Abstract
The dorsal raphe nucleus (DR) has a topographic neuroanatomy consistent with the idea that different parts of this nucleus subserve different functions. Here we use dual in situ hybridization to describe the rostral-caudal neurochemical distribution of three major cell groups, serotonin (5-hydroxytryptamine; 5-HT), γ-aminobutyric acid (GABA), and catecholamine, and their relative colocalization with each other and mRNA encoding four different receptor subtypes that have been described to influence DR responses, namely, 5HT-1A, α1b adrenergic (α1b ADR), and corticotropin-releasing factor type 1 (CRF-R1) and 2 (CRF-R2) receptors. Serotonergic and GABAergic neurons were distributed throughout the rostral-caudal extent of the DR, whereas catecholaminergic neurons were generally restricted to the rostral half of the nucleus. These phenotypes essentially represent distinct cell populations, because the neurochemical markers were rarely colocalized. Both 5HT-1A and α1b ADR mRNA were highly expressed throughout the DR, and the vast majority of serotonergic neurons expressed both receptors. A smaller percentage of GABAergic neurons also expressed 5HT-1A or α1b ADR mRNA. Very few catecholaminergic cells expressed either 5HT-1A or α1b ADR mRNA. CRF-R1 mRNA was detected only at very low levels within the DR, and quantitative colocalization studies were not technically feasible. CRF-R2 mRNA was mainly expressed at the middle and caudal levels of the DR. At midlevels, CRF-R2 mRNA was expressed exclusively in serotonin neurons, whereas, at caudal levels, approximately half the CRF-R2 mRNA was expressed in GABAergic neurons. The differential distribution of distinct neurochemical phenotypes lends support to the idea of functional differentiation of the DR.
Indexing terms: GABA, tyrosine hydroxylase, serotonin, corticotropin, dual in situ hybridization
The dorsal raphe nucleus (DR) contains the highest concentration of serotonin neurons in the brain and has extensive ascending projections that innervate most forebrain structures (Steinbusch, 1981). These projection neurons have been shown to regulate a wide variety of physiological responses and behaviors, including sleep–wake states, feeding, nociception, neuroendocrine responses, and motor activities (Jacobs and Azmitia, 1992). In addition, increasing evidence has implicated serotonin in affective conditions, such as depression and anxiety (Kahn et al., 1988; Graeff et al., 1996).
However, the DR is not a homogeneous structure, and serotonin neurons have been classified into subpopulations according to their electrophysiological properties in behaving animals. For example, the activity of type I neurons that make up the majority of serotonergic neurons is correlated with the degree of behavioral arousal and motor activity (Jacobs and Fornal, 1999). In contrast, the firing rate of the small group of type II serotonergic neurons, found at the caudal interface of the DR and median raphe nucleus, does not correlate with changes in arousal or activity (Rasmussen et al., 1984). Although substantial numbers of DR neurons contain serotonin, many other neurotransmitters are also present, including γ-aminobutyric acid (GABA), dopamine, glutamate, and corticotropin-releasing factor (CRF), and the distribution of cellular neurochemical phenotypes varies across the rostral-caudal extent of the nucleus (Gamrani et al., 1979; Stratford and Wirtshafter, 1990; Jacobs and Azmitia, 1992; Commons et al., 2003). In addition, both afferent and efferent projections exhibit a distinct topographic arrangement (Kohler and Steinbusch, 1982; Van Bockstaele et al., 1993; Peyron et al., 1996, 1998). Together these observations are consistent with the idea that distinct regions of the DR may subserve different functions.
Experimental support for this idea has recently been obtained with the animal model of learned helplessness. In this model, animals that experience uncontrollable shock exhibit a behavioral profile different from that of animals that have control over and can escape an equivalent shock, including an interference with subsequent escape behavior (Overmier and Seligman, 1967; Irwin et al., 1980), increased conditioned fear responses (Osborne et al., 1975), and increased anxiety (Short and Maier, 1993). Converging evidence suggests that the expression of learned helplessness following an uncontrollable stressor requires activation of the serotonergic neurons of the DR, specifically, in the caudal half of the nucleus, and interventions that reduce that activation prevent the development and/or expression of learned helplessness behaviors (Maier et al., 1993, 1994, 1995; Maswood et al., 1998; Grahn et al., 1999; Greenwood et al., 2003). Administration of CRF into the caudal, but not rostral, DR impeded escape behavior and increased conditioned fear in a manner similar to uncontrollable stress (Hammack et al., 2002). Furthermore, the behavioral consequences of exposure to uncontrollable shock were prevented by administration of a CRF receptor antagonist into the DR before exposure to the shock (Hammack et al., 2002). Use of more selective agonists and antagonists revealed that these effects were due to activation of the CRF-R2 receptor (Hammack et al., 2003). Consistent with these effects is the observation that CRF increases serotonergic activity in a subpopulation of cells in the caudal DR (Lowry et al., 2000). In contrast, the activity of serotonergic cells in the rostral to mid-DR is reportedly inhibited by CRF (Kirby et al., 2000).
Despite the growing evidence for rostral-caudal and dorsal-ventral variations in DR responses, relatively little is known about the relative distribution and colocalization patterns of neurotransmitters and receptors in the DR. The present dual in situ hybridization study was therefore carried out to assess whether there was a rostral-caudal difference in the distribution of mRNA encoding the CRF receptors (types 1 and 2) in the DR and to determine whether they are expressed in serotonergic, GABAergic, or catecholaminergic neurons. In addition, the distribution and colocalization pattern of the α1b adrenergic receptor (α1b ADR) mRNA was determined, because noradrenergic afferents to the DR exert a tonic excitatory effect on serotonergic neurons of the DR through an α1 ADR mechanism (Baraban and Aghajanian, 1980a,b, 1981; Yoshimura et al., 1985), intra-DR administration of a selective α1 ADR antagonist prevents the behavioral consequences of uncontrollable stress exposure (Grahn et al., 2002), and mRNA for the α1b subtype is highly expressed in the rat DR (Day et al., 1997). Finally, the colocalization pattern of serotonin 1A (5HT-1A) receptor mRNA was determined, because this autoreceptor plays a vital role in the direct inhibition of serotonergic activity (Sprouse and Aghajanian, 1987), because its activation can prevent and reverse the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock (Maier et al., 1995), and because its expression on nonserotonergic neurons has been reported recently (Kirby et al., 2003). The distribution of mRNA encoding these receptors in serotonergic, GABAergic, and catecholaminergic neurons at four rostral-caudal levels, and dorsal, ventral, and lateral divisions of the DR is described. The differential distribution patterns observed provide additional evidence that the DR has a functional topographic organization.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan, Indianapolis, IN), weighing approximately 300 g were used (N = 15). Rats were housed in groups of three or four under standard laboratory conditions, with free access to food and water. Rats were maintained on a 12-hour:12-hour light: dark cycle, with lights on at 08:00. Animals were allowed to acclimate to these conditions for at least 1 week before they were killed by rapid decapitation, between 1 and 3 hours after lights on, as approved by The University of Colorado at Boulder Institutional Animal Care and Use Committee, in conformation with NIH guidelines.
Dual in situ hybridization
The brains were removed and frozen in isopentane at −30°C to −40°C and stored at −80°C. Sections of 10 μm were cut on a cryostat (Leica model 1850) through the rostral-caudal extent of the DR. Tissue was thaw mounted onto polylysine-coated slides, air dried, and stored at −80°C until processing for in situ hybridization. The method for dual in situ hybridization has been described previously (Day et al., 1999).
cRNA probes complementary to the following rat mRNAs were used: serotonin transporter (5HT-T), was used as a marker for serotonin neurons (courtesy of Dr. Stanley J. Watson, University of Michigan; 490 mer: 828–1,318); glutamic acid decarboxylase isoforms 65 and 67 (GAD 65/67) were used concurrently as a marker for GABAergic neurons [courtesy of Dr. N.J. Killataratne, University of California, Los Angeles; GAD-65 (230 mer): 1,736–1,966; GAD-67 (210 mer): 1,836–2,046]; tyrosine hydroxylase (TH) was used as a marker for catecholaminergic neurons (courtesy of Dr. Stanley J. Watson, University of Michigan; 300 mer); CRF-R1 (the first probe used was courtesy of Dr. A. Seasholtz, University of Michigan; 367 mer: 1–367; a second, full-length probe (1.3 kb) was subsequently used, courtesy of Dr. P.E. Sawchenko, Salk Institute); CRF-R2 (courtesy of Dr. Robert Thompson, University of Michigan; 461 mer); α1b ADR (courtesy of Dr. Stanley J. Watson, University of Michigan; 766 mer: 144–910); and 5HT-1A receptor (courtesy of Dr. Stanley J. Watson, University of Michigan; 910 mer: 333–1,243). Control experiments using “sense” probes or pretreatment of tissue with 200 μg/ml RNase A, 37°C for 1 hour, prior to hybridization indicated that the labeling observed with the “antisense” probes was specific (data not shown). For the full-length CRF-R1 probe, an additional in situ hybridization experiment was performed as follows. Sections were treated under standard conditions or adjacent sections were deproteinated with proteinase K (PK), 1 μg/ml, for 10 minutes at 37°C, to aid in probe penetration. In this experiment, additional sections (with or without PK pretreatment) were run for dual in situ hybridization with digoxigenin 5HT-T (see below).
The distribution of each mRNA was determined by using a radioactive probe, labeled with [35S]UTP (Amersham Biosciences Corp., Piscataway, NJ) or [35S]UTP and -CTP for CRF-R1 and -R2, which were less abundant. For dual in situ hybridization, digoxigenin (DIG)-UTP-labeled probes (Roche Diagnostics Corp, Indianapolis, IN; 5HT-T, GAD65/67, or TH) were used in combination with 35S-labeled probes (5HT-T, GAD65/67, TH, 5HT-1A, α1b ADR, CRF-R1, or CRF-R2). To visualize labeled cells, after exposure to X-ray film, sections were dipped in liquid emulsion (Ilford KD-5; Polysciences Inc., Warrington, PA) and stored in light-tight boxes for 1 week (5HT-T), 3 weeks (GAD65/67; TH), 6 weeks (5HT-1A; α1b ADR), or 3 months (CRF-R1, CRF-R2). The relatively lengthy exposure times were used because sections were developed on ice (D-19; Kodak, Rochester, NY; diluted 1:1 with distilled water) to prevent an increase in nonradioactive background, and this results in a lower silver grain signal. Sections were then dehydrated and coverslipped in a xylene-based mounting medium (Permount; Fisher Scientific, Pittsburgh, PA). To increase the chances of observing CRF-R1 mRNA, the sections that were processed in the additional in situ hybridization experiment (singly labeled or dually labeled with DIG-5HT-T, with or without PK pretreatment) were developed at room temperature after a 2-month exposure.
Data analysis
The cellular distribution was determined with a Nikon Eclipse 800 microscope. Nonradioactive probes (5HT-T, GAD65/67, or TH) were visualized under brightfield as a blue/purple precipitate, whereas the radioactive probes (5HT-T, GAD65/67, TH, 5HT-1A, α1b ADR, CRF-R1, or CRF-R2) were visualized under darkfield by silver grain distribution. Sections through the DR (100-μm intervals) were analyzed to determine the extent of colocalization. Schematic diagrams of the DR are shown in Figure 1. The DR was divided into four separate levels: rostral (approximately −7.2 to −7.4 mm bregma), midrostral (approximately −7.6 to −7.8 mm bregma), midcaudal (approximately −8.0 to −8.2 mm bregma), and caudal (approximately −8.4 to −8.6 mm bregma). These levels were largely determined by the distribution of the 5HT-T mRNA, which was determined for each animal studied, and the white matter distribution. Nissl-stained sections were of limited used, probably because the tissue was fresh frozen rather than perfused. For the rostral and caudal levels, sections were further divided into dorsal and ventral parts; for the midrostral and midcaudal levels, sections were divided into dorsal, ventral, and lateral wings (Fig. 2). At least two sections per animal per level were analyzed. For each combination of probes, sections from three to five animals were analyzed. Cell profile counts were determined at ×20, with the aid of an eyepiece grid centered over each area. It should be noted that cell counts do not represent the absolute number of cells within a particular DR area. Rather, cells were counted to obtain an approximation of the relative degree of colocalization of two mRNAs. Although the relative abundance of each probe across areas was not quantified absolutely, a qualitative account is given in Results, and the cell counts given in Table 1 provide an approximation of the relative abundance.
Fig. 1.

Schematic coronal diagrams showing the rat dorsal raphe nucleus from rostral through caudal levels (A–H). Numbers refer to the distance from bregma. Colocalization cell counts were taken from sections at 200-μm intervals, with counts from two sections averaged for each of four levels: rostral (approximately −7.2 mm and −7.4 mm), midrostral (approximately −7.6 mm and −7.8 mm), midcaudal (approximately −8.0 and −8.2 mm), and caudal (approximately −8.4 mm and −8.6 mm). 3, Oculomotor nucleus; 4, trochlear nucleus; A8, A8 dopamine cells; Aq, aqueduct (Sylvius); Atg, anterior tegmental nucleus; CLi, caudal linear nucleus of the raphe; DLPAG, dorsolateral PAG; DR, dorsal raphe; DRC, DR caudal; DRD, DR dorsal; DRI, DR interfascicular; DRV, DR ventral; DRVL, DR ventrolateral; DTgP, dorsal tegmental nucleus, pericentral; LDTg, laterodorsal tegmental nucleus; LPAG, lateral PAG; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; Pa4, paratrochlear nucleus; PAG, periaqueductal gray; PMnR, paramedian raphe nucleus; PnO, pontine reticular nucleus, oral part; PnR, pontine raphe nucleus; scp, superior cerebellar peduncle; SPTg, subpedencular tegmental nucleus; ts, tectospinal tract; VLPAG, ventrolateral PAG; VTg, ventral tegmental nucleus; xscp, decussation of the scp. Adapted, with permission, from Paxinos and Watson (1998).
Fig. 2.

Darkfield digital photomicrographs of serotonin transporter mRNA to illustrate the approximate areas used for microscopic cellular analysis (for each probe combination), at rostral (A; approximately −7.2 to −7.4 mm relative to Bregma), midrostral (B; Mid-R, approximately −7.6 to −7.8 mm relative to Bregma), midcaudal (C; Mid-C, approximately −8.0 to −8.2 mm relative to Bregma), and caudal (D; approximately −8.4 mm to −8.6 mm relative to Bregma) levels. D, dorsal; V, ventral; L, lateral. Scale bar = 500 μm.
Table 1.
Expression of 5HT-1A, α1b Adrenergic, and CRF-R2 Receptor mRNA in Serotonergic, GABAergic, and Catecholaminergic Cells of the Rat DR, Determined by Dual In Situ Hybridization1
| No. DIG only | No. 35S only | No. doubles | Percentage double w.r.t. Dig | Percentage double w.r.t. 35S | |
|---|---|---|---|---|---|
| DIG-5HT-T + 35S-5HT-1A (n = 4) | |||||
| Rostral: dorsal | 1.1 ± 0.3 | 4.9 ± 0.4 | 44.4 ± 5.5 | 97.4 ± 0.7 | 89.9 ± 0.6 |
| Rostral: ventral | 0.6 ± 0.2 | 6.4 ± 0.8 | 44.4 ± 6.0 | 98.5 ± 0.5 | 86.6 ± 3.0 |
| Mid-R: dorsal | 0.4 ± 0.2 | 2.0 ± 0.4 | 65.8 ± 2.6 | 99.5 ± 0.3 | 97.1 ± 0.5 |
| Mid-R: ventral | 0.3 ± 0.1 | 1.8 ± 0.5 | 83.1 ± 2.7 | 99.7 ± 0.2 | 97.9 ± 0.6 |
| Mid-R: lateral | 0.4 ± 0.2 | 4.0 ± 0.8 | 38.3 ± 1.6 | 99.1 ± 0.4 | 90.4 ± 2.1 |
| Mid-C: dorsal | 0.6 ± 0.4 | 1.0 ± 0.2 | 72.4 ± 4.7 | 99.2 ± 0.5 | 98.6 ± 0.4 |
| Mid-C: ventral | 0.5 ± 0.2 | 0.4 ± 0.1 | 82.6 ± 8.9 | 99.3 ± 0.3 | 99.5 ± 0.2 |
| Mid-C: lateral | 0.1 ± 0.1 | 2.4 ± 0.5 | 34.3 ± 4.1 | 99.8 ± 0.2 | 93.0 ± 2.2 |
| Caudal: dorsal | 0.6 ± 0.5 | 2.9 ± 0.6 | 48.5 ± 1.7 | 98.7 ± 1.0 | 94.4 ± 1.2 |
| Caudal: ventral | 0.5 ± 0.2 | 1.5 ± 0.5 | 36.0 ± 2.4 | 98.7 ± 0.5 | 96.2 ± 0.9 |
| DIG-GAD65/67 + 35S-5HT-1A (n = 4) | |||||
| Rostral: dorsal | 37.4 ± 3.0 | 45.8 ± 8.1 | 7.5 ± 1.1 | 16.9 ± 2.8 | 14.5 ± 1.5 |
| Rostral: ventral | 29.3 ± 3.6 | 37.4 ± 2.8 | 6.1 ± 0.5 | 17.8 ± 1.9 | 14.1 ± 0.7 |
| Mid-R: dorsal | 22.5 ± 2.4 | 61.3 ± 5.5 | 2.3 ± 0.5 | 9.7 ± 2.9 | 3.5 ± 0.7 |
| Mid-R: ventral | 19.0 ± 2.5 | 73.1 ± 10.9 | 1.1 ± 0.3 | 5.9 ± 2.0 | 1.4 ± 0.2 |
| Mid-R: lateral | 57.7 ± 6.2 | 40.6 ± 2.5 | 4.8 ± 0.3 | 8.0 ± 1.2 | 10.6 ± 0.8 |
| Mid-C: dorsal | 19.4 ± 1.9 | 74.1 ± 9.1 | 0.9 ± 0.6 | 4.0 ± 2.4 | 1.0 ± 0.5 |
| Mid-C: ventral | 15.3 ± 1.4 | 71.8 ± 7.9 | 0.6 ± 0.2 | 3.8 ± 1.3 | 0.8 ± 0.3 |
| Mid-C: lateral | 57.5 ± 7.7 | 37.9 ± 3.0 | 3.8 ± 0.8 | 6.0 ± 0.6 | 9.4 ± 2.6 |
| Caudal: dorsal | 26.3 ± 3.4 | 35.8 ± 5.8 | 2.1 ± 0.4 | 7.6 ± 1.4 | 5.6 ± 0.7 |
| Caudal: ventral | 31.4 ± 4.9 | 22.6 ± 1.7 | 1.6 ± 0.2 | 5.5 ± 1.5 | 6.6 ± 0.8 |
| DIG-TH + 35S-5HT-1A (n = 4) | |||||
| Rostral: dorsal | 16.5 ± 2.9 | 31.0 ± 3.5 | 0.5 ± 0.2 | 2.8 ± 1.2 | 1.7 ± 0.9 |
| Rostral: ventral | 13.3 ± 3.9 | 27.6 ± 3.0 | 0.1 ± 0.1 | 1.5 ± 1.5 | 0.5 ± 0.5 |
| Mid-R: dorsal | 8.8 ± 2.7 | 36.1 ± 3.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: ventral | 4.6 ± 3.1 | 50.0 ± 7.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: lateral | 2.1 ± 0.6 | 25.7 ± 2.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: dorsal | 2.5 ± 0.3 | 32.0 ± 4.8 | 0.3 ± 0.1 | 12.2 ± 5.4 | 1.0 ± 0.4 |
| Mid-C: ventral | 0.0 ± 0.0 | 38.8 ± 6.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: lateral | 0.8 ± 0.1 | 22.7 ± 2.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Caudal: dorsal | 0.4 ± 0.2 | 19.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Caudal: ventral | 0.0 ± 0.0 | 24.8 ± 2.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| DIG-5HT-T + 35S-α1b (n = 4) | |||||
| Rostral: dorsal | 0.7 ± 0.2 | 14.2 ± 3.2 | 53.3 ± 4.6 | 98.8 ± 0.2 | 79.1 ± 4.1 |
| Rostral: ventral | 0.3 ± 0.2 | 12.5 ± 3.3 | 31.3 ± 9.9 | 99.2 ± 0.4 | 68.7 ± 7.1 |
| Mid-R: dorsal | 0.6 ± 0.3 | 22.8 ± 4.2 | 84.9 ± 4.2 | 99.3 ± 0.3 | 78.9 ± 3.8 |
| Mid-R: ventral | 0.5 ± 0.5 | 6.5 ± 0.8 | 82.9 ± 12.0 | 99.5 ± 0.5 | 92.3 ± 1.6 |
| Mid-R: lateral | 0.3 ± 0.1 | 16.8 ± 3.4 | 26.8 ± 2.2 | 99.1 ± 0.6 | 60.6 ± 7.2 |
| Mid-C: dorsal | 1.3 ± 0.3 | 12.3 ± 2.0 | 77.1 ± 4.6 | 98.4 ± 0.3 | 86.6 ± 1.5 |
| Mid-C: ventral | 1.5 ± 0.6 | 4.9 ± 0.9 | 84.0 ± 11.6 | 98.3 ± 0.6 | 93.9 ± 1.7 |
| Mid-C: lateral | 0.6 ± 0.4 | 8.8 ± 1.4 | 38.5 ± 5.5 | 98.5 ± 0.8 | 81.3 ± 1.7 |
| Caudal: dorsal | 0.5 ± 0.3 | 7.5 ± 1.1 | 60.3 ± 3.4 | 99.2 ± 0.4 | 88.9 ± 1.4 |
| Caudal: ventral | 0.9 ± 0.5 | 5.4 ± 1.3 | 55.8 ± 7.5 | 98.1 ± 1.2 | 90.9 ± 2.2 |
| DIG-GAD65/67 + 35S-α1b (n = 4) | |||||
| Rostral: dorsal | 19.2 ± 5.3 | 61.2 ± 11.1 | 11.7 ± 2.3 | 38.8 ± 1.9 | 16.9 ± 4.0 |
| Rostral: ventral | 11.0 ± 2.1 | 36.3 ± 10.5 | 12.5 ± 1.8 | 53.4 ± 7.0 | 27.5 ± 4.2 |
| Mid-R: dorsal | 8.0 ± 0.8 | 79.1 ± 5.8 | 8.0 ± 0.9 | 49.9 ± 2.3 | 9.4 ± 1.4 |
| Mid-R: ventral | 11.1 ± 1.5 | 83.5 ± 9.7 | 7.9 ± 0.5 | 42.1 ± 3.8 | 9.0 ± 1.2 |
| Mid-R: lateral | 31.1 ± 4.2 | 33.5 ± 1.2 | 22.2 ± 3.1 | 41.6 ± 1.0 | 39.4 ± 2.9 |
| Mid-C: dorsal | 8.6 ± 0.4 | 70.1 ± 5.2 | 7.3 ± 1.1 | 44.8 ± 4.5 | 9.6 ± 1.9 |
| Mid-C: ventral | 8.6 ± 0.9 | 75.8 ± 6.8 | 4.5 ± 1.3 | 32.8 ± 7.6 | 5.6 ± 1.7 |
| Mid-C: lateral | 33.8 ± 4.2 | 37.7 ± 2.9 | 13.1 ± 1.3 | 28.3 ± 2.4 | 25.9 ± 2.3 |
| Caudal: dorsal | 25.5 ± 6.7 | 51.1 ± 2.9 | 3.8 ± 1.1 | 16.9 ± 7.7 | 6.7 ± 1.5 |
| Caudal: ventral | 22.1 ± 7.7 | 39.5 ± 3.3 | 3.1 ± 0.7 | 17.8 ± 7.7 | 7.5 ± 1.6 |
| DIG-TH + 35S-α1b (n = 4) | |||||
| Rostral: dorsal | 12.1 ± 2.2 | 36.4 ± 6.0 | 0.9 ± 0.6 | 5.2 ± 3.0 | 2.5 ± 1.4 |
| Rostral: ventral | 7.3 ± 1.9 | 34.3 ± 8.2 | 2.1 ± 0.7 | 23.0 ± 3.3 | 6.7 ± 1.7 |
| Mid-R: dorsal | 7.6 ± 2.7 | 48.1 ± 3.3 | 0.5 ± 0.3 | 7.3 ± 5.2 | 0.9 ± 0.5 |
| Mid-R: ventral | 4.1 ± 2.5 | 47.4 ± 3.0 | 0.9 ± 0.4 | 20.4 ± 10.7 | 1.8 ± 0.8 |
| Mid-R: lateral | 1.9 ± 0.6 | 27.1 ± 1.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: dorsal | 1.3 ± 0.5 | 41.8 ± 5.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: ventral | 0.0 ± 0.0 | 32.9 ± 2.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: lateral | 0.1 ± 0.1 | 20.0 ± 3.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Caudal: dorsal | 0.1 ± 0.1 | 20.3 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Caudal: ventral | 0.0 ± 0.0 | 15.4 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| DIG-5HT-T + 35S-CRF-R2 (n = 4) | |||||
| Rostral: dorsal | 40.8 ± 5.8 | 0.0 ± 0.0 | 1.6 ± 0.4 | 3.7 ± 0.5 | 100.0 ± 0.0 |
| Rostral: ventral | 37.4 ± 7.1 | 0.0 ± 0.0 | 0.8 ± 0.3 | 1.6 ± 0.6 | 100.0 ± 0.0 |
| Mid-R: dorsal | 56.1 ± 6.9 | 0.0 ± 0.0 | 5.6 ± 1.7 | 9.0 ± 2.0 | 100.0 ± 0.0 |
| Mid-R: ventral | 85.0 ± 2.9 | 0.0 ± 0.0 | 9.3 ± 1.3 | 9.8 ± 1.2 | 100.0 ± 0.0 |
| Mid-R: lateral | 34.9 ± 4.8 | 0.0 ± 0.0 | 1.7 ± 0.3 | 4.8 ± 0.8 | 100.0 ± 0.0 |
| Mid-C: dorsal | 49.0 ± 4.1 | 0.0 ± 0.0 | 14.3 ± 1.9 | 22.7 ± 3.1 | 100.0 ± 0.0 |
| Mid-C: ventral | 57.5 ± 6.2 | 0.3 ± 0.1 | 6.5 ± 1.0 | 10.3 ± 1.2 | 95.5 ± 2.6 |
| Mid-C: lateral | 35.4 ± 6.2 | 0.4 ± 0.2 | 2.3 ± 0.5 | 6.9 ± 2.5 | 85.9 ± 6.5 |
| Caudal: dorsal | 30.1 ± 8.4 | 5.4 ± 1.0 | 4.9 ± 1.4 | 16.9 ± 6.5 | 46.0 ± 6.9 |
| Caudal: ventral | 35.9 ± 3.8 | 5.5 ± 1.5 | 1.6 ± 0.5 | 4.4 ± 1.4 | 24.7 ± 10.0 |
| DIG-GAD65/67 + 35S-CRF-R2 (n = 5) | |||||
| Rostral: dorsal | 26.9 ± 3.0 | 1.2 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Rostral: ventral | 33.3 ± 5.8 | 0.8 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: dorsal | 13.9 ± 1.6 | 7.4 ± 1.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: ventral | 16.6 ± 2.3 | 9.3 ± 3.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: lateral | 41.0 ± 2.3 | 3.6 ± 1.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: dorsal | 14.5 ± 3.8 | 16.8 ± 2.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: ventral | 16.7 ± 2.8 | 12.0 ± 1.4 | 0.5 ± 0.5 | 1.7 ± 1.7 | 2.9 ± 2.9 |
| Mid-C: lateral | 55.9 ± 7.6 | 3.8 ± 0.7 | 0.7 ± 0.5 | 0.8 ± 0.6 | 11.6 ± 9.1 |
| Caudal: dorsal | 40.1 ± 8.6 | 5.3 ± 1.0 | 5.7 ± 1.4 | 12.4 ± 1.0 | 51.2 ± 8.2 |
| Caudal: ventral | 29.4 ± 2.4 | 4.1 ± 1.1 | 6.4 ± 1.1 | 17.9 ± 2.9 | 66.2 ± 8.7 |
| DIG-TH + 35S-CRF-R2 (n = 4) | |||||
| Rostral: dorsal | 18.5 ± 0.9 | 2.5 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Rostral: ventral | 15.4 ± 3.2 | 1.9 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: dorsal | 16.9 ± 2.7 | 10.4 ± 3.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: ventral | 6.5 ± 2.6 | 10.5 ± 2.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-R: lateral | 3.8 ± 0.3 | 3.8 ± 1.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: dorsal | 5.8 ± 1.8 | 19.3 ± 3.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: ventral | 0.0 ± 0.0 | 17.8 ± 3.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mid-C: lateral | 0.7 ± 0.3 | 4.7 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Caudal: dorsal | 0.1 ± 0.1 | 10.5 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Caudal: ventral | 0.0 ± 0.0 | 10.8 ± 2.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Dual in situ hybridization was used to determine the distribution and relative colocalization of 5HT-1A (serotonin 1A receptor), α1b (α1B adrenergic receptor) and CRF-R2 (corticotropin releasing factor receptor type 2) mRNA in serotonergic (expressing serotonin transporter (5HT-T) mRNA), GABAergic (expressing glutamic acid decarboxylase isoforms 65 or 67 (GAD65/67) mRNA) or catecholaminergic (expressing tyrosine hydroxylase (TH) mRNA) cells through the rostral-caudal extent of the rat dorsal raphe nucleus. Cells were defined as being labeled for the digoxigenin probe only (Dig), radioactive probe only (35S) or both probes (double). Numbers in the first 3 columns refer to the average number of cells counted per section analyzed. At least 2 sections were analyzed per rat for each region, and the data averaged to obtain a single value for each animal. These single values were then averaged. “n” refers to the number of animals analyzed (not the number of sections). Note: Cells were counted with the aid of a grid in order to calculate the percentage of double-labeled cells, and do not represent absolute numbers of cells in a given region. However, since the grid used was identical between counts, the numbers give an approximation for relative cell counts for each probe. The percentage colocalization was calculated separately for each animal and then averaged across animals. Hence the numbers in the last 2 columns have not been derived directly from the numbers in the first 3 columns. w.r.t.—with respect to
Digital photography
Images in black-and-white mode were captured from the Nikon Eclipse 800 microscope with a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI). Separate images of darkfield and brightfield were captured for dual in situ hybridization studies. Photographic montages were created using Adobe Photoshop 5.5 for PC. For all figures, brightness and contrast were adjusted to best represent the data. For Figures 3 and 4 (low magnification darkfield), the individual figures were adjusted for “input levels” that adjusted the brightness of the midtone gray levels without altering shadows and highlights. This helped to compensate for a somewhat uneven darkfield illumination at this low magnification. For dual in situ hybridization (see Figs. 5–9), darkfield images were layered over brightfield images with a 50% opacity to allow both images to be seen concurrently. In addition, the darkfield image underwent a single “sharpen” filtering to try to provide more contrast between the silver grains and the underlying DIG-labeled cells.
Fig. 3.

Darkfield digital photomicrographs showing the distribution of serotonin transporter (5HT-T; A–D); glutamic acid decarboxylase isoforms 65 and 67, used as a marker for γ-aminobutyric acid (GAD65/67; E–H); and tyrosine hydroxylase, used as a marker for catecholamines (TH; I–L), mRNAs in the rat DR. The following levels are shown: rostral (approximately −7.2 to −7.4 mm relative to bregma; A,E,I), midrostral (Mid-R, approximately −7.6 to −7.8 mm relative to bregma; B,F,J), midcaudal (Mid-C, approximately −8.0 to −8.2 mm relative to bregma; C,G,K), and caudal (approximately −8.4 mm to −8.6 mm relative to bregma; D,H,L). Aq, aqueduct; mlf, medial longitudinal fasciculus; PAG, periaqueductal gray; scp, superior cerebellar peduncle; xscp, decussation of superior cerebellar peduncle. Scale bar = 500 μm.
Fig. 4.
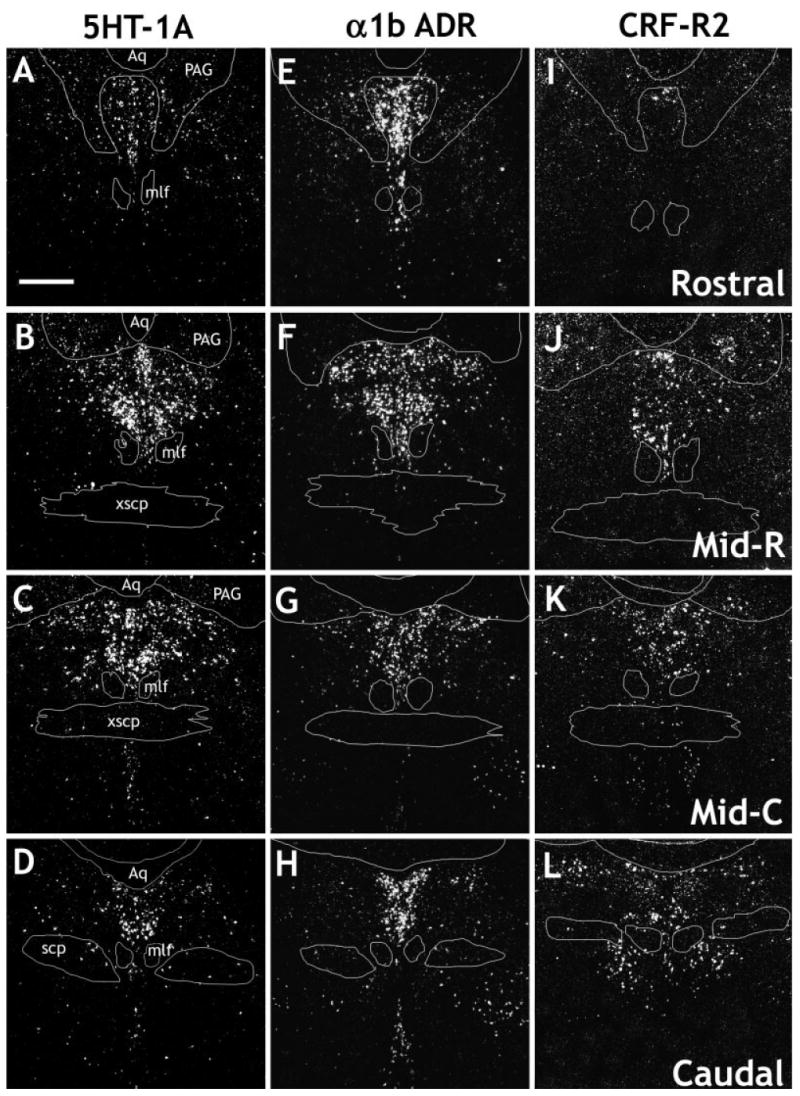
Darkfield digital photomicrographs showing the distribution of the serotonin 1A receptor (5HT-1A; A–D), α1b adrenergic receptor (α1b; E–H)m and corticotropin-releasing factor type 2 receptor (CRF-R2; I–L) mRNAs in the rat DR. The following levels are shown: rostral (approximately −7.2 to −7.4 mm relative to bregma; A,E,I), midrostral (Mid-R, approximately −7.6 to −7.8 mm relative to bregma; B,F,J), midcaudal (Mid-C, approximately −8.0 to −8.2 mm relative to bregma; C,G,K), and caudal (approximately −8.4 mm to −8.6 mm relative to bregma; D,H,L). Aq, aqueduct; mlf, medial longitudinal fasciculus; PAG, periaqueductal gray; scp, superior cerebellar peduncle; xscp, decussation of superior cerebellar peduncle. Scale bar = 500 μm.
Fig. 5.
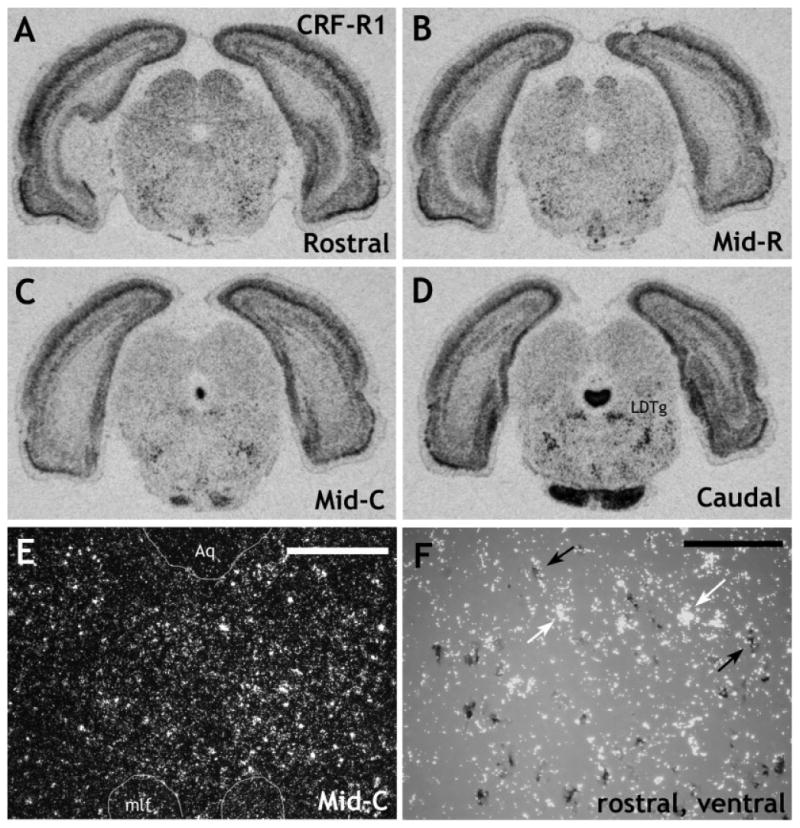
Expression of CRF-R1 mRNA, determined using a full-length riboprobe, in the rat DR. X-ray images at rostral (A), midrostral (B), midcaudal (C), and caudal (D) levels. Although significant expression of CRF-R1 mRNA was detected in several brain regions, including the LDTg, very little CRF-R1 mRNA was detected in the DR. E: Darkfield photomicrograph taken at a midcaudal level, midline, to show the relatively low levels of CRF-R1 mRNA in the DR. F shows DIG-5HT-T with [35S]CRF-R1 at a rostral level, ventral part. DIG-labeled cells were photographed under brightfield illumination (dark gray cells), whereas 35S-labeled cells were photographed under darkfield illumination (clusters of white grains). The photographs were combined in Adobe Photoshop (see Materials and Methods). Black arrows denote double-labeled cells, and white arrows denote single-labeled cells. Although levels of CRF-R1 mRNA expression were not high enough to perform a systematic analysis, it appears that CRF-R1 mRNA is expressed at low levels in both a few 5HT-T and non-5HT-T mRNA containing cells. LDTg, lateral dorsal tegmental nucleus; Aq, aqueduct; mlf, medial longitudinal fasciculus. Scale bars = 400 μm in E; 100 μm in F.
Fig. 9.

Digital photomicrographs showing the relative colocalization of corticotropin-releasing factor type 2 receptor (CRF-R2) mRNA with mRNA markers for serotonin or GABA. DIG-labeled cells were photographed under brightfield illumination (dark gray cells), whereas 35S-labeled cells were photographed under darkfield illumination (clusters of white grains). The photographs were combined in Adobe Photoshop (see Materials and Methods). Black arrows denote double-labeled cells, and white arrows denote single-labeled cells. A: Shows DIG-5HT-T with [35S]CRF-R2 midcaudal, dorsal part. Note the extensive colocalization, although several cells that express 5HT-T mRNA do not express CRF-R2 mRNA. B: Shows DIG-GAD65/67 (glutamic acid decarboxylase, isoforms 65 and 67, used as a marker for GABAergic cells) with [35S]CRF-R2 at a midrostral level, lateral part. Note that there is no colocalization at this level. C: Shows DIG-GAD65/67 (glutamic acid decarboxylase, isoforms 65 and 67, used as a marker for GABAergic cells) with [35S]CRF-R2 at a midcaudal level, ventral part. Note that there is significant colocalization at this level, although many cells remain singly labeled. For orientation, the single-labeled CRF-R2 cells, white grains, denoted by white arrows on the right of the image are approximately at the midline of the ventral DR. Scale bar = 100 μm.
Results
Technical note
It should be noted that the nonradioactive in situ hybridization technique is generally less sensitive than the radioactive technique, so the digoxigenin-labeled cell population tends to be underrepresented. Ideally, both combinations of radioactive and nonradioactive probes would be analyzed, but the abundance of several of the receptor probes was not sufficient to obtain reliable labeling with the nonradioactive in situ hybridization technique. However, dual in situ hybridization of the following probe combinations in the DR revealed that, for the phenotype markers for serotonin (5HT-T), GABA (GAD65/67), and catecholamine (TH) neurons, the DIG-labeled probes detected the vast majority of the cells detected by the more sensitive radioactive method. Specifically, for [35S]5HT-T with DIG-5HT-T, 100% of cells labeled with [35S]5HT-T were also positive for DIG-5HT-T. For [35S]GAD65/67 with DIG-GAD65/67, 98.7% of cells labeled with [35S]GAD65/67 also were labeled with DIG-GAD65/67. For [35S]TH with DIG-TH, 95.5% of cells labeled with [35S]TH were also labeled with DIG-TH. Hence we are confident that the data reported reflect the majority of the cell population that can be detected with an in situ hybridization method.
General distribution patterns
5HT-T mRNA was abundant throughout the rostral-caudal extent of the DR, in all subdivisions of the nucleus (Fig. 3A–D). In the dorsal and ventral aspects of the nucleus, the labeling was centered along the midline, and there was relatively less dense expression in the lateral wings. In contrast, GAD65/67 mRNA expression, which was also abundant throughout the DR, tended to flank the dorsal and ventral 5HT-T mRNA expression, with few cells in the vicinity of the midline, so that the distribution was mainly just lateral to the midline (Figs. 3E–H, 5A). Even in the ventral DR, which has a high density of closely packed serotonergic cells, it is clear that significant numbers of GABAergic cells are present in the immediate lateral region. In particular, a high density of GABAergic cells was present in the lateral wings. Overall, fewer cells expressed TH (Fig. 3I–L), and these cells were most abundant at the rostral level, with expression tapering off over the midrostral and midcaudal levels, so that virtually no TH-positive cells were observed at the caudal level.
Both 5HT-1A (Fig. 4A–D) and α1b ADR (Fig. 4E–H) receptor mRNAs were relatively highly expressed at all levels and in all subdivisions of the DR. In contrast, the CRF receptors showed a relatively discrete profile. Expression of CRF-R2 mRNA (Fig. 4I–L) was lower than that of 5HT-1A or α1b ADR mRNA. Very few cells expressing CRF-R2 mRNA were observed at the rostral level. The density increased over the midrostral level, was maximal at the midcaudal level, and was still relatively high at the caudal level. At the midrostral level of the DR, a significant number of cells expressing CRF-R2 mRNA in the ventral subdivision were observed in the interfascicular part of the nucleus. At the midcaudal and caudal levels, expression became more diffuse, with scattered cells also observed in the immediate surrounding region, for example, along the edge of the aqueduct (Aq). These additional cells were not included in the cell counts. Although it has been reported that CRF-R1 is present in the DR, under these experimental conditions, the 367 mer probe detected very few cells in the DR, except for those interspersed in the lateral wings, mostly at the midcaudal level (data not shown). Although these cells were clearly within the confines of the DR, as defined by the distribution of the 5HT-T, we believe that they are most likely part of the rostral extension of the lateral dorsal tegmental nucleus (LDTg). This is because 1) they appeared to be a part of a continuous collection of cells that clearly were part of the LDTg at more caudal levels and 2) they were not colocalized with 5HT-T, GAD65/67, or TH mRNA (data not shown). Because of the discrepancy between these data and immunohistochemical data [which has shown abundant CRF-R1 protein in cell bodies of the DR (Chen et al., 2000; Lowry et al., 2002; Roche et al., 2003)], we subsequently used a probe directed against the full length of the CRF-R1 receptor that has been reported to detect low levels of CRF-R1 mRNA in the DR (Van Pett et al., 2000). This probe was used under standard hybridization conditions or with an additional pretreatment with PK (see Materials and Methods). This latter probe was more sensitive than the 367 mer probe in that some additional cells were detected scattered throughout the DR; however, expression levels of CRF-R1 mRNA remained low in the DR (Fig. 5A–E). Pretreatment with PK did not increase the signal-to-noise ratio; in fact, the signal was slightly poorer in these sections compared with the standard conditions (data not shown).
Colocalization studies
By using reciprocal combinations of DIG- and 35S-labeled 5HT-T, GAD65/67, and TH cRNA probes, it was determined that serotonin, GABA, and catecholamines are very rarely colocalized at any rostral-caudal level or subdivision of the DR. Overall, colocalization was less than 0.5% for each combination of probes (Fig. 6). Thus, markers for each cell phenotype essentially define a distinct cell population of the DR.
Fig. 6.
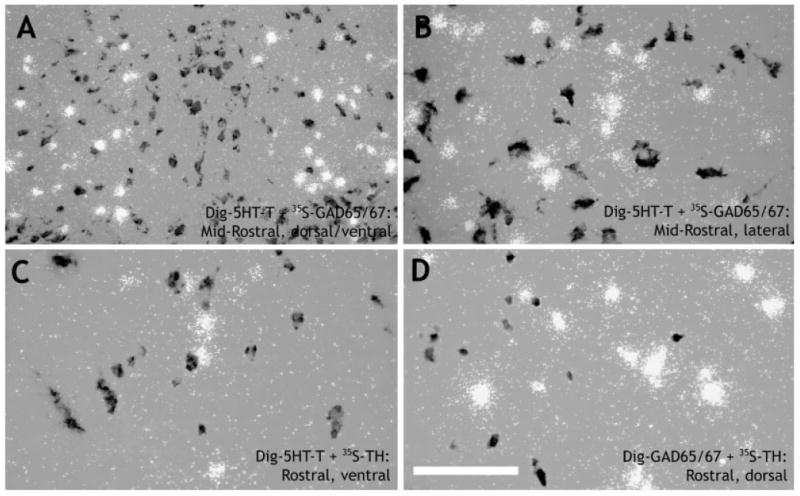
Digital photomicrographs showing that serotonin, GABA, and catecholamines are rarely expressed in the same cell in the rat DR. DIG-labeled cells were photographed under brightfield illumination (dark gray cells), whereas 35S-labeled cells were photographed under darkfield illumination (clusters of white grains). The photographs were combined in Adobe Photoshop (see Materials and Methods). A,B: Show DIG-5HT-T (serotonin transporter, used as a marker for serotonergic cells) and [35S]GAD65/67 (glutamic acid decarboxylase, isoforms 65 and 67, used as a marker for GABAergic cells) at a midrostral level. Note that, for the dorsal and ventral DR, GABAergic cells largely flank the midline distribution of serotonergic cells (A), whereas, in the lateral wings, the distribution is more intermingled (B). C: Shows DIG-5HT-T and [35S]TH (used as a marker for catecholamines) at a rostral level, ventral part. D: Shows DIG-GAD65/67 and [35S]TH at a rostral level, dorsal part. Scale bar = 100 μm in D (applies to B–D); 200 μm for A.
As expected, 5HT-1A receptor mRNA was expressed on virtually all identified serotonin neurons in the DR, regardless of rostrocaudal level or subdivision (Table 1, Fig. 7A). In addition, a small percentage (3.8–17.8%) of GABAergic neurons expressed the 5HT-1A receptor (Table 1, Fig. 7B). A clear difference in subdivision colocalization was not apparent, although the rostral level and the lateral wings tended to exhibit slightly higher levels of colocalization of 5HT-1A receptor with GAD65/67 mRNA (Table 1). 5HT-1A receptor mRNA was rarely colocalized with TH mRNA (Table 1).
Fig. 7.

Digital photomicrographs showing the relative colocalization of serotonin 1A receptor (5HT-1A) mRNA with mRNA markers for serotonin (A) or GABA (B). DIG-labeled cells were photographed under brightfield illumination (dark gray cells), whereas 35S-labeled cells were photographed under darkfield illumination (clusters of white grains). The photographs were combined in Adobe Photoshop (see Materials and Methods). Black arrows denote double-labeled cells, and white arrows denote single-labeled cells. A: Shows DIG-5HT-T (serotonin transporter, used as a marker for serotonergic cells) with [35S]5HT-1A at a midrostral level, ventral part. Note the extensive colocalization, although a few cells expressing 5HT-1A mRNA did not express 5HT-T mRNA. B: Shows DIG-GAD65/67 (glutamic acid decarboxylase, isoforms 65 and 67, used as a marker for GABAergic cells) with [35S]5HT-1A at a midrostral level, dorsal/lateral part. A few cells expressed both GAD65/67 and 5HT-1A mRNAs. Scale bar = 100 μm.
Virtually all serotonin neurons of the DR also expressed α1b ADR mRNA, regardless of rostrocaudal level or subdivision (Table 1, Fig. 8A). A moderate number (∼40%; Table 1, Fig. 8B) of GABA neurons also expressed α1b ADR mRNA, with the rate of colocalization tending to decline at caudal levels (∼20%; Table 1). A very small percentage of TH-positive cells also expressed α1b ADR mRNA, and these were localized mainly ventrally in the rostral half of the nucleus (Table 1).
Fig. 8.
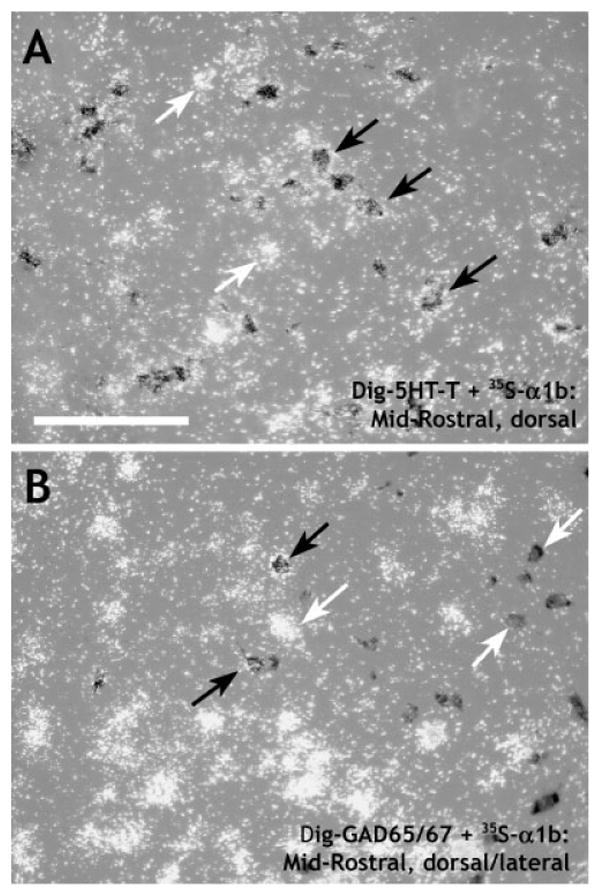
Digital photomicrographs showing the relative colocalization of α1b adrenergic receptor (α1b) mRNA with mRNA markers for serotonin (A) or GABA (B). DIG-labeled cells were photographed under brightfield illumination (dark gray cells), whereas 35S-labeled cells were photographed under darkfield illumination (clusters of white grains). The photographs were combined in Adobe Photoshop (see Materials and Methods). Black arrows denote double-labeled cells, and white arrows denote single-labeled cells. A: Shows DIG-5HT-T (serotonin transporter, used as a marker for serotonergic cells) with [35S]α1b at a midrostral level, dorsal part. Note the extensive colocalization, although a number of cells expressing α1b mRNA did not express 5HT-T mRNA. B: Shows DIG-GAD65/67 (glutamic acid decarboxylase, isoforms 65 and 67, used as a marker for GABAergic cells) with [35S]α1b at a midrostral level, dorsal/lateral part. Although many cells were singly labeled, a number of cells expressed both GAD65/67 and α1b mRNAs. Scale bar = 100 μm.
Dual in situ hybridization with a probe directed against the full-length CRF-R1 receptor with 5HT-T mRNA suggested that both a few 5HT-T mRNA-containing cells and a few non-5HT-T mRNA-containing cells expressed CRF-R1 mRNA (Fig. 5F). However, expression levels of CRF-R1 mRNA were so low that we were unable to perform a quantitative analysis with any confidence. No further attempts were made to colocalize CRF-R1 mRNA with GAD65/67 or TH mRNAs. CRF-R2 mRNA, however, showed a distinct rostral-caudal pattern of colocalization. At rostral and midrostral levels, expression of CRF-R2 mRNA was exclusively confined to the serotonin cells of the DR (Table 1, Fig. 9A,B). However, at increasingly more caudal levels, the CRF-R2 receptor was increasingly expressed in GABAergic cells, with approximately 50% of CRF-R2 mRNA expressed in GABAergic cells at the caudal level (Table 1, Fig. 9C). In addition, there was substantial colocalization of CRF-R2 and GAD65/67 mRNAs ventral to the DR at midcaudal to caudal levels. The double-labeled cells formed a bilateral strip on either side of the midline and are probably part of the paramedian raphe nucleus, also known as the lateral part of the superior central nucleus (Swanson, 1992). Finally, clusters of cells doubly labeled for CRF-R2 and GAD65/67 mRNAs were observed immediately lateral to the ventral DR at caudal levels, which appeared to be part of the dorsal tegmental nucleus, pericentral region (DTgP). No colocalization of CRF-R2 mRNA and TH mRNA was observed (Table 1).
Discussion
General distribution patterns: serotonin, GABA, catecholamines
The general distribution patterns reported here are largely in agreement with previous studies. The widespread distribution of serotonergic neurons, throughout all subdivisions and at all levels, has been widely reported (Steinbusch, 1981; Steinbusch et al., 1981; Kirifides et al., 2001; Serrats et al., 2003). In contrast to that found in primates, in which most serotonergic neurons are found in the ventrolateral part (Hornung and Fritschy, 1988; McLaughlin et al., 1996; Charara and Parent, 1998), the distribution of serotonergic neurons in rats is centered along the midline of the DR. Early immunohistochemical studies had suggested that GABAergic and serotonergic neurons were intermingled throughout the DR (Nanopoulos et al., 1982; Belin et al., 1983). However, more recent immunohistochemical studies have suggested that GABA is expressed in the periphery of the nucleus (Ford et al., 1995; Stamp and Semba, 1995). These latter studies are in better agreement with the present data demonstrating that GABAergic neurons mostly flank the midline serotonergic distribution in dorsal and ventral regions, whereas these two subtypes of neurons are more evenly distributed within the lateral wings. Several studies have shown both TH mRNA and TH-like immunoreactivity in the rat DR (Descarries et al., 1986; Seroogy et al., 1989; Stratford and Wirtshafter, 1990; Kirifides et al., 2001). The pattern of TH mRNA is similar to that seen previously for protein (Kirifides et al., 2001), with a largely medial and rostral distribution. However, we were able to detect some cells that expressed TH mRNA in the lateral wings, albeit relatively few, in contrast to that reported for the protein (Kirifides et al., 2001).
General distribution patterns: 5HT-1A, α1b, CRF-R1, and CRF-R2 receptors
As expected, 5HT-1A and α1b ADR mRNAs were relatively abundant throughout the rostral-caudal extent and in all subdivisions of the DR (Wright et al., 1995; Day et al., 1997). In contrast, by using a 367 mer probe, we were unable to detect CRF-R1 mRNA reliably within the boundaries of the DR. The use of a probe against the full-length CRF-R1 mRNA was more sensitive and did detect some CRF-R1-positive cells scattered throughout the DR. The relative lack of CRF-R1 mRNA in the DR is somewhat surprising. For example, intra-DR administration of CRF in anesthetized rats has been reported to have inhibitory effects on DR neurons, at least at lower doses, and a selective CRF-R1 antagonist blocked these effects (Kirby et al., 2000). Immunohistochemical studies have demonstrated a substantial level of CRF-R1-like immunoreactivity in the DR of both the mouse (Chen et al., 2000) and the rat (Lowry et al., 2002; Roche et al., 2003). In contrast, in situ hybridization studies have been unable to detect CRF-R1 mRNA in mouse (Van Pett et al., 2000) and at best demonstrated low CRF-R1 mRNA levels in rat, even with a full-length cRNA probe (this study; Bonaz and Rivest, 1998; Van Pett et al., 2000). It is not clear at the present time why there is such a large discrepancy between mRNA and protein levels, but it does not appear to be due solely to a difference in cell body vs. terminal distribution, because immunohistochemical studies have detected numerous CRF-R1-positive cell bodies within the DR. Although appropriate controls were performed in the immunohistochemical studies, it is possible that the antibodies used in the immunohistochemical studies are cross-reacting to some extent with CRF-R2. There is the possibility that the mRNA pool is very stable with a very high translation rate that may result in the apparent mismatch between mRNA and protein levels. Finally, the possibility of a third CRF receptor that is also recognized by the antibodies used in the immunohistochemical studies cannot be ruled out. The possibility of a third CRF receptor has been raised previously with respect to other brain areas that are highly responsive to CRF but do not express significant levels of either CRF-R1 or CRF-R2 mRNA, such as the central nucleus of the amygdala (Chalmers et al., 1995; Bittencourt and Sawchenko, 2000; Van Pett et al., 2000).
CRF-R2 mRNA showed the greatest rostral-caudal differential in distribution, and the data are consistent with a topographic functional organization of the CRF system. The effect of CRF on DR activity has been the subject of increased attention recently. Aside from the inhibitory effect of CRF on serotonergic neurons described above, in vitro electrophysiological evidence has shown that CRF has a mainly excitatory effect on a subpopulation of serotonergic neurons, situated predominantly in the ventral and interfascicular regions of the caudal half of the DR (Lowry et al., 2000). Prior isolation housing and 5 days of repeated restraint stress enhanced the effects of CRF on serotonergic neurons in this region, suggesting that these neurons may be important in the modulation of stress responses (Lowry et al., 2000). The facts that 1) only a subset of DR neurons was responsive to CRF, 2) these neurons predominated in a relatively discrete region within the DR, and 3) the responsiveness of these neurons changed after exposure to stress suggested that the DR may have a more topographic functional organization than was previously thought (Rueter et al., 1997; Jacobs and Fornal, 1999). From the pattern of mRNA distribution, it seems likely that the excitatory effect on the caudal serotonergic neurons observed by Lowry and colleagues (2000) was mediated by CRF type 2 receptors. These data are consistent with behavioral studies that have also suggested a topographic functional organization for CRF responsiveness, with CRF mimicking the effect of inescapable shock on escape behavior and fear conditioning when injected into the caudal half of the DR, but not the rostral half (Hammack et al., 2002). This effect appears to be mediated by CRF type 2 receptors, and intra-DR administration of a specific CRF-R2 antagonist blocks the behavioral consequences of inescapable shock (Hammack et al., 2003).
Colocalization studies: serotonin, GABA, and catecholamines
The data presented in the current study demonstrate that serotonin and GABA are rarely coexpressed in the same neuron in any subdivision of the rat DR. Although early studies had suggested significant colocalization of GABA and serotonin in the DR (Nanopoulos et al., 1982; Belin et al., 1983; Harandi et al., 1987), our data are in good agreement with a more recent immunohistochemical study that showed virtually no colocalization of these neurotransmitters in the rat DR (Stamp and Semba, 1995). This is consistent with the idea that intrinsic GABAergic neurons form local circuits to inhibit serotonergic neuron activity (Liu et al., 2000). The lack of colocalization of TH mRNA with serotonin transporter mRNA is also in good agreement with immunohistochemical data (Kirifides et al., 2001). The present data further demonstrate that GABA and catecholamines rarely coexist in the rat DR. To our knowledge, this has not been shown directly before, although similar results have been obtained in medullary catecholaminergic regions (Stornetta and Guyenet, 1999). Hence, it appears that, for the most part, serotonergic, GABAergic, and catecholaminergic neurons form discrete neuronal populations in the rat DR.
Colocalization studies: 5HT-1A, α1b, CRF-R1, and CRF-R2 receptors in identified neurons
The widespread expression of 5HT-1A receptor mRNA on virtually every serotonergic neuron is consistent with its role as an inhibitory autoreceptor (Sprouse and Aghajanian, 1987; Stamford et al., 2000). Recent electrophysiological and immunohistochemical evidence has indicated that the 5HT-1A receptor is expressed on nonserotonergic neurons also (Kirby et al., 2003). Data presented here are consistent with this idea and demonstrate that one population of nonserotonergic neurons that expresses 5HT-1A receptor mRNA is GABAergic. Approximately 100% of 5HT-1A receptor mRNA was colocalized in either serotonin- or GABAergic cells, so it is likely that the GABAergic expression represents most, if not all, the nonserotonergic 5HT-1A receptor colocalization. The general pattern of nonserotonergic 5HT-1A receptor distribution was somewhat similar between the studies, with the highest number of cells observed in the lateral wings. However, the extent to which the 5HT-1A receptor is colocalized in serotonin vs. nonserotonin cells is different between the studies. Specifically, we found that 5HT-1A receptor mRNA was expressed in virtually every serotonergic neuron, irrespective of subdivision, whereas Kirby and colleagues (2003) found a significant number of serotonergic neurons that did not express the 5HT-1A receptor, particularly in the lateral wings and caudal dorsal subdivision. Similarly, greater numbers (up to 40–50%, depending on the subdivision) of 5HT-1A receptors were expressed on nonserotonergic neurons (Kirby et al., 2003) than were found in the current study (up to 10–15%, depending on the subdivision). The reasons for these differences are not clear at present but they may simply reflect a difference in sensitivity between immunohistochemical and in situ hybridization techniques. Despite the relatively low colocalization rate, the presence of 5HT-1A receptors on GABAergic as well as serotonergic neurons of the DR should be considered when defining a role for this receptor as purely an autoreceptor for DR serotonergic neurons.
Noradrenergic afferents to the DR exert a tonic excitatory effect on serotonergic neurons of the DR through an α1 ADR mechanism (Baraban and Aghajanian, 1980a,b, 1981; Yoshimura et al., 1985). The presence of α1b ADR mRNA on virtually every serotonergic neuron throughout the DR makes this receptor subtype a good candidate for this response. However, the presence of α1b ADR mRNA on a significant proportion of GABAergic DR cells suggests that GABAergic neurons may also be activated by noradrenergic input. It is not clear at present whether this input results in tonic excitation, as has recently been suggested (Kirby et al., 2003), but it raises the possibility that, under some circumstances, activation of the α1b ADR subtype in the DR could actually result in inhibition of serotonergic neurons through local GABAergic circuits.
Dual in situ hybridization with a full-length probe for CRF-R1 mRNA and a DIG-labeled probe for 5HT-T mRNA suggested that both a few 5HT-T mRNA-containing cells and a few non-5HT-T mRNA-containing cells expressed CRF-R1 mRNA. However, because of the low levels of this receptor mRNA within individual cells, a quantitative analysis could not be performed with any confidence, and no further attempts were made to colocalize CRF-R1 mRNA with GAD65/67 or TH mRNAs. As was discussed above, previous immunohistochemical studies have detected much higher levels of CRF-R1 protein in the DR, which has been shown to be colocalized with serotonin (Lowry et al., 2002) or with GABA (Roche et al., 2003).
The colocalization pattern of CRF-R2 mRNA showed the highest topographic variation, being expressed exclusively in serotonergic cells at rostral to midrostral levels but becoming increasingly expressed in GABAergic cells at increasingly caudal levels of the DR. In addition, clusters of cells doubly labeled for CRF-R2 and GAD65/67 mRNA were observed in the immediate vicinity of the caudal DR in the DTgP and also widely in the paramedian raphe in the caudal half of the nucleus. To our knowledge, CRF-R2 mRNA expression has not been observed in the DTgP previously (Van Pett et al., 2000), and colocalization of CRF-R2 with a marker for cholinergic neurons would be useful in confirming this observation. The functional significance of a shift in colocalization patterns at more caudal levels is not clear, and this can make interpretation of data obtained from intra-DR injection studies more difficult. For example, Hammack and colleagues (2002, 2003) have found the greatest effects of CRF and urocortin II (a selective CRF-R2 agonist) in producing interference with escape behavior and potentiation of fear conditioning to occur with injection in the caudal half of the DR. Injection of specific CRF-R2 antagonists into the same region also inhibits the interference of escape behavior and potentiation of fear conditioning following exposure to uncontrollable stress (Hammack et al., 2003). CRF-R2 receptors are highly expressed in this region, and most are expressed in serotonergic neurons. This is consistent with the idea that activation of CRF-R2 receptors is important in mediating the changes in serotonergic function in the DR that are thought to be critical for producing the behavioral consequences of uncontrollable stress (learned helplessness). The present data suggest that this may be a direct effect mediated via CRF-R2 receptors on serotonergic DR neurons. However, the fact that some effective injections were centered at caudal levels, where an increasing number of neurons are doubly labeled for GAD65/67 and CRF-R2, suggests that a role for CRF-R2 and GABA in these behavioral effects cannot be excluded. Overall, the data presented here provide further evidence for the neurochemical heterogeneity of the rat DR and lend support to the idea that the DR has a functional topographic organization.
Acknowledgments
The authors thank Dr. Jose Amat and Scott Nebel for their assistance at the microscope.
Grant sponsor: National Institute of Mental Health; Grant number: MHO65327 (S.C.); Grant number: MHO50479 (S.F.M.).
Literature Cited
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980a;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of serotonergic neuronal firing by alpha-adrenoceptor antagonists: evidence against GABA mediation. Eur J Pharmacol. 1980b;66:287–294. doi: 10.1016/0014-2999(80)90461-6. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Noradrenergic innervation of serotonergic neurons in the dorsal raphe: demonstration by electron microscopic autoradiography. Brain Res. 1981;204:1–11. doi: 10.1016/0006-8993(81)90646-6. [DOI] [PubMed] [Google Scholar]
- Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol. 1998;275:R1438–1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Parent A. Chemoarchitecture of the primate dorsal raphe nucleus. J Chem Neuroanat. 1998;15:111–127. doi: 10.1016/s0891-0618(98)00036-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 [CRF(1)]-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- Descarries L, Berthelet F, Garcia S, Beaudet A. Dopaminergic projection from nucleus raphe dorsalis to neostriatum in the rat. J Comp Neurol. 1986;249:511–520. 484–485. doi: 10.1002/cne.902490407. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Gamrani H, Calas A, Belin MF, Aguera M, Pujol JF. High resolution radioautographic identification of [3H]GABA labeled neurons in the rat nucleus raphe dorsalis. Neurosci Lett. 1979;15:43–48. doi: 10.1016/0304-3940(79)91527-1. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Hammack SE, Will MJ, O'Connor KA, Deak T, Sparks PD, Watkins LR, Maier SF. Blockade of alpha1 adrenoreceptors in the dorsal raphe nucleus prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behav Brain Res. 2002;134:387–392. doi: 10.1016/s0166-4328(02)00061-x. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi M, Aguera M, Gamrani H, Didier M, Maitre M, Calas A, Belin MF. Gamma-aminobutyric acid and 5-hydroxytryptamine inter-relationship in the rat nucleus raphe dorsalis: combination of radioautographic and immunocytochemical techniques at light and electron microscopy levels. Neuroscience. 1987;21:237–251. doi: 10.1016/0306-4522(87)90336-8. [DOI] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM. Serotoninergic system in the brainstem of the marmoset: a combined immunocytochemical and three-dimensional reconstruction study. J Comp Neurol. 1988;270:471–487. doi: 10.1002/cne.902700402. [DOI] [PubMed] [Google Scholar]
- Irwin J, Suissa A, Anisman H. Differential effects of inescapable shock on escape performance and discrimination learning in a water escape task. J Exp Psychol Anim Behav Process. 1980;6:21–40. [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Kahn RS, van Praag HM, Wetzler S, Asnis GM, Barr G. Serotonin and anxiety revisited. Biol Psychiatry. 1988;23:189–208. doi: 10.1016/0006-3223(88)90091-1. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirifides ML, Simpson KL, Lin RC, Waterhouse BD. Topographic organization and neurochemical identity of dorsal raphe neurons that project to the trigeminal somatosensory pathway in the rat. J Comp Neurol. 2001;435:325–340. doi: 10.1002/cne.1033. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the midbrain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hathway NJA, Lightman SL. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. Distribution of corticotropin-releasing factor receptor 1 (CRFR1)- and CRFR2-immunoreactivity in limbic and caudal brainstem raphe nuclei. [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Kalman BA, Grahn RE. Chlordiazepoxide microinjected into the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behav Neurosci. 1994;108:121–130. doi: 10.1037//0735-7044.108.1.121. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin DP, Little KY, Lopez JF, Watson SJ. Expression of serotonin transporter mRNA in human brainstem raphe nuclei. Neuropsychopharmacology. 1996;15:523–529. doi: 10.1016/S0893-133X(96)00093-0. [DOI] [PubMed] [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982;232:375–389. doi: 10.1016/0006-8993(82)90281-5. [DOI] [PubMed] [Google Scholar]
- Osborne FH, Mattingly BA, Redmon WK, Osborne JS. Factors affecting the measurement of classically conditioned fear in rats following exposure to escapable vs. inescapable signaled shock. J Exp Psychol Anim Behav Process. 1975;1:364–373. doi: 10.1037//0097-7403.1.4.364. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Seligman ME. Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol. 1996;364:402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Heym J, Jacobs BL. Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats. Exp Neurol. 1984;83:302–317. doi: 10.1016/S0014-4886(84)90100-6. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Schalling M, Brene S, Dagerlind A, Chai SY, Hökfelt T, Persson H, Brownstein M, Huan R, Dixon J, Filer D, Schlessinger D, Goldstein M. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res. 1989;74:149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- Serrats J, Artigas F, Mengod G, Cortes R. GABAB receptor mRNA in the raphe nuclei: co-expression with serotonin transporter and glutamic acid decarboxylase. J Neurochem. 2003;84:743–752. doi: 10.1046/j.1471-4159.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacol Biochem Behav. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality? Trends Neurosci. 2000;23:459–465. doi: 10.1016/s0166-2236(00)01631-3. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol. 1981;77:157–174. [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol. 1999;407:367–380. [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res. 1990;511:173–176. doi: 10.1016/0006-8993(90)90239-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Higashi H, Nishi S. Noradrenaline mediates slow excitatory synaptic potentials in rat dorsal raphe neurons in vitro. Neurosci Lett. 1985;61:305–310. doi: 10.1016/0304-3940(85)90481-1. [DOI] [PubMed] [Google Scholar]


