Abstract
Carcinoid tumors are rare neuroendocrine tumors with a predilection for the gastrointestinal tract. Protein kinase D (PKD), a novel serine/threonine protein kinase, has been implicated in the regulation of transport processes in certain cell types. We have reported an important role for PKD in stimulated peptide secretion from a human (BON) carcinoid cell line; however, the role of PKD isoforms, including PKD2, in the proliferation and invasion of carcinoid tumors remains unclear. In the present study, we found that overexpression of PKD2 by stable transfection of BON cells with PKD2-wild type (PKD2WT) significantly increased proliferation and invasion compared to cells transfected with PKD2-kinase dead (PKD2KD) or pcDNA3 (control). Similarly, inhibition of PKD2 activity with small interfering RNA (siRNA) significantly decreased proliferation and invasion compared to cells transfected with non-targeting control (NTC) siRNA. These data support an important role for PKD2 in carcinoid tumor progression. Targeted inhibition of the PKD family may prove to be a novel treatment option for patients with carcinoid tumors.
Keywords: protein kinase D2 (PKD2), carcinoid, BON cell line, small interfering RNA (siRNA), neuroendocrine
INTRODUCTION
Carcinoid tumors, rare neuroendocrine tumors with a predilection for the gastrointestinal tract [1], are a morphologically and physiologically diverse subset of tumors, synthesizing a variety of bioactive substances, including serotonin, histamine, chromogranin A, prostaglandins, and intestinal hormones [2]. The release of these amines and peptides may cause a devastating sequelae known as carcinoid syndrome in the presence of liver metastasis or retroperitoneal disease, with characteristic symptoms such as cutaneous flushing, abdominal cramping, diarrhea, bronchospasm and tricuspid valve thickening [3–5]. Carcinoids are generally slow-growing, indolent neoplasms, but multicentricity and early metastasis occur in a subset of aggressive tumors. Factors thought to regulate growth and stimulate invasion of carcinoid tumors include gastrin, serotonin, hepatocyte growth factor (HGF), and insulin growth factor (IGF), among others [6]. Factors found to inhibit tumor secretion, growth, and metastasis include the inhibitory hormone somatostatin, α-interferon, and the inhibitor of polyamine biosynthesis, α-difluoromethylornithine (DFMO) [5, 7–10]. However, the molecular mechanisms linking growth factor signaling to carcinoid growth and invasion are poorly understood.
Protein kinase D (PKD), a novel family of diacylglycerol (DAG)-stimulated serine/threonine protein kinases which includes PKD1, PKD2, and PKD3, lies downstream of PKCs in a novel signal transduction pathway [11–13]. PKD family proteins have recently been implicated in myriad fundamental biological processes, including Golgi organization, hormone secretion, vesicle transport, proliferation, tumor metastasis, and immune responses [14]. Like PKD1, which is known to be activated by upstream PKC isoforms, PKD2 is also activated through the induction of PKC. Known stimulators of PKD2 activity include phorbol esters, growth factors, and neuropeptides such as gastrin, bombesin, and neurotensin [13, 15, 16], suggesting an important role for PKD2 in growth factor-mediated cell proliferation. Preliminary evidence suggests an important role for PKD2 in the regulation of tumor cell survival [12]; however, the mechanisms contributing to PKD2-mediated cell cycle regulation have not yet been delineated.
Progress in the management of carcinoid tumors has been hampered by the relative rarity of the disease and the lack of carcinoid tumor models to evaluate tumor behavior. In this regard, the novel BON cell line, established from a human pancreatic carcinoid tumor and characterized in our laboratory [5], represents one of the only carcinoid cell lines in the world and has proven to be a valuable in vitro and in vivo carcinoid tumor model. BON cells display morphological and physiological characteristics consistent with the carcinoid phenotype, including the presence of numerous dense core granules and the expression and secretion of chromogranin A, serotonin, pancreastatin, and other peptides. The BON cell line is especially useful in the delineation of mechanisms underlying tumor hormone secretion, growth, and invasion, and serves as a novel model for carcinoid behavior [5, 9–11]. We have previously utilized the cell line to study the effects of various agents on tumor growth in vivo, and have defined an important role for PKD in neurotensin secretion in vitro. The purpose of our current study was to determine the role of the PKD2 isoform on BON cell growth and invasion.
MATERIALS AND METHODS
Reagents and antibodies
The anti-PKD2 polyclonal antibodies were from Abcam (Cambridge, MA). The anti-GST-FITC antibody was from Alpha Diagnostic International (San Antonio, TX). The wild-type and kinase-dead GST-PKD2 constructs were from Dr. Vivek Malhotra (University of California San Diego). PKD2 and non-targeting siRNA were synthesized by custom SMARTPool siRNA Design Service of Dharmacon (Lafayette, CO). The enhanced chemiluminescence (ECL) system for Western immunoblot analysis was from Amersham Biosciences (Piscataway, NJ). Immun-Blot polyvinylidene difluoride (PVDF) membranes were from Bio-Rad (Hercules, CA). Cell lysis buffer was from Cell Signaling (Danvers, MA). Tissue culture media and reagents were from Invitrogen (Carlsbad, CA). Cell Proliferation MTT assay was from Roche (Indianapolis, IN). BD Biocoat Matrigel Invasion assay was from BD Biosciences (San Jose, CA).
Cell culture and establishment of stable cell lines
BON cells are maintained in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and nutrient mixture, F12K, supplemented with 5% fetal bovine serum in 5% CO2 at 37°C. BON cell clones expressing GST-PKD2WT (wild type) and GST-PKD2KD (kinase dead) were established in our laboratory. Briefly, parental BON cells were co-transfected with pcDNA3 (vector), GST-PKD2WT, or GST-PKD2KD by electroporation. Stably transfected clones were selected in medium containing 800 μg/ml of G418 (Cellgro). Individual G418-resistant clones were isolated using trypsin, transferred, and subcultured. Stable cell clones were screened by immunofluorescent staining using anti-GST-FITC antibody and fluorescent microscopy. Once established, clones were maintained in media containing 400 μg/ml of G418.
siRNA transfection
Non-targeting control (NTC) or PKD2 siGENOME siRNA (200nM) was transfected into BON cells by electroporation (400 V, 500 μfarads) using GenePulser XCell (Bio-Rad). Cells were allowed to grow in media for 24 h and were subsequently trypsinized for use in MTT or invasion assays.
Protein preparation and Western blotting
After treatment or transfection, Western immunoblot analyses were performed as described previously [17]. Cells were incubated with lysis buffer at 4°C for 30 min. Lysates were clarified by centrifugation (10,000 × g for 30 min at 4°C) and protein concentrations determined using the method of Bradford [18]. Briefly, total protein (60 μg) was resolved on a 10% Nu-PAGE Bis-Tris gel and transferred to PVDF membranes. Filters were incubated overnight at 4°C in blotting solution (Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween 20), followed by a 1 h incubation with primary antibodies. Filters were washed three times in a blocking solution and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. After three additional washes, the immune complexes were visualized by ECL detection.
MTT assay
MTT assay was performed as previously described [19]. Briefly, after treatment or transfection, cells were washed with PBS, counted, and diluted to 50,000 cells/ml. Cells (5,000 cells/well) were plated in duplicate in 96 well plates. After 24 h of incubation in 5% CO2 at 37°C, MTT labeling reagent (10 μl) was added, followed by the addition of 100 μl ofsolubilization solution 4 h later. The production of blue formazan produced by viable cells was measured on a microplate reader at an absorbance of 570 nm (against a reference of 650 nm) per the manufacturer’s protocol. Experiments were performed in triplicate.
Invasion assay
Matrigel invasion assay was performed as previously described [20]. Matrigel inserts (3 per cell line) were rehydrated by adding 0.5 ml of DMEM/F12 50/50 media without FBS to the insert and well and incubating in 5% CO2 at 37°C for 2 h. Media was removed, and 0.75 ml DMEM/F12 containing 5% FBS was added to each well; IGF-1 (10ng/ml) was added to media for invasion assay of siRNA-transfected cells. Cell suspensions in FBS-free DMEM/F12 culture media containing 50,000 cells/ml were prepared, and 0.5 ml was added to each insert. Chambers were then placed in 5% CO2 at 37°C for 24 h. Non-invading cells were removed by scrubbing with a cotton tipped swab, the membrane was placed in 500 μl of methanol at room temperature for 10 min, and then transferred to 500 μl of 1% crystal violet for 15 min to stain. Total number of cells invading each membrane was then counted.
Statistical analysis
Both absorbance from BON-MTT assay and the number of cells invading Matrigel membrane were analyzed using one-way classification analysis of variance. Groups were assessed at the 0.05 of significance. Fisher’s least significant difference procedure was used for multiple comparisons with Bonferroni adjustment for the number of comparisons. All statistical computations were conducted using the SAS® system, Release 9.1 [21].
RESULTS
Expression of PKD2WT increases proliferation of BON cells
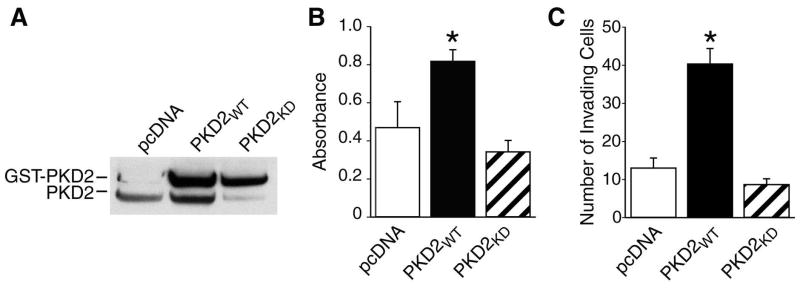
Since the role of the PKD2 isoform in proliferation and invasion of BON cells remains unclear, we first determined the effect of PKD2 overexpression on BON cell proliferation. BON cells were co-transfected with pcDNA3, GST-PKD2WT, or GST-PKD2KD by electroporation, and stably transfected clones were selected in medium containing G418. Expression of endogenous PKD2 and GST-PKD2 was assessed by Western blotting (Fig. 1A). After plating the cells in duplicate, MTT assay was then performed, and cell proliferation determined at 24 h by measurement of blue formazan production by microplate reader. Compared with cells transfected with pcDNA3, cells transfected with GST-PKD2WT demonstrated a greater than 2-fold increase in proliferation (Fig. 1B). There was not a statistically significant difference in proliferation of GST-PKD2KD cell clones compared with cells transfected with pcDNA3. The experiment was repeated in triplicate with identical results.
Fig. 1. GST-PKD2WT expression leads to a significant increase in proliferation and invasion of BON cell clones.
BON cells were stably co-transfected with pcDNA3 (vector), GST-PKD2WT, or GST-PKD2KD by electroporation. A. BON cell clones were lysed and Western blot was performed using anti-PKD2 antibody. B. Following stable transfection, MTT assay was performed. Cells were plated in duplicate; MTT labeling reagent was added, followed by the addition of solubilization solution and measurement of blue formazan production. C. Following stable transfection, Matrigel invasion assay of stable BON cell clones was performed. Cells were plated in triplicate in Matrigel invasion chambers; 5% FBS served as a chemoattractant. Data represent means of triplicate determinations ± SD; * = p<0.05 vs. pcDNA.
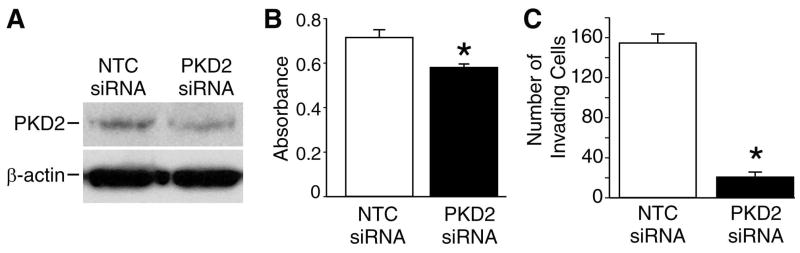
To confirm the importance of the PKD2 isoform on BON cell proliferation and invasion, we next transfected BON cells with either non-targeting control (NTC) or PKD2 siRNA; 24 h after transfection, cells were replated in duplicate for MTT assay, and proliferation was assessed 24 h later (48 h following transfection). Knockdown of PKD2 expression was confirmed by Western blotting (Fig. 2A). Consistent with our previous findings, inhibition of PKD2 with siRNA led to significantly decreased proliferation relative to cells transfected with NTC siRNA (Fig. 2B).
Fig. 2. Transfection with PKD2 siRNA significantly decreases proliferation and invasion of BON cells.
Non-targeting control (NTC) or PKD2 siGENOME siRNA (200nM) was transfected into BON cells by electroporation. A. Cells were lysed and Western blot was performed using anti-PKD2 antibody. β-actin was used as a loading control (bottom row). B. MTT assay was performed 24 h following transfection; MTT labeling reagent was added, followed by the addition of solubilization solution and measurement of blue formazan production. C. Following transfection with NTC or PKD2 siRNA, Matrigel invasion assay was performed. Cells were plated in triplicate in Matrigel invasion chambers; 5% FBS and IGF-1 (10ng/ml) served as chemoattractants. Data represent means of triplicate determinations ± SD; * = p<0.05 vs. NTC siRNA.
Expression of PKD2WT increases invasion of BON cells
Next, BON cells stably transfected with pcDNA3, GST-PKD2WT, or GST-PKD2KD were plated in triplicate in Matrigel invasion chambers; invasion was assessed after 24 h. Similar to our previous findings, GST-PKD2WT BON cells demonstrated significantly increased invasion of Matrigel (40 ± 5 cells/chamber) when compared with control cells (13.3 ± 1.2 cells/chamber) (Fig. 1C). There was not a statistically significant difference in proliferation of GST-PKD2KD cell clones (8 ± 1.5 cells/chamber) compared with cells transfected with pcDNA3. These results demonstrate that overexpression of PKD2 leads to increased proliferation invasion of the BON cell line.
To further confirm these results, BON cells transfected with NTC or PKD2 siRNA were plated in triplicate in Matrigel invasion chambers, and invasion was assessed after 24 h (48 h following transfection). BON cells transfected with PKD2 siRNA demonstrated significantly decreased invasion of Matrigel (21 ± 2) relative to cells transfected with NTC siRNA (154 ± 13) (Fig. 2C). Taken together, our results suggest an important role for the PKD2 isoform in BON cell invasion.
DISCUSSION
In our current study, we have demonstrated that the stable overexpression of PKD2 leads to both increased proliferation and invasion of the BON endocrine cell line. To corroborate these findings, BON cells were transfected with NTC or PKD2 siRNA; inhibition of PKD2 led to a decrease in proliferation and invasion relative to control cells. Therefore, we conclude that PKD2 is an important regulator of BON cell proliferation and invasion.
The members of the PKD family of serine/threonine kinases exhibit a high degree of homology, particularly in the catalytic domain, which distinguishes them from members of the protein kinase C (PKC) family [22]. PKD1 is the most clearly characterized isoform of the PKD family, and has been implicated in a variety of physiological processes, such as Golgi function, vesicular transport, cell proliferation, and apoptosis [22, 23]. Less is known about the function of the PKD2 isoform. Originally described by Sturany et al [15], PKD2 is highly expressed in a wide variety of human and murine tissues and cell lines and is particularly localized to highly proliferative tissues such as colonic mucosa and testis. Known stimulators of PKD2 activity include phorbol esters, growth factors, and neuropeptides such as gastrin, bombesin, and neurotensin [13, 15, 16]. Given the importance of these factors to biological processes such as secretion, growth, and transformation, it suggests that PKD2 may play an important role in cell proliferation and invasion. Mihailovic et al [12] found that PKD2 is the major PKD isoform expressed in Bcr-Abl+ human myeloid leukemia cells and that PKD2 is a novel mediator of NF-qB activation in these cells, further supporting a role for PKD2 in neoplastic transformation. However, evidence for a direct role of PKD2 in the proliferation and invasion of other types of cancers is lacking.
Studies using BON cells have characterized a number of regulators of carcinoid growth and secretion. For example, gastrin, nerve growth factor, and fibroblast growth factor (FGF) stimulate growth in a nonautocrine manner, while serotonin and insulin-like growth factor (IGF)-1, produced and secreted by BON cells, act as autocrine regulators of growth [6, 24–26]. These findings demonstrate an important relationship between BON secretion and proliferation. We have reported an important role for PKD1 in the regulation of neurotensin secretion from BON cells [11]. We speculate that, in addition to roles in hormone production, storage, and secretion, PKD may regulate proliferation and cell migration through the actions of secreted products or via direct downstream cell signaling.
Growth factors such as gastrin and IGF-1 stimulate PKD activity [6, 25]. In addition to its role in gastric acid secretion, gastrin plays an important role in tumor physiology, as it has been shown to regulate tumor growth and cell invasion [27]. There is a correlation between hypergastrinemia and the occurrence of gastric carcinoid tumors, as well as gastric and colonic adenocarcinoma [7]. In vitro, gastrin stimulates growth of BON cells in a dose-dependent fashion [6]; however, while gastrin acts as an autocrine growth factor for some carcinoid tumors, BON cells do not produce gastrin [6, 9]. Auer et al [22] recently demonstrated that gastrin, signaling through the cholecystokinin (CCK)B receptor of AGS-B gastric cancer cells, induces rapid PKD2 nuclear accumulation, suggesting an important functional role for PKD2 in the nucleus. Therefore, gastrin-mediated BON cell proliferation may be attributable in part to PKD2 signaling. Like gastrin, IGF-1 is a potent growth factor implicated in multistage carcinogenesis and tumor cell proliferation; PKD is a critical participant in IGF-1-mediated migration and invasion [28–30]. BON cells express functionally active IGF-1 receptors and secrete IGF-1, suggesting an autocrine action for this factor [6]. Future studies will better delineate the effects of various growth factors, such as gastrin and IGF-1, on PKD2 expression and activation in BON cells.
In conclusion, our data demonstrate an important role for PKD2 in the proliferation and invasion of the BON carcinoid cell line. By targeting tumor secretion, growth, and invasion, inhibitors of PKD may prove to be novel therapeutic agents for the management of carcinoid tumors.
Acknowledgments
The authors thank Dr. Kathleen O’Connor for thoughtful comments, Zobeida Cruz-Monserrate for technical assistance, Emily Bayne and Andrea Ramirez for maintenance of the BON cell line, Karen Martin for manuscript preparation, and Tatsuo Uchida for statistical analysis. This work was supported by grants R01DK48498, R37AG10885, P01DK35608, and T32DK07639 from the National Institutes of Health and a Jeane B. Kempner Scholar award (to LNJ).
Footnotes
This paper was presented, in part, at the annual meeting of the American Gastroenterological Association (May 20-25, 2006, Los Angeles, CA).
References
- 1.Woodside KJ, Townsend CM, Jr, Mark Evers B. Current management of gastrointestinal carcinoid tumors. J Gastrointest Surg. 2004;8:742–756. doi: 10.1016/j.gassur.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Thompson GB, van Heerden JA, Martin JK, Jr, Schutt AJ, Ilstrup DM, Carney JA. Carcinoid tumors of the gastrointestinal tract: presentation, management, and prognosis. Surgery. 1985;98:1054–1063. [PubMed] [Google Scholar]
- 3.Moertel CG, Sauer WG, Dockerty MB, Baggenstoss AH. Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901–912. doi: 10.1002/1097-0142(196109/10)14:5<901::aid-cncr2820140502>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Jackson L, Evers BM. Small bowel carcinoid tumors. In: Cameron JL, editor. Current Surgical Therapy. Mosby; Philadelphia: 2006. in press. [Google Scholar]
- 5.Evers BM, Ishizuka J, Townsend CM, Jr, Thompson JC. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci. 1994;733:393–406. doi: 10.1111/j.1749-6632.1994.tb17289.x. [DOI] [PubMed] [Google Scholar]
- 6.Hofsli E, Thommesen L, Yadetie F, Langaas M, Kusnierczyk W, Falkmer U, Sandvik AK, Laegreid A. Identification of novel growth factor-responsive genes in neuroendocrine gastrointestinal tumour cells. Br J Cancer. 2005;92:1506–1516. doi: 10.1038/sj.bjc.6602535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HJ, Evers BM, Banker NA, Hellmich MR, Townsend CM., Jr A Functional In Vitro Model to Examine Signaling Mechanisms in Gastrin-Mediated Human Cell Growth. J Gastrointest Surg. 1997;1:69–77. doi: 10.1007/s11605-006-0012-z. [DOI] [PubMed] [Google Scholar]
- 8.Parekh D, Ishizuka J, Townsend CM, Jr, Haber B, Beauchamp RD, Karp G, Kim SW, Rajaraman S, Greeley G, Jr, Thompson JC. Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas. 1994;9:83–90. doi: 10.1097/00006676-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Evers BM, Townsend CM, Jr, Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101:303–311. doi: 10.1016/0016-5085(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 10.Evers BM, Hurlbut SC, Tyring SK, Townsend CM, Jr, Uchida T, Thompson JC. Novel therapy for the treatment of human carcinoid. Ann Surg. 1991;213:411–416. doi: 10.1097/00000658-199105000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, O’Connor KL, Hellmich MR, Greeley GH, Jr, Townsend CM, Jr, Evers BM. The role of protein kinase D in neurotensin secretion mediated by protein kinase C-alpha/-delta and Rho/Rho kinase. J Biol Chem. 2004;279:28466–28474. doi: 10.1074/jbc.M314307200. [DOI] [PubMed] [Google Scholar]
- 12.Mihailovic T, Marx M, Auer A, Van Lint J, Schmid M, Weber C, Seufferlein T. Protein kinase D2 mediates activation of nuclear factor kappaB by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res. 2004;64:8939–8944. doi: 10.1158/0008-5472.CAN-04-0981. [DOI] [PubMed] [Google Scholar]
- 13.Sturany S, Van Lint J, Gilchrist A, Vandenheede JR, Adler G, Seufferlein T. Mechanism of activation of protein kinase D2(PKD2) by the CCK(B)/gastrin receptor. J Biol Chem. 2002;277:29431–29436. doi: 10.1074/jbc.M200934200. [DOI] [PubMed] [Google Scholar]
- 14.Papazyan R, Rozengurt E, Rey O. The C-terminal tail of protein kinase D2 and protein kinase D3 regulates their intracellular distribution. Biochem Biophys Res Commun. 2006;342:685–689. doi: 10.1016/j.bbrc.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Sturany S, Van Lint J, Muller F, Wilda M, Hameister H, Hocker M, Brey A, Gern U, Vandenheede J, Gress T, Adler G, Seufferlein T. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J Biol Chem. 2001;276:3310–3318. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- 16.Rey O, Yuan J, Rozengurt E. Intracellular redistribution of protein kinase D2 in response to G-protein-coupled receptor agonists. Biochem Biophys Res Commun. 2003;302:817–824. doi: 10.1016/s0006-291x(03)00269-9. [DOI] [PubMed] [Google Scholar]
- 17.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Kang J, Hu W, Evers BM, Chung DH. Geldanamycin decreases Raf-1 and Akt levels and induces apoptosis in neuroblastomas. Int J Cancer. 2003;103:352–359. doi: 10.1002/ijc.10820. [DOI] [PubMed] [Google Scholar]
- 20.Farrow B, Rychahou P, O’Connor KL, Evers BM. Butyrate inhibits pancreatic cancer invasion. J Gastrointest Surg. 2003;7:864–870. doi: 10.1007/s11605-003-0031-y. [DOI] [PubMed] [Google Scholar]
- 21.SAS, I. SAS/STAT 9.1 User’s Guide. Cary, NC: 2004. [Google Scholar]
- 22.Auer A, von Blume J, Sturany S, von Wichert G, Van Lint J, Vandenheede J, Adler G, Seufferlein T. Role of the regulatory domain of protein kinase D2 in phorbol ester binding, catalytic activity, and nucleocytoplasmic shuttling. Mol Biol Cell. 2005;16:4375–4385. doi: 10.1091/mbc.E05-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rykx A, De Kimpe L, Mikhalap S, Vantus T, Seufferlein T, Vandenheede JR, Van Lint J. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 24.Townsend CM, Jr, Ishizuka J, Thompson JC. Studies of growth regulation in a neuroendocrine cell line. Acta Oncol. 1993;32:125–130. doi: 10.3109/02841869309083900. [DOI] [PubMed] [Google Scholar]
- 25.von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E, Wiedenmann B, Boehm BO, Adler G, Seufferlein T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–4581. [PubMed] [Google Scholar]
- 26.Ishizuka J, Beauchamp RD, Townsend CM, Jr, Greeley GH, Jr, Thompson JC. Receptor-mediated autocrine growth-stimulatory effect of 5-hydroxytryptamine on cultured human pancreatic carcinoid cells. J Cell Physiol. 1992;150:1–7. doi: 10.1002/jcp.1041500102. [DOI] [PubMed] [Google Scholar]
- 27.Chiu T, Rozengurt E. CCK2 (CCK(B)/gastrin) receptor mediates rapid protein kinase D (PKD) activation through a protein kinase C-dependent pathway. FEBS Lett. 2001;489:101–106. doi: 10.1016/s0014-5793(01)02076-2. [DOI] [PubMed] [Google Scholar]
- 28.Qiang YW, Yao L, Tosato G, Rudikoff S. Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood. 2004;103:301–308. doi: 10.1182/blood-2003-06-2066. [DOI] [PubMed] [Google Scholar]
- 29.Stracke ML, Engel JD, Wilson LW, Rechler MM, Liotta LA, Schiffmann E. The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. J Biol Chem. 1989;264:21544–21549. [PubMed] [Google Scholar]
- 30.Brooks PC, Klemke RL, Schon S, Lewis JM, Schwartz MA, Cheresh DA. Insulin-like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo. J Clin Invest. 1997;99:1390–1398. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]