Abstract
Objectives
We determined nasopharyngeal colonization rates and antibiotic resistance patterns of Streptococcus pneumoniae isolated from Guatemalan children and determined risk factors for colonization and antibiotic nonsusceptibility.
Methods
Isolates were obtained from Guatemala City children 5 to 60 months of age attending public and private outpatient clinics and daycare centers during August 2001–June 2002 and outpatient clinics during November 2005–February 2006. Minimal inhibitory concentrations of penicillin, trimethoprim-sulfamethoxazole (TMS), cefotaxime, and erythromycin were determined using E-test.
Results
The overall nasopharyngeal colonization rate for S. pneumoniae was 59.1%. From 2001/2 to 2005/6 TMS nonsusceptibility increased from 42.4% to 60.8% (p<0.05) in public clinics and from 51.4% to 84.0% (p=0.009) in private clinics and penicillin nonsusceptibility increased from 1.5% to 33.3% in public clinics (p<0.001). Reported antibiotic use was not strictly associated with nonsusceptibility to that same antibiotic. Resistance to three or four antibiotics increased in public clinics from 2001/2 (0%) to 2005/6 (10.7%; p<0.001). Risk factors for nasopharyngeal colonization with penicillin- or TMS-nonsusceptible S. pneumoniae were low family income, daycare center attendance, and recent penicillin use.
Conclusions
Increasing antibiotic nonsusceptibility rates in nasopharyngeal S. pneumoniae isolates from Guatemalan children reflect worldwide trends. Policies encouraging more judicious use of TMS should be considered.
Keywords: antibiotic resistance, erythromycin, cefotaxime, macrolide, cephalosporin, risk factors
Antibiotic-resistant Streptococcus pneumoniae have emerged as a problem in many countries,1, 2 necessitating changes to more costly antibiotics which developing countries often cannot afford.3 Although some infections with pneumococci of intermediate resistance (MIC 0.1–1.0 ug/ml) can be treated with high doses of penicillin, bacteria with a higher degree of resistance (MIC >1.0 ug/ml) are often resistant to other antibiotics, including cephalosporins.4 There is limited information on distribution of antibiotic-resistant S. pneumoniae in the developing world, although the prevalence of S. pneumoniae of decreased susceptibility is increasing.5-9
In most developing countries, the availability and use of antibiotics differs dramatically among different socioeconomic strata. Children from higher socioeconomic groups are often prescribed broad-spectrum antibiotics while children in lower economic classes generally have access to only a small number of inexpensive antimicrobials. The “no-control” policy of antibiotic prescriptions in most developing countries has accelerated the emergence of multiple-resistant diarrheal pathogens and could be a catalyst for the emergence of antibiotic-resistant S. pneumoniae.
Nasopharyngeal (NP) colonization with S. pneumoniae precedes invasive infection, and the nasopharynx is likely the site where selection and transmission of resistant strains occurs.10, 11 Although a number of studies have examined serotype distributions and antibiotic susceptibility patterns of invasive S. pneumoniae isolates in Latin America,8, 12-15 few investigations of NP isolates have been conducted in this region. The aims of the current study were to determine nasopharyngeal colonization rates and antibiotic resistance patterns of S. pneumoniae isolated from Guatemalan children and determine risk factors for colonization and antibiotic nonsusceptibility.
Materials and Methods
Study Population
This study was approved by the Institutional Review Boards at the Johns Hopkins Bloomberg School of Public Health and the Universidad del Valle. Nasopharyngeal isolates were obtained in two studies. Children who had not received antibiotics in the previous 2 weeks were eligible. Children 5 to 60 months of age (study NP2001/2) were enrolled between August 2001 and June 2002 at a public hospital outpatient clinic, a public daycare center, four private daycare centers, and two private pediatric clinics. A second group of children (study NP2005/6) ≤24 months of age was enrolled between November 2005 and February 2006 at the same public hospital outpatient clinic and three private pediatric clinics. Administration of the pneumococcal 7-valent conjugate vaccine (Prevenar; Wyeth Pharmaceuticals, Philadelphia, PA, USA) at 2, 4, and 6 months of age has been available in the private pediatric clinics since 2003, but is uncommonly used due to cost. This vaccine has not yet been introduced in the public sector. Data were not collected on vaccination history. Written informed consent was obtained from a parent or guardian prior to enrollment. A standardized questionnaire including demographics, potential risk factors, and antibiotic use (as reported by respondent) was administered to the parent or guardian of each child.
Nasopharyngeal Sampling
Nasopharyngeal secretions were obtained by inserting a sterile calcium alginate tipped swabs (Calgiswab Type 1; Spectrum Laboratories, Houston TX) into the nasopharynx to a depth of 3–4 cm. Swabs were streaked immediately onto blood agar plates (trypticase soy base with 5% sheep blood) supplemented with gentamicin (Becton Dickinson, Cockeysville, MD), refrigerated, and transported to the laboratory within 4 hours of procurement. Streaked plates were incubated at 34–35 °C in 5% CO2 atmosphere for 24 to 48 hours. Colonies of alpha-hemolysis were evaluated for bacterial gram stain and morphology and optochin disk susceptibility. All isolates were stored in duplicate frozen in glycerol citrate at -70 °C. Serotyping was later performed by latex agglutination using standard sera (Statens Serum Institut, Copenhagen, Denmark) and confirmed by the Quellung reaction.
Optochin and oxacillin disk screening
Presumptive identification of S. pneumoniae was made by susceptibility to optochin. Plates were incubated in a CO2 atmosphere for 18–24 hours. Alpha-hemolytic strains with a zone of inhibition of growth ≥15 mm in diameter were considered pneumococci. No alpha-hemolytic strains with zones of inhibition ranging between 9 and 13 mm were found.
All S. pneumoniae isolates were inoculated into trypticase soy broth (0.5 McFarland standard) and plated on Mueller-Hinton blood agar plates with a 1-μg oxacillin disk, and incubated at 37 °C for 18 to 24 hours. Isolates exhibiting <20-mm-diameter zones around the oxacillin disk were considered potentially penicillin non-susceptible and underwent further susceptibility confirmation. Isolates with a ≥20-mm zone of inhibition were considered to be susceptible to penicillin.
Minimal Inhibitory Concentration Testing
Minimal inhibitory concentrations (MIC) of selected antibiotics [penicillin, cefotaxime, erythromycin, and trimethoprim-sulfamethoxazole (TMS)] were determined using E-test. Purity of isolates was assured by streaking frozen isolates for single colonies on trypticase soy agar containing 5% sheep blood and incubating at 34 to 35 °C in 5% CO2 atmosphere for 24 to 48 hours. A final bacterial density of McFarland 108, was inoculated into Mueller-Hinton 5% sheep blood agar where E-test strips were placed and incubated for 20 hours at 34 to 35 °C in a 5% CO2 atmosphere. S. pneumoniae isolates were defined as susceptible, intermediate, or resistant according to E-test manufacturer specifications (Table 1) and National Committee on Clinical Laboratory Standards criteria. Intermediate and resistant isolates were combined (nonsusceptible group) for analyses. Due to limited funds, a stratified random selection of 150 of the 478 isolates was selected for serotyping in the NP2001/2 study. Serotypes/groups were determined for 76 isolates from the NP2005/6 study; 4 isolates could not be recovered from frozen samples for serotyping.
Table 1.
E-Test Interpretations.
| Antibiotic | Susceptible | Intermediate | Resistant |
|---|---|---|---|
| Penicillin | ≤0.06 μg/ml | 0.12–1 μg/ml | ≥2 μg/ml |
| Cefotaxime | ≤1 μg/ml | 2 μg/ml | ≥4 μg/ml |
| Erythromycin | ≤0.5 μg/ml | 1 μg/ml | ≥2 μg/ml |
| Trimethoprim-sulfamethoxazole | ≤0.5 μg/ml | 1–2 μg/ml | ≥4 μg/ml |
Data Analysis
Statistical analyses were performed with the Statistical Package for Social Sciences (SPSS-PC version 10. Chicago, Illinois). Proportions were compared using 2-tailed Chi-square test with Yates' correction or Fisher's exact tests. The Student t-test was used to compare group means. Non-parametric variables were compared using the Mann-Whitney test. Univariate and multivariate logistic regression models were used to identify risk factors for NP carriage of S. pneumoniae and to compare risk factors between children with isolates susceptible to penicillin and TMS and those with isolates nonsusceptible to these antibiotics. The independent variables for each analysis were individual exposure to antibiotics, age, gender, number of people per bedroom, household income, tobacco exposure, use of a wood stove in the home, previous episodes of illnesses, and daycare center attendance. For all statistical analyses, a significance level of p<0.05 will be used to reject the null hypothesis.
Results
A total of 751 children were enrolled in the NP2001/2 study: 199 (26.5%) from a public hospital outpatient clinic, 190 (25.3%) from a public daycare center, 177 (23.6%) from four private daycare centers, and 185 (24.6%) from two private pediatric clinics. A total of 200 children were enrolled in the NP2005/6 study: 100 (50.0%) from the public hospital outpatient clinic and 100 (50.0%) from three private pediatric clinics. Children enrolled from public daycare centers and clinics were more likely than those enrolled from private daycare centers and clinics to belong to households with low family income, to have wood stoves in the home, and have ≥2 people per bedroom (Table 2). When compared to children from higher-income households, children in lower-income households were significantly more likely to have received penicillin or TMS and less likely to have received cephalosporins or macrolides (Table 3).
Table 2.
Population characteristics by site.
| 2001/2 Study | 2005/6 Study | |||||
|---|---|---|---|---|---|---|
| Daycare Centers | Clinics | Clinics | ||||
| Public
(n = 190) |
Private
(n = 177) |
Public
(n = 199) |
Private
(n = 185) |
Public
(n = 100) |
Private
(n = 100) |
|
| Male (%) | 94 (50) | 91 (51) | 115 (58) | 102 (55) | 48 (48) | 54 (54) |
| Mean age, months (SE) | 40 (1) | 38 (1) | 26 (1) | 28 (1) | 13.4 (0.51) | 14.5 (0.60) |
| Attend daycare (%) | 190 (100) | 177 (100) | 9 (4) | 74 (40) | 6 (6.0) | 17 (17) |
| Family income <$400/month (%) | 189 (99) | 17 (10)* | 198 (99) | 14 (8)* | 96 (96) | 15 (15)* |
| Woodstove (vs. gas/electric) | 11 (6) | 0 (0)* | 56 (28) | 2 (1)* | 19 (19) | 0 (0)* |
| Number/bedroom >= 2 | 147 (88) | 39 (22)* | 172 (86) | 47 (25)* | 37 (57) | 22 (24)* |
| Antibiotics in previous 3 months (%) | ||||||
| None | 86 (45) | 77 (44) | 96 (48) | 97 (52) | 33 (33) | 63 (63)* |
| Penicillin | 84 (44) | 72 (41) | 83 (42) | 49 (27)† | 48 (48) | 14 (14)* |
| TMS | 5 (3) | 9 (5) | 10 (5) | 10 (5) | 24 (24) | 2 (2)* |
| Cephalosporins | 15 (8) | 24 (14) | 11 (6) | 25 (14)§ | 9 (9) | 7 (7) |
| Macrolides | 4 (2) | 22 (12)* | 9 (5) | 25 (14)† | 7 (7) | 11 (11) |
TMS, trimethoprim-sulfamethoxazole
p<0.001 for public vs. private
p=0.001 for public vs. private
p=0.007 for public vs. private
Table 3.
Antibiotic use* in previous 3 months by family income.
| Family Income | p-value | ||
|---|---|---|---|
| <$400/month, n (%) | >$400/month, n (%) | ||
| Penicillin | 228 (43.1%) | 122 (28.9%) | <0.001 |
| TMS | 41 (7.8%) | 19 (4.5%) | 0.04 |
| Cephalosporins | 41 (7.8%) | 50 (11.8%) | 0.035 |
| Macrolides | 23 (4.3%) | 55 (13%) | <0.0001 |
TMS, trimethoprim-sulfamethoxazole
Antibiotic use data were collected by parent/guardian report.
Overall, 562 (59.1%) children were colonized with S. pneumoniae; colonization rates were consistently lower in private settings than in public settings. Colonization rates in private clinics decreased from 2001/2 to 2005/6 (Table 4). Colonization rates during 2001/2, for which data collection spanned 11 months (August–June), did not show seasonal differences (62.0% for the rainy season vs. 68.3% for the dry season).
Table 4.
Nasopharyngeal carriage rates by study site.
| Study | N | Carriage of Streptococcus pneumonia, n (%) |
|---|---|---|
| 2001/2 | ||
| Daycare Centers | ||
| Privatea | 177 | 113 (63.8) |
| Publica | 190 | 160 (84.2) |
| Clinics | ||
| Privateb,d | 185 | 85 (45.9) |
| Publicb | 199 | 120 (60.3) |
| 2005/6* | ||
| Privatec,d | 100 | 28 (28.0) |
| Publicc | 100 | 56 (56.0) |
| Total, all studies | 951 | 562 (59.1) |
2005/6 data were collected from clinics only.
p<0.001
p=0.004
p<0.001
p<0.003
E-test results were available for 554 of the 562 isolates. Of these, a total of 347 (62.6%), 149 (26.9%), and 155 (28.0%) isolates were nonsusceptible to TMS, penicillin, and erythromycin, respectively. Among these nonsusceptible isolates, 51.0%, 41.6%, and 99.4%, respectively, were fully resistant to TMS, penicillin, and erythromycin. All 18 cefotaxime nonsusceptible isolates were of intermediate resistance. During the 2001/2 period, a greater percentage of isolates were nonsusceptible to TMS during the rainy season (36.8%) than the dry season (22.8%; p=0.004), and more isolates were nonsusceptible to penicillin, erythromycin, and TMS during the dry season (29.4%) than the rainy season (12.3%; p<0.001). However, when the data were stratified by public versus private settings, these differences were no longer significant.
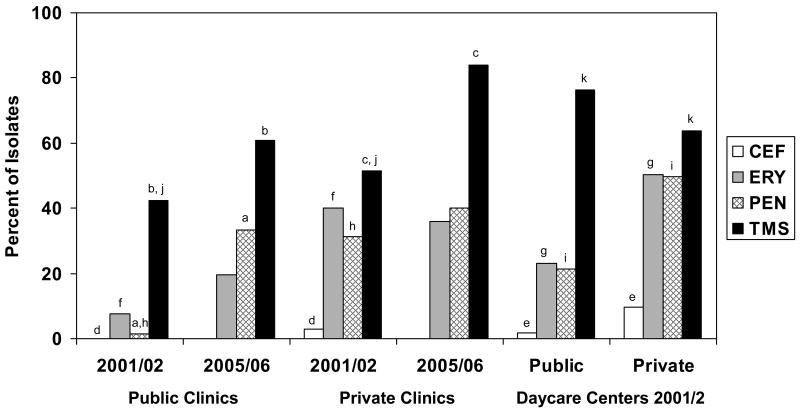
Nonsusceptibility of isolates to TMS increased from 2001/2 to 2005/6 in both public and private clinics (Figure 1). The percentage of isolates nonsusceptible to penicillin increased from 2001/2 to 2005/6 in the public clinics. Combined data from the public and private clinics showed increases in the percentage of isolates that were nonsusceptible to TMS (45.5% in 2001/2 vs. 68.4% in 2005/6; p=0.0025) and penicillin (11.9% in 2001/2 vs. 35.5% in 2005/6; p<0.001). This shift reflected a significant increase in the percentage of isolates with intermediate resistance to TMS (28.2% in 2001/2 to 46.1% in 2005/6) and penicillin (13.4% in 2001/2 to 30.3% in 2005/6). When data from both time periods were combined, a greater percentage of isolates from private clinics than from public clinics were nonsusceptible to penicillin (35% vs. 15.4%, respectively; p=0.003) and erythromycin (38.3% vs. 12.8%, respectively; p<0.001).
Figure 1.
Proportion of Streptococcus pneumoniae isolates from children attending daycare centers and clinics nonsusceptible to cefotaxime, erythromycin, penicillin, and trimethoprim-sulfamethoxazole, 2001/2 and 2005/6. CEF, cefotaxime; ERY, erythromycin; PEN, penicillin; TMS, trimethoprim-sulfamethoxazole. ap<0.001; bp<0.05; cp=0.009; dp=0.028; ep=0.003; fp<0.001; gp<0.001; hp<0.001; ip<0.001; jp=0.004.
The percentage of isolates nonsusceptible to each of the four antibiotics was higher among S. pneumoniae isolates obtained in 2001/2 from children in private clinics than isolates obtained in public settings (Figure 1). TMS nonsusceptibility was higher in isolates from children attending public daycare centers than for isolates from children in private daycare centers. When the data from private and public settings were combined, higher percentages of isolates from daycare centers than clinics were nonsusceptible to TMS (71.1% vs. 49.3%, respectively; p<0.001), penicillin (33.0% vs. 15.6%, respectively; p<0.001), and erythromycin (34.4% vs. 20.5%, respectively; p<0.001).
Overall, 113 of 554 (20.4%) isolates were nonsusceptible to multiple (3 or 4) antibiotics; multidrug nonsusceptibility rates were not different for the two time periods (20.9% in 2001/2 vs. 17.1% in 2005/6). Among isolates obtained in 2001/2, multidrug nonsusceptibility rates were higher in private than public daycare centers (41.6 vs. 15.6%, respectively; p<0.001) and private versus public clinics (32.9% vs. 0%, respectively; p<0.001). Nonsusceptibility to multiple antibiotics increased in public clinics from 2001/2 (0%) to 2005/6 (10.7%; p<0.001).
After adjustment for other factors, each one-month increase in age was associated with a 2% decrease in risk of S. pneumoniae colonization (Table 5). Other factors significantly associated with colonization were family income <$400 per month, daycare center attendance (public or private), and presence of a wood stove in the home. Among children colonized with S. pneumoniae, risk factors for carriage of penicillin nonsusceptible isolates were young age, daycare center attendance (public or private), and penicillin use in the previous 3 months. Family income <$400 per month and ≥2 people per bedroom were protective against colonization with a penicillin-nonsusceptible isolate. Risk factors for carriage of TMS nonsusceptible isolates were daycare center attendance (public or private), and penicillin use in the previous 3 months; income <$400 per month was protective.
Table 5.
Multivariate logistic regression analysis of risk factors for colonization with Streptococcus pneumoniae, penicillin-nonsusceptible S. pneumoniae, and trimethoprim-sulfamethoxazole-nonsusceptible S. pneumoniae.
| S. pneumoniae* | Penicillin-nonsusceptible S. pneumoniae† | TMS-nonsusceptible S. pneumoniae† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uncolonized | Colonized | OR (95% CI) | p-value | PS | PN | OR (95% CI) | p-value | TMSS | TMSN | OR (95% CI) | p-value | |
| Age in months | 0.98 (0.97-0.99) | 0.001 | .98 (.96-.99) | <0.001 | -- | -- | ||||||
| Income <$400/month | 171/389 (44.0) | 358/562 (63.7) | 1.7 (1.3-2.4) | 0.001 | 293/405 (72.6) | 58/149 (38.9) | .33 (.17-.60) | <0.001 | 140/207 (67.6) | 212/347 (61.1) | .68 (.49-.95) | 0.022 |
| Daycare | ||||||||||||
| Private preschool | 108/389 (27.8) | 159/562 (28.3) | 2.6 (1.7-4.0) | <0.001 | 86/405 (21.2) | 74/149 (49.0) | 2.4 (1.3-4.4) | 0.004 | 55/207 (26.6) | 104/347 (30.0) | 1.7 (1.2-2.4) | 0.002 |
| Public daycare center | 34/389 (8.7) | 170/562 (30.2) | 5.4 (3.3-8.9) | <0.001 | 134/405 (33.1) | 36/149 (24.2) | 3.1 (1.6-6.0) | 0.001 | 42/207 (20.3) | 128/347 (36.9) | 3.8 (2.4-6.0) | <0.001 |
| Penicillin in last 3 months | 138/405 (34.0) | 65/149 (43.6) | 1.6 (1.1-2.5) | <0.001 | 66/207 (31.9) | 137/347 (39.5) | 1.5 (1.1-2.2) | 0.025 | ||||
| Number/bedroom ≥2 | 152/361 (42.1) | 312/547 (57.0) | -- | -- | 261/393 (66.4) | 49/147 (33.3) | .42 (.26-.68) | <0.001 | ||||
| Wood stove (vs. gas or electric) | 24/389 (6.2) | 64/562 (11.4) | 2.0 (1.2-3.5) | 0.012 | ||||||||
TMS, trimethoprim-sulfamethoxazole; PS, penicillin-susceptible; PN, penicillin-nonsusceptible; TMSS, trimethoprim-sulfamethoxazole-susceptible; TMSN, trimethoprim-sulfamethoxazole-nonsusceptible
Sex, smoker in the house, prior antibiotic use, and prior illness were also included in initial model but were not significant.
Sex, smoker in the house, prior illness, trimethoprim-sulfamethoxazole use, erythromycin use, and cefotaxime use were also included in initial model but were not significant.
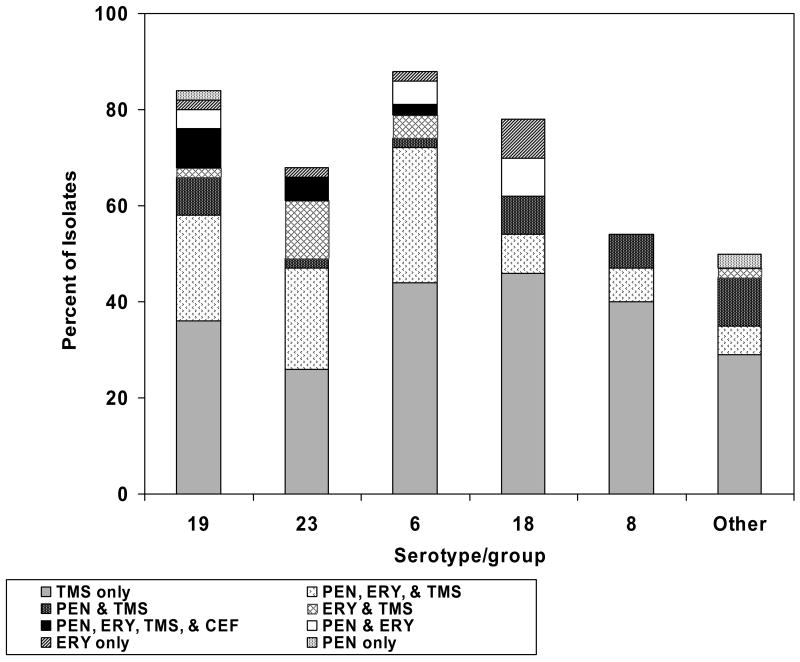
The five most common serotypes/groups isolated were 19, 23, 6, 18, and 8. Less than 25% of serotypes/groups 19, 6, and 18 were susceptible to all 4 antibiotics tested. Nonsusceptibility to all 4 antibiotics tested was present in 2%–8% of isolates in the three serotypes/groups most commonly isolated (19, 23, and 6; Figure 2). There were no significant differences in distribution of serotypes/groups or in distribution of antibiotic nonsusceptibility patterns between the time periods.
Figure 2.
Percentage of Streptococcus pneumoniae isolates nonsusceptible to various antibiotics by serotype/group. CEF, cefotaxime; ERY, erythromycin; PEN, penicillin; TMS, trimethoprim-sulfamethoxazole.
Discussion
More than one half of Guatemalan children 5 to 60 months of age were colonized with S. pneumoniae, within the wide range of colonization rates (14% to 61%) reported for children less than 5 years of age in Latin America.5, 16-19 The increasing nonsusceptibility of isolates to TMS and penicillin across time has been noted in other countries.1, 3, 5, 20-27 The risk factors for NP colonization and colonization with penicillin nonsusceptible S. pneumoniae including age, daycare center attendance, crowding, and income are consistent with findings in other countries.5, 26, 28-38 Use of a wood stove in the home as the primary method of cooking was also associated with increased rates of NP S. pneumoniae colonization. Other studies have shown associations between childhood respiratory tract infection rates and wood stove use in the home.39-42
Antibiotic nonsusceptibility rates in S. pneumoniae isolates are commonly thought to be associated with antibiotic exposure.43-48 In our study, according to antibiotic use reported by parents, exposures to penicillin and TMS were higher in public settings, and cephalosporin and macrolide exposures were higher in private settings. In private settings in 2001/2, nonsusceptibility to penicillin, erythromycin, and cefotaxime was higher than in public settings. Interestingly, although penicillin use was higher among children from public settings, penicillin resistance was higher among children attending private clinics. Also in 2001/2, nonsusceptibility to TMS, penicillin, and erythromycin was higher in daycare centers than clinics, although only reported penicillin use was higher in private daycares. Furthermore, after adjustment for factors such as income, daycare center attendance, and age, recent penicillin use was a risk factor for colonization with penicillin- or TMS-nonsusceptible isolates, but TMS use was not found to be a significant risk factor for carriage of nonsusceptible isolates. Thus, our data suggest that antibiotic nonsusceptibility of S. pneumoniae isolates is not strictly associated with antibiotic use. Antibiotic use data reported by parents could be inaccurate.49, 50 Furthermore, antibiotic nonsusceptibility is not always a clear one-to-one relationship between antibiotic exposure and resistance to that same antibiotic, but is likely complicated by bacterial acquisition of plasmids that often confer resistance to multiple antibiotics.51-53 Finally, macrolide use in the private clinics may have contributed to penicillin resistance despite low levels of reported penicillin use.54-56
S. pneumoniae serotypes/groups 19, 23, and 6 were the most commonly isolated and a small percentage of each group was nonsusceptible to all four antibiotics tested. If these same resistance patterns were found in isolates associated with invasive disease, treatment options would potentially be limited. The three most commonly isolated serotypes/groups are included in the PCV-7 vaccine, but this vaccine is not used routinely in Guatemala. Efforts to increase control, regulation, and more judicious use of antibiotics likely aid in reducing or reversing the rise in antibiotic-resistant S. pneumoniae45, 47, 57, 58 and are needed in Guatemala. Efforts should be made to address the problem of over-the-counter antibiotic sales in Guatemala, especially the sale of single doses rather than full courses. Control of antibiotic overuse and misuse in Guatemala will require programs aimed at prescribers, dispensers, and consumers.
The World Health Organization recommends TMS use as part of its Integrated Management of Childhood Illness program aimed at improving prevention and management of major childhood illnesses in developing countries. Our data along with data from other countries worldwide3, 5, 21-23, 25, 27 demonstrating increases in TMS resistance of S. pneumoniae indicate that policies encouraging the use of TMS may have adverse consequences. In the face of rising resistance to TMS, revised recommendations encouraging more judicious use of TMS should be considered.
Acknowledgments
This study was funded in part by grants from GlaxoSmithKline, a subcontract from The Johns Hopkins University with funds provided by The Boards of the Global Alliance for Vaccines and Immunizations and the Vaccine Fund (GAVI) (“Agency”), as well as independent funds. Support for Erica L. Dueger was provided by a grant from Fogarty International Research Scientist Development Award (Grant number KO1 TW06659).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum PC. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15(1):77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JP, 3rd, Zhanel GG. Escalation of antimicrobial resistance among Streptococcus pneumoniae: implications for therapy. Semin Respir Crit Care Med. 2005;26(6):575–616. doi: 10.1055/s-2005-925524. [DOI] [PubMed] [Google Scholar]
- 3.Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5(8):481–93. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci. 1999;354(1384):777–85. doi: 10.1098/rstb.1999.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa TJ, Rupa R, Guerra H, et al. Penicillin resistance and serotypes/serogroups of Streptococcus pneumoniae in nasopharyngeal carrier children younger than 2 years in Lima, Peru. Diagn Microbiol Infect Dis. 2005;52(1):59–64. doi: 10.1016/j.diagmicrobio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Mendes C, Marin ME, Quinones F, et al. Antibacterial resistance of community-acquired respiratory tract pathogens recovered from patients in Latin America: results from the PROTEKT surveillance study (1999-2000) Braz J Infect Dis. 2003;7(1):44–61. doi: 10.1590/s1413-86702003000100006. [DOI] [PubMed] [Google Scholar]
- 7.Saldias PF, Flores SL, Torres MC, Garcia CP, Diaz FA. Antimicrobial susceptibility of Streptococcus pneumoniae in pediatric and adult population from Santiago, 1997-2003. Rev Med Chil. 2005;133(1):42–9. doi: 10.4067/s0034-98872005000100006. [DOI] [PubMed] [Google Scholar]
- 8.Hortal M, Ruvinsky R, Rossi A, et al. Impact of Streptococcus pneumoniae on pneumonia in Latin American children. SIREVA-Vigia Group. Rev Panam Salud Publica. 2000;8(3):185–95. doi: 10.1590/s1020-49892000000800006. [DOI] [PubMed] [Google Scholar]
- 9.Hortal M, Lovgren M, de la Hoz F, et al. Antibiotic resistance in Streptococcus pneumoniae in six Latin American countries: 1993-1999 surveillance. Microb Drug Resist. 2001;7(4):391–401. doi: 10.1089/10766290152773400. [DOI] [PubMed] [Google Scholar]
- 10.Ghaffar F, Friedland IR, McCracken GH., Jr Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr Infect Dis J. 1999;18(7):638–46. doi: 10.1097/00006454-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Evans N, O'Dempsey TJ, Baldeh I, et al. Nasopharyngeal carriage of pneumococci in Gambian children and in their families. Pediatr Infect Dis J. 1996;15(10):866–71. doi: 10.1097/00006454-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Castaneda E, Leal AL, Castillo O, et al. Distribution of capsular types and antimicrobial susceptibility of invasive isolates of Streptococcus pneumoniae in Colombian children. Pneumococcal Study Group in Colombia. Microb Drug Resist. 1997;3(2):147–52. doi: 10.1089/mdr.1997.3.147. [DOI] [PubMed] [Google Scholar]
- 13.Castanheira M, Gales AC, Mendes RE, Jones RN, Sader HS. Antimicrobial susceptibility of Streptococcus pneumoniae in Latin America: results from five years of the SENTRY Antimicrobial Surveillance Program. Clin Microbiol Infect. 2004;10(7):645–51. doi: 10.1111/j.1469-0691.2004.00872.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia S, Levine OS, Cherian T, Gabastou JM, Andrus J. Pneumococcal disease and vaccination in the Americas: an agenda for accelerated vaccine introduction. Rev Panam Salud Publica. 2006;19(5):340–8. doi: 10.1590/s1020-49892006000500007. [DOI] [PubMed] [Google Scholar]
- 15.Zemlickova H, Crisostomo MI, Brandileone MC, et al. Serotypes and clonal types of penicillin-susceptible streptococcus pneumoniae causing invasive disease in children in five Latin American countries. Microb Drug Resist. 2005;11(3):195–204. doi: 10.1089/mdr.2005.11.195. [DOI] [PubMed] [Google Scholar]
- 16.Inostroza J, Trucco O, Prado V, et al. Capsular serotype and antibiotic resistance of Streptococcus pneumoniae isolates in two Chilean cities. Clin Diagn Lab Immunol. 1998;5(2):176–80. doi: 10.1128/cdli.5.2.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laval CB, de Andrade AL, Pimenta FC, et al. Serotypes of carriage and invasive isolates of Streptococcus pneumoniae in Brazilian children in the era of pneumococcal vaccines. Clin Microbiol Infect. 2006;12(1):50–5. doi: 10.1111/j.1469-0691.2005.01304.x. [DOI] [PubMed] [Google Scholar]
- 18.Rey LC, Wolf B, Moreira JL, Milatovic D, Verhoef J, Farhat CK. Antimicrobial susceptibility and serotypes of nasopharyngeal Streptococcus pneumoniae in children with pneumonia and in children attending day-care centres in Fortaleza, Brazil. Int J Antimicrob Agents. 2002;20(2):86–92. doi: 10.1016/s0924-8579(02)00128-0. [DOI] [PubMed] [Google Scholar]
- 19.Solorzano-Santos F, Ortiz-Ocampo LA, Miranda-Novales MG, Echaniz-Aviles G, Soto-Nogueron A, Guiscafre-Gallardo H. Prevalence of Streptococcus pneumoniae serotypes on nasopharyngeal colonization in children of Mexico City. Salud Publica Mex. 2005;47(4):276–81. doi: 10.1590/s0036-36342005000400004. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson P, Laurell MH. Impact of socioeconomic factors and antibiotic prescribing on penicillin-non-susceptible Streptococcus pneumoniae in the city of Malmo. Scand J Infect Dis. 2005;37(67):436–41. doi: 10.1080/00365540510037795. [DOI] [PubMed] [Google Scholar]
- 21.Arason VA, Sigurdsson JA, Erlendsdottir H, Gudmundsson S, Kristinsson KG. The role of antimicrobial use in the epidemiology of resistant pneumococci: A 10-year follow up. Microb Drug Resist. 2006;12(3):169–76. doi: 10.1089/mdr.2006.12.169. [DOI] [PubMed] [Google Scholar]
- 22.Hogberg L, Ekdahl K, Sjostrom K, et al. Penicillin-resistant pneumococci in Sweden 1997-2003: increased multiresistance despite stable prevalence and decreased antibiotic use. Microb Drug Resist. 2006;12(1):16–22. doi: 10.1089/mdr.2006.12.16. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DM, Stilwell MG, Fritsche TR, Jones RN. Emergence of multidrug-resistant Streptococcus pneumoniae: report from the SENTRY Antimicrobial Surveillance Program (1999-2003) Diagn Microbiol Infect Dis. 2006;56(1):69–74. doi: 10.1016/j.diagmicrobio.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Felmingham D, White AR, Jacobs MR, et al. The Alexander Project: the benefits from a decade of surveillance. J Antimicrob Chemother. 2005;56 2:ii3–ii21. doi: 10.1093/jac/dki297. [DOI] [PubMed] [Google Scholar]
- 25.Quinones-Falconi F, Calva JJ, Lopez-Vidal Y, Galicia-Velazco M, Jimenez-Martinez ME, Larios-Mondragon L. Antimicrobial susceptibility patterns of Streptococcus pneumoniae in Mexico. Diagn Microbiol Infect Dis. 2004;49(1):53–8. doi: 10.1016/j.diagmicrobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Volonakis K, Souli M, Kapaskelis A, et al. Evolution of resistance patterns and identification of risk factors for Streptococcus pneumoniae colonisation in daycare centre attendees in Athens, Greece. Int J Antimicrob Agents. 2006;28(4):297–301. doi: 10.1016/j.ijantimicag.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhanel GG, Palatnick L, Nichol KA, Bellyou T, Low DE, Hoban DJ. Antimicrobial resistance in respiratory tract Streptococcus pneumoniae isolates: results of the Canadian Respiratory Organism Susceptibility Study, 1997 to 2002. Antimicrob Agents Chemother. 2003;47(6):1867–74. doi: 10.1128/AAC.47.6.1867-1874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchisio P, Esposito S, Schito GC, Marchese A, Cavagna R, Principi N. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children: implications for the use of heptavalent pneumococcal conjugate vaccine. Emerg Infect Dis. 2002;8(5):479–84. doi: 10.3201/eid0805.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neto AS, Lavado P, Flores P, et al. Risk factors for the nasopharyngeal carriage of respiratory pathogens by Portuguese children: phenotype and antimicrobial susceptibility of Haemophilus influenzae and Streptococcus pneumoniae. Microb Drug Resist. 2003;9(1):99–108. doi: 10.1089/107662903764736409. [DOI] [PubMed] [Google Scholar]
- 30.Zemlickova H, Urbaskova P, Adamkova V, Motlova J, Lebedova V, Prochazka B. Characteristics of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus isolated from the nasopharynx of healthy children attending day-care centres in the Czech Republic. Epidemiol Infect. 2006;134(6):1179–87. doi: 10.1017/S0950268806006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu SS, Ho PL, Chow FK, Yuen KY, Lau YL. Nasopharyngeal carriage of antimicrobial-resistant Streptococcus pneumoniae among young children attending 79 kindergartens and day care centers in Hong Kong. Antimicrob Agents Chemother. 2001;45(10):2765–70. doi: 10.1128/AAC.45.10.2765-2770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelstein JA, Huang SS, Daniel J, et al. Antibiotic-resistant Streptococcus pneumoniae in the heptavalent pneumococcal conjugate vaccine era: predictors of carriage in a multicommunity sample. Pediatrics. 2003;112(4):862–9. doi: 10.1542/peds.112.4.862. [DOI] [PubMed] [Google Scholar]
- 33.Russell FM, Carapetis JR, Ketaiwai S, et al. Pneumococcal nasopharyngeal carriage and patterns of penicillin resistance in young children in Fiji. Ann Trop Paediatr. 2006;26(3):187–97. doi: 10.1179/146532806X120273. [DOI] [PubMed] [Google Scholar]
- 34.Kellner JD, Ford-Jones EL. Streptococcus pneumoniae carriage in children attending 59 Canadian child care centers. Toronto Child Care Centre Study Group. Arch Pediatr Adolesc Med. 1999;153(5):495–502. doi: 10.1001/archpedi.153.5.495. [DOI] [PubMed] [Google Scholar]
- 35.Nasrin D, Collignon PJ, Wilson EJ, Pilotto LS, Douglas RM. Antibiotic resistance in Streptococcus pneumoniae isolated from children. J Paediatr Child Health. 1999;35(6):558–61. doi: 10.1046/j.1440-1754.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- 36.Regev-Yochay G, Raz M, Shainberg B, et al. Independent risk factors for carriage of penicillin-non-susceptible Streptococcus pneumoniae. Scand J Infect Dis. 2003;35(4):219–22. doi: 10.1080/00365540310000319. [DOI] [PubMed] [Google Scholar]
- 37.Petrosillo N, Pantosti A, Bordi E, et al. Prevalence, determinants, and molecular epidemiology of Streptococcus pneumoniae isolates colonizing the nasopharynx of healthy children in Rome. Eur J Clin Microbiol Infect Dis. 2002;21(3):181–8. doi: 10.1007/s10096-001-0689-6. [DOI] [PubMed] [Google Scholar]
- 38.Tomasson G, Gudnason T, Kristinsson KG. Dynamics of pneumococcal carriage among healthy Icelandic children attending day-care centres. Scand J Infect Dis. 2005;37(67):422–8. doi: 10.1080/00365540510035346. [DOI] [PubMed] [Google Scholar]
- 39.Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55(6):518–32. doi: 10.1136/thorax.55.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey MR, Boleij JS, Smith KR, Wafula EM. Indoor air pollution in developing countries and acute respiratory infection in children. Lancet. 1989;1(8635):427–9. doi: 10.1016/s0140-6736(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 41.Mishra V. Indoor air pollution from biomass combustion and acute respiratory illness in preschool age children in Zimbabwe. Int J Epidemiol. 2003;32(5):847–53. doi: 10.1093/ije/dyg240. [DOI] [PubMed] [Google Scholar]
- 42.Mishra V, Retherford RD. Cooking smoke increases the risk of acute respiratory infection in children. Natl Fam Health Surv Bull. 1997;(8):1–4. [PubMed] [Google Scholar]
- 43.Arason VA, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. Bmj. 1996;313(7054):387–91. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melander E, Ekdahl K, Jonsson G, Molstad S. Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr Infect Dis J. 2000;19(12):1172–7. doi: 10.1097/00006454-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Seppala H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337(7):441–6. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 46.Granizo JJ, Aguilar L, Casal J, Garcia-Rey C, Dal-Re R, Baquero F. Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and beta-lactam consumption in Spain (1979-1997) J Antimicrob Chemother. 2000;46(5):767–73. doi: 10.1093/jac/46.5.767. [DOI] [PubMed] [Google Scholar]
- 47.Baquero F, Baquero-Artigao G, Canton R, Garcia-Rey C. Antibiotic consumption and resistance selection in Streptococcus pneumoniae. J Antimicrob Chemother. 2002;50 S2:27–37. doi: 10.1093/jac/dkf504. [DOI] [PubMed] [Google Scholar]
- 48.Fedler KA, Biedenbach DJ, Jones RN. Assessment of pathogen frequency and resistance patterns among pediatric patient isolates: report from the 2004 SENTRY Antimicrobial Surveillance Program on 3 continents. Diagn Microbiol Infect Dis. 2006;56(4):427–36. doi: 10.1016/j.diagmicrobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Sombrero L, Sunico ME, Quiambao B, et al. Reliability of parental history of antibiotic use for Filipino children admitted with acute lower respiratory tract infection. Am J Trop Med Hyg. 1999;60(3):397–9. doi: 10.4269/ajtmh.1999.60.397. [DOI] [PubMed] [Google Scholar]
- 50.Catalano M, Almiron MA, Romeo AM, Caruso E, Murtagh P, Harisiadi J. Comparison between parental report and results of microbiologic agar assay for presence of antibiotic in urine of Argentinian children with acute lower respiratory tract infection. Rev Infect Dis. 1990;12 8:S998–1000. doi: 10.1093/clinids/12.supplement_8.s998. [DOI] [PubMed] [Google Scholar]
- 51.Livermore DM. Bacterial resistance: origins, epidemiology, and impact. Clin Infect Dis. 2003;36 1:S11–23. doi: 10.1086/344654. [DOI] [PubMed] [Google Scholar]
- 52.McDermott PF, Walker RD, White DG. Antimicrobials: modes of action and mechanisms of resistance. Int J Toxicol. 2003;22(2):135–43. doi: 10.1080/10915810305089. [DOI] [PubMed] [Google Scholar]
- 53.DeNap JC, Hergenrother PJ. Bacterial death comes full circle: targeting plasmid replication in drug-resistant bacteria. Org Biomol Chem. 2005;3(6):959–66. doi: 10.1039/b500182j. [DOI] [PubMed] [Google Scholar]
- 54.Farrell DJ, Jenkins SG, Brown SD, Patel M, Lavin BS, Klugman KP. Emergence and spread of Streptococcus pneumoniae with erm(B) and mef(A) resistance. Emerg Infect Dis. 2005;11(6):851–8. doi: 10.3201/eid1106.050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrell DJ, Morrissey I, Bakker S, Morris L, Buckridge S, Felmingham D. Molecular epidemiology of multiresistant Streptococcus pneumoniae with both erm(B)- and mef(A)-mediated macrolide resistance. J Clin Microbiol. 2004;42(2):764–8. doi: 10.1128/JCM.42.2.764-768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins SG, Farrell DJ, Patel M, Lavin BS. Trends in anti-bacterial resistance among Streptococcus pneumoniae isolated in the USA, 2000-2003: PROTEKT US years 1-3. J Infect. 2005;51(5):355–63. doi: 10.1016/j.jinf.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Cohen R. Approaches to reduce antibiotic resistance in the community. Pediatr Infect Dis J. 2006;25(10):977–80. doi: 10.1097/01.inf.0000239271.10784.1e. [DOI] [PubMed] [Google Scholar]
- 58.Drusano GL, Louie A, Deziel M, Gumbo T. The crisis of resistance: identifying drug exposures to suppress amplification of resistant mutant subpopulations. Clin Infect Dis. 2006;42(4):525–32. doi: 10.1086/499046. [DOI] [PubMed] [Google Scholar]