Abstract
Background
Elevated total homocysteine (tHcy), a risk factor for many chronic diseases, can be remethylated to methionine by folate. Alternatively, tHcy can be metabolized by other 1-carbon nutrients, ie, betaine and its precursor, choline.
Objective
We aimed to assess the association between the dietary intakes of betaine and choline and the concentration of tHcy.
Design
We conducted a cross-sectional analysis in 1477 women by using linear regression models to predict mean fasting tHcy by intakes of of betaine and choline.
Results
tHcy was 8% lower in the highest quintile of total betaine + choline intake than in the lowest quintile, even after control for folate intake (P for trend = 0.07). Neither choline nor betaine intake individually was significantly associated with tHcy. Choline from 2 choline-containing compounds, glycerophosphocholine and phosphocholine, was inversely associated with tHcy. These inverse associations were more pronounced in women with folate intake < 400 μg/d than in those with intakes ≥400 μg/d (P for interaction = 0.03 for phosphocholine) and in moderate alcohol drinkers (≥15 g/d) than in nondrinkers or light drinkers (<15 g/d) (P for interaction = 0.02 for glycerophosphocholine and 0.04 for phosphocholine). The strongest dose response was seen in women with a low-methyl diet (high alcohol and low folate intake) (P for interaction = 0.002 for glycerophosphocholine and 0.001 for phosphocholine).
Conclusions
Total choline + betaine intake was inversely associated with tHcy, as was choline from 2 water-soluble choline-containing compounds. Remethylation of tHcy may be more dependent on the betaine pathway when methyl sources are low as a result of either inadequate folate intake or heavier alcohol consumption.
Keywords: Betaine, choline, folate, homocysteine, alcohol, low-methyl diet
INTRODUCTION
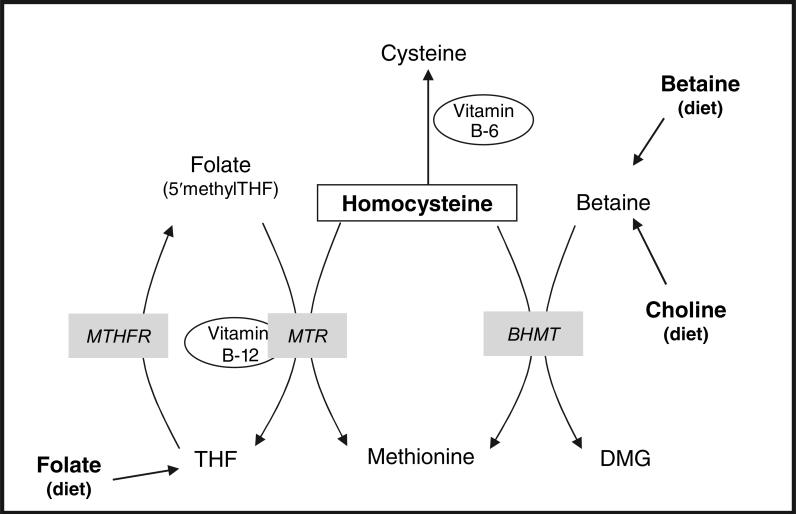
High total homocysteine (tHcy) concentrations have been associated with a greater risk of many chronic diseases, such as cardiovascular disease (1), cancer (2), cognitive decline (3), and bone fractures (4). Both dietary folate intake and folic acid supplementation reduce tHcy concentrations (5, 6); however, homocysteine also can be remethylated to methionine through a betaine-dependent pathway (Figure 1).
FIGURE 1.
Homocysteine remethylation by the folate and betaine pathways. THF, tetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase reductase; BHMT, betaine-homocysteine methyltransferase; DMG, dimethylglycine. Dietary folate can be converted into THF, which is metabolized by the MTHFR enzyme into 5,10-MTHF. In this form, folate can serve as a methyl donor to homocysteine by the MTR enzyme and its cofactor vitamin B-12. In this process, methionine is formed and 5,10-MTHF is metabolized back to THF, which may reenter the metabolic cycle. Betaine, which is obtained directly in the diet or through oxidation of dietary choline, can remethylate homocysteine with the BHMT enzyme, forming DMG as a byproduct.
The betaine-dependent remethylation pathway may become more crucial when folate availability is diminished (7), as a result of either low intake of folate or lower utilization of available folate pools. Additional alcohol consumption interferes with folate metabolism, potentially through the inhibition of methionine synthase, which remethylates homocysteine to methionine (8, 9). Chronic alcoholics have significantly higher plasma tHcy concentrations than do nondrinkers (10, 11). Moderate alcohol consumption in combination with low folate intake or a methyl-deficient diet has been associated with higher plasma tHcy, as well as greater risks of breast and colon cancer (12–14). Betaine may serve as the main methyl donor when the folate-dependent pathway is impaired.
Betaine can be obtained in the diet or through oxidation of its precursor choline. Choline is found in foods predominantly as phosphatidylcholine, which is commonly known as lecithin, but it is found in other forms within the diet. These choline-containing compounds are all interchangeable within the body, but the conversion of choline to betaine is irreversible (15).
Supplementation with either betaine (16, 17) or choline (18) reduces tHcy concentrations, but the doses of betaine (6 g/d) and choline (2.6 g/d) used in these short-term feeding studies are much greater than the amounts typically consumed in free-living populations. Plasma betaine is also inversely associated with plasma tHcy (19). Few studies have evaluated the intake of choline or betaine in humans, because of the lack of food-composition data for these nutrients. The food-composition database for choline and betaine was established a few years ago (20) and is now available for use in epidemiologic studies.
We examined the associations between intakes of betaine and its precursor, choline, and fasting tHcy concentrations. We also assessed whether these associations varied by intake of folate, alcohol, or a methyl-deficient diet.
SUBJECTS AND METHODS
The Nurses' Health Study
The Nurses' Health Study (NHS) is a prospective cohort of 121 700 female nurses aged 30−55 y at baseline in 1976. Participants biennially provided information on lifestyle and disease status via self-administered questionnaires. Between 1989 and 1990, 32 826 women in this cohort provided a blood sample. Methods for blood collection were described in detail elsewhere (21). The women in this analysis were healthy control subjects, were not currently using exogenous hormones, and provided a fasting blood specimen from separate nested case-control studies of cardiovascular disease (22), breast cancer (23), and colon neoplasia (24). Participants had no history of cancer (other than nonmelanoma skin cancer), stroke, myocardial infarction, angina, or revascularization surgery before the date of blood draw.
The Nurses' Health Study 2
The Nurses' Health Study 2 (NHS2) is a prospective cohort of 116 671 female nurses aged 25−42 y at baseline in 1989. Blood samples were obtained in 1996−1998 from 29 611 women. Methods of obtaining health and disease status information and of obtaining blood samples were similar to methods used in the NHS. In the present analysis, we included healthy controls from a nested case-control study of breast cancer. We also included a subset of healthy women, previously sampled on the basis of self-reported alcohol use, for a study of the association between alcohol and biological markers of cardiovascular disease. Further details on the selection process were published elsewhere (25). All women selected for this analysis were premenopausal, were not using exogenous hormones, provided a fasting blood specimen, and had no history of CVD, diabetes mellitus, gastric or duodenal ulcer, liver or gallbladder disease, or cancer (other than nonmelanoma skin cancer) before the date of the blood draw.
Assessment of diet
Dietary information is obtained every 4 y through a 131-item semiquantitative food-frequency questionnaire (FFQ). The reproducibility and validity of the FFQ have been documented elsewhere (26, 27). The participants were asked to indicate how often, on average, they had consumed specific food items during the previous year. Average nutrient intake was calculated from the FFQ by using nutrient values obtained from the Harvard University Food Composition Database, which is based on information from the US Department of Agriculture (USDA) and other sources.
Information on the choline and betaine concentrations of individual food items was obtained from the USDA database (Internet: http://www.ars.usda.gov/ba/bhnrc/ndl) and from values published by Zeisel et al (20). Foods contained the choline metabolite betaine, as well as multiple choline-containing compounds, which included water-soluble compounds (ie, free choline, glycerophosphocholine, and phosphocholine) and lipid-soluble compounds (ie, phosphatidylcholine and sphingomyelin). Total choline intake was the sum of free choline plus choline from each of the choline-containing compounds.
To minimize misclassification, we averaged nutrients calculated from the 3 most recent FFQs (1984, 1986, and 1990 for NHS and 1991, 1995, and 1999 for NHS2). Results were not appreciably different when we used only the most recent FFQs. All nutrient intakes were adjusted for total energy by using the residual method (28). Total intake of all vitamins was the sum of food and supplement sources.
Assays for plasma markers
Because this analysis was based on samples from several data-sets, some analytes were measured at separate laboratories by using different assay methods. Therefore, in all analyses, we controlled for laboratory batch. For samples from NHS and the breast cancer controls from NHS2, tHcy was measured by using HPLC at the Jean Meyer USDA Human Nutrition Research Center on Aging (Tufts University, Boston, MA). For the other NHS2 samples, tHcy concentrations were measured by using an immunoassay on an IMx analyzer (Abbott Laboratories, Abbott Park, IL). Quality-control samples (5%−10% of all samples), obtained from a plasma pool of healthy volunteers, were given indicator identifications and interspersed randomly among the specimens. The CVs were <10% for tHcy.
Exclusions
We excluded from our analysis women for whom information on nutrient intake or tHcy concentration was missing; we restricted our analysis to women who reported that they had fasted for ≥6 h before their blood samples were drawn. When assessing the interaction between alcohol and betaine or choline, we also excluded women for whom information on alcohol intake was missing (n = 42).
The final population for analysis consisted of 1477 women (867 in the NHS and 510 in the NHS2). The Institutional Review Board of the Harvard School of Public Health approved the study protocol.
Statistical analysis
We used linear regression to calculate least-squares mean concentrations of fasting tHcy (μmol/L) within each quintile of nutrient intake. Robust variance estimates were used to allow for valid statistical inference of linear regression models without the need for normal distribution assumptions (29). In multivariate models, we adjusted for age, smoking status, menopausal status, laboratory batch, coffee, total calories, alcohol, and the intakes of methionine, folate, and riboflavin. Further adjustment for the intakes of vitamins B-6 and B-12 did not appreciably alter the results. The intake of neither vitamin B-6 nor B-12 was significantly associated with tHcy in the population. Tests for linear trend were conducted by assigning the median value for each quintile of intake and treating this new variable as continuous.
To assess effect modification by folate intake, we created categorical interaction variables by cross-classifying folate intake (dichotomized by a cutoff of 400 μg/d) and choline or betaine intake (in quintiles). We entered 9 dummy variables into the model with a single referent category (highest quintile of choline or betaine intake and high folate intake). To test formally for interaction, we created a product term of categorized folate intake (binary) and the choline predictor (continuous) and used a likelihood ratio test, comparing the model with and without the interaction term. We performed similar analyses to examine effect modification by alcohol (0, 0.1−14.9, and ≥15 g alcohol/d) and methyl diets (low-methyl: ≥15 g alcohol/d and < 400 μg folate/d; high-methyl: 0 g alcohol/d and ≥ 400 μg folate/d; and intermediate-methyl). Statistical analyses were conducted by using SAS software (version 9; SAS Institute, Cary, NC). All P values are 2-tailed.
RESULTS
The top 5 food sources by percentage contribution to overall intakes of betaine and choline are shown in Table 1. The most common food sources were similar in the 2 populations of women. Both animal and plant-based products were sources of choline from the water-soluble compounds glycerophosphocholine, phosphocholine, and free choline, whereas animal products were the main source of choline from the lipid-soluble compounds phosphatidylcholine and sphingomyelin. Animal products were the main source of total choline, because the most common form of choline in the diet is phosphatidylcholine. Betaine and choline were not highly correlated (r = 0.14, P < 0.0001), because betaine is found mainly in grain products. The foods with the highest concentration of betaine are wheat bran (1339 mg/100 g) and wheat germ (1241 mg/100 g) (20).
TABLE 1.
Food sources (% contribution to total intake) of betaine, choline, and choline-containing compounds within the Nurses' Health Study (NHS) and NHS21
| NHS |
NHS2 |
|||
|---|---|---|---|---|
| Food source | Proportion | Food source | Proportion | |
| % | % | |||
| Betaine | Spinach | 31.3 | Spinach | 24.8 |
| Cold cereal | 10.6 | Dark bread | 11.9 | |
| Pasta | 9.6 | Pasta | 11.6 | |
| Dark bread | 7.6 | Pretzels | 9.4 | |
| White bread | 6.0 | Cold cereal | 7.9 | |
| Total choline | Milk | 17.9 | Milk | 12.0 |
| Chicken | 12.3 | Beef | 11.0 | |
| Beef | 11.2 | Chicken | 10.4 | |
| Eggs | 7.4 | Eggs | 8.2 | |
| Pork | 3.5 | Pork | 4.1 | |
| Free choline | Coffee | 12.6 | Milk | 10.2 |
| Milk | 8.9 | Coffee | 8.8 | |
| Potatoes | 7.5 | Potato | 5.7 | |
| Chicken | 4.0 | Tomatoes | 4.82 | |
| White fish | 3.1 | Beans | 2.9 | |
| Choline from glycerophosphocholine | Milk | 39.0 | Milk | 38.4 |
| White fish | 6.8 | Yogurt | 6.9 | |
| Coffee | 4.8 | Pork | 4.9 | |
| Pork | 4.3 | Coffee | 3.6 | |
| Yogurt | 3.7 | White fish | 3.0 | |
| Choline from phosphocholine | Milk | 24.8 | Milk | 26.5 |
| Chicken | 12.3 | Chicken | 12.2 | |
| Broccoli | 10.7 | Broccoli | 9.5 | |
| Tomatoes | 8.22 | Tomatoes | 7.32 | |
| Potatoes | 3.5 | Potatoes | 2.9 | |
| Choline from phosphatidylcholine | Beef | 18.7 | Beef | 18.7 |
| Chicken | 17.3 | Egg | 16.4 | |
| Eggs | 14.4 | Chicken | 14.9 | |
| Pork | 5.0 | Pork | 5.9 | |
| Liver | 3.53 | Bran muffin | 3.0 | |
| Choline from sphingomyelin | Chicken | 38.7 | Chicken | 35.2 |
| Beef | 19.2 | Beef | 19.4 | |
| Milk | 9.7 | Milk | 10.0 | |
| Eggs | 5.4 | Eggs | 6.5 | |
| Pork | 3.8 | Pork | 4.8 | |
Data are from the most recent food-frequency questionnaire for NHS (1990) and NHS2 (1999).
Includes tomato sauce and tomato juice.
Includes chicken and beef liver.
Among the 1477 women in this analysis, the median intake of total choline and of betaine was 323 and 189 mg/d, respectively. Overall, women who consumed more total choline tended to exercise more, smoke less, and have higher intakes of folate and other B vitamins than did women who consumed less total choline (Table 2). For most characteristics, the trends across quintiles of betaine intake followed a similar pattern. However, some characteristics had slightly different patterns. For example, women tended to be younger across increasing quintiles of betaine intake, but not choline intake. BMI tended to be lower across increasing quintiles of betaine intake, but higher with greater choline intake.
TABLE 2.
Selected characteristics among 1477 women by quintile of energy-adjusted total choline and betaine intakes1
| Total choline intake (mg/d)2 |
Betaine intake (mg/d) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Quintile 1 <283 | Quintile 3 311−334 | Quintile 5 >361 | P for trend | Quintile 1 <139 | Quintile 3 173−206 | Quintile 5 >252 | P for trend | |
| Median intake (mg/d) | 265 | 323 | 385 | 116 | 189 | 295 | ||
| Age (y)3 | 52 ± 0.64 | 52 ± 0.6 | 54 ± 0.6 | 0.14 | 55 ± 0.5 | 52 ± 0.6 | 49 ± 0.6 | < 0.001 |
| BMI (kg/m2) | 24.6 ± 0.3 | 25.3 ± 0.3 | 25.5 ± 0.3 | < 0.001 | 25.6 ± 0.3 | 24.6 ± 0.3 | 24.7 ± 0.3 | < 0.001 |
| Smoker (%) | 14 | 14 | 11 | 0.63 | 17 | 13 | 6 | < 0.001 |
| Physical activity (MET-h/wk) | 14.1 ± 1.0 | 17.4 ± 1.1 | 21.0 ± 1.5 | < 0.001 | 12.6 ± 0.9 | 18.8 ± 1.2 | 22.4 ± 1.6 | < 0.001 |
| Systolic blood pressure (mm Hg) | 123 ± 0.1 | 123 ± 0.1 | 124 ± 0.1 | 0.30 | 123 | 123 | 123 | 0.11 |
| Regular aspirin use (%) | 32 | 34 | 30 | 0.88 | 28 | 36 | 34 | 0.19 |
| Postmenopausal (%) | 41 | 41 | 40 | 0.34 | 41 | 42 | 42 | 0.99 |
| Nutrient intake | ||||||||
| Betaine (mg/d) | 181 ± 4.2 | 209 ± 4.8 | 215 ± 5.1 | < 0.001 | 113 ± 1.0 | 189 ± 0.6 | 315 ± 4.1 | < 0.001 |
| Choline (mg/d) | 258 ± 1.4 | 323 ± 0.4 | 402 ± 3.0 | < 0.001 | 314 ± 3.6 | 323 ± 2.9 | 339 ± 3.8 | < 0.001 |
| Folate (μg/d) | 397 ± 10.3 | 445 ± 11.7 | 526 ± 13.9 | < 0.001 | 361 ± 9.7 | 452 ± 10.6 | 537 ± 13.6 | < 0.001 |
| Riboflavin (mg/d) | 4.3 ± 0.5 | 4.7 ± 0.4 | 6.5 ± 0.5 | < 0.001 | 3.7 ± 0.3 | 5.6 ± 0.4 | 5.7 ± 0.5 | 0.001 |
| Vitamin B-6 (mg/d) | 7.7 ± 1.0 | 8.6 ± 1.2 | 12.7 ± 1.4 | < 0.001 | 8.3 ± 1.2 | 7.7 ± 0.8 | 12.4 ± 1.5 | 0.03 |
| Vitamin B-12 (μg/d) | 9.7 ± 1.1 | 10.8 ± 0.5 | 14.7 ± 0.7 | < 0.001 | 10.3 ± 0.6 | 12.2 ± 1.0 | 12.8 ± 0.7 | 0.003 |
| Methionine (g/d) | 1.5 ± 0.01 | 1.8 ± 0.01 | 2.1 ± 0.02 | < 0.001 | 1.8 ± 0.02 | 1.8 ± 0.02 | 1.9 ± 0.02 | < 0.001 |
| Coffee (cups/d) | 1.7 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 0.003 | 1.9 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.81 |
| Alcohol (g/d) | 6.5 ± 0.6 | 7.7 ± 0.6 | 5.8 ± 0.6 | 0.28 | 4.9 ± 0.6 | 7.3 ± 0.7 | 6.5 ± 0.5 | 0.30 |
n = 295 in each quintile of each intake.
Total choline intake was the sum of free choline and choline from glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin.
All characteristics other than age were standardized for age.
x̄ ± SE (all such values).
After adjustment for age, both betaine intake and total choline intake were inversely associated with fasting tHcy (P for trend < 0.001 for both) (Table 3). Adjustment for diet and other lifestyle factors did not greatly attenuate these associations. However, after further adjustment for intake of folate and riboflavin, neither betaine nor total choline was associated with fasting tHcy (P for trend = 0.21 for betaine and 0.44 for choline). We also examined the sum of choline + betaine intake, the total source of methyl donors for the betaine-dependent pathway, which metabolically may be the most relevant measure. Total intake of choline + betaine was inversely associated with tHcy, even after adjustment for folate and riboflavin (Table 3). In multivariate models, tHcy was 8% lower in the highest quintile of choline + betaine intake than in the lowest quintile (P = 0.07).
TABLE 3.
Adjusted mean values of total homocysteine (tHcy) among 1477 women by quintile (Q) of energy-adjusted betaine, total choline, and total choline + betaine intakes1
| Nutrient intake |
Percentage change (Q1—Q5) | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend2 | ||
| % | |||||||
| Betaine3 | |||||||
| Median intake (mg/d) | 116 | 157 | 189 | 224 | 295 | ||
| Range | < 139 | 140−173 | 173−206 | 207−252 | >252 | ||
| Age-adjusted tHcy | 11.5 ± 0.34 | 10.6 ± 0.25 | 10.7 ± 0.25 | 10.1 ± 0.25 | 10.1 ± 0.35 | −12 | 0.001 |
| Model 16 | 11.5 ± 0.3 | 10.6 ± 0.25 | 10.7 ± 0.25 | 10.1 ± 0.25 | 10.2 ± 0.35 | −11 | 0.003 |
| Model 27 | 11.2 ± 0.3 | 10.4 ± 0.25 | 10.8 ± 0.2 | 10.2 ± 0.23 | 10.5 ± 0.3 | −6 | 0.21 |
| Total choline8,9 | |||||||
| Median intake (mg/d) | 265 | 297 | 323 | 345 | 385 | ||
| Range | < 283 | 283−310 | 311−334 | 334−361 | >361 | ||
| Age-adjusted tHcy | 11.4 ± 0.3 | 10.7 ± 0.25 | 10.7 ± 0.25 | 10.2 ± 0.25 | 10.1 ± 0.35 | −11 | < 0.001 |
| Model 16 | 11.4 ± 0.3 | 10.6 ± 0.25 | 10.6 ± 0.2 | 10.3 ± 0.25 | 10.1 ± 0.35 | −11 | 0.003 |
| Model 27 | 11.0 ± 0.3 | 10.4 ± 0.2 | 10.6 ± 0.2 | 10.4 ± 0.2 | 10.6 ± 0.3 | −4 | 0.44 |
| Choline + betaine (mg/d)10 | |||||||
| Median intake (mg/d) | 409 | 471 | 516 | 561 | 646 | ||
| Range | < 446 | 446−494 | 494−539 | 539−596 | >597 | ||
| Age-adjusted tHcy | 11.8 ± 0.3 | 10.7 ± 0.25 | 10.6 ± 0.35 | 9.9 ± 0.25 | 10.0 ± 0.35 | −15 | < 0.001 |
| Model 16 | 11.8 ± 0.3 | 10.6 ± 0.25 | 10.7 ± 0.35 | 9.9 ± 0.25 | 10.0 ± 0.35 | −15 | < 0.001 |
| Model 27 | 11.3 ± 0.3 | 10.4 ± 0.25 | 10.7 ± 0.3 | 10.1 ± 0.25 | 10.4 ± 0.35 | −8 | 0.07 |
n = 295 in each quintile unless stated otherwise.
Linear regression coefficient for continuous variable was based on the median value from each quintile.
n = 296 in Q2 and Q4.
x̄ ± SEM (all such values).
Significantly different from Q1 according to linear regression models, P < 0.05 (Wald test).
Model 1 was adjusted for age, smoking status, menopausal status, laboratory batch, coffee, total calories, and intakes of methionine and alcohol.
Model 2 was adjusted as Model 1 plus for intakes of folate and riboflavin.
n = 296 in Q2 and Q4.
Total choline intake was the sum of free choline and choline from glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin.
n = 296 in Q1 and Q5.
Choline from glycerophosphocholine and that from phosphocholine were inversely associated with tHcy (Table 4). The tHcy concentration was 17% lower in the highest quintile of glycerophosphocholine intake than in the lowest quintile, after adjustment for potential confounders (model 1). This association was attenuated but remained statistically significant after adjustment for the intakes of folate and riboflavin (10% lower in the highest than in the lowest quintile). Similarly, tHcy was 17% lower in the highest quintile of phosphocholine intake than in the lowest quintile and 8% lower after adjustment for folate and riboflavin. Free choline was not associated with lower tHcy concentrations after adjustment for folate or B vitamins (P for trend = 0.47). The lipid-soluble choline-containing compounds tended toward a positive association with tHcy (P = 0.10 for phosphatidylcholine and 0.02 for sphingomyelin), although, after control for folate and riboflavin, these positive associations were no longer significant.
TABLE 4.
Adjusted mean values of total homocysteine (tHcy) by quintile (Q) of energy-adjusted choline-containing compounds1
| Nutrient intake |
Percentage change (Q1—Q5) | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend2 | ||
| % | |||||||
| Water-soluble | |||||||
| Choline from glycerophosphocholine3 | |||||||
| Median intake (mg/d) | 34.3 | 43.6 | 51.1 | 61.7 | 80.0 | ||
| Range | < 39.3 | 39.4−47.3 | 47.4−56.3 | 56.3−68.9 | >68.9 | ||
| Age-adjusted tHcy | 11.6 ± 0.34 | 11.1 ± 0.3 | 10.5 ± 0.25 | 10.4 ± 0.35 | 9.5 ± 0.25 | −18 | < 0.001 |
| Model 16 | 11.6 ± 0.3 | 11.0 ± 0.3 | 10.5 ± 0.25 | 10.3 ± 0.35 | 9.6 ± 0.25 | −17 | < 0.001 |
| Model 27 | 11.1 ± 0.3 | 10.8 ± 0.3 | 10.5 ± 0.2 | 10.6 ± 0.3 | 10.0 ± 0.25 | −10 | < 0.01 |
| Choline from phosphocholine8 | |||||||
| Median intake (mg/d) | 10.2 | 12.5 | 14.3 | 16.6 | 20.3 | ||
| Range | < 11.5 | 11.5−13.4 | 13.4−15.4 | 15.4−18.0 | > 18.0 | ||
| Age-adjusted tHcy | 11.6 ± 0.3 | 11.2 ± 0.3 | 10.9 ± 0.3 | 9.9 ± 0.25 | 9.4 ± 0.25 | −19 | < 0.001 |
| Model 16 | 11.5 ± 0.3 | 11.2 ± 0.3 | 10.9 ± 0.3 | 9.9 ± 0.25 | 9.5 ± 0.25 | −17 | < 0.001 |
| Model 27 | 10.9 ± 0.3 | 11.0 ± 0.3 | 11.0 ± 0.3 | 10.1 ± 0.25 | 10.0 ± 0.25 | −8 | 0.001 |
| Free choline9 | |||||||
| Median intake (mg/d) | 58.5 | 67.5 | 73.8 | 80.8 | 92.8 | ||
| Range | < 63.6 | 63.6−71.0 | 71.0−77.4 | 77.4−85.8 | >85.9 | ||
| Age-adjusted tHcy | 11.0 ± 0.2 | 10.8 ± 0.2 | 10.7 ± 0.3 | 10.3 ± 0.25 | 10.2 ± 0.25 | −7 | 0.009 |
| Model 16 | 11.3 ± 0.3 | 10.9 ± 0.2 | 10.6 ± 0.3 | 10.2 ± 0.35 | 10.0 ± 0.35 | −12 | < 0.001 |
| Model 27 | 10.7 ± 0.3 | 10.6 ± 0.2 | 10.8 ± 0.3 | 10.4 ± 0.2 | 10.5 ± 0.3 | −2 | 0.47 |
| Lipid-soluble | |||||||
| Choline from phosphatidylcholine10 | |||||||
| Median intake (mg/d) | 120 | 140 | 157 | 175 | 207 | ||
| Range | < 131 | 131−148 | 148−164 | 164−187 | > 187 | ||
| Age-adjusted tHcy | 10.7 ± 0.3 | 10.7 ± 0.2 | 10.4 ± 0.3 | 10.4 ± 0.2 | 10.8 ± 0.3 | 1 | 0.99 |
| Model 16 | 10.3 ± 0.3 | 10.6 ± 0.2 | 10.5 ± 0.3 | 10.7 ± 0.2 | 11.0 ± 0.3 | 7 | 0.10 |
| Model 27 | 10.5 ± 0.3 | 10.5 ± 0.2 | 10.5 ± 0.3 | 10.5 ± 0.2 | 11.0 ± 0.3 | 5 | 0.17 |
| Choline from sphingomyelin11 | |||||||
| Median intake (mg/d) | 13.4 | 16.1 | 18.2 | 20.4 | 23.7 | ||
| Range | < 14.9 | 14.9−17.2 | 17.2−19.3 | 19.3−21.9 | >21.9 | ||
| Age-adjusted tHcy | 10.8 ± 0.3 | 10.8 ± 0.3 | 10.5 ± 0.3 | 10.5 ± 0.2 | 10.5 ± 0.3 | −3 | 0.32 |
| Model 16 | 9.9 ± 0.3 | 10.4 ± 0.3 | 10.4 ± 0.3 | 11.0 ± 0.35 | 11.3 ± 0.45 | 14 | 0.02 |
| Model 27 | 10.5 ± 0.4 | 10.6 ± 0.3 | 10.3 ± 0.3 | 10.7 ± 0.3 | 11.0 ± 0.3 | 5 | 0.39 |
n = 295 in each quintile unless stated otherwise.
Linear regression coefficient for continuous variable was based on the median value from each quintile.
n = 296 in Q3 and Q4.
x̄ ± SEM (all such values).
Significantly different from Q1 according to linear regression models, P < 0.05 (Wald test).
Model 1 was adjusted for age, smoking status, menopausal status, laboratory batch, coffee, total calories, and intakes of methionine and alcohol.
Model 2 was adjusted as model 1 plus for intakes of folate and riboflavin.
n = 297 in Q1, 293 in Q2, and 296 in Q3 and Q4.
n = 296 in Q1, Q3, and Q4 and 294 in Q2.
n = 296 in Q2 and Q5.
n = 297 in Q3 and Q5 and 293 in Q4.
The intake of folate modified the association between choline from phosphocholine intake and tHcy concentration (P for interaction = 0.03) (Table 5). A greater intake of phosphocholine was associated with significantly lower tHcy concentrations only in women who had low folate intake (ie, <400 μg/d). The tHcy concentration was 15% lower in the highest quintile of phosphocholine intake than in the lowest quintile among women with low folate intake; among women with high folate intake (≥400 μg/d), tHcy was 4% lower in the same comparison. The tHcy concentration did not differ significantly between folate groups within the highest quintile of phosphocholine intake. When we further adjusted for folate intake—to remove any potential residual confounding within strata of folate—the results did not change (data not shown). This interaction was not significant for choline from glycerophosphocholine (P for interaction = 0.15).
TABLE 5.
Adjusted mean values of total homocysteine (tHcy) among 1477 women by quintile (Q) of energy-adjusted glycerophosphocholine and phosphocholine within categories of folate intake and alcohol intake1
| Nutrient intake |
Percentage change (Q1—Q5) | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for interaction | ||
| % | |||||||
| Folate intake (μg/d) | |||||||
| Choline from glycerophosphocholine2 | |||||||
| Median intake (mg/d) | 34.3 | 43.6 | 51.1 | 61.7 | 80.0 | ||
| <400 μg/d (n = 731) | 12.0 ± 0.53,4 | 11.2 ± 0.44 | 10.8 ± 0.3 | 11.4 ± 0.54 | 10.2 ± 0.3 | −15 | 0.15 |
| ≥400 μg/d (n = 746) | 10.1 ± 0.3 | 10.5 ± 0.34 | 10.2 ± 0.3 | 10.0 ± 0.3 | 9.7 ± 0.3 | −4 | |
| Choline from phosphocholine2 | |||||||
| Median intake (mg/d) | 10.2 | 12.5 | 14.3 | 16.6 | 20.3 | ||
| <400 μg/d (n = 731) | 11.7 ± 0.44 | 11.8 ± 0.54 | 11.5 ± 0.54 | 10.3 ± 0.3 | 9.9 ± 0.4 | −15 | 0.03 |
| ≥400 μg/d (n = 746) | 10.1 ± 0.3 | 10.1 ± 0.3 | 10.5 ± 0.34 | 9.9 ± 0.2 | 9.7 ± 0.3 | −4 | |
| Alcohol intake (g/d) | |||||||
| Choline from glycerophosphocholine5 | |||||||
| Median intake (mg/d) | 34.3 | 43.6 | 51.1 | 61.7 | 80.0 | ||
| 0 g/d (n = 471) | 10.3 ± 0.3 | 10.5 ± 0.5 | 10.3 ± 0.3 | 10.5 ± 0.4 | 9.8 ± 0.3 | −5 | |
| 0.1−14.9 g/d (n = 768) | 11.0 ± 0.4 | 10.5 ± 0.2 | 10.4 ± 0.3 | 10.7 ± 0.4 | 10.1 ± 0.3 | −8 | 0.02 |
| ≥15 g/d (n = 196) | 14.2 ± 1.66 | 12.7 ± 1.6 | 11.4 ± 0.7 | 11.1 ± 0.5 | 10.3 ± 0.6 | −27 | |
| Choline from phosphocholine5 | |||||||
| Median intake (mg/d) | 10.2 | 12.5 | 14.3 | 16.6 | 20.3 | ||
| 0 g/d (n = 471) | 9.8 ± 0.3 | 10.7 ± 0.4 | 11.3 ± 0.56 | 9.8 ± 0.3 | 9.6 ± 0.3 | −2 | |
| 0.1−14.9 g/d (n = 768) | 10.9 ± 0.46 | 10.5 ± 0.2 | 11.1 ± 0.56 | 10.3 ± 0.2 | 10.0 ± 0.3 | −8 | 0.04 |
| ≥15 g/d (n = 196) | 13.1 ± 1.06 | 13.3 ± 1.56 | 10.9 ± 0.5 | 10.5 ± 0.5 | 10.7 ± 0.6 | −18 | |
| Free choline5 | |||||||
| Median intake (mg/d) | 58.5 | 67.5 | 73.8 | 80.8 | 92.8 | ||
| 0 g/d (n = 471) | 10.1 ± 0.3 | 10.3 ± 0.3 | 10.8 ± 0.4 | 9.7 ± 0.3 | 10.6 ± 0.6 | 5 | |
| 0.1−14.9 g/d (n = 768) | 10.5 ± 0.4 | 10.7 ± 0.3 | 10.2 ± 0.3 | 10.6 ± 0.4 | 10.7 ± 0.3 | 2 | 0.07 |
| ≥15 g/d (n = 196) | 14.8 ± 2.36 | 11.1 ± 0.8 | 13.7 ± 1.86 | 11.5 ± 0.66 | 10.6 ± 0.4 | −28 | |
Linear regression model was adjusted for age, smoking status, menopausal status, laboratory batch, coffee, total calories, and intakes of methionine and riboflavin.
Also adjusted for alcohol intake.
x̄ ± SEM (all such values).
Significantly different from Q5 of choline-containing compound and folate ≥400 μg/d category based on linear regression models, P < 0.05 (Wald test).
Also adjusted for folate intake.
Significantly different from Q1 of choline-containing compound and 0 g/d alcohol based on linear regression models, P < 0.05 (Wald test).
Alcohol also modified the association between choline from glycerophosphocholine and tHcy (P for interaction = 0.02) (Table 5). High intake of glycerophosphocholine was associated with lower tHcy only among women in the top category of alcohol consumption (≥15 g alcohol/d). Among women in that category, tHcy was 27% lower in the highest quintile of glycerophosphocholine intake than in the lowest quintile. Among light drinkers and nondrinkers, tHcy was 8% and 5% lower in the highest quintile of glycerophosphochline intake than in the lowest quintile. Alcohol intake also modified the relations of choline from phosphocholine (P for interaction = 0.04) and free choline (P for interaction = 0.07) with tHcy (Table 5).
The intake of choline from glycerophosphocholine and that from phosphocholine were most strongly associated with tHcy among women who had a low-methyl diet (P for interaction = 0.002 for glycerophosphocholine and 0.001 for phosphocholine) (Figure 2). Among women with low-, intermediate-, and high-methyl diets, tHcy was 39%, 8%, and 1% lower in the highest quintile of glycerophospholine intake than in the lowest quintile and 18%, 9%, and 4% lower in the highest quintile of phosphocholine intake than in the lowest quintile.
FIGURE 2.

Adjusted mean plasma total homocysteine (tHcy) among 1477 women according to quintiles of energy-adjusted glycerophosphocholine and phosphocholine intake by methyl diet status (◆, high-methyl diet: 0 g/d alcohol + ≥400 μg/d folate; n = 227; ■, intermediate-methyl diet, n = 1126; ▲, low-methyl diet: ≥15 g/d alcohol + < 400 μg/d folate; n = 82). Mean tHcy values were calculated by linear regression models adjusted for age, smoking status, menopausal status, laboratory batch, coffee, total calories, and intakes of methionine and riboflavin. The SEs of mean tHcy within quintiles of glycerophosphocholine intake ranged from 0.4 to 0.6 for a high-methyl diet, from 0.2 to 0.3 for an intermediate-methyl diet, and from 1.3 to 2.8 for a low-methyl diet. The SEs of mean tHcy within quintiles of phosphocholine intake ranged from 0.4 to 0.6 for a high-methyl diet, from 0.2 to 0.4 for an intermediate-methyl diet, and from 1.0 to 3.0 for a low-methyl diet. Methyl diet status (in 3 categories) × glycerophosphocholine (continuous variable using median of quintiles) interaction, P = 0.002. Methyl diet status (in 3 categories) × phosphocholine (continuous variable using median of quintiles) interaction, P = 0.001. *Significantly different from the first quintile of the choline-containing compound within the low-methyl diet group based on linear regression models, P < 0.05 (Wald test).
The association between free choline and tHcy was somewhat modified by alcohol intake, but we did not observe any significant interactions between free choline and either folate intake or methyl diet status. The associations between tHcy and intakes of betaine, choline, choline + betaine, or choline from lipid-soluble choline compounds were not modified by folate intake, alcohol consumption, or low-methyl diets (data not shown).
DISCUSSION
In this cross-sectional analysis of 1477 healthy women, the sum of total choline + betaine intake was inversely associated with fasting tHcy, even after adjustment for other tHcy predictors, including folate. Individually, intakes of betaine and total choline were not associated with tHcy concentrations. Choline from 2 choline-containing compounds, glycerophosphocholine and phosphocholine, was significantly associated with lower tHcy, and these associations were modified by intakes of folate and alcohol. The association between total choline + betaine and tHcy concentrations was not modified by the intake of alcohol or folate.
Betaine intake was not associated with fasting tHcy in our population after adjustment for folate intake. In previous trials, supplementation with 1.5 g betaine/d (30) but not with 1 g betaine /d (31) significantly lowered fasting tHcy. Within the highest quintile, the median intake of betaine in this population was ≈300 mg/d, which may be too low to elicit a homocysteine-lowering response. Betaine intake may be associated with lower tHcy in a population with a higher intake of betaine. Betaine supplementation lowers postmethionine load tHcy more effectively than it lowers fasting tHcy (32), and plasma betaine was a stronger determinant of postmethionine load tHcy than was plasma folate (33). The betaine pathway may be more important in reducing increases in tHcy after a meal, when tHcy flux in the liver is high, whereas the folate pathway may be more crucial in maintaining fasting tHcy. The betaine-homocysteine methyltransferase enzyme in the betaine pathway is located mainly in human liver and kidney cells (34), whereas methylenetetrahydrofolate reductase in the folate pathway is located in most human tissues throughout the body (35).
Total choline was not associated with tHcy in the population of the present study. Although men on a choline-deficient diet (50 mg/d) in an earlier study had significantly elevated tHcy (36), the present study did not show significantly higher tHcy within the lowest quintile of total choline intake. The intake of phosphatidylcholine also was not associated with tHcy in this population. Choline supplementation in the form of phosphatidylcholine reduced fasting tHcy by 18% in a recent randomized trial (18). The supplement contained 2.6 g choline/d (as phosphatidylcholine), which was much higher than the intake of phosphatidylcholine among the women in the present study (median: 157 mg/d).
The intake of both betaine and choline was inversely associated with tHcy within the Framingham Offspring Study, a population that included men and women (37). However, there was a significant interaction by sex: men had stronger inverse associations than did women. A recent study in 903 Dutch women found no significant association between dietary intake of betaine or choline and tHcy (38). Results from animal studies suggest that females may have significantly greater endogenous phosphatidylcholine synthesis than do males (39). Men may be more dependent on exogenous sources of choline and betaine than are women.
Choline from 2 water-soluble compounds, glycerophosphocholine and phosphocholine, significantly predicted fasting tHcy. Choline from glycerophosphocholine and that from phosphocholine were strongly correlated with each other (r = 0.7, P < 0.0001) and therefore may provide similar information. We had no evidence to combine these nutrients a priori, and therefore we chose to present the results of these 2 nutrients separately. Absorption of the choline-containing compounds differs; the water-soluble compounds are absorbed in the portal circulation and go directly to liver, whereas the lipid-soluble compounds are absorbed via thoracic duct, which bypasses the liver (40). Although they are interchangeable in the body (41), the various choline compounds may have different metabolic fates when ingested. In addition to oxidation into betaine, choline is required for VLDL synthesis (as phosphatidylcholine) and neurotransmitter function (as acetylcholine) (41). Metabolic studies of the specific choline compounds may be warranted, because the differences in the kinetics, metabolism, and bioavailability from food are not well known.
We observed stronger associations of choline from glycerophosphocholine and phosphocholine with tHcy in women with low intakes of folate than in those with high intakes. Stratification by alcohol intake in women with low folate resulted in an even stronger dose response between the choline-containing compounds and tHcy. Among women with adequate folate intake, the same associations with tHcy were more modest. In previous studies, plasma betaine was most strongly associated with fasting and postmethionine loading tHcy among patients with low plasma folate (33, 42). The addition of betaine supplementation to a folic acid treatment had no additional effect on fasting tHcy in patients with hyperhomocysteinemia (43–45).
Betaine-dependent remethylation may compensate if the folate remethylation pathway is impaired because these 2 pathways are interrelated (46). In human and animal studies, a folate-deficient diet lowered choline stores, presumably because of an increased demand for choline as a methyl donor (7, 47). Choline concentrations were restored after folate repletion. Folate supplementation also increased plasma betaine (19). On the other hand, choline deficiency reduces folate stores, which are also restored through choline repletion (48).
The betaine pathway may be influenced by alcohol, a known folate inhibitor. In animal studies, alcohol administration increases activity in the betaine-homocysteine methyltransferase enzyme (49), which is responsible for donating the methyl group from betaine to tHcy (Figure 1). Betaine supplementation also prevents an ethanol-induced rise in tHcy and an ethanol-induced reduction in S-adenosylmethionine (9, 50), a metabolite of methionine, which acts as a methyl donor in DNA methylation.
The adequate intake of choline has been set at 425 mg/d for women and 550 mg/d for men (51). In the population of the present study, the cutoff for the 95th percentile of choline was 411 mg/d, which suggests that most of women within this population are not meeting the suggested intake. The intake of betaine from foods in the present population was lower than the previous estimates of 0.5−2 g/d (15). However, the median intake for both choline and betaine in the present population is consistent with values from other cohorts in the United States—313 mg for choline and 208 mg for betaine (37)—and in the Netherlands—300 mg for choline and 241 mg for betaine (38). In these cohorts, both nutrients were calculated with the use of the new USDA nutrient database (20). The use of self-reported FFQs may have led to an underestimation of the absolute intake of these nutrients. Although we may have underestimated the absolute intake, the FFQ used in this study was designed to rank participants, and thus it is likely that we properly discriminated subjects into the highest and lowest categories of intakes.
In conclusion, we found that intake of betaine + total choline was associated with lower tHcy. We also found that choline from 2 water-soluble choline-containing compounds, glycerophosphocholine and phosphocholine, was inversely associated with tHcy, which was most pronounced among women with a methyl-deficient diet. Further studies are needed to understand the differences in the metabolism and absorption of dietary sources of specific choline compounds. Although the betaine-remethylation pathway can compensate for the folate-remethylation pathway when folate availability is reduced, adequate intake of both nutrients may be most important in relation to chronic diseases.
Acknowledgments
The authors' responsibilities were as follows—SEC: design of the study, analysis of data, and writing of the manuscript; EBR: securing funding, design of the study, analysis of data, and writing of the manuscript; and ELG, SEH, SHZ, LWD, and WCW: critical review of the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Supported by grants no. CA87969, CA50385, CA100971, and AA11181 from the National Institutes of Health; by Merck Research Laboratories (for the plasma assays); by institutional training grant no. HL07575 from the National Heart, Lung, and Blood Institute (to SEC); and by grants no. DK55865 and AG09525 from the National Institutes of Health (to SHZ).
REFERENCES
- 1.Clarke R, Collins R, Lewington S, et al. Homocysteine and risk of ischemic heart disease and stroke—a meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 2.Wu LL, Wu JT. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta. 2002;322:21–8. doi: 10.1016/s0009-8981(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 4.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–41. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer IA, van Dusseldorp M, West CE, et al. Dietary folate from vegetables and citrus fruits decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J Nutr. 1999;129:1135–9. doi: 10.1093/jn/129.6.1135. [DOI] [PubMed] [Google Scholar]
- 6.Homocysteine Lowering Trialists' Collaboration Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 7.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129:712–7. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- 8.Barak AJ, Beckenhauer HC, Tuma DJ. Methionine synthase. a possible prime site of the ethanolic lesion in liver. Alcohol. 2002;26:65–7. doi: 10.1016/s0741-8329(01)00201-4. [DOI] [PubMed] [Google Scholar]
- 9.Barak AJ, Beckenhauer HC, Kharbanda KK, Tuma DJ. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol. 2001;25:77–81. doi: 10.1016/s0741-8329(01)00168-9. [DOI] [PubMed] [Google Scholar]
- 10.Cravo ML, Gloria LM, Selhub J, et al. Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B-12, and vitamin B-6 status. Am J Clin Nutr. 1996;63:220–4. doi: 10.1093/ajcn/63.2.220. [DOI] [PubMed] [Google Scholar]
- 11.Blasco C, Caballeria J, Deulofeu R, et al. Prevalence and mechanisms of hyperhomocysteinemia in chronic alcoholics. Alcohol Clin Exp Res. 2005;29:1044–8. doi: 10.1097/01.alc.0000169265.36440.ee. [DOI] [PubMed] [Google Scholar]
- 12.Chiuve SE, Giovannucci EL, Hankinson SE, et al. Alcohol intake and methylenetetrahydrofolate reductase polymorphism modify the relation of folate intake to plasma homocysteine. Am J Clin Nutr. 2005;82:155–62. doi: 10.1093/ajcn.82.1.155. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132(suppl):2350S–5S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 14.Baglietto L, English DR, Gertig DM, Hopper JL, Giles GG. Does dietary folate intake modify effect of alcohol consumption on breast cancer risk? Prospective cohort study. BMJ. 2005;331:807. doi: 10.1136/bmj.38551.446470.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–49. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 16.Brouwer IA, Verhoef P, Urgert R. Betaine supplementation and plasma homocysteine in healthy volunteers. Arch Intern Med. 2000;160:2546–7. doi: 10.1001/archinte.160.16.2546-a. [DOI] [PubMed] [Google Scholar]
- 17.Schwab U, Torronen A, Toppinen L, et al. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002;76:961–7. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- 18.Olthof MR, Brink EJ, Katan MB, Verhoef P. Choline supplemented as phosphatidylcholine decreases fasting and postmethionine-loading plasma homocysteine concentrations in healthy men. Am J Clin Nutr. 2005;82:111–7. doi: 10.1093/ajcn.82.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Melse-Boonstra A, Holm PI, Ueland PM, Olthof M, Clarke R, Verhoef P. Betaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrations. Am J Clin Nutr. 2005;81:1378–82. doi: 10.1093/ajcn/81.6.1378. [DOI] [PubMed] [Google Scholar]
- 20.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–7. doi: 10.1093/jn/133.5.1302. (Published erratum appears in J Nutr 2003;133:2918−9.) [DOI] [PubMed] [Google Scholar]
- 21.Hankinson SE, London SJ, Chute CG, et al. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35:2313–6. [PubMed] [Google Scholar]
- 22.Shai I, Stampfer MJ, Ma J, et al. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis. 2004;177:375–81. doi: 10.1016/j.atherosclerosis.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SM, Willett WC, Selhub J, et al. Plasma folate, vitamin B6, vitamin B12, and homocysteine and risk of breast cancer. J Natl Cancer Inst. 2003;95:373–80. doi: 10.1093/jnci/95.5.373. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-I and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–9. [PubMed] [Google Scholar]
- 25.Kroenke CH, Chu NF, Rifai N, et al. A cross-sectional study of alcohol consumption patterns and biologic markers of glycemic control among 459 women. Diabetes Care. 2003;26:1971–8. doi: 10.2337/diacare.26.7.1971. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–7. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 27.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of a expanded self-administered semi-quantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 28.Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 29.White HA. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–38. [Google Scholar]
- 30.Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003;133:4135–8. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- 31.Alfthan G, Tapani K, Nissinen K, Saarela J, Aro A. The effect of low doses of betaine on plasma homocysteine in healthy volunteers. Br J Nutr. 2004;92:665–9. doi: 10.1079/bjn20041253. [DOI] [PubMed] [Google Scholar]
- 32.Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291–5. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 33.Holm PI, Ueland PM, Vollset SE, et al. Betaine and folate status as cooperative determinants of plasma homocysteine in humans. Arterioscler Thromb Vasc Biol. 2005;25:379–85. doi: 10.1161/01.ATV.0000151283.33976.e6. [DOI] [PubMed] [Google Scholar]
- 34.Heil SG, Lievers KJ, Boers GH, et al. Betaine-homocysteine methyltransferase (BHMT): genomic sequencing and relevance to hyperhomocysteinemia and vascular disease in humans. Mol Genet Metab. 2000;71:511–9. doi: 10.1006/mgme.2000.3078. [DOI] [PubMed] [Google Scholar]
- 35.Gaughan DJ, Barbaux S, Kluijtmans LA, Whitehead AS. The human and mouse methylenetetrahydrofolate reductase (MTHFR) genes: genomic organization, mRNA structure and linkage to the CLCN6 gene. Gene. 2000;257:279–89. doi: 10.1016/s0378-1119(00)00392-9. [DOI] [PubMed] [Google Scholar]
- 36.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–4. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho E, Zeisel SH, Jacques P, et al. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83:905–11. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2007 Mar 21; doi: 10.1038/sj.ejcn.1602725. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 39.Noga AA, Vance DE. Insights into the requirement of phosphatidylcholine synthesis for liver function in mice. J Lipid Res. 2003;44:1998–2005. doi: 10.1194/jlr.M300226-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr. 1996;64:572–6. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 41.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 42.Holm PI, Bleie O, Ueland PM, et al. Betaine as a determinant of post-methionine load total plasma homocysteine before and after B-vitamin supplementation. Arterioscler Thromb Vasc Biol. 2004;24:301–7. doi: 10.1161/01.ATV.0000114569.54976.31. [DOI] [PubMed] [Google Scholar]
- 43.Bostom AG, Shemin D, Nadeau MR, et al. Short term betaine therapy fails to lower elevated fasting total plasma homocysteine concentrations in hemodialysis patients maintained on chronic folic acid supplementation. Atherosclerosis. 1995;113:129–32. doi: 10.1016/0021-9150(94)05466-v. [DOI] [PubMed] [Google Scholar]
- 44.van Guldener C, Janssen MJ, de Meer K, Donker AJ, Stehouwer CD. Effect of folic acid and betaine on fasting and postmethionine-loading plasma homocysteine and methionine levels in chronic haemodialysis patients. J Intern Med. 1999;245:175–83. doi: 10.1046/j.1365-2796.1999.00430.x. [DOI] [PubMed] [Google Scholar]
- 45.McGregor DO, Dellow WJ, Robson RA, Lever M, George PM, Chambers ST. Betaine supplementation decreases post-methionine hyperhomocysteinemia in chronic renal failure. Kidney Int. 2002;61:1040–6. doi: 10.1046/j.1523-1755.2002.00199.x. [DOI] [PubMed] [Google Scholar]
- 46.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132(suppl):2333S–5S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 47.Kim YI, Miller JW, da Costa KA, et al. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J Nutr. 1994;124:2197–203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- 48.Varela-Moreiras G, Selhub J, da Costa KA, Zeisel SH. Effect of chronic choline deficiency in rats on liver folate content and distribution. J Nutr Biochem. 1992;3:519–22. [Google Scholar]
- 49.Barak AJ, Beckenhauer HC, Tuma DJ. Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol. 1996;13:483–6. doi: 10.1016/0741-8329(96)00040-7. [DOI] [PubMed] [Google Scholar]
- 50.Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res. 1993;17:552–5. doi: 10.1111/j.1530-0277.1993.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 51.Zeisel SH. Choline: an essential nutrient for humans. Nutrition. 2000;16:669–71. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]