Abstract
Both nitric oxide and asymmetrical dimethylarginine (ADMA) play a critical role in the regulation of cerebral blood flow, though their neuroprotective and cytotoxic effects are still under investigation. In this study we found that nitrate/nitrite (NOx) levels in plasma, ischemic brain tissue, and cerebrospinal fluid (CSF) increased significantly 24h after 2h transient middle cerebral artery occlusion (MCAO) in rats. ADMA levels were unchanged in plasma, but decreased significantly in CSF 24h following MCAO. The CSF ADMA/NOx ratio decreased markedly following ischemia. Rats protected by expression of the chaperonin GroEL or its folding deficient mutant D87K had lower plasma NOx levels at 24h reperfusion. ADMA, NO, and their ratio in CSF merit further study as biomarkers for ischemic brain injury.
Keywords: NO, ADMA, CSF, focal ischemia, rat, GroEL
Introduction
Stroke is the third leading cause of death and the leading cause of neurological disability in the United States. To date, three isoforms of nitric oxide synthase (NOS; eNOS, nNOS, iNOS) have been characterized in brain. In addition to a central role in cerebral vasodilation, nitric oxide (NO) has both neuroprotective and cytotoxic effects in the setting of cerebral ischemia, depending on which isoform is active, the cell type in which NO is produced, and the time following onset of ischemia [24]. Immediately after ischemia, NO release from eNOS in the vessel wall is protective by promoting vasodilatation; infarct area is increased in eNOS deficient mice [9, 15]. However, after ischemia develops, NO produced by overactivation of neuronal NOS [5, 10] and later, NO release by inducible NOS contribute to brain damage [8, 17]. Neuronal NOS knockout mice are resistant to global and focal cerebral ischemia, confirming a role for neuronal NOS in exacerbating stroke [8, 10]. In addition to effects on vasodilatation and generation of free radicals, recent work also suggests a role for NO as an essential mediator in neurogenesis, and a dual role for NO also exists in adult neurogenesis [1, 2].
Asymmetric dimethylarginine (ADMA) has been identified as an endogenous inhibitor of NOS and elevated ADMA levels have been associated with an increased risk of stroke and transient ischemia attacks [20, 23]. Both in vitro and animal studies [6, 21], as well as a human volunteer study [13] showed that ADMA increased vascular tone in cerebral blood vessels. Jung et al reported that cerebrospinal fluid (CSF) levels of ADMA correlated strongly with the degree of arteriographic vasospasm in a primate model of subarachnoid hemorrhage [12]. To date, acute changes in ADMA in focal ischemia have not been studied.
GroEL is a bacterial chaperonin which prevents protein aggregation and facilitates protein folding. We previously demonstrated that expression of GroEL or the folding deficient mutant GroEL-D87K in rat brain reduced infarct volume following middle cerebral artery occlusion (MCAO) [22]. We postulated that NOx and ADMA might be useful biomarkers for cerebral ischemia. We tested this in normal animals and in animals protected by expression of GroEL to reduce injury for the same duration of occlusion. We also compared NOx and ADMA levels in brain tissue and CSF in normal animals subjected to focal ischemia.
Materials and Methods
Materials
Experimental procedures and animal protocols
All experiments were performed according to protocols approved by the Stanford University Administrative Panel on Laboratory Animal Care and followed NIH guidelines.
Groups
Sprague-Dawley male rats weighing between 280 and 310 g were divided into 5 groups: GroEL-wild type (G-WT) transfected ischemic, GroEL-D87K (G-D87K) transfected ischemic, LXSN control transfected ischemic, and untransfected ischemic (ISC). In addition several animals were subjected to sham surgery, comprising a non-ischemic control group (Sham). CSF and brain tissue were harvested in the ISC and Sham groups for NOx and ADMA analysis.
Transfection and expression of GroEL or GroEL-D87K
Transfection and expression of G-WT or the folding deficient mutant G-D87K was achieved by stereotaxic injection of an expression plasmid encoding the gene of interest under control of the murine leukemia virus LTR promoter or just the expression plasmid without added coding sequence, LXSN, for the transfection control, mixed with the cationic liposomal transfection reagent DOTAP (Roche, Diagnostika GmbH, Germany) into the lateral cerebral ventricle 24 h prior to subjecting the animals to MCAO [22].
Middle Cerebral Artery Occlusion (MCAO) and evaluation of outcome
Animals were initially anesthetized with 3% isoflurane in a mixture of 30% oxygen (O2) and 70% nitrous oxide (N2O) and maintained with 1.0–2.0% isoflurane in O2:N2O (30:70) during surgical procedures. A femoral artery was cannulated for continuous monitoring of arterial blood pressure and heart rate. A rectal temperature probe was used to monitor temperature during the procedure and a heating pad was used to maintain the rectal temperature at 37±0.5°C. The left carotid artery was exposed, and a 3-0 monofilament nylon suture with the tip rounded by a flame was inserted 19 to 20 mm to occlude the origin of the left middle cerebral artery as previously described [22]. After 2 h MCAO, the animals were reanesthetized and the suture was removed. Following 24 h reperfusion, the neurological deficit score was assessed as previously described [22], and then rats were anesthetized for sampling of blood and CSF followed by sacrifice. Infarct volume was determined with TTC staining and corrected for edema as described previously [22].
Plasma samples
Blood samples were collected from the tail vein before MCAO, at the end of 2 h MCAO, and after 24 h reperfusion in BD Vacutainer tubes (Fisher Scientific, Pittsburgh, PA, USA) containing buffered sodium citrate as an anticoagulant and centrifuged at 3000 rpm for 10 min at 4°C. Plasma was then filtered using Amicon Ultra-4 30 kDa filter units (Millipore, Billerica, MA, USA), and kept in aliquots at −80°C until assayed for NOx and ADMA.
CSF samples
The animals from the ischemic and sham groups were anesthetized with isoflurane then placed on a stereotaxic frame (David Kopf, Tujunga, CA) to fix the head in a flexed position. CSF was aspirated from the cisterna magna with a 33 g needle [19], and filtered.
Tissue samples
Animals from the ischemic and sham groups were perfused with cold saline, brains were harvested and placed on a dry ice and alcohol slurry. The cortex was dissected from the ischemic hemisphere (Ipsi), the contralateral (Contra) hemisphere, and from sham surgery hemispheres (Sham). Tissue was stored at −80°C until processed.
Measurement of NOx levels
Cold tissue lysis buffer containing 50 mM Hepes, pH 7.4, 1 mM dithiothreitol, 1 mM EDTA, 0.32 M glucose, 10 µg per ml leupeptin, 10 µg per ml soybean trypsin inhibitor, 2 µg per ml aprotinin, 1 mM phenylmethylsulfonyl fluoride (Sigma, Louis, MO, USA); 3 ml/g was added to the frozen tissue samples, which were then sonicated on ice twice for 10 seconds each. The homogenates were centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was then centrifuged at 100,000 × g for 30 min. The resulting supernatant was ultrafiltered using Microcon YM-30 30 kDa molecular weight cut-off filters from Millipore, and kept in aliquots at −80°C. Protein concentration in tissue samples was determined using the BCA protein assay (Bio-Rad, Hercules, CA, USA). NOx in tissue samples was normalized to protein.
The concentration of NOx in peripheral blood plasma and brain cortical tissue was determined in duplicate using a commercially available kit from Cayman Chemical (Ann Arbor, Michigan USA). The concentration of NOx was measured colorimetrically using a multimode microtiter plate reader (Molecular Devices, Sunnyvale, CA) with a 540 nm filter.
Measurement of ADMA levels
ADMA concentration in peripheral blood plasma, CSF, and brain tissue was measured with a highly sensitive ELISA kit from DLD (Diagnostica GmbH, Germany). The intensity of the color reaction is inversely proportional to the amount of ADMA in the sample and is measured by reading the optical density at 450 nm with a microtiter plate reader (Tecan GENios, Durham, NC, USA) [11]. The ADMA in tissue samples was normalized to protein.
Statistical analysis
Data are given as means ± SEM. All samples were measured in duplicate. Statistical analysis was performed using analysis of variance followed by 2-tailed t test for comparisons of two groups or ANOVA followed by Student Neuman Keuls correction for multiple comparisons when more than two groups were compared. Differences were considered statistically significant if p < 0.05.
Results
Neurological score and infarct area
The neurological deficit score was significantly lower in GroEL overexpressing rats (1.9±0.4 G-WT and 2.1±0.2 G-D87K, vs 2.6±0.7 LXSN and 2.7±0.9 ISC, p < 0.05). The infarct area was also significantly smaller (32.6±6.8% G-WT and 35.1±5.3% G-D87K, vs 53.7±5.6% LXSN and 48.9±8.6 % ISC, p < 0.05) consistent with our previous results [22].
Plasma NOx and ADMA levels
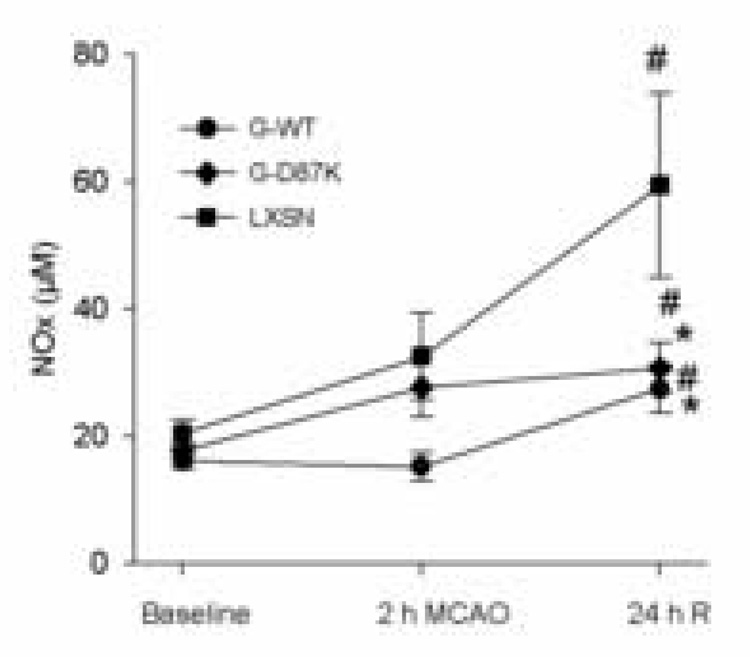
Plasma NOx levels already showed some increase by the end of the 2 h MCAO in some groups, and increased significantly in all groups at 24 h reperfusion (Fig 1). The neuroprotective effect of G-WT or G-D87K expression was associated with lower peripheral plasma NOx after 24 h reperfusion compared with the control transfected group, LXSN. Plasma ADMA levels were similar in all ischemic groups at baseline and did not vary significantly following ischemia (Table 1).
Figure 1.
NOx levels in plasma following MCAO. Blood samples were collected from three groups of rats (G-WT, n=8; G-D87K, n=10, LXSN, n=6) before MCAO (Baseline), at the end of 2 h MCAO, and after 24 h reperfusion (24 h R). The concentration of NOx in plasma was determined in duplicate. # compared to baseline within the same group, * compared to LXSN, (P < 0.05).
Table.
Peripheral blood plasma ADMA levels (µM)
| baseline | 2h | 24h | |
|---|---|---|---|
| G-WT (n=7) | 0.51±0.07 | 0.44±0.07 | 0.46±0.09 |
| G-D87 (n=9) | 0.44±0.06 | 0.44±0.05 | 0.47±0.04 |
| LXSN (n=4) | 0.44±0.08 | 0.48±0.04 | 0.41±0.03 |
There are no statistically significant differences between groups.
NOx and ADMA levels in cortex
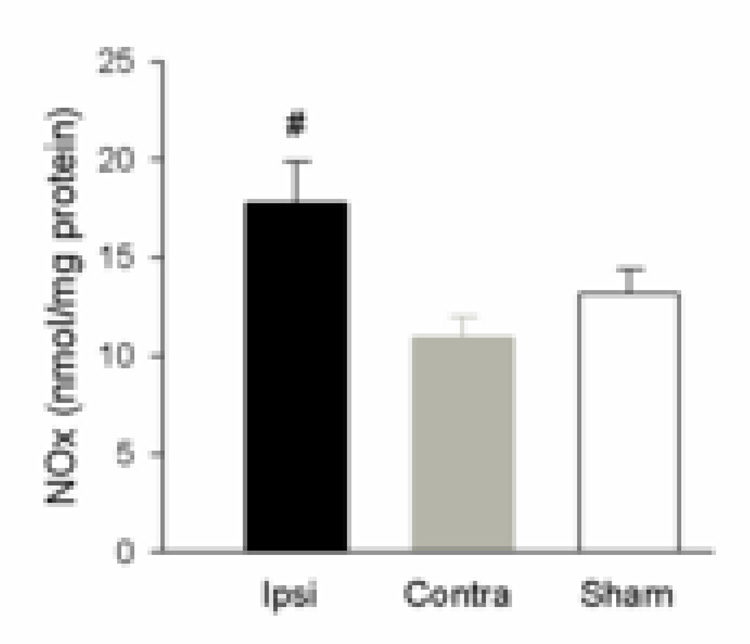
Ischemic cortical brain tissue (Ipsi) NOx was significantly higher than contralateral cortex (Contra) at 24 h following MCAO (p < 0.05), also higher than in sham cortex, but the trend did not reach significance (fig 2). ADMA levels were not significantly different in Ipsi (0.111±0.045 nmol/mg protein, n=7) compared to Contra (0.0788±0.018, n=8) and Sham (0.0830±0.055, n=4).
Figure 2.
Brain tissue NOx levels in cortex with MCAO. Brain tissue from ischemic cortex (Ipsi, n=7), contralateral cortex (Contra, n=7) and the cortex from animals that had sham surgery (Sham, n=4) was assayed for NOx, and normalized to protein. The level of NOx in tissue was determined in duplicate. NOx in ischemic cortex (Ipsi) was significantly higher than contralateral cortex (Contra) (#, P < 0.05).
NOx and ADMA levels in CSF
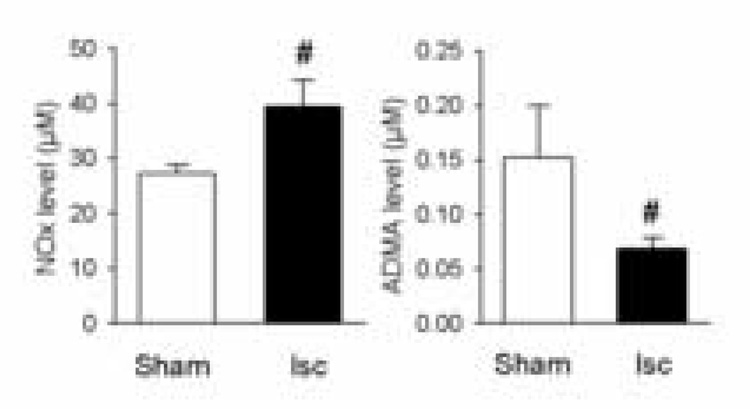
The NOx level in CSF at 24 h reperfusion after MCAO (Fig 3) was significantly higher than in the sham group (p < 0.05). In contrast, the CSF ADMA level following MCAO was significantly lower than that in the sham group (p < 0.05). The ADMA/NO ratio in CSF was much lower in the MCAO group (0.18±0.03%) than in the normal control group (0.54±0.13%, P=0.034, n=4).
Figure 3.
NOx and ADMA levels in CSF. At 24 h reperfusion after MCAO, CSF was aspirated from the cisterna magna and the NOx and ADMA levels were assayed. NOx was significantly higher in the ischemic compared to the non-ischemic sham group (n=4, #, p < 0.05). The CSF ADMA level following MCAO was significantly reduced following ischemia (n=4, #, P < 0.05).
Discussion
We find that NOx levels are increased in the brain after ischemic injury, consistent with results from other laboratories in animal studies [7, 14] and in patients following stroke [4]. The increase in cerebral NOx is reflected by increases in CSF and plasma NOx. The increase in plasma NOx with cerebral ischemia is attenuated by expression of either the wild type chaperonin GroEL or its folding deficient point mutant –D87K, both of which reduce infarct volume [22]. Finally, the NOS inhibitor ADMA is reduced in the CSF following ischemia.
The reduction in CSF ADMA level with ischemia was striking. A reduction to 30% of the value in sham animals was observed. It is likely that this reduction in CSF ADMA has physiological effects, based on our previous work [3]. In a transgenic mouse model we overexpressed the gene encoding dimethylarginine dimethylaminohydrolase (DDAH), the enzyme that metabolizes ADMA. Plasma ADMA was reduced 50%, plasma NOx doubled, and systemic vascular resistance decreased [3].
The reduction in CSF ADMA could reduce cerebrovascular resistance and improve blood flow. On the other hand, a reduction in ADMA could promote the activity of iNOS and nNOS, and enhance formation of cytotoxic free radicals (peroxynitrite and superoxide anion) [24]. Intriguingly, whereas we observed a marked reduction in the CSF ADMA levels, the ADMA levels in the cortex did not significantly change. Thus, neuronal ADMA may blunt the detrimental rise in neuronal NO after ischemic injury. The delayed increase in NOx levels in the brain after cerebral ischemia may be injurious, or at the very least reflect injury. The Gro-EL transfected animals manifested less injury, which was associated with an attenuated rise in NOx levels with ischemia. As suggested by prior work, specific targeting of NOS isoforms can have therapeutic effects [9, 10].
Earlier work looked at S-100b and neuron specific enolase as biomarkers for stroke [16], and a recent study suggests that combinations of markers may be more reliable than individual markers [18]. While changes in peripheral blood NOx at 24 hr reflected the extent of injury and the neurological score, we were not able to detect changes in ADMA in serum or brain tissue. While we observed a trend to increased serum NOx already at 2 h, it was only significant in one of the ischemic groups at that timepoint. It would be useful to determine a more detailed time course of changes in NOx in serum to identify the earliest time at which it is reliably elevated. Both NOx and ADMA showed measurable changes in CSF with ischemia, and the ratio is even more sensitive to change. It will be important to investigate the relative time course of changes in markers in CSF compared to serum to identify which has the earliest reliable change. Availability of a peripherally detectable biomarker for stroke could more quickly clarify the diagnosis in some cases. A useful biomarker should appear quickly after the insult, remain elevated, and its amount should correlate with the extent of injury. Thus the possibility that serum NOx and the CSF ADMA:NOx ratio might be useful biomarkers of cerebral ischemia is worth further study. The combined increase in NOx and decrease in ADMA suggests marked activation of NOS occurs.
Conclusion
NOx levels in plasma, ischemic cortex, and CSF were significantly increased after MCAO at 24 hrs reperfusion in the rat. The ADMA level in CSF decreased following ischemia in concert with an increase in NOx, leading to a marked reduction in ADMA/NOx ratio in CSF. Animals protected by gene therapy with GroEL showed a significantly smaller increase in plasma NOx at 24 hrs reperfusion, correlated with the reduced injury. Currently, reliable and predictive biomarkers for ischemic brain injury are still being sought. ADMA and NOx may be useful biomarkers in this setting.
Acknowledgments
Dr. Cooke is the inventor of patents owned by Stanford University for diagnostic and therapeutic applications of the NOS pathway from which he receives royalties.
Sponsorship: Supported in part by NIH grants GM 49831 and NS 37520 to RGG and HL-63685, HL-75774, CA098303 to JPC, and the Tobacco Related Disease Research Program Grant 11RT-0147 to JPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cardenas A, Moro MA, Hurtado O, Leza JC, Lizasoain I. Dual role of nitric oxide in adult neurogenesis. Brain Res. Brain Res. Rev. 2005;50:1–6. doi: 10.1016/j.brainresrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J. Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 4.El Kossi MM, Zakhary MM. Oxidative stress in the context of acute cerebrovascular stroke. Stroke. 2000;31:1889–1892. doi: 10.1161/01.str.31.8.1889. [DOI] [PubMed] [Google Scholar]
- 5.Endres M, Scott G, Namura S, Salzman AL, Huang PL, Moskowitz MA, Szabo C. Role of peroxynitrite and neuronal nitric oxide synthase in the activation of poly(ADP-ribose) synthetase in a murine model of cerebral ischemia-reperfusion. Neurosci. Lett. 1998;248:41–44. doi: 10.1016/s0304-3940(98)00224-9. [DOI] [PubMed] [Google Scholar]
- 6.Faraci FM, Brian JE, Jr., Heistad DD. Response of cerebral blood vessels to an endogenous inhibitor of nitric oxide synthase. Am. J. Physiol. 1995;269:H1522–H1527. doi: 10.1152/ajpheart.1995.269.5.H1522. [DOI] [PubMed] [Google Scholar]
- 7.Gamez A, Carbonell T, Rama R. Does nitric oxide contribute to iron-dependent brain injury after experimental cerebral ischaemia? J. Physiol. Biochem. 2003;59:249–254. doi: 10.1007/BF03179881. [DOI] [PubMed] [Google Scholar]
- 8.Huang PL. Lessons learned from nitric oxide synthase knockout animals. Semin. Perinatol. 2000;24:87–90. doi: 10.1016/s0146-0005(00)80064-6. [DOI] [PubMed] [Google Scholar]
- 9.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J. Cereb. Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 11.Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, Patterson AJ, Kimoto M, Blau HM, Cooke JP. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005;111:1431–1438. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 12.Jung CS, Iuliano BA, Harvey-White J, Espey MG, Oldfield EH, Pluta RM. Association between cerebrospinal fluid levels of asymmetric dimethyl-L-arginine, an endogenous inhibitor of endothelial nitric oxide synthase, and cerebral vasospasm in a primate model of subarachnoid hemorrhage. J. Neurosurg. 2004;101:836–842. doi: 10.3171/jns.2004.101.5.0836. [DOI] [PubMed] [Google Scholar]
- 13.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Boger SM. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37:2024–2029. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- 14.Kumura E, Kosaka H, Shiga T, Yoshimine T, Hayakawa T. Elevation of plasma nitric oxide end products during focal cerebral ischemia and reperfusion in the rat. J. Cereb. Blood Flow Metab. 1994;14:487–491. doi: 10.1038/jcbfm.1994.60. [DOI] [PubMed] [Google Scholar]
- 15.Lo EH, Hara H, Rogowska J, Trocha M, Pierce AR, Huang PL, Fishman MC, Wolf GL, Moskowitz MA. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke. 1996;27:1381–1385. doi: 10.1161/01.str.27.8.1381. [DOI] [PubMed] [Google Scholar]
- 16.Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- 17.Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, Laskowitz DT, Valkirs GE, Buechler KF. Early biomarkers of stroke. Clin. Chem. 2003;49:1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- 19.Takemoto Y. An improved method for using cisternal cerebrospinal fluid in conscious rats for application in the measurement of catecholamines. Jpn. J. Physiol. 1991;41:665–669. doi: 10.2170/jjphysiol.41.665. [DOI] [PubMed] [Google Scholar]
- 20.Wanby P, Teerlink T, Brudin L, Brattstrom L, Nilsson I, Palmqvist P, Carlsson M. Asymmetric dimethylarginine (ADMA) as a risk marker for stroke and TIA in a Swedish population. Atherosclerosis. 2006;185:271–277. doi: 10.1016/j.atherosclerosis.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Dayal M, Ouyang YB, Sun Y, Yang CF, Frydman J, Giffard RG. Chaperonin GroEL and its mutant D87K protect from ischemia in vivo and in vitro. Neurobiol. Aging. 2006;27:562–569. doi: 10.1016/j.neurobiolaging.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Yoo JH, Lee SC. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis. 2001;158:425–430. doi: 10.1016/s0021-9150(01)00444-0. [DOI] [PubMed] [Google Scholar]
- 24.Yun HY, Dawson VL, Dawson TM. Nitric oxide in health and disease of the nervous system. Mol. Psychiatry. 1997;2:300–310. doi: 10.1038/sj.mp.4000272. [DOI] [PubMed] [Google Scholar]