Abstract
IL-2-dependent activated cells undergo apoptotic death when IL-2 is withdrawn either in vitro or after in vivo cell transfer. To attempt to sustain their survival after IL-2 withdrawal, melanoma-reactive human T lymphocytes were retrovirally transduced with an exogenous human IL-2 gene. Transduced PBMC and cloned CD8+ T cells produced IL-2 and maintained viability after IL-2 withdrawal. Upon restimulation, IL-2 transductants proliferated in the absence of exogenous IL-2 and could be actively grown, and their survival could be maintained without added IL-2 for over 8 wk. PBMCs similarly transduced with a control vector did not produce IL-2 and failed to proliferate in the absence of IL-2. A CD8+ T cell clone, when transduced with an IL-2 gene, manifested the same phenotypes as PBMCs in the absence of exogenous IL-2. Furthermore, an Ab reactive with the α-chain of IL-2R complex reduced the viability mediated by IL-2 secretion of the IL-2 transductants. Moreover, transduction of an IL-2 gene did not affect the high degree of recognition and specificity of transductants against melanoma targets. These tumor-reactive IL-2 transductants may be valuable for in vitro studies and for improved adoptive transfer therapies for patients with metastatic melanoma.
Multiple factors regulate lymphocyte survival, including the availability of appropriate growth factors, the activation status of the cell, as well as the competing influences of the proliferative and the apoptotic signals resulting from cell activation in the presence of a variety of cytokines (1, 2). These factors can be manipulated in vitro and can result in the sustained survival and growth of lymphocytes in culture (3). It has, however, been extremely difficult to control lymphocyte survival and growth following transfer of cells to intact hosts in vivo. This may severely limit the ability of the adoptively transferred lymphocytes to mediate tumor regression (4).
Lymphocyte survival in vivo is dependent on appropriate stimulation, but also on the availability of sufficient local concentrations of appropriate growth factors such as IL-2 (5). To sustain the survival of activated lymphocytes following in vivo administration, it has been necessary to supply an exogenous source of IL-2, although the systemic toxicity of IL-2 severely limits the amount of this cytokine that can be given (6). Although multiple studies of the adoptive transfer of activated lymphocytes with specific function to humans with HIV infection and cancer have been attempted, the limited survival of these cells in vivo has severely compromised their function (4, 7–11). One potential solution to this problem is the introduction of genes into lymphocytes that can result in either the regulated or constitutive production of appropriate growth signals that might obviate the need for the administration of potentially toxic levels of administered cytokines.
Introduction of an exogenous IL-2 gene to a murine CD4 T cell line led to IL-2 secretion and growth of that cell line independent of added IL-2 in vitro (12). Tumor cells of a variety of histologies when modified by an IL-2 gene could produce IL-2, and such modified cells have been used as tumor vaccines in at least 24 current human gene therapy trials (13). However, there have been no convincing data that IL-2 gene modification can be achieved in human primary T cells, especially CD8+ T cells that are used in adoptive transfer therapy for patients with cancer. One report described the transfection of the IL-2 gene into human tumor-infiltrating lymphocytes (TILs)2 isolated from pleural effusions of advanced lung cancer patients (14), although few data were presented concerning the production of IL-2 and the function of these modified cells.
It is against this background that we undertook studies to introduce an exogenous IL-2 gene into specific tumor-reactive lymphocytes in an attempt to enhance their survival and reduce their dependence on the exogenous administration of IL-2.
Materials and Methods
Construction of a retroviral plasmid containing an IL-2 gene
To construct a retrovirus to deliver the IL-2 gene to primary T lymphocytes, we used a pSAMIL-2EN plasmid (kindly provided by P. Hwu, Surgery Branch, National Institutes of Health, Bethesda, MD), which contained an IL-2 cDNA A50. A PCR reaction was performed using pSAMIL-2EN as a template to obtain a 490-bp fragment. Primers used to generate this fragment were: 1) 5′-GGAGGCCTGGATCCATGTACAGGATG CAACTCCT-3′, and 2) 5′-GGGTCGACGGATCCTCAAGTTAGTGTT GAGATGA-3′. Primer 2 inserted a SalI restriction site (italicized) at one terminus (translational start and stop codons of the IL-2 gene are underlined). This fragment was directionally cloned into the SrfI (Stratagene, La Jolla, CA) and SalI (Promega, Madison, WI) sites of the plasmid pGCIR ESYFP (15) (kindly provided by G. Costa, Stanford University, Stanford, CA), resulting in the final bicistronic construct, pIL-2-IRES-YFP, whose structure was confirmed by the nucleotide sequencing from 5′ to 3′ long terminal repeats. Yellow fluorescent protein (YFP, a variant of enhanced green fluorescent protein) as a marker was used in our study to facilitate the evaluation of transduction efficiency and for positive selection by FACS sorting. The expression of the IL-2 gene in the pIL-2-IRES-YFP is under the viral 5′ long terminal repeat promoter control, and the translation of YFP is facilitated by the internal ribosomal entry site (IRES). This obviated the theoretical concern of promoter competition if an internal promoter were used for the expression of the marker gene (12, 17).
Construction of pseudotyped retroviruses
The control vector (IRES-YFP, referred to as YFP) and vector containing the IL-2 gene (IL-2-IRES-YFP, referred to as IL-2YFP) were prepared in parallel. Two packaging cell lines, Phoenix E and PT 67, were grown in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% FCS (HyClone Laboratories, Logan, UT) in a 37°C humidified incubator with 5% CO2. Ecotropic Phoenix E was purchased from the American Type Culture Collection (Manassas, VA) with the permission of G. Nolan (Stanford University), and amphotropic PT 67 was purchased from Clon-tech (Palo Alto, CA). Twenty micrograms of plasmid DNA were mixed with the GeneJammer transfection reagent (Stratagene) and transfected to 2 × 106 Phoenix E cells, according to manufacturer’s instruction. At 48 h post-transfection, the culture medium was used immediately to infect 1 × 107 PT 67 cells in the presence of 8 μg/ml Polybrene. Forty-eight hours postinfection, PT 67 cells were detached from the flask by digestion with trypsin/EDTA (Biofluids, Rockville, MD) and prepared for FACS analysis by a FACScan flow cytometer (BD Biosciences, Mountain View, CA). YFP+ (FITC channel) cells were sterilely sorted twice on a FACSorterPlus (BD Biosciences) (18). The expression of the IL-2 gene from the stable IL-2YFP vector-producing PT 67 line was confirmed by the presence of from 5000 to 8000 IU/ml IL-2 present in the supernatant, as detected by ELISA (Endogen, Woburn, MA). Retroviral titer was determined as described (19). Consistently, supernatants of 0.5–1.7 × 106 TU/ml were obtained.
In vitro stimulation of PBMCs with gp100:209–217 (210 M) (referred to as 209-2M) peptide
Cryopreserved PBMCs obtained after the eighth weekly s.c. injection with 209-2M peptide (IMDQVPFSV) (in IFA) of the patient RP with metastatic melanoma (20) were thawed in complete medium (CM) consisting of RPMI 1640 (Life Technologies) supplemented with 10 mM HEPES buffer, 100 U/ml penicillin and 100 μg/ml streptomycin (Biofluids), 20 μM 2-ME, and 10% heat-inactivated freshly pooled normal human male serum (Biochemed Pharmacologicals, Winchester, VA), and plated at 3 × 106 in 2 ml CM with 1 μM 209-2M peptide. On the next day and every 3 subsequent days, IL-2 (kindly supplied by Chiron, Emeryville, CA) was added to the cultures to a final concentration of 300 IU/ml. Cultures were maintained at the cell density of 0.7–1 × 106/ml. Peptide 209-2M (IMDQVPFSV) is a modified immunodominant epitope from melanoma differentiation Ag, gp100, spanning aa 209–217. This altered peptide with a methionine substituting natural threonine at position 2 (thus referred to as 209-2M) was shown to have much higher degree of recognition in binding to HLA-A2 molecule and more immunogenic than native peptide. CTLs elicited by 209-2M also recognize the native peptide 209-pulsed T2 cells (23).
CD8+ T cell clone (D4F12)
This clone was isolated from a TIL bulk culture obtained from a metastatic lesion of the patient MD, and was kindly provided by M. Dudley and the TIL laboratory (Surgery Branch, National Cancer Institute). The D4F12 clone was grown in 50/50 medium consisting of a 1:1 volume ratio of CM and AIM V medium (Life Technologies) supplemented with 300 IU/ml IL-2. As described below, this clone was expanded by the rapid expansion protocols (REPs) with irradiated (35 Gy) allogeneic feeders and OKT3. On day 7 of REP 3, the transduction was performed.
Transduction
Transductions were performed in wells of 6- or 24-well plates (Becton Dickinson) coated with 10 μg/cm2 Retronectin (Takara, Shuzo, Otsu, Shiga, Japan), according to the manufacturer’s instruction. Two million PBMC or D4F12 cell pellets were resuspended with 8 ml freshly prepared YFP or IL-2YFP retroviral supernatants, respectively. IL-2 was added to YFP supernatants to a final concentration of 7200 IU/ml. No exogenous IL-2 was added to IL-2YFP supernatants, which already contained comparable level of IL-2. This cell-viral supernatant mixture was then applied to a Retronectin-coated well and incubated at 37°C, 5% CO2 for 6 h. A total of four retroviral transductions in the same Retronectin-coated wells was performed in 2 consecutive days. At the end of the second day, the transduced cells were washed twice with CM, resuspended in CM for PBMCs, or 50/50 medium for D4F12, respectively supplemented with 300 IU/ml IL-2. The cultures were maintained at the cell density of 0.7–1 × 106/ml at 37°C, 5% CO2. On day 4 –5 posttransduction, transduction efficiency was determined as the percentage of YFP+ cells by a FACScan (FITC channel). These YFP+ cells were sterilely sorted on a FACStarPlus sorter. Sorted transductants were incubated at 37°C, 5% CO2 in CM or 50/50 medium supplemented with IL-2 at a concentration of 300 IU/ml.
Microwell viability and IL-2 production assay
Sorted transductants were washed three times with CM. Cells of 0.5 × 105 in 100 μl CM or 50/50 media were placed in wells of 96-well U-bottom plate (Costar, Corning, NY) uncoated or coated with anti-CD3 (OKT3; Orthoclone, Ortho-biotech, Raritan, NJ) and anti-CD28 (BD PharMingen, San Diego, CA) in the absence or presence of IL-2 at a concentration of 300 IU/ml. Wells were coated with OKT3 and anti-CD28, as described (21). As indicated, anti-CD25 (an Ab reactive with the α-chain of the IL-2R complex, 5–10 μg/ml) or a control isotope Ab (5–10 μg/ml) (gifts from Y. Tagaya, Metabolism Branch, National Cancer Institute) was added to the no IL-2 condition. Two days later, 50 μl media were removed from the wells lacking added IL-2 and assayed for IL-2 by ELISA. These wells were replaced with 50 μl fresh media without IL-2. Also on day 2, cells from OKT3- and anti-CD28-coated wells were transferred to regular wells (nonspecific TCR restimulation for 2 days only). Fresh IL-2 (300 IU/ml) was added to +IL-2 condition every 3 days. On day 6, the cell clusters at the bottom of all wells were photographed. On day 7, viable cells were scored using CellTiter 96 Aqueous One solution cell proliferation assay [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophe-nyl)-2H-tetrazolium, inner salt] (MTS); Owen’s reagent) viability assay; Promega], according to the manufacturer’s instruction. Included in the assay were standards of viable cells with known numbers, as determined by trypan blue (BioWhittaker, Walkersville, MD) exclusion, and counted. A standard curve was generated, and viable cell equivalents were calculated from this curve.
Rapid expansion protocol (REP)
Cells were expanded, as described previously (8), with minor modifications. A total of 1 × 105 sorted PBMC transductants was added to 25 ml CM without IL-2 in a 25-cm2 tissue culture flask containing 2.5 × 107 irradiated (35 Gy) allogeneic PBMCs and OKT3 at 30 ng/ml. IL-2 was added to some flasks on the next day to a final concentration of 300 IU/ml. Twenty milliliters of media were removed on day 7 and replaced with fresh CM with or without IL-2 (300 IU/ml). On day 9, cells from some flasks were rigorously washed three times and resuspended in CM without IL-2. Viable cells of each condition were counted by trypan blue exclusion, and the total number of viable cells was plotted against time. To test for the existence of an IL-2 autocrine loop, cells from the no IL-2 condition were washed twice with CM and incubated with anti-CD25 or a control isotope Ab at a concentration of 5–10 μg/ml at 37°C, 5% CO2 in the wells of a 48-well plate. Forty-eight hours after incubation, the supernatants were harvested and the IL-2 level was assayed by ELISA. For REPs performed in the wells of 24-well plates (Costar), 1 × 105 effector T cells were incubated with 1 × 106 irradiated (35 Gy) allogeneic PBMCs in 2 ml media containing 30 ng/ml OKT3.
Measurement of melanoma-specific T cell reactivity (20)
A total of 5 × 104 cells of the sorted transductants or D4F12 was cocultured with 1 × 105 target cells in the microwells of 96 U-bottom plates in a final volume of 200 μl. Cocultures were incubated at 37°C in 5% CO2 overnight. One hundred thirty microliters of the supernatant were harvested, and the IFN-γ released by reactive T cells was analyzed by ELISA (Endogen). Target cells used were T2 cells (HLA-A2+, Tap-deficient line derived from a B-T cell hybrid (22)) pulsed with peptide gp100:209–217 (209 peptide) at concentration of 1, 0.01, and 0.0001 μM, respectively; T2 cells pulsed with 1 μM MART-1:27-35 (MART-1); and melanoma cell lines 526 mel (HLA-A2+) and 888 mel (HLA-A2−). Positive IFN-γ values were defined as at least 100 pg/ml and at least twice background.
Results
Transduction of PBMCs
Cryopreserved PBMCs obtained from patient RP after eight weekly injections of 209-2M peptide were thawed, stimulated with the immunizing peptide, and transduced with retroviruses. To determine an optimal time point for transduction, a time course of T cell surface marker and transduction efficiency was investigated (Table I). At the indicated times after peptide stimulation, cells were transduced with either YFP control vector or IL-2YFP vector supernatant on Retronectin-coated plates (21, 31). Four days post-transduction, equal portions of cells were taken for analysis of T cell surface markers and for transduction efficiency. As shown in Table I, CD8+ cells increased from day 6 to day 9 after peptide stimulation (52% to 64%). A significant increase in transduction efficiency was seen for both control vector (YFP) and the IL-2YFP vector over time, with the highest transduction efficiency seen on day 5 after peptide stimulation. The vast majority of YFP+ cells were CD8+ (78–96%), as expected by the preferential proliferation of CTL precursors after a class I-restricted peptide stimulation. When transduction was performed on day 6 after peptide stimulation in another independent experiment, the efficiency was 19% for control YFP vector and 6.3% for IL-2YFP vector, respectively. Therefore, day 6 postpeptide stimulation was chosen for later experiments.
Table I.
T cell profile and transduction efficiency of PBMCs post-peptide 209-2M stimulationa
| Transduction on Days Post-Peptide Stimulation | Transduction Efficiency (%)
|
||||||
|---|---|---|---|---|---|---|---|
| T Cell Surface Markers (%)
|
YFP vector
|
IL-2YFP vector
|
|||||
| CD3+ | CD4+ | CD8+ | YFP+ | CD8+YFP+ | YFP+ | CD8+YFP | |
| 2 | 63.47 | 31.73 | 52.63 | 5 | 4.8 | 2.35 | 2 |
| 3 | 65.32 | 26.1 | 62.54 | 10 | 9.65 | 3.52 | 2.88 |
| 5 | 67.5 | 25.9 | 64.2 | 18.61 | 14.6 | 6.98 | 5.47 |
PBMCs were transduced at the time indicated post-peptide stimulation. Four days post-transduction, equal portions of cells were incubated with PE-conjugated Abs to determine T cell surface markers, the percent of which were determined by FACS (PE channel). Cells were also analyzed for the percent of YFP+ and YFP+/CD8+ to determine the transduction efficiency.
The transduction efficiency was lower for the IL-2YFP vector when compared with that of the control vector, although similar retroviral titers were used for these transductions (data not shown). We interpreted this difference as a result of relatively inefficient capping-independent translation of YFP related to the IRES in the cells transduced by the IL-2YFP vector. Thus, this lower percentage of YFP+ cells probably underestimated the proportion of IL-2YFP transductants since some transduced cells might not have expressed YFP. A transduction efficiency of 6.5% was obtained when a different vector, human telomerase reverse transcriptase-YFP with an equivalent titer, was used for PBMC transduction on day 5 after peptide stimulation in the same experiment as shown in Table I, suggesting that the lower efficiency seen in the IL-2YFP transduction was not due to a deleterious effect of the IL-2 gene expression.
IL-2YFP PBMC transductants were able to secrete IL-2 and maintain their viability in the absence of exogenous IL-2 upon restimulation
We next examined the growth and phenotypes of the transduced cells in the absence of added IL-2 in the tissue culture media. PBMCs were stimulated with 209-2M peptide, and 6 days later transductions were performed. The transduction efficiency was determined 5 days later by scoring YFP+ cells (28.9% for YFP vector and 16.7% for IL-2YFP vector), followed by sterile sorting of YFP+ cells (Fig. 1). The sorting efficiency for YFP and IL-2YFP was 93.8% and 76.3%, respectively. These sorted cells were assayed for their ability to produce IL-2 and for their growth and viability in microwells of 96 U-bottom well plates.
FIGURE 1.
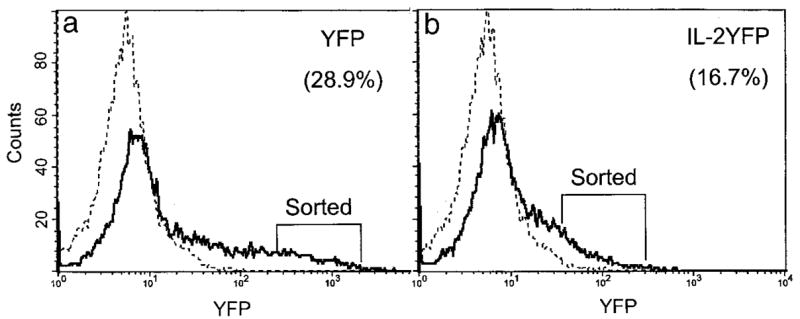
Transduction efficiency for PBMC transductants. PBMCs were stimulated with peptide 209-2M. IL-2 (300 IU/ml) was added on the next day. Five days later, transduction with the indicated retroviral supernatant was performed on Retronectin-coated plates on 2 consecutive days. Transductants were maintained in complete medium supplemented with fresh IL-2 every 3 days (300 IU/ml). Seven days posttransduction (day 13 post-peptide stimulation), transduction efficiency was determined by a FACS flow cytometer as the percentage of YFP+ cells of that transductant, and indicated in parentheses. Dashed line, untransduced cells; solid line, transduced cells. a, YFP transductants; b, IL-2YFP transductants. YFP+ cells as indicated from each transductant were sterilely sorted on a FACSorterPlus.
No IL-2 production could be detected either for control vector (YFP) or IL-2YFP vector transductants 2 days after sorted transductants were placed in medium containing no IL-2, in the absence of OKT3 and anti-CD28 stimulation (data not shown). There was also no IL-2 detectable in cultures of the YFP transductants or untransduced PBMCs after stimulation with OKT3 and anti-CD28. In contrast, with OKT3 and anti-CD28 restimulation, 240 ± 15 (SEM) pg/ml IL-2 was detected in the medium of the IL-2YFP transductants (0.5 ± 105 cells in a volume of 200 μl). Because medium was removed 2 days after restimulation, the level of IL-2 detected here represented an accumulated amount, reflecting a steady state between IL-2 production and active consumption by the cells.
In the absence of IL-2 and no stimulation with OKT3 and anti-CD28, there was no difference in the size of cell clusters in untransduced or YFP or IL-2YFP cells (Fig. 2a). Furthermore, all cells had lost their viability in the MTS viability assay (Fig. 2c). However, with the continuing presence of IL-2 in media, all groups retained their viability (Fig. 2, b and d).
FIGURE 2.
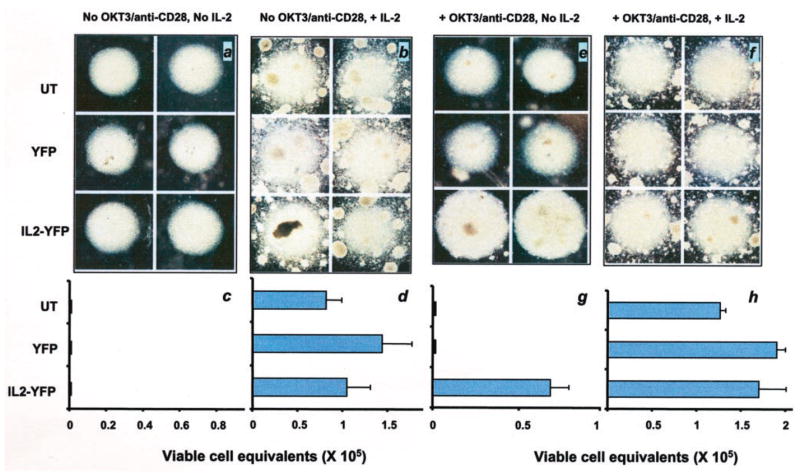
IL-2YFP transductants maintained their viability in the absence of exogenous IL-2 upon restimulation with anti-CD3 and anti-CD28. Duplicates of each sorted transductant (YFP or IL-2YFP) or untransduced (UT) PBMC were washed extensively to remove any residual IL-2, resuspended in complete medium with (b, d, f, and h) or without IL-2 (a, c, e, and g), and replated in regular wells (a–d) or wells previously coated with anti-CD3 and anti-CD28 Abs on a 96 U-bottom microwell plate (e–h). On day 2, cells from Ab-coated wells were transferred to regular wells (restimulation for only 2 days). On day 5, cell clusters at the bottom of all microwells were photographed (a, b, e, and f), and an MTS viability assay was performed for each transductant (c, d, g, and h). Shown here are the mean ± SEM of two independent wells for each transductant or untransduced PBMC (c, d, g, and h). Without restimulation, all cells lost their viability. Upon restimulation with anti-CD3 and anti-CD28, IL-2YFP transductants maintained their viability in the absence of exogenous IL-2.
In sharp contrast, with restimulaton in the absence of exogenous IL-2, cell clusters of IL-2YFP transductants were significantly larger than control vector transductants or untransduced PBMCs. Only IL-2YFP transductants were viable in the MTS assay (Fig. 2, e and g). When IL-2 was present in the media, there were no differences seen in cell clusters and viability for all transductants (Fig. 2, b and d, f and h). Thus, it appeared that the expression of the IL-2 gene by the IL-2YFP transductants could sustain their survival when restimulated with OKT3 and anti-CD28 in the absence of added IL-2.
A second round of stimulation was then performed. The IL-2YFP transductants that had been stimulated with OKT3/anti-CD28 in the absence of IL-2 from a replica plate, as shown in Fig. 2e, were washed twice and subjected to similar restimulation, as described in Fig. 2. As controls, untransduced and YFP transductants from the +IL-2 +OKT3/anti-CD28 condition from a replica plate as shown in Fig. 2f were also restimulated.
As shown in Fig. 3, the same patterns of results were obtained: only IL-2YFP transductants were viable in the absence of IL-2 (a and c), and no significant difference was seen when IL-2 was present in the medium (b and d).
FIGURE 3.
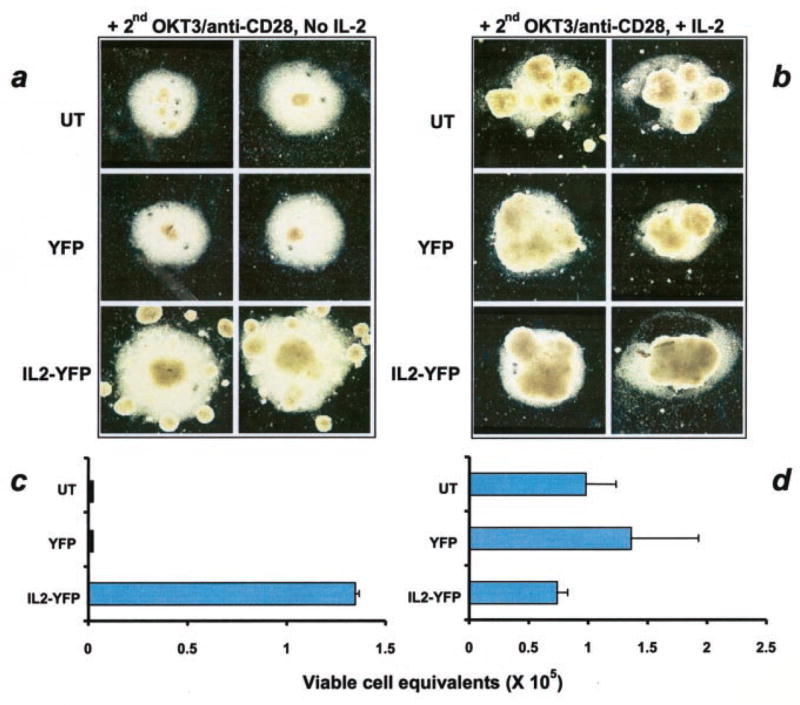
IL-2 transductants maintained their viability in the absence of exogenous IL-2 upon second restimulation with anti-CD3 and anti-CD28. Replicates of IL-2 transductants from the no IL-2 condition from a replica plate as shown in Fig. 2e, and YFP transductants and untransduced PBMC from +IL-2 condition from a replica plate as shown in Fig. 2f were subjected to the same experiment as described in the legend to Fig. 2. a and b, Photographs of cell clusters taken on day 5; and c and d, values of calculated viable cell equivalents.
These results demonstrated that upon restimulation, IL-2YFP transductants were able to secrete IL-2 and retain their viability in the absence of added IL-2. Untransduced or vector alone YFP transductants could not do so under the same conditions.
IL-2YFP PBMC transductants were able to proliferate in the absence of exogenous IL-2 upon restimulation
To determine whether the IL-2YFP-transduced cells were capable of actively proliferating in the absence of IL-2, a REP experiment was performed. IL-2YFP transductants were obtained from the no IL-2 condition of a second stimulation from a replica plate, as shown in Fig. 3a. YFP transductants were obtained from a cell culture that had been maintained after sorting without any restimulation in the presence of IL-2. Both transductants (1 × 105 each) were washed twice to remove any residual IL-2 and subjected to the REP protocol with soluble OKT3 and irradiated allogeneic PBMCs in wells of a 24-well plate with or without exogenous IL-2 in the culture medium. On day 14 of this experiment, viable cells were counted by trypan blue exclusion.
YFP transductants did not proliferate at all in the absence of IL. In contrast, IL-2YFP transductants proliferated and expanded 10.4-fold in the absence of IL-2. In the presence of IL-2, YFP transductants expanded 230-fold, and IL-2 transductants expanded 135-fold.
These results established that IL-2-sorted transductants were able to proliferate in the absence of exogenous IL-2 upon restimulation. The difference between YFP and IL-2YFP transductants in the presence of IL-2 might be due to greater growth potential of YFP transductants because they had not been restimulated before this experiment.
Transduction of an IL-2 gene into cells with antitumor reactivity did not affect tumor recognition of transductants
IL-2YFP and YFP transductants, as shown in Fig. 1, were maintained in the presence of IL-2 after sorting. On day 14 after sorting, they were analyzed for the release of IFN-γ in a coculture assay with tumor targets. Both transductants exhibited high degree of recognition and specificity for 209 peptide-pulsed T2 cells (Table II, experiment 1). As a further test, the transduced PBMCs were cloned two days after sorting. The resultant clones (P209IL-2YFP clone 3 and P209YFP clone 29) were expanded in a REP protocol in the presence of IL-2. On day 10 of this REP experiment, an independent coculture assay with the target cells as shown in Table II was performed and the IFN-γ released by these clones was assayed by ELISA. These clones showed high degree of recognition and specificity for 209 peptide and recognized melanoma tumor targets in an HLA-A2-restricted fashion (Table II, experiment 2). More importantly, in another independent experiment (Fig. 4, middle), IL-2YFP transductants that proliferated in the absence of IL-2 specifically recognized 209 peptide-pulsed T2 cells (Table II, experiment 3). Thus, transduction of either a reporter gene or an IL-2 with the reporter gene did not affect tumor recognition of transductants.
Table II.
Transduction of an IL-2 gene into cells with antitumor reactivity did not affect tumor recognition of transductantsa
| Effectors (IFN-γ pg/ml)
|
|||||
|---|---|---|---|---|---|
| Expt. 1 (sorted PBMC transductants)
|
Expt. 2 (P209 clones)
|
||||
| Targets | IL-2YFP | YFP | IL-2YFP #3 | YFP #29 | Expt. 3 (IL-2YFP transductants (proliferated in the no IL-2 condition)) |
| T2 pulsed with peptide | |||||
| MART-1 (1 μM) | 1 | 0 | 1 | 0 | 6 |
| 209 (1 μM) | >900 | >900 | 4255 | 1870 | >900 |
| 209 (0.01 μM) | 915 | 339 | |||
| 209 (0.0001 μM) | 23 | 3 | |||
| Tumors cell line | |||||
| 526 (A2+) | 1032 | 437 | |||
| 888 (A2−) | 3 | 2 | |||
| No targets | 2 | 6 | |||
Experiment 1: The indicated PBMC transductants (14 days after sorting and maintained in the presence of IL-2) were cocultured overnight with the targets as shown. IFN-γ release was determined by ELISA (>900 pg/ml offscale). Experiment 2: P209IL-2YFP and YFP clones were derived from transduced PBMCs as shown in experiment 1. These clones were expanded in a REP experiment in the presence of IL-2 and were assayed at day 10 of the REP for the IFN-γ release in an independent coculture experiment. The levels of the IFN-γ released were quantified by serial dilution of the coculture media in an ELISA. Experiment 3: cells were obtained from the no IL-2 condition (Fig. 4, middle) in a REP experiment on day 14. Antitumor T cells (4 × 104) were incubated with 1 × 105 T2 cells pulsed with peptides as shown. After overnight coculture, the IFN-γ released was assayed as described above (>900 offscale).
FIGURE 4.
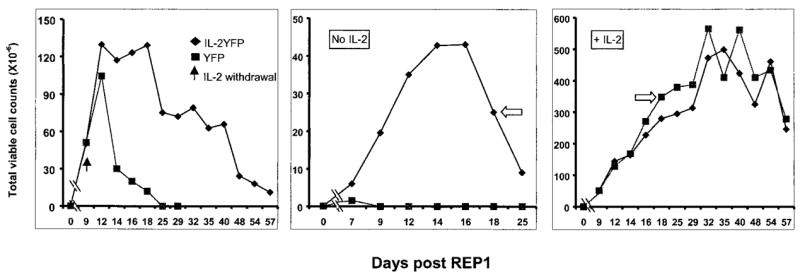
IL-2YFP transductants maintained their viability after IL-2 withdrawal when they were actively proliferating. IL-2YFP-sorted transductants and YFP-unsorted transductants were obtained as described in Results. A total of 1 × 105 cells of each transductant as indicated was subjected to a REP experiment. At the days indicated, viable cell counts were determined by trypan blue exclusion. Left, IL-2 was added on day 1 and every 3 days; then on day 9, cells were extensively washed and resuspended in CM without IL-2 (black arrow). Middle, No IL-2 from the start. Right, IL-2 added from the start (300 IU/ml) and every 3 days after. White arrows denote cells used for REP2 (Fig. 5).
IL-2YFP transductants maintained their viability after IL-2 withdrawal when they were actively proliferating
We next examined the viability of IL-2YFP transductants when IL-2 was withdrawn from the culture medium when cells were actively dividing. To investigate this, an independent transduction experiment was performed. Bulk PBMCs from patient RP were stimulated with peptide 209-2M, and transduced with IL-2YFP retroviral vector (transduction efficiency 6.3%) and positively selected on a FACS sorter, as described. Cells were also transduced with control vector, YFP (transduction efficiency 19.7%), but not sorted. Cells were washed twice to remove residual IL-2 and subjected to a REP protocol. Three conditions were included in this experiment: no IL-2; +IL-2; and +IL-2 until day 9. Cells were counted on the indicated days (Fig. 4) by trypan blue exclusion.
Upon withdrawal of IL-2 from the medium on day 9, control cells (YFP) decreased dramatically in their viability 5 days later and there were no viable cells left at day 25 (Fig. 4, left). In contrast, IL-2YFP transductants maintained their growth peak for a week before decreasing in viability after IL-2 withdrawal, and maintained at this level for another 2 wk before dying. In cultures that underwent a REP in the absence of IL-2 from the onset of the culture, IL-2YFP transductants could proliferate in the absence of exogenous IL-2 (Fig. 4, middle). At the peak of their growth, 400-fold expansion was obtained. Control YFP transductants did not proliferate at all, confirming the result described earlier. In the presence of IL-2, there was no difference seen for the growth of YFP transductants when compared with IL-2YFP transductants (Fig. 4, right).
To extend these results, a second REP was performed. IL-2YFP transductants that had grown in the absence of exogenous IL-2 were obtained from the cells shown in Fig. 4, middle, on day 18 (indicated by an empty arrow). Control YFP cells were obtained from the added IL-2 condition on day 18 (Fig. 4, right, indicated by a white arrow). The cells were stimulated in a REP experiment (REP2) using the same conditions as shown in Fig. 4. For this experiment, the cultures were coded and counted in a blinded fashion. Withdrawal of IL-2 from the medium on day 9 resulted in a rapid decrease in viability of YFP transductants, although the IL-2YFP transductants grew and maintained viability for 3 wk (Fig. 5, left). In contrast, in the complete absence of added IL-2, IL-2YFP transductants proliferated again, and a higher fold expansion of IL-2YFP transductants was seen (900-fold at the peak, Fig. 5, middle). This could be attributed to selection of more IL-2-expressing transductants during the first REP. During the first REP, IL-2YFP transductants may have been positively selected in the absence of IL-2. This was supported by the observation that at day 17 of the REP2 culture, in the absence of IL-2, almost all IL-2YFP transductants were YFP+ cells (thus containing IL-2 gene), compared with approximately 80% YFP+ cells on day 13 of REP1 (data not shown). Furthermore, a longer and a higher level of viability was seen for IL2YFP transductants after IL-2 withdrawal during the REP2 (compare the left panels of Figs. 4 and 5).
FIGURE 5.
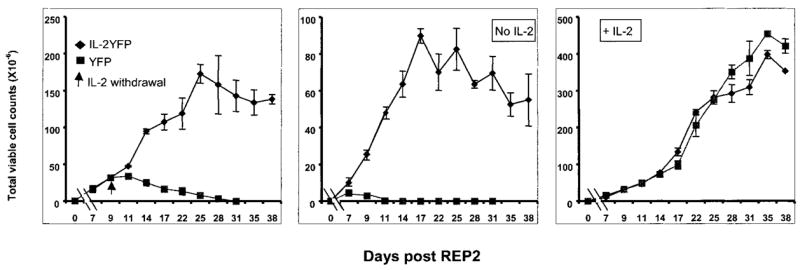
IL-2YFP transductants maintained their viability after IL-2 withdrawal upon second restimulation (REP2). IL-2YFP-sorted transductants and YFP-unsorted transductants were obtained, as indicated by empty arrows in Fig. 4. Replicates of 1 × 105 cells of each transductant as indicated were subjected to a second REP experiment. At the days indicated, viable cell counts were determined by trypan blue exclusion. Shown here are the average of cell counts from two independent 25-cm2 flasks for each transductant with SE. Left, IL-2 was added on day 1 and every 3 days; on day 9, cells were extensively washed and resuspended in CM without IL-2 (indicated by an arrow). Middle, No IL-2 from the start. Right, IL-2 added from the start (300 IU/ml) and every 3 days after.
Existence of an autocrine loop of IL-2 during the growth of IL-2YFP transductants in the absence of exogenous IL-2
To test the secretion of IL-2 during the peak of their proliferation and whether an autocrine loop of IL-2 existed by the IL-2YFP transductants, the following experiment was performed. On day 9 of the second REP from the previous experiment, IL-2 secretion from cells from IL-2YFP transductants (Fig. 5, middle) in the absence of IL-2 was compared with control cells from YFP transductants grown in the presence of IL-2 (Fig. 5, right). Cells were washed twice to remove IL-2, resuspended in medium without IL-2, and plated in the presence of IgG2a, anti-CD25, or no Ab. Two days after incubation, the IL-2 present in the media was assayed by ELISA.
At the peak of their growth, there was no IL-2 detectable in the medium from IL-2 transductants, possibly due to active consumption of IL-2 by these transductants (data not shown). When the α-chain of the IL-2R was blocked by anti-CD25 Ab, 155 ± 3 (SEM) pg/ml IL-2 could be detected in the medium of IL-2YFP transductants (3.5 × 105 cells in a volume of 500 μl). This production of IL-2 and its consumption by the same cells indicated the presence of an autocrine loop. There was no detectable IL-2 in any condition from YFP transductants (data not shown).
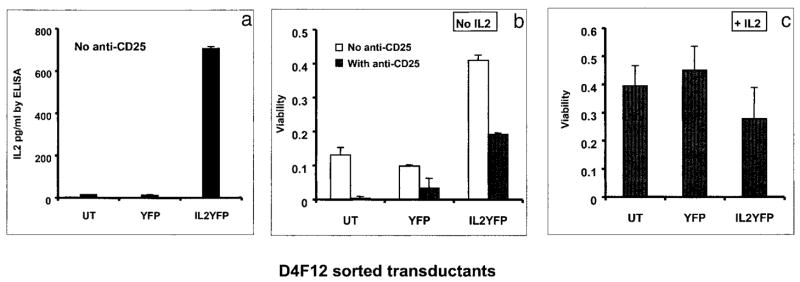
CD8+ T cell clone when transduced with an exogenous IL-2 gene secreted IL-2 and survived in the absence of IL-2
One plausible explanation for the observed phenotype of IL-2 PBMC transductants (IL-2 secretion, active proliferation, and prolonged survival upon IL-2 withdrawal) when compared with control YFP transductants is that some individual cells in the bulk culture of IL-2YFP transductants might have been inherently capable of better growth. To test this possibility, we transduced a CD8+ T cell clone with the IL-2YFP vector and the control YFP vector, respectively. Transductants were sorted by a FACS sorter, and their properties were analyzed in the absence of added IL-2. In this case, all transductants were derived from one clone, and any difference seen between IL-2 transductants vs control YFP vector transductants could be ascribed to the expression of the IL-2 gene. In addition, the protocol developed for the transduction of clones could potentially be useful for clinical application.
Experiments to determine an optimal time to transduce clones showed that on days 7 and 8 of the REP, transduction efficiency was better than on later days (data not shown). Thus, the CD8+ T cell clone, D4F12 reactive with the gp100:209–217 peptide (8), was transduced on days 7 and 8 of REP3. Transduction efficiency was 21.4% for the YFP vector and 10.3% for the IL-2 YFP vector. Six days after transduction, YFP-expressing transductants were sorted and tested for viability and IL-2 production.
Without restimulation, there was no IL-2 detectable in the medium of either untransduced, YFP, or IL-2YFP transductants (data not shown). However, upon restimulation with OKT3 and anti-CD28, IL-2 transductants secreted 700 ± 30 (SEM) pg/ml IL-2 (0.5 × 105 cells in 200 μl). No IL-2 was detected in cultures of untransduced or control vector transductants (Fig. 6a). When assayed for viability upon restimulation 6 days after being maintained in the absence of IL-2, IL-2YFP transductants exhibited significantly higher viability than untransduced or YFP transductants (p = 0.0249 by Kruskal-Wallis statistical test). This viability was partially blocked by anti-CD25 Ab, indicating that IL-2 was responsible for this viability (Fig. 6b). Low viability was seen for untransduced and YFP transductants presumably due to endogenous IL-2 secretion after restimulation (8), which could be detected in the presence of anti-CD25 (data not shown). This low level of viability was also reversed by anti-CD25. There were no differences observed in cell viability when IL-2 was present in the media (Fig. 6c). These results reproduced those previously described for bulk PBMC transductants.
FIGURE 6.
IL-2 gene-transduced CD8+ T cell clone, D4F12, produced IL-2 and maintained a longer survival in the absence of IL-2. Replicates of 0.5 × 105 cells of each sorted transductant or untransduced (UT) D4F12 as shown were washed extensively to remove any residual IL-2, resuspended in 50/50 medium with or without IL-2, and replated in wells previously coated with anti-CD3 and anti-CD28 Abs, on a 96 U-bottom microwell plate. On day 2, 0.5 volume of medium was removed from the no IL-2 condition and replaced with 0.5 volume of medium without IL-2. Cells were transferred to regular wells (restimulation for only 2 days). IL-2 was added again to +IL-2 condition on day 3. In the conditions indicated, anti-CD25 was added from the onset of the experiment at 10 μg/ml. On day 5, an MTS viability assay was performed for each well. a, IL-2 ELISA in the absence of anti-CD25; b and c, MTS cell viability assays. Viability is expressed as the value of OD average − OD medium; OD average ± SEM, an average from OD492 of two independent wells of each transductant; OD medium, an average of OD readings from wells containing medium alone; b, no IL-2; c, +IL-2 (300 IU/ml).
The ability of D4F12 transductants to recognize melanoma Ags was not affected. IL-2 transductants could recognize T2 cells pulsed with 0.01 μM 209 peptide with a high level of IFN-γ release (872 pg/ml), and did not recognize an irrelevant peptide, melanoma Ag recognized by T cell-1-pulsed T2 cells (Table III). Specific recognition of melanoma tumor cell lines was also seen. The transductants recognized HLA A2+ 526 mel, but not the A2− tumor cell line (888 mel) (Table III).
Table III.
IL-2 gene-transduced D4F12 exhibited highly specific recognition against melanomaa
| Targets | IFN-γ Release (pg/ml) |
|---|---|
| T2 pulsed with peptide | |
| MART-1 (1 μM) | 33 |
| 209 (1 μM) | 5040 |
| 209 (0.01 μM) | 872 |
| 209 (0.0001 μM) | 66 |
| Tumor cell line | |
| 526mel (A2+) | 1650 |
| 888mel (A2−) | 100 |
| No stimulator | 46 |
IL-2-transduced, sorted D4F12 were cocultured in wells of a U-bottom 96-well plate with target cells as indicated. Sixteen hours later, culture medium was harvested and assayed for IFN-γ by ELISA.
These results demonstrated that upon restimulation, the CD8+ T cell clone transduced with IL-2YFP produced IL-2 and maintained a longer in vitro survival when compared with untransduced or YFP transductants. When the same experiment was repeated, IL-2YFP-transduced D4F12 exhibited sustained viability for 2 wk after IL-2 withdrawal (data not shown). This prolonged viability was due to the effect of IL-2 encoded by the transgene since untransduced cells and YFP transductants did not exhibit this effect, and Ab reactive with the IL-2R on the IL-2YFP transductants could reverse the effects.
Discussion
In this study, we have described an approach to retrovirally transduce human primary T lymphocytes that exhibited avid specific melanoma Ag and tumor recognition. Cells transduced with a gene encoding IL-2 could produce IL-2 and grow in the absence of IL-2 upon restimulation. Furthermore, upon withdrawal of IL-2 from the medium during their logarithmic growth phase, IL-2 transductants sustained a longer survival than control transductants that were similarly manipulated. We further demonstrated that this is specific to the effect of IL-2 expressed by the transgene by the following lines of evidence: 1) YFP transductants did not produce IL-2 and failed to proliferate in the absence of IL-2; 2) there existed an IL-2 autocrine loop by IL-2 transductants; 3) a CD8+ T cell clone, when transduced with an IL-2 gene, manifested the same phenotypes as PBMCs in the absence of exogenous IL-2; and 4) an anti-IL-2R Ab (anti-CD25) reduced the viability mediated by IL-2 secretion of the IL-2 transductants. More importantly, the transduction procedure was not detrimental to the high degree of recognition and specificity of transductants against melanoma.
In this work, we used the 209-2M peptide (23), a specific melanoma peptide Ag to stimulate PBMCs from peptide-immunized patients before retroviral transduction. CTL precursors with Ag recognition were specifically enriched by such a stimulus, as evident by the high percentage of CD8+ cells that were transduced and the specific melanoma reactivity of the sorted transductants (Tables I and II). The 10A1 envelope from the PT 67 packaging cells used in this study may have contributed to this, since it has been shown that pseudotyped retroviruses expressing this envelope protein have tropism for CD8+ T cells when compared with other envelope proteins (16). This is probably related to the binding of the 10A1 envelope protein to both the A-murine leukemia virus receptor (Pit2) and the gibbon ape leukemia virus (Pit1) receptor for cell entry (24). To validate this approach, bulk-sorted PBMC transductants (YFP and IL-2YFP, respectively) were cloned using T cell cloning methods described previously (3, 8). Clones with highly avid tumor recognition were screened and subjected to FACS analyses to score for YFP positivity. It was found that almost all YFP+ clones were tumor reactive in an HLA-A2 restriction fashion (data not shown), reinforcing the notion that peptide-reactive CTLs preferentially proliferated during peptide stimulation and were subsequently transduced. A similar approach was used by others to selectively transduce EBV-specific T cells with genes encoding a selectable marker and HSV thymidine kinase after PBMC precursors were stimulated with autologous EBV-transformed B cells (25). The procedure described in this study represents a novel and efficient way to introduce exogenous genes to tumor Ag-specific T lymphocytes, and may be useful for cells reactive with tumor types other than melanoma.
The other major finding of our work was the prolonged viability of IL-2 transductants in the absence of exogenous IL-2. As shown in Figs. 4 (left) and 5 (left), withdrawal of IL-2 from the culture medium during logarithmic growth resulted in a drastic decrease in the viability of mock transductants. In contrast, provision of an exogenous IL-2 gene to these cells maintained their viability for more than 3 wk in vitro. Moreover, with restimulation, IL-2 transductants could be actively grown for 8 wk in the absence of added IL-2 (Figs. 4, middle, and 5, middle). We attribute this to the existence of an IL-2 autocrine loop; IL-2 transductants constitutively produced IL-2 to support their own growth and/or survival. This finding could have profound clinical implications for the development of cell transfer therapies for patients with cancer. In our prior studies of cell transfer in murine tumor models, we have shown that the concurrent administration of exogenous IL-2 is essential for the effective elimination of invasive tumors (26). Similarly in the human, we have shown that the survival of transferred lymphocytes may be limited by the inability of humans to tolerate the high doses of exogenous IL-2 required to sustain the survival of IL-2-dependent cells (8). The constitutive IL-2 expression of IL-2 transductants described in this work could substantially prolong the survival of transferred cells in vivo and enhance their antitumor activity. In addition, IL-2 produced locally by these cells at the tumor site might be used by other T cells, lymphokine-activated killer and NK cells, etc., thus augmenting the effect. This may limit the need for the administration of high-dose IL-2 and reduce its toxicities. Further clinical research is planned to investigate the fate and impact of these IL-2 gene-modified cells when transferred to patients with metastatic melanoma.
The production of IL-2, and the proliferation and prolonged viability of IL-2 transductants depend on restimulation of these transductants. These results are in agreement with other reports that expression of transgenes in primary T cells is positively correlated with the activation status of the transductants. Pollok et al. (21) described that primary human T cells when transduced with a mB7-1 gene could enhance the transgene expression by repeated stimulation with anti-CD3 and anti-CD28, even at 31 days post-transduction. In a gene therapy feasibility trial for HIV in which a dominant-negative mutant of HIV rev protein was controlled by a retroviral vector, it was shown that only highly activated, transduced cells were resistant to HIV replication (27). The fact that IL-2 expression by transductants depends on restimulation may have in vivo implications; the presence of tumor Ag(s) in the local milieu of the tumor site may serve as a continuous stimulus for IL-2 production, which may ultimately enhance their antitumor activity.
The detectable level of IL-2 produced by IL-2 transductants was limited (from 155 to 700 pg/ml) possibly due to the active consumption of IL-2 by the cells. The anti-CD25 Ab used in these experiments to block IL-2 consumption may not have been completely efficient in blocking the IL-2 autocrine loop of these cells, which were activated by peptide stimulation, followed by nonspecific TCR restimulation with OKT3 and anti-CD28. These stimuli are known to up-regulate CD25 expression. Furthermore, IL-2 itself can induce CD25 up-regulation by binding to the intermediate affinity β-chain of the receptor complex (28). All these factors make it difficult to measure the true amount of IL-2 produced by IL-2 transductants, although the local concentration of IL-2 in the surrounding milieu of the lymphocytes in vivo at the tumor site may be much higher than the amount of IL-2 released into a relatively large culture medium. This low level of IL-2 produced in vitro was reflected in a lower level of growth of these transductants in the absence of exogenous IL-2, when compared with 300 IU/ml IL-2 present in the medium (Fig. 4, middle vs right and Fig. 5, middle vs right). In addition, as shown in Figs. 4, middle, and 5, middle, IL-2 transductants eventually stopped proliferating after 16–17 days in cultures not supplied with exogenous IL-2 without further stimulation. However, with the supplement of 300 IU/ml IL-2, the same transductants continued to proliferate for 29–35 days. In a murine model reported by our group, the sperm whale myoglobin-specific CD4+ T cell line 14.1, retrovirally transduced with a human cDNA IL-2 gene, remained IL-2 independent without further stimulation (12). Our results of limited proliferation of IL-2 transductants are also inconsistent with other reports of transduction of an exogenous IL-2 gene to murine T cells. Yamada et al. (29) constructed a pZipSV retroviral vector containing a human IL-2 cDNA. Transduction of this vector to an IL-2-dependent murine T cell line, CTLL2, led to the expression of IL-2. Transductants not only proliferated in vitro in the absence of IL-2, but also developed tumors (lymphomas) in nude and syngeneic mice. In another study reported by Karasuyama (30), a cDNA expression vector BCMGNeo.mIL-2 was constructed in which a murine IL-2 cDNA was driven by a CMV promoter. This construct was used to transfect an IL-2-dependent murine Th cell line, HT-2. It was shown that stable transfectants were able to secrete high amounts of murine IL-2 and to proliferate autonomously without exogenous IL-2. Furthermore, high IL-2-producing transfectants were tumorigenic in nude mice. There are at least two explanations for the discrepancy between these murine data and the results described in this study. First, gene expression in mouse cell lines might be different from that in the normal human primary T cells used in this study. Second, murine T cell lines used in the above studies might contain many genetic alterations that may permit higher expression of IL-2 and might have predisposed them to become easily immortalized and tumorigenic upon transduction or transfection. Despite these dissimilarities, it is apparent that opportunities exist to improve upon expression of the IL-2 gene in our system. These may include utilization of a different pseudotyping envelope protein, a different vector, a different promoter to drive the expression of IL-2, or addition of an IL-2R α-chain gene to enhance the impact of the IL-2 produced. Our current efforts also focus on the transduction of other cytokine and immortalizing genes that are involved in the maintenance and survival of lymphocytes into melanoma-reactive human lymphocytes.
The IL-2 gene-modified lymphocytes described in this study can provide a valuable tool both for in vitro studies and for adoptive transfer therapy to patients with melanoma. The principle developed from this study, i.e., sustaining lymphocyte survival while maintaining their antitumor activity and specificity by gene modification, may be useful for the immunotherapy of patients with cancer and viral infections.
Acknowledgments
We thank Dr. Mark Dudley for excellent advice in T cell culture and T cell cloning and insightful discussions; Arnold Mixon and Shawn Farid for their excellent technical support for FACS analyses and Arnold Mixon for FACS sorting; Dr. John Wunderlich and the TIL laboratory of the Surgery Branch for provision of T cells; Yong Li for help with nucleotide sequencing; Dr. Patrick Hwu for helpful discussion and providing the pSAMIL-2EN plasmid; Dr. Jehad Charo for helpful discussions; Dr. Gina Costa for providing pGCIRESYFP vector; and Dr. Paul Robbins and Dr. Richard Morgan for insightful discussions.
Footnotes
Abbreviations used in this paper: TIL, tumor-infiltrating lymphocyte; CM, complete medium; IRES, internal ribosomal entry site; MTS, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt]; REP, rapid expansion protocol; YFP, yellow fluorescent protein.
References
- 1.Sprent J. T-cell survival and the role of cytokines. Immunol Cell Biol. 2001;79:199. doi: 10.1046/j.1440-1711.2001.00999.x. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105. [PubMed] [Google Scholar]
- 3.Riddell SR, Greeberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL, et al. Gene transfer into humans: immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. N Engl J Med. 1988;319:1676. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose in-terleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodie SJ, Patterson BK, Lewinsohn DA, Diem K, Spach D, Greenberg PD, Riddell SR, Corey L. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Invest. 2000;105:1407. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Dudley M, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, et al. Adoptive transfer of cloned, melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Riddell SR, Warren EH, Gavin MA, Akatsuka Y, Lewinsohn D, Mutimer H, Cooper L, Topp MS, Bonini C, Greenberg PD. Immunotherapy of human viral and malignant diseases with genetically modified T-cell clones. Cancer J Sci Am. 2000;6(Suppl 3):S250. [PubMed] [Google Scholar]
- 10.Rosenberg SA. Progress in human tumor immunology and immunotherapy. Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA. Cell transfer therapy: clinical implications: melanoma. In: Rosenberg SA, editor. Principles and Practice of Biologic Therapy of Cancer. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 323–333. [Google Scholar]
- 12.Treisman JT, Hwu P, Minamoto S, Shafer GE, Cowherd R, Morgan RA, Rosenberg SA. Interleukin-2-transduced lymphocytes grow in an autocrine fashion and remain responsive to antigen. Blood. 1995;85:139. [PubMed] [Google Scholar]
- 13.Rosenberg SA, Blaese RM, Brenner MK, Deisseroth AB, Ledley FD, Lotze MT, Wilson JM, Nabel GJ, Cornetta K, Economou JS, et al. Human gene marker/therapy clinical protocols. Hum Gene Ther. 2000;11:919. doi: 10.1089/10430349950016465. [DOI] [PubMed] [Google Scholar]
- 14.Tan Y, Xu M, Wang W, Zhang F, Li D, Xu X, Gu J, Hoffman RM. IL-2 gene therapy of advanced lung cancer patients. Anticancer Res. 1996;16:1993. [PubMed] [Google Scholar]
- 15.Costa GL, Benson JM, Seroogy CM, Achacoso P, Fathman CG, Nolan GP. Targeting rare populations of murine antigen-specific T lymphocytes by retroviral transduction for potential application in gene therapy for autoimmune disease. J Immunol. 2000;164:3581. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- 16.Uckert W, Becker C, Gladow M, Klein D, Kammertoens T, Pedersen L, Blankenstein T. Efficient gene transfer into primary human CD8+ T lymphocytes by MuLV-10A1 retrovirus pseudotype. Hum Gene Ther. 2000;11:1005. doi: 10.1089/10430340050015310. [DOI] [PubMed] [Google Scholar]
- 17.Ghattas IR, Sanes JR, Majors JE. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991;11:5848. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uckert W, Pedersen L, Gunzburg W. Green fluorescent protein retroviral vector. In: Walther W, Stein U, editors. Gene Therapy of Cancer: Methods and Protocols. Humana Press; Totowa: 2000. pp. 275–285. [Google Scholar]
- 19.Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollok KE, Hanenberg H, Noblitt TW, Schroeder WL, Kato I, Emanuel D, Williams DA. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol. 1998;72:4882. doi: 10.1128/jvi.72.6.4882-4892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salter RD, Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant T×B cell hybrid. EMBO J. 1986;5:943. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539. [PubMed] [Google Scholar]
- 24.Miller AD. Cell-surface receptors for retroviruses and implications for gene transfer. Proc Natl Acad Sci USA. 1996;93:11407. doi: 10.1073/pnas.93.21.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koehne G, Gallardo HF, Sadelain M, O’Reilly RJ. Rapid selection of antigen-specific T lymphocytes by retroviral transduction. Blood. 2000;96:109. [PubMed] [Google Scholar]
- 26.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 27.Plavce I, Agarwal M, Ho KE, Pineda M, Auten J, Baker J, Matsuzaki H, Escaich S, Bonyhadi M, Bohnlein E. High transdominant RevM10 protein levels are required to inhibit HIV-1 replication in cell lines and primary T cells: implication for gene therapy of AIDS. Gene Ther. 1997;4:128. doi: 10.1038/sj.gt.3300369. [DOI] [PubMed] [Google Scholar]
- 28.Waldmann TA. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- 29.Yamada G, Kitamura Y, Sonoda H, Harada H, Taki S, Mulligan RC, Osawa H, Diamantstein T, Yokoyama S, Taniguchi T. Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J. 1987;6:2705. doi: 10.1002/j.1460-2075.1987.tb02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karasuyama H, Tohyama N, Tada T. Autocrine growth and tumor-igenicity of interleukin 2-dependent helper T cells transfected with IL-2 gene. J Exp Med. 1989;169:13. doi: 10.1084/jem.169.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Movassagh M, Boyer O, Burland MC, Leclercq V, Klatzmann D, Lemoine FM. Retrovirus-mediated gene transfer into T cells: 95% transduction efficiency without further in vitro selection. Hum Gene Ther. 2000;11:1189. doi: 10.1089/10430340050015239. [DOI] [PubMed] [Google Scholar]