Abstract
Choline is an essential nutrient required for methyl group metabolism, but its role in carcinogenesis and tumor progression is not well understood. By utilizing a population-based study of 1508 cases and 1556 controls, we investigated the associations of dietary intake of choline and two related micronutrients, methionine and betaine, and risk of breast cancer. The highest quintile of choline consumption was associated with a lower risk of breast cancer [odds ratio (OR): 0.76; 95% confidence interval (CI): 0.58−1.00] compared with the lowest quintile. Two putatively functional single nucleotide polymorphisms of cholinemetabolizing genes, PEMT −774G>C (rs12325817) and CHDH +432G>T (rs12676), were also found be related to breast cancer risk. Compared with the PEMT GG genotype, the variant CC genotype was associated with an increased risk of breast cancer (OR: 1.30; 95% CI: 1.01−1.67). The CHDH minor T allele was also associated with an increased risk (OR: 1.19; 95% CI: 1.00−1.41) compared with the major G allele. The BHMT rs3733890 polymorphism was also examined but was found not to be associated with breast cancer risk. We observed a significant interaction between dietary betaine intake and the PEMT rs7926 polymorphism (Pinteraction=0.04). Our findings suggest that choline metabolism may play an important role in breast cancer etiology.—Xu, X., Gammon, M. D., Zeisel, S. H., Lee, Y. L., Wetmur, J. G., Teitelbaum, S. L., Bradshaw, P. T., Neugut, A. I., Santella, R. M., Chen, J. Choline metabolism and risk of breast cancer in a population-based study.
Keywords: methyl diet, methylation, genetic polymorphism, phosphatidylethanolamine N-methyltransferase, choline dehydrogenase, betaine-homocysteine methyltransferase
Methyl folate, methionine, and choline are the major sources of methyl groups in the human diet (1, 2). Metabolites of these micronutrients have an essential role in forming the universal methyl donor S-adenosylmethionine (SAM). SAM donates its labile methyl group in more than 80 biological methylation reactions, including the methylation of DNA, RNA, and protein.
Folate-mediated one-carbon metabolism has been studied with respect to breast cancer in several epidemiologic studies. Although not entirely consistent, both folate intake and genetic variations in genes of one-carbon metabolism have been associated with risk of breast cancer (3, 4). We previously reported such relationships in the Long Island Breast Cancer Study Project (LIBCSP) (5, 6). We found an inverse association between B vitamin intake and breast cancer risk among nonsupplement users. We also observed that a functional polymorphism, 677C>T in methylenetetrahydrofolate reductase (MTHFR rs1801133), a key one-carbon-metabolizing gene, was independently associated with an increased risk of breast cancer. Further, a significant interaction between this polymorphism and folate intake was apparent.
Choline is necessary for the structure and function of all cells and is crucial for sustaining life (7). It comes from diet or is synthesized de novo. Betaine (N,N,N-trimethylglycine) is the substrate for betaine-homocysteine methyltransferase (BHMT), acting as a methyl donor for methylating homocysteine. Betaine also can be obtained from food or from choline metabolism in liver and kidney; the latter conversion is modulated by choline concentration (8). Methionine is one of the essential dietary amino acids for humans and is the precursor for SAM. The tight interrelationship among these dietary methyl sources makes it important to assess them together when studying diet and its association with disease outcome (1).
Humans require additional choline from dietary sources besides the de novo synthesis in the body. A wide range of foods, including meat (e.g., chicken and beef liver), dairy products (e.g., eggs), vegetables (e.g., spinach), and baking products (e.g., wheat germ), are sources of choline, methionine, and betaine in the human diet (9). However, daily requirements for some of these nutrients are not well defined. Dietary deficiency of choline in humans results in fatty liver, liver damage, and muscle damage (10, 11). The most recent results from the Nurses’ Health Study showed that increasing choline intake was associated with an elevated risk of colorectal adenoma, whereas an inverse relationship was observed for betaine intake (12); moreover, total choline and betaine intake was inversely associated with total homocysteine levels in the same population (13).
Key enzymes involved in this pathway (Fig. 1) are phosphatidylethanolamine N-methyltransferase (PEMT), choline dehydrogenase (CHDH), and betaine-homocysteine methyltransferase (BHMT). PEMT catalyzes the only reaction for de novo synthesis of choline in the body via methylation of phosphatidylethanolamine to form phosphatidylcholine, using SAM as the methyl donor. CHDH catalyzes the oxidation of choline to betaine via a betaine aldehyde intermediate. BHMT catalyzes the synthesis of methionine from betaine and homocysteine. Functional genetic variations exist in these genes in humans. A polymorphism of the PEMT gene (rs7946) results in an amino acid substitution (V175M) and is associated with diminished enzyme activity and may confer susceptibility to nonalcoholic fatty liver disease (14). Women with genotypes CC or CG of another single nucleotide polymorphism (SNP) in the 5′-untranslated region of PEMT, −744G>C (rs12325817) are more susceptible to choline deficiency and to development of fatty liver compared with those with the GG genotype (15). Two SNPs in the coding region of the CHDH gene (+318A>C, rs9001 and +432G>T, rs12676) have been shown to associate with altered susceptibility to choline deficiency (15). BHMT has a nonsynonymous SNP (+742G>A, rs3733890) in its exon 6, and it has been suggested that altered function of BHMT may result in elevated homocysteine levels (16).
Figure 1.
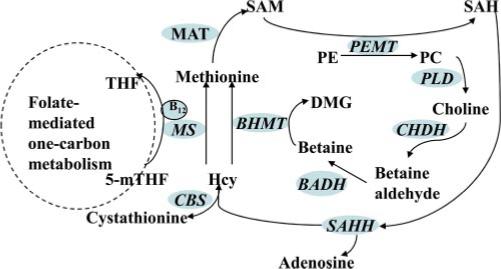
Schematic illustration for choline metabolism. Folate, choline, and methionine metabolism interact at the point at which homocysteine (Hcy) is converted to methionine. Folate mediates the generation of methionine using the methyl group, i.e., 5-methyltetrahydrofolate (5-mTHF), which is derived de novo from the one-carbon pool; alternatively, the methyl group of choline can be made available on conversion to betaine for the methylation of homocysteine. CHDH converts choline to betaine aldehyde, which can then be oxidized in the mitochondria or cytoplasm to betaine. BHMT catalyzes the methylation of homocysteine, using betaine as the methyl donor. SAM is converted following the regeneration of methionine by methionine adenosyltransferase (MAT). THF, tetrahydrofolate; MS, methionine synthase; SAH, S-adenosyl-l-homocysteine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PLD, phospholipase D; SAHH, S-adenosyl homocysteine hydrolase; BADH, betaine aldehyde dehydrogenase; DMG, N,N-dimethylglycine; CBS, cystathionine β-synthase.
Herein, we used the LIBCSP, a population-based case-control study, to investigate choline, methionine, and betaine intake and putatively functional variations of genes of choline metabolism, PEMT, CHDH, and BHMT, in relation to breast cancer risk.
MATERIALS AND METHODS
Study population
The main purpose of the LIBCSP was to investigate whether environmental factors, specifically polycyclic aromatic hydro-carbons and organochlorine pesticides, were associated with breast cancer risk. Details of the study design have been described in detail previously (17–19). In brief, case participants were women residing in Nassau and Suffolk counties of Long Island, New York, with newly diagnosed in situ or invasive breast cancer between August 1, 1996, and July 31, 1997. Community-based control participants were identified through random-digit dialing for those younger than 65 yr and through the Center for Medicare and Medicaid Services rosters for those 65 yr and older. Eligible controls were women who resided in the same Long Island counties as the cases, but who had no personal history of breast cancer and were frequency matched to the expected age distribution of case women by 5-yr age group. Interview response rates among eligible case and control participants were 82.1% (n=1,508) and 62.8% (n=1,556), respectively. Overall, 94% of the case participants and 93% of the control participants were Caucasians and ranged in age from 20 to 98 yr.
Data collection
The case-control questionnaire was administered shortly after identification of eligible case and control participants and assessed information on known and suspected risk factors for breast cancer, including passive and active cigarette smoking, lifetime alcohol use, menstrual and reproductive histories, exogenous hormone use, body size by decade of adult life, lifetime participation in recreational activities, prior medical history, and family history of breast cancer (http://epi.grants.cancer.gov/LIBCSP/projects/Questionnaire.html). In-person interviews were completed for 82.1% of case participants (n=1508) and 62.8% of control participants (n=1556). Ninety-eight percent of participants also completed a modified Block food frequency questionnaire (FFQ), which assessed intake of more than 100 food items in the year before the interview (19). The frequency and portion size data were translated to daily intakes of nutrients from both dietary and supplement sources using the National Cancer Institute's Diet-Sys version 3 for folate. A previously described protocol (9) and database from the U.S. Department of Agriculture (http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choline.html) were used to assess intakes of choline, methionine, and betaine. Habitual use of multivitamin supplements was also obtained from the FFQ. Among those who completed an interview, 2243 donated blood samples (1102 case and 1141 control participants) (17) and stored DNA samples were available for 1065 case participants (70.6% of case group) and 1109 control participants (71.3% of control group). As previously reported (17), an increased risk of breast cancer was found to be associated with lower parity, late age at first birth, little or no breastfeeding, a family history of breast cancer, and increasing income and education. Associations were similar when the analyses were restricted to respondents who donated blood (17, 18). The study protocol was approved by the institutional review boards of the collaborating institutions.
Genotyping
DNA was isolated from blood specimens using the methods described previously (18). Genotyping of PEMT rs7946, CHDH rs12676, and CHDH rs9001 were performed using the TaqMan 5′ allelic discrimination assay (Applied Biosystems, Foster City, CA, USA). The assay identification numbers were C_9245965_10 for PEMT rs7946, C_7553898_10 for CHDH rs12676, and C_7553897_10 for CHDH rs9001. Two probes for allelic discrimination were labeled with fluorescent dyes VIC and 6-carboxyfluorescein, respectively. Polymerase chain reactions (PCRs) were performed following the manufacturer's instruction. Genotyping of each sample was automatically analyzed by SDS 2.0 software (Applied Biosystems) for allelic discrimination. Blank (water) controls were included in each 384-well plate for quality control.
Genotyping of PEMT rs12325817 was performed by PCR-restriction fragment length polymorphism (RFLP) using the following primers: forward, 5′-ACTTCCTGGGTTGAAGCGATTCTC-3′; and reverse, 5′-TTTATTCTCTGGCCGTGCCCAG-3′. The 224-bp PCR products were digested with BsmBI (New England Biolabs Inc., Ipswich, MA, USA), thereby cutting the wild-type G allele into two products of 132 and 92 bp. Products were size-fractionated on a 2.5% agarose gel and visualized with ethidium bromide. The BHMT rs3733890 genotyping was performed at BioServe Biotechnologies (Laurel, MD, USA) using high-throughput matrix-assisted laser desorption/ionization time-of-flight as described elsewhere (6). To assure genotype accuracy, ∼200 randomly selected samples were regenotyped with a PCR-RFLP method for the CHDH rs12676 polymorphism using the following primers: forward, 5′-AGTCATCTCATTCCCCTCCGTGGATCAGA-3′; and reverse, 5′-TAGCACCAGTTGTACCTGTCGTCGCACA-3′. The 370-bp PCR products were digested with BssHII (New England Biolabs), thereby cutting the wild-type G allele into two products of 281 and 89 bp.
The mean call rate was 98%; the main reason for unascertained genotypes was insufficient DNA. For ∼10% of the study population, random duplicates were included as quality control samples; the concordance rate was higher than 99% for all polymorphisms in this study. All laboratory personnel were blinded to the case-control as well as quality control status of the specimens.
Statistical analysis
Unconditional logistic regression was used to estimate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for the association between the dietary nutrient intake and polymorphisms and breast cancer (20, 21). Dietary nutrient intakes were categorized on the basis of the distribution among the control participants. Age at reference (date of diagnosis for case group and date of identification for control group) was included in all models as a continuous variable. Energy intake (continuous) was included in models developed to evaluate the effects of dietary intake. Tests for trend were undertaken by treating each categorized variable as a continuous term and entering the variable into a logistic regression model. When all three methyl-related nutrients were analyzed together, dietary methyl status was modeled as a three-level categorical variable (low, intermediate, and high). Low methyl status was defined as intakes below the median level among control participants for total folate, choline, and methionine. High methyl status was defined as intakes at or above the median levels for all three nutrients. The rest of the study population was considered as intermediate. Joint effects of alcohol intake and choline, methionine, and betaine intake with respect to breast cancer risk were also investigated.
Hardy-Weinberg equilibrium (HWE) was tested with the Pearson goodness-of-fit statistic (22) for polymorphisms in the study to compare the observed and expected genotype frequencies among case and control participants, respectively. Dominant, recessive, and additive models were examined to evaluate the associations between genetic polymorphisms and breast cancer risk. Stratified analyses were performed with respect to multivitamin use (yes/no), menopausal status (pre/postmenopausal), breast cancer type (invasive/in situ), hormone replacement treatment (HRT) use (yes/no), and oral contraceptive (OC) use (yes/no). Effect modification on the multiplicative scale for these variables was evaluated using the log likelihood ratio test to compare the difference of log likelihood statistics for a model with or without a cross-product term (21).
We evaluated age-adjusted models for potential confounders including family history of breast cancer in a first-degree relative, history of benign breast disease, education, body mass index (BMI) at age 20, BMI at diagnosis, alcohol drinking, parity, lactation history, use of contraceptives, use of HRT, age at menarche, age at first birth, smoking status, mammography history, and race. If adding a covariate to the logistic regression model changed the effect estimate by 10% or more, the covariate was considered a confounder (20). None of the covariates tested met this criterion; thus, only the results of the age-adjusted model are presented.
Potential gene-environment interactions were tested on a multiplicative scale by performing likelihood ratio tests to compare the difference of log likelihood statistics for a model with or without a cross-product term for two main effect variables (21).
Linkage disequilibrium between two polymorphisms of the same gene was calculated as D′, which ranges from 0 (no linkage disequilibrium) to 1 or −1 (complete linkage disequilibrium) (23). The EH linkage utility program (http://www.genemapping.cn/eh.htm) (24) was used to determine χ2 statistics and P values for tests of allelic association between polymorphic markers.
All statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC, USA).
RESULTS
In our study population, the mean and median intakes were 326 and 321 mg/day for choline, 1.01 and 0.94 g/day for methionine, and 138 and 114 mg/day for betaine, respectively. Among all control particpants in our study, the top five contributors of choline were coffee (42.5%), eggs (9.5%), skim milk (5.6%), 2% milk (3.6%), and orange juice (3.3%). The top five contributors of betaine were cooked spinach (23.7%), bran cereal (16.2%), spaghetti (11.4%), dark bread (10.6%), and white bread (10.3%), whereas top sources of methionine were coffee (15.8%), skim milk (8.8%), 2% milk (5.5%), fish other than tuna (5.2%), and tuna (5.1%). As shown in Table 1, a reduced breast cancer risk was observed in the highest quintile of choline consumption with borderline significance (OR: 0.76; 95% CI: 0.58−1.00). The inverse associations between choline intake and breast cancer risk did not vary substantially with menopausal status (pre- vs. postmenopausal) or cancer type (invasive vs. in situ) (data not shown). Methionine and betaine intakes were not associated with breast cancer risk in our analysis (Table 1). Because folate, methionine, and choline are tightly linked as methyl donors, we further examined the three levels of overall dietary methyl status (described in the Materials and Methods section) in relation to breast cancer risk; however, no significant associations were observed (data not shown). Modification of the folate-breast cancer relationship by alcohol intake has been reported previously (4). In this study, alcohol did not modify the risk of breast cancer associated with choline, methionine, and betaine. No joint effect between alcohol and any of the three nutrient intakes was observed with respect to breast cancer risk.
TABLE 1.
Associations of daily intake of choline, methionine, and betaine with risk of breast cancer in the LIBCSP, 1996−1997
| Category | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ptrend |
|---|---|---|---|---|---|---|
| Choline | ||||||
| mg/day | <196.5 | 196.5−280.5 | 280.5−362.9 | 362.9−455.8 | >455.8 | |
| Case | 314 | 296 | 293 | 336 | 240 | |
| Control | 304 | 304 | 305 | 304 | 305 | |
| OR and 95% CI | 1.00 (ref) | 0.91 (0.73−1.15) | 0.91 (0.72−1.15) | 1.04 (0.82−1.31) | 0.76 (0.58−1.00)* | 0.29 |
| Methionine | ||||||
| g/day | <0.65 | 0.65−0.86 | 0.86−1.06 | 1.06−1.34 | >1.34 | |
| Case | 316 | 300 | 301 | 288 | 274 | |
| Control | 305 | 303 | 305 | 304 | 305 | |
| OR and 95% CI | 1.00 (ref) | 0.97 (0.77−1.23) | 0.98 (0.77−1.26) | 0.96 (0.73−1.26) | 0.94 (0.69−1.30) | 0.73 |
| Betaine | ||||||
| mg/day | <66.4 | 66.4−97.2 | 97.2−133.0 | 133.0−201.0 | >201.0 | |
| Case | 319 | 285 | 270 | 320 | 285 | |
| Control | 305 | 303 | 306 | 303 | 305 | |
| OR and 95% CI | 1.00 (ref) | 0.92 (0.73−1.16) | 0.87 (0.69−1.11) | 1.06 (0.83−1.36) | 0.96 (0.74−1.24) | 0.83 |
ORs were adjusted for age and daily energy intake; absolute nutrient intake values were categorized according to quintiles among controls. ref, referent.
P < 0.05.
Table 2 summarizes the associations of breast cancer and five polymorphisms in three choline/betaine-metabolizing genes. All genotype distributions were in agreement with HWE among controls except for the CHDH rs12676 polymorphism (PHWE=0.02). However, genotyping results using two different methods were 100% in concordance for the randomly selected ∼200 samples. Two SNPs were independently associated with breast cancer risk in our study population. The minor C allele of the PEMT rs12325817 polymorphism was associated with increased breast cancer risk in a dose-dependent fashion (Ptrend=0.04) (Table 2). Compared with individuals with the GG genotype, those with the CC genotype had an age-adjusted OR of 1.30 (95% CI: 1.01−1.67). The minor T allele of the CHDH rs12676 polymorphism was associated with an increased breast cancer risk. Compared with the GG genotype, T allele carriers have 19% increased risk (95% CI: 1.00−1.41). However, when the GT and TT genotypes were analyzed separately, a significant association was only observed for the GT heterozygous group (Table 2). The BHMT rs3733890 polymorphism has been reported previously in this population, and it was not associated with breast cancer risk (6). We also examined the gene-cancer relationship stratified by multivitamin use (yes vs. no), menopausal status (pre- vs. postmenopausal), and cancer type (invasive vs. in situ). Comparable results were observed (data not shown).
TABLE 2.
Associations of choline-metabolizing gene SNPs and breast cancer risk
| Gene and SNP | Genotype | Case (%) | Control (%) | OR (95% CI)a | Ptrend |
|---|---|---|---|---|---|
| PEMT rs7946 | TT | 462 (44.9) | 483 (45.0) | 1.00 (ref) | 0.65 |
| TC | 438 (42.5) | 462 (43.0) | 1.00 (0.83−1.20) | ||
| CC | 130 (12.6) | 129 (12.0) | 1.09 (0.83−1.44) | ||
| TC/CC | 568 (55.1) | 591 (55.0) | 1.02 (0.86−1.21) | ||
| C allele frequency | 0.339 | 0.335 | |||
| PHWE | 0.10 | 0.25 | |||
| PEMT rs12325817 | GG | 310 (30.0) | 359 (33.1) | 1.00 (ref) | 0.04# |
| GC | 512 (49.5) | 533 (49.2) | 1.12 (0.92−1.37) | ||
| CC | 213 (20.6) | 192 (17.7) | 1.30 (1.01−1.67)* | ||
| GC/CC | 725 (70..0) | 725 (66.9) | 1.17 (0.97−1.41) | ||
| C allele frequency | 0.453 | 0.423 | |||
| PHWE | 0.95 | 0.81 | |||
| CHDH rs12676 | GG | 514 (48.9) | 580 (53.1) | 1.00 (ref) | 0.16 |
| GT | 444 (42.2) | 410 (37.5) | 1.23 (1.02−1.47)* | ||
| TT | 94 (8.9) | 103 (9.4) | 1.05 (0.77−1.42) | ||
| GT/TT | 538 (51.1) | 513 (46.9) | 1.19 (1.00−1.41)* | ||
| T allele frequency | 0.300 | 0.282 | |||
| PHWE | 0.89 | 0.02 | |||
| CHDH rs9001 | AA | 822 (79.0) | 847 (78.2) | 1.00 (ref) | 0.80 |
| AC | 199 (19.1) | 216 (19.9) | 0.97 (0.78−1.20) | ||
| CC | 19 (1.8) | 20 (1.9) | 0.99 (0.52−1.87) | ||
| AC/CC | 218 (21.0) | 236 (21.8) | 0.97 (0.79−1.20) | ||
| C allele frequency | 0.114 | 0.118 | |||
| PHWE | 0.09 | 0.16 | |||
| BHMT rs3733890b | GG | 510 (48.1) | 530 (47.8) | 1.00 (ref) | 0.61 |
| GA | 443 (41.8) | 456 (41.2) | 1.01 (0.84−1.21) | ||
| AA | 108 (10.2) | 122 (11.1) | 0.90 (0.67−1.20) | ||
| GA/AA | 551 (51.9) | 578 (52.2) | 0.98 (0.83−1.18) | ||
| A allele frequency | 0.311 | 0.316 | |||
| PHWE | 0.42 | 0.11 |
ref, referent.
OR adjusted for age.
Published previously in Xu et al. (6).
P = 0.04 (multiplicative interaction);
P < 0.05.
Because the PEMT rs12325817 (−774G>C) SNP is in the promoter region, which has an estrogen response element (25), this SNP may alter estrogen responsiveness (15). We explored whether the association of this SNP and breast cancer risk was modified by exogenous hormone use. When stratified by use of HRT, the PEMT polymorphism was associated with breast cancer risk only among HRT users (Ptrend=0.04). Compared with individuals with the GG genotype, those with the GC or GG genotype had an age-adjusted OR of 1.41 (95% CI: 0.97−2.04) or 1.56 (95% CI: 0.99−2.46), respectively. The effect of this polymorphism did not differ by the use of OCs.
A high degree of linkage disequilibrium was observed between the CHDH rs12676 and rs9001 polymorphisms (D′=−0.98, P<0.001). The negative sign of the D′ indicates that G the alleles of rs12676 and the C allele of rs9001 were linked. The two PEMT polymorphisms were partially linked (D′=−0.47, P<0.001). When combined genotypes were examined, none of the combined genotypes were significantly associated with breast cancer risk (data not shown).
We examined the gene-environment interactions between each of the five SNPs and choline/methionine/betaine intake in relation to breast cancer (Table 3). Among all combinations, only the interaction between PEMT rs7946 and betaine intake was significant (Pinteraction=0.04). An almost 2-fold increase of risk was observed among women with the variant genotype and lowest betaine intake (OR: 1.90; 95% CI: 1.14−3.16).
TABLE 3.
Interactions of PEMT rs7946 and betaine intake in relation to breast cancer risk
| Betaine intake |
|||
|---|---|---|---|
| PEMT rs7946 | High | Medium | Low |
| TT | |||
| Case/control | 149/163 | 152/168 | 151/147 |
| OR (95% CI)a | 1.00 (ref.) | 1.00 (0.73−1.36) | 1.11 (0.81−1.53) |
| TC | |||
| Case/control | 163/150 | 130/143 | 140/162 |
| OR (95% CI)a | 1.22 (0.89−1.67) | 0.99 (0.72−1.38) | 0.94 (0.68−1.29) |
| CC | |||
| Case/control | 45/46 | 34/51 | 50/30 |
| OR (95% CI)a | 1.09 (0.68−1.75) | 0.76 (0.47−1.25) | 1.90 (1.14−3.16)* |
Unconditional logistic regression adjusted for age. P = 0.04 for multiplicative interaction;
P < 0.05.
DISCUSSION
Our examination of a population-based sample of case and control participants of the LIBSCP revealed that breast cancer risk was reduced 24% among women with a high dietary intake of choline and was increased 30% among women homozygous for the minor allele of PEMT rs12325817, a gene of choline metabolism. We also noted a significant interaction between betaine intake and the PEMT rs7946 polymorphism. To the best of our knowledge, this is the first study on choline metabolism and breast cancer. The population-based study design, in which cases encompassed a broad range of ages and were drawn from a defined geographic area, yields results that are more generalizable than a series of cases from a narrow age range or from a single institution. In addition, the relatively large sample size allows multiple risk factors to be taken into consideration in studying associations, with the ability to conduct stratified analyses and adjustment in multivariate models.
Choline is an essential nutrient required for one-carbon metabolism, structural integrity and signaling functions of cell membranes, and neurotransmitter synthesis (7). Cancers usually exhibit a significantly altered choline phospholipid metabolite profile that is characterized by elevations of phosphocholine and total choline-containing compounds compared with those in normal tissues (26, 27). Although animal studies have implied a causal relationship between choline deficiency and carcinogenesis (28, 29), its role in human carcinogenesis is not clear.
In a case-control study conducted by the March of Dimes in Berkley, California, low dietary intake of choline in pregnant women significantly increased neural tube and oral cleft defects in babies (30, 31). This population-based study (∼800 subjects) complements smaller clinical studies in which 60 or so subjects developed organ dysfunction when deprived of choline (32). The lack of additional studies may be due in part to the fact that a food composition database for choline and betaine has not been available until recently (9). The general intake level of choline of the U.S. population is not known, and only adequate intakes of ∼550 mg/day for men and ∼425 mg/day for women have been estimated (33). Two recent epidemiological studies examined choline and betaine intake at the population level. One is the Nurses’ Health Study, in which the median intakes of total choline and of betaine were 323 and 189 mg/day, respectively (12, 13). The other is the European Prospective Investigation into Cancer and Nutrition (EPIC) study, in which the mean choline intake was 300 mg/day and mean betaine intake was 214 mg/day (34).
We previously reported on folate-mediated one-carbon metabolism in relation to breast cancer risk in this population (5, 6). The pathways of choline and one-carbon metabolism intersect at the formation of methionine from homocysteine, which makes choline and folate metabolism closely interrelated. Folate mediates the generation of methionine from homocysteine using 5-methyltetrahydrofolate, which is derived de novo from the one-carbon pool; alternatively, a methyl group from choline, via the intermediate betaine, can be used for the methylation of homocysteine. SAM is formed from methionine catalyzed by methionine adenosyltransferase (Fig. 1). Our current analysis suggests an inverse association between choline intake and breast cancer risk. Lower choline availability may tilt the SAM pool balance and consequently induce aberrant DNA methylation. Animal studies have suggested that choline deficiency has cancer-initiating and cancer-promoting activities, given the fact that choline-deficient rats had a higher incidence of spontaneous hepatocarcinoma and were more sensitive to carcinogens (28). Aberrant DNA methylation may be the underlying mechanism, as rats with a choline-deficient diet have hypomethylation of CpG sites of c-myc along with overexpression of this gene (29). Results from the Nurses’ Health Study showed that higher choline intake was associated with an elevated risk of colorectal adenoma (12), and the positive association persisted after adjustment for multiple dietary factors and when assessed for different sizes and sites of the adenoma. The different relation between choline intake and disease outcome could be due to the different etiology of breast cancer and colorectal adenoma.
We found that one SNP (rs12325817) of PEMT was associated with an increased breast cancer risk in our study population. PEMT is responsible for endogenous biosynthesis of the choline moiety and this activity is increased by estrogen treatment (25). The rs12325817 (−774G>C) SNP resides in the promoter region of PEMT, which was proposed to have an estrogen response element. Thus, this SNP may alter estrogen responsiveness of the promoter (15). This mechanism was supported by our observation that a stronger PEMT-breast cancer relationship was observed among HRT users.
We also observed that carriers of the minor T allele of the CHDH rs12676 (+432G>T) SNP had a modest increase in breast cancer risk. CHDH converts choline to betaine aldehyde, which is then oxidized to betaine, a methyl donor for homocysteine. A previous study showed that this SNP was associated with increased susceptibility to choline deficiency (15). This SNP produces an amino acid substitution that could alter the enzyme activity and consequently affect the methyl moiety availability for the methylation reaction. However, we should interpret this finding with caution as the genotype distribution in our control group was not in agreement with HWE, even though we have excluded genotyping error as an underlying cause.
Although neither PEMT rs7946 nor betaine intake was independently related to breast cancer risk in this study population, a significant gene-environmental interaction was found. Women carrying the variant allele with low betaine intake have an ∼2-fold increased risk. The underlying mechanism is not clear. If this finding is substantiated, it implies that the effect of genotype could be modified by lifestyle such as dietary intake. The genetic effect is only obvious when the betaine intake is suboptimal (low). However, such an observation could be a chance finding. We should interpret the result with caution, and replication from other large studies is warranted.
We did not measure choline or betaine levels in peripheral blood. Such measurements, although biologically relevant, may only reflect short-term dietary intake and do not accurately reflect long-term exposure (35). For this reason estimates based on a FFQ were considered to be the best estimates of dietary intake of choline and betaine. A potential limitation of the Block FFQ used in our study is its lack of complete assessment of commonly consumed products that are choline-rich. The current nutrient databases for choline and betaine are limited but suitable for FFQ diet intake analyses. One study showed that a 3-day food record underestimated choline intake by 25% compared with direct measurements from food intake (36). It is likely that FFQ estimates were similarly low. However, the ranking of intakes was unlikely to change between methods of assessment as the Block FFQ was shown to be a valid and reliable dietary assessment tool for estimating usual food intake and ranking individuals into categories of intake of micronutrients (37, 38). Future studies should incorporate newly developed and commonly consumed choline-rich products in the FFQs or other dietary history assessment tools to enhance coverage.
In summary, we found that dietary choline intake was inversely associated with breast cancer risk. Two SNPs of PEMT and CHDH were also associated with breast cancer risk. Results from our study indicate that choline metabolism may play an important role in breast cancer etiology, and further investigation is needed to clarify the underlining mechanisms.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (CA109753 to J.C.; DK55865 to S.Z.) and in part by grants from the U.S. Department of Defense (BC031746), the National Cancer Institute, and the National Institute of Environmental Health Sciences (UO1CA/ES66572, UO1CA66572, P30ES09089, and P30ES10126) and by the University of North Carolina Clinical Nutrition Research Unit (DK56350) and the Center for Environmental Health and Susceptibility (ES10126). X.X. is a recipient of predoctoral traineeship award W81XWH-06-1-0298) of the Department of Defense Breast Cancer Research Program.
REFERENCES
- 1.Niculescu MD. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 2.Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- 3.Lewis SJ, Harbord RM, Harris R, Smith GD. Meta-analyses of observational and genetic association studies of folate intakes or levels and breast cancer risk. J. Natl. Cancer Inst. 2006;98:1607–1622. doi: 10.1093/jnci/djj440. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. J. Natl. Cancer Inst. 2007;99:64–76. doi: 10.1093/jnci/djk006. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Gammon MD, Chan W, Palomeque C, Wetmur JG, Kabat GC, Teitelbaum SL, Britton JA, Terry MB, Neugut AI, Santella RM. One-carbon metabolism, MTHFR polymorphisms, and risk of breast Cancer. Cancer Res. 2005;65:1606–1614. doi: 10.1158/0008-5472.CAN-04-2630. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Gammon MD, Zhang H, Wetmur JG, Rao M, Teitelbaum SL, Britton JA, Neugut AI, Santella RM, Chen J. Polymorphisms of one-carbon metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis. 2007;28:1504–1509. doi: 10.1093/carcin/bgm061. [DOI] [PubMed] [Google Scholar]
- 7.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu. Rev. Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 8.Ueland PM, Holm PI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med. 2005;43:1069–1075. doi: 10.1515/CCLM.2005.187. [DOI] [PubMed] [Google Scholar]
- 9.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 10.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- 11.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 12.Cho E, Willett WC, Colditz GA, Fuchs CS, Wu K, Chan AT, Zeisel SH, Giovannucci EL. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl. Cancer Inst. 2007;99:1224–1231. doi: 10.1093/jnci/djm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiuve SE, Giovannucci EL, Hankinson SE, Zeisel SH, Dougherty LW, Willett WC, Rimm EB. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr. 2007;86:1073–1081. doi: 10.1093/ajcn/86.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J, da Costa KA, Fischer LM, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005;19:1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg IS, Park E, Ballman KV, Berger P, Nunn M, Suh DS, Breksa AP, 3rd, Garrow TA, Rozen R. Investigations of a common genetic variant in betaine-homocysteine methyltransferase (BHMT) in coronary artery disease. Atherosclerosis. 2003;167:205–214. doi: 10.1016/s0021-9150(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 17.Gammon MD, Neugut AI, Santella RM, Teitelbaum SL, Britton JA, Terry MB, Eng SM, Wolff MS, Stellman SD, Kabat GC, Levin B, Bradlow HL, Hatch M, Beyea J, Camann D, Trent M, Senie RT, Garbowski GC, Maffeo C, Montalvan P, Berkowitz GS, Kemeny M, Citron M, Schnabe F, Schuss A, Hajdu S, Vincguerra V, Collman GW, Obrams GI. The long island breast cancer study project: Description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res. Treat. 2002;74:235–254. doi: 10.1023/a:1016387020854. [DOI] [PubMed] [Google Scholar]
- 18.Gammon MD, Santella RM, Neugut AI, Eng SM, Teitelbaum SL, Paykin A, Levin B, Terry MB, Young TL, Wang LW, Wang Q, Britton JA, Wolff MS, Stellman SD, Hatch M, Kabat GC, Senie R, Garbowski G, Maffeo C, Montalvan P, Berkowitz G, Kemeny M, Citron M, Schnabel F, Schuss A, Hajdu S, Vinceguerra V. Environmental toxins and breast cancer on long island. I. polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol. Biomarkers Prev. 2002;11:677–685. [PubMed] [Google Scholar]
- 19.Gaudet MM, Britton JA, Kabat GC, Steck-Scott S, Eng SM, Teitelbaum SL, Terry MB, Neugut AI, Gammon MD. Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol. Biomarkers Prev. 2004;13:1485–1494. [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S. Modern Epidemiology. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- 21.Hosmer DW. Applied Logistic Regression. Wiley; New York: 1989. [Google Scholar]
- 22.Cox DG, Canzian F. Genotype transposer: automated genotype manipulation for linkage disequilibrium analysis. Bioinformatics. 2001;17:738–739. doi: 10.1093/bioinformatics/17.8.738. [DOI] [PubMed] [Google Scholar]
- 23.Lewontin RC. On measures of gametic disequilibrium. Genetics. 1988;120:849–852. doi: 10.1093/genetics/120.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terwilliger JD, Ott. J. Handbook of Human Genetic Linkage. Johns Hopkins University Press; Baltimore: 1994. [Google Scholar]
- 25.Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J. Cell. Biochem. 2003;90:525–533. doi: 10.1002/jcb.10659. [DOI] [PubMed] [Google Scholar]
- 27.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7:1109–1123. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 28.Newberne PM, Rogers AE. Labile methyl groups and the promotion of cancer. Annu. Rev. Nutr. 1986;6:407–432. doi: 10.1146/annurev.nu.06.070186.002203. [DOI] [PubMed] [Google Scholar]
- 29.Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn. J. Cancer Res. 1999;90:909–913. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 31.Zeisel SH, Mar MH, Zhou Z, da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 32.Fischer LM, Dacosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yates AA, Schlicker SA, Suitor CW. Dietary reference intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J. Am. Diet. Assoc. 1998;98:699–706. doi: 10.1016/S0002-8223(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 34.Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur. J. Clin. Nutr. 2007 doi: 10.1038/sj.ejcn.1602725. [E-pub ahead of print] doi: 10.1038/sj.ejcn.1602725. [DOI] [PubMed] [Google Scholar]
- 35.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer LM, Scearce JA, Mar MH, Patel JR, Blanchard RT, Macintosh BA, Busby MG, Zeisel SH. Ad libitum choline intake in healthy individuals meets or exceeds the proposed adequate intake level. J. Nutr. 2005;135:826–829. doi: 10.1093/jn/135.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 38.Potischman N, Swanson CA, Coates RJ, Weiss HA, Brogan DR, Stanford JL, Schoenberg JB, Gammon MD, Brinton LA. Dietary relationships with early onset (under age 45) breast cancer in a case-control study in the United States: influence of chemotherapy treatment. Cancer Causes Control. 1997;8:713–721. doi: 10.1023/a:1018475203820. [DOI] [PubMed] [Google Scholar]


