Abstract
It is well established that the central nucleus of the amygdala (CEA) is involved in responses to stress, fear and anxiety. Many studies have used c-fos expression to map the brain's response to processive stress, but curiously the CEA generally is not highly activated. We have previously shown that exposure to a novel vs. home environment reduces amphetamine-induced activation of the lateral CEA (CEAl) and the oval nucleus of the bed nucleus of the stria terminalis (BSTov). This is consistent with the idea that processive stress inhibits neurons in these nuclei. We have tested this hypothesis by exposing rats to noise, at a range of intensities from non-stressful to stressful, or to restraint conditions, immediately after a remote injection of amphetamine, 2 mg/kg i.p., or interleukin-1β (IL-1β) 0.5 μg/kg i.p. (used to obtain a level of c-fos mRNA against which to measure inhibition). In keeping with our hypothesis, amphetamine- or IL-1β-induced c-fos and zif-268 mRNA were significantly decreased in the CEAl and BSTov under conditions of loud noise or restraint stress compared with control conditions. This inhibition does not require a stress-induced rise in corticosterone because data were similar in animals that had been adrenalectomized with a low-dose corticosterone replacement. As both the CEAl and BSTov are highly γ-aminobutyric acid (GABA) -ergic and project to the medial CEA (CEAm), their inhibition potentially causes an increased input to the CEAm. As the CEAm is a major output nucleus of the amygdala, this could have important consequences within the neural circuitry controlling responses to processive stress.
Keywords: amphetamine, bed nucleus of the stria terminalis, corticotropin-releasing hormone, enkephalin, fos, interleukin-1β
Introduction
The central nucleus of the amygdala (CEA) has repeatedly been shown in a variety of models to be important for the expression and integration of behavioral and autonomic responses to aversive stimuli (Kapp et al., 1982; Davis, 1992; Gallagher & Chiba, 1996; Maren & Fanselow, 1996; LeDoux, 2000). Although, in general, less is known about the bed nucleus of the stria terminalis (BST), it too has been shown to be important for autonomic, neuroendocrine and somatomotor responses during emotional behaviors (Casada & Dafny, 1991; Gray et al., 1993; Dunn & Williams, 1995; Herman & Cullinan, 1997). The amygdala and BST are highly interconnected, and distinct subregions have often been associated under an ‘extended amygdala’ concept, primarily based on afferent and efferent projections, morphological features and neurochemical phenotypes (Alheid et al., 1995). Under this system of categorization, the lateral CEA (CEAl) and oval nucleus of the BST (BSTov; Ju & Swanson, 1989; Ju et al., 1989; Swanson, 1992), also known as the dorsolateral BST (Moga et al., 1989), are parts of the central extended amygdala due to their highly similar neuroanatomical features.
The expression of c-fos mRNA, Fos protein or other immediate-early genes has been used widely as a tool to assess neuronal activation by processive stressors. Processive stressors have been defined as stimuli that do not present a direct physiological threat to the organism, but elicit a hypothalamic–pituitary–adrenal (HPA) axis response via higher order processing involving limbic and forebrain structures (Herman & Cullinan, 1997). This is in contrast to systemic stressors that pose a direct physiological threat to the organism and activate the HPA axis through direct innervation of the paraventricular nucleus of the hypothalamus (PVN) via inputs from the brainstem, for example. Although processive stressors can elicit a small c-fos induction in the CEA, given the involvement of the CEA in fear and anxiety responses, the levels of expression are surprisingly meager in this nucleus and the BSTov. For example, loud noise stress (Campeau & Watson, 1997; Dayas et al., 2001), restraint (Cullinan et al., 1995; Dayas et al., 2001), forced swim (Cullinan et al., 1995; Dayas et al., 2001), foot-shock (Pezzone et al., 1992; Imaki et al., 1993), open-field (Emmert & Herman, 1999), novelty (Day et al., 2001) and even fear conditioning (Pezzone et al., 1992; Beck & Fibiger, 1995; Campeau et al., 1997) induce relatively low levels of c-fos in the CEA and BSTov (but see Honkaniemi, 1992; Kollack-Walker et al., 1997; Radulovic et al., 1998; Holahan & White, 2004; Scicli et al., 2004). This raises the question, if the CEA or BSTov are involved in responses to processive stress, is c-fos a poor marker of activation in these areas? This seems unlikely as many other stimuli, for example amphetamine, cocaine, interleukin-1β (IL-1β), cholecystokinin, dexfenfluramine, flesinoxan, imipramine, citalopram, chlordiazepoxide, diazepam, ethanol, nicotine and clozapine, result in very strong c-fos expression in the CEAl and BSTov (Li & Rowland, 1994; Chang et al., 1995; Compaan et al., 1996; Matta et al., 1997; Ryabinin et al., 1997; Day et al., 1999a; Hitzemann & Hitzemann, 1999; Javed et al., 1999; Morelli & Pinna, 1999; Morelli et al., 1999; Day et al., 2001). Given that these regions readily express c-fos and that processive stressors activate c-fos mRNA and protein in a vast number of other brain areas, it is possible that neurons of the BSTov and CEA are actively inhibited by processive stress. One way in which this could be determined would be to analyse the response to a stimulus that activates these regions in the presence or absence of processive stress. If processive stress inhibits neurons of the CEA and BSTov, then a decreased response to the stimulus would be predicted, relative to the non-stress condition. Supporting this is the observation that exposure to a novel environment, that is considered to be a processive stressor (Hennessy & Levine, 1978; Badiani et al., 1998; Emmert & Herman, 1999), decreases amphetamine-induced c-fos mRNA expression in the CEAl and BSTov by approximately 50% compared with home-cage conditions (Day et al., 2001).
To determine whether this inhibition generalizes to other processive stressors and other stimuli that activate the CEA and BSTov, we determined the effect of increasing intensities of noise, from non-stressful to stressful, or the effect of restraint on levels of amphetamine- or IL-β-induced c-fos mRNA expression in the CEA and BSTov by semiquantitative in situ hybridization. Amphetamine was chosen as the initial stimulus because an inhibition of amphetamine-induced c-fos mRNA in the BSTov and CEA has previously been demonstrated for novelty stress (Day et al., 2001). IL-1β was chosen as an alternative stimulus because it increases c-fos mRNA expression in cells of the BSTov and CEAl that have the same neurochemical phenotype as those activated by amphetamine, but likely acts via a different mechanism (Day et al., 1999a, 2001). The requirement of a stress-induced rise in corticosterone was also tested in adrenalectomized animals with low-level corticosterone replacement. The data support the hypothesis that processive stress inhibits neurons of the CEAl and BSTov, and that this is a neuronally mediated effect. Preliminary data have been reported previously (Day et al., 2004).
Materials and methods
Animals
Adult male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) weighing 200–250 g at the time of arrival in the colony were used. Animals were initially housed in groups of three–four in plastic cages, and were maintained on a 12 : 12 h light : dark cycle (lights on at 08.00 h) under conditions of constant temperature and humidity. Animals were allowed free access to food and water at all times and were allowed to acclimatize for at least 7 days before any experimental manipulations. Experiments were run between 08.00 h and 12.00 h when circulating levels of ACTH and corticosterone are low. All procedures were approved by The University of Colorado at Boulder Institutional Animal Care and Use Committee, in conformation with NIH guidelines.
Surgery
Animals requiring i.p. injections were implanted with an i.p. catheter to allow remote injection, and reduce the acute stress of handling and injection procedures at the time of the experiment. The surgical procedure used has been described before (Day & Akil, 1999), with minor modifications outlined below. Briefly a 22-cm piece of silastic tubing (0.020 inch internal diameter; 0.037 inch external diameter; Dow Corning, Midland, MI, USA) was used as a catheter and was introduced into the peritoneal cavity under sodium pentobarbital general anesthesia. It was secured at the peritoneal wall with sutures around a double silicon ring on the catheter, 3 cm from the end, as previously described. The catheter was then exteriorized with the aid of a trochar at the neck region. Instead of being left loose, the catheter was connected to a cannula connector pedestal (Plastics One, Roanoke, VA, USA) and secured to the skull with screws and cranioplastic cement (Plastics One, Roanoke, VA, USA). A dust cap was used to keep the tubing clean. Animals were then housed individually in plastic shoebox cages (22 × 43 × 21 cm) with sawdust bedding on the floor, and allowed to recover for at least a week before the experiment.
General experimental procedure for noise exposure
The night before the experiment, the animals were transported from the colony room to the behavioral testing suite. Animals were first weighed and then placed, within their home-cage, in one of eight ventilated soundproofed enclosures located in an adjacent room. The enclosure consisted of two wooden boxes (0.75 inch plywood board) with the outer chamber lined with 0.5 inch foam insulation (Celotex®). The internal dimensions of the inner box were depth 38 cm × width 60 cm × height 38 cm. Each enclosure was fitted with a single 6 inch × 9 inch Optimus speaker (#12-1769-120 W RMS) fixed in the middle of the ceiling. Lighting was provided by a fluorescent lamp (15 W) located in the upper left corner of the chamber that was automatically turned off at 20.00 h and on at 08.00 h. Noise was produced by a General Radio (#1381) solid-state random-noise generator with the bandwidth set at 2 Hz–50 kHz. The output of the noise generator was fed to power amplifiers (Pyramid Studio Pro #PA-600X), the outputs of which fed the speakers. Noise intensity was measured by placing a Radio Shack Realistic Sound Level Meter (A scale; #33-2050) in the animal's home cages at several locations and taking an average of the readings. Preliminary studies using a range of noise intensities determined that levels of 85 dBA (Sound Pressure Level) or above (up to 110 dBA) were stressful, as determined by a significant increase in plasma ACTH and corticosterone levels (data not shown). The effect on plasma ACTH and corticosterone was intensity dependent, with exposure to 85 dBA leading to a smaller ACTH and corticosterone response than exposure to 90 dBA, etc. Corticosterone data from this experimental apparatus were similar to those observed previously (Campeau & Watson, 1997). Levels up to and including 80 dBA were not considered stressful using these criteria. The background noise level within the apparatus was approximately 60 dBA, generated mostly by the ventilation fans. This compares with a background noise level of 55 dBA in the general colony room.
After the rat was placed in the soundproofed enclosure, the catheter was connected to an external 1 mL syringe filled with sterile 0.9% saline via a metal spring-covered connector assembly (Plastics One, Roanoke, VA, USA), a cage-top-mounted fluid swivel (Instech Laboratories, Plymouth Meeting, PA, USA) and saline-filled PE tubing that passed outside the box enclosure allowing remote injection. A 1-mL volume of saline was injected to check the patency of the catheter and that there were no leaks. Animals remained in their home-cages in the soundproofed enclosures overnight and had free access to food and water.
The following morning, without being disturbed, animals were injected remotely with the appropriate drug, followed by a 0.6-mL vehicle flush. (The total volume of the PE tubing/catheter was 0.5 mL.) Animals were then immediately exposed to noise of varying intensities, from 60 dBA to 110 dBA for up to 90 min (depending on the experiment, see below). At the end of the noise exposure, animals were disconnected from the swivel, removed from the cage and rapidly decapitated in an adjacent room. The time between the end of the noise and decapitation was less than 2 min. Trunk blood was collected in Vacutainer® tubes containing EDTA and plasma used for determining corticosterone levels. Brains were removed, frozen in isopentane cooled to between −30 °C and −40 °C, and stored at −80 °C until processing for in situ hybridization.
Experiment 1: to compare the expression pattern of c-fos mRNA in the BSTov, CEA and PVN following loud noise or restraint stress, compared with amphetamine administration
This experiment was run for illustrative purposes only, to demonstrate: (i) the relative lack of c-fos mRNA expression in the CEA and BSTov following processive stress, compared with a systemic injection of amphetamine; and (ii) the low baseline expression of c-fos mRNA in these regions. The stressors chosen for these experiments were loud noise and restraint. Both of these stimuli are well characterized laboratory stressors thought to belong to the processive class of stressors (Herman & Cullinan, 1997), and result in a constellation of behavioral, endocrine and autonomic responses consistent with a classical stress response (Yano & Harada, 1980; De Boer et al., 1989; Segal et al., 1989; Overton et al., 1991; Helmstetter & Bellgowan, 1994; Campeau & Watson, 1997; Ginsberg et al., 2003). Animals (n = 12) underwent surgery to implant an i.p. catheter (n = 4) or were left unoperated (n = 8), and were housed individually. One week later and the afternoon before the experiment, the animals were transported to the behavioral suite. Half of the unoperated animals (n = 4) remained in the room in their home-cages. The other unoperated animals (n = 4) were placed in the soundproofed boxes as described above. The animals with i.p. catheters (n = 4) were also placed in the soundproofed boxes and were connected to the injection system as described above. The following morning animals were exposed to the following treatment conditions for 30 min: (i) unoperated plus background (60 dBA) noise, n = 2; (ii) unoperated plus 100 dBA noise, n = 2; (iii) a remote saline injection via the i.p. catheter (background noise), n = 2; (iv) a remote amphetamine injection (d-amphetamine sulphate salt; Sigma, St. Louis, MO, USA), 2 mg/mL/kg via the i.p. catheter (background noise), n = 2; (v) unoperated and unhandled (naïve), n = 2; (vi) unoperated plus restraint stress within the home cage, n = 2. Restraint stress consisted of placing each animal in a clear Plexiglas tube (23.5 cm in length and 7 cm in diameter) with tails protruding (Ginsberg et al., 2003). The size of the tube restricted movement in all directions, but did not interfere with breathing. After 30 min, animals were killed by decapitation in an adjacent room. Trunk blood was taken for analysis of plasma corticosterone and brains were taken for in situ hybridization for c-fos mRNA.
Experiment 2: to test if a stressful level of loud noise inhibits the BSTov and CEAl by determining levels of amphetamine-induced c-fos or zif-268 mRNA expression under background or stressful noise conditions
Rats (n = 12) were implanted with an i.p. catheter as described above. The experiment was run in two cohorts, n = 6 as described above. All animals received a remote injection of 2 mg/kg amphetamine via the i.p. catheter and were immediately exposed to a stressful level of noise (100 dBA; n = 5) or background noise conditions (60 dBA; n = 7) for 50 min. The 50 min time point was chosen because amphetamine-induced c-fos mRNA expression under home vs. novel conditions was significantly different at this time (Day et al., 2001). Treatment conditions were randomized across cohorts.
Experiment 3: to determine the time course for loud noise stress inhibition of amphetamine-induced c-fos mRNA in the BSTov and CEAl
Rats (n = 42) were implanted with an i.p. catheter as described above. The experiment was run in six cohorts, n = 4 or 8. Animals (n = 36) received a remote injection of 2 mg/kg amphetamine via the i.p. catheter and were immediately exposed to a stressful level of noise (100 dBA) or background noise conditions (60 dBA) for 30, 60 or 90 min; n = 6 per group, except 90′/100 dBA noise, n = 5, due to a catheter failure. The other six animals received an equivalent volume of saline by remote injection via the i.p. catheter, and were exposed to background noise conditions (60 dBA) for 30 min to give a background measure for c-fos mRNA expression.
Experiment 4: to test which noise intensities inhibit amphetamine-induced c-fos mRNA expression in the BSTov and CEAl
Rats (n = 36) were implanted with an i.p. catheter as described above. The experiment was run in five cohorts, n = 4 or n = 8. All animals received a remote injection of amphetamine, 2 mg/kg, via the i.p. catheter and were immediately exposed to background (60 dBA), non-stressful (70, 80 dBA) or stressful (90, 100 or 110 dBA) levels of noise for 30 min, based on the results from Experiment 2; n = 6 per group. Treatment conditions were randomized across cohorts. In this experiment, tissue was not cut accurately for the BSTov and 14 additional animals were run over two cohorts to obtain at least six per group (all treatment conditions were run). Hence for the BSTov data, n = 6–8 per group.
Experiment 5: to test if restraint stress inhibits amphetamine-induced c-fos mRNA expression in the BSTov and CEAl
Rats (n = 12) were implanted with an i.p. catheter, as described above. The afternoon before the experiment, animals were transported to the behavioral suite, weighed and replaced in their home-cages. The following morning all animals were injected remotely with amphetamine, 2 mg/kg via the i.p. catheter. Immediately after the injection, animals remained unhandled (n = 6) or were subjected to restraint stress for 30 min within the home-cage (n = 6), as described in Experiment 1. Animals were killed by decapitation 30 min after the amphetamine injection. Trunk blood was collected for analysis of plasma corticosterone and the brain removed for analysis of c-fos mRNA expression by in situ hybridization.
Experiment 6: to test if loud noise stress inhibits IL-1β-induced c-fos mRNA expression in the BSTov and CEA
Rats (n = 12) were implanted with an i.p. catheter, as described above. The afternoon before the experiment, animals were transported to the behavioral suite, weighed and replaced in their home-cages. The following morning all animals were injected remotely with IL-1β, 0.5 μg/kg in 0.9% saline containing 0.01% rat serum albumin (Day & Akil, 1999) via the i.p. catheter. Immediately after the injection, animals remained under background noise conditions (60 dBA; n = 6) or were subjected to conditions of loud noise stress (100 dBA) for 30 min within the home-cage (n = 6), as described above. Animals were killed by decapitation 30 min after the IL-1β injection. Trunk blood was collected for analysis of plasma corticosterone and the brain removed for analysis of c-fos mRNA expression by in situ hybridization.
Experiment 7: to test if inhibition of amphetamine-induced c-fos mRNA expression in the BSTov and CEAl by loud noise stress is dependent on a stress-induced rise in corticosterone
Rats (n = 24) were implanted with an i.p. catheter, as described above. During the same surgery, animals underwent bilateral adrenalectomy (ADX) via the dorsal approach, or sham surgery, as described previously (Day et al., 1999b). A 10-mg, 21-day release pellet of corticosterone (Innovative Research of America, Sarasota, FL, USA) was placed subcutaneously at the nape of the neck of adrenalectomized animals, which we have previously shown produces low levels of corticosterone (< 5 μg/dL; Day et al., 1999b). Corticosterone was replaced at low levels to prevent many of the very deleterious effects of adrenalectomy, but to provide lower levels than the stress-induced rise in corticosterone observed after loud noise stress (> 20 μg/dL; Experiment 1 and preliminary data, not shown). Animals were allowed to recover for at least a week before the experiment, which was run in three cohorts. All animals received a remote injection of amphetamine, 2 mg/kg, via the i.p. catheter and were immediately exposed to background (60 dBA) or stressful (100 dBA) levels of noise for 30 min; n = 6 per group. Treatment conditions were randomized across cohorts.
In situ hybridization
The method for in situ hybridization has been described previously (Day & Akil, 1996). Briefly, 10-μm sections were cut on a cryostat (Leica model 1850) through the extent of the BSTov, hypothalamus and CEA, thaw-mounted onto polylysine-coated slides and stored at −80 °C. [35S]-UTP-labeled riboprobes against c-fos mRNA (680 mer; courtesy of Dr T. Curran, St Jude Children's Hospital, Memphis, TN, USA) or zif-268 mRNA (also known as EGR-1 or NGFI-A; 205 mer; courtesy of Dr J. Milbrandt, Washington University School of Medicine, St Louis, MO, USA) were generated using standard transcription methods. For each experiment, all sections for a given brain region were run simultaneously. Sections through the extent of the region to be analysed, at 60-μm (BSTov and PVN) or 120-μm (CEA) intervals, were fixed in 4% paraformaldehyde (1 h), acetylated in 0.1 m triethanolamine with 0.25% acetic anhydride (10 min) and dehydrated through graded alcohols. Sections were hybridized overnight at 55 °C with a [35S]-labeled riboprobe diluted in hybridization buffer containing 50% formamide, 10% dextran sulphate, 2 × saline sodium citrate, 50 mm phosphate-buffered saline, pH 7.4, 1 × Denhardt's solution and 0.1 mg/mL yeast tRNA. The following day, sections were treated with RNase A, 200 μg/mL at 37 °C (1 h), and washed to a final stringency of 0.1 × saline sodium citrate at 65 °C (1 h). Dehydrated sections were exposed to X-ray film (BioMax MR; Eastman Kodak, Rochester, NY, USA) for up to 2 weeks and the films analysed as described below. Selected sections were dipped in photographic emulsion (Ilford K5D, Polysciences, Warrington, PA, USA) for qualitative analysis. Emulsion-dipped sections were stored at 4 °C for up to 1 month, before developing (1 : 1 water : D19; Eastman Kodak, Rochester, NY, USA). Sections were coverslipped with a xylene-based mounting medium for qualitative and quantitative analysis at the microscope (Nikon Eclipse 800).
Semi-quantitative X-ray film analysis
Levels of c-fos or zif-268 mRNA were analysed by computer-assisted optical densitometry. Analysis was performed blind to the treatment conditions. Anatomical landmarks were based on the white matter distribution of the unstained tissue section, according to a standard rat brain atlas (Swanson, 1992). The anterior commissure was the primary landmark used for the BSTov, the optic tract was used for the PVN, and the optic tract and boundary of the basolateral amygdaloid complex were used for the CEA. From emulsion-dipped sections (Fig. 6) it was clear that there was little c-fos mRNA expression in the CEAm, and there was a clear demarcation at the cellular level between the lateral part of the CEA and adjacent areas (amygdalostriatal transition area and basolateral amygdaloid complex). In these frozen sections, it was not possible to distinguish the capsular CEA from the CEAl, and it is probable that some of the induced c-fos mRNA is expressed in this subdivision also. Overall, the relatively discrete expression of amphetamine-and IL-1β-induced c-fos mRNA in these brain areas facilitated analysis from X-ray film. Brain section images were captured digitally (CCD camera, model XC-77; Sony, Tokyo, Japan), and the relative optical density of the X-ray film was determined using Scion Image version 4.0 for PC. A macro was written (Dr S. Campeau) that enabled signal above background to be determined automatically. For each section, a background sample was taken over an area of white matter, and the signal threshold was calculated as mean grey value of background + 3.5 SD. The section was automatically density sliced at this value, so that only pixels with grey values above these criteria were included in the analysis. The rostral-caudal extent of each nucleus was analysed (at 60-μm intervals for the BSTov and PVN and 120-μm intervals for the CEA). Because there were no significant differences between the weights of the animals between treatment groups, and because the rostral-caudal extent of the nucleus was analysed, it was assumed that the areas of the brain regions analysed would not be significantly different. The peak of c-fos expression was included in the analysis, with the top three (BSTov and PVN) or five (CEA) integrated density values for each hemisphere averaged to obtain a single value for each animal. Integrated density reflects both the signal intensity and the number of pixels above assigned background (mean signal above background × number of pixels above background).
Fig. 6.
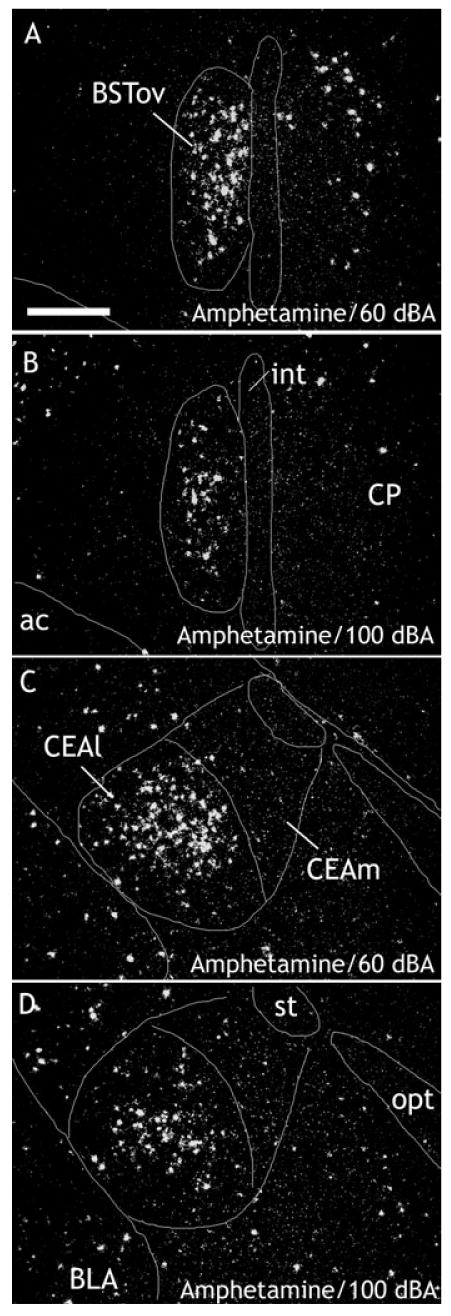
Photomicrographs to illustrate the distribution of c-fos mRNA within the BSTov (A and B) and CEA (C and D) 30 min after an amphetamine injection, 2 mg/kg i.p. under conditions of background (60 dBA; A and C) or stressful (100 dBA; B and D) white noise. Note that within the CEA, amphetamine-induced c-fos mRNA expression is largely restricted to the lateral division. Abbreviations: ac, anterior commissure; BLA, basolateral nucleus of the amygdala; BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; CP, caudate putamen; int, internal capsule; opt, optic tract; PVN, paraventricular nucleus of the hypothalamus; st, stria terminalis. Scale bar, 300 μm.
Semi-quantitative analysis from emulsion-dipped sections
In order to verify the validity of the integrated density measure from X-ray film described above, sections from the BSTov from Experiment 4 (the effect of noise intensity on amphetamine-induced c-fos mRNA expression) were analysed from emulsion-dipped sections. For each animal, an observer blind to the experimental condition analysed one section at the peak of c-fos mRNA expression in the BSTov. A total of seven animals in the ‘amphetamine + 60 dBA’ group and eight animals in the ‘amphetamine + 100 dBA’ group were analysed. The total number of cells expressing c-fos mRNA within the boundaries of the BSTov was counted bilaterally under a 20 × objective. The average number of silver grains per cell was counted manually for 10 cells selected at random for each hemisphere (20 cells total) under a 60 × objective. For each animal, the average number of grains per cell was multiplied by the total number of cells counted to obtain the total grains per animal.
Corticosterone radioimmunoassay
The method for detecting levels of plasma corticosterone has been described previously (Day & Akil, 1996). Briefly, 5 μL plasma in 0.1 m sodium phosphate buffer was heated at 70 °C for 30 min to remove it from its binding protein. The plasma was incubated in duplicate overnight with an antibody against corticosterone (courtesy of Dr S.J. Watson, University of Michigan, Ann Arbor, USA) and [3H]-corticosterone (Amersham Biosciences, Piscataway, NJ, USA). The following day the samples were charcoal (1%) extracted with 0.1% dextran. Levels of corticosterone were calculated by comparison with a standard curve generated concurrently.
Statistical analysis
Data were analysed by one-way (Experiments 2–6) or two-way (Experiment 7) anova followed by Fisher's protected least significant difference (PLSD) post-hoc multiple comparisons test. In addition, a linear trend analysis was performed for Experiment 4. Significance was set at P < 0.05.
Results
Experiment 1: to compare the expression pattern of c-fos mRNA in the BSTov, CEA and PVN following loud noise or restraint stress, compared with amphetamine administration
The results from this experiment are shown in Fig. 1. As expected, c-fos mRNA expression was very low in most brain areas for all control conditions: background noise (60 dBA), naïve and remote saline injection. In contrast, exposure to 30 min of processive stress, either loud noise (100 dBA) or restraint, resulted in widespread c-fos mRNA expression, as previously reported. These regions included the PVN, the central driver of the HPA axis response. However, it is notable that the BSTov and CEA expressed very low levels of c-fos mRNA following either loud noise exposure or restraint stress. In contrast, a remote injection of amphetamine, 2 mg/kg i.p., resulted in robust c-fos mRNA expression in both the BSTov and CEA. Despite producing a high level of plasma corticosterone (24.5 and 39.4 μg/dL), amphetamine administration did not induce c-fos mRNA in the PVN. Corticosterone levels in the other conditions were as follows: saline i.p., 0.5 and 2.8 μg/dL; naïve, 2.2 and 0.9 μg/dL; restraint, 20.2 and 25.8 μg/dL; 60 dBA, 0.2 and 0.5 μg/dL; and 100 dBA, 28.8 and 34.7 μg/dL.
Fig. 1.
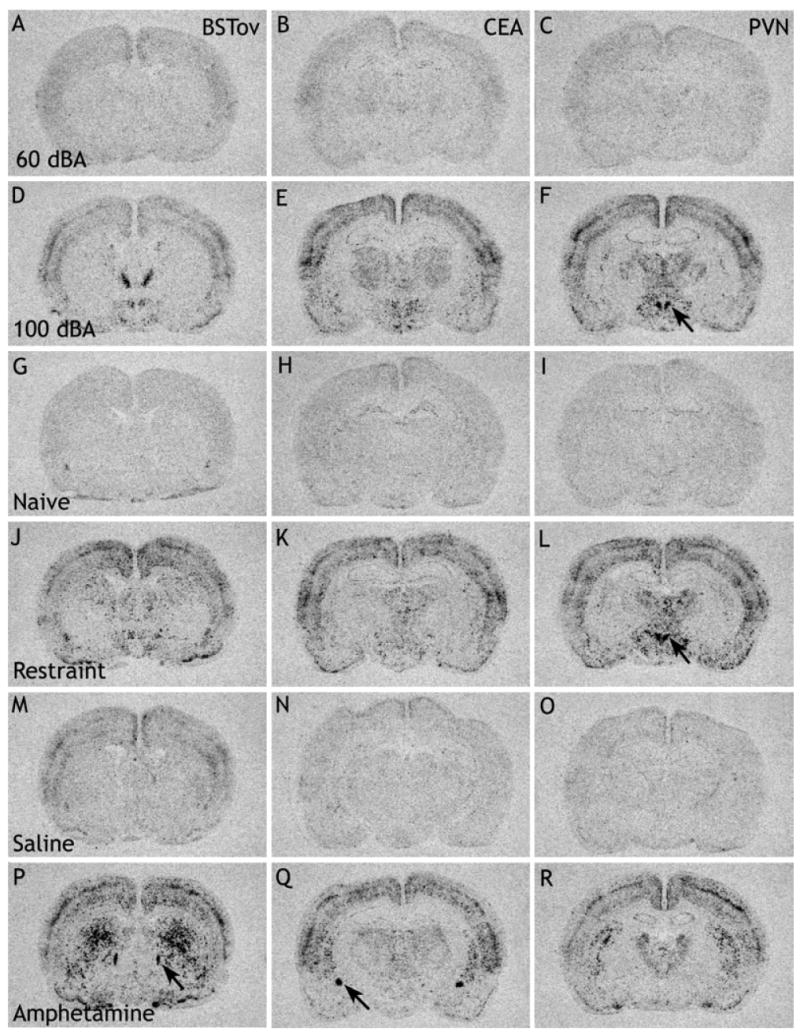
Digital images of X-ray films to show c-fos mRNA in the BSTov (A, D, G, J, M and P), CEA (B, E, H, K, N and Q) and PVN (C, F, I, L, O and R) after exposure to background noise conditions (60 dBA; A–C), stressful noise conditions (30 min at 100 dBA; D–F), unhandled cage control (naïve; G–I), restraint stress for 30 min (J–L), a remote injection of saline (M–O) or a remote injection of amphetamine, 2 mg/kg i.p. (P–R). Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus. Arrows indicate these respective regions. Note the very low level of c-fos mRNA in the BSTov and CEA of noise- and restraint-stressed animals.
Experiment 2: to test if a stressful level of loud noise inhibits the BSTov and CEAl by determining levels of amphetamine-induced c-fos and zif-268 mRNA expression under background or stressful noise conditions
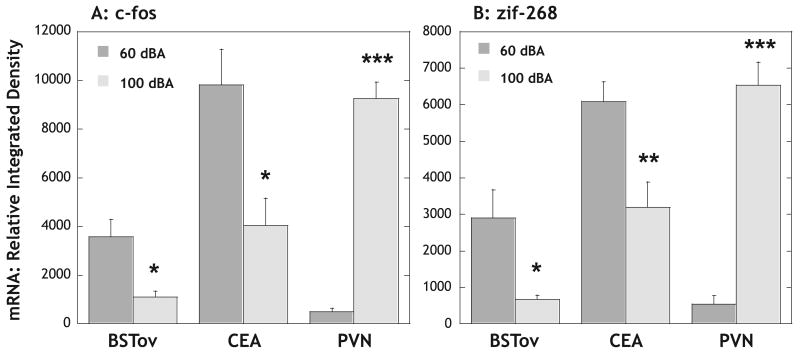
Administration of amphetamine under stressful noise conditions (100 dBA) resulted in significantly higher (P < 0.01) levels of corticosterone (40.0 μg/dL; n = 5) at 50 min, compared with administration under background (60 dBA) noise conditions (19.3 μg/dL; n = 7). As expected, amphetamine administration under background noise conditions resulted in robust c-fos mRNA expression in both the BSTov and CEA at 50 min (Fig. 2A). In contrast, under background noise conditions, amphetamine administration resulted in very little c-fos mRNA expression in the PVN of the hypothalamus (Fig. 2A), despite the high levels of corticosterone. When amphetamine was administered under stressful (100 dBA) noise conditions, levels of c-fos mRNA were significantly lower in both the BSTov (∼ 70%; P < 0.05) and CEA (∼ 60%; P < 0.01) relative to amphetamine administered under background noise conditions (Fig. 2A). In contrast, levels of amphetamine-induced c-fos mRNA in the PVN were approximately 18-fold higher under stressful (100 dBA) vs. background (60 dBA) noise conditions (P < 0.001; Fig. 2A). This effect was not limited to c-fos mRNA expression, because similar data were obtained for zif-268 mRNA expression in the BSTov (P < 0.05), CEA (P < 0.01) and PVN (P < 0.001; Fig. 2B).
Fig. 2.
Graphs to show the relative levels of (A) c-fos and (B) zif-268 mRNA in the BSTov, CEA and PVN, 50 min after a remote injection of amphetamine (2 mg/kg i.p.) under conditions of background (60 dBA) or stressful (100 dBA) white noise. Values represent means + SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with respect to the relevant 60 dBA group. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
Experiment 3: to determine the time course for loud noise stress inhibition of amphetamine-induced c-fos mRNA in the BSTov and CEAl
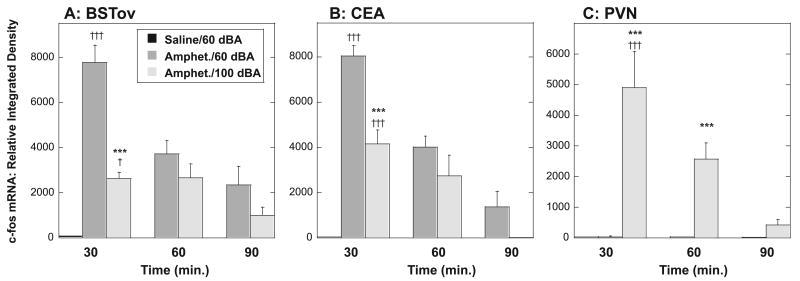
One animal in the 90-min stressful noise (100 dBA) group was excluded because of catheter failure. Under background noise conditions, a saline injection resulted in very low levels of plasma corticosterone (3.2 ± 1.0 μg/dL) and c-fos mRNA in the BSTov, CEA and PVN (Fig. 3). Under background noise conditions (60 dBA), amphetamine resulted in significantly elevated levels of plasma corticosterone that was highest at 30 min (29.2 ± 5.5 μg/dL; P < 0.001), and declined over time (60 min, 18.5 ± 6.3 μg/dL; 90 min, 9.1 ± 3.2 μg/dL). Amphetamine administered under stressful noise conditions (100 dBA) resulted in significantly higher corticosterone levels at 60 min, P < 0.05 compared with 60 dBA condition (30 min, 39.8 ± 4.9 μg/dL; 60 min, 32.7 ± 3.2 μg/dL and 90 min, 9.3 ± 3.4 μg/dL). Under background noise conditions (60 dBA), amphetamine increased c-fos mRNA expression in both the BSTov (Fig. 3A) and CEA (Fig. 3B) compared with saline injection at 30 min (P < 0.001). Levels of amphetamine-induced c-fos mRNA were highest at 30 min, and were lower at 60 and 90 min. At all time points, amphetamine-induced c-fos mRNA in the BSTov and CEA tended to be lower in the groups exposed to stressful levels of noise (100 dBA) compared with background noise conditions (60 dBA), and this was highly significant at the 30-min time point (P < 0.001). Amphetamine administered under background (60 dBA) noise conditions did not increase c-fos mRNA in the PVN above control levels at any time point studied (Fig. 3C). In contrast, when amphetamine was administered under stressful noise conditions (100 dBA), levels of c-fos mRNA were increased in the PVN at the 30- and 60-min time points (P < 0.001). Because levels of amphetamine-induced c-fos mRNA were highest at 30 min, and the reduction by exposure to 100 dBA noise was highly significant (P < 0.001) for both the BSTov (−66%) and CEA (−48%), this time point was chosen for Experiments 4–7.
Fig. 3.
Graphs to show the relative levels of c-fos mRNA in the (A) BSTov, (B) CEA and (C) PVN at 30, 60 or 90 min after a remote injection of amphetamine (2 mg/kg i.p.) under conditions of background (60 dBA) or stressful (100 dBA) white noise. The level of c-fos mRNA 30 min after a saline injection under background noise conditions is shown to illustrate baseline c-fos mRNA levels in this experimental set-up. Values represent means + SEM. ***P < 0.001 with respect to the time-equivalent 60 dBA group. †P < 0.05, †††P < 0.001 with respect to the 30 min saline/60 dBA group. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
Experiment 4: to test which noise intensities inhibit amphetamine-induced c-fos mRNA expression in the BSTov and CEAl
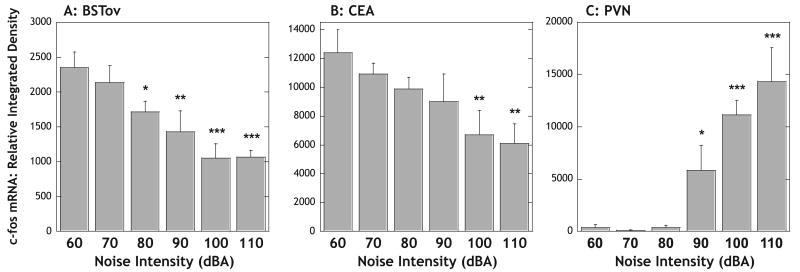
Levels of c-fos mRNA in both the BSTov (Fig. 4A) and CEA (Fig. 4B) showed a dose-related pattern (linear trend analysis, P < 0.001 for both regions), with increasing noise intensities leading to increased inhibition of amphetamine-induced c-fos mRNA expression in these regions. We have shown in preliminary studies that in this model of noise exposure, levels of 85 dBA and above are stressful, as determined by increased levels of plasma ACTH or corticosterone. In the current study, plasma levels of corticosterone were not a good measure to affirm which noise levels produced a central stress response, because amphetamine increases plasma corticosterone levels under background noise conditions. Indeed, at this 30-min time point, there were no significant differences between corticosterone levels for any of the groups: 60 dBA, 37.1 ± 6.1 μg/dL; 70 dBA, 37.3 ± 9.1 μg/dL; 80 dBA, 38.5 ± 5.8 μg/dL; 90 dBA, 45.2 ± 6.1 μg/dL; 100 dBA, 49.0 ± 5.3 μg/dL; 110 dBA, 48.2 ± 3.2 μg/dL. However, c-fos mRNA expression in the PVN suggested that as expected, noise levels of 60, 70 and 80 dBA were not stressful, because c-fos mRNA levels were very low (Fig. 3C). In contrast, animals exposed to noise levels of 90, 100 or 110 dBA had significantly increased levels of c-fos mRNA in the PVN compared with background noise conditions (90 dBA, P < 0.05; 100 and 110 dBA, P < 0.001; Fig. 4C), consistent with data obtained in animals exposed to these noise levels without amphetamine injection (data not shown). Digital X-ray images of sections labeled for c-fos mRNA at the level of the BSTov, CEA and PVN from the 60 and 100 dBA groups are shown in Fig. 5. Higher magnification images from photographic emulsion-dipped sections are shown for the BSTov and CEA in Fig. 6. Amphetamine-induced c-fos mRNA expression was mainly located in the lateral portion of the CEA (CEAl; Fig. 6). As was noted above, it was not possible from the sections to determine the boundaries of the capsular division of the CEA, and our assigned boundaries of the CEAl likely include this subdivision also.
Fig. 4.
Graphs to show the relative levels of c-fos mRNA in the (A) BSTov, (B) CEA and (C) PVN 30 min after a remote injection of amphetamine (2 mg/kg i.p.) under conditions of background (60 dBA) or increasing intensities of white noise. Values represent means + SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with respect to the relevant 60 dBA group. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
Fig. 5.
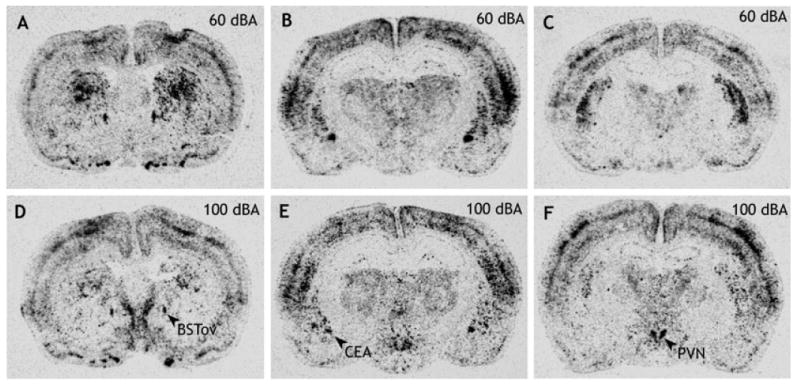
Digital images of X-ray films to show c-fos mRNA in the BSTov (A and D), CEA (B and E) and PVN (C and F) 30 min after a remote injection of amphetamine (2 mg/kg i.p.) under conditions of background (60 dBA; A–C) or stressful (100 dBA; D–F) levels of white noise. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
Quantitative microscopic analysis of emulsion-dipped BSTov sections from the ‘amphetamine + 60 dBA’ (control; n = 7) and ‘amphetamine + 100 dBA’ (stressed; n = 8) groups demonstrated a highly significant decrease in amphetamine-induced c-fos mRNA expression in the stressed group compared with control group for all measures. Specifically, there was a significant decrease (P < 0.001) in the mean number of cells counted bilaterally within the boundaries of the BSTov (amphetamine + 60 dBA group, 113.0 ± 4.3 cells; amphetamine + 100 dBA group, 74.3 ± 3.5 cells). In addition, there was a significant (P < 0.001) decrease in the average number of silver grains per cell (amphetamine + 60 dBA group, 134.7 ± 4.7 grains/cell; amphetamine + 100 dBA group, 84.0 ± 2.3 grains/cell). When the average number of grains per cell was multiplied by the average number of cells counted, there was a highly significant (P < 0.001) 59% decrease in the amphetamine-induced c-fos mRNA in the stress vs. control group (amphetamine + 60 dBA group, 15,288 ± 919 total grains; amphetamine + 100 dBA group, 6262 ± 440 total grains). This is very similar to the results obtained for these groups using the integrated density measure from X-ray film, which showed a 55% decrease in amphetamine-induced c-fos mRNA expression in the 100 dBA compared with 60 dBA group [obtained by averaging the highest three integrated density values for each hemisphere (i.e. six values per animal) to obtain a single value per animal]. We therefore take these results to indicate real differences in cell number and level of mRNA per cell that are best characterized with the integrated density measure.
Experiment 5: to test if restraint stress inhibits amphetamine-induced c-fos mRNA expression in the BSTov and CEAl
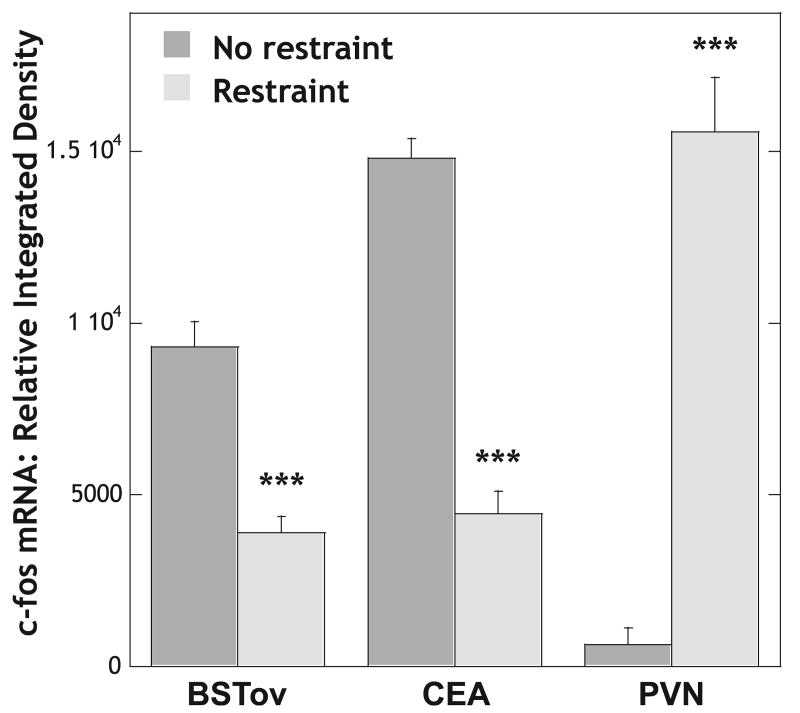
Animals that were injected remotely with amphetamine, 2 mg/kg i.p., under control conditions, had high levels of plasma corticosterone 31.7 ± 9.4 μg/dL (n = 6), high levels of c-fos mRNA in the BSTov and CEA, but low levels of c-fos mRNA in the PVN (Fig. 7). As was observed for noise stress, animals that were restrained for 30 min immediately after the amphetamine injection had significantly lower levels of c-fos mRNA in both the BSTov (P < 0.001) and CEA (P < 0.001), but significantly higher levels of c-fos mRNA in the PVN (P < 0.001; Fig. 7), compared with control conditions. There was no difference in corticosterone levels between the two groups, with the amphetamine plus restraint group having a level of 30.2 ± 2.0 μg/dL (n = 6).
Fig. 7.
Graph to show the relative levels of c-fos mRNA in the BSTov, CEA and PVN 30 min after a remote injection of amphetamine (2 mg/kg i.p.) under home-cage or restraint stress conditions. Values represent means + SEM. ***P < 0.001 with respect to control values. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
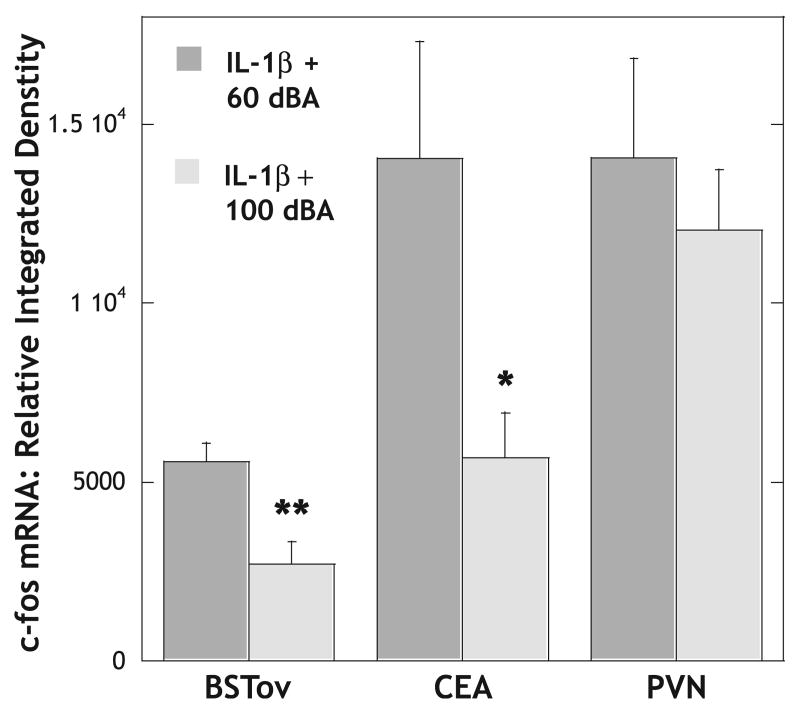
Experiment 6: to test if loud noise stress inhibits IL-1β-induced c-fos mRNA expression in the BSTov and CEA
One animal in the IL-1β + 60 dBA noise group had to be excluded due to catheter failure. Animals that were injected remotely with IL-1β, 0.5 μg/kg i.p., under background noise conditions, had high levels of plasma corticosterone 39.0 ± 0.8 μg/dL (n = 5) and high levels of c-fos mRNA in the BSTov, CEA and PVN (Fig. 8). As was observed with an injection of amphetamine, animals that were subjected to loud noise (100 dBA) for 30 min immediately after the IL-1β injection had significantly lower levels of c-fos mRNA in both the BSTov (P < 0.01) and CEA (P < 0.05; Fig. 8). Levels of c-fos mRNA in the PVN were not significantly different compared with control noise conditions (Fig. 8). There was no difference in corticosterone levels between the two groups, with the IL-1β plus 100 dBA noise group having a level of 43.8 ± 2.0 μg/dL (n = 6).
Fig. 8.
Graph to show the relative levels of c-fos mRNA in the BSTov, CEA and PVN 30 min after a remote injection of IL-1β (0.5 μg/kg i.p.) under 60 dBA (control) or 100 dBA (stressful) noise conditions. Values represent means + SEM. *P < 0.05; **P < 0.01 with respect to control values. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
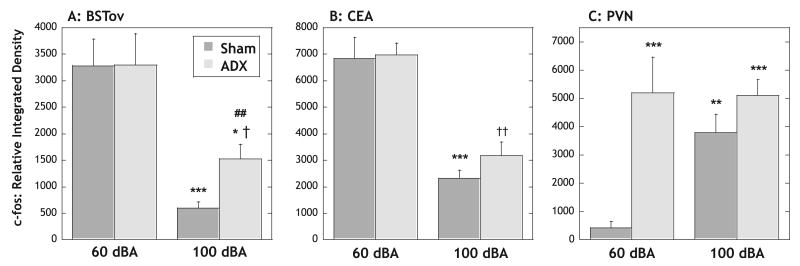
Experiment 7: to test if inhibition of amphetamine-induced c-fos mRNA expression in the BSTov and CEAl by loud noise stress is dependent on a stress-induced rise in corticosterone
Two animals in each of the ADX groups had high corticosterone levels after exposure to amphetamine under either background (16.2 and 23.3 μg/dL) or 100 dBA (24.5 and 24.1 μg/dL) noise conditions, and were excluded from the study. Animals that had undergone sham surgery had robust levels of corticosterone (27.5 ± 2.0 μg/dL) after amphetamine administration under 60 dBA noise conditions. Administration of amphetamine to sham-operated animals under 100 dBA noise conditions resulted in a significantly higher level of plasma corticosterone (34.9 ± 2.7 μg/dL) compared with the 60 dBA condition (P < 0.05). In contrast, under both the 60 and 100 dBA noise conditions, animals that had undergone bilateral adrenalectomy with implantation of a 10-mg, 21-day release corticosterone pellet had significantly lower levels of corticosterone (P < 0.001) compared with sham-operated animals (ADX/amphetamine/60 dBA, 4.0 ± 0.2 μg/dL; ADX/amphetamine/100 dBA, 2.4 ± 0.2 μg/dL; n = 4 per group).
In sham-operated animals, levels of amphetamine-induced c-fos mRNA in both the BSTov and CEAl were similar to that seen previously, with robust levels under 60 dBA conditions, and significantly reduced levels under stressful 100 dBA noise conditions (BSTov, 82% reduction, P < 0.001, Fig. 9A; CEA, 66% reduction, P < 0.001, Fig. 9B). A broadly similar profile was seen in animals that had undergone ADX with a low-dose corticosterone replacement. In these animals, amphetamine induced a similar level of c-fos mRNA in the BSTov and CEAl under background (60 dBA) conditions, compared with sham animals. As was observed in sham-operated animals, the amphetamine-induced c-fos mRNA levels were significantly reduced in adrenalectomized/corticosterone-replaced animals under stressful (100 dBA) noise conditions, compared with the 60 dBA conditions (BSTov, 54% reduction, P < 0.05; CEA, 55% reduction, P < 0.01). However, in the BSTov, the reduction in amphetamine-induced c-fos mRNA observed in the stress condition was not as marked in adrenalectomized animals compared with sham animals, with the levels of c-fos mRNA significantly higher under 100 dBA noise conditions in adrenalectomized/corticosterone-replaced, compared with sham-operated animals (P < 0.001).
Fig. 9.
Graphs to show the effect of adrenalectomy and low-dose corticosterone replacement (ADX) vs. sham surgery on the relative levels of c-fos mRNA in the (A) BSTov, (B) CEA and (C) PVN 30 min after a remote injection of amphetamine (2 mg/kg i.p.) under conditions of background (60 dBA) or stressful (100 dBA) levels of white noise. Values represent means + SEM. *P < 0.05; **P < 0.01; ***P < 0.001 with respect to the 60 dBA sham group; †P < 0.05; ††P < 0.01 with respect to the 60 dBA ADX group; ##P < 0.01 with respect to the 100 dBA sham group. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus.
An interesting pattern of c-fos mRNA expression emerged in the PVN (Fig. 9C). In sham-operated animals, amphetamine administration resulted in very little c-fos mRNA in the PVN under background (60 dBA) conditions, whereas exposure to the stressful level of noise (100 dBA) resulted in high levels of c-fos mRNA in the PVN, as was observed in Experiments 2–4 (P < 0.01). In contrast, in adrenalectomized animals with a low-dose corticosterone replacement, amphetamine administration resulted in high levels of c-fos mRNA expression in the PVN, irrespective of the noise level (P < 0.001 for both 60 and 100 dBA adrenalectomized/corticosterone-replaced groups compared with sham/60 dBA condition).
Discussion
The data presented in this study support the hypothesis that processive stress actively inhibits cells of the CEAl and BSTov, with exposure to stressful levels of noise or restraint decreasing the amphetamine- or IL-1β-induced expression of c-fos and zif-268 mRNA in these regions, compared with control conditions. The intensity-related nature of the response suggests that it depends on the degree of the stress experienced. Finally, a stress-induced rise in corticosterone is not required for this inhibition, consistent with the idea that this is a neuronally mediated effect. Further support for the generality of stress inhibition of the BSTov and CEAl comes from the observation that a third processive stressor, exposure to a novel environment, also decreases amphetamine-induced c-fos mRNA expression in the CEAl and BSTov (Day et al., 2001). In addition, diazepam-induced zif-268 mRNA in the CEAl was inhibited more than 50% by a single mild footshock exposure in a one-trial fear-conditioning paradigm (Malkani & Rosen, 2000). Taken together, these data suggest that the phenomenon of inhibition of the BSTov and CEA is common to many, if not all, processive stressors.
Functional relevance of c-fos mRNA expression
It is generally accepted that the magnitude of c-fos mRNA expression reflects the strength of a stimulus (for review see Hoffman & Lyo, 2002). Hence, a decrease in induced c-fos mRNA expression in a given region implies that the strength of the incoming signal to that region is decreased. It should be noted, however, that this reflects a decrease in activated signal transduction pathways, and does not necessarily reflect the extent of depolarization of the cells. Hence, the current data indicate that exposure to processive stress results in a decreased input to cells of the BSTov and CEAl. However, the consequences of this decreased input remain speculative.
Potential pathways for neuronal inhibition
There have recently been several reports dissociating the functional role of the amygdala and BST (Lee & Davis, 1997; Walker & Davis, 1997; Gewirtz et al., 1998), leading to the suggestion that the BST may be more closely involved in anxiety, whereas the CEA may be more important for fear responses (reviewed in Davis, 1998; Davis & Shi, 1999). However, even if the functional relevance of these systems may ultimately differ, there are many similarities in the afferent connections to the CEAl and BSTov and, thus far, the effect of a diverse range of stimuli (psychostimulant, immune, antidepressant, neuroleptic, anxiolytic, etc.) that activate the CEAl (as determined by c-fos expression) has been largely mirrored in the BSTov, including the neurochemical phenotype that is activated (e.g. Li & Rowland, 1994; Chang et al., 1995; Compaan et al., 1996; Matta et al., 1997; Ryabinin et al., 1997; Day et al., 1999a; Hitzemann & Hitzemann, 1999; Javed et al., 1999; Morelli & Pinna, 1999; Morelli et al., 1999; Day et al., 2001). As was observed previously with novelty (Day et al., 2001), the effect of loud noise or restraint to inhibit the amphetamine- or IL-1β-induced c-fos response was similar in both the CEAl and BSTov. This is consistent with the idea that the processive stress inhibition of the CEAl and BSTov is dependent on a common input.
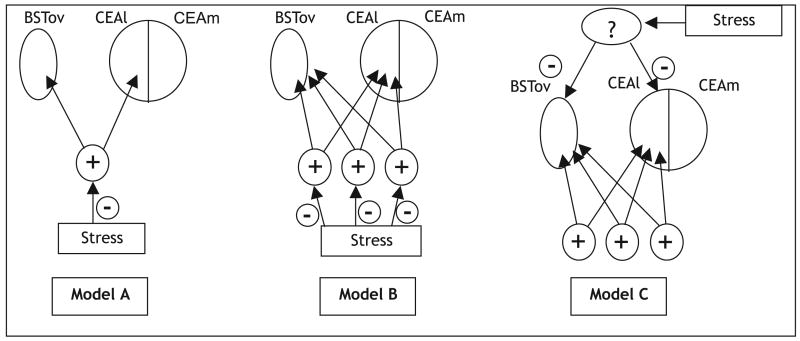
There are three possible ways in which this could occur (Fig. 10). Model A assumes that stress exerts inhibition at one or more points along a single activating pathway. If a number of diverse stimuli are similarly inhibited by stress, as is supported by current evidence (present study; Malkani & Rosen, 2000; Day et al., 2001), this would not be a good model, as the stimuli likely use different pathways of activation. Model B asserts that the stimuli do use different neuronal circuitry to activate the BSTov and CEA, but that stress acts separately on all these different routes to dampen the activation. In the more parsimonious Model C, stress directly inhibits the BSTov and CEAl via an independent pathway than the activating stimuli, resulting in a ‘competition’ for activation vs. inhibition of the CEAl and BSTov. If processive stress does result in a direct inhibition of the BSTov and CEAl, a likely candidate for this inhibition would be γ-aminobutyric acid (GABA), and recent evidence has indicated that both functional GABAA and GABAC-like receptors are present in the CEAl (Delaney & Sah, 1999; Pirker et al., 2000; Delaney & Sah, 2001).
Fig. 10.
Schematic diagram to show possible mechanisms for processive stress inhibition of c-fos mRNA expression (−) in the BSTov and CEAl by an activating stimulus (+). Model A: activating stimuli use a single pathway to stimulate the CEAl and BSTov, and processive stress inhibits neurons along this activational pathway. Model B: activating stimuli use different pathways to stimulate the CEAl and BSTov, and processive stress inhibits neurons along these different activational pathways. Model C, on which our working hypothesis is based: activating stimuli use different pathways to stimulate the CEAl and BSTov, but stress directly inhibits the CEAl and BSTov through a separate pathway, thus setting up a competition between activation and inhibition. Abbreviations: BSTov, bed nucleus of the stria terminalis, oval subdivision; CEAl/CEAm, central nucleus of the amygdala, lateral/medial division; PVN, paraventricular nucleus of the hypothalamus.
It is not known which areas of the brain could be responsible for such an inhibition. The BSTov projects heavily to the CEAm, but does not provide significant input directly to the CEAl (Dong et al., 2001a), and therefore does not seem to be a good candidate for direct regulation of responses of the CEAl. There are strong topographic GABAergic projections from the CEAl to the BST (Nitecka & Ben-Ari, 1987; Sun et al., 1991; Petrovich & Swanson, 1997; Day et al., 1999a), and at first glance this would appear to be a candidate for inhibition of the BSTov. However, because processive stress decreases the activation of CEAl, there would likely be a smaller inhibitory drive to the BSTov from this region, and so it does not seem likely that the CEAl is responsible for the decreased response within the BSTov. The CEAm does not project to the CEAl (Jolkkonen & Pitkänen, 1998), but has some direct projections to the BSTov, that are also likely GABAergic, and remains a potential candidate for stress inhibition of the BSTov (Day et al., 1999a; Dong et al., 2001b). However, if the processive stress inhibition of the CEAl and BSTov is dependent on a common input, of particular interest are the potentially shared afferent inputs from various cortical (Hurley et al., 1991; Sun et al., 1994; McDonald & Mascagni, 1997; McDonald, 1998), thalamic (Moga et al., 1995) and brainstem regions (Krukoff et al., 1993; Sim & Joseph, 1994).
Potential functional relevance: role of CRH and enkephalin
The functional significance of processive stress inhibition of the CEAl and BSTov is not clear. However, both of these regions provide inhibitory input to the CEAm (Sun & Cassell, 1993; Petrovich & Swanson, 1997; Jolkkonen & Pitkänen, 1998; Day et al., 1999a; Dong et al., 2001a). The CEAm is the major output nucleus of the amygdala, and one current theory proposes that this region elicits an output that reflects the sum of amygdaloid activity (Pitkanen et al., 1997). Hence, inhibition of the CEAl and BSTov has the potential to increase overall amygdaloid output, and facilitate somatomotor, autonomic, visceromotor and neuroendocrine responses to processive stress. In addition, the CEA receives direct cortical and subcortical inputs, and may encode simple stimulus–response Pavlovian associations independently of amygdaloid input, and thus its inhibition by stress, may also directly affect this type of learning (Cardinal et al., 2002). The functional relevance of inhibition of the CEAl and BSTov is complicated by the fact that there are two distinct GABAergic cell populations in these regions, containing either enkephalin or CRH (Day et al., 1999a), and there is evidence to suggest that these cell populations have differential efferent projection patterns. For example, CRH-containing neurons have been reported to innervate brainstem regions such as the central grey, parabrachial nucleus and dorsal vagal complex, whereas enkephalin-containing neurons do not (Veening et al., 1984; Moga & Gray, 1985; Gray & Magnuson, 1987, 1992; Moga et al., 1989). Enkephalin on the other hand has been reported to be present in projections from the CEAl to the BST (Palkovits et al., 1981; Rao et al., 1987). However, it is not known which of these cell groups innervate the CEAm. We have previously demonstrated that amphetamine and IL-1β selectively activate the enkephalinergic cells of the CEAl and BSTov (Day et al., 1999a, 2001) but, to our knowledge, the neurochemical identity of cells of the BSTov and CEA that are activated by diazepam has not been established. Therefore, the processive stress inhibition of the CEAl and BSTov has been demonstrated for the enkephalinergic cell population only. Evidence suggests that enkephalin in these areas is anxiolytic. For example, knockout mice that lack preproenkephalin-derived peptides exhibit higher anxiety levels (Konig et al., 1996), whereas overexpression of proenkephalin in the CEA results in a potentiation of the anxiolytic effect of benzodiazepines (Kang et al., 1999). Recently it has been suggested that regulation of enkephalin in the CEA by fear may act as a ‘gain control’ for amygdaloid output (Petrovich & Swanson, 1997; Petrovich et al., 2000). In contrast, extra-hypothalamic CRH has been implicated in stress, fear and anxiety responses (Sutton et al., 1982; Dunn & Berridge, 1990; Gray & Bingaman, 1996). For example, an intracerebroventricular injection of CRH results in a similar behavioral profile to conditioned fear (Liang et al., 1992). Levels of CRH mRNA in the CEA and BST have been shown to increase as the levels of plasma corticosterone increase (Watts & Sanchez-Watts, 1995), and this has been suggested to be a way in which events would more likely be perceived as fearful (Schulkin et al., 1998). It would be of great interest to determine whether the inhibitory effect of processive stress on neurons of the CEAl and BSTov is specific for enkephalinergic cells, or whether a similar effect is observed in neurons expressing CRH.
In conclusion, the data presented in the current study support the hypothesis that processive stress inhibits neurons of the CEAl and BSTov. Because these neurons are GABAergic and project to the CEAm, the major output nucleus of the amygdala, their inhibition could lead to an increased amygdaloid output and a facilitation of responses to processive stress. It is conceivable that this is one mechanism whereby stress precipitates or exacerbates symptoms associated with a variety of mood disorders.
Acknowledgments
This work was supported by the University of Colorado start-up funds (S.C.), NARSAD Young Investigator Award (S.C.), NIMH 67988 (H.E.W.D.) and NIMH 65327 (S.C.). The authors thank Dr Robert Spencer for the use of his scintillation counter and restrainers for these experiments.
Abbreviations
- ADX
adrenalectomy
- BST
bed nucleus of the stria terminalis
- CEA
central nucleus of the amygdala
- GABA
γ-aminobutyric acid
- HPA
hypothalamic–pituitary–adrenal
- IL-1β
interleukin-1β
- PVN
paraventricular nucleus of the hypothalamus
References
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 495–578. [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CHM, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Casada J, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Compaan JC, Groenink L, van der Gugten J, Maes RA, Olivier B. 5-HT1A receptor agonist flesinoxan enhances Fos immunoreactivity in rat central amygdala, bed nucleus of the stria terminalis and hypothalamus. Eur J Neurosci. 1996;8:2340–2347. doi: 10.1111/j.1460-9568.1996.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H. Evidence that cholecystokinin receptors are not involved in the hypothalamic-pituitary-adrenal response to intraperitoneal administration of interleukin-1beta. J Neuroendocrinol. 1999;11:561–568. doi: 10.1046/j.1365-2826.1999.00358.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Badiani A, Uslaner JM, Oates MM, Vittoz NM, Robinson TE, Watson SJ, Jr, Akil H. Environmental novelty differentially affects c-fos mRNA expression induced by amphetamine or cocaine in subregions of the bed nucleus of the stria terminalis and amygdala. J Neurosci. 2001;21:732–740. doi: 10.1523/JNEUROSCI.21-02-00732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Expression of alpha (1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci. 1999b;19:10098–10106. doi: 10.1523/JNEUROSCI.19-22-10098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999a;413:113–128. [PubMed] [Google Scholar]
- Day HEW, Nebel S, Sasse SK, Campeau S. Psychological Stress Inhibition of the Central Extended Amygdala. Society for Neuroscience Meeting; San Diego, CA, USA. 2004. p. 425.1. [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol Behav. 1989;45:789–795. doi: 10.1016/0031-9384(89)90296-5. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Sah P. GABA receptors inhibited by benzodiazepines mediate fast inhibitory transmission in the central amygdala. J Neurosci. 1999;19:9698–9704. doi: 10.1523/JNEUROSCI.19-22-09698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Sah P. Pathway-specific targeting of GABA (A) receptor subtypes to somatic and dendritic synapses in the central amygdala. J Neurophysiol. 2001;86:717–723. doi: 10.1152/jn.2001.86.2.717. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nucleus of the stria terminalis. Brain Res Rev. 2001b;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001a;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dunn A, Berridge C. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Williams TJ. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. J Comp Neurol. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA. The amygdala and emotion. Curr Opin Neurobiol. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Gray T, Bingaman E. The amygdala: corticotropin-releasing factor, steroids and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gray T, Magnuson D. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Gray T, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Hypoalgesia in response to sensitization during acute noise stress. Behav Neurosci. 1994;108:177–185. doi: 10.1037//0735-7044.108.1.177. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Levine S. Sensitive pituitary-adrenal responsiveness to varying intensities of psychological stimulation. Physiol Behav. 1978;21:295–297. doi: 10.1016/0031-9384(78)90083-5. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hitzemann B, Hitzemann R. Chlordiazepoxide-induced expression of c-Fos in the central extended amygdala and other brain regions of the C57BL/6J and DBA/2J inbred mouse strains: relationships to mechanisms of ethanol action. Alcohol Clin Exp Res. 1999;23:1158–1172. [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all ‘Fos-ed out’? J Neuroendocrinol. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Holahan M, White NM. Amygdala c-fos induction corresponds to unconditioned and conditioned aversive stimuli but not freezing. Behav Brain Res. 2004;152:109–120. doi: 10.1016/j.bbr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J. Colocalization of peptide- and tyrosine hydroxylase-like immunoreactivities with Fos-immunoreactive neurons in rat central amygdaloid nucleus after immobilization stress. Brain Res. 1992;598:107–113. doi: 10.1016/0006-8993(92)90173-7. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Javed A, Kamradt MC, Van de Kar LD, Gray TS. D-Fenfluramine induces serotonin-mediated Fos expression in corticotropin-releasing factor and oxytocin neurons of the hypothalamus, and serotonin-independent Fos expression in enkephalin and neurotensin neurons of the amygdala. Neuroscience. 1999;90:851–858. doi: 10.1016/s0306-4522(98)00523-5. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson L. Studies on the cellular architecture of the bed nucleus of the stria terminalis in the rat. I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson L, Simerly R. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat. II. Chemoarchitecture. J Comp Neurol. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Kang W, Wilson SP, Wilson MA. Changes in nociceptive and anxiolytic responses following herpes virus-mediated preproenkephalin overexpression in rat amygdala are naloxone- reversible and transient. Ann N Y Acad Sci. 1999;877:751–755. doi: 10.1111/j.1749-6632.1999.tb09316.x. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Res. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre- proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30:163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH, Rowland NE. Cholecystokinin- and dexfenfluramine-induced anorexia compared using devazepide and c-fos expression in the rat brain. Regul Pept. 1994;50:223–233. doi: 10.1016/0167-0115(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Differential expression of EGR-1 mRNA in the amygdala following diazepam in contextual fear conditioning. Brain Res. 2000;860:53–63. doi: 10.1016/s0006-8993(00)01976-4. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow M. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Matta SG, Valentine JD, Sharp BM. Nicotinic activation of CRH neurons in extrahypothalamic regions of the rat brain. Endocrine. 1997;7:245–253. doi: 10.1007/BF02778147. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris-leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Morelli M, Pinna A. Antidepressants and atypical neuroleptics induce Fos-like immunoreactivity in the central extended amygdala. Ann N Y Acad Sci. 1999;877:703–706. doi: 10.1111/j.1749-6632.1999.tb09306.x. [DOI] [PubMed] [Google Scholar]
- Morelli M, Pinna A, Ruiu S, Del Zompo M. Induction of Fos-like-immunoreactivity in the central extended amygdala by antidepressant drugs. Synapse. 1999;31:1–4. doi: 10.1002/(SICI)1098-2396(199901)31:1<1::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol. 1987;266:45–55. doi: 10.1002/cne.902660105. [DOI] [PubMed] [Google Scholar]
- Overton JM, Kregel KC, Davis-Gorman G, Seals DR, Tipton CM, Fisher LA. Effects of exercise training on responses to central injection of CRF and noise stress. Physiol Behav. 1991;49:93–98. doi: 10.1016/0031-9384(91)90237-i. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Epelbaum J, Gros C. Met-enkephalin concentrations in individual brain nuclei of ansa lenticularis and stria terminalis transected rats. Brain Res. 1981;216:203–209. doi: 10.1016/0006-8993(81)91290-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Scicli AP, Thompson RF, Swanson LW. Associative fear conditioning of enkephalin mRNA levels in central amygdalar neurons. Behav Neurosci. 2000;114:681–686. doi: 10.1037//0735-7044.114.4.681. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Pezzone M, Lee WS, Hoffman G, Rabin B. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res. 1992;597:41–50. doi: 10.1016/0006-8993(92)91503-7. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA (A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Relationship between Fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao ZR, Yamano M, Shiosaka S, Shinohara A, Tohyama M. Origin of leucine-enkephalin fibers and their two main afferent pathways in the bed nucleus of the stria terminalis in the rat. Exp Brain Res. 1987;65:411–420. doi: 10.1007/BF00236314. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Scicli AP, Petrovich GD, Swanson LW, Thompson RF. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behav Neurosci. 2004;118:5–14. doi: 10.1037/0735-7044.118.1.5. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, Swick D. Audiogenic stress response: behavioral characteristics and underlying monoamine mechanisms. J Neural Transm. 1989;75:31–50. doi: 10.1007/BF01250642. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA. Efferents of the opiocortin-containing region of the commissural nucleus tractus solitarius. Peptides. 1994;15:169–174. doi: 10.1016/0196-9781(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull. 1991;27:651–662. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol. 1994;340:43–64. doi: 10.1002/cne.903400105. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Veening J, Swanson L, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. J Physiol (Lond) 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Harada M. Activation of autonomic nervous system during exposure of rats to restraint and water immersion stress. II. Comparison with restraint stress. J Pharmacobiodyn. 1980;3:11–16. doi: 10.1248/bpb1978.3.11. [DOI] [PubMed] [Google Scholar]