Abstract
Recent microarray studies with stringent validating criteria identified Bcl-2-associated athanogene (BAG1) as a target for the actions of medications that are mainstays in the treatment of bipolar disorder (BPD). BAG1 is a Hsp70/Hsc70-regulating cochaperone that also interacts with glucocorticoid receptors (GRs) and attenuates their nuclear trafficking and function. Notably, glucocorticoids are one of the few agents capable of triggering both depressive and manic episodes in patients with BPD. As a nexus for the actions of glucocorticoids and bipolar medications, we hypothesized that the level of BAG1 expression would play a pivotal role in regulating affective-like behaviors. This hypothesis was investigated in neuron-selective BAG1 transgenic (TG) mice and BAG1 heterozygous knockout (+/−) mice. On mania-related tests, BAG1 TG mice recovered much faster than wild-type (WT) mice in the amphetamine-induced hyperlocomotion test and displayed a clear resistance to cocaine-induced behavioral sensitization. In contrast, BAG1+/− mice displayed an enhanced response to cocaine-induced behavioral sensitization. The BAG1 TG mice showed less anxious-like behavior on the elevated plus maze test and had higher spontaneous recovery rates from helplessness behavior compared with WT mice. In contrast, fewer BAG1+/− mice recovered from helplessness behavior compared with their WT controls. BAG1 TG mice also exhibited specific alterations of hippocampal proteins known to regulate GR function, including Hsp70 and FKBP51. These data suggest that BAG1 plays a key role in affective resilience and in regulating recovery from both manic-like and depression-like behavioral impairments.
Keywords: FKBP51, lithium, valproate, mood disorders, resilience
Bipolar disorder (BPD) is one of the most severely debilitating medical illnesses and affects the lives and functioning of millions worldwide. A number of studies indicate that for a large percentage of patients, outcome is quite poor (1), with high rates of relapse, chronicity, lingering residual symptoms, subsyndromes, cognitive and functional impairment, psychosocial disability, and diminished well being (2–4). Suicide is estimated to be the cause of death in up to 15% of individuals with BPD, and in addition to suicide, many other deleterious health-related effects are increasingly being recognized (5). In light of this tremendous morbidity and mortality, it is striking that no new treatments have been developed specifically for BPD in 40 years. With the exception of lithium, every alternative treatment currently used for BPD was initially developed for other illnesses (most notably epilepsy or schizophrenia) and then subsequently used in BPD (1, 6). This lack of specific medication development for BPD is undoubtedly due, at least in part, to the fact that our understanding of the underlying neurobiology of the disorder is in its infancy.
Syndromically and symptomatically, BPD is a complex illness encompassing various degrees of disturbances of emotions, behavior, thought and cognition, and hedonic and motoric drive (1). Indeed, the hallmark of BPD appears to be the predilection to “overshoot” into full-blown affective episodes in the face of stressors (6). Thus, a number of stressors (including psychological, hormonal, and pharmacological) that induce modest, transient perturbations in healthy individuals are capable of inducing full-blown, sustained mood episodes in individuals with a bipolar diathesis. At present, the precise cellular mechanisms underlying this loss of homeostasis and clinical manifestation of affective symptomatology, that is, mania and/or depression, remain to be fully elucidated. However, it is noteworthy that considerable clinical data have shown that glucocorticoids are one of the few agents capable of triggering both depressive and manic episodes in patients with BPD (reviewed in ref. 6).
Of relevance to the ability of glucocorticoids to trigger both manic and depressive episodes, Wei et al. (7) recently examined the behavioral phenotype of glucocorticoid receptor (GR)-overexpressing transgenic (TG) mice. These mice displayed a significant increase in depression-like behaviors relative to wild-type (WT) (control) mice. Additionally, the mice showed enhanced sensitization to cocaine and were also supersensitive to antidepressants. Together, these intriguing data suggest that these mice show an enhanced propensity toward the development of both depression-like and manic-like episodes, thereby recapitulating certain facets of BPD.
Moreover, studies have shown that two pharmacologic agents that are the mainstay of treatment for BPD, lithium and valproate (VPA), regulate the expression of a protein known to modulate GR function (8), despite the fact that these agents have highly dissimilar chemical structures (a monovalent cation and a fatty acid). Specifically, chronic lithium or VPA, when administered in therapeutically relevant paradigms, robustly up-regulate the GR chaperone protein BAG1 (8). Consistent with the known actions of BAG1 on GR trafficking and function, previous studies have demonstrated that lithium/VPA-induced BAG1 up-regulation attenuates GR nuclear translocation and also attenuates the activity of a reporter gene driven by GRs (8). Notably, these effects were only observed when using high (pathophysiologic) glucocorticoid concentrations; GR function at lower glucocorticoid levels was unaffected by BAG1 up-regulation (8).
In view of (i) the role of GR overexpression in modulating affective symptomatology observed in BPD; (ii) the role of BAG1 in attenuating GR function at high glucocorticoid concentrations; and (iii) the fact that two of the mainstays in the long-term treatment of BPD up-regulate BAG1 levels, we used BAG1-overexpressing mice (9) to investigate the hypothesis that elevated BAG1 expression would attenuate stress-responsive and affective-like behaviors. We found that BAG1 overexpression is associated with enhanced recovery from both manic-like and depression-like behavioral impairments. Furthermore, we also found that BAG heterozygous knockout (+/−) mice showed somewhat of an opposite picture in selected tests. While further investigation is clearly required to substantiate the clinical relevance of BAG1 function in the potential treatment of BPD, these findings suggest the possibility of new therapies aimed at enhancing BAG1 function.
Results
General Physical and Behavioral Assessments of Mice with Neuron-Specific Overexpression of BAG1.
Consistent with previous data (10), BAG1 TG mice displayed clear hippocampal expression of recombinant BAG1 without significant change of the endogenous BAG1 levels in total tissue homogenates and cellular fractions of cytoplasm, nuclei, and mitochondria [supporting information (SI) Fig. S1A]. Previous immunocytochemical studies have further demonstrated selective BAG1 increases in hippocampal, striatal, and cortical neurons (10). The BAG1 TG mice were viable, fertile, and devoid of any gross anatomical abnormalities. They exhibited normal performance on a variety of neurological and sensory tests (data not shown) as well as locomotor activity (Fig. S1). These data suggest that BAG1 TG mice do not have obvious motor, sensory, or learning and memory impairments.
General Physical and Behavioral Assessments of BAG1+/− Mice.
The major purpose of this work was to investigate the effects of BAG1 overexpression on affective-like behaviors. However, BAG1+/− mice of C57BL/6J background were also used in selected tests. The mice showed ≈50% reduced BAG1 levels (Fig. S2). The BAG1+/− mice were viable, fertile, devoid of any gross anatomical abnormalities, and exhibited normal performance on a variety of neurological and sensory tests (data not shown). Furthermore, the locomotor activities of BAG1+/− mice in the open field test were similar to those of WT mice (Fig. S1E).
Decreased Anxiety-Like Behaviors in BAG1 TG Mice.
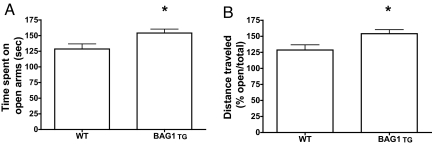
The elevated plus maze test (EPM) is a commonly used test to monitor anxiety-like behavior. The maze offers the subject two choices: stay in walled (or closed) arms or explore in open arms, with rodents generally spending more time in the closed arms. Treatment with anxiolytic agents increases animal activity in the open arms (11). Although mice from both genotypes entered the open arms with similar frequency (data not shown), BAG1 TG mice spent significantly more time (Fig. 1A) and traveled significantly greater distances (Fig. 1B) in the open arms than WT mice. No significant differences were detected in total arm entries and total distance traveled in both arms, indicating that the increased activity of BAG1 TG mice in the open arms was not due to hyperlocomotion. One plausible interpretation of the data is that BAG1 TG mice are more tolerant to stressful conditions (open arms) while being more exploratory.
Fig. 1.
Increased activity of BAG1 TG mice in the open arms of elevated plus maze (EPM). EPM was performed under ambient light (20 lux) as detailed in SI Methods. The time spent in open arms (A) and the ratio of travel distance (open/total arms) for 5 min (B) were plotted. Data represent mean ± SEM (n = 12 mice per group); *, P < 0.05, Student's t test.
Facilitated Recovery from Severe Stress-Induced Depression-Like Behavior in BAG1 TG Mice.
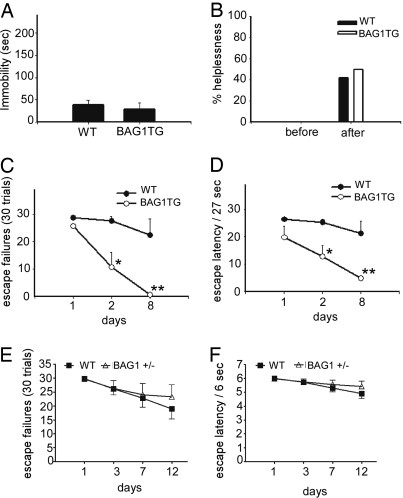
The forced swim test (FST) is widely used as a test of behavioral despair (12). It is used to monitor the behavioral effects of antidepressants and to screen agents that produce antidepressant-like effects (12). During the test, a mouse is forced to spend 6 min in an inescapable tank of water. A mouse is scored as immobile when it suspends all of its efforts to escape in the last 4 min of the test. A potential limitation of this test is that acute treatment with antidepressants effectively reduces immobility (in contrast to the chronic administration required for therapeutic efficacy in patients). BAG1 TG mice displayed no significant difference in immobility times in the test (Fig. 2A).
Fig. 2.
Facilitated spontaneous recovery of BAG1 TG mice in learned-helplessness paradigm. (A) Forced swim test in BAG1 TG mice. No significant genotype difference in immobility times was detected. (B) Learned-helplessness paradigm. No genotype difference was detected in the number of BAG1 TG versus WT mice that became helpless. (C) BAG1 TG helpless mice were assessed again on days 2 and 8. Significantly fewer helpless mice were found in the BAG1 TG mouse group. (D) There was a smaller percentage of recovered-helplessness mice in the BAG1+/− mice group. However, no significant differences between BAG1+/− and C57BL/6J WT mice were detected on number of escape failures. (E) Shortened escape latencies were found in the BAG1 TG mouse group. (F) No significant differences were found in escape latencies between BAG1+/− and WT mice at assessment days 1, 3, 7, and 12. For BAG1 TG vs. WT mice, data represent mean ± SEM (n = 6∼13 mice per group); *, P < 0.05; **, P < 0.01, compared with WT on the same day, two-way ANOVA, Tukey's post hoc test.
The learned-helplessness paradigm is another behavioral despair test involving severe stress (13). The typical paradigm consists of the induction of helplessness, as well as initial and follow-up helplessness assessment sessions. During the induction session on day 0, animals are given a series of light-cued electrical foot shocks while the gate that allows potential escape is closed. During the assessment sessions, animals receive a series of cued shocks with the gate open to allow potential avoidance or escape from the shock (by passing through the gate). The number of escape failures and latencies to escape are recorded as the readout measures. Mice that fail to escape at least 20 of 30 cued shocks are considered to have developed helplessness. Helpless mice can spontaneously recover over a period of days or weeks (14). In follow-up assessments, subchronic, but not acute or subacute, treatment with antidepressants facilitate escape for helpless mice (15).
Here, mice of both genotypes displayed no significant outcome differences in the initial assessments conducted 1 day after the induction session (Fig. 2B). However, the BAG1 TG mice that had developed helplessness behavior showed a markedly rapid spontaneous recovery over a week-long period (as indicated by significant differences in escape failures and latencies) (Fig. 2 C and E).
To ensure that this enhanced recovery was not due to a memory deficit, active and passive avoidance tests were undertaken. No significant differences were detected in the active and passive avoidance tests (Fig. 2 B and C), suggesting that BAG1 mediates behavioral recovery from severe stress.
Recovery from Severe Stress-Induced Depression-Like Behavior in BAG1+/− Mice.
Both BAG1+/− mice and their WT controls displayed no significant outcome differences in the initial assessments conducted 1 day after the induction session (data not shown). The BAG1+/− mice that had developed helplessness behavior showed modestly delayed spontaneous recovery at day 12 of the assessment period (as indicated by escape failures and latencies); however, these delayed recoveries did not reach statistical significance (Fig. 2 D and F). Notably, fewer BAG1+/− mice (22%) spontaneously recovered from the helplessness behavior than their WT controls (43%).
Facilitated Recovery from Psychomotor Stimulant-Induced Locomotion in BAG1 TG Mice.
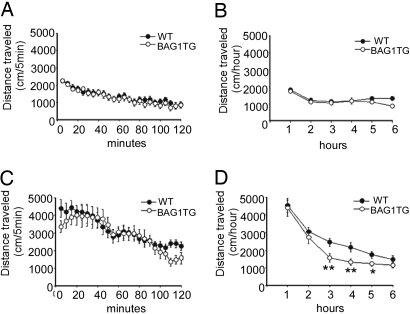
Psychostimulants such as amphetamine cause mania in human subjects with a personal or family history of mood disorders and worsen manic manifestation in hypomanic patients (16). Amphetamine-induced hyperlocomotion has been viewed as an experimental model of mania in rodents, with supporting evidence that lithium and VPA treatment attenuates amphetamine-induced locomotion in this model (17). No significant difference in activity was detected between the genotypes after saline injection (Fig. 3 A and C). Amphetamine injection (i.p. 2 mg/kg and 5 mg/kg) significantly increased locomotion in both genotypes (Fig. 3 B and D). At 2 mg/kg amphetamine, both genotypes returned to baseline within 2 h, and no significant difference was detected in distance traveled between mice of different genotypes (Fig. 3B). At the higher dose, BAG1 TG mice returned to baseline much earlier than the WT mice (Fig. 3D). These effects of BAG1 overexpression are unlikely to be confounded by general locomotion, because no significant baseline locomotion differences were seen between mice from the two genotypes (Fig. 3 A and C). These data imply that BAG1 also facilitates behavioral recovery from amphetamine-induced changes in a model of mania. It remains possible that these mice show altered amphetamine metabolism; however, the resilience observed in both pharmacologic and nonpharmacologic paradigms is unlikely to be due to pharmacokinetic effects.
Fig. 3.
Accelerated recovery and sensitization resilience of BAG1 TG mice in psychostimulant models of mania: amphetamine. (A–D) Amphetamine-induced hyperlocomotion. Locomotor activities were monitored for 2 h before (A) and after (C) 2 mg/kg amphetamine, and for 6 h before (B) and after (D) 5 mg/kg amphetamine injection. In the experiments with 2 mg/kg amphetamine, locomotor activity was reduced to baseline levels within 2 h, and there was no significant genotype difference (C). In the experiments with 5 mg/kg amphetamine, BAG1 TG mice showed facilitated reduction in locomotion (D), and genotype differences were significant.
Reduction of Cocaine-Induced Behavioral Sensitization in BAG1 TG Mice.
The cocaine-induced behavioral sensitization paradigm is another experimental model of mania that mimics the course of BPD (18). In this paradigm, animals receive repeated injections of psychostimulants over a period of several days or weeks, and the animal's locomotor responses to the injections are gradually intensified. Such an intensified response can last for days or weeks without additional injections (19). Reports show that administration of lithium during the psychostimulant sensitization process attenuates the gradual increases in motor response (20).
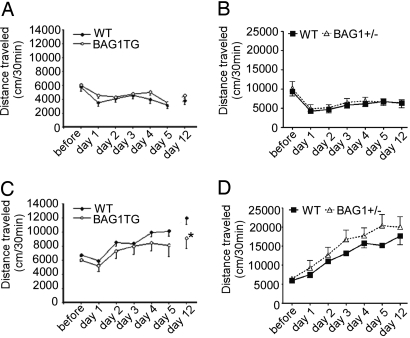
To evaluate the effect of BAG1 overexpression on the behavioral sensitization paradigm, 20 mg/kg cocaine or saline was repeatedly administered for 5 consecutive days, and the mice were challenged with the same dose of cocaine 1 week later. Gradual increases in locomotor activity were observed in both groups, but no significant differences were seen between the genotype groups over 5 days of cocaine administration (Fig. 4 B and D). On the challenge test 1 week later, a significant increase in locomotor activity was detected compared with the first day of injection, indicating the development of behavioral sensitization (Fig. 4 B and D). Notably, this sensitization was significantly lower in BAG1 TG mice (Fig. 4C). Saline treatment did not induce changes in locomotor activity (Fig. 4 A and C). These results indicate that mice overexpressing BAG1 are resistant to cocaine-sensitized behavior in a model of mania.
Fig. 4.
Blunted response of BAG1 TG mice but increased sensitization of BAG1+/− mice in the cocaine-induced behavioral sensitization mode of mania. Locomotor activity was monitored after repeated injections of saline [for BAG1 TG mice (A) and for BAG1+/− (B)] or cocaine [20 mg/kg i.p. for BAG1 TG (C); 10 mg/kg i.p. for BAG1+/− mice (D)] for 5 consecutive days. A challenge test with the same dose of cocaine was performed 1 week after the last cocaine injection. Repeated injections with cocaine, but not saline, caused gradual locomotion increases in WT, BAG1 TG mice and BAG1+/− mice (A–D). The repeated cocaine injection-induced locomotion increases were significantly less in BAG1 TG mice than FVB/n WT mice (C) (n = 8–12 mice per group; *, P < 0.05; **, P < 0.01 compared with WT at the same time block, two-way ANOVA, Tukey's post hoc test). The repeated cocaine injection-induced locomotion increases were significantly greater in BAG1+/− mice than C57BL/6J mice (D) [F (1, 56) = 6.924, P = 0.0110]. Data represent mean ± SEM.
Enhancement of Cocaine-Induced Behavioral Sensitization in BAG1+/− Mice.
To evaluate the effect of reduced BAG1 levels on the behavioral sensitization paradigm, BAG1+/− mice were repeatedly administered cocaine (10 mg/kg) or saline for 5 consecutive days and then challenged with the same dose of cocaine 1 week later. Gradual increases in locomotor activity were observed in both BAG1+/− and WT mice, but no significant differences were seen between the genotype groups over 5 days of cocaine administration (10 mg/kg). On the challenge test 1 week later, a significant increase in locomotor activity was detected compared with the first day of injection, indicating the development of behavioral sensitization (Fig. 4D). Notably, this sensitization was significantly higher in BAG1+/− mice compared with their WT controls (Fig. 4D). These results indicate that mice with lower levels of BAG1 show an enhanced sensitivity to behavioral sensitization with cocaine.
Hippocampal Neurochemical Changes in BAG1 TG Mice.
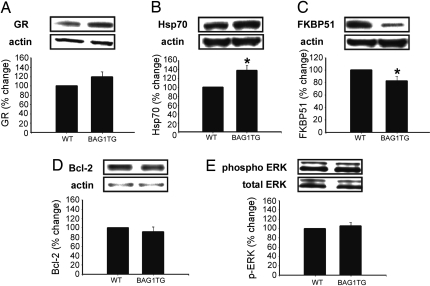
The hippocampus is one of the brain regions involved in mood regulation and behavioral response to stress and glucocorticoids; furthermore, mood stabilizers robustly up-regulate BAG1 expression in hippocampus compared with other brain regions (8). To explore the in vivo relationship of BAG1 to GR function, we monitored levels of BAG1-interacting molecules in this brain region. In this regard, hippocampal GR translocation is known to be regulated via interactions with BAG1, Hsp70, and FKBP51. Notably, BAG-1 is known to stabilize Hsp70 levels in the brain (9, 21). Similar to BAG1, FKBP-51 is known to inhibit GR function (22). In addition, some studies suggest that inhibition of GR activity (as would be expected in BAG1 TG mice) might be expected to result in a compensatory reduction in FKBP-51 levels. Therefore, we quantified levels of GR, Hsp70, and FKBP51 in BAG1 TG mice. Furthermore, cytosolic BAG1 also interacts with Raf-1 (thereby promoting activation of ERK MAP kinases) and with Bcl-2 (thereby promoting cell survival) (8, 9, 23, 24). We therefore also quantified total ERK levels, pERK1/2 levels (known to represent the activated form of ERK MAP kinases), and Bcl-2 levels.
As predicted by BAG1's stabilizing effects on Hsp70 (11), mice overexpressing BAG1 displayed significantly higher hippocampal levels of Hsp70 (Fig. 5). BAG1-overexpressing mice also displayed significantly lower levels of FKBP51, consistent with compensatory adaptations (22) (Fig. 5). No changes were observed in total GR protein levels. Similarly, the hippocampal levels of Bcl-2, ERK, or pERK did not significantly differ between the BAG1 TG and WT groups.
Fig. 5.
Neurochemical changes induced by BAG1 overexpression. Significant genotype effects were observed in levels of Hsp70 (B) and FKBP51 (C), but not GR (A), Bcl-2 (D), or ERK (E). Data represent mean ± SEM of percentage change compared with WT mice; *, P < 0.05, Student's t test.
Because the BAG1 TG mice displayed changes in behavioral response to psychostimulants (Figs. 3 and 4), we examined the striatal levels of proteins known to be involved in dopamine neurotransmission and intracellular signaling. No significant differences were observed between BAG1 TG and WT mice with respect to levels of dopamine transporter (DAT), catechol-O-methyltransferase (COMT), and transcription factors previously implicated in control of expression of these genes: Fos B and phospho-CREB (cAMP-responsive element binding protein) (Fig. S3).
Discussion
In this report, we present evidence that the level of neuronal BAG1 expression plays an important role in regulating affective-like behaviors, a finding with translational implications for BPD. In BPD, both physical and psychosocial stresses are capable of destabilizing the illness and inducing sustained manic or depressive episodes. Thus, a number of stressors that induce modest, transient perturbations in healthy individuals are capable of inducing sustained mood episodes in individuals with a bipolar diathesis. The primary function of BAG1 concerns its interaction with Hsp70 family molecular chaperones, and thus BAG1 can be considered to be an anti-cell stress protein. BAG1 displays a variety of cytoprotective activities and effects on signal transduction, transcription, and cell-surviving pathways, all suggestive of a role in overcoming cellular stress (reviewed in refs. 9, 24, and 25).
BAG1 is known to interact with GRs and attenuate their nuclear trafficking and function (23, 26). In this context, it is noteworthy that considerable clinical data have shown that glucocorticoids are one of the few agents capable of triggering both depressive and manic episodes in patients with BPD (reviewed in ref. 6). Furthermore, GR-overexpressing mice show an enhanced predilection toward the development of both depression-like and manic-like episodes (7). Finally, chronic treatment with lithium or VPA, which enhance recovery from spontaneous as well as stress-induced affective episodes (discussed in ref. 27), robustly up-regulate the GR chaperone protein BAG1 (8). In the present work, we demonstrated a role for neuronal BAG1 overexpression in regulating recovery from manic-like and depression-like behavioral states that are known to be enhanced by stress. In keeping with its role in regulating GR nuclear translocation and transactivation, we also demonstrated that BAG1 overexpression resulted in higher hippocampal levels of Hsp70 and lower levels of FKBP51 without changing overall GR levels or levels of Bcl-2, ERK, or pERK. Our findings thus provide additional support for an important role of GRs in mania and depression, although other GR-independent functions of BAG1 may also contribute.
Therefore, therapies aimed at enhancing BAG1 function may ultimately be capable of treating both the manic and depressive phases of BPD. In this regard, the up-regulation of BAG1 by mood stabilizers represents a finding that meets several important criteria for assessing potential clinical therapeutic relevance (8). Specifically, (i) the effect of mood stabilizers on BAG1 protein expression and GR trafficking are common to both lithium and VPA; (ii) BAG1 up-regulation by lithium and VPA occurs in the hippocampus, a brain region known to be involved in critical affective neuronal circuits; (iii) BAG1 up-regulation occurs in the CA3 subfield, a region where robust protection against stress-induced apical dendritic retraction by lithium was recently observed (VPA was not examined in that study) (28); (iv) the effect of lithium and VPA on BAG1 and GR trafficking/function occurs at therapeutic concentrations both in vivo and in vitro; (v) similar to the clinical therapeutic effects, the changes in BAG1 protein expression and GR trafficking/function are observed only after chronic (and not after acute) administration; (vi) the effects are specific to the mood stabilizers lithium and VPA; chronic administration of an antidepressant, a psychostimulant, or an antipsychotic does not affect BAG1; and (vii) the effects of BAG1 overexpression on GR function were manifest at high (pathophysiologic) glucocorticoid levels (8).
The BAG1 TG mice appeared normal in terms of physical appearance and performance on a variety of neurological, sensory, locomotor, and learning and memory tests. On behavioral tests relevant to affective-like behavior, these mice showed increased exploratory activity in relatively stressful conditions, enhanced spontaneous recovery from helplessness, faster recovery from amphetamine-induced hyperactivity, and resistance to cocaine-induced behavioral sensitization (Figs. 1–4). Collectively, the behavioral pattern of BAG1 TG mice suggests a phenotype associated with enhanced recovery from certain affective-like behaviors associated with BPD. In contrast, we found that the BAG1+/− mice showed an enhanced sensitivity to cocaine (Fig. 4). Furthermore, fewer BAG1+/− recovered spontaneously from helplessness (Fig. 2 D and F).
Because BAG1 emerged as a candidate gene from previous microarray studies of lithium and VPA, the question of whether or not rodents treated with lithium and VPA show behavioral similarities to BAG1 TG mice is important. Despite some methodological differences between previous pharmacologic studies and this report, the overall data do suggest strong similarities between lithium-treated (and to a lesser extent VPA-treated) mice and BAG1 TG mice (reviewed in ref. 6).
Studies of suppression of GR action by the BAG1 proteins have identified two primary modes of action: (i) inhibition of GR translocation to the nucleus and (ii) a more direct nuclear action at the level of regulation of the transactivation function of the receptor (23, 29). Consistent with this mode of action, we have observed in studies with lithium and VPA an attenuation of GR nuclear translocation and an inhibition of the activity of a reporter gene driven by GRs (8). Notably, these effects were only observed when using high (pathophysiologic) glucocorticoid concentrations. Similarly, GR function at lower glucocorticoid levels was unaffected by BAG1 up-regulation (8). Chronic lithium and VPA (both of which up-regulate BAG1) have been shown to enhance recovery from both the depressive and manic episodes associated with exogenous or endogenous (i.e., Cushing disease) elevations of glucocorticoids (discussed in ref. 6). The data presented here suggest that the interaction between GRs and BAG1 contributes to enhanced recovery from manic- and depression-like behavioral impairments observed in TG mice overexpressing BAG1 in neurons.
Because BAG1 regulates GR function primarily by attenuating its nuclear translocation and transactivation functions, it is not altogether surprising that we did not observe changes in total GR protein levels. However, we did observe alterations in the levels of two GR chaperone proteins: Hsp70 and FKBP51. We found that BAG1-overexpressing mice had higher hippocampal levels of Hsp70. BAG1 is known to bind to and stabilize Hsp70, thereby leading to Hsp70 accumulation in cells (10). Notably, BAG1-induced activation of Hsp70 has been postulated to be important for neuroprotection (26). Thus, BAG1-overexpressing TG animals have increased levels of Hsp70 and exhibit relative resistance to in vitro and in vivo insults (10, 26).
With respect to FKBP51, this immunophilin is known to attenuate GR function (22). It is a potent inhibitor of GR binding, and glucocorticoid resistance in three New World primates is associated with overexpression of FKBP51 (22). Most recently, it has been demonstrated that a combination of a transcriptionally incompetent GRs and overexpression of the GR cochaperone FKBP51 (22) contributes markedly to glucocorticoid resistance in squirrel monkeys. Together, the data suggest that the decreases in hippocampal FKBP51 observed in BAG1-overexpressing mice may well represent a compensatory change in response to attenuated GR function.
Overall, this work shows that brain overexpression of BAG1, a previously identified biochemical target for structurally dissimilar mood stabilizers, is associated with enhanced recovery from certain affective states. We are not aware of any human genetic studies that implicate BAG1 as a gene associated with mood disorders. However, BAG1 is a chaperone protein that interacts with GRs, Hsp70, and, thus, FKBP5. Binder et al. (30) investigated single nucleotide polymorphisms (SNPs) in the FKBP5 gene (6p21.321.2) and seven other genes thought to play a role in HPA axis regulation. Two SNPs in FKBP5 were associated with rate of treatment response in two independent samples. Most recently, a completely independent study replicated an association between FKBP5 markers and treatment response to antidepressant treatment when using the categorical “responder” and “remitter” outcomes (31). Indeed, this study also found an association between the FKBP5 gene and depression itself. Finally, very recent data have also shown that variants in the FKBP5 gene are associated with an increased likelihood of developing PTSD (32); these findings fit nicely with the putative role of these molecules in mediating affective resilience. Ongoing studies are attempting to elucidate the complex interactions between FKBP5 and BAG1 in the pathophysiology and treatment of mood disorders.
In conclusion, the data presented here suggest that therapies designed to enhance BAG1 function may lead to treatments for both the manic and depressive phases of BPD, as well as modulating the effects of stress. Further investigation of the role of BAG1 will help clarify the mechanisms of interaction between mood stabilizers and glucocorticoids in mood disorders.
Materials and Methods
Animals.
Mice expressing FLAG-mouse BAG-1 (mBAG-1) under the control of the neuron specific enolase promoter and WT FVB/n mice used in the study are described in a previous report (10). All these mice had the same genetic background and were bred in-house. BAG1 heterozygous knockout (+/−) mice were generated as described (25) and backcrossed to mice of C57BL/6J (Jackson Laboratories) background for seven generations. All animals were housed under constant temperature (22 ± 1°C) and 12 h light/dark cycle with access to water and food ad libitum. All of the behavioral experiments were performed between 8 and 11 weeks of age. All animal treatments, procedures, and care were approved by the National Institute of Mental Health Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals.
General Evaluation.
Assessment of physical appearance, growth and development, and neurological and sensory functions were performed following guidelines established by Crawley (33).
Behavioral Tests.
The open-field test (OFT), the active and passive avoidance tests, the EPM, the FST, the learned-helplessness paradigm, the amphetamine-induced hyperlocomotion test, and cocaine-induced behavioral sensitization were conducted in WT and BAG1 TG mice as specified in SI Methods. The Ethovision system (Noldus) was used to analyze data from the EPM, the FST, the amphetamine-induced hyperlocomotion test, and cocaine-induced behavioral sensitization. The learned-helplessness paradigm and cocaine-induced behavioral sensitization were also conducted in WT and BAG1+/− mice as specified in SI Methods. The Clever Systems (CleverSys., Inc.) was used to analyze data from the cocaine-induced behavioral sensitization experiment.
Hippocampal Cellular Fractionations, Striatal Sample Preparation, and Immunoblotting.
Hippocampus and striatum were removed from drug-naïve WT and BAG1 TG mice, and the cellular fractionation of hippocampal samples was conducted as specified in SI Methods. Frozen striatal tissues were homogenized in lysing buffer containing protease inhibitor and phosphatase inhibitor cocktails (Sigma) and then spun at 12,000 × g for 10 min to remove debris. Immunoblotting of fractionated hippocampal samples and striatal protein extracts was conducted as described previously (34), using antibodies from Santa Cruz Biotechnology for BAG-1, Hsp70, Bcl-2, DAT, Fos B, and COMT, from Abcam for GRs and FKBP51, and from Cell Signaling for phospho-ERK, total-ERK, and phospho-CREB. An equal amount of total protein from different animals was loaded on the gel for comparison. The amount of protein loaded on the gel was within a linear detection range for the protein being detected. The immunocomplex was detected by using chemiluminescence (ECL kit; GE Healthcare). Quantitation of the immunoblots was performed by densitometric scanning of the film by using a Kodak Image Station (4000R Digital Imaging System). An aliquot of pooled “standard” mouse brain (hippocampus) was run on one lane of each gel. Data were normalized against the standard mouse brain to minimize between-blot variability.
Statistical Analysis.
All results are expressed as mean ± SEM. Statistical comparisons were performed with the use of 1- or 2-way ANOVA with Tukey's post hoc test or a Student t test to identify significant differences. In all cases, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
Ioline Henter provided outstanding editorial assistance. C. L. Kress provided expert animal husbandry. This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services (H.K.M. and G.C.) and Grant CA-67329 (to J.C.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803736105/DCSupplemental.
References
- 1.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Fagiolini A, et al. Functional impairment in the remission phase of bipolar disorder. Bipolar Disord. 2005;7:281–285. doi: 10.1111/j.1399-5618.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 3.Revicki DA, Matza LS, Flood E, Lloyd A. Bipolar disorder and health-related quality of life: Review of burden of disease and clinical trials. Pharmacoeconomics. 2005;23:583–594. doi: 10.2165/00019053-200523060-00005. [DOI] [PubMed] [Google Scholar]
- 4.Tohen M, et al. The McLean–Harvard First-Episode Mania Study: Prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- 5.Kupfer DJ. The increasing medical burden in bipolar disorder. J Am Med Assoc. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar and Recurrent Unipolar Disorders. New York: Oxford Univ Press; 2007. [Google Scholar]
- 7.Wei Q, et al. Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. Proc Natl Acad Sci USA. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kermer P, et al. Bag1 is a regulator and marker of neuronal differentiation. Cell Death Differ. 2002;9:405–413. doi: 10.1038/sj.cdd.4400972. [DOI] [PubMed] [Google Scholar]
- 10.Kermer P, et al. BAG1 over-expression in brain protects against stroke. Brain Pathology. 2003;13:495–506. doi: 10.1111/j.1750-3639.2003.tb00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurt M, Arik AC, Celik S. The effects of sertraline and fluoxetine on anxiety in the elevated plus-maze test in mice. J Basic Clin Physiol Pharmacol. 2000;11:173–180. doi: 10.1515/jbcpp.2000.11.2.173. [DOI] [PubMed] [Google Scholar]
- 12.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berlin) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 13.Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- 14.Chourbaji S, et al. Learned helplessness: Validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behavior, CRE-binding activity and BDNF level in learned helplessness rats. Eur J Pharmacol. 2004;498:135–142. doi: 10.1016/j.ejphar.2004.07.084. [DOI] [PubMed] [Google Scholar]
- 16.Camacho A, Akiskal HS. Proposal for a bipolar-stimulant spectrum: Temperament, diagnostic validation and therapeutic outcomes with mood stabilizers. J Affect Disord. 2005;85:217–230. doi: 10.1016/j.jad.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Post RM, Weiss SR. Sensitization, kindling, and anticonvulsants in mania. J Clin Psychiatry. 1989;50(Suppl):23–30. discussion 45–27. [PubMed] [Google Scholar]
- 19.Post RM, Weiss SR, Pert A. Cocaine-induced behavioral sensitization and kindling: implications for the emergence of psychopathology and seizures. Ann N Y Acad Sci. 1988;537:292–308. doi: 10.1111/j.1749-6632.1988.tb42114.x. [DOI] [PubMed] [Google Scholar]
- 20.Gould TD, et al. Beta-Catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 21.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Schneikert J, Hubner S, Martin E, Cato AC. A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J Cell Biol. 1999;146:929–940. doi: 10.1083/jcb.146.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takayama S, et al. Cloning and functional analysis of BAG-1: A novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- 25.Gotz R, et al. Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci. 2005;8:1169–1178. doi: 10.1038/nn1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liman J, et al. Interaction of BAG1 and Hsp70 mediates neuroprotectivity and increases chaperone activity. Mol Cell Biol. 2005;25:3715–3725. doi: 10.1128/MCB.25.9.3715-3725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan PX, et al. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 29.Kanelakis KC, et al. Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J Biol Chem. 1999;274:34134–34140. doi: 10.1074/jbc.274.48.34134. [DOI] [PubMed] [Google Scholar]
- 30.Binder EB, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 31.Lekman M, et al. The FKBP5-gene in depression and treatment response: An association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J Am Med Assoc. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Yuan PX, Jiang YM, Huang LD, Manji HK. Lithium increases tyrosine hydroxylase levels both in vivo and in vitro. J Neurochem. 1998;70:1768–1771. doi: 10.1046/j.1471-4159.1998.70041768.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.