Abstract
Background
HIV-infection and disease are accompanied by decreases in the absolute number and function of Vγ2Vδ2 T cells, suggesting that this subset may play an important role in controlling disease. We performed a cross-sectional study on HIV-1-infected former blood donors (FBDs) and assessed the association betweenVγ2Vδ2 T cells and markers of disease progression.
Methods
Changes in Vγ2Vδ2 T cell count and function were compared among HIV infected individuals and healthy donors using Mann–Whitney tests. The relationships between Vγ2Vδ2 T cells, plasma viral load, and CD4 T-cell counts were analyzed using the Spearman correlation.
Results
We found significant positive correlations between CD4 T-cell counts and both total Vγ2Vδ2 T cells (P<0.0001) and functional (IPP-responsive) Vγ2Vδ2 T cells (P<0.0001), and significant reverse correlations between viral load and both total Vγ2Vδ2 T cells (P<0.05) and functional Vγ2Vδ2 T cells (P<0.05).
Conclusions
The association of Vγ2Vδ2 T cells with disease progression in 146 HIV+ participants supports a view that intact Vγ2Vδ2 T cell populations are important for controlling HIV disease.
Keywords: HIV-1, disease progression, Vγ2Vδ2 T cells, association
Introduction
Human gamma delta (γδ) T cells comprise on average 3% of the total peripheral blood T cell +population. Of these γδ T cells, a majority express the Vγ2Vδ2 receptor and around 75% have the Vγ2-Jγ1.2 rearrangement [1]. In contrast to αβT cells, the γδ subset generally lacks CD4 or CD8 expression and recognizes non-peptidic microbial antigens in a major histocompatibility complex (MHC)-unrestricted manner, without antigen processing by professional antigen-presenting cells (APC) [2–5]. Similar to natural killer (NK) cells, Vγ2Vδ2 T cells express MHC-I receptors, including the inhibitory CD94/NKG2 complexes and also express killer immunoglobulin-like receptors [6, 7]. Vγ2Vδ2 T cells produce tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) [8] and β-chemokines (MIP-α, MIP-β, and RANTES) [9, 10] in response to stimulatory ligands [11]. Because Vγ2Vδ2 T cells are broadly reactive against various intracellular pathogens, it is probable that Vγ2Vδ2 T cells do not respond to specific viral antigens, but rather to host molecules, such as phosphoantigens, and/or MHC products induced or modified by viral infections [12]. In vitro, Vγ2Vδ2 T cells display both proliferative and lytic responses to human immunodeficiency virus (HIV)-infected cells [13] and activated Vγ2Vδ2 T cells can suppress HIV replication by releasing soluble factors including β-chemokines [9].
Alterations of γδ T-cell distribution have been previously reported in the peripheral blood of HIV-infected persons, including a dramatic reduction in the absolute number of Vγ2Vδ2 T cells [14]. In most HIV-infected subjects, damage to theVγ2Vδ2 T cells also eliminates the response to phosphoantigen stimulation [15, 16]. These changes occur before a significant decline in the CD4+ T cell subset [15] and are among the earliest defects in cellular immunity after infection with HIV. However, it is still not clear whether damage to Vγ2Vδ2 T cells is associated with disease progression in HIV infection. To address the question, we analyzed the Vγ2Vδ2 T cells from a cohort [17] of 146 people with chronic untreated HIV infection in Anhui Province, China.
Material and method
Study subjects
A group of former blood/plasma donors (FBD) became infected with HIV between 1992 and 1995 because of unregulated commercial blood/plasma collection; our volunteers were recruited from local clinics in Fuyang City (Anhui province, China). Previous baseline investigation showed that their ages ranged from 27 to 65 years old and they were comprised of about a half male and a half female study subjects. No injecting drug usage was identified and all participants are naïve to antiretroviral therapy (ART). Informed consent was obtained from all subjects. The study protocol was sequentially approved by the National Institute of Health, USA, the Institutional Review Board (IRB) of China Center for AIDS/STD Control and Prevention and the IRB of Anhui Provincial Center for Disease Control and Prevention, respectively. Details on HIV testing, sample collection, peripheral blood mononuclear cell (PBMC) isolation, and other relevant information were described previously [17].
In vitro stimulation of Vγ2Vδ2 T cells and effector function assessment
Peripheral blood mononuclear cells were isolated from EDTA anti-coagulated blood by Ficoll-Hypaque (Pharmacia) gradient centrifugation. The PBMC were cultured at 5×105 cells/well in complete RPMI 1640 medium (supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 100 U/mL penicillin-100 mg/mL streptomycin) in 96-well round-bottomed plates (Corning) and stimulated in vitro for 10 hours with either medium alone or 15 μM isopentenyl pyrophosphate (IPP; Sigma) or 10 μg/ml phytohemagglutinin (PHA). Brefeldin A (BFA, Sigma) was added 3 hours before staining for cytokine production. IPP selectively stimulates T cells expressing the Vγ2Vδ2 T cell receptor.
Flow cytometry
Unless noted otherwise, cells were stained with fluorophore-conjugated monoclonal antibodies from BD Biosciences. CD3, CD4, and CD8 T cell were measured with a FACSCalibur (Becton Dickinson) TruCount tube, multi-color antibody (CD3/8/45/4). Results were analyzed by Multiset software. To determine the frequency of circulating Vγ2Vδ2 T cells, 3×105~5×105 cells were washed, resuspended in 50–100 oL of RPMI 1640, and stained with mouse anti–human Vγ9–fluorescein isothiocyanate (FITC) clone 7A5 (Pierce Biotechnology) (note that Vγ9 and Vγ2 are alternate names for the same chain of the T cell receptor), mouse anti–human CD3–allophycocyanin (APC) clone UCHT1 and isotype controls, including rabbit anti–mouse IgG1–FITC clone X40, and IgG1-APC clone X40. For detecting intracellular IFN-γ, IPP-stimulated cells were stained with Vγ9-FITC, fixed, permeabilized, and incubated for 45 min at 4°C with mouse anti–human IFNγ–PE clone 4S.B3. Intracellular staining solutions were obtained from the Cytofix/Cytoperm Kit (BD Biosciences). Data for at least 5×104 lymphocytes (gated on the basis of forward- and side-scatter profiles) were acquired for each sample on a FACS Calibur flow cytometer (BD Biosciences). Total Vγ2Vδ2 T cell and IFN-γ+ Vγ2Vδ2 T cell counts were determined based on their percentage among CD3 T cell and CD3 T cell counts. All samples were analyzed using FCSExpress software (version 3; De Novo Software).
Viral load testing
Plasma viral loads were analyzed by the Roche Cobas Amplicor 2.0 assay (Roche Diagnostics), which has a lower limit for detection of 50 copies/ml.
Statistical analysis
Spearman rank-correlation and Mann–Whitney tests were performed using GraphPad Prism version 5. All tests were two-tailed and P-values of P<0.05 were considered significant.
Results
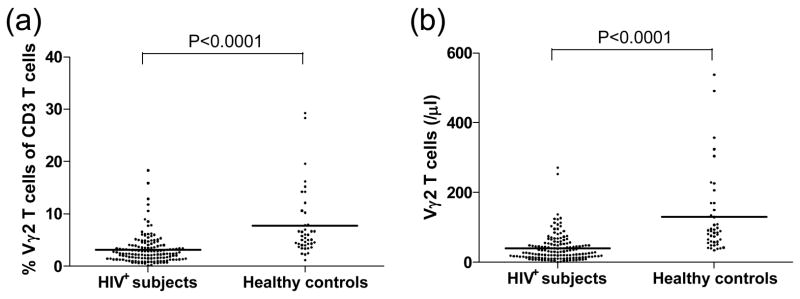
We compared Vγ2Vδ2 T cells in 146 HIV infected individuals and 42 healthy (HIV−) donors. Consistent with previous studies, both the percentage among total CD3 T cells and absolute counts of Vγ2Vδ2 T cells were significantly decreased after HIV infection (P<0.0001, Fig. 1). Furthermore, we found a significant positive correlation between Vγ2Vδ2 T cell count and functional (IPP-responsive) Vγ2Vδ2 T cells (P<0.0001, Fig. 2), indicating that the quantity and quality of Vγ2Vδ2 T cells were damaged simultaneously.
Figure 1. Alteration of Vγ2Vδ2 T cells in the peripheral blood of HIV-1-infected persons.
The percentage of Vγ2Vδ2 T cells among total CD3 T cells and absolute counts of Vγ2Vδ2 T cells in HIV-1-infected persons (n=146) significantly decreased compared with healthy donors (n=42). Statistical comparisons were made using the Mann-Whitney test.
Figure 2. Association of Vγ2Vδ2 T cells and functional Vγ2Vδ2 T cells.
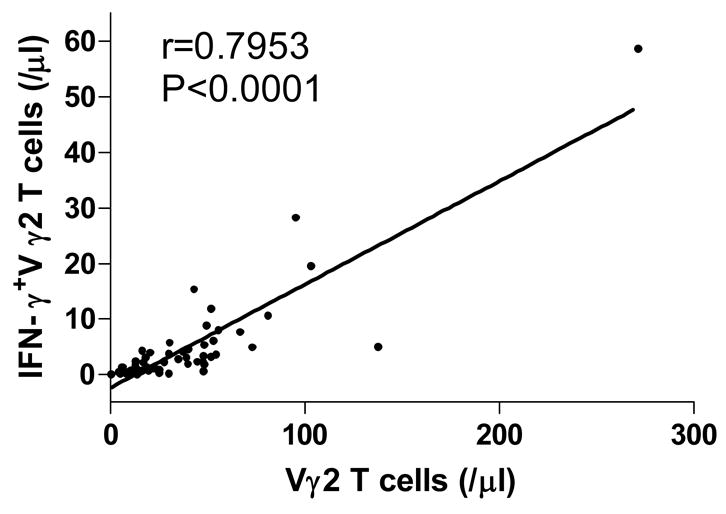
Positive relationship between Vγ2Vδ2 T cells and functional (IPP-responsive) Vγ2Vδ2 T cells; correlation statistics were analyzed using the spearman correlation.
Next, we assessed the relationship between Vγ2Vδ2 T cells, plasma viral load, and CD4 T-cell counts—the latter two being predictors of HIV disease progression. We found a significant positive correlation between CD4 T-cell counts and Vγ2Vδ2 T cells (n=146; P<0.0001; Fig. 3a), and an inverse correlation between viral load and Vγ2Vδ2 T cells (n=146; P<0.05; Fig. 3b). If the HIV+ participants were divided into 4 groups based on CD4+ T-cell counts (cells/μl): CD4<200, 200≤CD4<350, 350≤CD4<500 and CD4≥500, and Vγ2Vδ2 T cells of different subgroups were compared, we found that the subgroups with higher CD4+ T-cell counts also have more Vγ2Vδ2 T cells. The statistical significance is detailed in Figure 3c. The subgroups characteristics, such as the number of patients in each subgroup, CD4+ T-cell counts, CD4/CD8 ratios and HIV-1 plasma viral loads are described in Table 1. We also assessed the Vγ2Vδ2 T cells in healthy controls with stratified CD4+ T cells (Table 2). We did not find correlations between CD4+ T cells and Vγ2Vδ2 T cells in healthy controls.
Figure 3. Vγ2Vδ2 T cells are associated with HIV disease progression.
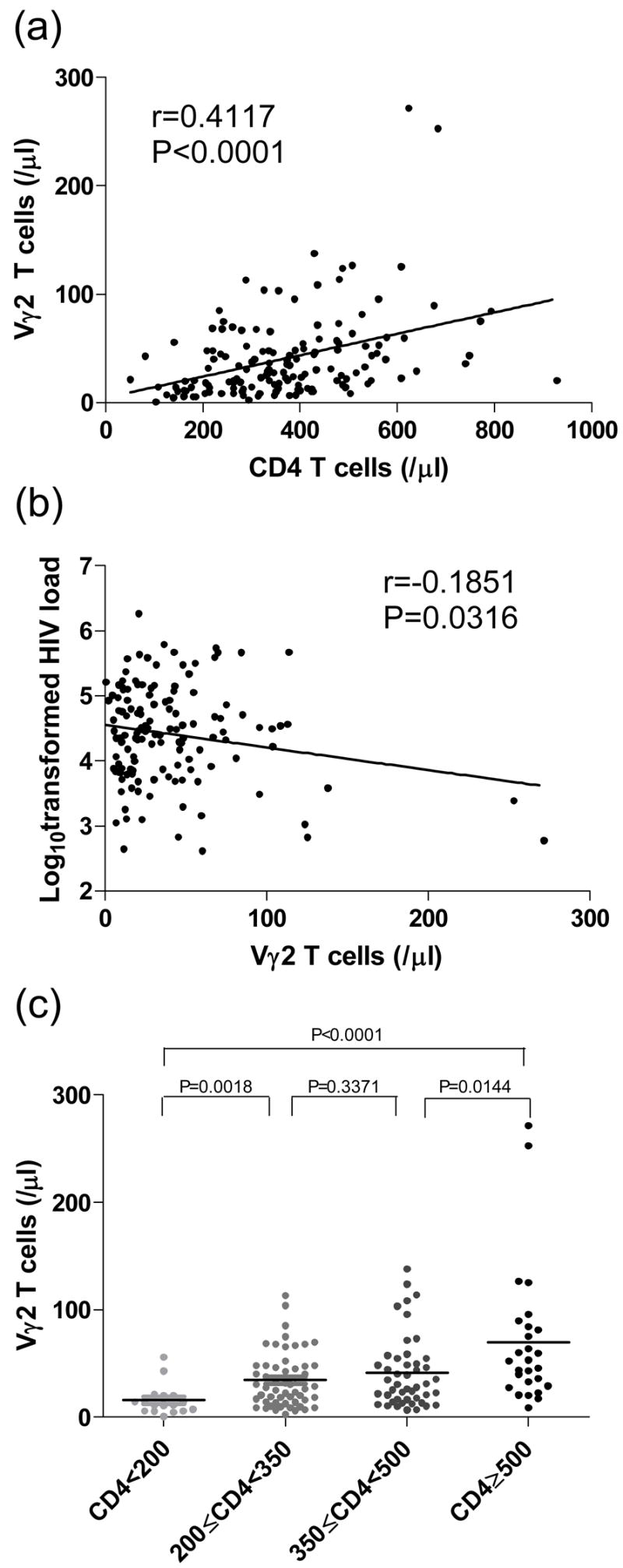
(a) Positive correlation between Vγ2Vδ2 T cells and CD4 T cells; (b) Inverse correlation between Vγ2Vδ2 T cells and viral load (log10); (c) Significant differences of Vγ2Vδ2 T cells between subgroups divided by CD4 T-cell counts. Correlation statistics for (a) and (b) were analyzed using the spearman correlation; statistical comparisons for (c) were made using the Mann-Whitney test.
Table 1.
Laboratory characteristics among subgroups of HIV-1-positive FBDs with stratified CD4 T-cell counts (/μl)
| Characteristics | CD4<200 | 200≤CD4<350 | 350≤CD4<500 | CD4≥500 |
|---|---|---|---|---|
| Cases (%) | 17 (11.6) | 55 (33.7) | 47 (32.2) | 27 (18.5) |
| CD4+ counts (cells/μl) | 141.8±37.8 | 278.7±46.1 | 418.3±44 | 612±105.6 |
| CD4+ percent (%) | 15.3±5.9 | 21.3±5.9 | 26.2±7.3 | 28.5±5.4 |
| CD4+/CD8+ | 0.27±0.12 | 0.43±0.19 | 0.61±0.28 | 0.67±0.23 |
| Viral loads (lg copies/ml) | 4.87±0.55 | 4.51±0.67 | 4.31±0.73 | 4.01±0.9 |
Table 2.
Vγ2 T cells in healthy controls with stratified CD4 T cells (/μl)
| CD4<600 | 600≤CD4<800 | CD4≥800 | |
|---|---|---|---|
| Cases (%) | 16 (38) | 18 (43) | 8 (19) |
| CD4+ counts (cells/μl) | 522.8±60.2 | 657.5±62.4 | 1130.4±147.8 |
| Vγ2+ counts (cells/μl) | 129.9±124.5 | 130.2±129.1 | 126.8±87.4 |
| Vγ2+/CD3+ (%) | 8.6±7.2 | 7.9±6.5 | 5.7±3.6 |
Phosphoantigen stimulation is considered a model for the normal response to pathogen infection, as these compounds are present in mycobacterial cell extracts and are also produced as metabolites of stressed cells [12]. Thus we examined the phosphoantigen (IPP)-responsive Vγ2Vδ2 T cells measured by IFN-γ expression after IPP stimulation, from 66 HIV-infected individuals. We assessed the relationships between IPP-responsive Vγ2Vδ2 T cells, plasma viral load, and CD4 T-cell counts. We found a significant positive correlation between CD4 T-cell counts and IPP-responsive Vγ2Vδ2 T cells (P<0.0001; Fig. 4a), and an inverse correlation between viral load and IPP-responsive Vγ2Vδ2 T cells (P<0.05; Fig. 4b). We also found that functional Vγ2Vδ2 T-cells between different subgroups divided by CD4 T-cell counts as described above are significantly different (P<0.05) (Fig. 4c). The subgroups with higher CD4+ T-cell counts have much more functional Vγ2Vδ2 T cells.
Figure 4. Functional Vγ2Vδ2 T cells are associated with HIV disease progression.
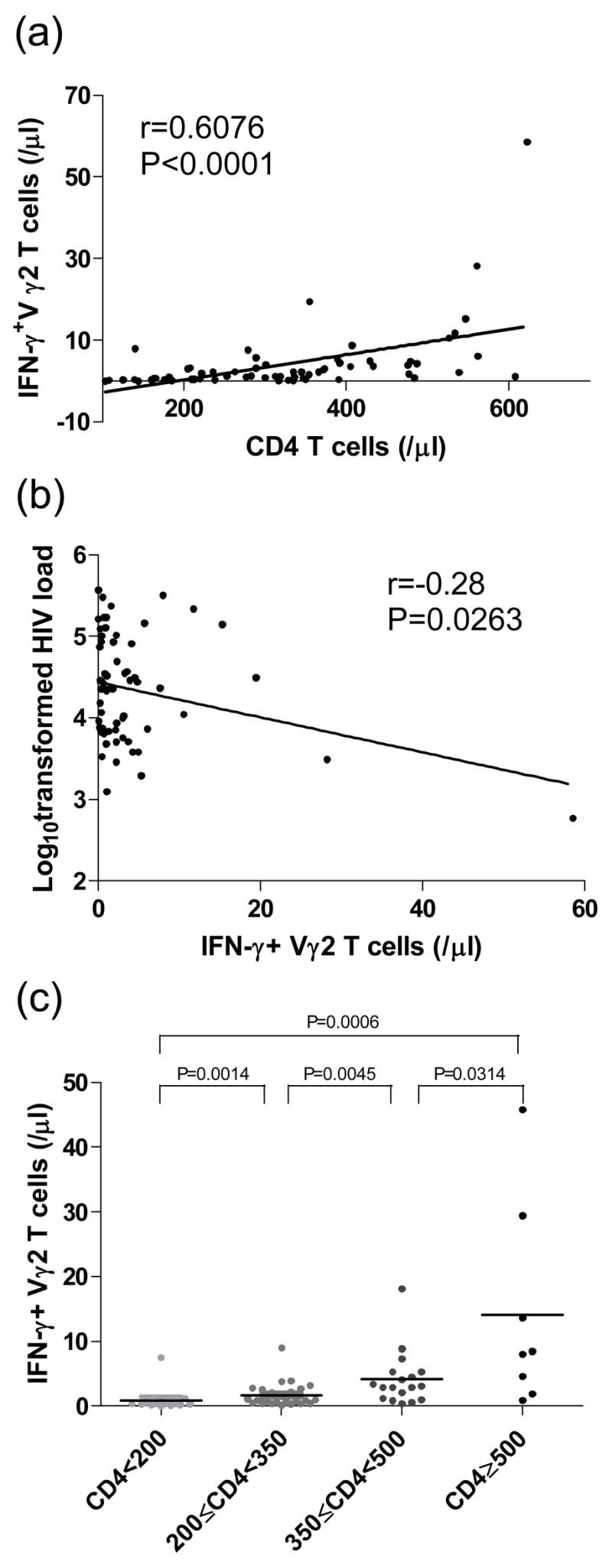
(a) Positive correlation between IPP-responsive functional Vγ2Vδ2 T cells and CD4 T cells; (b) Inverse correlation between IPP-responsive functional Vγ2Vδ2 T cells and viral load (log10); (c) Significant differences of functional Vγ2Vδ2 T cells between subgroups divided by CD4 T-cell counts. Correlation statistics for (a) and (b) were analyzed using the spearman correlation; statistical comparisons for (c) were made using the Mann-Whitney test.
We also analyzed the data to assess the effect of Vγ2Vδ2 T cells on CD4 T cells during HIV disease. We found that CD4 T cells of the group with higher Vγ2Vδ2 T cells (n=38) decreased more slowly than that of the group with lower Vγ2Vδ2 T cell (n=50). And at each time point, the CD4 T cells of the group with higher Vγ2Vδ2 T cell were significantly higher than that of the group with lower Vγ2Vδ2 T cell (P<0.0001; Fig. 5).
Figure 5. The effect of Vγ2Vδ2 T cells on CD4 T cells during HIV disease.
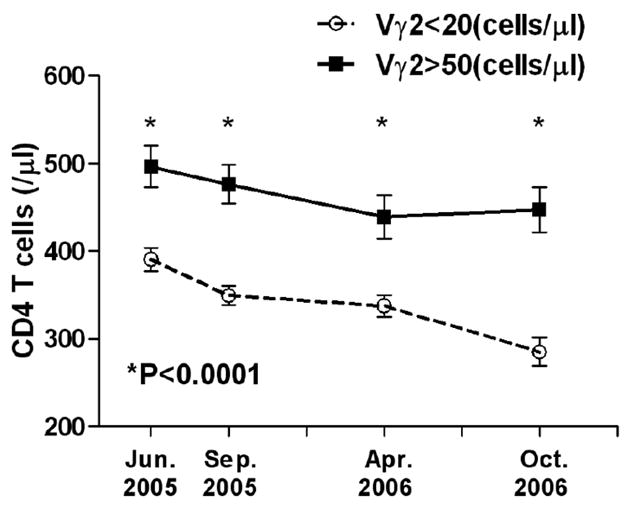
CD4 T cells of the group with higher Vγ2Vδ2 T cells (n=38) decreased more slowly than that of the group with lower Vγ2Vδ2 T cell (n=50). And at each time point, the CD4 T cells of the group with higher Vγ2Vδ2 T cell were significantly higher than that of the group with lower Vγ2Vδ2 T cell. Statistical comparisons were made using the Mann-Whitney test.
Collectively these data indicated that Vγ2Vδ2 T cell count and functionality are strongly associated with HIV infection and might play an important role in controlling disease progression.
Discussion
In the present study, we found strong correlations between Vγ2Vδ2 T cells and disease progression among HIV+ individuals in China, indicating that this T cell subset may be directly involved in the natural resistance to HIV infection. Although the in vivo mechanism of Vγ2Vδ2 T cells in the pathogenesis and progression of HIV infection is unclear at present and requires further investigation, the accumulated knowledge up to date in this area allows us to hypothesize a reasonable scenario to explain the phenomena we observed. Very importantly, this unusual group represents individuals infected at roughly the same time and with very similar virus strains as a result of using contaminated blood drawing equipment. Studies of this unique cohort offer great potential to understand fundamental aspects of HIV pathogenesis.
Data presented here show a clear association between Vγ2Vδ2 T cells, their functional responses to phopshoantigen stimulation and markers of HIV disease progression. This is the first reported study of Vγ2Vδ2 T cells among HIV-infected individuals in China and is the largest single study group analyzed so far. There was a clear association between advancing disease, measured by CD4 T cell count, and Vγ2Vδ2 T cell count and function. The data on healthy Chinese donors agrees well with normal Vγ2Vδ2 T cell values that have been published for European and North American populations. In terms of HIV+ groups, the characteristics of this cross-section of HIV+ individuals is similar to what has been observed for HIV+ patients in the U.S. (Bordon J, et al.). The major difference is that the Chinese cohort allows us to observe a group of people infected at around the same time, with a relatively homogeneous virus strain. Thus, the current studies add to our growing knowledge, but bring an important new dimension to this problem.
These data argue that the Vγ2Vδ2 T cell subset is impacted early in disease, because all HIV+ individuals showed some degree of debilitation. Damage to this subset is also progressive as shown by the declining Vγ2Vδ2 T cell counts and function that are worse with successively lower CD4+ T cell counts. Damage to the Vγ2Vδ2 T cells is related to vial replication, as shown by the correlation between vRNA levels and Vγ2Vδ2 T cell counts or function. However, Vγ2Vδ2 T cell lack the CD4 receptor for virus are generally considered non-permissive for HIV infection. Thus, the extent of damage depends on the degree of virus replication but occurs by an indirect mechanism that does not involve direct HIV infection and replication in Vγ2Vδ2 T cells.
The mechanism for Vγ2Vδ2 T cell depletion remains unknown. A preliminary study in SIV-infected nonhuman primates showed an initial increase in γδT cells (up to 300% of starting levels within a few weeks), followed by the precipitous decline that characterizes persistent SIV or HIV infection [18]. Apparently, virus infection alters cell metabolism or cell surface characteristics that are detected by Vγ2Vδ2 T cells resulting in their stimulation and proliferation. This is consistent with other examples where γδ T cells are among the earliest responders to virus infection. Their ability to secrete cytokines including Interferon-gamma [8], provides for immunity against vaccinia infection in mice, where γδ T cells are the principal mechanism for resistance against fatal disease [19]. We showed recently that vaccinia inhibits human Vγ2Vδ2 T cell responses to phosphoantigens [20], This type of immune evasion mechanism likely indicates that Vγ2Vδ2 T cells are important for vaccinia resistance in human beings as was seen in mice. During HIV infection, evasion from the Vγ2Vδ2 T cell response appears to occur by specific depletion of this cell population, rending the cells non-responsive to phosphoantigen stimulus. Although we cannot yet prove their role in HIV disease, the pattern of depletion, the relationship to disease progression and the fact that depletion is common to all persons with HIV infection support the idea that Vγ2Vδ2 T cells are part of cellular immunity against HIV.
Studies conducted primarily in mice highlighted several ways that γδ T cells participate in viral immunity. The vaccinia example mentioned above showed that early γδ T cell responses with production of interferon-gamma, provided for resistance to fatal disease in mice [20]. In other studies with vaccinia and vesicular stomatitis virus, γδ T cells were important for developing virus neutralizing antibodies. When CD4 T cell were depleted, murine γδ T cells were sufficient for immunoglobulin class switching to produce neutralizing IgG [21]. HIV disease is characterized by a loss of capacity for Type 1 immune responses and a failure to produce high titer neutralizing antibody. The deficit in Vγ2Vδ2 T cells might contribute to both of these important disease mechanisms.
The importance of Vγ2Vδ2 T cells in containing HIV infection and disease progression make it possible to treat AIDS by recovery Vγ2Vδ2 T cells. Long term treatment with highly active antiretroviral therapies (HAART) lead to partial recovery of Vγ2Vδ2 T cell in one study [22] and to little or no increase in a second study that used specimens from the Multicenter AIDS Cohort Study (Hebbeler, et al., submitted). Consequently, studies aimed at stimulating and stabilizing the Vγ2Vδ2 T cell subset might contribute to efforts at reconstituting immunity in persons with HIV but successful control of viremia through HAART. Preliminary studies performed in monkeys suggested a protective effect of activated γδ T cells in SIV infection, substantially improving in vivo both clinical and virological parameters [23].
Acknowledgments
We would like to thank Yanming Wan, Lianxing Liu, Xianggang Huang, Ting Zhu and Chao Wu for their kind technical support, and also to acknowledge all study participants for their contribution. We dedicate this paper to the memory of Fabrizio Poccia, PhD.
Financial support. This project was supported by the CIPRA Program granted by the National Institute of Health (No. 1 U19 AI51915-02) and PHS grants CA113261 and AI51212 (C.D.P.).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vgamma2-Jgamma1.2/Vdelta2 T-cell receptors. Immunology. 2001 Sep;104(1):19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constant P, Davodeau F, Peyrat MA, et al. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science (New York, NY. 1994 Apr 8;264(5156):267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH. gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proceedings of the National Academy of Sciences of the United States of America. 1996 Mar 19;93(6):2272–9. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 5.Poccia F, Gougeon ML, Bonneville M, et al. Innate T-cell immunity to nonpeptidic antigens. Immunology today. 1998 Jun;19(6):253–6. doi: 10.1016/s0167-5699(98)01266-3. [DOI] [PubMed] [Google Scholar]
- 6.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V gamma 9V delta 2 T lymphocytes. J Immunol. 1997 Dec 15;159(12):6009–17. [PubMed] [Google Scholar]
- 7.Battistini L, Borsellino G, Sawicki G, et al. Phenotypic and cytokine analysis of human peripheral blood gamma delta T cells expressing NK cell receptors. J Immunol. 1997 Oct 15;159(8):3723–30. [PubMed] [Google Scholar]
- 8.Lang F, Peyrat MA, Constant P, et al. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995 Jun 1;154(11):5986–94. [PubMed] [Google Scholar]
- 9.Poccia F, Battistini L, Cipriani B, et al. Phosphoantigen-reactive Vgamma9Vdelta2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. The Journal of infectious diseases. 1999 Sep;180(3):858–61. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 10.Tikhonov I, Deetz CO, Paca R, et al. Human Vgamma2Vdelta2 T cells contain cytoplasmic RANTES. International immunology. 2006 Aug;18(8):1243–51. doi: 10.1093/intimm/dxl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thedrez A, Sabourin C, Gertner J, et al. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunological reviews. 2007 Feb;215:123–35. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZW, Letvin NL. Vgamma2Vdelta2+ T cells and anti-microbial immune responses. Microbes and infection/Institut Pasteur. 2003 May;5(6):491–8. doi: 10.1016/s1286-4579(03)00074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace M, Bartz SR, Chang WL, Mackenzie DA, Pauza CD, Malkovsky M. Gamma delta T lymphocyte responses to HIV. Clinical and experimental immunology. 1996 Feb;103(2):177–84. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autran B, Triebel F, Katlama C, Rozenbaum W, Hercend T, Debre P. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clinical and experimental immunology. 1989 Feb;75(2):206–10. [PMC free article] [PubMed] [Google Scholar]
- 15.Poccia F, Boullier S, Lecoeur H, et al. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996 Jul 1;157(1):449–61. [PubMed] [Google Scholar]
- 16.Wallace M, Scharko AM, Pauza CD, et al. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Molecular medicine (Cambridge, Mass. 1997 Jan;3(1):60–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Xu JQ, Wang JJ, Han LF, et al. Epidemiology, clinical and laboratory characteristics of currently alive HIV-1 infected former blood donors naive to antiretroviral therapy in Anhui Province, China. Chinese medical journal. 2006 Dec 5;119(23):1941–8. [PubMed] [Google Scholar]
- 18.Gan YH, Pauza CD, Malkovsky M. Gamma delta T cells in rhesus monkeys and their response to simian immunodeficiency virus (SIV) infection. Clinical and experimental immunology. 1995 Nov;102(2):251–5. doi: 10.1111/j.1365-2249.1995.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001 Jun 1;166(11):6784–94. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Deetz CO, Zapata JC, et al. Vaccinia virus inhibits T cell receptor-dependent responses by human gammadelta T cells. The Journal of infectious diseases. 2007 Jan 1;195(1):37–45. doi: 10.1086/509823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proceedings of the National Academy of Sciences of the United States of America. 1998 Feb 3;95(3):1160–5. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordon J, Evans PS, Propp N, Davis CE, Jr, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the V gamma 2 T cell receptor repertoire. The Journal of infectious diseases. 2004 Apr 15;189(8):1482–6. doi: 10.1086/382961. [DOI] [PubMed] [Google Scholar]
- 23.Gougeon ML, Malkovsky M, Casetti R, Agrati C, Poccia F. Innate T cell immunity to HIV-infection. Immunotherapy with phosphocarbohydrates, a novel strategy of immune intervention? Vaccine. 2002 May 6;20(15):1938–41. doi: 10.1016/s0264-410x(02)00070-1. [DOI] [PubMed] [Google Scholar]