Abstract
Background Context
Total disc replacements (TDRs) have been used to reduce pain and preserve motion. However, the comparison of polyethylene wear following long-term implantation to those tested using an in vitro model had not yet been investigated.
Purpose
The purpose of this study was to correlate wear and damage patterns in retrieved TDRs with motion patterns observed in a clinically validated in vitro lumbar spine model. We also sought to determine whether one-sided wear and motion patterns were associated with greater in vivo wear.
Study Design
This two-part study combined the evaluation of retrieved total disc replacements with a biomechanical study using human lumbar spines.
Patient Sample
38 CHARITÉ lumbar artificial discs were retrieved from 32 patients (24 female, 75%) after 7.3 years average implantation (range: 1.8 to 16.1y). The components were implanted at L2/L3 (n=1), L3/L4 (n=2), L4/L5 (n=20), and L5/S1 (n=15). All the implants were removed due to intractable back pain and/or facet degeneration. In addition, they were removed due to subsidence (n=10), anterior migration (n=3), core dislocation (n=2), lateral subluxation (n=1), endplate loosening (n = 2), and osteolysis (n=1). In parallel, 7 new implants were evaluated at L4-L5 and 13 implants at L5-S1 in an in vitro lumbar spine model.
Outcome Measures
Retrieval analysis included evaluation of clinical data, dimensional measurements and assessment of the extent and severity of PE surface damage mechanisms. In vitro testing involved the observation of motion patterns during physiological loading.
Methods
For the retrievals, each side of the PE core was independently analyzed at the rim and dome for the presence of machining marks, wear, and fracture. 35 cores were further analyzed using MicroCT to determine whether the wear was one-sided, or symmetrically distributed. For the in vitro study the new implants were tested under physiologic loads (flexion-extension with a compressive follower preload) using a validated cadaveric lumbar spine model. The center of the prosthesis was 2 mm posterior to the mid-point of the vertebral body endplate in mid-sagittal plane. Motion patterns of the in vitro-tested implants were tracked using sequential video-flouroscopy.
Results
Substantial variability was observed in the wear patterns of the retrievals. 15/35 retrieved cores (43%) displayed one-sided wear patterns. The median dome penetration was 0.2 mm (range: 0.06 to 0.9 mm) and the median penetration rate was 0.04 mm/y (range: 0.01 to 0.2 mm/y). No significant difference in penetration or penetration rate was observed between retrievals with one-sided and symmetric wear patterns (p >0.05). Significant correlations were observed between implantation time and penetration (rho = 0.46, p = 0.004) and penetration rate (rho = −0.48, p = 0.003). In the in vitro study, there was clear visual evidence of motion at both articulations in 8/20 implantations. In additional 8/20 cases, there was some evidence of motion at both articulations; however, the predominant motion occurred at the top articulation. In 4/20 implantations motion could be visually detected only at the top articulation. Core entrapment and pinching was observed in 7/20 cases as the segment was extended, and was associated with visual evidence of core bending or deformation in 5/20 cases.
Keywords: total disc arthroplasty, total disc replacement, lumbar spine, CHARITÉ, degenerative disc disease, artificial disc, in vitro motion response, wear, surface damage, fracture, impingement, ultra, high molecular weight polyethylene, UHMWPE
Introduction
Total disc replacement (TDR) is a technology to reduce pain and preserve motion in a degenerated symptomatic functional spinal unit. One of the basic tenets of disc arthroplasty, first postulated by Fernström in the 1960s [1], is that motion preservation would hopefully forestall, or even prevent, an accelerated, degenerative cascade of adjacent levels associated with spinal fusion. Despite decades of interest in this topic, it still remains to be seen whether the central premise of lumbar disc arthroplasty will be supported by clinical findings.
Historically, a limiting factor for spine arthroplasty has been the longevity of the bearing, both in terms of its ability to preserve long-term motion at the treated level, as well as in terms of its durability, wear resistance, and biocompatibility [2]. In the lumbar spine, the issues of long-term functional mobility and durability are paramount, as these constructs are currently implanted by an anterior approach [3]. As a result, the revision surgery to remove a failed lumbar total disc replacement represents both an arduous undertaking for the surgeon and a potentially life threatening risk for the patient [4–9].
Although many designs of lumbar disc replacement have been developed, as of yet, very few have established a long-term track record of clinical use [2]. The design of one lumbar disc arthroplasty, the CHARITÉ Artificial Disc, previously referred to as the SB III Charité Artificial Disc, has remained conceptually unchanged since its European commercial introduction in 1989, providing nearly two decades of clinical experience with this implant system [3]. Borrowing successful arthroplasty concepts from total hip and knee replacement, the CHARITÉ consists of two cobalt chrome alloy endplates, with a mobile bearing core fabricated from ultra-high molecular weight polyethylene (hereafter, polyethylene) [3]. Despite its long-term use, until recent years few studies have been published to elucidate the biomechanical function and clinical wear characteristics of mobile bearing, metal-on-polyethylene total disc arthroplasty [6, 9–16]. As an example of a disc technology with the potential for long-term clinical survival in the lumbar spine [17–19], the CHARITÉ provides an opportunity for research to improve existing wear test methodologies that are designed to predict such behavior [15]. It was in this context that we undertook to critically examine the in vivo wear performance of explants and further validate an established biomechanical testing protocol for artificial discs.
Previous research using five cadaveric spines has shown that motion patterns of spinal segments implanted with the CHARITÉ differ qualitatively from the intact lumbar spine [13]. Furthermore, both one-sided and symmetric motion patterns were observed for the mobile polyethylene core during previous in vitro testing [13]. When the core motion was symmetric, motion occurred between both the superior and inferior endplates and the core, suggesting that the center of rotation was observed closer to the center of the artificial disc (Fig. 1). When the core motion was one-sided, it occurred exclusively between the superior endplate and the polyethylene core, suggesting that the center of rotation was located caudal with respect to the inferior endplate (Fig. 1). It has also been previously observed that CHARITÉ motions from an in vitro spine model were consistent with clinical radiographic observations [13]. Previous analyses of retrieved CHARITÉ polyethylene cores showed evidence of dome wear and/or rim fracture, mechanisms previously observed in hip and knee replacements, respectively [6, 9, 14–16]. However, the ramifications and unifying elements of these diverse motion and damage patterns were previously unexplored.
Figure 1.
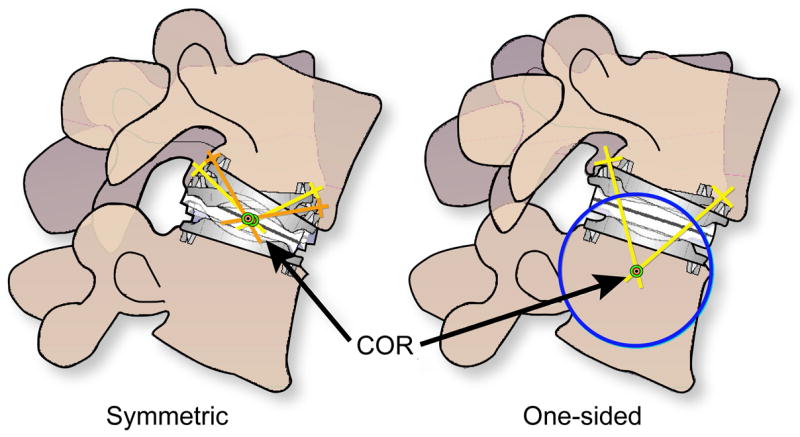
Implications of symmetric vs. one-sided motion patterns for the center of rotation in a mobile bearing TDR. A symmetric wear pattern suggests that the center of rotation is near the center of the mobile bearing. In a one-sided wear pattern, in which motion occurs predominantly between the superior endplate and the superior face of the core, the center of rotation is expected below the inferior endplate.
In the simplest theoretical model for wear, the magnitude of wear is proportional to the product of load (i.e., applied forces) and sliding distance (i.e., joint motion) [20]. This theory, known among scientists who study wear as “Archard’s Law,” has been validated for polyethylene total hip replacement components [21]. Because the domed surfaces of TDR have previously been shown to exhibit hip-like wear in vivo [15, 16], Archard’s law is a reasonable starting point for a conceptual model of dome wear in total disc arthroplasty. According to Archard’s wear theory, in order to predict the amount of wear in a disc arthroplasty, with all other factors being equal (i.e., lubrication conditions, friction, etc.), we would need to understand both the magnitude of the force and the joint motion.
In an individual disc replacement, we can take advantage of the fact that the resultant force of the superior endplate pressing on the superior face of the core is equal and opposite to the resultant force of the inferior endplate pressing on the inferior face of the core, thanks to Newton’s Third Law of Motion, which applies because the core is effectively at static equilibrium in vivo (i.e., quasi-static). Therefore, if we combine Archard’s wear theory and Newton’s third law, we can theorize that if both sides of a disc prosthesis exhibit substantially different magnitudes of wear, the mechanism responsible for such an observation would be different magnitudes and/or qualities of motion on both sides of the core with respect to their endplates, because the resultant forces at both interfaces are equal in magnitude, but opposite in direction.
The purpose of this study was to correlate wear and fracture patterns in retrieved total disc replacements with motion patterns observed in a clinically validated in vitro lumbar spine model. Specifically, we sought to address the following three research questions: (1) Do findings from preclinical biomechanical testing provide insight into the long-term clinical wear behavior of artificial discs?; (2) Are the wear patterns in explanted polyethylene cores one-sided or symmetrical, as suggested by the previously observed motion patterns illustrated in Figure 1?; and (3) Are components with one-sided wear patterns also associated with a greater magnitude of in vivo wear? Because the retrieval and biomechanical research of the investigators is still ongoing, a secondary objective for this study was to provide an update to prior reports [13, 16] using the latest data currently available. By studying the correlation between in vitro motion patterns and the wear results from retrieval analysis for the same design, we sought to establish the ability of preclinical testing to predict clinical wear patterns for total disc arthroplasty. The ultimate goal of this research is to provide a unified and expanding framework for validation of experimental and analytical preclinical test methods for motion preservation technologies using the long-term results derived from retrieval analysis. The overall hypothesis for our work is that preclinical test methods validated by retrieval analysis can predict the long-term clinical behavior of total disc replacements.
Methods and Materials
Retrieval Analysis
We have previously reported on the wear behavior and clinical details for the first 21 explants in our international, multi-center artificial disc repository [16]. At present, the implant collection has grown to 38 artificial discs that were explanted from 32 patients (female=24, male=8) undergoing TDR revision surgery. The clinical details for the 17 recently explanted discs are summarized in Table 1, whereas the same information for the first 21 cases has been published in Table 1 in the previous study [16].
Table 1.
Clinical information for all retrieved TDRs.
| Implant | Age at Implantation | Gender | Primary Diagnosis | Level | Implant Fixation Method | Year of Index Surgery | Year of Removal Surgery | Endplate Size | Implantation Time (years) |
|---|---|---|---|---|---|---|---|---|---|
| Sal 002 | 45 | M | Disc degeneration | L4/L5 | Noncoated | 1997 | 2006 | 3 | 8.3 |
| Sal 003 | 45 | M | Disc degeneration | L5/S1 | Noncoated | 1997 | 2006 | 3 | 8.3 |
| Maa019 | 72 | F | Disc degeneration | L4/L5 | Noncoated | 1990 | 2006 | 2 | 16.1 |
| Maa020 | 43 | F | Disc degeneration after herniation | L4/L5 | Coated | 2002 | 2006 | 4 | 3.3 |
| Maa021 | 43 | F | Disc degeneration after herniation | L5/S1 | Coated | 2002 | 2006 | 4 | 3.3 |
| Maa022 | 47 | F | Disc degeneration | L4/L5 | Coated | 2001 | 2006 | 3 | 5.3 |
| Maa023 | 45 | F | Disc degeneration | L5/S1 | Coated | 2001 | 2006 | 3 | 4.6 |
| BR 001 | 43 | F | Painful disc | L5/S1 | Noncoated | 2004 | 2006 | 2 | 2.0 |
| Maa024 | 42 | F | Disc degeneration | L4/L5 | Noncoated | 2000 | 2006 | 4 | 6.2 |
| Med001 | 50 | F | Disc degeneration, Back Pain | L5/S1 | Noncoated | 2000 | 2005 | 3 | 4.8 |
| Sal 004 | 44 | M | Disc degeneration | L4/L5 | Noncoated | 1998 | 2006 | 3 | 7.8 |
| Sal 005 | 44 | M | Disc degeneration | L5/S1 | Noncoated | 1998 | 2006 | 4 | 7.8 |
| Maa025 | 45 | F | Disc degeneration post herniated disc surgery | L4/L5 | Noncoated | 1993 | 2006 | 3 | 13.6 |
| Maa026 | 42 | F | Disc degeneration | L4/L5 | Noncoated | 1996 | 2006 | 2 | 10.7 |
| BR-002 | 46 | F | Disc degeneration | L4/L5 | Noncoated | 2004 | 2006 | 3 | 2.2 |
| Sal 006 | 44 | F | Disc degeneration, Back Pain | L5/S1 | Noncoated | 2001 | 2006 | 2 | 5.7 |
| Maa027 | 52 | F | Disc degeneration L3/L4 | L3/L4 | Coated | 2003 | 2007 | 4 | 4.1 |
| Implant | Revision Reason(s) | Complications | Osteolysis? | ||||||
| Sal 002 | Wire breakage; pain; superior endplate subsidence | Subsidence | No | ||||||
| Sal 003 | Pain | No | |||||||
| Maa019 | Progressive anterior migration; pressure against the aorta; back and leg pain | Anterior Migration | No | ||||||
| Maa020 | Severe back pain; lateral subsidence | Subsidence | No | ||||||
| Maa021 | Severe back pain; facet degeneration | No | |||||||
| Maa022 | Severe pain; height loss posteriorly at core; facet joint arthrosis (L4-L5/L5-S1); adjacent disc (L3-L4) degeneration | No | |||||||
| Maa023 | Severe back and leg pain; multiple disc degeneration above prosthesis; lateral displacement (upper endplate in relation to lower endplate) | No | |||||||
| BR 001 | Painful prosthesis; superior endplate subsidence | Subsidence | No | ||||||
| Maa024 | Persisting pain after posterior pedicle screw fixation and fusion L4-L5/L5-S1 | No | |||||||
| Med001 | Pain | No | |||||||
| Sal 004 | Pain; wire breakage | No | |||||||
| Sal 005 | Pain | No | |||||||
| Maa025 | Severe back and leg pain; wire breakage | No | |||||||
| Maa026 | Pain | No | |||||||
| BR-002 | Pain | No | |||||||
| Sal 006 | Pain; subsidence | Subsidence | No | ||||||
| Maa027 | Persisting pain in lower back and both legs; no bony fusion; prosthesis in excessive extension | No | |||||||
The average patient age at the time of implantation was 43 years (range: 22 to 72 years). The artificial discs were all of the SB III CHARITÉ design and were all manufactured by Link (this design is currently produced by DePuy Spine, Raynham, MA). 28/38 implants were retrieved in the Netherlands, 6/38 were retrieved in England, three implants were retrieved in the United States, and one in Germany. 36/38 implants were implanted in routine clinical practice and were not part of a randomized clinical trial. 2/3 implants from the United States were enrolled in the IDE clinical trial.
The artificial discs were implanted an average of 7.3 years (range 1.8 to 16.1 years) at L2/L3 (n=1), L3/L4 (n=2), L4/L5 (n=20), and L5/S1 (n=15) between 1989 and 2004. All the implants were removed because of intractable back pain and/or facet degeneration. In addition, they were removed due to subsidence (n=10), anterior migration (n=3), core dislocation (n=2), lateral subluxation (n=1), endplate loosening (n = 2), and osteolysis (n=1). The one case with osteolysis was diagnosed using computed tomography studies of the lumbosacral spine, and confirmed histologically upon revision.
The cores were cleaned and examined at magnifications of up to 40x with optical microscopy. Because machining marks are on the order of 5 μm in height, their presence was used to identify worn and unworn regions at the rim and dome of the implants. The rim of the cores were also studied specifically for evidence of burnishing, plastic deformation, or the presence of radial and transverse cracks, as described previously [16].
Dome penetration, which reflects the combined effects of creep and wear, was determined by subtracting the as-retrieved maximum dome-to-dome thickness of the explant from the maximum theoretical dome-to-dome thickness for the appropriate nominal, implant height size. Using Link’s historical design drawings, we confirmed that the nominal implant height size was equal to the maximum theoretical dome-to-dome thickness. Because the original thickness of each retrieved core was not measured prior to implantation, and was hence unknown, we confirmed using the design drawings that our approach resulted in a conservative, and systematic overestimate of dome penetration, with an absolute uncertainty of approximately 0.1 mm. We were unable to accurately quantify the dome thickness in one retrieval, due to iatrogenic damage at that location. Thus, our dome penetration measurements were performed in 37 cores using a calibrated digital micrometer (± 0.001 mm accuracy).
To determine the magnitude and symmetry of endplate penetration at the dome, the cores were scanned using a microCT (Scanco Medical AG, Bassersdorf, Switzerland) using a 0.018 mm voxel resolution. The midline microCT section was analyzed using a commercially available software packages (ANALYZE and NIH IMAGEJ) to determine the symmetry of endplate penetration. We measured the overall dome-to-dome height or thickness of the explant, as well as the two midline-to-dome heights (h1 and h2) with respect to the midline or equator, defined through the center of the rim (Fig. 2). To validate our retrieval measurements, we compared the dome-to-dome height of each explant measured using the MicroCT with calibrated micrometer measurements.
Figure 2.
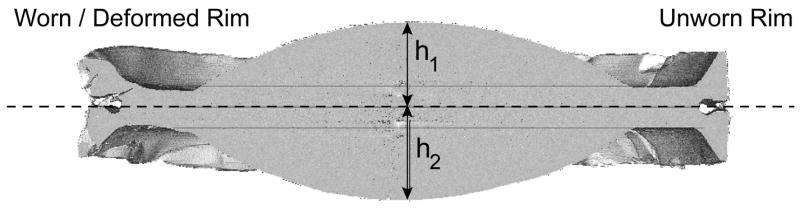
MicroCT analysis of symmetry of dome penetration in a mobile polyethylene core. In the mid-section of the core, a reference axis is defined through the middle of the unworn rim. The symmetry of endplate penetration was characterized by the symmetry ratio, h2/h1 assuming h1 ≥ h2. This ratio is defined such that when h1 = h2, the symmetry ratio is 1.00.
We used each patient as their own control for a normalized analysis of wear symmetry. The symmetry of endplate penetration was characterized by the symmetry ratio, h2/h1 assuming h1 ≥ h2. This ratio is defined such that when h1 = h2, the symmetry ratio is 1.00. By using a normalized symmetry ratio to quantify the wear pattern at the core, we sought to limit variability in absolute midline-to-dome height measurements that could be caused by differences in disc forces applied by different patients. Assuming a worst case scenario, in which the smallest sized core (7.5 mm) had 100% of the manufacturing tolerances located at one pole and 0% at the other, we calculated that a Symmetry Ratio (SR) of less than 0.95 could be unambiguously interpreted as one-sided (i.e., asymmetric) in vivo wear.
We classified implants as exhibiting one-sided wear (SR ≤ 0.95) or symmetric wear patterns (SR>0.95). For both the dome and the rim, we compared the implantation time, penetration and penetration rates for explanted cores with one-sided and symmetric wear patterns using Wilcoxon nonparametric tests (p<0.05 for significance).
Rim penetration was calculated as the difference in the measured rim thickness in worn and unworn regions. For both the dome and the rim, we used Spearman’s correlation to evaluate relationships between implantation time, penetration, penetration rate, and symmetry ratio, and absolute asymmetry. A p value of 0.05 was taken as significant and all analyses were performed using JMP statistical software (SAS Institute, Cary NC).
In Vitro Biomechanical Testing
We have previously reported on the motion response of 10 new implants in five human cadaveric spines (5 at L5-S1 and 5 at L4-L5) [13]. To date, our experience has grown to 20 CHARITÉ discs that were implanted in 13 human cadaveric lumbar spines.
Thirteen human cadaveric lumbar spines (9 males, 4 females; age: 48.4±8.6 years) were used. Twenty new implants were evaluated (L4-L5: 7 and L5-S1: 13). These specimens were tested under physiologic loads: flexion (8 Nm) and extension (6 Nm) moments with compressive follower preload of 400 N. The specifics of this testing protocol has been published previously by O’Leary et al [13]. The center of the prosthesis was 2 mm posterior to the mid-point of the vertebral body endplate in the mid-sagittal plane. Segmental motions were measured optoelectronically. Motions between prosthesis endplates and core were visually assessed using sequential digital video-fluoroscopy (GE OEC 9800 Plus digital fluoroscopy machine) over the full range of motion in flexion and extension.
Results
Retrieval Analysis
Substantial variability was observed in the wear and damage patterns of the retrievals. The patterns of wear, surface damage and quantification of penetration at the dome and rim of the 38 implants are summarized in Table 2.
Table 2.
Wear and surface damage assessments from all retrieved TDRs.
| Implant | Implantation Time (years) | Initial Dome Height (mm) | Dome Penetration (mm) | Dome Penetration Rate (mm/yr) | Maximum Rim Thickness (mm) | Rim Penetration (mm) | Rim Penetration Rate (mm/y) |
|---|---|---|---|---|---|---|---|
| Dal001 | 2.9 | 9.5 | 0.06 | 0.02 | 2.9 | 0.05 | 0.02 |
| Maa001 | 11.0 | 11.5 | 0.18 | 0.02 | 4.9 | 0.14 | 0.01 |
| Maa002 | 9.2 | 9.5 | 0.34 | 0.04 | 2.8 | -- | -- |
| Maa003 | 6.2 | 11.5 | 0.11 | 0.02 | 5.0 | 0.42 | 0.07 |
| Maa004 | 12.7 | 11.5 | 0.21 | 0.02 | 5.0 | 0.63 | 0.05 |
| Maa005 | 12.7 | 9.5 | 0.21 | 0.02 | 2.9 | 0.23 | 0.02 |
| Maa006 | 6.5 | 9.5 | 0.92 | 0.14 | 2.8 | -- | -- |
| Maa007 | 4.2 | 9.5 | 0.32 | 0.08 | 2.9 | 0.80 | 0.19 |
| Maa008 | 4.9 | 7.5 | 0.23 | 0.05 | 0.9 | -- | -- |
| Maa009 | 10.2 | 11.5 | 0.54 | 0.05 | 4.8 | 0.09 | 0.01 |
| Maa010 | 8.5 | 11.5 | 0.37 | 0.04 | 4.8 | 0.02 | 0.00 |
| Maa011 | 3.1 | 8.5 | 0.14 | 0.05 | 2.0 | 0.05 | 0.02 |
| Maa012 | 3.1 | 8.5 | 0.11 | 0.03 | 1.9 | 0.06 | 0.02 |
| Maa013 | 12.7 | 11.5 | 0.36 | 0.03 | 4.9 | 0.10 | 0.01 |
| Maa014 | 13.5 | 11.5 | 0.83 | 0.06 | 4.8 | 0.26 | 0.02 |
| Maa015 | 16.0 | 11.5 | 0.55 | 0.03 | 4.8 | 0.50 | 0.03 |
| Maa016 | 6.5 | 9.5 | 0.10 | 0.02 | 2.9 | 0.63 | 0.10 |
| Maa017 | 6.5 | 9.5 | 0.34 | 0.05 | 3.1 | 0.50 | 0.08 |
| Sal 001 | 1.8 | 8.5 | 0.23 | 0.13 | 2.0 | -- | -- |
| Til 001 | 1.8 | 8.5 | 0.25 | 0.14 | 1.9 | 0.05 | 0.03 |
| Maa018 | 10.6 | 9.5 | 0.33 | 0.03 | 2.9 | 0.06 | 0.01 |
| Sal 002 | 8.3 | 9.5 | 0.68 | 0.08 | 2.7 | -- | -- |
| Sal 003 | 8.3 | 9.5 | 0.43 | 0.05 | 2.9 | 0.05 | 0.01 |
| Maa019 | 16.1 | 11.5 | 0.58 | 0.04 | 4.9 | 0.81 | 0.05 |
| Maa020 | 3.3 | 8.5 | 0.25 | 0.07 | 1.8 | 0.26 | 0.08 |
| Maa021 | 3.3 | 8.5 | 0.29 | 0.09 | 1.9 | 0.10 | 0.03 |
| Maa022 | 5.3 | 9.5 | 0.22 | 0.04 | 3.0 | 0.39 | 0.07 |
| Maa023 | 4.6 | 9.5 | 0.23 | 0.05 | 3.0 | 0.03 | 0.01 |
| BR 001 | 2.0 | 8.5 | 0.17 | 0.09 | 2.0 | 0.72 | 0.36 |
| Maa024 | 6.2 | 9.5 | 0.23 | 0.04 | 2.9 | 0.03 | 0.00 |
| Med001 | 4.8 | -- | -- | -- | -- | -- | -- |
| Sal 004 | 7.8 | 9.5 | 0.23 | 0.03 | 2.9 | 0.29 | 0.04 |
| Sal 005 | 7.8 | 9.5 | 0.12 | 0.01 | 2.9 | 0.02 | 0.00 |
| Maa025 | 13.6 | 11.5 | 0.61 | 0.04 | 4.8 | 0.10 | 0.01 |
| Maa026 | 10.7 | 9.5 | 0.25 | 0.02 | 2.7 | 0.39 | 0.04 |
| BR-002 | 2.2 | 8.5 | 0.39 | 0.18 | 1.8 | 0.03 | 0.01 |
| Sal 006 | 5.7 | 8.5 | 0.19 | 0.03 | 1.9 | 0.76 | 0.13 |
| Maa027 | 4.1 | 8.5 | 0.21 | 0.05 | 1.9 | 1.53 | 0.36 |
| Implant | Radial Cracks? | Transverse Cracks? | Fractured Wire? | Rim Intact? | PE Locked? | Comments | |
| Dal001 | No | Yes | No | Yes | -- | ||
| Maa001 | Yes | No | No | Yes | Yes | ||
| Maa002 | No | Yes | Yes | No | -- | Anterior and lateral iatrogenic rim damage; posterior rim intact | |
| Maa003 | No | No | No | Yes | Yes | ||
| Maa004 | Yes | Yes | Yes | Yes | Yes | Third-body rim damage from wire | |
| Maa005 | Yes | Yes | Yes | Yes | Yes | ||
| Maa006 | Yes | Yes | Yes | No | Yes | Full thickness rim fracture | |
| Maa007 | No | No | No | Yes | Yes | ||
| Maa008 | No | Yes | Yes | Yes | Yes | Full thickness rim fracture | |
| Maa009 | Yes | No | No | Yes | No | ||
| Maa010 | Yes | No | No | Yes | No | ||
| Maa011 | Yes | Yes | No | Yes | Yes | ||
| Maa012 | No | No | No | Yes | No | ||
| Maa013 | Yes | Yes | No | Yes | No | ||
| Maa014 | Yes | Yes | Yes | Yes | Yes | Delamination at dome | |
| Maa015 | Yes | Yes | Yes | Yes | Yes | ||
| Maa016 | Yes | No | No | Yes | Yes | ||
| Maa017 | No | No | Yes | Yes | Yes | ||
| Sal 001 | No | Yes | Yes | No | Yes | Full thickness rim fracture; third body wire damage to dome | |
| Til 001 | No | No | No | Yes | No | ||
| Maa018 | No | No | No | Yes | Yes | ||
| Sal 002 | Yes | Yes | Yes | No | -- | Extensive Rim Damage | |
| Sal 003 | No | No | No | Yes | No | ||
| Maa019 | Yes | Yes | No | Yes | Yes | ||
| Maa020 | No | No | No | Yes | No | ||
| Maa021 | No | No | No | Yes | Yes | ||
| Maa022 | Yes | No | No | Yes | Yes | ||
| Maa023 | Yes | No | No | Yes | No | ||
| BR 001 | Yes | No | No | Yes | Yes | ||
| Maa024 | No | No | No | Yes | No | ||
| Med001 | No | No | Yes | Yes | Yes | Iatrogenic damage | |
| Sal 004 | No | No | Yes | Yes | Yes | ||
| Sal 005 | No | No | No | Yes | Yes | ||
| Maa025 | Yes | No | Yes | Yes | No | ||
| Maa026 | Yes | No | No | Yes | No | ||
| BR-002 | No | No | No | Yes | No | ||
| Sal 006 | No | No | No | Yes | Yes | ||
| Maa027 | Yes | Yes | No | Yes | Yes | ||
On average, we observed a 0.02 mm (i.e., within one voxel) difference between the dome-to-dome height measured by MicroCT and the calibrated micrometer, but the difference was not significant (p > 0.05). The symmetry ratio of the retrievals was found to vary between 0.83 and 1.00 (Fig. 3). The majority of retrievals exhibited symmetric wear patterns (20/35, 57%), characterized by symmetry ratios between 0.96 and 1.00 (mean: 0.98). 15/35 retrieved cores (43%) displayed one-sided wear patterns, with symmetry ratios ranging between 0.83 and 0.95 (mean: 0.92). No correlations were observed between symmetry ratio and implantation time, penetration, or penetration rate at either rim or dome (p > 0.05). We similarly found no significant associations between symmetry ratio and clinical factors, such as the number of previous surgeries of the patient, or the reasons for removal (p > 0.05). When we compared inserts categorically according to their wear pattern (one-sided vs. symmetric), we likewise found no significance difference in penetration (Fig. 4), nor in penetration rate, implantation time, or other clinical factors, such as reason for revision.
Figure 3.
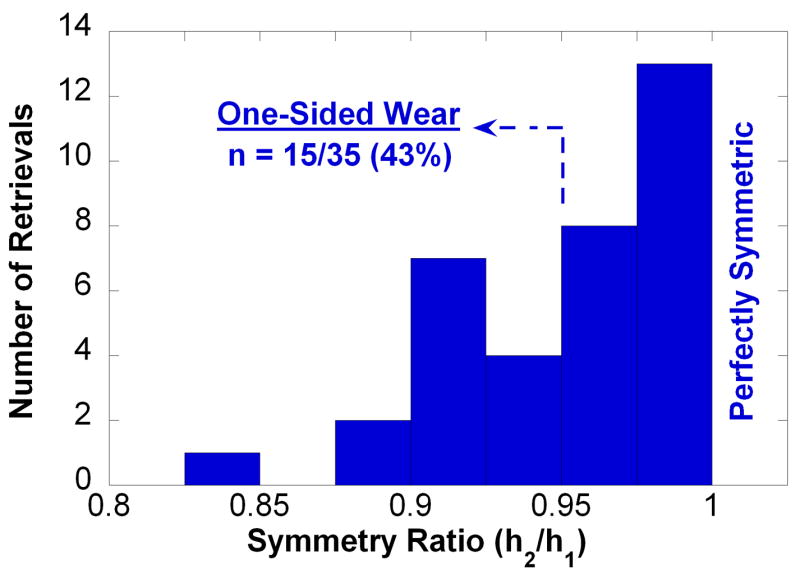
Histogram of symmetry ratio calculated from MicroCT in 35 retrieved polyethylene cores. Note that a ratio of 1.0 corresponds to perfect symmetry in the penetration of both domes with respect to the equatorial axis shown in Figure 2.
Figure 4.
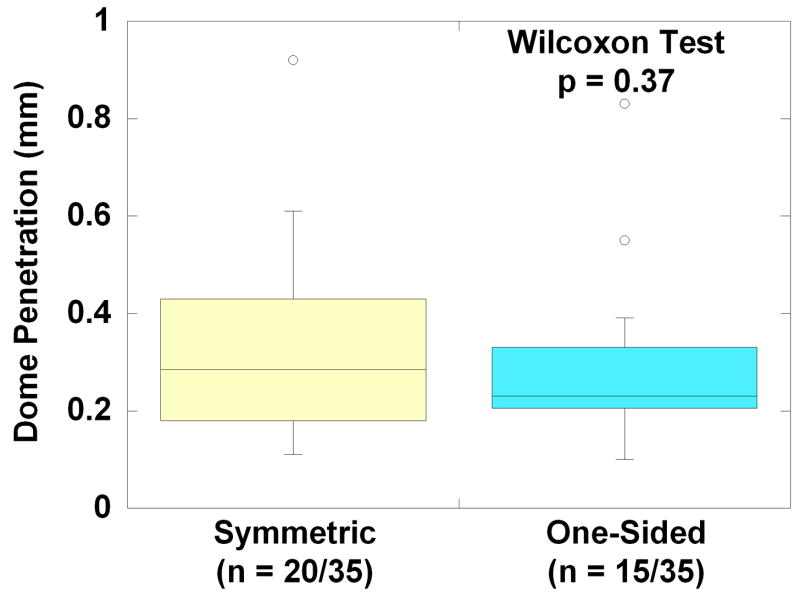
Box plot comparisons of dome penetration for retrieved polyethylene cores classified with symmetric (n = 20/35) or one-sided penetration (n = 15/35). There was no significant difference in penetration between the two groups, based on the Wilcoxon test (p = 0.37).
Among the retrievals, machining marks were worn away from the domes on both sides of the implants in 29/38 cases (76%). In 4/38 cases machining marks were still present on both sides of the dome, and in 5/38 cases machining marks were present on only one side. The implantation time for 4 cores with machining marks on both sides of the dome ranged between 2.0 and 4.9 years (mean: 3.2 years), whereas the implants with no machining marks had been implanted between 1.8 and 16.1 years (mean: 8.1 years). The 5 cores with machining marks visible on only one side were implanted 2.2 to 11.0 years (mean 6.0 years).
The average and the median penetration measured in the center of the dome were 0.32 mm and 0.25 mm, respectively (range: 0.06 to 0.92 mm). There was a positive correlation between dome penetration and implantation time (Spearman’s rho = 0.46, p = 0.004, Fig. 5A). The average and the median penetration rate measured in the center of the dome were 0.05 mm/y and 0.04 mm/y, respectively (range: 0.01 to 0.18 mm/y, B). A negative correlation was observed between endplate penetration rate and implantation time (rho = −0.48, p = 0.003, Fig. 5B).
Figure 5.
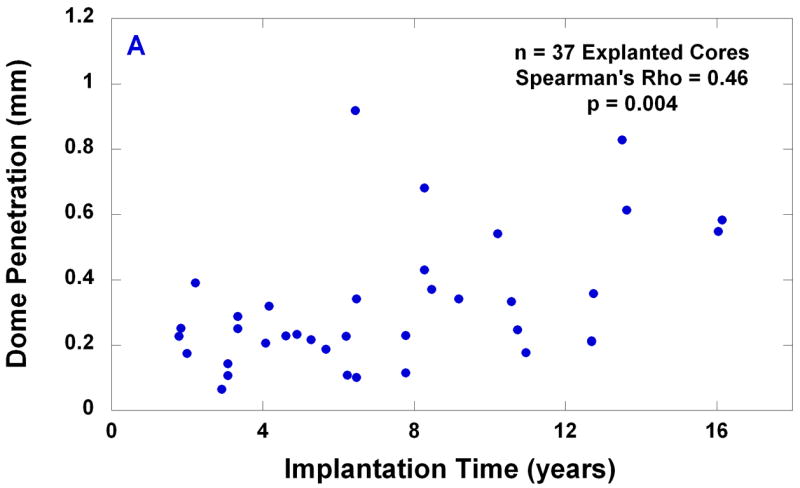
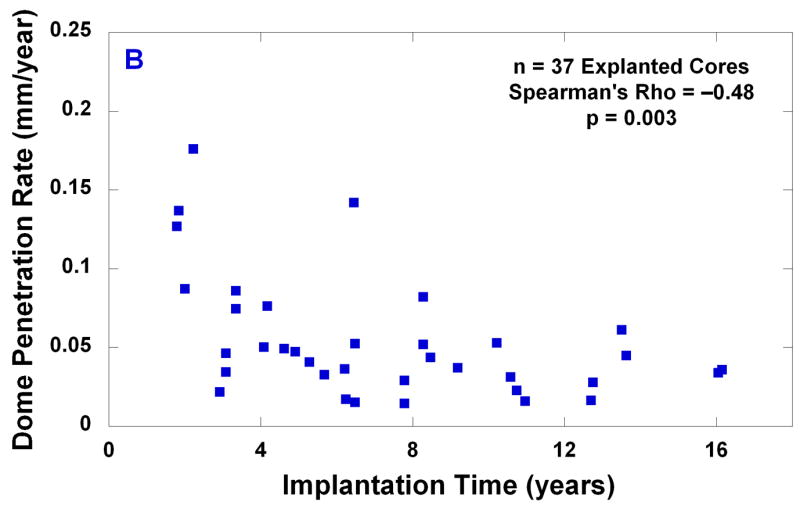
(A) Dome penetration vs. implantation time; and (B) dome penetration rate vs. implantation time for 37 explanted cores.
All of the retrieved cores had at least microscopic evidence of rim contact, but considerable variation in the extent of rim wear and damage was observed (Table 2). Overall, the rim was found to be macroscopically intact in 34/38 of the retrieved cores. Incidental, transient rim impingement was noted in 12/35 (34%) of the retrievals and generally associated with mild burnishing and minimal plastic deformation (Fig. 6A). In these cases, the evidence of transient rim contact was consistent with a mobile core that was free to axially rotate. Chronic rim impingement was noted in 23/35 cases (66%) and was associated with localized burnishing and plastic deformation (Fig. 6B). The cases of chronic rim impingement appeared consistent with an immobile core that was locked or pinched in place, and therefore unable to axially rotate.
Figure 6.
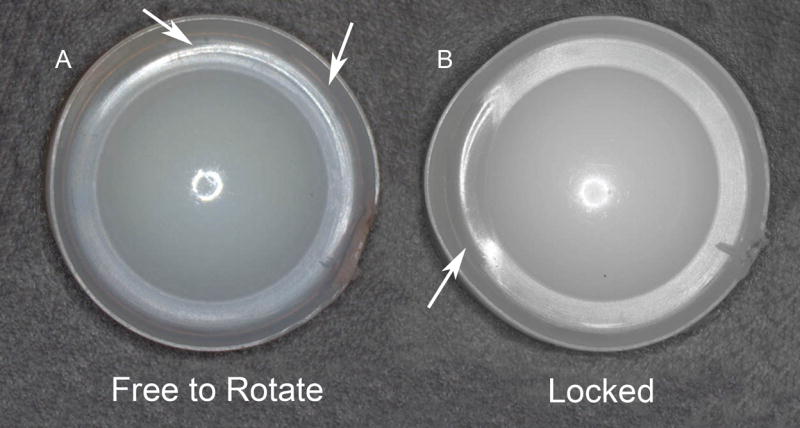
(A) Transient rim impingement generally associated with mild burnishing and minimal plastic deformation consistent with a mobile core that was free to axially rotate. (B) Chronic rim impingement associated with localized burnishing and plastic deformation consistent with an immobile core that was locked or pinched in place, and therefore unable to axially rotate.
Full-thickness rim fracture was observed in four of the retrieved cores, and the wire marker was fractured in 13/38. 14/38 had evidence of transverse cracks, and 19/38 had evidence of radial rim cracks. The morphology of transverse and radial cracks was diverse, but consistent with previous reports [15, 16].
In Vitro Biomechanical Testing
CHARITÉ TDR increased the flexion-extension range of motion of lumbar segments. Under 400 N preload, the range of motion increased from intact values of 8.3 ± 3.9 to 11.1 ± 2.4 degrees at L5-S1 (p<0.05) and from 7.3 ± 2.2 to 10.5 ± 2.4 degrees at L4-L5 (p<0.05).
Because the CHARITÉ prosthesis has a mobile core with two articulating surfaces, angulation between the upper and lower endplates can be the result of angulation between the upper endplate and the core (top articulation), angulation between the lower endplate and the core (bottom articulation), or angulation at both articulations. In 8/20 implantations (L5-S1: 5/13, L4-L5: 3/7) there was clear visual evidence of motion at both articulations. In additional 8/20 cases (L5-S1: 5/13, L4-L5: 3/7), there was some evidence of motion at both articulations; however, the predominant motion occurred at the top articulation. Finally, in 4/20 implantations (L5-S1: 3/13, L4-L5: 1/7) motion could be visually detected only at the top articulation. Core entrapment and pinching was observed in 7/20 cases (L5-S1: 6/13, L4-L5: 1/7) as the segment was extended, and was associated with visual evidence of core bending or deformation in 5/20 cases (L5-S1: 4/13, L4-L5: 1/7). The core was restored to its original shape when the extension loading was removed and no visual evidence of permanent deformation was seen when the cores were explanted at the end of the test protocol.
Discussion
In total disc arthroplasty, as in hip and knee replacement, a broad distribution of wear patterns and wear magnitudes is encountered clinically. Similarly, a distribution of motion patterns was also observed from in vitro biomechanical testing. For this reason, we sought regions of overlap, both qualitatively and quantitatively, between the distribution of motion patterns observed in vitro, and the wear patterns from clinical retrievals. Overall, we found one-sided motion and core entrapment detected during the in vitro testing were generally consistent with one-sided wear and chronic rim impingement observed from the retrieval analysis.
As a mobile bearing, it has remained open to debate whether in vivo wear for this design is distributed evenly on both superior and inferior surfaces of the core. Conceptually, this evenly-distributed motion pattern appears to have been intended by the designers of the implant system [3]. This scenario was observed in the majority of retrieved polyethylene cores (20/35, 57%), as well as in 40–80% of the in vitro tests.
The tendency of polyethylene cores to undergo one-sided wear in vivo, as opposed to dual-sided wear, was determined from our MicroCT analysis, and further confirmed by our assessment of machining marks. However, the extent of asymmetric wear was typically very small, and could only be quantified using a novel MicroCT-based method we developed and validated specifically for this purpose. Analysis of the presence or absence of machining marks, the benchmark for microscopically low wear, revealed one-sided wear in dome of only 5/38 cases. Although analysis of the presence of machining marks confirmed that one-sided wear was indeed possible in TDR, even for cores that had been implanted for up to 11 years, this methodology underestimated the prevalence of one-sided wear clinically, because only approximately the first 5 μm of wear can be appreciated using this technique. Because of the approximate nature of the machining marks analysis, we place greater emphasis on the MicroCT-based analysis of core height differences, which can detect greater magnitudes of wear.
The observation of core entrapment and pinching of the core by the endplate during the in vitro experiments was also consistent with observations of chronic rim impingement in the majority of the retrievals studied. Although the in vitro tests were clearly predictive of this in vivo damage mode, chronic rim damage occurred in the majority of retrievals, but core entrapment and pinching of the core by the endplate was observed in only in 7/20 implants tested in vitro. The reason for this discrepancy is likely due to the clinical subsidence or migration of the endplates, which occurred in 13/38 of the implants after 2.0 to 16.1 years of implantation. If we excluded these complications from among the cases of severe rim contact, we obtain a closer correlation between the prevalence of impingement between the retrievals and in vitro experiment.
Prolonged impingement of the endplates on the core was associated with full-thickness rim fracture in 4/38 cases, as well as radial and transverse cracking. Preliminary analyses suggest that post-irradiation oxidation, whether due to shelf aging, or due to exposure to oxygen sources in vivo, is correlated, at least in part, to the rim fracture mechanisms [22]. Because chronic impingement appears to be necessary to produce the transverse, but not radial, cracks observed clinically, rim fracture is a multi-factorial phenomenon, with likely implant size, material, and clinical factors playing a role in its manifestation. A more thorough treatment of the subject of polyethylene oxidation in total disc arthroplasty is beyond the scope of the current study and will be addressed in a separate paper.
Although the wear results from the current study are consistent with our previous research on this topic, the larger sample size lends greater significance to many of the prior findings. Many of the same concerns and limitations associated with interpreting dome penetration were discussed previously and remain relevant for the current study. With the recent availability of design drawings from the manufacturer, we are able to interpret previous findings in the context of initial dimensional uncertainty from tolerance variation. It is clear that, for the majority of the retrievals available for study, the maximum endplate penetration (up to 0.9 mm) is substantially greater than the uncertainty in tolerances. Our current experience with a greater number of retrievals further reinforces our recommendation to characterize penetration at the dome and rim using our currently reported methodologies.
In light of the number of disc replacement technologies currently in development, it remains crucial that preclinical test methods, if they are to be relied upon in an absolute sense, be validated using available clinical evidence from designs with a proven long-term clinical track record. If in vitro test methods cannot be shown to reproduce the same mechanisms that occur in vivo, then relying on such methods, even for the purposes of making Device A-to- Device B comparisons, could provide misleading results. In the present study, we observed that the spectrum of motion results from a specific, in vitro biomechanical study of the spine that incorporated a compressive follower preload provided insight into the wear patterns observed in clinical retrievals. Although other protocols have previously been used to provide biomechanical characterization for relative comparison purposes, the authors are unaware of another study demonstrating a correlation between biomechanical test procedures, clinical radiographic findings, and the clinical wear patterns from retrievals.
The correlations we have observed in the present study, while encouraging, are thus far limited by experience to a specific biomechanical test procedure, validated by retrievals of a single artificial design, albeit one with nearly two decades of clinical use. Additional research is needed, not only to better understand the distribution of performance with the current mobile bearing design, but also to correlate the in vitro biomechanics with retrieval analysis of other, fixed center-of-rotation artificial disc designs.
Conclusions
This is the first study to directly compare the long-term PE wear and damage mechanisms in TDR retrievals with the motion patterns generated by a validated in vitro cadaveric testing model. The retrievals exhibited wear patterns consistent with the core entrapment and one-sided motion patterns observed in the in vitro testing. Our results provide the foundation for further development of testing protocols to replicate, and ultimately help prevent, wear and damage observed in many of the retrievals reported here.
Key Points
One-sided motion and core entrapment detected during in vitro testing were generally consistent with one-sided wear and chronic rim impingement observed from the retrieval analysis.
Transient rim impingement was generally associated with mild burnishing and minimal plastic deformation consistent with a mobile core that was free to axially rotate, whereas chronic rim impingement was associated with localized burnishing and plastic deformation consistent with an immobile core that was locked or pinched in place, and therefore unable to axially rotate.
This is the first study to compare the long-term PE wear and damage mechanisms in total disc replacement (TDR) retrievals with the motion patterns generated by a validated in vitro cadaveric testing model.
Acknowledgments
The authors are grateful to Drs. John Peloza, United States; Jan de Waal Malefijt, Netherlands; Ferdinand Krappel, Germany; and their patients for sending retrievals to our repository. Special thanks are due to Anton Bowden, Exponent, for assistance with the MicroCT analysis aspects of this study. We also thank DePuy Spine for providing access to the historical manufacturing drawings used by Link to produce the CHARITÉ Artificial Disc. Parts of this study were made possible by institutional funding from DePuy Spine (Raynham, MA); Medtronic, Spinal and Biologics (Memphis, TN); and by an R01 Grant AR47904 from the National Institutes of Health.
References
- 1.Fernström U. Arthroplasty with intercorporal endoprothesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154–159. [PubMed] [Google Scholar]
- 2.Kurtz SM. Total disc arthroplasty. In: Kurtz SM, Edidin AA, editors. Spine Technology Handbook. New York: Academic Press; 2006. pp. 1–10. [Google Scholar]
- 3.Büttner-Janz K, Hochschuler SH, McAfee PC. The artificial disc. Berlin: Springer; 2003. [Google Scholar]
- 4.van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. Journal of spinal disorders & techniques. 2003 Aug;16(4):369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 5.McAfee PC, Geisler FH, Scott-Youn M, editors. Roundtables in Spine Surgery: Complications and Revision Strategies in Lumbar Spine Arthroplasty. St. Louis: Quality Medical; 2005. www.qualitymedicalpublishing.com/ssrtables.php (priority code: dpssrt) [Google Scholar]
- 6.Kurtz SM, Peloza J, Siskey R, Villarraga ML. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005 May–Jun;5(3):344–350. doi: 10.1016/j.spinee.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Stieber JR, Donald GD., 3rd Early failure of lumbar disc replacement: case report and review of the literature. Journal of spinal disorders & techniques. 2006 Feb;19(1):55–60. doi: 10.1097/01.bsd.0000163414.53732.a3. [DOI] [PubMed] [Google Scholar]
- 8.Wagner WH, Regan JJ, Leary SP, Lanman TH, Johnson JP, Rao RK, et al. Access strategies for revision or explantation of the Charite lumbar artificial disc replacement. J Vasc Surg. 2006 Dec;44(6):1266–1272. doi: 10.1016/j.jvs.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 9.van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijn L. Polyethylene wear debris and long-term clinical failure of the Charite disc prosthesis: a study of 4 patients. Spine. 2007 Jan 15;32(2):223–229. doi: 10.1097/01.brs.0000251370.56327.c6. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham BW. Basic scientific considerations in total disc arthroplasty. Spine J. 2004 Nov–Dec;4(6 Suppl):219S–230S. doi: 10.1016/j.spinee.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham BW, Dmitriev AE, Hu N, McAfee PC. General principles of total disc replacement arthroplasty: seventeen cases in a nonhuman primate model. Spine. 2003 Oct 15;28(20):S118–124. doi: 10.1097/00007632-200310151-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham BW, Gordon JD, Dmitriev AE, Hu N, McAfee PC. Biomechanical evaluation of total disc replacement arthroplasty: an in vitro human cadaveric model. Spine. 2003 Oct 15;28(20):S110–117. doi: 10.1097/01.BRS.0000092209.27573.90. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary P, Nicolakis M, Lorenz MA, Voronov LI, Zindrick MR, Ghanayem A, et al. Response of Charite total disc replacement under physiologic loads: prosthesis component motion patterns. Spine J. 2005 November – December;5(6):590–599. doi: 10.1016/j.spinee.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 14.David T. Revision of a Charite artificial disc 9.5 years in vivo to a new Charite artificial disc: case report and explant analysis. Eur Spine J. 2005 Jun;14(5):507–511. doi: 10.1007/s00586-004-0842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz S, Siskey R, Ciccarelli L, van Ooij A, Peloza J, Villarraga M. Retrieval analysis of total disc replacements: Implications for standardized wear testing. Journal of ASTM International. 2006;3(6):1–12. [Google Scholar]
- 16.Kurtz SM, van Ooij A, Ross ERS, de Waal Malefijt J, Peloza J, Ciccarelli L, et al. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7(1):12–21. doi: 10.1016/j.spinee.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Lemaire JP, Carrier H, Ali el HS, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. Journal of spinal disorders & techniques. 2005 Aug;18(4):353–359. doi: 10.1097/01.bsd.0000172361.07479.6b. [DOI] [PubMed] [Google Scholar]
- 18.Putzier M, Funk JF, Schneider SV, Gross C, Tohtz SW, Khodadadyan-Klostermann C, et al. Charite total disc replacement--clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006 Feb;15(2):183–195. doi: 10.1007/s00586-005-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine. 2007 Mar 15;32(6):661–666. doi: 10.1097/01.brs.0000257554.67505.45. [DOI] [PubMed] [Google Scholar]
- 20.Archard JF. Contact and rubbing of flat surfaces. J Appl Phys. 1953;24:981–988. [Google Scholar]
- 21.Maxian TA, Brown TD, Pedersen DR, McKellop HA, Lu B, Callaghan JJ. Finite element analysis of acetabular wear. Validation, and backing and fixation effects. Clin Orthop. 1997;(344):111–117. [PubMed] [Google Scholar]
- 22.Kurtz SM, van Ooij A, Ross ERS, de Waal J, Isaza J, Peloza J, et al. Clinical significance of polyethylene oxidation for total disc arthroplasty. Transactions of the 53rd Orthopedic Research Society. 2007;32:1130. [Google Scholar]