To the Editor
Human chronic myelogenous leukemia (CML) and a subset of acute lymphocytic leukemia (ALL) are caused by expression of Bcr-Abl, a fusion gene generated by reciprocal t(9;22)(q34;q11) chromosome translocation. Bcr-Abl-positive leukemias are characterized by premature release of myeloid and lymphoid lineage cells from bone marrow, followed by expansion of these cells in peripheral blood and infiltration of organs such as spleen, liver, and lung. The progression of these diseases involves not only accelerated cell proliferation and enhanced cell survival, but also increased cell motility and active invasion of leukemic cells through blood vessel and matrix barriers. The mechanism by which Bcr-Abl induces increased motility and invasion of leukemic cells is not completely understood.
Membrane-type 1 matrix metalloproteinase (MT1-MMP) is a member of transmembrane metalloprotein which is responsible for the degradation of a variety of extracellular matrix (ECM)1. MT1-MMP is also an activator of proMMP2 as well as proMMP13 and is a key regulator of cell migration and invasion1,2. In migrating cells, MT1-MMP is enriched in migration structures such as lamellipodia, whereas in some tumor cells it is found to localize to a specialized migrating/invasive structure named invadopodium3. This polarized membrane distribution of MT1-MMP is believed to be critical for cell migration and tumor metastasis. Although available evidence suggests that the interactions of MT1-MMP with transmembrane adhesion molecules and filamentous actin (F-actin) may contribute to its polarized distribution in tumor cells1, the signaling pathways leading to these interactions remain to be elucidated.
Previously, we found that the expression of Bcr-Abl in murine pro-B cell line Ba/F3 induces the assembly of an abnormal F-actin-enriched structure at the sites adjacent to membrane4. This abnormal structure is also enriched with adhesion molecules such as β1-integrin. Given the importance of actin cytoskeleton and transmembrane adhesion molecules in regulation of subcellular distribution of MT1-MMP1, we set forth to test if Bcr-Abl-induced formation of the F-actin rich structures affects the membrane distribution of MT1-MMP. Furthermore, because Bcr-Abl-induced formation of the F-actin rich structures is dependent on Abl interactor 1(Abi1), a key regulator of actin polymerization, we asked if this pathway plays a role in regulation of membrane distribution of MT1-MMP in Bcr-Abl-positive leukemic cells.
Mononuclear white blood cells isolated from Bcr-Abl-positive CML patients were stained with TRITC-conjugated phalloidin and analyzed by confocal microscopy for F-actin cytoskeleton. Cells isolated from CML patients displayed the actin-enriched structures similar to those found in Bcr-Abl-transformed Ba/F3 cells (Figure 1A, compare middle panel to lower panel). In contrast, cells isolated from a Bcr-Abl-negative human blood sample showed no such structures (Figure 1A, upper panel). Indirect immuno-fluorescence staining followed by confocal microscopy analysis revealed that the polarized F-actin structure was enriched not only with Abl tyrosine kinases (Figure 1B, left panel), but also the β1-integrin (Figure 1B, middle panel) and the MT1-MMP (Figure 1B, right panel).
Figure 1. Leukemia cells isolated from CML patient display abnormal F-actin rich structures enriched with Bcr-Abl, β1-integrin, and MT1-MMP.
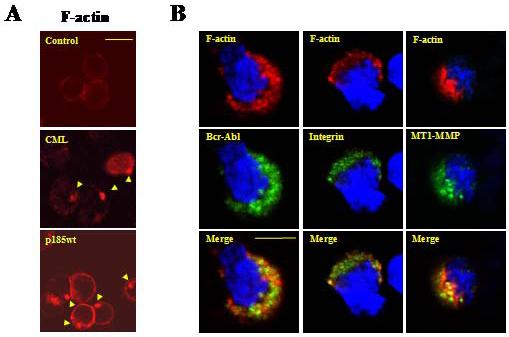
(A) Mononuclear cells isolated from a Bcr-Abl-positive CML patient (CML) and a Bcr-Abl-negative control human sample (control) were stained with TRITC-conjugated phalloidin and analyzed by confocal microscopy for actin cytoskeleton structure. A murine pro-B cell line Ba/F3 transformed by p185wt (p185wt) was also stained and analyzed. Arrows indicated F-actin rich structures; Bar: 10 μm. (B) Mononuclear cells isolated from a CML patient were probed with the anti-Abl (left panel), anti-β1integrin (middle panel), and anti-MT1-MMP (right panel) antibodies. This was followed by staining with FITC-conjugated secondary antibody. Cells were then counterstained with TRITC-conjugated phalloidin and DAPI to visualize F-actin and nuclei, respectively. The pictures were captured by two-photon confocal microscopy. Bar: 5 μm.
To determine if the polarized distribution of MT1-MMP is induced by Bcr-Abl, we examined the subcellular localization of MT1-MMP in Ba/F3 cells as well as in Ba/F3 cells transformed by wild type p185Bcr-Abl (p185wt). A polarized distribution of MT1-MMP around the F-actin rich structures was observed in p185wt cells, but not Ba/F3 cells, suggesting that the expression of Bcr-Abl is a causative event for the polarized distribution of MT1-MMP (Supplementary Figure 1A). To confirm that the polarized distribution of MT1-MMP is induced by Bcr-Abl, we also generated a fusion gene encoding for MT1-MMP with green fluorescence protein (GFP) tag at its C-terminus. The fusion gene (gfp-mt1-mmp) was introduced into Ba/F3 cells as well as the p185wt cells and the expression of GFP-MT1-MMP in these cells was determined by Western blot analysis (Supplementary Figure 1B). Fluorescence microscopy analysis revealed that the GFP-MT1-MMP displayed a polarized distribution around F-actin rich structures in p185wt cells, but not Ba/F3 cells (Supplementary Figure 1C). Together, our data indicates that Bcr-Abl induces a translocation of MT1-MMP to the F-actin rich structures in leukemic cells.
To test if the Abi1 pathway is required for Bcr-Abl to induce the polarized distribution of MT1-MMP, we made use of p185ΔSH3ΔC, a mutant Bcr-Abl defective in signaling to Abi14. The Ba/F3 cells expressing GFP-MT1-MMP were transformed by p185wt or p185ΔSH3ΔC and the expression of the wild type and mutant Bcr-Abl proteins was confirmed by Western blot analysis (Supplementary Figure 2A). Expression of p185wt, but not p185ΔSH3ΔC, induced the F-actin rich structures and recruitment of GFP-MT1-MMP to these structures (Figure 2A, right panel), suggesting that the polarized distribution of MT1-MMP requires Abi1 signaling. This notion is further supported by the data obtained from a complementary approach. In this experiment, sequence specific small hairpin RNA (shRNA) was used to knock down the expression of Abi1 in p185wt cells that express GFP-MT1-MMP (Supplementary Figure 2B). The subcellular distribution of GFP-MT1-MMP was then examined by fluorescence microscopy. We found that the knockdown of Abi1 expression significantly impaired Bcr-Abl-induced polarized distribution of GFP-MT1-MMP (Figure 2B).
Figure 2. Abi1 pathway is required for Bcr-Abl to induce a polarized distribution of MT1-MMP.
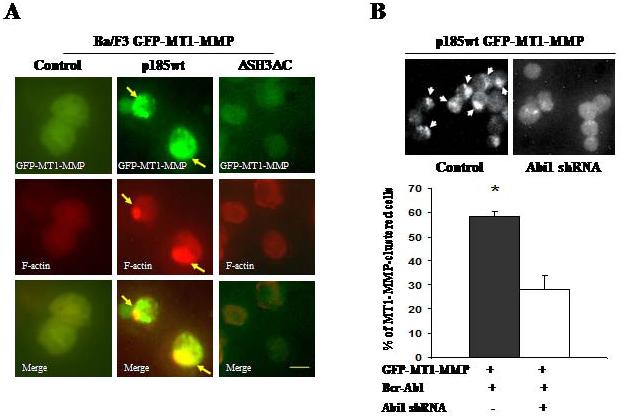
(A) Ba/F3 cells expressing GFP-MT1-MMP were transfected with plain vector (control) or vectors expressing either the wild type Bcr-Abl (p185wt) or mutant Bcr-Abl with deletion of SH3 domain and C-terminal proline-rich sequences (ΔSH3ΔC). The expression of Bcr-Abl proteins was confirmed by Western blotting (Supplementary Figure 2A). GFP-MT1-MMP was visualized by fluorescence microscopy. Arrows indicate polarized distribution of MT1-MMP. Bar: 5 μm. (B) Ba/F3 cells expressing both p185wt and GFP-MT1-MMP were transduced with either control retrovirus (control) or the retrovirus expressing the sequence specific Abi1 shRNA (Abi1 shRNA). The knockdown of Abi1 expression was confirmed by Western blotting as shown in Supplementary Figure 2B. The cells with polarized distribution of GFP-MT1-MMP were visualized by fluorescence microscopy (upper panel) and were counted (lower panel, represented as average percentage of three randomly picked areas). * P<0.01.
Recent studies have shown that MT1-MMP plays a critical role in regulation of human leukocyte migration5-7. The observations that Bcr-Abl/Abi1 pathway induces a polarized distribution of MT1-MMP in leukemic cells raised the question as to whether the MT1-MMP is involved in Bcr-Abl-stimulated leukemic cell migration. To test this we knocked down the expression of MT1-MMP in p185wt cells by shRNA-mediated gene silencing. The level of MT1-MMP transcript decreased by more than 90% in p185wt cells transfected by MT1-MMP shRNA (p185wt-MMP kd cells), as determined by real time quantitative PCR (Figure 3A, lower panel). A reduction in overall enzyme activity of MT1-MMP in p185wt-MMP kd cells was also suggested by gelatin zymography analysis of the level of proMMP2, which is a substrate of MT1-MMP and is cleaved to an active form by MT1-MMP. As shown in Figure 3A (upper panel), while most, if not all, of MMP2 existed as the cleaved active form in control p185wt cells, a large proportion of proMMP2 was observed in p185wt-MMP kd cells. Correlating with the reduction of MT1-MMP mRNA level and enzyme activity, transmigration of p185wt-MMP kd cells through fibronectin coated membrane decreased, as compared to that of control p185wt cells (Figure 3B). Thus, our studies suggest that MT1-MMP may play a role in Bcr-Abl- stimulated leukemic cells migration.
Figure 3. Knockdown of MT1-MMP expression impaired Bcr-Abl-stimulated cell migration.
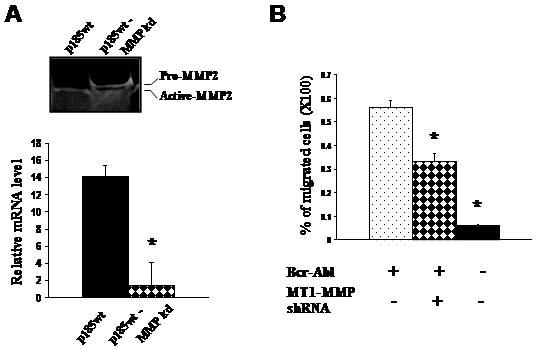
(A) Expression of MT1-MMP shRNA resulted in a decrease in mRNA level (lower panel) and proteolytic activity (upper panel) of MT1-MMP in p185wt cells. Ba/F3 cells transformed by p185wt were transduced with control retrovirus (p185wt) or the retrovirus expressing the sequence specific MT1-MMP shRNA (p185wt MMP kd). The proteolytic activity in conditioned medium was analyzed by gelatin zymography and the relative level of MT1-MMP mRNA was analyzed by real time quantitative PCR and the data represents the mean +/- s.d. of triplicate experiments. * P<0.001. (B) Knockdown of MT1-MMP expression impaired the ability of p185wt cells to transmigrate through fibronectin coated membrane. Data represents the mean +/- s.d. of triplicate experiments. * P<0.001.
In this report we identified MT1-MMP as a novel downstream target of Bcr-Abl/Abi1 signaling. We show that by activating the Abi1 pathway, Bcr-Abl induces a translocation of MT1-MMP to a membrane-associated structural complex enriched with F-actin and adhesion molecules. Recruitment of MT1-MMP to invadopodia in invasive tumor cells has been reported and is thought to play an important role in tumor metastasis, as it may enhance local ECM degradation and therefore, facilitate tumor cell migration and invasion1, 3, 8. Our studies suggest that a similar mechanism may also be utilized by Bcr-Abl-positive leukemic cells. It is notable that the F-actin rich structure induced by Bcr-Abl shares some similarities with invadopodia in that they both appear as a membrane-associated F-actin rich complex consisting of multiple structural/regulatory proteins involved in the regulation of actin cytoskeleton assembly, cell adhesion, and ECM degradation. We believe that the studies presented here warrant further investigation of this membrane-associated structural complex found in Bcr-Abl-positive leukemic cells.
Supplementary Material
Acknowledgements
This work was supported by NIH/NCI grant R01 CA094921 (Z. Dai) and NIH/NIDDK grant K01 DK067191 (Y. Tao).
References
- 1.Itoh Y, Seiki M. MT1-MMP: A potent modifier of petricellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 2.Cowell S, Knäuper V, Stewart ML, D’Ortho MP, Stanton H, Hembry RM, et al. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J. 1998;331:453–458. doi: 10.1042/bj3310453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann N Y Acad Sci. 1999;878:361–71. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Clough N, Sun X, Yu W, Abbott BL, Hogan CJ, et al. Bcr-Abl induces abnormal cytoskeleton remodeling, beta1 integrin clustering and increased cell adhesion to fibronectin through the Abl interactor 1 pathway. J Cell Sci. 2007;120:1436–46. doi: 10.1242/jcs.03430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matías-Román S, Gálvez BG, Genís L, Yáñez-Mó M, de la Rosa G, Sánchez-Mateos P, et al. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood. 2005;105:3956–3964. doi: 10.1182/blood-2004-06-2382. [DOI] [PubMed] [Google Scholar]
- 6.Yang MX, Qu X, Kong BH, Lam QL, Shao QQ, Deng BP, et al. Membrane type 1-matrix metalloproteinase is involved in the migration of human monocyte-derived dendritic cells. Immunol Cell Biol. 2006;84:557–562. doi: 10.1111/j.1440-1711.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 7.Sithu SD, English WR, Olson P, Krubasik D, Baker AH, Murphy G, et al. Membrane-type 1-Matrix Metalloproteinase Regulates Intracellular Adhesion Molecule-1 (ICAM-1)-mediated Monocyte Transmigration. J Biol Chem. 2007;282:25010–25019. doi: 10.1074/jbc.M611273200. [DOI] [PubMed] [Google Scholar]
- 8.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


