Abstract
The present study used isobaric tags for relative and absolute quantitation (iTRAQ) to identify novel targets in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. The expression of 41 proteins was significantly altered in the inflamed spinal cord. Twenty of these are implicated in EAE for the first time and many have previously been shown to play a role in antigen processing, inflammation, neuroprotection, or neurodegeneration.
Keywords: EAE, multiple sclerosis, iTRAQ, mass spectrometry, spinal cord
Introduction
Multiple Sclerosis is an autoimmune, inflammatory, demyelinating, and neurodegenerative disease of the central nervous system (CNS) leading to permanent neurological deficits. Experimental autoimmune encephalomyelitis (EAE) is one of the best-characterized animal models of multiple sclerosis. Although the histopathology of these diseases is well-defined, the molecular mechanisms underlying neural deficits remain elusive. This issue is of critical importance for the characterization of novel targets for therapeutic interventions.
Earlier studies investigating the molecular processes underlying neural dysfunction in EAE and multiple sclerosis focused on select proteins or pathways by use of conventional biochemical or molecular approaches. However, in recent years, the advent of new techniques such as gene microarray analysis presented unique opportunities for the large-scale delineation of changes in gene expression. In fact, a number of reports used microarray analysis to provide global perspectives on the differential gene expression profiles in the CNS during EAE and multiple sclerosis.1-3 These investigations also shed light into molecular commonalities between the human disease and the rodent models. However, changes in mRNA expression may not always reflect the actual alterations in the levels of functional proteins. Yet, comprehensive surveys on differential protein expression in these diseases have been sparse. New proteomic approaches have increasingly received attention as effective tools for the identification of diagnostic and prognostic biomarkers or for the broad mapping of proteins in pathological conditions. Therefore, approaches such as two-dimensional gel electrophoresis coupled with mass spectrometry or iTRAQ may provide both complementary as well as novel, large-scale information about modifications in protein expression.4-6 To date, there is only limited number of comprehensive proteomic studies on multiple sclerosis and EAE. These investigations characterized proteins in the cerebrospinal fluid or serum of multiple sclerosis patients,7-10 delineated the profile of autoantibodies in EAE employing protein microarrays,11 and analyzed protein expression in the CNS of mice affected by EAE as compared to controls.12 The current investigations used the iTRAQ technology to define differential protein expression in the lumbar spinal cord during acute EAE in the Lewis rat. We focused on the lumbar spinal cord because it is the CNS region most affected in our experimental model.
The iTRAQ approach is a mass spectrometry (MS)-based proteomics method which enables simultaneous quantification of proteins obtained from the tissue of four distinct animals. All proteins are first digested by trypsin into peptides, and a small molecule termed “iTRAQ tag” is covalently added onto the peptides via primary amines, which include peptide N-termini and lysine side chains.13 When labeled peptides are analyzed in a tandem mass spectrometer (MS/MS), a “signature ion” derived from the iTRAQ tags can be observed in a mass spectrum for quantification purposes. There are currently four versions of iTRAQ tags that can be used to label peptides from four different sources. When the mixture of labeled peptides is analyzed by MS/MS, relative ion intensities for the four signature ions with mass/charge (m/z) 114, 115, 116, and 117 are used to indicate relative changes in protein expression. The key feature of the iTRAQ tags is that they are isobaric, containing combinations of stable isotopes with identical total molecular mass. This elegant design ensures that iTRAQ-labeled peptides are treated as equal for chromatographic separation yet can be quantified by MS/MS analysis of the signature ions.14 In recent years, iTRAQ has been successfully used for diverse applications including identification of biomarkers and characterization of dynamic phosphorylation events.15-18
The aim of the present study was to delineate proteomic changes in the lumbar spinal cord during acute EAE in order to identify novel targets which merit further investigations and could potentially be useful for the design of new therapeutic interventions.
Materials and Methods
Induction of EAE
Eight-week old female Lewis rats were immunized with myelin basic protein emulsified in Complete Freund's Adjuvant (CFA), or CFA/vehicle as described before.3 Clinical symptoms were scored as follows: 1, tail weakness; 2, hindlimb weakness; 3, hindlimb paralysis; 4, quadriplegia; and 5, moribund. Animals exhibiting hindlimb paralysis (clinical score 3) were euthanized by exposure to CO2. The spinal cords were dissected, meninges were carefully removed, and the tissues were thoroughly rinsed with saline to remove blood. The lumbar spinal cord was immediately frozen on dry ice and conserved at −80 °C until use. All animal handling protocols were performed according to institutional guidelines.
Protein Extraction and iTRAQ Labeling
Lumbar spinal cords obtained from two CFA-treated controls and two rats affected by EAE were used for iTRAQ analysis. Fifteen milligrams of spinal cord tissue was homogenized in 300 μL of lysis buffer consisting of 25 mM triethylammonium bicarbonate, 20 mM Na2CO3, and 2 μL of protease inhibitor cocktail (Sigma, St Louis, MO). After centrifugation at 19 000g for 30 min, the supernatants were collected and adjusted to pH 8.0 with HEPES. Protein concentrations were measured using Bradford assay. Ninety micrograms of soluble proteins from each sample was sequentially reduced, alkylated, digested, and labeled with the four iTRAQ reagents (termed here tags 114, 115, 116, and 117) according to the manufacturer's instructions (Applied Biosystems, ABI, Framingham, MA). Samples derived from two different control spinal cords were labeled with tag 114 and tag 115, whereas samples obtained from two individual EAE spinal cords were labeled with tag 116 and tag 117. The labeled peptides were combined and separated by strong cation exchange chromatography followed by reversed phase (RP) liquid chromatography as described previously.19
Mass Spectrometry
The peptides eluted from the RP column were mixed via a micro-tee fitting in a 1:2 ratio with Matrix-Assisted Laser Desorption Ionization (MALDI) matrix consisting of 6 mg/mL α-cyano-4-hydroxycinnamic acid in 60% acetonitrile, 0.1% trifluoroacetic acid, 5 mM ammonium monobasicphosphate, 50 fmol/μL each of glu-fibrinogen peptide (m/z 1570.677) and adrenocorticotrophin hormone fragment 18–39, (m/z 2465.199), as internal calibrants. The mixture was spotted at 10 s intervals with a Probot spotting device (Dionex, Sunnyvale, CA) onto Opti-TOF MALDI target plates (ABI). Peptide analysis was performed on a 4700 Proteomics Analyzer tandem mass spectrometer (ABI) in a data-dependent fashion, where MS spectra (m/z 800–3000) were acquired in positive ion mode with internal mass calibration. The 10 most intense MS ions (S/N ratio >50) per spot were selected for subsequent MS/MS analysis in 1 keV mode. Each spectrum was averaged over 3000 laser shots and smoothed with the Savitsky-Golay algorithm (fwhm = 9, polynomial order = 4).
Protein Database Search and Bioinformatics
GPS Explorer software (v. 3.5, ABI) was used to process the MS/MS spectra and to submit peak lists to MASCOT (v. 1.9) search engine for peptide identification. The following search parameters were used: trypsin with one missed cleavage, mass tolerance of 100 ppm for the precursors, and 0.3 Da for the MS/MS ions. Labeling of lysines and peptide N-termini by the iTRAQ reagent and alkylation of cysteines by methane-methylthiosulfonate were set as fixed modifications, while oxidized methionines and iTRAQ-labeled tyrosines were set as variable modifications. MS/MS data were searched against rodent proteins in Swiss-Prot database (v. 46). In the event that one spectrum matched to a mouse sequence, the sequence was searched in Basic Local Alignment Search Tool (BLAST) against rat sequences in the International Protein Index (IPI) database to find the corresponding rat protein (http://www.ebi.ac.uk/IPI/). Only peptides identified with confidence interval (C.I.) values ≥95% were used for protein identification and quantification. If the peptides were shared by multiple proteins, they were assigned by GPS Explorer to the corresponding top ranked proteins. To reduce false identification, we chose to report only proteins containing at least two matched peptides.
Protein Quantification
Changes in the expression of peptides and proteins (EAE/control ratios) were calculated as described previously.19 In brief, the iTRAQ report peak areas (RPAs) corresponding to quantification ions m/z 114–117 were extracted from the raw spectra and corrected for isotopic carryover using GPS Explorer. Assuming comparable overall protein concentration in each sample, individual RPA was normalized according to the population median for each sample. Protein expression ratios were computed as the average of selected corresponding peptide ratios using a bioinformatics workflow described previously.19 Protein expression ratio distributions between the animals were summarized in Supporting Information Table 1. SC (control to control protein ratio standard deviation) = 0.25 and SE (EAE to EAE protein ratio standard deviation) = 0.31, and the pooled within group standard deviation SP is computed as 0.28 by the formula below:
(Sp is the pooled standard deviation, ni is the sample size of the ith group, si is the standard deviation of the ith group, and k is the number of groups being combined.)
Table 1.
Proteins Implicated in Multiple Sclerosis and Its Animal Models
| protein | accession number |
peptide count |
ratio | p-value | multiple sclerosis |
EAE |
|---|---|---|---|---|---|---|
| Apolipoprotein D | P23593 | 3 | 1.4 | 9.1E-3 | Reindl et al.71 Chabas et al.72 |
Alt et al.60 |
| Apolipoprotein E | P02650 | 5 | 2.5 | 3.0E-3 | Carlin et al.73 Hammack et al.8 |
Alt et al.60 |
| Ca2+/Calmodulin kinase IIα | P11275 | 2 | 0.6 | 3.1E-2 | Lock et al.1 | |
| Cathepsin B | P00787 | 3 | 1.5 | 3.3E-2 | Bever et al.74 Bever and Garver75 |
|
| Ceruloplasmin | P13635 | 7 | 2.8 | 2.4E-3 | Hunter et al.76 | Alt et al.60 |
| Complement C3 | P01026 | 12 | 2.2 | 3.8E-3 | Gay48 | Alt et al.60 Ibrahim et al.39 |
| Cystatin C | P14841 | 2 | 2.5 | 3.0E-2 | Irani et al.77 Tajouri et al.38 |
|
| Excitatory amino acid trasporter 2 | P31596 | 6 | 0.7 | 2.6E-2 | Pitt et al.47 Vallejo-Illarramendi et al.78 |
Ohgoh et al.79 |
| Fibronectin | P04937 | 5 | 2.6 | 3.3E-2 | Sobel and Mitchell80 Van Horssen et al.81 |
Ibrahim et al.39 |
| Filamin A | IPI00409539 | 2 | 1.8 | 4.4E-2 | Lock et al.1 | Ibrahim et al.39 |
| Hemopexin | P20059 | 7 | 2.1 | 8.3E-3 | Hammack et al.8 | |
| α2-HS Glycoprotein | P24090 | 3 | 2.3 | 1.0E-2 | Donelan et al.82 | |
| IgG-2A chain C region | P20760 | 2 | 3.2 | 2.2E-2 | Lock et al.1 | |
| L-Plastin | Q5XI38 | 6 | 2.3 | 1.5E-2 | Alt et al.60 | |
| α2-Macroglobulin | P06238 | 2 | 2.3 | 3.8E-2 | Jensen et al.44 | Ibrahim et al.39 |
| Moesin | O35763 | 6 | 1.5 | 3.1E-2 | Tajouri et al.40 | Ibrahim et al.39 |
| Peptidyl-prolyl cis-trans isomerase B | P24368 | 2 | 1.4 | 4.2E-2 | Ramanathan et al.83 | |
| Proteasome activating complex subunit | Q63797 | 2 | 2.6 | 2.7E-3 | Ibrahim et al.39 | |
| Serum albumin | P02770 | 30 | 3.4 | 1.4E-2 | Hammack et al.8 | Juhler et al.84 |
| Serotransferrin | P12346 | 20 | 2.5 | 1.4E-2 | LeVine et al.85 Tajouri et al.38 |
|
| Vimentin | P31000 | 10 | 1.5 | 5.5E-3 | Holley et al.86 Yamada et al.87 |
For each protein, a p-value was also generated via Student's t test. The significantly changed proteins met two criteria (1) t test p-values ≤ 0.05, and (2) protein ratios ≥1.4-fold increase or ≤0.7-fold decrease (i.e., ratio outside of 1.65 SP, corresponding to the top 5% increased and bottom 5% decreased proteins20 in EAE versus control groups).
Western Blot Analysis
Ten to thirty micrograms of protein extracted from the lumbar spinal cord of controls and EAE rats (n = 3) was subjected to 10% SDS-PAGE or 3–8% NuPAGE Novex Tris-acetate gel (Invitrogen, Carlsbad, CA), depending on the molecular weight of the protein of interest. The proteins were electroblotted onto immobilon-P (Millipore, Billerica, MA) or polyvinylidene difluoride membranes (PVDF, Invitrogen). The membranes were probed with antibodies against proteasome activating complex subunit (PA28, Santa Cruz Biotechnology, Santa Cruz, CA, 1:200 dilution), protein phosphatase 2A inhibitor (I2PP2A, Santa Cruz, 1:200 dilution), apolipoprotein E (ApoE, Abcam, Inc., Cambridge, MA, 1:2000 dilution), vimentin (BD Biosciences, San Jose, CA; 1:2000 dilution), Ca2+/calmodulin kinase IIα (CaMKIIα, Abcam, Inc., 1:2000 dilution), moesin (Abcam, Inc., 1:500 dilution), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Research Diognostics, Inc., Concord, MA, 1:2000 dilution). Secondary antibody-coupled signals were detected using the ECL chemiluminescence method (Perkin-Elmer, Boston, MA). Quantification of band densities was performed using Quantity One software (v. 4.3.1, Bio-Rad, Hercules, CA).
Immunohistochemistry
Control and EAE rats were perfused with saline followed by 4% paraformaldehyde in phosphate buffer (pH 7). The tissue was post-fixed, cryoprotected, frozen on dry ice, and sectioned on a cryostat. Ten μm transverse spinal cord sections, obtained from control and EAE rats (clinical score 3), were mounted side by side on the same slide. The sections were blocked in phosphate buffered saline (PBS; 0.1 M, pH 7.4), containing 15% heat inactivated serum and 0.3% Triton X-100, for 1 h at room temperature. They were then incubated with the anti-PA28 antibody (1:200 dilution), and either the Neu N antibody (Chemicon, Temecula CA; 1:500 dilution) or the ED-1 antibody (Serotec, Raleigh NC; 1:200) which are markers specific for neurons and activated microglia/macrophages, respectively. Incubation was performed overnight at 4 °C. The sections were then washed with PBS and incubated with FITC-labeled anti-rabbit IgG (Vector, Burlingame, CA; 1:200 dilution) and biotinylated anti-mouse IgG (Vector, 1:200 dilution) for 1 h at room temperature. After PBS washes, Avidin-Texas Red (4 μg/mL) was applied for 20 min at room temperature. The sections were washed in PBS and coverslipped using Vectashield (Vector). Three controls and three EAE rats were used for these investigations.
Results
Identification of Differentially Expressed Proteins in EAE
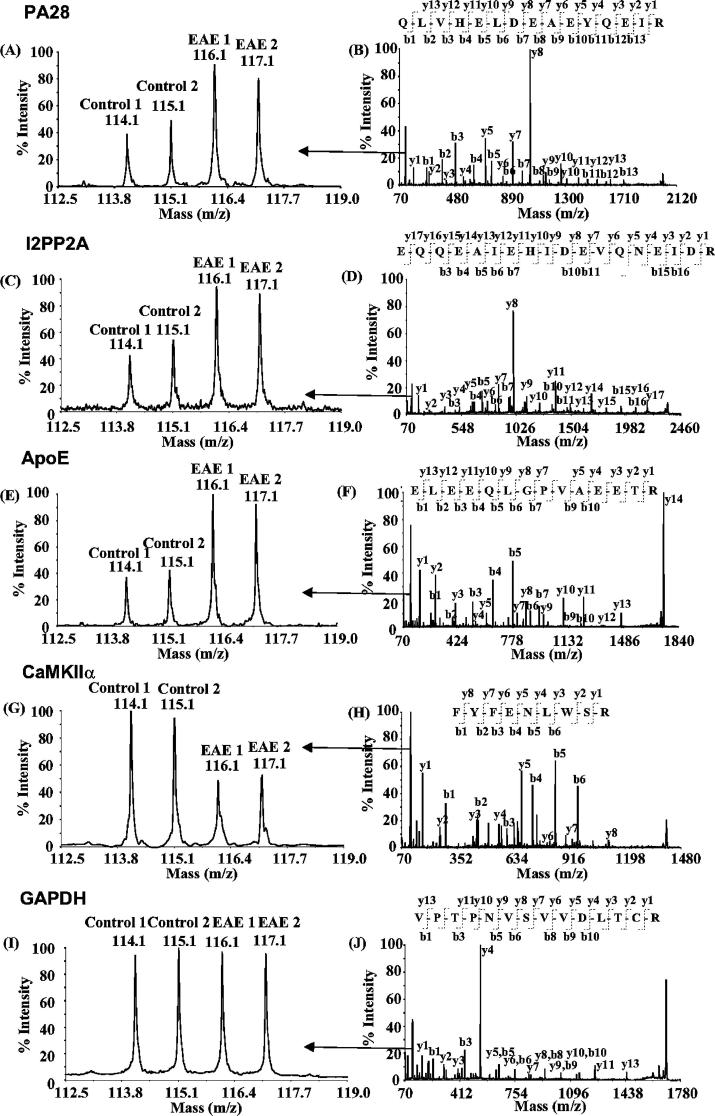
To delineate the profiles of differentially expressed proteins, we individually labeled 90 μg of digested proteins obtained from EAE and control rat spinal cords with the four iTRAQ reagents. Identification of proteins was based on the presence of at least two peptides with C.I. values at or above 95%. In total, 510 proteins were identified from 2488 unique peptide sequences deduced from 13 834 MS/MS spectra. To estimate the false positive rate for protein identification, all spectra were searched against a decoy database with all Swiss-Prot sequences reversed. Sixteen peptides from 8 proteins were identified, corresponding to an estimated false positive rate of 3.1%.21 Five representative MS/MS spectra of the peptides corresponding to PA28 (Q63797), I2PP2A (Q63945), ApoE (P02650), CaMKIIα (P11275), and GAPDH (P04797) are shown in Figure 1. The corresponding peptide sequences were manually confirmed using Data Explorer software (ABI). The quantification of the peak areas of the reporter ions, m/z 114 and 115, or 116 and 117, in the MS/MS spectra illustrate the changes in the abundance of the peptide in control versus EAE proteins. The levels of several proteins including PA28; a key constituent of the immunoproteasome (Figure 1A,B); I2PP2A, an important protein phosphatase inhibitor (Figure 1C,D); and ApoE, a protein involved in lipid uptake, transport, and delivery (Figure 1E,F), were significantly increased. In contrast, the levels of other proteins including CAMKIIα were either reduced (Figure 1G,H), or, as in the case of GAPDH, unaltered (Figure 1I,J).
Figure 1.
MS/MS spectra of selected peptides. PA28 (A and B), I2PP2A (C and D), and ApoE (E and F) were upregulated, whereas CaMK II α (G and H) was down-regulated in the spinal cord of rats exhibiting EAE. GAPDH (I and J) was not changed. Peptide sequences were deduced from the MS/MS spectra (B, D, F, H, and J) based on the observation of continuous series of either N-terminal (b series) or C-terminal (y series) ions. The peak areas of iTRAQ quantification ions, m/z 114–117 were used to measure the relative abundance of individual peptides (A, C, E, G, and I).
Using a bioinformatics workflow which we developed previously,19 we identified 41 differentially expressed proteins in EAE. Comparison with other published reports indicated that 21 of these proteins have previously been implicated in either EAE and/or multiple sclerosis (Table 1). These earlier observations were mostly based on microarray analysis and other conventional biochemical approaches. Notably, we have also identified 20 differentially expressed proteins which are ascribed to EAE for the first time (Table 2). These proteins, which play roles in inflammation, neuronal protection or damage, cytoskeletal integrity, and protein synthesis, may be targets for future investigations.
Table 2.
Proteins Not Previously Ascribed to Multiple Sclerosis and EAE
| protein | accession number |
peptide count |
ratio | p-value |
|---|---|---|---|---|
| 1. Inflammation | ||||
| Annexin III | P14669 | 3 | 2.8 | 4.4E-3 |
| Contrapsin-like protease inhibitor 3 | P05544 | 2 | 3.1 | 7.7E-3 |
| 78 kDa Glucose-regulated protein | P06761 | 9 | 1.4 | 3.7E-2 |
| α1-Inhibitor III | P14046 | 12 | 2.9 | 2.2E-2 |
| Lysozyme C | Q05820 | 2 | 5.7 | 1.4E-2 |
| Nucleophosmin | P13084 | 2 | 2.1 | 3.8E-2 |
| T-Kininogen | P01048 | 3 | 2.6 | 2.5E-3 |
| 2. Neuronal Protection and Damage | ||||
| Hepatoma-derived growth factor | Q8VHK7 | 2 | 1.4 | 3.3E-2 |
| Phosphatase 2A inhibitor | Q63945 | 2 | 1.9 | 3.9E-2 |
| Protein disulfide isomerase | P04785 | 7 | 1.5 | 2.5E-3 |
| Talin 1 | IPI00362014 | 2 | 1.6 | 3.0E-2 |
| 3. Cytoskeletal Integrity and Interactions with Membrane | ||||
| Coactosin-like protein | IPI00365323 | 2 | 1.6 | 2.0E-2 |
| myosin heavy chain A (non-muscle) | Q62812 | 8 | 1.4 | 9.3E-3 |
| 4. Protein Synthesis | ||||
| EF-hand containing protein 2 | IPI00200410 | 4 | 1.4 | 3.5E-3 |
| Eukaryotic initiation factor 4A-I | IPI00369618 | 6 | 1.4 | 1.8E-2 |
| 40S Ribosomal protein S18 | P62271 | 2 | 1.8 | 1.1E-2 |
| 40S Ribosomal protein SA | P38983 | 4 | 1.6 | 4.8E-3 |
| 60S Ribosomal protein L10 | Q6PDV7 | 2 | 1.6 | 7.8E-3 |
| 5. Other | ||||
| Cytosol aminopeptidase | IPI00471530 | 4 | 1.4 | 2.0E-2 |
| α-1B-Glycoprotein | Q9EPH1 | 2 | 3.4 | 1.9E-2 |
Corroboration of the Results by Western Blot Analysis
To corroborate the results obtained by the iTRAQ approach, we quantified the levels of six differentially expressed proteins, PA28, I2PP2A, ApoE, vimentin, CaMKIIα, and moesin, in EAE and control spinal cord by Western blot analysis. GAPDH was used as a control for sample loading and other experimental variations. Antibodies against PA28 (∼33 kDa), I2PP2A (∼37 kDa), CaMKIIα (∼59 kDa), and GAPDH (∼40 kDa) visualized bands of expected molecular weights bothȈin controls and EAE samples (Figure 2A). The signals obtained with the antibodies against ApoE and vimentin were below detection level in controls but clearly discernible in EAE spinal cord extracts and corresponded to anticipated molecular weights (∼38 and 57 kDa, respectively). In the case of moesin, in addition to a prominent band at the expected molecular weight (∼77 kDa), a weaker band of ∼50 kDa was visible only in EAE but not control samples. This new band might be derived from the proteolysis of moesin or may be a moesin isoform which is induced in the EAE spinal cord. Alternatively, it may represent changes due to differential RNA splicing. Measurement of band densities by use of Quantity One software (Bio-Rad) indicated a 4.4- and 2.8-fold increase in PA28 and I2PP2A levels, respectively, and a 0.6-fold decrease in CaMKIIα levels (Figure 2B). The fold-increase in ApoE and vimentin could not be determined since signal was not measurable in controls. Although there was no significant augmentation in the levels of the ∼77 kDa moesin band in EAE versus controls, when the 77 and 50 kDa bands were taken together, a significant 22% increase was observed in EAE (Control: 100 ± 8%; EAE 122 ± 3.8%, p < 0.03 by Student's t test). In contrast, GAPDH levels were not altered in EAE. Thus, Western blot analysis confirmed the results produced by iTRAQ analysis, although the fold changes obtained by the two approaches were slightly different from each other.
Figure 2.
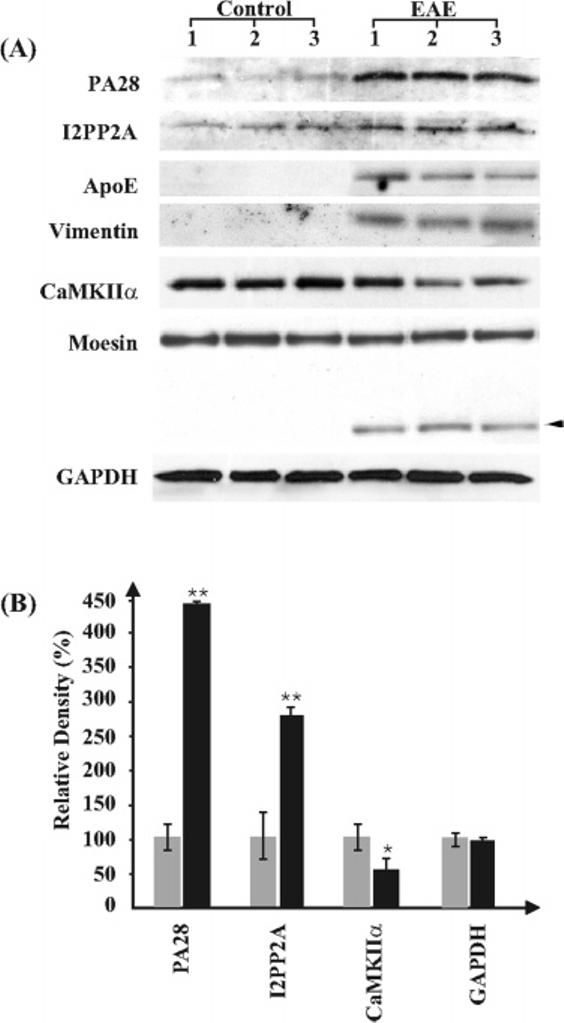
Western blot analysis of select proteins which are differentially expressed in the rat spinal cord during EAE. (A) Representative blots showing bands at molecular weights corresponding to PA28 (∼33 kDa), I2PP2A (∼37 kDa), ApoE (∼38 kDa), vimentin (∼57 kDa), CaMKIIα (∼59 kDa), and GAPDH (∼40 kDa). The antibody to moesin detected two bands at 77∼and ∼50 kDa (arrowhead). The lower band was present only in the EAE spinal cord extracts. Each lane represents protein obtained from the lumbar spinal cord of distinct animals. (B) Graphic representation of the results obtained by Western blots after quantification of band densities using Quantity One software (Bio-Rad). Gray columns are controls and black columns are EAE samples. GAPDH was used as a control for experimental variations including sample loading, n = 3. Significantly different from controls * p < 0.05 and ** p < 0.02 by Student's t test.
Expression and Localization of PA28 in the Spinal Cord
To further validate the results obtained by iTRAQ and Western blots and to determine the cellular localization of PA28, we performed immunocytochemistry (Figure 3). We focused on PA28 because studies suggest that it plays critical roles in antigen processing, an important step associated with antigen presentation. It is believed that presentation of antigen by microglia/macrophages to T cells is a major contributor to EAE pathogenesis.
Figure 3.
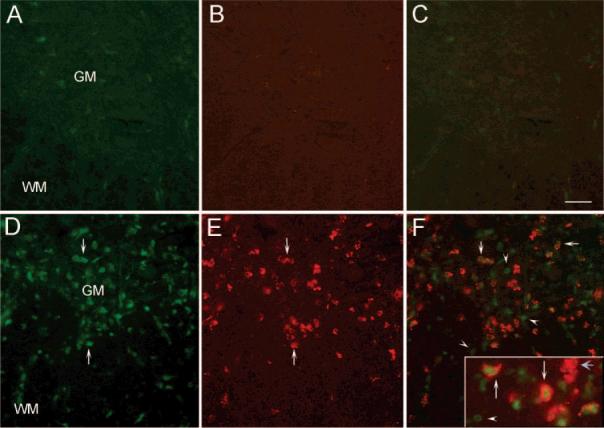
Expression of PA28 in activated microglia. (A) A transverse section of control spinal cord revealed very low PA28 staining only in occasional cells. (B) Labeling of the same section with the ED-1 antibody revealed the absence of activated microglia in controls. (C) The combined picture of PA28 and ED-1 staining. (D) In contrast, a marked increase in cells expressing PA28 was seen in spinal cord sections obtained from EAE rats (arrows). (E) As expected, there was also a noticeable increase in ED-1 positive cells which may either be activated microglia or infiltrating peripheral macrophages (arrows). (F) Combination of the two staining showed co-localization of ED-1 and PA28 immunoreactivity (arrows) indicating induction of PA28 in activated microglia or macrophages. However, there were also PA28 positive cells, which were not ED-1 positive (arrowheads), and ED-1 positive cells which were not PA28 positive (blue arrow in inset). Inset is the high magnification picture of an area in the spinal cord showing the details of the co-localization. N = 3; GM: Gray matter, WM: White matter. Bar represents 100 μm for A–F and 60 μm for inset.
PA28 immunoreactivity was low in sections obtained from control spinal cords and could be observed only in occasional cells in the white or gray matter (Figure 3A). In contrast, there was a pronounced increase in PA28 positive cells both in the gray and white matter of EAE spinal cords (Figure 3D). As some of the immunoreactivity appeared to be associated with small cells morphologically similar to macrophages or activated microglia, we labeled the same sections with the ED-1 antibody, a marker for activated but not resting microglia and macrophages. As expected, ED-1 did not label cells in the control spinal cord (Figure 3B). However, a sharp increase in ED-1 positive cells was observed in the EAE spinal cord (Figure 3E). A vast majority of these cells were also positive for PA28 in EAE but not control spinal cords (Figure 3C,F). However, there were also some PA28 negative and ED-1 positive cells as well as many PA28 positive and ED-1 negative cells. These results indicate that PA28 expression is upregulated only in a subpopulation of activated microglia or infiltrating macrophages. Moreover, some additional cell type(s) also express increased PA28 levels. Double labeling of sections with anti-PA28 and Neu N antibody, a neuronal marker, ruled out the possibility of expression in neurons as the two labels were not co-localized (Figure 4).
Figure 4.
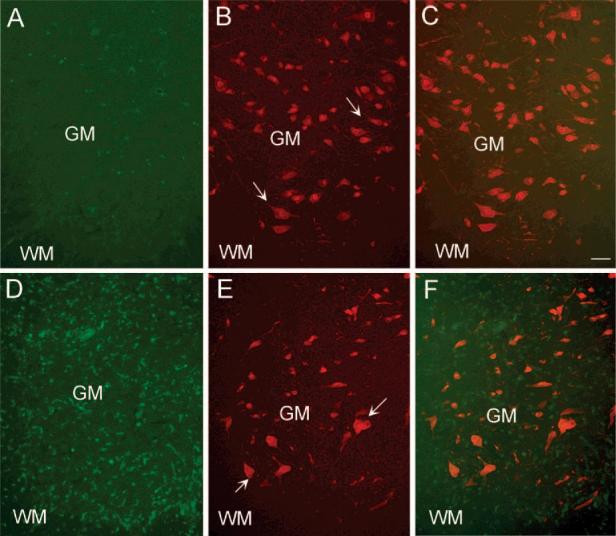
Absence of co-localization of PA28 with neurons in the spinal cord during EAE. (A) A control section showing a ventral region in the lumbar spinal cord comprising both the white matter (WM) and gray matter (GM). PA28 staining was mostly at background level as also shown in Figure 3. (B) Neu N positive neurons in the same section shown in panel A. Arrows point at some examples of Neu N positive cells. (C) The combined picture of PA28 and Neu N staining. (D) Low magnification picture of a transverse spinal cord section obtained from an EAE rat (clinical score 3) showing the dramatic increase in PA28 positive cells both in white and in gray matter. (E) Neu N immunoreactive cells in the same section presented in panel D. Arrows point at some examples. (F) The combined picture of PA28 and Neu N staining. Note that the staining is not co-localized, indicating the absence of PA28 in neuronal cells. Bar represents 100 μm. N = 3.
Discussion
iTRAQ analysis of the spinal cord in acute EAE revealed a complex and functionally diverse profile of 41 differentially expressed proteins. Some of these proteins including serotransferrin, serum albumin, complement C3, α2-macroglobulin, ceruloplasmin, and hemopexin are abundant in blood. The increase in the levels of these proteins may be due to the opening of the blood–brain barrier and the subsequent serum extravasation (Table 1). Other differentially expressed proteins have been implicated in inflammation (Table 2), and the increase in their expression may be the consequence of infiltration of inflammatory cells or glial activation. In addition, a few proteins have previously been associated with either neurodegeneration or neuroprotection, highlighting the concomitant occurrence of both beneficial and deleterious mechanisms in autoimmune demyelinating disease of the CNS (Table 1).
Among the differentially expressed proteins, some have been formerly associated with multiple sclerosis and/or its animal models, whereas others are implicated for the first time in EAE. The confirmation of earlier published findings by iTRAQ validates the usefulness of this approach for the characterization of novel targets in this animal model.
Corroboration of Previous Findings by iTRAQ Analysis
iTRAQ analysis detected 21 differentially expressed proteins which have been previously ascribed to multiple sclerosis, other EAE models or both (Table 1), primarily by use of microarray analysis or other conventional approaches. Some of these proteins play a role in neuroprotection and others in neural damage.
Among proteins involved in neuroprotection are apolipoprotein D (ApoD) and apolipoprotein E (ApoE),22,23 whose levels were increased by 1.4- and 2.5-fold, respectively. This lipoprotein family is involved in lipid uptake, transport, and delivery as well as cholesterol metabolism, processes necessary for myelin repair. In addition, they have been implicated in neuronal growth and axonal regeneration,24-26 immunomodulation,27,28 and antioxidant activity.29,30 Administration of ApoE to EAE animals attenuates symptoms, pro-inflammatory cytokine production, demyelination, and lymphocyte influx, and enhances remyelination.31 In agreement with these findings, EAE disease course, CNS lesions, and lymphocyte proliferation are exacerbated in ApoE-knockout mice.28 The ApoE4 allele is associated with clinical severity,32 disease progression,33 brain atrophy, and axonal damage in multiple sclerosis patients,34-36 although this link has recently been disputed.37 Taken together, these findings suggest that the elevated levels of apolipoprotein may be a compensatory strategy which promotes neuroprotective and reparative processes in EAE.
We found that moesin protein levels in the EAE spinal cord were 1.5-fold higher than controls, as assessed by iTRAQ analysis. This is in agreement with microarray studies showing an increase in moesin transcript levels in multiple sclerosis and in a mouse EAE model.38,39 However, the results of Western blots indicate that the increase is mostly due to the appearance of a new band of lower molecular weight, only in the EAE spinal cord. As the identity of this band is not yet elucidated, further studies are necessary to determine the significance of the changes observed. Induction of moesin isoforms in EAE may reflect an attempt by damaged axons to regenerate, as this protein has been implicated in the regenerative response of neurons to injury40 and axonal growth.41 Alternatively, if the band of lower molecular weight is the result of proteolytic cleavage, the changes may be associated with axonal damage rather than regeneration.
α2-Macroglobulin (α2-M), a proteinase inhibitor, has been shown to attenuate EAE symptoms when administered exogenously and may exert its beneficial effects by neutralizing proteinases involved in tissue damage or by directly interfering with antigen recognition due to its ability to bind myelin basic protein.42 In agreement with our studies showing a significant increase in α2-M protein levels, an upregulation in α2-M transcript levels in relapsing-remitting EAE has been reported.39 A rise in transformed α2-M has also been observed in the plasma of multiple sclerosis patients.43,44
In contrast, the reduction in the levels of excitatory amino acid transporter 2 (EAAT-2) may be associated with glutamate excitotoxicity, a trigger that causes damage to both axons and oligodendrocytes.45,46 Our findings are in agreement with the decrease in EAAT-2 mRNA and protein expression observed in acute and chronic multiple sclerosis lesions.47 These latter studies indicate that in normal brain, the predominant cell type expressing EAAT-2 are oligodendrocytes and that the reduction in EAAT-2 in multiple sclerosis lesions occurs in these cells.47
Other harmful mechanisms may involve increases in IgG. A significant increase in IgG-2A chain C was observed in our model. Cerebrospinal fluid and plaques of multiple sclerosis patients contain high IgG concentrations. Traditionally, the presence of IgG in multiple sclerosis has been associated with myelin opsonization, tissue destruction, and monocyte activation. IgG associates with complement C3, another protein upregulated in EAE as indicated by our analysis. Such IgG-C3 immune complexes have been identified in very early multiple sclerosis lesions48 and may promote demyelination.49
Potential Role of PA28 in Activated Microglia and Macrophages
One of the interesting findings of this study was the dramatic increase in PA28 protein expression and the association of this increase with activated microglia, the antigen presenting cells of the CNS or with infiltrating macrophages. The consequence of elevated PA28 levels in microglia during EAE is not known. It has been shown that PA28 is expressed in antigen presenting cells of the immune system.50,51 Interferon-γ, as well as TNF-α, two cytokines which play important roles in EAE and multiple sclerosis, regulate PA28 levels.52,53 PA28 plays a critical role in MHC class I antigen processing54 and activates the proteasome complex to produce peptides which are presented to T-cells via MHC I. Therefore, it is possible that the elevated PA28 expression in activated microglia or macrophages is related to the generation of antigenic peptides for presentation to T-cells. Microglia found in multiple sclerosis lesions express high MHC II levels, suggesting that microglia-mediated antigen presentation occurs primarily via MHC II.55 To our knowledge, the involvement of PA28 in MHC II mediated antigen presentation by microglia has not yet been described and should be further investigated. Interestingly, in sporadic Parkinson's disease, another neurodegenerative condition involving activated microglia, the levels of PA28 remained low in the diseased brain and were comparable to controls.56 One possible interpretation is that an increase in PA28 in microglia depends on the type of stimulus that induces cellular activation in distinct pathological conditions. Another interpretation is that elevated PA28 is primarily associated with infiltrating macrophages rather than activated microglia in EAE. As the ED-1 antibody cannot differentiate between the two cell types, this latter possibility cannot be ruled out. We found that cells other than microglia also expressed PA28 in the EAE spinal cord. The cells were not Neu N positive excluding the association of PA28 with neurons. Thus, PA28 positive and ED-1 negative cells may be dendritic cells which are known to express PA28 and infiltrate the spinal cord during EAE.
Identification of Differentially Expressed Proteins in EAE
The identification of proteins which have previously been implicated in multiple sclerosis and EAE by iTRAQ indicated that the approach may be useful to detect other proteins playing a role in EAE but not yet studied in this disease. The examples given below illustrate how the present results open new avenues for novel investigations.
Among the differentially expressed proteins, annexin III may be of special interest. We found that the levels of annexin III are significantly upregulated (2.8-fold) in the spinal cord during acute EAE. Although the contribution of annexin III to multiple sclerosis or EAE pathology remains undefined, a role for other annexin isoforms in these diseases has been reported. Annexins (I–V) are a family of structurally related calcium-dependent phospholipid binding proteins. Annexins I, II, IV, and V are upregulated in multiple sclerosis, and the expression of annexin I is increased in EAE.57-61 Annexin I plays an anti-inflammatory role by inhibiting phospholipase A262 and has been implicated in neuroprotection against excitotoxicity.63 Annexin I is localized to activated microglia and astrocytes found in EAE lesions, and administration of a peptide derived from annexin I attenuates the clinical severity of the disease.58 Annexin II mediates microglial activation induced by tissue plasminogen activator, a process that might have neurotoxic effects.64 Other roles attributed to annexins include suppression of autoimmune T-cell autoactivation,65 regulation of ion channels, and maintenance of extracellular matrix.66 In view of these findings, the involvement of annexin III in EAE or multiple sclerosis warrants further study. These future investigations may be especially important with respect to the role of microglia in axonal injury during multiple sclerosis and EAE as axonal transection induces a dramatic increase in annexin III in microglia.67
Finally, the increase in the levels of hepatoma-derived growth factor (HDGF) in EAE is noteworthy. Although HDGF is best known for its mitogenic effects on various cell types, recent studies indicate that it can act as a neurotrophic factor promoting neuronal survival, in vitro and in vivo, and is expressed in neuronal nuclei.68,69 Importantly, HDGF supports the survival of spinal cord motor neurons and enhances neurite extension in primary cultures.69 The expression of HDGF is increased in motor neurons in two animal models of motor neuron degeneration during presymptomatic stages. Therefore, it has been hypothesized that HDGF plays a neuroprotective role prior to the degeneration of motor neurons.69 The increase in this neurotrophic factor may be important in view of neuronal/axonal injury that occurs in the spinal cord during EAE.
Conclusions
Our data indicate that iTRAQ is an effective approach to study changes in complex biological processes related to neuroprotection, neuronal dysfunction, immune activation, and inflammation in EAE. The present study corroborated previous findings and identified a number of differentially regulated proteins which have been implicated in multiple sclerosis and/or other EAE models. Therefore, this method appears to be an efficient and reliable approach to detect novel molecular targets. However, iTRAQ may also have limitations in sensitivity. For example, we previously reported the differential expression of a number of neuronal proteins by a combination of microarray analysis, quantitative reverse transcription polymerase chain reaction (RT-PCR), and Western blots.3,70 The current proteomic analysis either did not detect some of these proteins or did not confirm their differential expression. Therefore, we conclude that the abundance of the proteins and the extent of the alterations in expression may be limiting factors for adequate quantification by this approach. Thus, it would be prudent to use this technique as a complementary method in conjunction with others such as microarray analysis and immunoblotting. Despite this, our initial discovery of modulated proteins not yet reported in the EAE or multiple sclerosis literature is significant. For future studies, it would be useful to investigate possible changes in these proteins in other EAE models and in post-mortem multiple sclerosis brain and spinal cord. Most importantly, in vivo and in vitro studies need to be performed to elucidate the role of these new targets in different pathological processes that take place in EAE and potentially multiple sclerosis.
Acknowledgment
This research is supported in part by the NIH grants NS046593 to H.L. and NS046363 to S.E.
Footnotes
Supporting Information Available: Tables showing the statistical analysis of specimen to specimen protein expression ratios and the identification of differentially expressed proteins in EAE. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8(5):500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 2.Mix E, Ibrahim S, Pahnke J, Koczan D, Sina C, Bottcher T, Thiesen HJ, Rolfs A. Gene-expression profiling of the early stages of MOG-induced EAE proves EAE-resistance as an active process. J. Neuroimmunol. 2004;151:158–70. doi: 10.1016/j.jneuroim.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Nicot A, Ratnakar PV, Ron Y, Chen CC, Elkabes S. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain. 2003;126:398–412. doi: 10.1093/brain/awg041. [DOI] [PubMed] [Google Scholar]
- 4.Chambers G, Lawrie L, Cash P, Murray GI. Proteomics: a new approach to the study of disease. J. Pathol. 2000;192(3):280–8. doi: 10.1002/1096-9896(200011)192:3<280::AID-PATH748>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Abdi F, Quinn JF, Jankovic J, McIntosh M, Leverenz JB, Peskind E, Nixon R, Nutt J, Chung K, Zabetian C, Samii A, Lin M, Hattan S, Pan C, Wang Y, Jin J, Zhu D, Li GJ, Liu Y, Waichunas D, Montine TJ, Zhang J. Detection of biomarkers with a multiplex quantitative proteomic platform in cerebrospinal fluid of patients with neurodegenerative disorders. J. Alzheimer's Dis. 2006;9(3):293–348. doi: 10.3233/jad-2006-9309. [DOI] [PubMed] [Google Scholar]
- 6.Newcombe J, Eriksson B, Ottervald J, Yang Y, Franzen B. Extraction and proteomic analysis of proteins from normal and multiple sclerosis postmortem brain. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2005;815(1–2):191–202. doi: 10.1016/j.jchromb.2004.10.073. [DOI] [PubMed] [Google Scholar]
- 7.Dumont D, Noben JP, Raus J, Stinissen P, Robben J. Proteomic analysis of cerebrospinal fluid from multiple sclerosis patients. Proteomics. 2004;4(7):2117–24. doi: 10.1002/pmic.200300715. [DOI] [PubMed] [Google Scholar]
- 8.Hammack BN, Fung KY, Hunsucker SW, Duncan MW, Burgoon MP, Owens GP, Gilden DH. Proteomic analysis of multiple sclerosis cerebrospinal fluid. Mult. Scler. 2004;10(3):245–60. doi: 10.1191/1352458504ms1023oa. [DOI] [PubMed] [Google Scholar]
- 9.Avasarala JR, Wall MR, Wolfe GM. A distinctive molecular signature of multiple sclerosis derived from MALDI-TOF/MS and serum proteomic pattern analysis: detection of three biomarkers. J. Mol. Neurosci. 2005;25(1):119–25. doi: 10.1385/JMN:25:1:119. [DOI] [PubMed] [Google Scholar]
- 10.Noben JP, Dumont D, Kwasnikowska N, Verhaert P, Somers V, Hupperts R, Stinissen P, Robben J. Lumbar cerebrospinal fluid proteome in multiple sclerosis: characterization by ultra-filtration, liquid chromatography, and mass spectrometry. J. Proteome Res. 2006;5(7):1647–57. doi: 10.1021/pr0504788. [DOI] [PubMed] [Google Scholar]
- 11.Robinson WH, Fontoura P, Lee BJ, de Vegvar HE, Tom J, Pedotti R, DiGennaro CD, Mitchell DJ, Fong D, Ho PP, Ruiz PJ, Maverakis E, Stevens DB, Bernard CC, Martin R, Kuchroo VK, van Noort JM, Genain CP, Amor S, Olsson T, Utz PJ, Garren H, Steinman L. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat Biotechnol. 2003;21:1033–9. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 12.Duzhak T, Emerson MR, Chakrabarty A, Alterman MA, Levine SM. Analysis of protein induction in the CNS of SJL mice with experimental allergic encephalomyelitis by proteomic screening and immunohistochemistry. Cell. Mol. Biol. (Paris) 2003;49(5):723–32. [PubMed] [Google Scholar]
- 13.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics. 2004;3(12):1154–69. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Shadforth IP, Dunkley TP, Lilley KS, Bessant C. i-Tracker: for quantitative proteomics using iTRAQ. BMC Genomics. 2005;6:145. doi: 10.1186/1471-2164-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSouza L, Diehl G, Rodrigues MJ, Guo J, Romaschin AD, Colgan TJ, Siu KW. Search for cancer markers from endometrial tissues using differentially labeled tags iTRAQ and cICAT with multidimensional liquid chromatography and tandem mass spectrometry. J. Proteome Res. 2005;4(2):377–86. doi: 10.1021/pr049821j. [DOI] [PubMed] [Google Scholar]
- 16.Jones AM, Bennett MH, Mansfield JW, Grant M. Analysis of the defense phosphoproteome of Arabidopsis thaliana using differential mass tagging. Proteomics. 2006;6(14):4155–65. doi: 10.1002/pmic.200500172. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wolf-Yadlin A, Ross PL, Pappin DJ, Rush J, Lauffenburger DA, White FM. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Mol. Cell. Proteomics. 2005;4(9):1240–50. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, D'Mello V, Deng L, Hu J, Ricardo M, Pan S, Lu X, Wadsworth S, Siekierka J, Birge R, Li H. A multiplexed proteomics approach to differentiate neurite outgrowth patterns. J. Neurosci. Methods. 2006;158(1):22–9. doi: 10.1016/j.jneumeth.2006.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Qian J, Borisov O, Pan S, Li Y, Liu T, Deng L, Wannemacher K, Kurnellas M, Patterson C, Elkabes S, Li H. Optimized proteomic analysis of a mouse model of cerebellar dysfunction using amine-specific isobaric tags. Proteomics. 2006;6(15):4321–34. doi: 10.1002/pmic.200600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinbaum DG, K. LL, Muller KE, Nizam A. Applied Regression Analysis and Other Multivariable Methods. Duxbury Press; Pacific Grove, CA: 1998. p. 712. [Google Scholar]
- 21.Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2003;2(1):43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 22.Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, Warner DS, Laskowitz DT. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–45. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Aono M, Laskowitz D, Warner DS, Pearlstein RD. Apolipoprotein E protects against oxidative stress in mixed neuronal-glial cell cultures by reducing glutamate toxicity. Neurochem. Int. 2004;44(2):107–18. doi: 10.1016/s0197-0186(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 24.Boyles JK, Zoellner CD, Anderson LJ, Kosik LM, Pitas RE, Weisgraber KH, Hui DY, Mahley RW, Gebicke-Haerter PJ, Ignatius MJ, et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J. Clin. Invest. 1989;83(3):1015–31. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264(5160):850–2. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 26.Vance JE, Campenot RB, Vance DE. The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim. Biophys. Acta. 2000;1486(1):84–96. doi: 10.1016/s1388-1981(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 27.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J. Cereb. Blood Flow Metab. 1998;18(5):465–71. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Karussis D, Michaelson DM, Grigoriadis N, Korezyn AD, Mizrachi-Koll R, Chapman S, Abramsky O, Chapman J. Lack of apolipoprotein-E exacerbates experimental allergic encephalomyelitis. Mult. Scler. 2003;9(5):476–80. doi: 10.1191/1352458503ms950oa. [DOI] [PubMed] [Google Scholar]
- 29.Shea TB, Rogers E, Ashline D, Ortiz D, Sheu MS. Apolipoprotein E deficiency promotes increased oxidative stress and compensatory increases in antioxidants in brain tissue. Free Radical Biol. Med. 2002;33(8):1115–20. doi: 10.1016/s0891-5849(02)01001-8. [DOI] [PubMed] [Google Scholar]
- 30.Ramassamy C, Krzywkowski P, Averill D, Lussier-Cacan S, Theroux L, Christen Y, Davignon J, Poirier J. Impact of apoE deficiency on oxidative insults and antioxidant levels in the brain. Brain Res. Mol. Brain Res. 2001;86(1–2):76–83. doi: 10.1016/s0169-328x(00)00268-0. [DOI] [PubMed] [Google Scholar]
- 31.Li FQ, Sempowski GD, McKenna SE, Laskowitz DT, Colton CA, Vitek MP. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J. Pharmacol. Exp. Ther. 2006;318(3):956–65. doi: 10.1124/jpet.106.103671. [DOI] [PubMed] [Google Scholar]
- 32.Masterman T, Zhang Z, Hellgren D, Salter H, Anvret M, Lilius L, Lannfelt L, Hillert J. APOE genotypes and disease severity in multiple sclerosis. Mult. Scler. 2002;8(2):98–103. doi: 10.1191/1352458502ms787oa. [DOI] [PubMed] [Google Scholar]
- 33.Chapman J, Vinokurov S, Achiron A, Karussis DM, Mitosek-Szewczyk K, Birnbaum M, Michaelson DM, Korczyn AD. APOE genotype is a major predictor of long-term progression of disability in MS. Neurology. 2001;56(3):312–6. doi: 10.1212/wnl.56.3.312. [DOI] [PubMed] [Google Scholar]
- 34.Enzinger C, Ropele S, Smith S, Strasser-Fuchs S, Poltrum B, Schmidt H, Matthews PM, Fazekas F. Accelerated evolution of brain atrophy and “black holes” in MS patients with APOE-epsilon 4. Ann. Neurol. 2004;55(4):563–9. doi: 10.1002/ana.20027. [DOI] [PubMed] [Google Scholar]
- 35.Fazekas F, Strasser-Fuchs S, Schmidt H, Enzinger C, Ropele S, Lechner A, Flooh E, Schmidt R, Hartung HP. Apolipoprotein E genotype related differences in brain lesions of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2000;69(1):25–8. doi: 10.1136/jnnp.69.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Stefano N, Bartolozzi ML, Nacmias B, Zipoli V, Mortilla M, Guidi L, Siracusa G, Sorbi S, Federico A, Amato MP. Influence of apolipoprotein E epsilon4 genotype on brain tissue integrity in relapsing-remitting multiple sclerosis. Arch. Neurol. 2004;61(4):536–40. doi: 10.1001/archneur.61.4.536. [DOI] [PubMed] [Google Scholar]
- 37.Burwick RM, Ramsay PP, Haines JL, Hauser SL, Oksenberg JR, Pericak-Vance MA, Schmidt S, Compston A, Sawcer S, Cittadella R, Savettieri G, Quattrone A, Polman CH, Uitdehaag BM, Zwemmer JN, Hawkins CP, Ollier WE, Weatherby S, Enzinger C, Fazekas F, Schmidt H, Schmidt R, Hillert J, Masterman T, Hogh P, Niino M, Kikuchi S, Maciel P, Santos M, Rio ME, Kwiecinski H, Zakrzewska-Pniewska B, Evangelou N, Palace J, Barcellos LF. APOE epsilon variation in multiple sclerosis susceptibility and disease severity: some answers. Neurology. 2006;66(9):1373–83. doi: 10.1212/01.wnl.0000210531.19498.3f. [DOI] [PubMed] [Google Scholar]
- 38.Tajouri L, Mellick AS, Ashton KJ, Tannenberg AE, Nagra RM, Tourtellotte WW, Griffiths LR. Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain Res. Mol. Brain Res. 2003;119(2):170–83. doi: 10.1016/j.molbrainres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim SM, Mix E, Bottcher T, Koczan D, Gold R, Rolfs A, Thiesen HJ. Gene expression profiling of the nervous system in murine experimental autoimmune encephalomyelitis. Brain. 2001;124(Pt 10):1927–38. doi: 10.1093/brain/124.10.1927. [DOI] [PubMed] [Google Scholar]
- 40.Haas MA, Vickers JC, Dickson TC. Binding partners L1 cell adhesion molecule and the ezrin-radixin-moesin (ERM) proteins are involved in development and the regenerative response to injury of hippocampal and cortical neurons. Eur. J. Neurosci. 2004;20(6):1436–44. doi: 10.1111/j.1460-9568.2004.03620.x. [DOI] [PubMed] [Google Scholar]
- 41.Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J. Cell Biol. 1998;143(2):443–55. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter N, Weston KM, Bowern NA. Suppression of experimental allergic encephalomyelitis by alpha 2-macroglobulin. Immunology. 1991;73(1):58–63. [PMC free article] [PubMed] [Google Scholar]
- 43.Gunnarsson M, Sundstrom P, Stigbrand T, Jensen PE. Native and transformed alpha2-macroglobulin in plasma from patients with multiple sclerosis. Acta Neurol. Scand. 2003;108(1):16–21. doi: 10.1034/j.1600-0404.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 44.Jensen PE, Humle Jorgensen S, Datta P, Sorensen PS. Significantly increased fractions of transformed to total alpha2-macroglobulin concentrations in plasma from patients with multiple sclerosis. Biochim. Biophys. Acta. 2004;1690(3):203–7. doi: 10.1016/j.bbadis.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000;6(1):67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 46.Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 2000;6(1):62–6. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- 47.Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61(8):1113–20. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 48.Gay FW. Early cellular events in multiple sclerosis. Intimations of an extrinsic myelinolytic antigen. Clin. Neurol. Neurosurg. 2006;108(3):234–40. doi: 10.1016/j.clineuro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody-and complement-mediated demyelination. Ann. Neurol. 1998;43(4):465–71. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 50.Macagno A, Gilliet M, Sallusto F, Lanzavecchia A, Nestle FO, Groettrup M. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur. J. Immunol. 1999;29(12):4037–42. doi: 10.1002/(SICI)1521-4141(199912)29:12<4037::AID-IMMU4037>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 51.Ossendorp F, Fu N, Camps M, Granucci F, Gobin SJ, van den Elsen PJ, Schuurhuis D, Adema GJ, Lipford GB, Chiba T, Sijts A, Kloetzel PM, Ricciardi-Castagnoli P, Melief CJ. Differential expression regulation of the alpha and beta subunits of the PA28 proteasome activator in mature dendritic cells. J. Immunol. 2005;174(12):7815–22. doi: 10.4049/jimmunol.174.12.7815. [DOI] [PubMed] [Google Scholar]
- 52.Rivett AJ, Bose S, Brooks P, Broadfoot KI. Regulation of proteasome complexes by gamma-interferon and phosphorylation. Biochimie. 2001;83(3–4):363–6. doi: 10.1016/s0300-9084(01)01249-4. [DOI] [PubMed] [Google Scholar]
- 53.Hallermalm K, Seki K, Wei C, Castelli C, Rivoltini L, Kiessling R, Levitskaya J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood. 2001;98(4):1108–15. doi: 10.1182/blood.v98.4.1108. [DOI] [PubMed] [Google Scholar]
- 54.Strehl B, Seifert U, Kruger E, Heink S, Kuckelkorn U, Kloetzel PM. Interferon-gamma, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol. Rev. 2005;207:19–30. doi: 10.1111/j.0105-2896.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 55.Bo L, Mork S, Kong PA, Nyland H, Pardo CA, Trapp BD. Detection of MHC class II-antigens on macrophages and microglia, but not on astrocytes and endothelia in active multiple sclerosis lesions. J Neuroimmunol. 1994;51:135–46. doi: 10.1016/0165-5728(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 56.McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp. Neurol. 2003;179(1):38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 57.Bolton C, Elderfield AJ, Flower RJ. The detection of lipocortins 1, 2 and 5 in central nervous system tissues from Lewis rats with acute experimental allergic encephalomyelitis. J. Neuroimmunol. 1990;29(1–3):173–81. doi: 10.1016/0165-5728(90)90160-o. [DOI] [PubMed] [Google Scholar]
- 58.Huitinga I, Bauer J, Strijbos PJ, Rothwell NJ, Dijkstra CD, Tilders FJ. Effect of annexin-1 on experimental autoimmune encephalomyelitis (EAE) in the rat. Clin. Exp. Immunol. 1998;111(1):198–204. doi: 10.1046/j.1365-2249.1998.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Craner MJ, Lo AC, Black JA, Baker D, Newcombe J, Cuzner ML, Waxman SG. Annexin II/p11 is up-regulated in Purkinje cells in EAE and MS. Neuroreport. 2003;14(4):555–8. doi: 10.1097/00001756-200303240-00005. [DOI] [PubMed] [Google Scholar]
- 60.Alt C, Duvefelt K, Franzen B, Yang Y, Engelhardt B. Gene and protein expression profiling of the microvascular compartment in experimental autoimmune encephalomyelitis in C57Bl/6 and SJL mice. Brain Pathol. 2005;15(1):1–16. doi: 10.1111/j.1750-3639.2005.tb00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elderfield AJ, Newcombe J, Bolton C, Flower RJ. Lipocortins (annexins) 1, 2, 4 and 5 are increased in the central nervous system in multiple sclerosis. J. Neuroimmunol. 1992;39(1–2):91–100. doi: 10.1016/0165-5728(92)90178-n. [DOI] [PubMed] [Google Scholar]
- 62.Kim SW, Rhee HJ, Ko J, Kim YJ, Kim HG, Yang JM, Choi EC, Na DS. Inhibition of cytosolic phospholipase A2 by annexin I. Specific interaction model and mapping of the interaction site. J. Biol. Chem. 2001;276(19):15712–9. doi: 10.1074/jbc.M009905200. [DOI] [PubMed] [Google Scholar]
- 63.Black MD, Carey F, Crossman AR, Relton JK, Rothwell NJ. Lipocortin-1 inhibits NMDA receptor-mediated neuronal damage in the striatum of the rat. Brain Res. 1992;585(1–2):135–40. doi: 10.1016/0006-8993(92)91198-n. [DOI] [PubMed] [Google Scholar]
- 64.Siao CJ, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J. Neurosci. 2002;22(9):3352–8. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gold R, Pepinsky RB, Zettl UK, Toyka KV, Hartung HP. Lipocortin-1 (annexin-1) suppresses activation of autoimmune T cell lines in the Lewis rat. J. Neuroimmunol. 1996;69(1–2):157–64. doi: 10.1016/0165-5728(96)00086-0. [DOI] [PubMed] [Google Scholar]
- 66.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6(6):449–61. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 67.Konishi H, Namikawa K, Kiyama H. Annexin III implicated in the microglial response to motor nerve injury. Glia. 2006;53(7):723–32. doi: 10.1002/glia.20327. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Z, Yamamoto Y, Sugai F, Yoshida K, Kishima Y, Sumi H, Nakamura H, Sakoda S. Hepatoma-derived growth factor is a neurotrophic factor harbored in the nucleus. J. Biol. Chem. 2004;279(26):27320–6. doi: 10.1074/jbc.M308650200. [DOI] [PubMed] [Google Scholar]
- 69.Marubuchi S, Okuda T, Tagawa K, Enokido Y, Horiuchi D, Shimokawa R, Tamura T, Qi ML, Eishi Y, Watabe K, Shibata M, Nakagawa M, Okazawa H. Hepatoma-derived growth factor, a new trophic factor for motor neurons, is up-regulated in the spinal cord of PQBP-1 transgenic mice before onset of degeneration. J. Neurochem. 2006;99(1):70–83. doi: 10.1111/j.1471-4159.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- 70.Nicot A, Kurnellas M, Elkabes S. Temporal pattern of plasma membrane calcium ATPase 2 expression in the spinal cord correlates with the course of clinical symptoms in two rodent models of autoimmune encephalomyelitis. Eur. J. Neurosci. 2005;21(10):2660–70. doi: 10.1111/j.1460-9568.2005.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reindl M, Knipping G, Wicher I, Dilitz E, Egg R, Deisen-hammer F, Berger T. Increased intrathecal production of apolipoprotein D in multiple sclerosis. J. Neuroimmunol. 2001;119(2):327–32. doi: 10.1016/s0165-5728(01)00378-2. [DOI] [PubMed] [Google Scholar]
- 72.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294(5547):1731–5. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 73.Carlin C, Murray L, Graham D, Doyle D, Nicoll J. Involvement of apolipoprotein E in multiple sclerosis: absence of remyelination associated with possession of the APOE epsilon2 allele. J. Neuropathol. Exp. Neurol. 2000;59(5):361–7. doi: 10.1093/jnen/59.5.361. [DOI] [PubMed] [Google Scholar]
- 74.Bever CT, Jr., Panitch HS, Johnson KP. Increased cathepsin B activity in peripheral blood mononuclear cells of multiple sclerosis patients. Neurology. 1994;44(4):745–8. doi: 10.1212/wnl.44.4.745. [DOI] [PubMed] [Google Scholar]
- 75.Bever CT, Jr., Garver DW. Increased cathepsin B activity in multiple sclerosis brain. J. Neurol. Sci. 1995;131(1):71–3. doi: 10.1016/0022-510x(95)00039-5. [DOI] [PubMed] [Google Scholar]
- 76.Hunter MI, Nlemadim BC, Davidson DL. Lipid peroxidation products and antioxidant proteins in plasma and cerebrospinal fluid from multiple sclerosis patients. Neurochem. Res. 1985;10(12):1645–52. doi: 10.1007/BF00988606. [DOI] [PubMed] [Google Scholar]
- 77.Irani DN, Anderson C, Gundry R, Cotter R, Moore S, Kerr DA, McArthur JC, Sacktor N, Pardo CA, Jones M, Calabresi PA, Nath A. Cleavage of cystatin C in the cerebrospinal fluid of patients with multiple sclerosis. Ann. Neurol. 2006;59(2):237–47. doi: 10.1002/ana.20786. [DOI] [PubMed] [Google Scholar]
- 78.Vallejo-Illarramendi A, Domercq M, Perez-Cerda F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol. Dis. 2006;21(1):154–64. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 79.Ohgoh M, Hanada T, Smith T, Hashimoto T, Ueno M, Yamanishi Y, Watanabe M, Nishizawa Y. Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002;125(1–2):170–8. doi: 10.1016/s0165-5728(02)00029-2. [DOI] [PubMed] [Google Scholar]
- 80.Sobel RA, Mitchell ME. Fibronectin in multiple sclerosis lesions. Am. J. Pathol. 1989;135(1):161–8. [PMC free article] [PubMed] [Google Scholar]
- 81.van Horssen J, Bo L, Vos CM, Virtanen I, de Vries HE. Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol. Exp. Neurol. 2005;64(8):722–9. doi: 10.1097/01.jnen.0000173894.09553.13. [DOI] [PubMed] [Google Scholar]
- 82.Donelan NR, Seader E, Poopatana CA, Harris VK, Sadiq S. Fetuin-A Levels Are Elevated in Cerebrospinal Fluid of Patients with Secondary Progressive Multiple Sclerosis; 58th American Academy of Neurology Annual Meeting, Selected Proteomics Abstracts; San Diego, CA. April 1–8, 2006.2006. [Google Scholar]
- 83.Ramanathan M, Weinstock-Guttman B, Nguyen LT, Badgett D, Miller C, Patrick K, Brownscheidle C, Jacobs L. In vivo gene expression revealed by cDNA arrays: the pattern in relapsing-remitting multiple sclerosis patients compared with normal subjects. J. Neuroimmunol. 2001;116(2):213–9. doi: 10.1016/s0165-5728(01)00308-3. [DOI] [PubMed] [Google Scholar]
- 84.Juhler M, Laursen H, Barry DI. The distribution of immunoglobulins and albumin in the central nervous system in acute experimental allergic encephalomyelitis. Acta Neurol. Scand. 1986;73(2):119–24. doi: 10.1111/j.1600-0404.1986.tb03251.x. [DOI] [PubMed] [Google Scholar]
- 85.LeVine SM, Lynch SG, Ou CN, Wulser MJ, Tam E, Boo N. Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Res. 1999;821(2):511–5. doi: 10.1016/s0006-8993(98)01360-2. [DOI] [PubMed] [Google Scholar]
- 86.Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol. Appl. Neurobiol. 2003;29(5):434–44. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 87.Yamada T, Kawamata T, Walker DG, McGeer PL. Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathol. 1992;84(2):157–62. doi: 10.1007/BF00311389. [DOI] [PubMed] [Google Scholar]