil Chemotaxis In Vitro and In Vivo. The Journal of Immunology. 2007;179:4161–4169. doi: 10.4049/jimmunol.179.6.4161. [DOI] [PubMed] [Google Scholar]