Abstract
The factors that mediate the increases in salt sensitivity of blood pressure with age remain to be clarified. The present study investigated (i) the effects of high NaCl intake on two Na pump inhibitors, endogenous ouabain (EO) and marinobufagenin (MBG) in middle-aged and older normotensive Caucasian women; and (ii) whether individual differences in EO and MBG are linked to variations in sodium excretion or salt sensitivity. A change from six days of a lower (0.7 mmol/kg/day) to six days of a higher (4 mmol/kg/day) NaCl diet elicited a sustained increase in MBG excretion that directly correlated with an increase in the fractional Na excretion, and was inversely related to age, and to an age-dependent increase in salt sensitivity. In contrast, EO excretion increased only transiently in response to NaCl loading, and did not vary with age, or correlate with fractional Na excretion or salt sensitivity. A positive correlation of both plasma and urine levels of EO and MBG during salt loading may indicate a casual link between two Na pump inhibitors in response to NaCl loading, as observed in animal models. A linear mixed-effects model demonstrated that age, dietary NaCl, renal MBG excretion, and body mass index were each independently associated with systolic blood pressure. Thus, a sustained increase in MBG in response to acutely elevated dietary NaCl is inversely linked to salt sensitivity in normotensive middle-aged and older women; and a relative failure of MBG elaboration by these older persons may be involved in the increased salt sensitivity with advancing age.
Keywords: NaCl-sensitivity of blood pressure, endogenous Na pump inhibitors, marinobufagenin, ouabain
Introduction
Numerous studies have reported that the magnitude of the systolic blood pressure (SBP) response to acute changes in dietary NaCl intake, i.e., salt sensitivity of blood pressure, increases with advancing age (16, 20, 27, 32-35). Specific determinants of the greater blood pressure response of older persons to dietary NaCl, however, remain to be identified.
NaCl ingestion results in an increase in plasma volume and natriuresis. It has been postulated for some time that endogenous substances (sodium pump inhibitors, SPI) are stimulated by increased Na intake, and increase natriuresis by inhibiting renal tubular Na pumps to prevent renal reabsorption of filtered Na (6, 18, 30). However, such substances were not purified adequately in early studies, and their assays were non-specific (17).
More recently, SPI assays have improved and become more specific (3, 14), and studies in various animal models and in humans have documented an increased elaboration of SPI in response to NaCl-loading. This research has focused largely on two SPI: an endogenous ouabain (EO) and a bufadienoide, marinobufagenin (MBG). Differences in the kinetics and tissue actions of these substances in response to NaCl loading have been demonstrated in animal models and in human salt sensitive hypertensive response (4, 7, 10, 19, 22, 28).
That age-associated differences in circulating endogenous Na pump inhibitors may be implicated in the age-associated increase in SBP and increased NaCl sensitivity of SBP in older humans has been suggested previously (21), but never tested. The goal of the present study was to investigate effects of a sub-acute change in dietary NaCl on urinary and plasma EO and MBG in middle-aged and older normotensive subjects, and to determine whether NaCl-induced individual differences in the levels of these substances are linked to variations in renal sodium excretion or salt sensitivity of SBP.
Methods
Subjects
Inclusion and exclusion criteria
MBG and EO were measured in a series of healthy Caucasian women, ages 40 - 70 years, who, as part of another study, were salt-restricted, then salt-loaded, to determine interactions among breathing patterns, pCO2, and arterial pressure (2). Determination of subject eligibility involved telephone interview, physical examination, and informed consent. Inclusion and exclusion criteria also include the following: all qualified candidates were normotensive (resting systolic blood pressure < 139 mmHg and resting diastolic blood pressure < 89 mmHg), had no history of respiratory, cardiovascular, liver or kidney disease, or diabetes, were free of cardiac organ damage, determined by electrocardiogram, and were free of kidney dysfunction as determined by plasma creatinine > 1.5. Subjects treated with estrogens or non-steroidal anti-inflammatory agents, or medications affecting sodium, potassium, calcium, water, hemodynamic or neural regulation (including diuretics, steroids, major tranquilizers, narcotics, or benzodiazepines) were also excluded, as were smokers or those with body mass index <19 or >30. The protocol of the study was approved by the Medstar Research Institute Institutional Review Board, and all study subjects signed informed consents.
Experimental design
The experiment consisted of a 12-day, outpatient, dietary intervention, including six days on a low sodium (0.7 mmol/kg/day), low potassium (0.7 mmol/kg/day) diet, followed immediately by six days on a high sodium (4 mmol/kg/day), low potassium (0.7 mmol/kg/day) diet. The average recommended dietary sodium intake in the American diet for an average-sized adult is about 1.4 mmol/kg/day (2,300 mg/day), whereas the average sodium intake in the United States is approximately 2.1 mmol/kg/day (3,500 mg/day) (27).
The high NaCl diet included supplementation with enteric-coated NaCl capsules (Slo-Sodium, CIBA-Geigy), ingested with each meal and at bedtime. Dietary intake of calories, protein, fat, and percentage of calories from fat was standardized per body weight. Meals were provided in order to facilitate compliance with the dietary regimen. Participants were instructed to eat all of the food provided to them and nothing else, and kept a food diary. Water was freely available but participants were limited to one cup of a caffeinated beverage per day. Body weight was recorded at each session. Participants were instructed to maintain their normal daily activities during the 12-day period.
Blood pressure and heart rate were monitored during 25-min seated rest in the clinic setting immediately before and after each six-day sodium diet condition. Blood pressure was recorded every six min during these sessions from an inflatable cuff attached to an automated oscillometric device (Spacelabs, Model 90207, Redmond, WA). Venous blood was drawn following the low and high sodium diets. Plasma aliquots were frozen at -80°C. Hematocrit, plasma sodium, potassium, calcium, magnesium, and creatinine were quantitated.
24-hour urine samples were obtained for each subject during the last day on both diets. In addition, in a preliminary experiment with a group of 8 subjects, 24-hour urine samples were collected on a daily basis during the high NaCl diet. Urine volume was determined, and aliquots frozen at -80°C. Sodium, potassium, and creatinine were quantitated.
Assays
Urinary and plasma SPI were measured using competitive fluoroimmunoassay, as reported previously in detail (10).
Calculations
Creatinine clearance (CrC), SPI clearance, Na filtered, and Fractional excretion of sodium (FENa) were calculated using the following equations:
CrC = uCr×uVol×1.73/(pCr×T×BSA), where uCr is urine creatinine concentration (mg/ml), uVol is urine volume (ml), pCr is plasma creatinine concentration (mg/ml), T is time (min). BSA (body surface area) = W0.425×H0.725×0.007184, where W is weight (kg), H is height (cm). BSA is expressed as m2. CrC is expressed as ml/min/1.73m2.
Clearances of MBG and EO were calculated with the same equation as creatinine clearance.
Na filtered = pNa×CrC, where pNa is plasma sodium concentration (mmol/ml), CrC is creatinine clearance (ml/min). Na filtered is expressed as mmol/min.
FENa = uNa×pCr×100/(pNa×uCr), where uNa and pNa are urine and plasma sodium concentrations (mmol/L), uCr and pCr are urine and plasma creatinine concentrations (mg/ml). FENa is expressed as %.
Statistical Methods
Data are presented as mean ± SEM. The effect of dietary Na intake on measured variables was determined by a paired t-test. Pearson's correlation coefficient was used to assess the significance of the associations between pairs of measured variables. Least squares linear regression analysis was used to obtain the best fitting line. A linear mixed effects model (24) was employed to describe the repeated measures data and to determine independent predictors of SBP and MBG. Mixed-effects models provide a flexible way of modeling repeated-measures data. The model contains both fixed- and random-effects. The fixed-effects provide population-average regression coefficients that describe the mean effect of the explanatory variables on the response variable. Random-effects allow each subject to deviate from the average. These random effects impose a correlation structure that take into account the association among the repeated observations within each subject. A backward elimination procedure was used to remove statistically non-significant variables until only statistically significant variables remained in the model.
Results
Thirty-six subjects met the initial screening criteria, and 33 completed the study. Data from 5 subjects were excluded from the analysis on the basis of incomplete 24-hour urine collection, resulting in a final total of 28 subjects. Mean age was 53 ± 1.6 years, mean height: 163.4 ± 1.3 cm, and mean BMI: 25.2 ± 0.6.
Table 1 lists mean values of measured parameters following six days of dietary NaCl restriction and six days of NaCl loading for the entire sample. After six days on high sodium diet, systolic pressure, pulse pressure, and body weight were higher, diastolic blood pressure was unchanged, and heart rate was lower, than on low salt diet. High dietary NaCl resulted in 35% increase in urine MBG, six fold increase in 24-hr urinary Na and fractional excretion of sodium (FENa), and 24% increase in urine volume. Plasma MBG and MBG clearance were both increased by 26%, while plasma EO and EO clearance were not different from low salt levels. The relatively low creatinine clearance in this study may be attributed, at least in part, to the age and sex of the subjects It is known that the creatinine clearance decreases with age, and is lower in women than in men (26). An “ideal” creatinine clearance calculation for our population, based on the formula from the study by Rowe et al. (26) using a “super-clean” population, is 91.7 ± 0.9 ml/min/1.73m2 only slightly higher than in our study (Table 1). Both plasma and urine MBG, and plasma and urine EO on a low sodium intake were correlated with each other (r = 0.67, p = 0.0001; r = 0.53, p = 0.008, respectively). In response to high NaCl intake (after 6 days), plasma MBG levels, urinary MBG levels, and MBG clearance increased compared to the low NaCl diet (Table 1). In contrast, neither plasma levels, nor the urine levels of EO, nor the clearance of EO, differed between low and high NaCl diets (Table 1); and there was no correlation between change in FENa and the urinary EO change (Table 2).
Table 1. Effect of dietary Na on measured variables.
| Low NaCl
Diet (LS) |
High NaCl
Diet (HS) |
Difference
HS-LS |
P* | |
|---|---|---|---|---|
| Body weight, kg | 67.6±1.7 | 68.3±1.8 | 0.68±0.26 | 0.013 |
| Systolic BP, mmHg | 116.9±1.9 | 121.4±2.5 | 4.48±1.25 | 0.001 |
| Mean BP, mmHg | 88.1±1.2 | 89.4±1.6 | 1.30±1.04 | NS |
| Diastolic BP, mmHg | 72.9±1.0 | 73.6±1.2 | 0.74±1.19 | NS |
| Pulse pressure, mmHg | 44.0±1.7 | 47.8±1.9 | 3.74±0.70 | <0.0001 |
| Heart rate, bpm | 69.7±1.4 | 66.0±1.3 | -3.72±0.77 | <0.0001 |
| Plasma Na, mmol/L | 140.9±0.5 | 143.2±0.4 | 2.29±0.33 | <0.0001 |
| Plasma K, mmol/L | 4.40±0.08 | 4.18±0.07 | -0.22±0.07 | 0.007 |
| Plasma Ca, mmol/L | 2.34±0.02 | 2.29±0.02 | -0.05±0.02 | 0.013 |
| Plasma Mg, mmol/L | 0.86±0.01 | 0.81±0.01 | -0.05±0.01 | <0.0001 |
| Hematocrit | 0.40±0.01 | 0.39±0.01 | -0.01±0.004 | 0.003 |
| Urine volume, ml/kg/24hr | 30.0±2.3 | 37.8±2.3 | 7.82±2.42 | 0.003 |
| Creatinine clearance,
ml/min/1.73m2 |
82.3±3.7 | 81.2±3.5 | -1.08±3.42 | NS |
| Urine Na, mEq/kg/24h | 0.55±0.06 | 3.48±0.17 | 2.92±0.19 | <0.0001 |
| Na filtered, mmol/min | 11.58±0.51 | 11.71±0.48 | 0.13±0.57 | NS |
| Fractional Na excretion, % | 0.23±0.03 | 1.42±0.07 | 1.20±0.07 | <0.0001 |
| Renal MBG excretion,
pmol/kg/24 hr |
27.0±2.4 | 35.9±2.6 | 8.86±2.35 | 0.001 |
| Renal EO excretion,
pmol/kg/24 hr |
8.9±0.9 | 9.4±1.5 | 0.76±1.19 | NS |
| Plasma MBG, nmol/L | 0.34±0.02 | 0.43±0.02 | 0.09±0.03 | 0.005 |
| Plasma EO, nmol/L | 0.19±0.03 | 0.17±0.03 | -0.02±0.02 | NS |
| MBG clearance,
ml/min/1.73m2 |
3.51±0.24 | 4.43±0.35 | 0.89±0.37 | 0.028 |
| EO clearance,
ml/min/1.73m2 |
2.96±0.35 | 3.90±0.73 | 1.16±0.63 | NS |
Data are means ± SE (n = 28). BP, blood pressure; MBG, marinobufagenin; EO, endogenous ouabain. P* - paired t-test (n = 28).
Table 2. Correlation between 24-hour urine levels of EO and other parameters measured.
| Total 24-hour urine EO | |||
|---|---|---|---|
| Low NaCl (LS) | High NaCl (HS) | Δ LS to HS | |
| R | R | R | |
| Age | -0.24 | -0.28 | -0.17 |
| Body weight | -0.42 * | -0.21 | 0.29 |
| BMI | -0.23 | -0.18 | -0.05 |
| SBP | -0.09 | -0.29 | -0.20 |
| MBP | 0.001 | -0.33 | -0.08 |
| DBP | 0.11 | -0.34 | -0.10 |
| PP | -0.17 | -0.14 | 0.14 |
| Urine Volume | 0.31 | 0.03 | -0.07 |
| Urine Na | 0.13 | 0.25 | 0.01 |
| Urine MBG | 0.54 ** | 0.52 ** | 0.11 |
| Plasma Na | -0.09 | 0.14 | 0.15 |
| Plasma MBG | 0.52 ** | 0.05 | -0.07 |
| Plasma EO | 0.44 * | 0.19 | -0.11 |
| Creatinine clearance | 0.30 | 0.25 | 0.08 |
| Na clearance | 0.04 | 0.22 | -0.01 |
| Na filtered | 0.29 | 0.24 | 0.08 |
| FENa | 0.02 | -0.09 | -0.01 |
Data are Pearson correlation coefficient (R); EO, endogenous ouabain; MBG, endogenous marinobufagenin; BMI, body mass index; SBP, DBP and MBP - systolic, diastolic, and mean blood pressure; PP, pulse pressure; FENa, fractional sodium excretion;
- P < 0.05;
- P < 0.01.
To detect day-to-day changes in SPI in humans in response to the NaCl loading protocol, we measured EO and MBG production daily following the increase in dietary NaCl in 8 subjects. Figure 1A shows that EO increased transiently, and then returned toward baseline four days after the initiation of the high NaCl diet. In contrast, MBG increased progressively, and its levels remained elevated six days after the initiation of NaCl loading (Fig. 1B).
Fig. 1. Renal excretion of sodium pump inhibitors.
Renal endogenous ouabain (EO, open triangles, A) and marinobufagenin (MBG, closed circles, B) in a subset of 8 subjects during 6 days on a high NaCl diet. Data are means ± SE. * = p<0.05 vs. day 0; mixed-effects analysis followed by the Bonferroni adjusted p-value test.
While neither the renal glomerular filtration rate, (i.e. 24 hr creatinine clearance), nor filtered Na were affected by dietary NaCl (Table 1), the total Na clearance at a given creatinine clearance markedly increased during high NaCl diet (Fig. 2A). Neither total Na clearance on the high Na diet, nor the change in Na clearance from the low-to-high Na diet correlated with the change in filtered Na. However, on the high NaCl diet, the increase in FENa, i.e., total urine Na/Na filtered, and the change in FENa elicited by the high NaCl diet, were both highly correlated with the change in total urinary Na (Fig. 2B). Thus, the large increase in urinary Na excretion on high Na diet was mediated by a mechanism distal to the glomerulus.
Fig. 2.
Correlation of creatinine clearance and Na clearance on a low NaCl (LS) and high NaCl (HS) diets (A); correlation of change in fractional excretion of Na and change in urine Na from LS to HS diet (B).
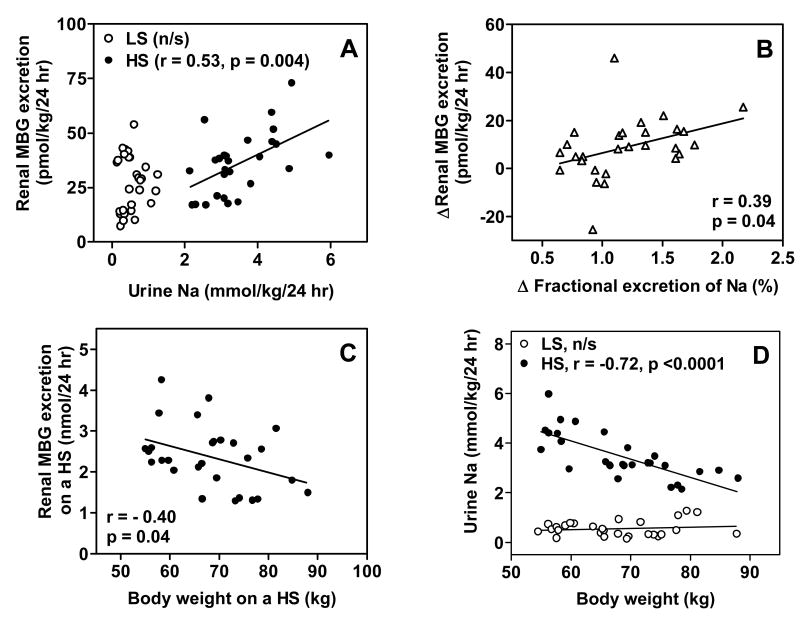
One renal tubular mechanism that affects the marked increase in the FENa during the high NaCl diet (Fig. 2B) is a NaCl-induced elaboration of MBG (Table 1), which predominantly inhibits the renal alpha-1 Na pump isoform (8). The relationship of total urinary Na as a function of total urinary MBG on low and high Na intake is illustrated in Figure 3A. During high NaCl diet, urinary Na was directly related to urinary MBG, but no relationship between the two was present during NaCl restriction. Figure 3B shows that the change in FENa among individuals in response to high NaCl diet was significantly correlated with the change in MBG. As with the change in urine Na in response to high NaCl diet, renal MBG excretion on the high salt diet was inversely related to body weight (Fig. 3C and 3D).
Fig. 3.
Correlations of urinary Na and marinobufagenin (MBG) excretion on a low NaCl (LS) and high NaCl (HS) diets (A); correlation of change in fractional excretion of Na and change in urinary MBG excretion from LS to HS diet (B); correlation of body weight and MBG excretion on a HS diet (C); correlation of body weight and urine Na on a both diets (D).
Next, we determined the association of NaCl loading with SBP, and whether a sodium-induced change in SBP was related to the change in MBG. Figure 4A shows that SBP was inversely related to urinary Na, but only on the high NaCl diet. Figure 4B shows that on high NaCl diet, the increased MBG was inversely related to SBP.
Fig. 4.
Correlations of systolic blood pressure (SBP) and urinary Na on a low NaCl (LS) and high NaCl (HS) diets (A); correlation of urinary marinobufagenin (MBG) excretion and SBP on HS diet (B).
The interaction between SPI, age, and salt sensitivity of SBP is illustrated in Figure 5. Figure 5A shows the NaCl-induced change in SBP as a function of age. Note that the response of SBP to NaCl loading varied among subjects: in most, it increased; in some, decreased, and, still in others, did not change. This highly variable response to an increase in dietary NaCl is a pattern common in prior studies (32). More importantly, Figure 5A shows that the change in SBP to NaCl loading varied directly with age, as also previously demonstrated (32).
Fig. 5.
Correlation of age and changes in systolic blood pressure (SBP) from low to high NaCl diets (A); correlation of age and renal marinobufagenin (MBG) excretion on a high NaCl (HS) diet (B).
That salt sensitivity of SBP varied directly with age (Fig. 5A), and that salt sensitivity of SBP varied inversely with MBG on the high NaCl diet (Fig. 4B), suggests that MBG production during NaCl loading might vary inversely with age. Figure 5B shows that urinary MBG on the high NaCl diet, indeed, was inversely related to age. Neither renal MBG clearance, nor renal EO clearance declined with age. Interestingly, the change in urine volume induced by the change in dietary NaCl tended to vary inversely with age (r = -0.36, p > 0.06).
In contrast to the relationship of MBG to urine Na, FENa, SBP, age (Fig. 3, 4, and 5B), EO was not related to any of these variables (Table 2).
Independent Determinants of SBP
The bivariate correlations illustrated in Figures 4 and 5 suggest that SBP is a function of dietary NaCl, age, and the natriuretic Na pump inhibitor, MBG. We employed a linear mixed-effects model to determine the independent impact of a number of measured variables on SBP. The initial model contained the following main effects: NaCl intake (High vs. Low), age, BMI (body mass index), BMI2, FENa, and Urine MBG, as well as a number of interactions of these terms. The final model is shown in Table 3a. The high correlation of the observed and fitted values indicates that the model provided a good fit to the data. In the final model, NaCl intake (Salt), age, BMI and uMBG were significantly and independently correlated with SBP. Two significant interactions remained in the model: FENa×uMBG, and BMI×age. While the main effect of FENa was not statistically significant, it was retained in the model since it had a statistically significant interaction (Table 3a) (24). After adjusting for other terms in the model, SBP was, on average, 8.6 mmHg higher at high salt than at low salt. The coefficient of the BMI×age interaction indicates that the effect of BMI on SBP was linked to age, and that as age increased the effect of BMI on SBP decreased. Finally, the coefficient of the FENa×uMBG interaction indicates that the effect of uMBG on SBP was linked to its effect on FENa.
Table 3.
| Table 3a. Final linear mixed-effects model of systolic blood pressure.
| ||
|---|---|---|
| Variable | Parameter Estimate | P |
| Intercept | -118.04 | |
| Age | 4.43 | 0.013 |
| Salt | 8.63 | 0.027 |
| BMI | 7.20 | 0.038 |
| FENa | 4.70 | 0.211 |
| uMBG | 0.004 | 0.030 |
| FENa×uMBG | -0.004 | 0.006 |
| BMI×Age | -0.14 | 0.035 |
|
| ||
| Variance (Error) | 18.38 | |
| Variance (Subjects) | 52.04 | |
| Corr (y, ŷ)* | 0.96 | |
| Table 3b. Final linear mixed-effects model of urine marinobufagenin (adjusted for body weight).
| ||
| Variable | Parameter Estimate | P |
|
| ||
| Intercept | 61.07 | |
| Salt (High) | 7.81 | 0.005 |
| Age | -0.76 | <0.0001 |
| uEO/Wt | 0.84 | 0.001 |
|
| ||
| Variance (Error) | 85.06 | |
| Variance (Subjects) | 3.76 | |
| Corr (y, ŷ)* | 0.75 | |
BMI, body mass index; FENa, fractional excretion of Na; uMBG, urine marinobufagenin.
ŷ represents the y values predicted from the Mixed-Effects model.
Wt, body weight; uEO, urine ouabain.
ŷ represents the y values predicted from the Mixed-Effects model.
Independent Determinants of Urine MBG
A second mixed-effects model for Urine MBG/Weight (Table 3b) contains main effects for NaCl intake, age, and Urine EO/Weight, and no interaction terms. The model showed that, after accounting for age and Urine EO/Weight, the indexed Urine MBG averaged 7.8 pmol/kg/24 hr higher at high, than at low, salt. After accounting for salt level and Urine EO/Weight, the indexed Urine MBG decreased 0.77 pmol/kg/24 hr per year of age. Interestingly, after accounting for age and salt level, the indexed Urine MBG increased by 0.84 pmol/kg/24 hr with each unit of indexed Urine EO.
Discussion
The present results are the first to demonstrate in normotensive humans that following a change from a lower to a higher NaCl diet, a sustained increase in MBG production occurs, and renal fractional NaCl excretion increases, and correlates directly with increased MBG excretion. In addition, this study found that the salt-induced increase in MBG was inversely related to age, and that the NaCl sensitivity of SBP was directly related to age, and inversely related to the NaCl-induced increase in MBG. A linear mixed effect model showed that, after accounting for age, dietary Na was an independent determinant of MBG, and that after accounting for the dietary NaCl, urine MBG declined with age.
In contrast to the sustained increase in MBG on high NaCl diet, EO levels in the present study increased only transiently (Fig. 1). This finding is consistent with a previous study with normotensive men, which showed that high NaCl intake increased plasma EO for three days followed by a decrease towards baseline on the day 5 (22). We have reported a similar pattern of MBG and EO response in experimental studies following acute and chronic NaCl loading of Dahl-S rats (10). Using selective in vivo immunoneutralization of EO and MBG with specific antibodies and blockade of AT1 receptors, we found that following NaCl loading of Dahl-S, a peak response of EO, a neurohormone, via activation of renin-angiotensin system stimulated adrenocortical production of MBG, a natriuretic and a vasoconstrictor (11). Although in the present study, EO levels after six days of salt loading did not correlate with urinary Na, SBP or age, in accord with our previous experimental findings, in the present study, plasma EO were positively correlated with plasma MBG levels after salt loading. Also the renal excretion of MBG and EO were positively correlated at high NaCl diet (Table 2). Thus, in NaCl-loaded human subjects, a causal link may exist between EO and MBG production. Additional studies of the relationship between SPI in salt-loaded humans are warranted.
SPI elaboration in response to high dietary NaCl ingestion can result in divergent effects on arterial pressure: natriuretic effects, due to direct action on the kidney to increase NaCl and H2O excretion, tending to reduce arterial pressure; and increases in vascular smooth muscle tone to increase arterial pressure (6, 18). Na pump inhibition in vascular smooth muscle leads to an increase in cell Ca2+ via enhanced influx or reduced efflux via the Na-Ca exchanger (5, 6). The net effect of SPI actions, i.e., natriuresis vs. vasoconstriction, on blood pressure, however, depends upon the level of sustained concentrations that are achieved following NaCl ingestion, the response (potency, and sensitivity) of the renal and arterial Na pumps to these endogenous SPI, and the levels, potency, and sensitivity of other natriuretic and vasoactive substances that respond to NaCl ingestion.
The vasoactive effects of SPI have been repeatedly documented with respect to NaCl-dependence of BP (6, 18, 30). Chronic NaCl loading in salt-sensitive rodent models with abnormal renal function results in sustained, elevated levels of MBG that mediate sustained, NaCl-dependent increases in arterial pressure (10). Specific antibodies against MBG prevent both the acute and sustained NaCl-induced increase in arterial pressure (10). A pro-hypertensive effect of SPI in response to salt loading in hypertensive prone rats has been repeatedly observed (9, 10, 12, 13, 29). Our results show that a sub-acute increase in dietary NaCl elicits a sustained increase in MBG that is directly related to an increase in renal FENa and inversely related to NaCl sensitivity of SBP in population of normotensive middle-age and older women. Lower MBG levels and a greater SBP salt sensitivity in the subjects in the present study, as well as high NaCl diet, age and MBG were independently associated with SBP level. Thus, the net response to an increase in MBG induced by six days of dietary NaCl in healthy, normotensive women is natriuretic. It should be noted that most of the participants in the present study were postmenopausal women. Menopause is associated with an increased salt-sensitivity of blood pressure, and with a reduction in the levels of another steroid hormone with natriuretic action, i.e., progesterone (25). The impact of postmenopausal hormonal status on MBG production merits further study. Moreover, subjects in the present study were normotensive, and our findings do not necessarily reflect salt sensitivity or MBG production in hypertensive patients.
In accordance with the concept of a natriuretic hormone (30, 31), we can speculate that lower MBG levels during the first few days of NaCl loading in some subjects can add to the deficit (acquired or inherited) in sodium excretion, with commensurate increases in total blood volume, and, as a result, in greater elevation in BP. Furthermore, a lack of MBG production following NaCl loading could result in a relatively high renotubular sodium pump activity. Indeed, activation of renal Na/K-ATPase was shown to contribute to hypertension in Milan hypertensive rats, and was attributed to mutation of the alpha adducin gene (15). A polymorphism of the adducin gene has been detected in 20% of human hypertensive population (23). Whether or not these individuals exhibit reduced MBG production remains to be investigated.
Perspectives
A relative failure to increase natriuretic MBG levels in response to NaCl-loading with increasing age could be a factor involved in the increase in NaCl sensitivity of SBP with aging. A similar pattern has been described in a previous observational study showing, that MBG excretion of more salt-sensitive African American participants was lower than that of less salt-sensitive non-African American subjects (1). The response to salt loading is complex, however, and includes the stimulation of other vasoactive and natriuretic substances, for example, ANGII, ANP, endothelin, and vasopressin. To evaluate the specific role of MBG in the context of these other physiological factors in regulation of natriuresis and BP additional studies are required.
Acknowledgments
We thank Beverly A. Parsons for her expert assistance with recruitment and care of the study subjects, and Alexandra Newman, Danielle Joseph and Chad Boily for outstanding technical support.
Sources of Funding
This study was supported by the Intramural Research Program of the National Institute on Aging, National institutes of Health.
References
- 1.Anderson DE, Scuteri A, Agalakova N, Parsons DJ, Bagrov AY. Racial differences in resting end-tidal CO2 and circulating sodium pump inhibitor. Am J Hypertens. 2001;14(8):761–767. doi: 10.1016/s0895-7061(01)02163-x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DE, Parsons BA, McNeely JD, Miller ER. Resting respiratory rate predicts salt sensitivity: results of a clinical feeding trial in women. J Amer Soc Hypertens. 2007;1(4):256–263. doi: 10.1016/j.jash.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagrov AY, Fedorova OV, Austin-Lane JL, Dmitrieva RI, Anderson DE. Endogenous marinobufagenin-like immunoreactive factor and Na,K ATPase inhibition during voluntary hypoventilation. Hypertension. 1995;26:781–788. doi: 10.1161/01.hyp.26.5.781. [DOI] [PubMed] [Google Scholar]
- 4.Bagrov AY, Fedorova OV. Cardenolide and bufadinenolide ligands of the sodium pump. How they work together in NaCl sensitive hypertension. Front Biosci. 2005;10:2250–2256. doi: 10.2741/1694. [DOI] [PubMed] [Google Scholar]
- 5.Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977;232:C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 7.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102(24):3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 8.Fedorova OV, Kolodkin NI, Agalakova NI, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension. 2001;37:462–466. doi: 10.1161/01.hyp.37.2.462. [DOI] [PubMed] [Google Scholar]
- 9.Fedorova OV, Dorofeeva NA, Lopatin DA, Lakatta EG, Bagrov AY. Phorbol diacetate potentiates Na(+)-K(+) ATPase inhibition by a putative endogenous ligand, marinobufagenin. Hypertension. 2002;39:298–302. doi: 10.1161/hy0202.104344. [DOI] [PubMed] [Google Scholar]
- 10.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation. 2002;105(9):1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 11.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signaling in NaCl-loaded Dahl-S rats. J Hypertens. 2005;23(8):1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. [DOI] [PubMed] [Google Scholar]
- 12.Fedorova OV, Kolodkin NI, Agalakova NI, Namikas AR, Bzhelyansky A, Louis J, Lakatta EG, Bagrov AY. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J Hypertens. 2005;23:835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 13.Fedorova OV, Agalakova NI, Lakatta EG, Bagrov AY. ANP differentially modulates matinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension. 2006;48(6):1160–1168. doi: 10.1161/01.HYP.0000248129.20524.d0. [DOI] [PubMed] [Google Scholar]
- 14.Ferrandi M, Manunta P, Balzan S, Hamlyn JM, Bianchi G, Ferrari P. Ouabain-like factor quantification in mammalian tissues and plasma: comparison of two independent assays. Hypertension. 1997;30:886–896. doi: 10.1161/01.hyp.30.4.886. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na +-K+- ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 2006;290:529–535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 16.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 17.Goto A, Yamada K, Yagi N, Yoshioka M, Sugimoto T. Physiology and pharmacology of endogenous digitalis-like factors. Pharmacol Rev. 1992;44(3):377–399. [PubMed] [Google Scholar]
- 18.Haddy FJ, Pamnani MB, Clough DL. Humoral factors and the sodium-potassium pump in volume expanded hypertension. Life Sci. 1979;24:2105–2117. doi: 10.1016/0024-3205(79)90108-5. [DOI] [PubMed] [Google Scholar]
- 19.Haddy FJ, Pamnani MB. Role of ouabain-like factors and Na-K-ATPase inhibitors in hypertension - some old and recent findings. Clin Exp Hypertens. 1998;20(56):499–508. doi: 10.3109/10641969809053228. [DOI] [PubMed] [Google Scholar]
- 20.Khaw KT, Barrett-Connor E. The association between blood pressure, age and dietary sodium and potassium: a population study. Circulation. 1988;77:53–61. doi: 10.1161/01.cir.77.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Lakatta EG. Mechanisms of Hypertension in the Elderly. J Am Geriatr Soc. 1989;37:780–790. doi: 10.1111/j.1532-5415.1989.tb02243.x. [DOI] [PubMed] [Google Scholar]
- 22.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R553–R559. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 23.Manunta P, Citterio L, Lanzani C, Ferrandi M. Adducin polymorphism and the treatment of hypertension. Pharmacogenomics. 2007;8:465–472. doi: 10.2217/14622416.8.5.465. [DOI] [PubMed] [Google Scholar]
- 24.Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. The American Statistician. 1997;51:338–343. [Google Scholar]
- 25.Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens. 2004;17:994–1001. doi: 10.1016/j.amjhyper.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. Age-adjusted standards for creatinine clearance. Ann Intern Med. 1976;84(5):567–569. doi: 10.7326/0003-4819-84-5-567. [DOI] [PubMed] [Google Scholar]
- 27.Sacks FM, Svetkey LP, Vollmer MW, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH, DASH Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 28.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293(2):C509–536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 29.Vu HV, Ianosi-Irimie MR, Pridjian CA, Whitbred JM, Durst JM, Bagrov AY, Fedorova OV, Pridjian G, Puschett JB. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol. 2005;25:520–528. doi: 10.1159/000088461. [DOI] [PubMed] [Google Scholar]
- 30.de Wardener HE, Clarkson EM. Concept of natriuretic hormone. Physiol Rev. 1985;65:658–759. doi: 10.1152/physrev.1985.65.3.658. [DOI] [PubMed] [Google Scholar]
- 31.de Wardener HE, MacGregor GA. Sodium and blood pressure. Curr Opin Cardiol. 2002;17:360–367. doi: 10.1097/00001573-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8 II:127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Weinberger MH. Salt sensitivity: does it play an important role in the pathogenesis and treatment of hypertension? Curr Opin Nephrol Hypertens. 1996;5(3):2095–2098. doi: 10.1097/00041552-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ye T, Liu ZQ, Mu JJ, Fu FH, Yang J, Gao BL, Zhang XH. Blood pressure changes with age in salt sensitive teenagers. Clin Med Sci J. 2004;19:248–251. [PubMed] [Google Scholar]