Abstract
In order to reproduce successfully, animals must integrate multiple environmental cues to synchronize breeding with favorable conditions. In temperate seasonally breeding rodents, photoperiod acts as the primary seasonal cue. Long days are associated with reproductive development and maturation of the gonads whereas short days induce gonadal regression. The neuropeptide kisspeptin has potent stimulatory effects on reproductive development. Kisspeptin potently stimulates GnRH release and kisspeptin expression co-varies with photoperiod in seasonally breeding animals. Here we tested the hypothesis that reproductive involution in response to inhibitory day lenghts results from reduced kisspeptin stimulation of the reproductive axis in seasonally breeding Siberian hamsters (Phodopus sungorus). If true, gonadal regrowth should be hastened by kisspeptin treatment in regressed hamsters and prevented in hamsters by treatment prior to and during regression. In Experiment 1 and Experiment 2 we tested the ability of kisspeptin to reverse gonadal regression. In Experiment 1, reproductively regressed hamsters received chronic kisspeptin via osmotic mini-pumps for 4 weeks. In Experiment 2, daily injections of kisspeptin were administered to regressed hamsters for 6 weeks. In Experiment 3, the ability of kisspeptin to block gonadal regression was tested; hamsters transferred to short days received daily injections of kisspeptin for 6 weeks. In all three studies, short day animals receiving exogenous kisspeptin did not differ from short-day controls. Collectively, these results provide evidence that mechanisms in addition to those that converge on the kisspeptin system are likely critical for seasonal changes in the reproductive axis.
Keywords: metastin, seasonal reproduction, gonadal recrudescence, GPR54, puberty
Introduction
Most animals experience temporal fluctuations in environmental conditions, and must integrate appropriate environmental signals to alter the activity of the reproductive neuroendocrine axis to time reproduction with favorable conditions (e.g., moderate ambient temperatures, reliable food resources). To maximize reproductive success, temperate animals often breed seasonally, limiting breeding to times of the year that ensure offspring birth in the spring and summer (Bronson, 1989). Although a wide range of factors change seasonally, most north-temperate species utilize photoperiod (day length) as the principal environmental signal indicating time of year (Bronson, 1989). Changes in photoperiod alter the activity of the hypothalamo-pituitary-gonadal (HPG) axis (Goldman, 2001), the endocrine axis that regulates reproductive function. The control of reproduction via the HPG axis has been well-characterized in many species: gonadotropin-releasing hormone (GnRH) is released from the hypothalamus and stimulates anterior pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH). LH and FSH, in turn, exert their actions on the gonads to regulate steroidogenesis and gametogenesis respectively.
Recently, the peptide products of the KiSS-1 gene, termed kisspeptins (i.e., kisspeptin-54, −14, −13, −10)(Kotani et al., 2001), have been identified as a potent positive regulator of hypothalamic GnRH release (reviewed by (Smith et al., 2006). This peptide activates the G-protein-coupled receptor 54 (GPR54)(Kotani et al., 2001; Ohtaki et al., 2001). GPR54 has been localized on a majority of GnRH neurons (Han et al., 2005; Irwig et al., 2004; Messager et al., 2005), and kisspeptin has been shown to potently stimulate GnRH and luteinizing hormone (LH) release, presumably via activation of GPR54 (Castellano et al., 2006; Dhillo et al., 2005; Gottsch et al., 2004; Han et al., 2005; Irwig et al., 2004; Mason et al., 2007; Matsui et al., 2004; Messager et al., 2005; Navarro et al., 2005; Patterson et al., 2006; Plant et al., 2006; Shahab et al., 2005; Thompson et al., 2004).
The majority of research on kisspeptin to date has focused on animals that maintain relatively continuous reproductive function (e.g., laboratory mice and rats, rhesus monkeys, humans). Seasonally breeding animals provide an excellent model to test the role of kisspeptin in regulating changes in reproductive status in adult animals, as they exhibit marked, natural changes in HPG axis function. Kisspeptin has been localized to the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus (Arc) of mammals (Smith and Clarke, 2007), and seasonal breeders display significantly different kisspeptin expression patterns (as measured by immunohistochemistry and in situ hybridization) in one or both of these nuclei in response to photoperiod or season (Siberian hamster:(Greives et al., 2007; Mason et al., 2007); Syrian hamster:(Revel et al., 2006); Sheep: (Smith et al., 2007). Interestingly, although marked photoperiod-induced changes in kisspeptin staining in the hypothalamus are observed, short-term administration of kisspeptin to both reproductive and non-reproductive hamsters and sheep elicits robust elevations in circulating levels of LH (Caraty et al., 2007; Greives et al., 2007), suggesting reproductive regression results from reductions in kisspeptin rather than sensitivity of GnRH neurons to this peptide.
Despite the observed photoperiodic changes in kisspeptin staining described previously, as well as the ability of short-term kisspeptin exposure to elicit an elevation in circulating LH in reproductively quiescent animals, the functional significance in seasonally breeding animals requires further exploration. In a recent study in anestrous sheep, intravenous (i.v.) infusion of kisspeptin transiently elevated circulating LH levels, inducing ovulation, before returning to baseline, (Caraty et al., 2007), and in Syrian hamsters, chronic intracerebroventricular (i.c.v.) exposure to kisspeptin across four weeks via osmotic mini-pump significantly increased gonadal mass in short-day housed, reproductively regressed animals, suggesting that kisspeptin is able to reverse reproductive status in these animals (Revel et al., 2006). In contrast, preliminary findings in a different hamster species, the Siberian hamster, reported by our laboratory indicate that chronic subcutaneous (s.c.) kisspeptin exposure via mini-pump does not alter LH concentrations or gonadal mass (Greives et al., 2006). Given the equivocal results from previous studies, we sought to further elucidate the source of these species differences in the functional role of kisspeptin.
The goal of the current study was to test the hypothesis that kisspeptin plays a significant functional role in maintaining and activating reproduction in response to changes in photoperiod in a seasonally breeding animal. We chose Siberian hamsters because they display marked changes in reproductive physiology in response to changing photoperiods (Goldman, 2001); animals held on long-day photoperiods (e.g., 16 h light/day) display functional reproductive physiology and behaviors whereas animals held on short-day photoperiods (e.g., 8 h light/day) have regressed gonads, basal levels of circulating sex steroids, and a significant reduction or absence of typical reproductive behaviors. The experiments in the current investigation sought to address the following questions: 1) is the maintenance of reproductive quiescence due to the suppression of kisspeptin, (i.e., can exogenous kisspeptin stimulate reproductive recrudescence), and 2) does the process of reproductive regression require reductions in kisspeptin, (i.e., will exogenous kisspeptin block regression)?
Materials and Methods
Animals and Housing Conditions
All animals were obtained from our breeding colony maintained at Indiana University, Bloomington. The progenitors of these animals were generously provided by Dr. Randy Nelson (Ohio State University) and Dr. Timothy Bartness (Georgia State University). All animals were initially group-housed (2–4 per cage with same sex siblings upon weaning at 18–20 days of age), held on a long-day photoperiod (light:dark 16:8). At the start of the experiments adult males (>60 d) were assigned to experimental groups, individually housed in polypropylene cages (27.8 × 17.5 × 13.0 cm) and provided ad libitum food (Purina Rat Chow) and tap water. All experimental procedures follow NIH guidelines for the Care and Use of Experimental Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC).
Experiment 1: Does chronic kisspeptin reverse gonadal regression?
Hamsters were housed in long-day (L:D 16:8)(n=6) or short-day (L:D 8:16)(n=20) photoperiods for 8 weeks. After 8 weeks in their respective photoperiods, all animals received a 28-day constant release subcutaneous osmotic mini-pumps (Alzet® Model 2004), which supplied a constant release of 10µM kisspeptin-10 [KiSS-1 (112–121)/ metastin (45–54)(human); Phoenix Pharmaceuticals, Inc. Belmont, CA], a commercially available product with known ability to stimulate the HPG axis(Greives et al., 2007), or vehicle (PBS) at a rate of 0.25µl/h, providing a dose of 0.003µg/h of kisspeptin. This concentration of kisspeptin was chosen to maintain consistency with the concentration of kisspeptin we used for peripheral (i.p) injections in previous studies (Greives et al., 2007; Mason et al., 2007) and to maintain consistency with our preliminary investigation on the effects of chronic kisspeptin on reproductive status (Greives et al., 2006). Long-day animals served as a control group and had a mini-pump filled with vehicle alone. Because long-day animals are already reproductive we chose not to administer kisspeptin to these animals. Short-day animals were implanted with mini-pumps that was either filled with kisspeptin-10 or PBS vehicle. Baseline blood samples were collected via puncture of the retro orbital sinus under light anesthesia prior to implantation and subsequent samples were taken after 14 and 28 days to measure circulating levels of LH. Blood was centrifuged at 2500 RPM for 30 min. and serum was collected and stored at −80°C until assayed for hormones. After the final blood sample was collected, the hamsters were weighed, then killed and necropsies were performed. Gonads were excised, cleaned of fat and connective tissue and weighed to the nearest 0.001g.
Importantly, short-day animals display a polymorphism in reproductive response to photoperiod, and were subdivided into responders and non-responders. Reproductive non-responders fail to respond to photoperiodic information and remain reproductively active despite exposure to short days (Kliman and Lynch, 1992; Lynch and Lynch, 1986; Prendergast et al., 2001); the remaining animals (i.e., responders), in contrast, display the typical gonadal regression in response to short days. Additionally, whereas short-day responsive (non-reproductive) animals molt their breeding season pelage and replace it with a thicker whiter fur, reproductive non-responders maintain their breeding season pelage. Pelage coloration was noted throughout the duration of the experiment. We designated hamsters as reproductive non-responders based on pelage color combined with final paired testes mass. Five short-day vehicle treated animals had paired testes weighing >0.1g and were deemed non-responders (mean responder paired testes mass: 0.047 ± 0.003g; mean non-responder paired testes mass 0.191 ± 0.025g). Only one out of 10 short-day kisspeptin-implanted animals had paired testes mass >0.1g and displayed no change in pelage, thus this animal was determined to be a non-responder (mean responder paired testes mass: 0.040 ± 0.002g: non-responder paired testes mass: 0.582g). As only one short-day non-responsive animal received a kisspeptin filled mini-pumps, precluding statistical comparisons, all six non-responders were excluded from further analysis, yielding three treatment groups: long-day vehicle (n= 6), short-day responder kisspeptin (n = 9), and short-day responder vehicle pump (n = 5).
Experiment 2: Does daily kisspeptin treatment reverse gonadal regression?
a. Effectiveness of a single injection of kisspeptin-10 to elicit a physiologically relevant LH elevation
We previously demonstrated that a series of four peripheral (i.p) injections of kisspetpin (one injection every half-hour), elicits a significant and robust elevation in circulating levels of LH (Greives et al., 2007). In order to facilitate consistent timing of administration of kisspetpin to several animals each day in the following two experiments, and reduce the stress of multiple injections we asked whether a single i.p injection elicits a similar LH response as the four-injection protocol previously employed (Greives et al., 2007). To this end, we administered long-day housed hamsters either a single 100µl i.p. injection of either vehicle (n=5) or 10µM kisspeptin in 0.1MPBS (1.3 µg kisspeptin/animal)(n=6). Blood samples were obtained as described in Experiment 1 prior to injection (baseline) and 30 minutes post-injection. Serum was collected and analyzed for LH via RIA.
To verify the effectiveness of a single administration of kisspeptin and to determine a physiologically relevant dose for short-day housed animals, short-day responsive (i.e., non-reproductive) male hamsters received a single 100µl injection of either vehicle (0.1M PBS)(n=5), 1µM (0.13µg kisspeptin/animal)(n=6) or 10µM kisspetpin-10 in 0.1M PBS (1.3 µg kisspeptin/animal)(n=5). Blood samples were obtained as described in Experiment 1 prior to injection (baseline) and 30 minutes post-injection. Serum was collected and analyzed for LH via RIA.
b. Administration of daily injections on gonadal recrudescence
A single peripheral, daily injection was administered for 6 weeks to adult male hamsters in an attempt to reverse gonadal regression. One single daily injection was administered based on the results of the pilot study described above. Furthermore, this decision was based on a previous finding that a single peripheral daily injection of the glutamate agonist NMDA was sufficient to block gonadal regression in Siberian hamsters (Ebling et al., 1995).
Hamsters were weighed and individually housed in either long- (n=10) or short-day (n = 35) photoperiods for eight weeks. After 8 weeks, body mass was measured again. Responsiveness to short-day photoperiods in these animals was assessed prior to manipulation via estimated testis volume (ETV) as previously described (Gorman and Zucker, 1995) and a significant reduction in body mass. Animals that lost >10% of their original body mass were deemed responsive to short-day photoperiods, all others were excluded from the remainder of the experiment. Short-day induced gonadal regression was confirmed in these individuals by measuring ETV, where ETV is equal to the product of the testis width squared times testis length (Gorman and Zucker, 1995). Short-day responsive hamsters had significantly smaller testes than long-day hamsters (mean long-day ETV: 684.5 ± 34.1; mean short-day responsive ETV: 137.2 ± 18.7) (one-way ANOVA: P < 0.05).
After confirmation of photoperiodic responsiveness, daily peripheral (i.p.) injections were administered for the following six weeks between 14:00–14:45 EST (1.25–2h prior to lights off in short-day animals and 5.25–6h prior to lights of in long-days animals). All long days animals received 100 µl of 0.1M PBS vehicle (n=10). Short-day regressed animals were provided with daily injections of 100µl of either 0.1M PBS vehicle (n=12) or 10µM kisspeptin-10 in 0.1M PBS, providing 1.3µg kisspeptin per animal (n=12). The day following the final administration of daily injections a blood sample was collected as described in Experiment 1 for analysis of circulating testosterone levels, final body masses recorded and necropsies were performed; gonads were excised, cleaned of fat and connective tissue and paired testes weighed to the nearest 0.001g.
Experiment 3: Does daily kisspeptin treatment block gonadal regression?
Hamsters were weighed and individually housed in either long- (n=10) or short-day (n=39) photoperiods. We attempted to identify short-day responsive animals before regression began, after two weeks in photoperiod. Previous data from our laboratory indicated that responsive animals display a decrease in body but not testis mass after 2 weeks in photoperiod (G.E. Demas unpublished data). No animals in the present study, however, displayed a significant decrease in body mass after two weeks in short-day photoperiods. Therefore, only animals that gained mass after two weeks in short-day photoperiod were excluded from the remainder of the experiment. As non-responsiveness is a genetically controlled trait (Lynch and Lynch, 1986), siblings were separated into each of the three treatments to reduce any potential bias of non-responsiveness and all animals were included in the final analysis.
After two weeks in photoperiod we initiated administration of daily peripheral (i.p.) injections, which continued for 6 weeks. Daily injections were administered between 14:00–14:45 EST (1.25–2h prior to lights off in short-day animals and 5.25–6h prior to lights off in long-days animals). All long-day animals served as controls and received a daily injection of 100µl 0.1M PBS vehicle (n = 10). Short-day animals received a daily injection of 100µl of either vehicle (n = 15) or 10µM kisspeptin-10 in 0.1M PBS, providing 1.3 µg kisspeptin per animal (n = 16). After 8 weeks in photoperiod (6 weeks of injections) a blood sample was collected the day after the final injection for analysis of circulating testosterone levels, final body mass recorded, and necropsies were performed. Gonads were excised, cleaned of fat and connective tissue and paired testes weighed to the nearest 0.001g.
Hormone Measurements
To investigate the efficacy of kisspeptin-10 to stimulate LH release after prolonged kisspeptin exposure, blood samples were collected from all animals in Experiment 1, and from a subset of animals from Experiment 2 and Experiment 3 thirty minutes after the final kisspeptin injection. Additionally, as the effect of kisspeptin injections on circulating FSH concentrations is unknown in this species, FSH concentrations were determined from the same samples used to measure LH in Experiment 2 and Experiment 3. All serum LH and FSH concentrations were measured in duplicate via a single radioimmunoassay (RIA) with reagents obtained from the National Institutes of Health based on a previous protocol (Chappell et al., 1997). The sensitivity for the LH assay was 0.01ng/tube and the intra-assay coefficient of variation was 8.57% for the low pool and 1.83% for the high pool. The sensitivity for the FSH assay was 0.05ng/tube, and the intra-assay coefficient of variation was 15.3% for the low pool and 8.3% for the high pool.
Serum testosterone was measured via a commercial EIA kit (Correlate-EIA Kit #900-065; Assay Designs, Ann Arbor, MI). Serum samples were diluted 1:20 and run in duplicate for each sample. The sensitivity of the assay was 3.82 pg/ml and the intra-assay coefficient of variation ranged from 0.7–9.5% and the inter-assay coefficient of variation was 8.9%. The antisera used in all assays were highly specific for the hormones measured, with low cross-reactivity with other hormones. All three assays have been previously validated for use in Siberian hamsters (Demas et al., 2004; Wolfe et al., 1995).
Statistical Analyses
Photoperiod and injection or mini-pump regime yielded three distinct treatment groups (i.e., long-day vehicle, short-day vehicle, short-day kisspeptin). Effect of treatment was analyzed using separate one-way ANOVAs. Pair-wise comparisons were probed using Tukey’s post-hoc tests. Paired t-tests were employed to validate the ability of a single 10µM kisspeptin injection in long days and either a single 10µM and 1µM kisspeptin injection in short days to elevate circulating levels of LH. We also investigated the ability of kisspeptin injections to elevate LH after 6 weeks of daily injections by comparing LH values on a subset of vehicle (n=4) or kisspeptin (n=6) injected animals from Experiment 2 and Experiment 3 after the final injection. As kisspeptin has been shown repeatedly to significantly elevate LH levels in our laboratory (e.g., (Greives et al., 2007), the effect of the type of injection on LH levels was compared using a one-tailed t-test. Additionally, as the effect of kisspeptin injections on FSH levels is unknown, a two-tailed t-test was used to compare vehicle versus kisspeptin injected animals; these samples were from the same animals in Experiment 2 and Experiment 3 used to determine LH values. Comparisons were deemed significant when p < 0.05. All analyses were performed on Minitab 14 for Windows.
Results
Experiment 1: Does chronic kisspeptin reverse gonadal regression?
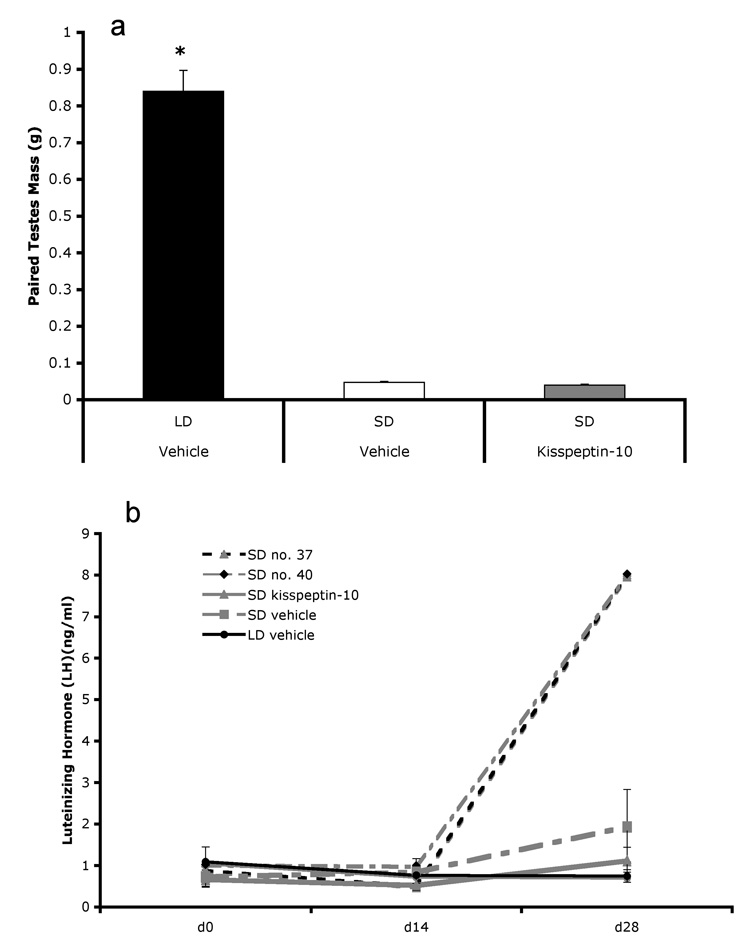
Twenty-eight days of kisspeptin via osmotic mini-pump did not induce gonadal recrudescence in short-day hamsters (Figure 1a). An overall effect of treatment was found (F2, 19 = 234.6, p < 0.001) with long-day hamsters having heavier paired testes than both short-day hamsters that received mini-pumps filled with vehicle (t = −17.35, p < 0.001) and short-day hamsters with kisspeptin mini-pumps (t = −20.12, p < 0.001). Short-day hamsters with kisspeptin mini-pumps did not have heavier paired testes (p > 0.05) than short-day animals receiving mini-pumps filled with vehicle. There was no effect of treatment on circulating levels of LH (p >0.05)(Figure 1b). Interestingly, two short-day individuals with kisspeptin mini-pumps displayed LH levels similar to all other animals at the start of the experiment and after 14 days with the mini-pump. After 28 days however, these individuals displayed greater than 4 times higher circulating LH concentrations compared with both other individuals in the kisspeptin mini-pump group, as well as both vehicle implanted groups. These individuals were included in all analyses, but have been separated out for graphical representation (Figure 1b). Photoperiod treatment significantly affected final body mass (F2,19 = 23.88, p < 0.001)(Table 1); long-day hamsters with vehicle mini-pumps were significantly heavier than both short-day vehicle mini-pump (t = −4.15, p = 0.002) and short-day kisspeptin mini-pump hamsters (t = −6.88, p < 0.001). Short-day hamsters with vehicle filled mini-pumps were not heavier than short-day hamsters with kisspeptin mini-pumps (p > 0.05).
Figure 1.
Chronic kisspeptin provided via osmotic mini-pump had no detectable effect on paired testis mass (a) or circulating levels of LH. Interestingly two of the nine animals (animal number 37 and 40) that received a kisspeptin-filled mini-pump displayed no difference in LH levels after 14 days, however after 28 days, these animals displayed elevated LH levels compared to all other groups (>4x’s higher)(b). * indicates p < 0.05.
Table 1.
Mean (± SEM) body mass in grams (g) at the conclusion of each experiment.
| Treatment Group | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| Long-day vehicle | 44.13 (±1.74)* | 42.77 (±1.37)* | 41.84 (±1.84) |
| Short-day vehicle | 35.68 (±1.92) | 34.09 (±1.30) | 36.09 (±1.41) |
| Short-day kisspeptin-10 | 31.93 (±0.62) | 30.78 (±1.00) | 38.49 (±1.76) |
indicates group significantly different from all other groups within experiment (p < 0.05).
Experiment 2: Does daily kisspeptin treatment reverse gonadal regression?
a. Ability of single injections of kisspeptin to elevate LH
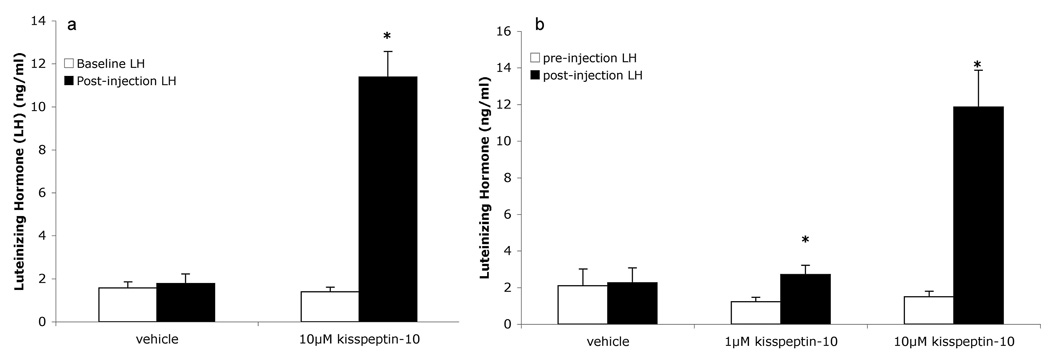
In long-day hamsters, a single 100µl injection of 10µM kisspeptin was effective in significantly elevating circulating levels of LH over baseline (T = −10.15, p < 0.001), while vehicle injection failed to elicit an LH elevation (p > 0.05)(Figure 2a).
Figure 2.
A single injection of 100µl of a 10µM solution of kisspetpin-10 significantly elevates circulating levels of LH in long-day hamsters (a), and an injection of 100µL of either a 1µM or 10µM solution significantly elevates circulating levels of LH in short-day hamsters (b). * indicates p < 0.05.
In short-day hamsters a single 100µl injection of either 1µM (T = −2.72, p = 0.04) or 10µM kisspeptin (T = −4.66, p = 0.01) was sufficient to significantly elevate circulating levels of LH over baseline. Vehicle injections failed to elevate circulating LH levels (p>0.05)(Figure 2b).
b. Ability of daily injections to reverse gonadal regression
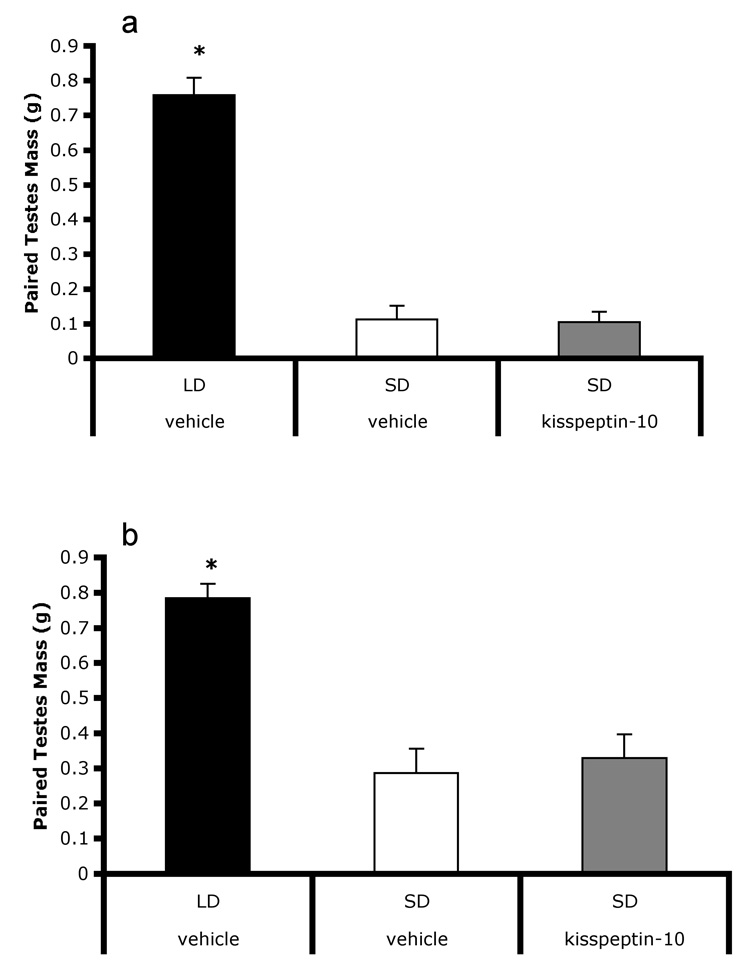
Six weeks of daily injections with kisspeptin did not induce gonadal recrudescence in regressed males (Figure 3a). There was an overall effect of treatment (F 2,33 = 85.3, p < 0.001), with long-day vehicle injected animals having heavier paired testes than both short-day vehicle (t = −11.43, p < 0.001) and short-day kisspeptin injected animals (t = −11.54, p < 0.001). Paired testes masses of short-day kisspeptin injected animals were not different from short-day vehicle injected animals (p > 0.05). Treatment significantly affected circulating testosterone levels (F2,33 = 15.336, P = 0.01)(Table 2); titers were significantly higher in long-day vehicle injected animals than short-day kisspeptin injected (p < 0.01) but not short-day vehicle injected animals (P > 0.05). Testosterone titers did not differ between short-day vehicle and short-day kisspeptin injected groups (p > 0.05). Photoperiod treatment significantly affected final body mass (F2,33 = 24.23, p < 0.001)(Table 1). Long-day vehicle injected hamsters were significantly heavier than both short-day vehicle injected (t = −4.93, p < 0.001) and short-day kisspeptin injected hamsters (t = −6.81, p < 0.001).
Figure 3.
Six weeks of daily injections of kisspeptin administered to fully regressed individuals did not lead to initiation of gonadal recrudescence (a), nor did six weeks of daily injections block regression in animals transferred to short-days (b). Short-day hamsters receiving kisspeptin did not differ short-day vehicle injected hamsters. Both groups displayed significantly smaller testes than long-day hamsters. * indicates p < 0.05.
Table 2.
Mean (± SEM) testosterone values (ng/ml) after 6 weeks of daily kisspeptin or vehicle injections (Experiment 2 and Experiment 3).
| Treatment Group | Experiment 2 | Experiment 3 |
|---|---|---|
| Long-day vehicle | 7.80 (±0.68)† | 9.40 (±2.49) |
| Short-day vehicle | 6.34 (±0.48) | 9.06 (±1.05) |
| Short-day kisspeptin-10 | 5.44 (±0.36)† | 5.43 (±0.70) |
indicates groups significantly differ from each other (p < 0.05).
Experiment 3: Does daily kisspeptin treatment block gonadal regression?
Six weeks of daily injections with kisspeptin did not block gonadal regression. There was an overall effect of photoperiod treatment on paired testes mass (F 2,40 =14.58, p < 0.001)(Figure 3b). Long-day vehicle injected animals had significantly heavier paired testes than both short-day vehicle injected (T = −5.01, p < 0.001) and short-day kisspeptin injected animals (T = −4.65, p = 0.001). Neither short-day vehicle injected nor short-day kisspeptin injected treatments differed (p > 0.05). Treatment did not significantly affect circulating testosterone levels (p > 0.05)(Table 2). Treatment tended to (p = 0.089), but did not significantly affect final body mass (Table 1).
LH and FSH response to kisspeptin after 6 weeks of daily injections
A single injection of kisspeptin analyzed in a subset of animals that had received six weeks of daily injections elicited an elevation in circulating levels of LH using a one-tailed t-test (T7 = 2.1, P < 0.04)(Table 3); a two-tailed t-test revealed a similar trend (T7 = 2.1, p = 0.07). The single injection of kisspeptin, however, did not lead to a difference in circulating FSH compared with vehicle-injected animals (T6 = −0.13, p > 0.05)(Table 3).
Table 3.
Mean (± SEM) luteinizing hormone (LH) titers (ng/ml) 30 minutes following a single injection of kisspeptin in animals that had received 6 weeks of daily kisspeptin injections.
| Injection | LH(ng/ml) | FSH(ng/ml) |
|---|---|---|
| Vehicle | 0.79 (±0.24) | 2.68 (±0.27) |
| Kisspeptin-10 | 1.66 (±0.34)* | 2.60 (±0.59) |
indicates p < 0.05
Discussion
The current set of studies sought to test the hypothesis that kisspeptin plays an important functional role in regulating reproductive status in a seasonally breeding animal. Specifically, we addressed the question of whether long-term kisspeptin exposure would up-regulate the HPG axis and alter reproductive status accordingly in the photoperiodic seasonally breeding animal, the Siberian hamster. Although kisspeptin exhibits pronounced seasonal changes associated with reproductive status, the present results, however, indicate that kisspeptin, administered either chronically with osmotic mini-pump, or via daily injections, did not alter reproductive status.
In Experiment 1, we demonstrated that chronic s.c. kisspeptin, provided peripherally at a constant rate of 0.003µg/h for four weeks via osmotic mini-pump, was insufficient to induce gonadal recrudescence or elevate circulating levels of LH in short-day regressed hamsters. This finding differs with results in Syrian hamsters where chronic kisspeptin induces gonadal regrowth and an elevation in testosterone secretion (Revel et al., 2006). It is interesting to note that kisspeptin immunoreactivity in short-day housed Siberian hamsters is downregulated in the AVPV, while an increase in kisspeptin immunoreactivity in the Arc is observed (Greives et al., 2007; Mason et al., 2007); in Syrian hamsters, kisspeptin has not been localized to the AVPV, and KiSS-1 mRNA in the Arc is downregulated in short-day housed animals (Revel et al., 2007). In the Syrian hamster study, kisspeptin was administered i.c.v. and at a significantly higher dose (3.3µg/h as opposed to 0.003µg/h in the current study), perhaps accounting for these differences. Notably, in rats chronic peripheral (s.c.) kisspeptin administration via mini-pump at a similar dose (2.71µg/h) to that used in Syrian hamsters resulted in testicular degeneration (Thompson et al., 2006). Consistent with results in rats, continuous i.v. infusion of kisspeptin to juvenile and adult rhesus monkeys, at a rate of 100µg/h for the juveniles and 200 or 400µg/h in the adult, leads to a significant desensitization of the GPR54 receptor, inhibiting an LH surge in response to a bolus injection of kisspeptin (Ramaswamy et al., 2007; Seminara et al., 2006). Thus, it is possible the continuous release of kisspeptin provided to Siberian hamsters, even at a lower dose than in the above studies, may have desensitized GPR54 receptors on GnRH neurons, prohibiting sufficient stimulation of the HPG axis. Although the current data do not allow us to address this question, a subset of our data may provide indirect support for this hypothesis; two hamsters that received kisspeptin mini-pumps displayed low levels of circulating LH after 14 days of implantation, similar to all other individuals, whereas on the 28th day these two animals displayed relatively elevated LH levels compared with control animals and the rest of the animals receiving kisspeptin (Fig 1b). The mini-pumps employed in the current investigation are designed to provide continuous release for a maximum of 28 days. Although only speculation, at the end of this 28-day period, the pump was likely delivering sporadic low doses of kisspeptin which may allow for re-sensitiztion of the GPR54 receptor and LH elevation.
A recent report in sheep indicates that continuous infusion of kisspeptin over 30–48 h stimulates ovulation in the majority of seasonally anestrous sheep (80–86% ovulation)(Caraty et al., 2007). Interestingly though, continuous infusion was unable to maintain elevated levels of LH, dropping after 24 h in kisspeptin infused sheep and displaying similar LH levels as vehicle infused animals after 48 h (Caraty et al., 2007). This finding demonstrates that, as suggested by the current study, kisspeptin can activate the HPG axis of non-reproductive animals. However hamsters require several weeks to allow for gonadal recrudescence (e.g., Anchordoquy and Lynch, 2000), and thus, one large LH surge is likely insufficient to fully activate quiescent gonads. Studies utilizing a wide range of doses of kisspeptin combined with efforts designed to test the effect of continuous versus sporadic kisspeptin on the HPG axis of both reproductively active and inactive adult animals across species with divergent life histories will be needed to clarify these possibilities.
Daily injections of kisspeptin were administered in Experiment 2 and Experiment 3 in an attempt to induce gonadal recrudescence (Experiment 2) or block gonadal regression (Experiment 3). Reproductive status, however, was not altered in response to daily kisspeptin injections in either experiment; hamsters did not have heavier paired testis masses or higher circulating testosterone titers than vehicle-treated controls. It is possible that the injections of kisspeptin were insufficient in either amount or number to maintain HPG activity in short-day animals. If true, multiple daily injections or higher doses of the hormone may be required to maintain HPG activity at sufficiently high levels to block or reverse gonadal regression. This possibility is unlikely for several reasons. First, a single daily peripheral injection of the glutamate agonist, NMDA, is sufficient to block gonadal regression in Siberian hamsters (Ebling et al., 1995), indicating the frequency of injections was adequate. Furthermore, data in the present study (Experiment 2a) confirmed that the dosage of kisspeptin (10µM) employed in Experiment 2b and Experiment 3 significantly elevates LH levels in both reproductive an underproductive male Siberian hamsters after a single injection, and kisspeptin injections an order of magnitude lower than those employed in Experiment 2b and Experiment 3 (i.e., 1µM) are sufficient to significantly elevate LH over baseline. After 6 weeks of daily injections, animals displayed markedly higher levels of circulating LH 30 minutes after kisspeptin injection, compared with animals receiving vehicle injections. The LH concentrations detected, however, were noticeably lower than those observed with a single injection (Experiment 2a) or using a multiple injection protocol (Greives et al., 2007), consistent with the observation in sheep, where anestrous females displayed a lower LH surge after multiple daily injections compared with the LH surge observed following the first injection (Caraty et al., 2007). Importantly, we sought to test our hypothesis using a physiologically relevant dose of kisspeptin. The LH levels induced by a single injection of 100µl of 10µM kisspeptin are similar to those observed in male Siberian hamsters in response to a relevant environmental stimulus (e.g., reproductive female scent)(Anand et al., 2002; Anand et al., 2004) and this injection generates LH levels slightly above levels induced by daily injections of 20 mg/kg NMDA, which are sufficient for maintaining the reproductive axis (Ebling et al., 1995). Future studies employing higher doses of kisspeptin will be needed to investigate if supraphysiological levels of kisspeptin are able to override inhibition likely generated by the inhibitory signal of short days.
The failure of kisspeptin to alter reproductive status may also be due to insufficient elevation in FSH, despite elevated levels of LH. FSH is known to be crucial for gonadal recrudescence in Siberian hamsters; gonadal recrudescence in response to long-day photoperiods is inhibited when FSH is suppressed (Milette et al., 1988; Wolfe et al., 1995). Indeed, in the current study there was no evidence of an elevation in FSH levels thirty minutes after a single injection of kisspeptin when compared with a vehicle injection. Although the current study had a relatively small sample size, a separate study from our laboratory with a larger sample size indicates a similar pattern, failing to find an effect of kisspeptin on circulating FSH levels thirty minutes post injection (T.J. Greives, C. Bergeon, and G.E. Demas unpublished data). While the ability of kisspeptin to stimulate LH is robust across taxa, studies investigating the effect of kisspeptin on FSH release have provided equivocal results. Although some studies indicate that kissepeptin induces FSH release, albeit with significantly less effect than on LH (Messager et al., 2005), others find no increase in FSH following kisspeptin administration (Patterson et al., 2006). If daily injections of kisspeptin did not elevate circulating FSH levels sufficiently then this likely prevented significant effects on gonadal morphology in hamsters exposed to short-days. These findings suggest the possibility of additional mechanisms regulating seasonal profiles of FSH.
In summary, long-term exogenous kisspeptin treatment did not alter reproductive status in hamsters housed in inhibitory short-day photoperiods. The mechanisms underlying the inability of prolonged kisspeptin exposure to alter reproductive status will help to guide future investigations into the regulation of the reproductive axis across taxa. Collectively, these data further our understanding of kisspeptin’s role in regulating seasonal reproduction, suggesting additional factors or multiple daily pulses are required to induce gonadal recrudescence in the presence of inhibitory environmental signals. Furthermore, the current data highlights the need for future investigations into the underlying differences in the ability of kisspeptin to alter reproductive status observed in Siberian and Syrian hamsters (Revel et al., 2006), which will provide important insights into the comparative neuroendocrinology and evolution of mechanisms regulating mammalian reproduction.
Acknowledgements
We would like to thank Devin Zysling, Melissa Scotti, Emily Chester, Christy Bergeon, Laura Garman, and Jill Lodde for animal assistance and Joel McGlothlin, Ellen Ketterson and Philip Quirk for discussion and comments on an earlier draft of this manuscript. The authors also wish to thank Brigitte Mann at Northwestern University for LH and FSH analysis. This work was supported by a SICB Grant-in-Aid (TJG), NIH/T32 HD049336-0 (TJG), NSF IOB:0543798 (GED), Indiana University Faculty Research Support Program (GED), NIH HD050470 (LJK), and the UC Berkeley Committee on research grant (LJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand S, et al. Differential regulation of luteinizing hormone and follicle-stimulating hormone in male Siberian hamsters by exposure to females and photoperiod. Endocrinology. 2002;143:2178–2188. doi: 10.1210/endo.143.6.8839. [DOI] [PubMed] [Google Scholar]
- Anand S, et al. Chemosensory stimulation of luteinizing hormone secretion in male Siberian hamsters (Phodopus sungorus) Biology of Reproduction. 2004;70:1033–1040. doi: 10.1095/biolreprod.103.019380. [DOI] [PubMed] [Google Scholar]
- Anchordoquy HC, Lynch GR. Timing of testicular recrudescence in Siberian hamsters is unaffected by pinealectomy or long-day photoperiod after 9 weeks in short days. Journal of Biological Rhythms. 2000;15:406–416. doi: 10.1177/074873000129001495. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian Reproductive Biology. 1989 [Google Scholar]
- Caraty A, et al. Kisspeptin Synchronizes Preovulatory Surges in Cyclical Ewes and Causes Ovulation in Seasonally Acyclic Ewes. Endocrinology. 2007;148:5258. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- Castellano JM, et al. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Molecular and Cellular Endocrinology. 2006;257–8:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Chappell PE, et al. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene 1. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Demas GE, et al. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiology & Behavior. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. Journal of Clinical Endocrinology and Metabolism. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Ebling FJP, et al. Effects of N-Methyl-D-Asparate (NMDA) on seasonal cycles of reproduction, body-weight and pelage color in the male siberian hamster. Journal of Neuroendocrinology. 1995;7:555–566. doi: 10.1111/j.1365-2826.1995.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. Journal of Biological Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Testicular regression and recrudescence without subsequent photorefractoriness in siberian hamsters. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1995;38:R800–R806. doi: 10.1152/ajpregu.1995.269.4.R800. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greives TJ, et al. Environmental control of kisspeptin: Implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Greives TJ, et al. Kisspeptin induces gonadotropin release in long- and short-day Siberian hamsters (phodopus sungorus) Pittsburgh, PA: Society for Behavioral Neuroendocrinology; 2006. [Google Scholar]
- Han SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. Journal of Neuroscience. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Kliman RM, Lynch GR. Evidence for genetic-variation in the occurrence of the photoresponse of the Djungarian hamster, Phodopus sungorus. Journal of Biological Rhythms. 1992;7:161–173. doi: 10.1177/074873049200700207. [DOI] [PubMed] [Google Scholar]
- Kotani M, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. Journal of Biological Chemistry. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Lynch GR, Lynch CB. Seasonal photoperiodism in the Djungarian hamster - a genetic component influences photoresponsiveness. Behavior Genetics. 1986;16:625–626. [Google Scholar]
- Mason AO, et al. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Hormones and Behavior. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, et al. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochemical and Biophysical Research Communications. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milette JJ, et al. The importance of follicle-stimulating-hormone in the initiation of testiculargGrowth in photostimulated Djungarian hamsters. Endocrinology. 1988;122:1060–1066. doi: 10.1210/endo-122-3-1060. [DOI] [PubMed] [Google Scholar]
- Navarro VM, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Patterson M, et al. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. Journal of Neuroendocrinology. 2006;18:349–354. doi: 10.1111/j.1365-2826.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- Plant TM, et al. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, et al. Photoperiodic polyphenisms in rodents: Neuroendocrine mechanisms, costs, and functions. Quarterly Review of Biology. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, et al. Effect of continuous intravenous administration of Human Metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult aale Rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- Revel FG, et al. Kisspeptin: A key link to seasonal breeding. Reviews in Endocrine & Metabolic Disorders. 2007;8:57–65. doi: 10.1007/s11154-007-9031-7. [DOI] [PubMed] [Google Scholar]
- Revel FG, et al. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Current Biology. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Seminara SB, et al. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): A finding with therapeutic implications. Endocrinology. 2006;147:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Shahab M, et al. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Clarke IJ. Kisspeptin expression in the brain: Catalyst for the initiation of puberty. Reviews in Endocrine & Metabolic Disorders. 2007;8:1–9. doi: 10.1007/s11154-007-9026-4. [DOI] [PubMed] [Google Scholar]
- Smith JT, et al. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Smith JT, et al. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Thompson EL, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. American Journal of Physiology-Endocrinology and Metabolism. 2006;291:E1074–E1082. doi: 10.1152/ajpendo.00040.2006. [DOI] [PubMed] [Google Scholar]
- Thompson EL, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. Journal of Neuroendocrinology. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Wolfe AM, et al. Blockade of Singular Follicle-Stimulating-Hormone Secretion and Testicular Development in Photostimulated Djungarian Hamsters (Phodopus-Sungorus) by a Gonadotropin-Releasing-Hormone Antagonist. Biology of Reproduction. 1995;53:724–731. doi: 10.1095/biolreprod53.3.724. [DOI] [PubMed] [Google Scholar]