Abstract
Many behavioral effects of neuroactive steroids are mediated by GABAA receptors; however, other receptors might be involved. Ethanol has a complex mechanism of action, and many of the same receptors have been implicated in the effects of neuroactive steroids and ethanol. The goal of this study was to determine whether actions of neuroactive steroids and ethanol at multiple receptors result in similar discriminative stimulus effects. Rats discriminated 5.6 mg/kg of pregnanolone while responding under a fixed-ratio 20 schedule of food presentation. Pregnanolone, flunitrazepam and pentobarbital produced >80% pregnanolone-lever responding. In contrast, neither morphine nor the negative GABAA modulator β-CCE substituted for pregnanolone up to doses that markedly decreased response rates. Ethanol substituted only in some rats; in other rats, ethanol produced <20% pregnanolone-lever responding up to rate-decreasing doses. Thus, substitution of positive GABAA modulators, and not morphine or β-CCE, for pregnanolone in all rats suggests that positive modulation of GABAA receptors is important in the discriminative stimulus effects of pregnanolone. Although pregnanolone might have actions at other receptors, in addition to actions at GABAA receptors, substitution of ethanol for pregnanolone only in some rats suggests that the mechanisms of action of pregnanolone and ethanol overlap but are not identical.
Keywords: pregnanolone, ethanol, drug discrimination, rats
The γ-aminobutyric acidA (GABAA) receptor complex has several modulatory sites through which drugs can alter the actions of GABA. In addition to benzodiazepine and barbiturate modulatory sites, which are targets for treatment of a variety of clinical disorders, there are distinct modulatory sites to which neuroactive steroids bind (Paul and Purdy, 1992). Like positive modulators acting at benzodiazepine sites, those acting at the neuroactive steroid site on GABAA receptors produce anxiolytic (e.g., Wieland et al., 1997), sedative (e.g., Lancel, 1999; Vanover et al., 1999) and anticonvulsant effects (e.g., Gasior et al., 2000; Kokate et al., 1994; Reddy and Rogawski, 2001). Despite these similarities in acute effects, a number of observations indicate that the effects of neuroactive steroids may not be identical to those of other positive GABAA modulators. For example, tolerance develops to benzodiazepines (Cesare and McKearney, 1980; McMillan, 1992; McMillan and Leander, 1978; Pugh et al., 1992); however, tolerance does not appear to develop to neuroactive steroids (Kokate et al., 1998; McMahon and France, 2002; Reddy and Rogawski, 2000). This difference in the chronic effects of positive GABAA modulators could be useful clinically. One disorder that can require daily use of benzodiazepines is insomnia and tolerance to their sedative effects can develop. Although sedative effects can be obtained in benzodiazepine-tolerant individuals by increasing the treatment dose, this strategy also increases the likelihood that dependence will develop and termination of treatment can be difficult if a withdrawal syndrome emerges. Clearly, the development of benzodiazepine tolerance and dependence decreases the clinical usefulness of these drugs. Differences in the chronic effects of benzodiazepines and neuroactive steroids might be exploited to reduce the impact of tolerance development associated with therapeutic use of benzodiazepines.
Although there are a number of possible explanations for these differences among positive GABAA modulators (e.g., GABAA receptor heterogeneity; Lambert et al., 2001; Mehta and Ticku, 1999), one likely possibility involves actions of neuroactive steroids at receptors other than the GABAA receptor complex (Rupprecht and Holsboer, 1999; but see also Lambert et al., 2001). This possibility is supported by results of drug discrimination studies in which positive GABAA modulators as well as drugs whose primary mechanism of action is at other receptors, such as NMDA, sigma, and 5-HT3 receptors, produce pregnanolone-lever responding in rats (Engel et al., 2001). Another drug that has complex mechanism of action is ethanol (Grant, 1994; Grant, 1999). The ethanol discriminative stimulus is similar to that of neuroactive steroids in that positive GABAA modulators as well as drugs whose primary mechanism of action is at other receptors, particularly NMDA receptors, can substitute for ethanol (Grant and Colombo, 1993a; Grant and Colombo, 1993b; Kostowski and Bienkowski, 1999). Generally, when two drugs share discriminative stimulus effects, substitution of one drug for the other will occur regardless of which drug used as the training drug; however, that is not necessarily true when one of those drugs is ethanol. Although benzodiazepines produce ethanol-lever responding, ethanol does not necessarily produce benzodiazepine-lever responding (De Vry and Slangen, 1986). One possibility that can account for results of these drug discrimination studies is that ethanol acts at multiple receptors to produce a compound discriminative stimulus with one component (i.e., positive modulation of GABAA receptors) overlapping with the actions of benzodiazepines (Grant and Colombo, 1993b; Stolerman and Olufsen, 2001). To the extent that actions of neuroactive steroids at multiple receptors also produce a compound discriminative stimulus, ethanol might be more likely to substitute for neuroactive steroids, as compared to benzodiazepines.
Similarities in mechanism of action of ethanol and neuroactive steroids might have other implications as well. For example, some investigators have proposed that endogenous neurosteroids may play a role in the effects of ethanol, suggesting that ethanol-induced changes in levels of endogenous neurosteroids contribute to its behavioral effects (Morrow et al., 2001). Regardless of whether ethanol acts directly at receptors or indirectly through release of neurosteroids, similarities in the effects produced by ethanol and neuroactive steroids suggest that neuroactive steroids might be more effective in treating ethanol withdrawal, as compared to benzodiazepines, which are the current drugs of choice; although both ethanol and benzodiazepines can modulate GABAA receptors, benzodiazepines do not have actions at other receptors that have been implicated in the behavioral effects of ethanol.
The purpose of the current studies was to determine whether the apparently complex mechanisms of action of neuroactive steroids and of ethanol result in similar discriminative stimulus effects. Drug discrimination procedures have been used extensively to determine the mechanism of action of drugs because they are pharmacologically selective (Shannon and Holtzman, 1976; Winger and Herling, 1982) and have contributed to the characterization of drugs with complex mechanisms of action (Grant and Colombo, 1993a; Grant and Colombo, 1993b; Koek et al., 2006). In these studies, ethanol was compared to the neuroactive steroid pregnanolone in rats discriminating pregnanolone.
Methods
Subjects
Twelve male Long-Evans hooded rats were housed individually in a humidity- and temperature-controlled room with a 12-h light/dark cycle; experiments were conducted during the light cycle. Water was available ad libitum in the home cage. Rats received food pellets during experimental sessions (Research Diets, Inc., New Brunswick, NJ) and rodent chow (Lab Diets, Brentwood, MO) in their home cage. The total amount of food provided was sufficient to maintain rats at 85% of the free-feeding weight, which was determined immediately before the start of the experiment. Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, Louisiana State University Health Sciences Center, and guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council [Department of Health, Education and Welfare, publication No. (NIH) 85−23, revised 1996].
Apparatus
During experimental sessions, rats were placed in six modular test chambers enclosed within sound-attenuating cubicles. Ventilation was provided by fans within each cubicle. Chambers were equipped with houselights, speakers, pellet troughs, pellet dispensers and response levers with stimulus lights located directly above each lever. White noise was present in the chambers to mask extraneous noise. An interface connected chambers to a computer that controlled experiments and recorded data using MED-PC/MEDSTATE NOTATION software (MED Associates, Inc., St. Albans, VT); responses were also recorded by cumulative recorders (Gerbrands Corp., Arlington, MA).
Procedure
Rats discriminated 5.6 mg/kg of pregnanolone while responding under a fixed-ratio 20 schedule of food presentation. One of the rats used in the current study was initially part of another group that discriminated 10 mg/kg of pregnanolone. That training dose was selected because it was the largest dose that did not have rate-decreasing effects in male Long-Evans rats responding on a single lever under a fixed-ratio 20 schedule of food presentation (Ginsburg et al., in press). With repeated administration of 10 mg/kg, dramatic rate-decreasing effects emerged which necessitated a decrease in training dose to 5.6 mg/kg. Sensitivity to the rate-decreasing effects of the smaller dose of pregnanolone did not change with repeated administration, regardless of whether rats initially discriminated 10 mg/kg or 5.6 mg/kg of pregnanolone. Similar rate-decreasing effects with repeated pregnanolone administration have been reported in mice discriminating a large dose of pregnanolone (Shannon et al., 2005).
Experimental sessions were 30 min in duration and began with a 10-min timeout period during which the chamber was dark and responses had no programmed consequence. Illumination of house lights signaled the end of the timeout period and the beginning of the response period, during which 20 responses on the lever designated correct by the injection given immediately before the session resulted in delivery of a 45-mg food pellet. During training sessions, either 5.6 mg/kg of pregnanolone or saline (i.p.) was administered prior to sessions. The lever designated correct following an injection of pregnanolone was counterbalanced among rats. Responses on the incorrect lever reset the response requirement on the correct lever. Rats could continue to respond and receive food pellets until the 20-min response period ended.
Training continued until the following criteria were satisfied for 9 of 10 sessions: ≥80% of total responses emitted on the injection-appropriate lever and fewer than 20 responses emitted on the incorrect lever prior to delivery of the first food pellet. Thereafter, test sessions were conducted twice each week as long as the testing criteria were satisfied during intervening training sessions; otherwise, test sessions were postponed until the criteria were satisfied for two consecutive training sessions. Test sessions were identical to training sessions except that a single dose of a test compound was administered before sessions and 20 consecutive responses on either lever resulted in the delivery of a food pellet. To determine the pharmacological selectivity of the pregnanolone discriminative stimulus, on separate occasions, rats received a single dose of pregnanolone, other positive GABAA modulators (flunitrazepam or pentobarbital), a negative GABAA modulator (β-CCE) or a drug whose primary mechanism of action does not involve GABAA receptors (morphine); each drug was administered immediately before sessions. Some drugs were studied in fewer than 12 rats; however, each rat received either flunitrazepam or pentobarbital (i.e., positive control) and either β-CCE or morphine (i.e., negative control). Similarities between the discriminative stimulus effects of ethanol and pregnanolone were determined by administering ethanol either immediately or 15 min before sessions. In a previous study in rats, maximum rate-decreasing effects were obtained when ethanol was administered immediately before sessions that used similar temporal parameters (Gerak et al., 2004); in the current study, ethanol was also administered 15 min before sessions to ensure that the maximum effects of ethanol were studied under these conditions.
Drugs
Pregnanolone (Steraloids, Inc., Newport, RI) was dissolved in 45% (w/v) hydroxypropyl-γ-cyclodextrin. Flunitrazepam and β-CCE (Sigma-Aldrich Co., St. Louis, MO) were dissolved in a vehicle containing 20% emulphor, 10% ethanol and 70% sterile water. Pentobarbital sodium (Sigma-Aldrich Co., St. Louis, MO) and morphine sulfate (Research Technology Branch, National Institute on Drug Abuse, Rockville, MD) were dissolved in sterile saline. Ethanol was diluted with saline to obtain a 20% (v/v) solution. Doses are expressed in terms of the forms listed above in mg/kg body weight. Drugs were administered i.p. typically in a volume of 1 ml/kg body weight with the exception of ethanol for which the 20% solution was used for all injections and the appropriate dose was administered by adjusting the volume of each injection. Each vehicle was also studied by administering a volume of vehicle equal to the largest volume of the appropriate drug.
Data Analyses
Control response rates were obtained for individual rats by averaging rates for 10 training sessions (± 1 S.E.M.) during which saline was administered and rats satisfied the testing criteria. Response rates for each subject were expressed as a percentage of the control rate and averaged across subjects. Mean response rates were also determined following administration of the training dose of pregnanolone. Differences in control response rates, obtained following administration of saline or 5.6 mg/kg of pregnanolone, were analyzed using a t-test (P<0.05). In addition, response rates obtained under each condition when stimulus control was initially established were compared to rates obtained under the same condition at the end of the experiment to determine whether there was a systematic change in response rates over the course of these studies. The percentage of responses on the pregnanolone lever (% pregnanolone-lever responding) and rates (% control) are plotted as a function of dose. To understand further individual differences in the discriminative stimulus and rate-decreasing effects of ethanol, rats were divided into groups based on lever selection following ethanol administration and data obtained in those two groups were plotted (Figure 3). Discrimination data are not included in the analyses when responses rates were decreased to <20% of control for an individual rat.
Figure 3.
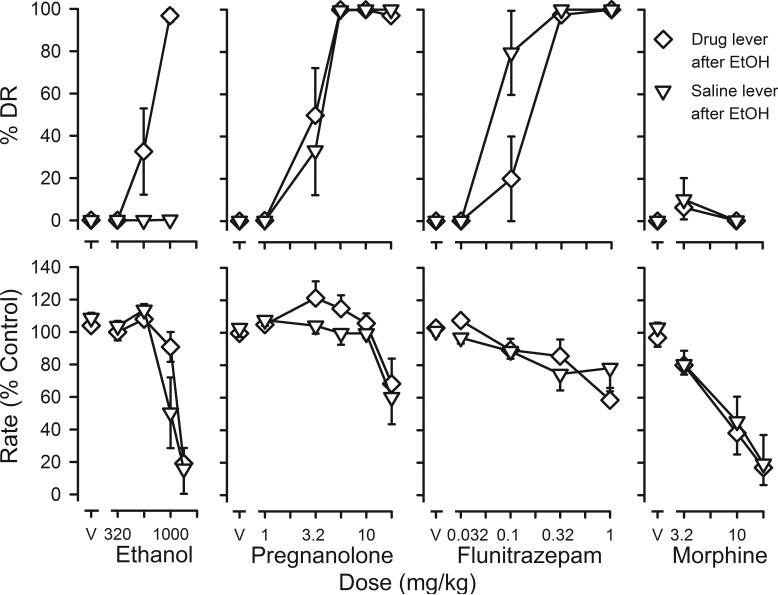
Discriminative stimulus and rate-decreasing effects of ethanol (left panels), pregnanolone (second to left panels), flunitrazepam (second to right panels) and morphine (right panels). Rats were divided into two groups based on lever selection following ethanol administration; data from rats in which ethanol substitutes for pregnanolone are shown in diamonds and data from rats in which ethanol does not substitute for pregnanolone are shown in inverted triangles. For ethanol and pregnanolone, each symbol represents data from 6 rats. For flunitrazepam, each symbol represents data from 5 rats. For morphine, diamonds represent 5 rats and inverted triangles represent 4 rats. See Figure 1 for other details.
Results
Stimulus control was considered adequate for testing after 68 (range: 11−149) training sessions in the 11 rats that only had 5.6 mg/kg of pregnanolone as a training dose. In the twelfth rat, 41 sessions were required to satisfy the testing criteria with the initial training dose of 10 mg/kg and an additional 38 training sessions were required to reestablish stimulus control when the training dose was decreased to 5.6 mg/kg. When saline was administered during training sessions, the mean (± 1 S.E.M.) response rate for the 12 rats was 2.79 ± 0.18 responses/sec. When rats received 5.6 mg/kg of pregnanolone during training sessions, the mean response rate was 3.01 ± 0.18 responses/sec; although rates were slightly higher following pregnanolone administration, as compared to rates obtained when saline was administered, rates under these two conditions were not statistically different. In addition, rates did not change significantly over the course of the experiment for either training condition.
When rats received pregnanolone vehicle immediately before sessions, they responded on the saline-appropriate lever and response rates were similar to rates obtained when saline was administered (solid circles above V, Figure 1). Pregnanolone dose-dependently increased responding on the pregnanolone lever with rats responding predominantly on the pregnanolone lever following administration of doses larger than 3.2 mg/kg (solid circles, upper panel, Figure 1). Doses of pregnanolone smaller than 17.8 mg/kg did not systematically alter response rates, and a dose of 17.8 mg/kg decreased responding to 65% of control (solid circles, lower panel, Figure 1).
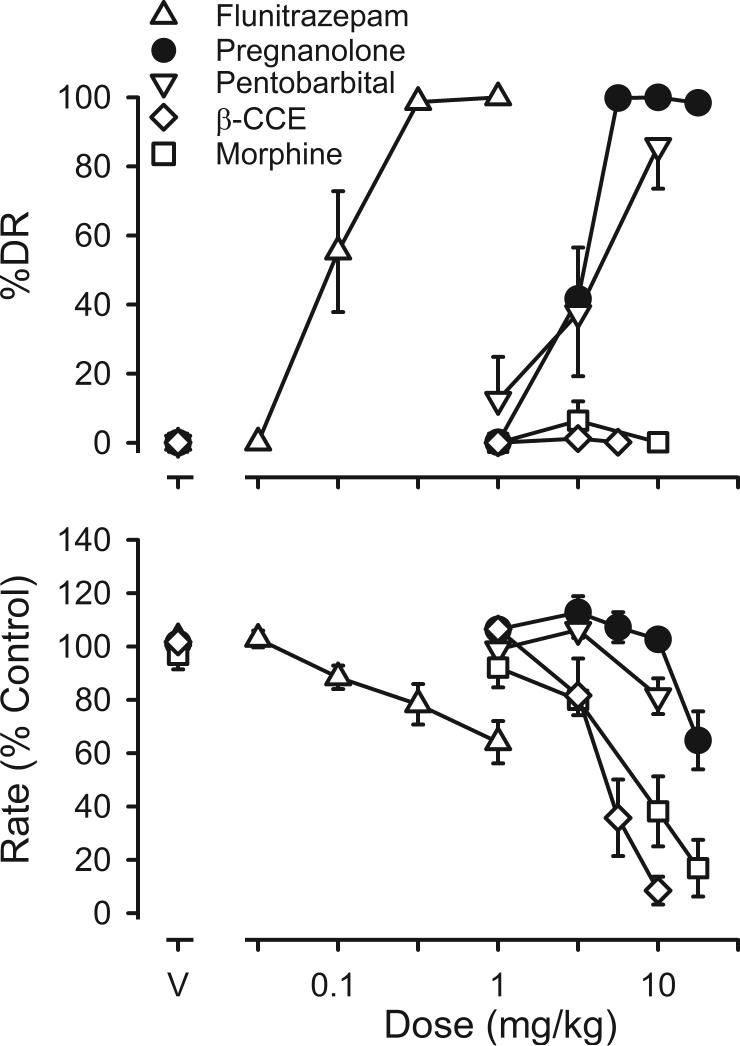
Figure 1.
Discriminative stimulus and rate-decreasing effects of pregnanolone (n=12), flunitrazepam (n=10), pentobarbital (n=8), β-CCE (n=8) and morphine (n=9) in rats discriminating pregnanolone while responding under a fixed-ratio 20 schedule of food presentation. Ordinates: top panel, percentage of total responses emitted on the drug (i.e., pregnanolone) lever (% DR ± 1 SEM); bottom panel, average rate expressed as a percentage of control response rates (± 1 SEM). Abscissa: dose in mg/kg. Points above V represent the effects of vehicle.
To determine the pharmacological selectivity of the pregnanolone discriminative stimulus, two other positive GABAA modulators were examined in rats discriminating pregnanolone. Both flunitrazepam and pentobarbital occasioned pregnanolone-lever responding with doses of flunitrazepam larger than 0.1 mg/kg and doses of pentobarbital larger than 3.2 mg/kg producing >80% responding on the pregnanolone lever (Figure 1). Flunitrazepam dose-dependently decreased response rates with the largest dose studied (1 mg/kg) decreasing rates to 64% of control. A dose of 10 mg/kg of pentobarbital had modest rate-decreasing effects. The pharmacological selectivity of the pregnanolone discriminative stimulus was further supported by the effects of the negative modulator β-CCE and morphine. Up to doses that markedly decreased responding, rats responded predominantly on the saline lever following administration of either drug (Figure 1).
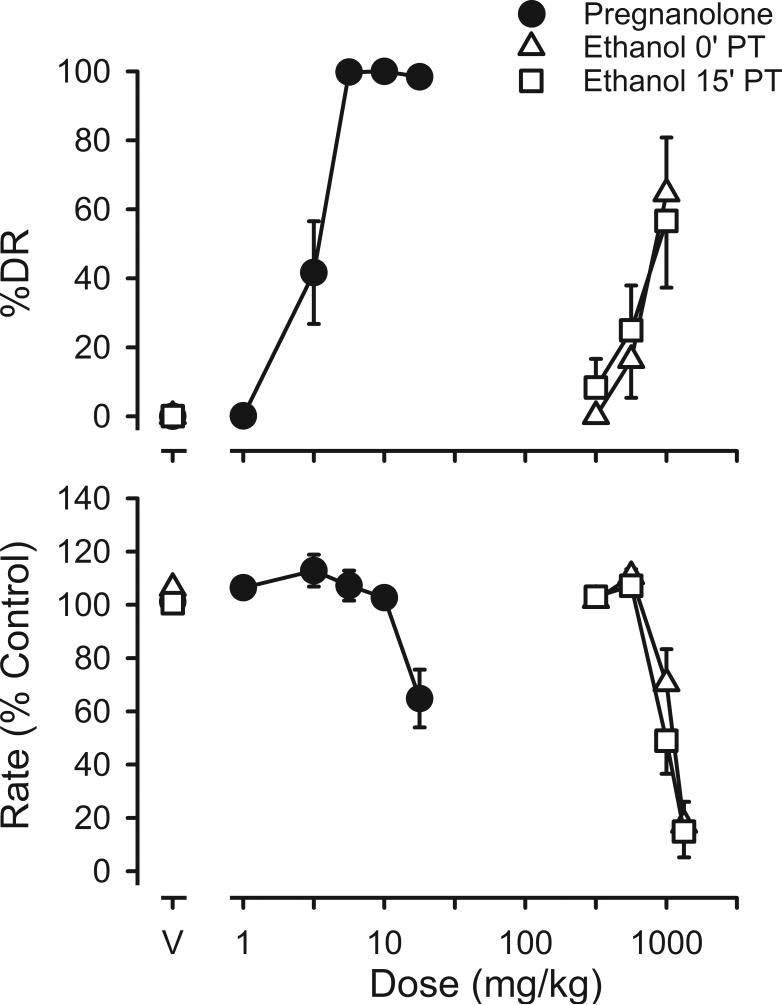
Ethanol dose-dependently increased responding on the pregnanolone lever; however, the mean percentage of responding that occurred on the pregnanolone lever was 65% when ethanol was administered immediately before sessions and 57% when ethanol was administered 15 min before sessions (Figure 2). These maximal effects were obtained with a dose of 1 g/kg of ethanol, and a dose of 1.33 g/kg of ethanol decreased responding to >20% of control, regardless of the interval between ethanol administration and sessions (Figure 2). Although the mean percentage of responding on the pregnanolone lever produced by ethanol was less than that produced by pregnanolone, flunitrazepam or pentobarbital, data from individual rats suggest that effects of ethanol were similar to those of other positive GABAA modulators in some rats. In fact, the 57−65% pregnanolone-lever responding that is observed following administration of 1 g/kg of ethanol results from 6 rats responding predominantly on the pregnanolone lever (diamonds, upper left panel, Figure 3) with the other 6 rats responding predominantly on the saline lever up to the dose of ethanol that decreased responding to <20% of control (inverted triangles, left panels, Figure 3). Of the 6 drugs studied in these rats, only ethanol produced qualitatively different effects in individual subjects. For example, pregnanolone and flunitrazepam produced >80% pregnanolone-lever responding in all rats studied and morphine produced predominantly saline-lever responding in all rats (Figure 3). Moreover, sensitivity to the discriminative stimulus or rate-decreasing effects of other drugs was not markedly different in rats that responded on the pregnanolone lever following ethanol administration, as compared to those that responded on the saline lever. Rate-decreasing effects of ethanol were evident at a smaller dose in rats that did not respond on the pregnanolone lever, with a dose of 1 g/kg of ethanol decreasing rates to <20% of control in 3 of the 6 rats. That dose did not alter response rates in any of the other 6 rats.
Figure 2.
Discriminative stimulus and rate-decreasing effects of ethanol administered immediately (n=12) or 15 min (n=12) before sessions. See Figure 1 for other details.
Discussion
Many behavioral effects of neuroactive steroids are similar to those produced by other GABAA modulators. For example, neuroactive steroids share discriminative stimulus effects with benzodiazepines (Ator et al., 1993; Engel et al., 2001; Vanover, 1997). In the current studies, the benzodiazepine flunitrazepam and the barbiturate pentobarbital produce pregnanolone-lever responding, supporting the role of GABAA receptors in the discriminative stimulus effects of pregnanolone. These studies also demonstrate that the negative GABAA modulator β-CCE does not produce pregnanolone-lever responding, indicating that positive modulation of GABAA receptors, rather than any action at modulatory sites, accounts for the discriminative stimulus effects of pregnanolone.
Despite similarities in the acute behavioral effects of positive GABAA modulators, including discriminative stimulus effects, neuroactive steroids appear to produce some effects that are not identical to those of benzodiazepines. There are a number of possible explanations for differences among positive modulators (e.g., Hosie et al., 2006; Lan et al., 1990; Shingai et al., 1991), one of which is that neuroactive steroids have actions at other receptor systems, such as NMDA receptors (Rupprecht and Holsboer, 1999); receptors other than GABAA receptors appear to contribute to the discriminative stimulus effects of pregnanolone at least under some conditions (Engel et al., 2001; Shannon et al., 2005). If these actions at multiple receptors distinguish neuroactive steroids from benzodiazepines, this complex mechanism of action of neuroactive steroids might produce a compound discriminative stimulus, which would be more similar to the compound stimulus produced by ethanol, as compared to the simpler discriminative stimulus produced by benzodiazepines. In the current study, ethanol produced pregnanolone-lever responding only in half of the rats whereas other positive GABAA modulators produced pregnanolone-lever responding in all rats. Thus, the discriminative stimulus effects of ethanol, and not those of any other drug studied, varied among subjects. Given that other positive GABAA modulators produced qualitatively similar discriminative stimulus effects among rats, differences among subjects suggest that the distinct components which contribute to the discriminative stimulus effects of ethanol (Grant and Colombo, 1993b; Stolerman and Olufsen, 2001) differ in their relative importance in individual subjects. For example, positive modulation of GABAA receptors likely plays a large role in the discriminative stimulus effects of ethanol in individuals that respond on the pregnanolone lever following ethanol administration whereas other receptors might be more important than GABAA receptors in rats that respond on the saline lever following ethanol.
That ethanol does not reliably substitute for positive GABAA modulators is well documented; in fact, individual differences, such as those observed in the current study, are not unusual. For example, in four monkeys discriminating the benzodiazepine midazolam, the maximum percentage of responding on the drug lever following ethanol administration is 0% in two monkeys, 63% in one monkey, and 93% in one monkey (McMahon and France, 2005). Similarly, in rats discriminating 0.3 mg/kg of diazepam, ethanol produces 55% responding on the diazepam lever with some rats responding predominantly on the diazepam lever and other rats responding predominantly on the vehicle lever; as the training dose of diazepam increases (1−6 mg/kg), diazepam-appropriate responding decreases (Shannon and Herling, 1983). Thus, qualitative differences among individual rats in the discriminative stimulus effects of ethanol, like those obtained in the current study, also occur in subjects discriminating a benzodiazepine, suggesting that the relative contribution of GABAA receptors to the discriminative stimulus effects of ethanol varies among subjects.
When a fixed-ratio schedule of food presentation is used in subjects discriminating a drug from its vehicle, the response is usually quantal in nature (Colpaert et al., 1976). Consequently, when a test drug substitutes for the training stimulus in all subjects, intermediate levels of drug-lever responding usually result from differences among subjects in their sensitivity to drugs with some subjects responding predominantly on the drug lever and other subjects responding predominantly on the vehicle lever at doses that, on average, produce partial drug-lever responding; larger doses produce predominantly drug-lever responding in all subjects. In contrast, other test drugs that produce drug-lever responding do not substitute for the training stimulus in all subjects; nevertheless, the response is still quantal, with subjects responding predominantly on either the drug- or saline-appropriate lever and intermediate levels of responding are obtained when data are averaged across subjects. Several mechanisms have been described to account for these individual differences which result in intermediate levels of responding (Colpaert, 1999); these mechanisms could also account for individual differences observed in the current study. For example, partial drug-lever responding can occur following administration of NMDA antagonists in subjects discriminating vehicle from one of a variety of drugs from pharmacologically diverse classes (Koek et al., 1995). One component of the discriminative stimulus effects of ethanol appears to be NMDA receptors (Grant and Colombo, 1993b; Stolerman and Olufsen, 2001), and one possible explanation for individual differences observed in the current study is that actions of ethanol at NMDA receptors result in intermediate levels of drug-lever responding.
In addition to these mechanisms described by Colpaert (1999), the current data suggest that the complexity of the mechanism of action of ethanol, rather than simply its actions at NMDA receptors, could account for individual differences. When the mechanism of action of the test compound overlaps with the training stimulus, some drug-lever responding would be expected; however, if their mechanisms of action are not identical, intermediate levels of responding are obtained (Stolerman and Olufsen, 2001). In the current study, actions of pregnanolone and ethanol at GABAA receptors likely result in drug-lever responding in some rats with actions of ethanol at other receptors accounting for differences among subjects. Thus, the ability of ethanol to substitute for pregnanolone might vary among individuals depending on the role of GABAA receptors in its behavioral effects, and these differences among rats could result in intermediate levels of pregnanolone-lever responding following ethanol administration.
In summary, under the experimental conditions established in these studies, the behavioral effects of pregnanolone appear to be mediated by GABAA receptors with no differences between pregnanolone and flunitrazepam detected. In contrast, although positive modulation of GABAA receptors is also important in the discriminative stimulus effects of ethanol (Kostowski and Bienkowski, 1999), this similarity between ethanol and pregnanolone is not sufficient to result in substitution in all rats. Although multiple receptor systems have been implicated in the behavioral effects of ethanol and neuroactive steroids, these actions at multiple receptors do not result in similar discriminative stimulus effects for these two drugs. Similarities in mechanism of action between neuroactive steroids and ethanol have led to speculation that the effects of ethanol might be linked to those of neuroactive steroids (Morrow et al., 2001; Rupprecht and Holsboer, 1999) with ethanol releasing endogenous neurosteroids which ultimately act at receptors to produce behavioral effects; however, differences between ethanol and pregnanolone observed in the current study suggest that the effects of ethanol are not dependent on endogenous neurosteroids.
Acknowledgements
These studies were supported by U.S. Public Health Service Grants DA017240 (LRG), DA12427 (PJW) and AA09803 (PJW and LRG). The authors wish to thank J. Hulst, M. Bertrand, and E. Sullivan for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA, Grant KA, Purdy RH, Paul SM, Griffiths RR. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241:237–43. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Cesare DA, McKearney JW. Tolerance to suppressive effects of chlordiazepoxide on operant behavior: lack of cross tolerance to pentobarbital. Pharmacol Biochem Behav. 1980;13:545–8. doi: 10.1016/0091-3057(80)90278-6. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–45. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. Theoretical and methodological considerations on drug discrimination learning. Psychopharmacologia. 1976;46:169–77. doi: 10.1007/BF00421388. [DOI] [PubMed] [Google Scholar]
- De Vry J, Slangen JL. Effects of training dose on discrimination and cross-generalization of chlordiazepoxide, pentobarbital and ethanol in the rat. Psychopharmacology. 1986;88:341–5. doi: 10.1007/BF00180836. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–95. [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–96. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Hicks AR, Winsauer PJ, Varner KJ. Interaction between 1,4-butanediol and ethanol on operant responding and the cardiovascular system. Eur J Pharmacol. 2004;506:75–82. doi: 10.1016/j.ejphar.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Gerak LR, McMahon LR, Roache JD. Neurosteroids in alcohol and substance use. In: Ritsner MS, Weizman A, editors. Neuroactive Steroids in Brain Function, Behavioral and Neuropsychiatric Disorders. Springer. in press. [Google Scholar]
- Grant KA. Emerging neurochemical concepts in the actions of ethanol at ligand- gated ion channels. Behav Pharmacol. 1994;5:383–404. doi: 10.1097/00008877-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–7. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993a;264:1241–7. [PubMed] [Google Scholar]
- Grant KA, Colombo G. Pharmacological analysis of the mixed discriminative stimulus effects of ethanol. Alcohol Alcohol Suppl. 1993b;2:445–9. [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Koek W, Chen W, Mercer SL, Coop A, France CP. Discriminative stimulus effects of gammahydroxybutyrate: role of training dose. J Pharmacol Exp Ther. 2006;317:409–17. doi: 10.1124/jpet.105.096909. [DOI] [PubMed] [Google Scholar]
- Koek W, Kleven MS, Colpaert FC. Effects of the NMDA antagonist, dizocilpine, in various drug discriminations: characterization of intermediate levels of drug lever selection. Behav Pharmacol. 1995;6:590–600. [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–9. [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, et al. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–8. [PubMed] [Google Scholar]
- Kostowski W, Bienkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17:63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- Lan NC, Chen JS, Belelli D, Pritchett DB, Seeburg PH, Gee KW. A steroid recognition site is functionally coupled to an expressed GABA(A)-benzodiazepine receptor. Eur J Pharmacol. 1990;188:403–6. doi: 10.1016/0922-4106(90)90201-8. [DOI] [PubMed] [Google Scholar]
- Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Acute and chronic effects of the neuroactive steroid pregnanolone on schedule-controlled responding in rhesus monkeys. Behav Pharmacol. 2002;13:545–55. doi: 10.1097/00008877-200211000-00004. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Combined discriminative stimulus effects of midazolam with other positive GABAA modulators and GABAA receptor agonists in rhesus monkeys. Psychopharmacology. 2005;178:400–9. doi: 10.1007/s00213-004-2022-4. [DOI] [PubMed] [Google Scholar]
- McMillan DE. Effects of drugs on behavior before and during chronic diazepam administration. Eur J Pharmacol. 1992;215:145–52. doi: 10.1016/0014-2999(92)90022-v. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Leander JD. Chronic chlordiazepoxide and pentobarbital interactions on punished and unpunished behavior. J Pharmacol Exp Ther. 1978;207:515–20. [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Pugh SL, Boone MS, Emmett-Oglesby MW. Tolerance, cross-tolerance and withdrawal in rats made dependent on diazepam. J Pharmacol Exp Ther. 1992;262:751–8. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–48. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–44. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–6. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Porcu P, Purdy RH, Grant KA. Characterization of the discriminative stimulus effects of the neuroactive steroid pregnanolone in DBA/2J and C57BL/6J inbred mice. J Pharmacol Exp Ther. 2005;314:675–85. doi: 10.1124/jpet.104.082644. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Herling S. Discriminative stimulus effects of diazepam in rats: evidence for a maximal effect. J Pharmacol Exp Ther. 1983;227:160–6. [PubMed] [Google Scholar]
- Shannon HE, Holtzman SG. Evaluation of the discriminative effects of morphine in the rat. J Pharmacol Exp Ther. 1976;198:54–65. [PubMed] [Google Scholar]
- Shingai R, Sutherland ML, Barnard EA. Effects of subunit types of the cloned GABAA receptor on the response to a neurosteroid. Eur J Pharmacol. 1991;206:77–80. doi: 10.1016/0922-4106(91)90149-c. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Olufsen K. Generalisation of ethanol with drug mixtures containing a positive modulator of the GABA(A) receptor and an NMDA antagonist. Neuropharmacology. 2001;40:123–30. doi: 10.1016/s0028-3908(00)00100-3. [DOI] [PubMed] [Google Scholar]
- Vanover KE. Discriminative stimulus effects of the endogenous neuroactive steroid pregnanolone. Eur J Pharmacol. 1997;327:97–101. doi: 10.1016/s0014-2999(97)89647-1. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Robledo S, Huber M, Wieland S, Lan NC, et al. Positive allosteric modulators of the GABA(A) receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology. 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- Wieland S, Belluzzi J, Hawkinson JE, Hogenkamp D, Upasani R, Stein L, et al. Anxiolytic and anticonvulsant activity of a synthetic neuroactive steroid Co 3−0593. Psychopharmacology. 1997;134:46–54. doi: 10.1007/s002130050424. [DOI] [PubMed] [Google Scholar]
- Winger G, Herling S. Discriminative stimulus effects of pentobarbital in rhesus monkeys: tests of stimulus generalization and duration of action. Psychopharmacology. 1982;76:172–6. doi: 10.1007/BF00435273. [DOI] [PubMed] [Google Scholar]