Abstract
CDK1 is a pivotal regulator of resumption of meiosis and meiotic maturation of oocytes. CDC25A/B/C are dual-specificity phosphatases and activate cyclin-dependent kinases (CDKs). Although CDC25C is not essential for either mitotic or meiotic cell cycle regulation, CDC25B is essential for CDK1 activation during resumption of meiosis. Cdc25a −/− mice are embryonic lethal and therefore a role for CDC25A in meiosis is unknown. We report that activation of CDK1 results in a maturation-associated decrease in the amount of CDC25A protein, but not Cdc25a mRNA, such that little CDC25A is present by metaphase I. In addition, expression of exogenous CDC25A overcomes cAMP-mediated maintenance of meiotic arrest. Microinjection of Gfp-Cdc25a and Gpf-Cdc25b mRNAs constructs reveals that CDC25A is exclusively localized to the nucleus prior to nuclear envelope breakdown (NEBD). In contrast, CDC25B localizes to cytoplasm in GV-intact oocytes and translocates to the nucleus shortly before NEBD. Over-expressing GFP-CDC25A, which compensates for the normal maturation-associated decrease in CDC25A, blocks meiotic maturation at MI. This MI block is characterized by defects in chromosome congression and spindle formation and a transient reduction in both CDK1 and MAPK activities. Last, RNAi-mediated reduction of CDC25A results in fewer oocytes resuming meiosis and reaching MII. These data demonstrate that CDC25A behaves differently during female meiosis than during mitosis, and moreover, that CDC25A has a function in resumption of meiosis, MI spindle formation and the MI-MII transition. Thus, both CDC25A and CDC25B are critical for meiotic maturation of oocytes.
Keywords: resumption of meiosis, meiotic maturation, mouse oocytes, CDC25A, CDC25B, CDK1, MAPK, spindle formation
Introduction
Meiotic maturation, which involves resumption of meiosis of prophase I-arrested oocytes, completion of the first meiotic division and arrest at metaphase II (MII), is controlled by the activity of the CDK1-cyclin B complex (Dekel, 2005; Kishimoto, 2005; Motlik and Kubelka, 1990). CDK1, in addition to being regulated by its association with a cyclin, is also negatively regulated by phosphorylation on T14 and Y15 that are located within the ATP-binding loop. The WEE1/MYT1 protein kinases mediate this phosphorylation, whereas CDC25 phosphatases are responsible for dephosphorylation of T14 and Y15, which in turn leads to CDK1 activation (Malumbres and Barbacid, 2005).
CDK1 activity in mouse oocytes is naturally inhibited by cAMP. cAMP produced by the oocyte (and not derived from the surrounding cumulus cells that are coupled to the oocyte via gap junctions) appears essential for maintenance of meiotic arrest because oocytes, which express adenylate cyclase 3 (AC3) (Horner et al., 2003), that either lack the G(s)-linked G-protein-coupled receptor (GPR3) (Freudzon et al., 2005; Mehlmann, 2005; Mehlmann et al., 2004) or in which GPR3 is inhibited by microinjected anti-GPR3 antibodies (Mehlmann et al., 2002) resume meiosis within the follicle. A maturation-associated decrease in cAMP that precedes NEBD (Schultz et al., 1983) is likely mediated by phosphodiesterase 3A (PDE3A) (Masciarelli et al., 2004). PKB/AKT kinase, which is important for CDK1 activation and involved in resumption of meiosis (Kalous et al., 2006), is responsible for PDE3A activation (Han et al., 2006). cAMP-mediated inhibition of maturation is likely mediated by PKA phosphorylation of WEE1B, an oocyte specific member of WEE1/MYT1 protein kinase family (Han et al., 2005). PKA also negatively phosphorylates Xenopus CDC25 (Duckworth et al., 2002) and probably mouse CDC25B (Han and Conti, 2006).
CDC25B phosphatase is essential for CDK1 activation, because oocytes obtained from Cdc25b −/− mice fail to activate CDK1 and resume meiosis, but do so following microinjection of Cdc25b mRNA (Lincoln et al., 2002). Surprisingly, CDC25C is dispensable for both mitotic and meiotic cell cycles (Chen et al., 2001). Cdc25a −/− mice exhibit an early embryonic lethality, which would be consistent with a critical function for mitotic cell cycle regulation (Ray et al., 2007). Little is known, however, about the role of CDC25A in oocyte maturation in vertebrates. Cdc25a mRNA is expressed in mouse oocytes (Wickramasinghe et al., 1995) but information regarding expression at the protein level is lacking. Microinjection of Xenopus Cdc25a mRNA into Xenopus oocytes induces resumption of meiosis more potently than microinjection of CDC25C mRNA (Okazaki et al., 1996). Microinjection of bacterially expressed human CDC25A induces resumption of meiosis of Xenopus oocytes but the oocytes are arrested at metaphase I-stage and do not reach metaphase II (Rime et al., 1994).
In somatic cells CDC25A regulates both G1/S and G2/M associated CDK-activities (Mailand et al., 2002; Molinari et al., 2000). In late G1, S and G2-phase CDC25A protein has a very short half-life (Mailand et al., 2002) as a consequence of CHK1-mediated phosphorylation during a normal cell cycle (Zhao et al., 2002). DNA damage or inhibiting DNA replication activates CHK1 and CHK2 kinases that then lead to rapid degradation of CDC25A, thereby preventing cell cycle progression (Falck et al., 2001; Mailand et al., 2000; Molinari et al., 2000; Zhao et al., 2002). In contrast, CDC25A is stabilized by CDK1 phosphorylation on at least two residues, S17 and S115, before entry to mitosis. This mitotic form of CDC25A is stable even after ionizing radiation, which induces double-strand DNA breaks (Mailand et al., 2002). Degradation of CDC25A at anaphase and early G1 mediates APC/CCDH1 activation. CDC25A contains a KEN-box that is essential for degradation mediated by the APC/C but mutations in the KEN-box mutation do not affect the stability of CDC25A protein in interphase protein (Donzelli et al., 2002).
We report here that CDC25A protein is present in fully-grown meiotically-competent oocytes (GV-stage). In contrast to CDC25B, which resides in the cytoplasm, CDC25A is a nuclear protein. Following NEBD, there is a maturation-associated decrease in the amount of CDC25A such that only small amounts of CDC25A are present in MI and MII eggs. Over-expressing CDC25A overcomes cAMP-mediated maintenance of meiotic arrest and RNAi-mediated CDC25A knock-down indicates a role for CDC25A in both resumption of meiosis and the MI-MII transition.
Materials and methods
Oocyte collection and culture, and RNA microinjection
Mouse ovaries were obtained from 3–4 week-old PMSG-primed (C57BL/6J X BALB/c) F1 hybrid female mice. Ovaries were transferred to bicarbonate-free minimal essential medium (Earle salt) at 37°C and supplemented with 3 mg/ml of polyvinylalcohol (PVA) and 25 mM Hepes (pH 7.3). To inhibit resumption of meiosis, the medium contained 0.1 mM 3-isobutyl-1-methyl-xanthine (IBMX). Oocytes were cultured in M-16 medium (M7292, Sigma Aldrich) at 37.5 °C in 5% CO2 in air.
Oocytes were microinjected with 5 pl of the RNA solution using a MIS-5000 micromanipulator (Burleigh, Exfo Life Sciences, USA) and PM 2000B4 microinjector (MicroData Instrument, USA). The microinjection medium was Whitten’s medium supplemented with 10 mM Hepes (pH 7.3) and 0.1 mM IBMX. Pipets for microinjection were made using P97 Pipette Puller (Sutter Instrument Company, USA).
In vitro mRNA and dsRNA production for microinjection
pCMV-SPORT6 vector containing mouse full-length Cdc25a (GenBank accession no. BC046296.1, MGC:66900, IMAGE:6401489), and pYX-ASC vector containing mouse full-length Cdc25b (GenBank accession no BC057568.1, MGC:66900, IMAGE:6401489) were purchased from imaGenes GmbH, Germany (formally RZPD German Resource Center for Genome Research).
Cdc25a and b mRNAs for microinjection were produced by in vitro transcription using mMESSAGE mMACHINE® T3 Kit (#1348, Ambion). To generate the template for transcription, full-length mouse Cdc25 a and b cDNAs were cloned into Spe1 site (for N-terminal GFP tags) of the pBluscript-GFP vector containing a T3-promoter and Xenopus globin 5′UTR, 3′UTR and Kozak sequences for high mRNA stability and efficient translation initiation, respectively. This vector was obtained from Martin Anger, University of Oxford, UK, and will be described elsewhere. For in vitro transcription, the vectors were linearized with Asc1. After in vitro transcription, mRNAs were immediately polyadenylated using the Poly(A) Tailing Kit (#AM1350, Ambion). mRNAs were purified using RNeasy Mini Kit (#74104, Qiagen). Gfp mRNA for control microinjection was transcribed from an empty pBluscript-GFP vector.
dsRNA was produced using MEGAscript® RNAi Kit (#AM1626, Ambion). A PCR strategy in which the T7 sites were added on both sides of the template was used to generate template for in vitro transcription. Primers for Cdc25a were AGGATCCTAATACGACTCACTATAGGGAGAAGCTGCTGGCGGACTGTC and ACTCGAGTAATACGACTCACTATAGGGAGACAAACAGCCCGCAACGAT, for control Gfp AGGATCCTAATACGACTAACTATAGGGAGAATGGTGAGCAAGGGCGAGGA and ACTCGAGTAATACGACTCACTATAGGGAGAGCGGCCGCTTTACTTGTACA. The length of the dsRNA fragment was 653 bp for Cdc25a and 712 bp for Gfp.
Both mRNA and dsRNA, in nuclease free water (#AM9939, Ambion), were aliquoted (5 μl) at 500 ng RNA/μl and stored at −80 °C until used for microinjection.
Total RNA isolation and mRNA quantification
Total RNA from 15 or 25 oocytes was isolated using Absolutely RNA® Microprep Kit (Stratagene) and eluted with 42 μl of elution buffer. External Gfp mRNA (0.04 pg Gfp mRNA per oocyte) was added to lysed oocytes to serve as a control for quantifying RNA recovery and normalizing the RT-PCR data to the exogenously added Gfp mRNA. The eluted total RNA (3 μl) was used in 10 μl reaction volume using onestep QuantiTect SYBR Green RT-PCR Kit (#204243, Qiagen). Primers were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, USA). The following gene-specific primers were used: Cdc25a primers located at 3′ end of mRNA: AGAACCCTATTGTGCCTACTG and TACTCATTGCCGAGCCTATC; Cdc25a primers located at 5′ end of mRNA were purchased (QuantiTect Primer Assay QT01058778, Qiagen); Gfp primers were TTCAAGATCCGCCACAAC and GACTGGGTGCTCAGGTAG. One-step real-time RT-qPCR was done using a Chromo4 Real-Time PCR detection System (Biorad), and passive ROX reference was used for reaction volume correction. The following one-step real-time qRT-PCR protocols were used, Cdc25a 3′end: 1. 50° C 30 min, 2. 95° C 15 min, 3. 94° C 15 sec, 4. 57°C 30 sec, 5. 72° C 30 sec, 6. 75° C 3 sec, 7. plate reading, 8. go to step 3 for 50 additional cycles; Cdc25a 5′end: 1. 50° C 30 min, 2. 95° C 15 min, 3. 94° C 15 sec, 4. 55° C 30 sec, 5. 72° C 30 sec, 6. 72° C 3 sec, 7. plate reading, 8. go to step 3 for 50 additional cycles; Gfp: 1. 50° C 30 min, 2. 95° C 15 min, 3. 94° C 15 sec, 4. 50°C 30 sec, 5. 72° C 30 sec, 6. 72° C 3 sec, 7. plate reading, 8. go to step 3 for 50 additional cycles. Following quantification of the PCR reaction product, the Tm of product was determined and the expected size of product was verified by electrophoresis in a 1% agarose gel. The product sizes and Tm’s were: Cdc25a 5′end, 124 bp, Tm=79.5°C; Cdc25a 5′end, 103 bp, Tm=77.5°C; and Gfp, 122 bp, Tm=85°C. Raw data, without smoothening and with a global minimum base line correction, were imported into Excel, where they were resized for amplitude normalization. Then data were fit into sigmoid curve using NCSS200 statistical software (NCSS, Utah, USA) and initial amount of mRNA was calculated (Liu and Saint, 2002). Cdc25a mRNA expression was normalized to Gfp mRNA and expressed into graphs in relative arbitrary units.
Immunological methods, antibodies and kinase assays
Standard immunoblot and imunofluorescence methods were used as previously described (Kalous et al., 2006). For CDC25A immunoblotting, mouse monoclonal DCS-122 antibody was used (Mailand et al., 2000). For microtubule visualization using imunofluorescence, mouse monoclonal anti-acetylated α-tubulin antibody (T7451, Sigma Aldrich) was used. Histone H1 and MAPK assays were performed as previously described (Kalous et al., 2006).
Results
CDC25A protein and mRNA expression during meiotic maturation
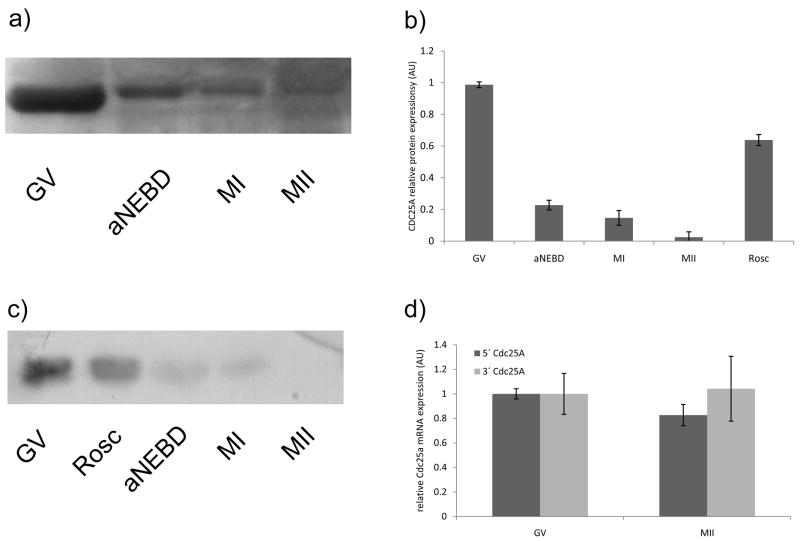
In somatic cells CDC25A protein expression gradually increases from late G1 and peaks at metaphase but after anaphase onset CDC25A is degraded because it is a target of the APC/CCDH1 complex (Donzelli et al., 2002; Mailand et al., 2002). In contrast to somatic cells, CDC25A protein displays a pronounced decrease shortly after NEBD (Fig. 1a and b) that is far in advance of anaphase onset, which typically occurs around 8–9 h following initiation of maturation in mouse. By MI the bulk of CDC25A has been degraded and progression to MII reveals is accompanied by a further decrease. The apparent decrease in electrophoretic mobility, which was not always seen, is likely due to a post-translational modification, e.g., phosphorylation, and could mark the protein for degradation.
Figure 1.
Expression of CDC25A protein and mRNA during meiotic maturation. a) At the indicated times oocytes were processed for and subjected to immunoblotting for CDC25A. GV-intact, aNEBD-(2 h), MI-(7h) and MII-(18h) stages. aNEBD; 2 h after transfer to IBMX-free medium, which corresponds to 1 h after NEBD. Each lane contained 400 oocytes/eggs. b) Quantification of immunoblots. The experiment was performed three times and the data are expressed as mean ± SEM. c) CDK1-dependent decrease in CDC25A protein. The samples were prepared as described in (a) but Roscovitine (0.025 mM, Rosc) was added after oocytes were transferred to IBMX-free medium. The experiment was done 3 times and quantification is included in b). Shown is a representative immunoblot; a loading control for Fig 1a and 1c) is included in Supplementary information d) Cdc25a mRNA expression in relative arbitrary units in GV-intact and MII-arrested eggs. Expression of both 5′ and 3′ ends of Cdc25a mRNA were normalized to exogenous Gfp mRNA, mRNA expression was measured in 4 independent experiments. The data are expressed as the mean ± SEM.
The maturation-associated decrease in CDC25A protein requires CDK1 activity (Fig. 1c). Addition of Roscovitine, a potent CDK1 inhibitor, markedly inhibited the decrease in CDC25A protein at a time when all control oocytes had undergone NEBD (i.e. 2 h after transfer to IBMX-free medium). Meiotic maturation triggers degradation of many maternal mRNAs (Su et al., 2007) and therefore, degradation of Cdc25a mRNA could underlie the observed decrease in CDC25A protein. Such is not the case, however, because there was no apparent change in Cdc25a mRNA during maturation (Fig. 1d), as assessed by qRT-PCR that analyzed both 5′ and 3′ ends of the mRNA.
Exogenous CDC25A overcomes cAMP-mediated inhibition of maturation
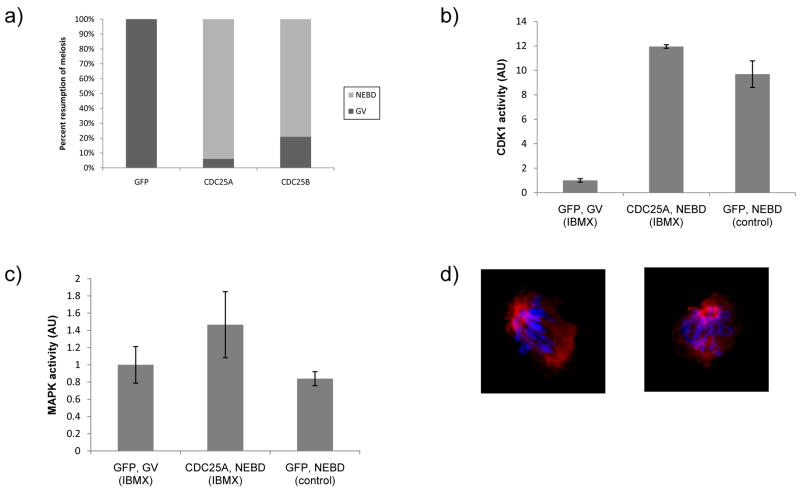
CDC25B, which can activate CDK1, is essential for initiation of maturation (Lincoln et al., 2002). Consistent with this finding is that over-expressing CDC25B in oocytes overcomes the ability of IBMX to inhibit maturation (Fig. 2a) with some 79% of the oocytes undergoing NEBD. CDC25A is a known positive regulator of both CDK2 (Blomberg and Hoffmann, 1999; Hoffmann et al., 1994) and CDK1 (Mailand et al., 2002). To ascertain whether CDC25A can activate CDK1 in mouse oocytes, we assessed the ability of microinjected GFP-Cdc25a mRNA to overcome inhibition of maturation by IBMX. Results of these experiments indicated that over-expression of CDC25A led to a high incidence of NEBD (Fig. 2a) and activation of CDK1, as well as MAPK (Fig. 2b and c). Eighteen h after Cdc25a mRNA microinjection, 94% of the injected oocytes underwent NEBD, whereas all of the control Gfp mRNA injected oocytes remained at the GV-stage. Noteworthy is that the level of CDC25A and B expression, as assessed by quantifying GFP fluorescence from the chimeric construct, was similar (52 ± 10 and 58 ± 18 arbitrary units, respectively). This finding minimizes the likelihood that higher levels of CDC25A expression accounted for its ability to induce NEBD when compared to CDC25B. Nevertheless, spindle formation and chromosome congression were impaired in oocytes over-expressing CDC25A (Fig. 2d). These results suggest that CDC25A could collaborate with CDC25B to initiate and drive maturation.
Figure 2.
Exogenous CDC25A overcomes cAMP-mediated GV-stage block. a) Oocytes were scored for NEBD 18 h following injection of mRNAs encoding the indicated Gfp-containing constructs. About 100 oocytes were scored for each group. CDK1 (b) and MAPK (c) activities in oocytes resuming meiosis 2 h after microinjection of Gpf-Cdc25a mRNA in IBMX-arrested oocytes (CDC25A, NEBD), and control Gfp mRNA injected oocytes maintained in IBMX-containing medium (GFP, GV) and those spontaneously maturing after transfer to IBMX-free medium at 2 h (GFP, NEBD). The experiment was conducted 3 times and the data are expressed as the mean ± SEM. d) Immunocytochemical analysis of spindle formation and chromosome congression in defective metaphase I oocytes that were microinjected with Gpf-Cdc25a mRNA. Red, acetylated α-tubulin; blue, DAPI for DNA staining; CDK1
CDC25A and CDC25B exhibit different localization during resumption of meiosis
In somatic cells CDC25A is mainly nuclear (Kallstrom et al., 2005) whereas CDC25B is mainly cytoplasmic at late G2 when a portion translocates to the nucleus (Kieffer et al., 2007). We therefore addressed CDC25A and CDC25B localization during spontaneous resumption of meiosis. GV-stage oocytes in IBMX-supplemented medium were microinjected with either Gfp-Cdc25a or Gfp-Cdc25b mRNAs and cultured for 60 min to allow protein expression. When a distinct GFP signal was detected, the oocytes were washed and transferred to IBMX-free medium to allow them to resume meiosis. Localization of the fusion proteins was monitored by imaging live cells during resumption of meiosis (Fig. 3).
Figure 3.
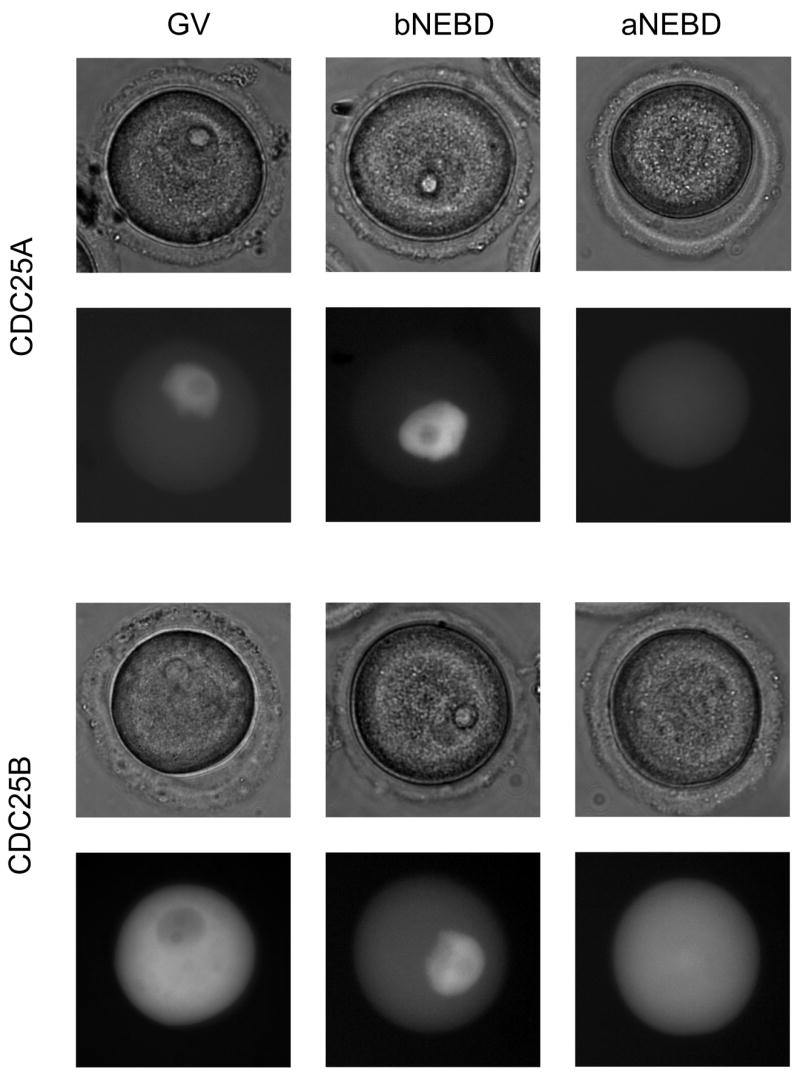
CDC25A and CDC25B exhibit different localization during resumption of meiosis. Localization of CDC25A and CDC25B in live oocytes after microinjection of Gfp-Cdc25a or Gfp-Cdc25b mRNA (150 ng/μl). GV-stage, shortly before (bNEBD) and shortly after (aNEBD) nuclear envelope break down. The experiment was performed 4 times and 120 oocytes were imaged. Shown are representative examples.
Prior to NEBD, CDC25A was localized to the nucleus, whereas CDC25B was essentially cytoplasmic (Fig. 3). Shortly before NEBD, CDC25A continues to remain nuclear, but a significant fraction of CDC25B translocates to the GV. Following NEBD, both GFP-CDC25A and GFP-CDC25B exhibit a diffuse localization throughout entire oocyte. Consistent with a decline of the endogenous CDC25A during meiotic maturation (Fig. 1a), is that GFP-CDC25A signal also decreases following NEBD. The initial differences in localization suggest that CDC25A and B are not fully redundant with respect to function, which is consistent with their functions in somatic cells in which CDC25A regulates early nuclear events and CDC25B early cytoplasmic (mainly centrosomal) mitotic events (Cazales et al., 2005; Dutertre et al., 2004; Lindqvist et al., 2005).
Maturation-associated decrease in CDC25A protein is essential for the MI – MII transition
The decrease in CDC25A after NEBD at prometaphase I (Fig. 1a) contrasts with its degradation at anaphase in somatic cells that is mediated by APC/CCDH1 (Donzelli et al., 2002; Mailand et al., 2002). To address the functional significance of the decrease in CDC25A during maturation we assessed the effect of over-expressing CDC25A to overcome the naturally occurring decrease.
GV-intact oocytes were microinjected with Gfp-Cdc25a or Gfp-Cdc25b mRNAs. The oocytes were cultured for 60 min in IBMX-containing medium to inhibit resumption of meiosis and provide time for protein expression. When a readily detectable GFP signal was observed, the oocyte were washed in IBMX-free medium and then cultured for 18 h and scored for their ability to reach MII, as assessed by the presence of a polar body. Control oocytes were microinjected with Gfp mRNA.
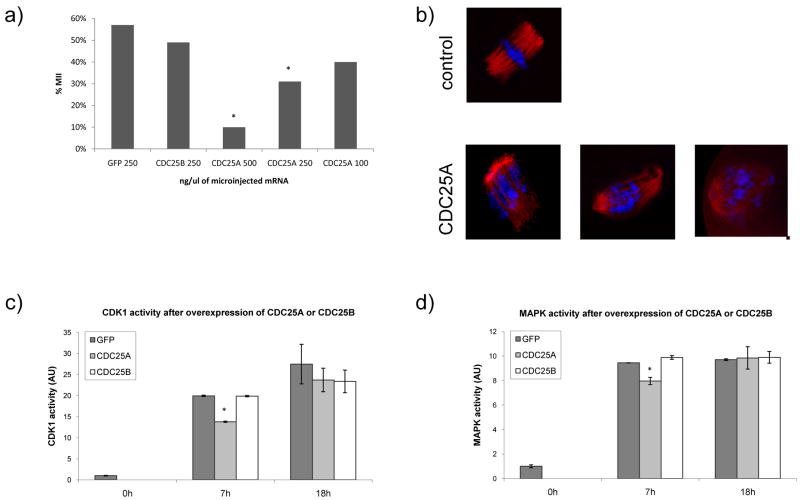
Control Gfp and Gfp-Cdc25b mRNA microinjected oocytes reached MII at 57% or 49%, respectively (Fig. 4a). In contrast, fewer Gpf-Cdc25a mRNA microinjected oocytes reached MII; only 10%, 31%, and 40% reached MII after microinjection of 500, 250, and 100 ng/μl of Gfp-Cdc25a mRNA, respectively. No effect of over-expression of CDC25A, CDC25B or GFP on NEBD was observed (data not shown). Similar levels of fluorescence were observed following injection of 500 ng/μl of Gpf-Cdc25a mRNA and 250 ng/μl Gpf-Cdc25b mRNA (89 ±26 and 95 ± 26 arbitrary units, respectively). This finding minimizes the likelihood that higher levels of CDC25A expression accounted for its ability to inhibit meiotic progression to MII when compared to CDC25B. The differences in concentration of the injected mRNAs required to achieve similar levels of fluorescence likely reflects the degradation of CDC25A that occurs during maturation.
Figure 4.
CDC25A protein degradation is essential for MI—MII transition. a) Concentration-dependent effect of microinjected Cdc25a mRNA on ability of oocytes to reach metaphase II by 18 h after transfer to IBMX-free medium. Gfp mRNA injected oocytes served as the control. Significant differences (p<0.05) in comparison to GFP controls are marked (*). About 50 oocytes in each group were scored; b) Immunocytochemical analysis of spindle formation and chromosome congression in control (Gfp mRNA injected) and Cdc25a mRNA-injected oocytes. The cells were processed for immunocytochemistry 7 h after microinjection; in both cases the injection solution was 250 ng/μl. Red, acetylated α-tubulin; blue, DAPI for DNA staining. About 75 of oocytes were injected and representative images are shown. CDK1 (c) and MAPK (d) activities at 7 h (MI in control GFP oocytes) and 18 h (MII in control GFP oocytes) after transfer to IBMX-free medium. In all cases the oocytes were injected with mRNAs at 250 ng/μl. Significant differences (p<0.05) in comparison to GFP controls at 7 h are marked (*). The experiment was performed three times and the data are expressed as mean ± SEM.
To ascertain at which stage of meiosis CDC25A over-expressing oocytes were arrested, injected oocytes were fixed and stained with an antibody to acetylated-α-tubulin and DAPI 7 h after initiation of maturation by transfer to IBMX-free medium (Fig 4b). Control Gfp mRNA-injected oocytes reached MI by this time and possessed well-formed spindles and with normal equatorial chromosome plates. In marked contrast, CDC25A over-expressing oocytes exhibited numerous spindle defects and problems with chromosome congression. These perturbations were not observed in CDC25B over-expressing oocytes that were indistinguishable from GFP over-expressing oocytes (data not shown).
Because CDC25A dephosphorylates not only CDKs but also MAPK (Wang et al., 2005), we assayed both CDK1 and MAPK activities in oocytes to explore whether MI-arrest of CDC25A over-expressing oocytes is associated with misregulation of these kinase activities. At 7 h after transfer to IBMX-free medium, when control oocytes (Gfp or Gfp-Cdc25b mRNA injected) were in MI (Fig. 4b), both CDK1 and MAPK activities were slightly but significantly reduced in Gfp-Cdc25a mRNA injected oocytes when compared to controls (Fig. 4c, d). This difference was not observed at 18 h, when control oocytes reached MII and CDC25A over-expressing oocytes were arrested still in meiosis I (Fig. 4c,d).
CDC25A is involved in both resumption of meiosis and MI—MII transition
The results described above suggest that degradation of CDC25A protein is required for the MI-MII transition. The experiments, however, do not address a role, if any, for CDC25A in resumption of meiosis, which clearly requires CDC25B (Lincoln et al., 2002). The absence of oocyte-specific conditional KO mice for Cdc25a and the early embryonic lethality of Cdc25a null mice preclude assessing such a role for CDC25A. To circumvent this problem, we used RNAi to decrease the amount of oocyte CDC25A protein.
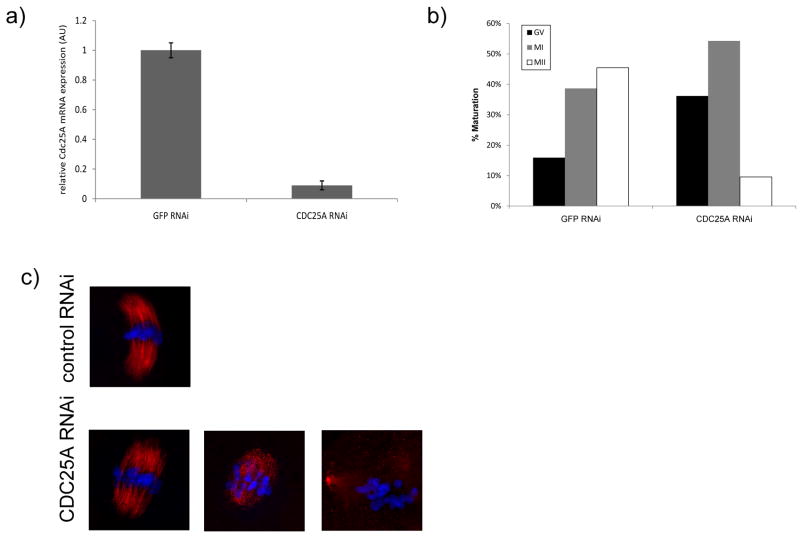
Oocytes were microinjected with long dsRNA and cultured in IBMX-containing medium for 24 h, which was sufficient to observe a 90% decrease in Cdc25a mRNA (Fig. 5a); essentially no degradation of Cdc25a mRNA was observed when oocytes were injected with long Gfp dsRNA. In addition, the long Cdc25a dsRNA was designed such that there was no region of homology in Cdc25b mRNA that was greater than 11 nucleotides. Further evidence for targeting specificity is that this approach can target specifically single family members (Ma et al., 2006; Yu et al., 2004). Following transfer to IBMX-free medium, the oocytes were cultured for 18 h and then scored for maturation (Fig. 5b). Compared to control injected oocytes in which only 16% failed to resume meiosis as evidenced by NEBD, 36% of oocytes injected with long Cdc25a dsRNA failed to resume meiosis. In addition, of those oocytes that did resume meiosis, only 10% reached MII, in contrast to 45% of their control injected counterparts.
Figure 5.
CDC25A is involved in resumption of meiosis and MI - MII transition. a) Effectiveness of RNAi-mediated knock-down of Cdc25a mRNA at 24 h in IBMX-supplemented medium after microinjection of long dsRNA Gfp RNAi (control) or long Cdc25a RNA. The experiment was performed 4 times and the data are expressed as the mean ± SEM. b) Meiotic maturation of control (GFP RNAi) and CDC25A RNAi oocytes scored 18 h after transfer to IBMX-free medium; ~100 oocytes for each group were scored. c) Immunocytochemical analysis of spindle formation and chromosome congression in GFP RNAi (control) and CDC25A RNAi oocytes at 7 h after transfer to IBMX-free medium. Red, acetylated α-tubulin; blue, DAPI for DNA staining. About 80 oocytes were injected and representative images are shown.
To characterize further the MI arrest of oocytes injected with long Cdc25a dsRNA, the oocytes were fixed and stained with an antibody to acetylated-tubulin antibody and DAPI 7 h after transfer to IBMX-free medium (Fig. 5c). Control oocytes (Gfp RNAi) exhibited normal MI spindles formation, where oocytes injected with long Cdc25a dsRNA exhibited two main phenotypes. Approximately half of the oocytes had normal a MI spindle with chromosomes aligned at the equatorial plate. The other half displayed defects with spindle formation and/or chromosomal congression.
Discussion
The early embryonic lethality of Cdc25a null embryos (Ray et al., 2007), coupled with the absence of a conditional, oocyte-specific Cdc25a knockout has precluded to date assessing the function of CDC25A during early development. Using complementary approaches of over-expressing and ablating CDC25A, we describe here the first evidence for a role of CDC25A in meiotic maturation.
Oocytes are arrested in the first meiotic prophase, which resembles arrest at the G2/M transition in the cell cycle. The presence of CDC25A in oocytes is consistent with accumulation of CDC25A as somatic cells enter M phase. In oocytes, CDC25B is essential for resumption of meiosis (Lincoln et al., 2002) and we show that over-expressing CDC25A overcomes IBMX-inhibition of oocyte maturation. In somatic cells CDC25A and B collaborate in mitotic events (Lindqvist et al., 2005), and over-expressing CDC25A induces mitotic events such as chromosome condensation (Mailand et al., 2000; Molinari et al., 2000). Thus, a competence of CDC25A and B to induce mitosis entry appears also conserved in meiosis entry (resumption of meiosis).
CDC25A degradation, which occurs in the absence of any apparent decrease in Cdc25a mRNA, is apparent within 1 h following nuclear envelope breakdown and therefore degradation precedes MI by several hours. In addition, there is little, if any, degradation of CDC25B during this early time. CDC25A degradation occurs much later in somatic cells and follows the metaphase to anaphase transition (Donzelli et al., 2002). CDC25B is also degraded in somatic cells after sister chromatid separation (Kieffer et al., 2007).
A functional APC/CCDH1 present in prometaphase oocytes (Marangos et al., 2007; Reis et al., 2007) may be responsible for the early degradation of CDC25A. In mouse oocytes, APC/CCDH1 is required for chromosome congression and targets destruction of CDC20, the other APC/C activator, thereby ensuring that CDC20 re-synthesis is required for meiotic progression. The switch from APC/CCDH1 to APC/CCDC20 is controlled by increased CDK1 activity and CDH1 loss (Reis et al., 2007). SCF/βTrCP, which mediates CDC25A degradation in interphase somatic cells (Donzelli et al., 2002), promotes degradation of EMI1 (early mitotic inhibitor 1) that inhibits the APC/C; EMI1 degradation is required for meiotic progression (Marangos et al., 2007). Both the APC/CCDH1 and SCF/βTrCP E3-ubiquitin ligases operate in prometaphase I oocytes (Marangos et al., 2007; Reis et al., 2007) but which, if any, of them is involved in CDC25A destruction in mouse oocytes is unresolved. It also should be noted that in Xenopus, CDC25A protein is absent in unfertilized eggs and its synthesis begins within 30 min of fertilization (Kim et al., 1999). The virtual absence of CDC25A in MII mouse eggs raises the interesting question when and how CDC25A accumulates following fertilization.
The maturation-associated decrease in CDC25A requires CDK1 activity because inhibiting CDK1 with Roscovitine prevents the decrease. This requirement for CDK1 for rapid CDC25A degradation stands in stark contrast to CDK1-mediated phosphorylation of CDC25A leading to its stability prior mitosis. In somatic cells, CDK1-dependent phosphorylation of CDC25A stabilizes CDC25A against activity of SCF/βTrCP, but not APC/CCDH1 (Mailand et al., 2002), which normally targets CDC25A during anaphase despite persisting CDK1 phosphorylation. As discussed above, APC/CCDH1 is a good candidate for the E3 ubiquitin ligase that targets CDC25A in prometaphase I oocytes. Moreover, because EMI1 destruction initiates immediately after GVBD (Marangos et al., 2007), EMI1 activity presumably continues to inhibit APC/CCDH1 following transferring oocytes from IBMX-supplemented medium into roscovitin-supplemented medium.
Over-expressing CDC25B overcomes IBMX-mediated meiotic arrest, a finding consistent with the essential role of CDC25B for resumption of meiosis (Lincoln et al., 2002). Likewise, over-expressing CDC25A also induced resumption of meiosis. This effect cannot be ascribed to expressing higher amounts of CDC25A than CDC25B and thereby dephosphorylating inappropriate substrates, because similar levels of expression were observed as assayed by measuring GFP fluorescence from the chimeric proteins. This finding provides the first evidence that CDC25A plays a role in oocyte maturation.
Although expressing CDC25A induces nuclear envelope breakdown in IBMX-arrested oocytes, entry into MI is abnormal and characterized by abnormal spindle formation and chromosome congression, with no apparent sign of exit from MI. A similar observation was made when CDC25B was over-expressed (data not shown). The inability of oocytes expressing either CDC25A or CDC25B to reach MII is probably a consequence of IBMX maintaining high concentrations of cAMP in these oocytes. Consistent with this interpretation is that injection of Cdc25b mRNA into Cdc25b null oocytes initiates resumption of meiosis, with oocytes emitting a polar body and arresting at MII with a normal spindle and congressed chromosomes in control IBMX-free medium (Lincoln et al., 2002).
CDC25A and CDC25B display different temporal and spatial patterns of localization. We find CDC25A is almost exclusively nuclear in GV-intact oocytes until NEBD occurs. In contrast CDC25B is essentially cytoplasmic and translocates to the GV just prior to nuclear envelope breakdown. A similar situation was observed for cyclin B that undergoes nuclear translocation just before NEBD (Reis et al., 2006). Previous reports on cell cycle-dependent localization of CDC25B in somatic cells were controversial (Baldin et al., 2002; Baldin et al., 2003; Davezac et al., 2000; Giles et al., 2003; Uchida et al., 2004a; Uchida et al., 2004b; Woo et al., 1999), but the recent results using time-lapse video microscopy of YFP-CDC25B expressed under control of the mouse minimal CDC25B promoter show translocation of cytoplasmic CDC25B into nucleus during the G2-M transition (Kieffer et al., 2007). In human somatic cells, cytoplasmic localization of CDC25B1 is due to its association with either 14-3-3beta or 14-3-3epsilon. This association requires S309 of CDC25B1; S309 resides within a putative 14-3-3 binding consensus sequence and is sufficient for cytoplasmic retention (Uchida et al., 2004a). Whether S309 plays a similar role in cytoplasmic localization of CDC25B in oocytes and whether its phosphorylation regulates CDC25B translocation into the nucleus shortly before GVBD will be the subject of future studies.
The differences in localization of the two phosphatases suggest that they have different substrates and non-redundant functions. Consistent with this proposal is that over-expression of CDC25A, but not CDC25B, inhibits meiotic progression to MII when oocytes are transferred to IBMX-free medium. The oocytes arrest at MI with an abnormal spindle and poorly congressed chromosomes. As described above, the difference in the effects of over-expressing CDC25A and CDC25B cannot be attributed to differences in expression. These results also indicate that CDC25A degradation is essential for the MI-MII transition. The rationale for conducting the experiment was that by over-expressing CDC25A that even though the endogenous and exogenous proteins would be degraded following nuclear envelope breakdown, the amount CDC25A activity would remain above a threshold. Interestingly, in these experiments activation of CDK1 and MAPK displayed a transient lag when compared to the increase in these activities following expression of CDC25B. CDC25A has many non-CDK substrates, including components of the MAPK pathway (Kar et al., 2002; Nemoto et al., 2004; Wang et al., 2005; Xia et al., 1999), and the small transient decrease of MAPK activity (Fig. 4d) may be an outcome of over-expressing CDC25A. The small transient decrease in CDK1 activity in these MI arrested oocytes may be significant, because in yeast and human, a slight decrease of CDK1 activity does not prevent metaphase spindle formation, but blocks the metaphase-anaphase transition (Lindqvist et al., 2007; Rudner et al., 2000).
Reducing the amount of CDC25A by RNAi prior to resumption of meiosis reveals a role for CDC25A not only in meiotic progression to MII but also in resumption of meiosis. Of those oocytes that resume meiosis, half of the MI-arrested oocytes exhibited an abnormal spindle. Thus, even though the maturation-associated degradation of CDC25A results in MI eggs containing very low amounts of CDC25A, these small amounts nevertheless appear critical for MI spindle formation and the MI-MII transition. It may seem paradoxical that CDC25A is required not only for initiation of maturation but that it must be degraded for a successful MI-MII transition. In fact, a similar situation is observed for EMI1, which has to be degraded during meiotic maturation via βTrCF-SCF but is still required for the MI-MII transition (Marangos et al., 2007). A possible resolution to this paradox couples the known role of CDC25A in CDK1 activation with two potentially separate and later functions. The decrease in CDC25A activity—CDC25A has a broad substrate spectrum—could modulate the time course and magnitude of MAPK activation after NEBD (Kar et al., 2002; Nemoto et al., 2004; Wang et al., 2005; Xia et al., 1999). In addition, CDC25A probably dephosphorylates other key substrates during/after NEBD that are required for a successful MI-MII transition and this dephosphorylation is not compensated by either the remaining CDC25B or C.
In summary, the results described here provide the first evidence for a critical role for CDC25A in both resumption and completion of meiosis during oocyte maturation. The functional inter-relationships in these processes between CDC25A and CDC25B remain to be resolved.
Supplementary Material
Acknowledgments
This research was performed in the frame of IRP IAPG No. AV0Z50450515. This study was supported by GACR No. 305/06/1413 and grant ME08030 (Czech-US scientific cooperation program). A. Š. was also supported by the grant 204/05/H023. Vladimir Baran was supported by VEGA 2/6176/26. RMS was supported by HD22681.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldin V, et al. Nuclear localization of CDC25B1 and serine 146 integrity are required for induction of mitosis. J Biol Chem. 2002;277:35176–82. doi: 10.1074/jbc.M204430200. [DOI] [PubMed] [Google Scholar]
- Baldin V, et al. PKB/Akt phosphorylates the CDC25B phosphatase and regulates its intracellular localisation. Biol Cell. 2003;95:547–54. doi: 10.1016/j.biolcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183–94. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazales M, et al. CDC25B phosphorylation by Aurora-A occurs at the G2/M transition and is inhibited by DNA damage. Cell Cycle. 2005;4:1233–8. doi: 10.4161/cc.4.9.1964. [DOI] [PubMed] [Google Scholar]
- Davezac N, et al. Regulation of CDC25B phosphatases subcellular localization. Oncogene. 2000;19:2179–85. doi: 10.1038/sj.onc.1203545. [DOI] [PubMed] [Google Scholar]
- Dekel N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endocrinol. 2005;234:19–25. doi: 10.1016/j.mce.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Donzelli M, et al. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 2002;21:4875–84. doi: 10.1093/emboj/cdf491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth BC, et al. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci U S A. 2002;99:16794–9. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117:2523–31. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- Falck J, et al. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–7. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- Freudzon L, et al. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol. 2005;171:255–65. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N, et al. 14-3-3 acts as an intramolecular bridge to regulate cdc25B localization and activity. J Biol Chem. 2003;278:28580–7. doi: 10.1074/jbc.M304027200. [DOI] [PubMed] [Google Scholar]
- Han SJ, Conti M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle. 2006;5:227–31. doi: 10.4161/cc.5.3.2395. [DOI] [PubMed] [Google Scholar]
- Han SJ, et al. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–6. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Han SJ, et al. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–25. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann I, et al. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994;13:4302–10. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner K, et al. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–96. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Chen MS, et al. Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol Cell Biol. 2001;21:3853–61. doi: 10.1128/MCB.21.12.3853-3861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallstrom H, et al. Cdc25A localisation and shuttling: characterisation of sequences mediating nuclear export and import. Exp Cell Res. 2005;303:89–100. doi: 10.1016/j.yexcr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Kalous J, et al. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell. 2006;98:111–23. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- Kar S, et al. EGFR-independent activation of ERK1/2 mediates growth inhibition by a PTPase antagonizing K-vitamin analog. J Cell Physiol. 2002;190:356–64. doi: 10.1002/jcp.10063. [DOI] [PubMed] [Google Scholar]
- Kieffer I, et al. Differential mitotic degradation of the CDC25B phosphatase variants. Oncogene. 2007 doi: 10.1038/sj.onc.1210596. [DOI] [PubMed] [Google Scholar]
- Kim SH, et al. A maternal form of the phosphatase Cdc25A regulates early embryonic cell cycles in Xenopus laevis. Dev Biol. 1999;212:381–91. doi: 10.1006/dbio.1999.9361. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Developmental biology: cell cycle unleashed. Nature. 2005;437:963–5. doi: 10.1038/437963a. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, et al. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–9. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, et al. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome. J Cell Biol. 2005;171:35–45. doi: 10.1083/jcb.200503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, et al. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol. 2007;5:e123. doi: 10.1371/journal.pbio.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Saint DA. Validation of a quantitative method for real time PCR kinetics. Biochem Biophys Res Commun. 2002;294:347–53. doi: 10.1016/S0006-291X(02)00478-3. [DOI] [PubMed] [Google Scholar]
- Ma J, et al. Basonuclin: a novel mammalian maternal-effect gene. Development. 2006;133:2053–62. doi: 10.1242/dev.02371. [DOI] [PubMed] [Google Scholar]
- Mailand N, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–9. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- Mailand N, et al. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002;21:5911–20. doi: 10.1093/emboj/cdf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–41. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Marangos P, et al. Prophase I arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APC(Cdh1) J Cell Biol. 2007;176:65–75. doi: 10.1083/jcb.200607070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciarelli S, et al. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005;288:397–404. doi: 10.1016/j.ydbio.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, et al. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–5. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–50. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Molinari M, et al. Human Cdc25 A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO Rep. 2000;1:71–9. doi: 10.1093/embo-reports/kvd018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlik J, Kubelka M. Cell-cycle aspects of growth and maturation of mammalian oocytes. Mol Reprod Dev. 1990;27:366–75. doi: 10.1002/mrd.1080270411. [DOI] [PubMed] [Google Scholar]
- Nemoto K, et al. Activation of the Raf-1/MEK/Erk kinase pathway by a novel Cdc25 inhibitor in human prostate cancer cells. Prostate. 2004;58:95–102. doi: 10.1002/pros.10292. [DOI] [PubMed] [Google Scholar]
- Okazaki K, et al. Isolation of a cDNA encoding the X enopus homologue of mammalian Cdc25A that can induce meiotic maturation of oocytes. Gene. 1996;178:111–4. doi: 10.1016/0378-1119(96)00344-7. [DOI] [PubMed] [Google Scholar]
- Ray D, et al. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res. 2007;67:6605–11. doi: 10.1158/0008-5472.CAN-06-4815. [DOI] [PubMed] [Google Scholar]
- Reis A, et al. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol. 2006;8:539–40. doi: 10.1038/ncb1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, et al. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol. 2007;9:1192–8. doi: 10.1038/ncb1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rime H, et al. Microinjection of Cdc25 protein phosphatase into Xenopus prophase oocyte activates MPF and arrests meiosis at metaphase I. Biol Cell. 1994;82:11–22. doi: 10.1016/0248-4900(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Rudner AD, et al. Cdc28 activates exit from mitosis in budding yeast. J Cell Biol. 2000;149:1361–76. doi: 10.1083/jcb.149.7.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, et al. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–73. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Su YQ, et al. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol. 2007;302:104–17. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, et al. Binding of 14-3-3beta but not 14-3-3sigma controls the cytoplasmic localization of CDC25B: binding site preferences of 14-3-3 subtypes and the subcellular localization of CDC25B. J Cell Sci. 2004a;117:3011–20. doi: 10.1242/jcs.01086. [DOI] [PubMed] [Google Scholar]
- Uchida S, et al. Nuclear export signal in CDC25B. Biochem Biophys Res Commun. 2004b;316:226–32. doi: 10.1016/j.bbrc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Cdc25A and ERK interaction: EGFR-independent ERK activation by a protein phosphatase Cdc25A inhibitor, compound 5. J Cell Physiol. 2005;204:437–44. doi: 10.1002/jcp.20297. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe D, et al. Two CDC25 homologues are differentially expressed during mouse development. Development. 1995;121:2047–56. doi: 10.1242/dev.121.7.2047. [DOI] [PubMed] [Google Scholar]
- Woo ES, et al. Cell cycle dependent subcellular distribution of Cdc25B subtypes. Oncogene. 1999;18:2770–6. doi: 10.1038/sj.onc.1202614. [DOI] [PubMed] [Google Scholar]
- Xia K, et al. Tyrosine phosphorylation of the proto-oncoprotein Raf-1 is regulated by Raf-1 itself and the phosphatase Cdc25A. Mol Cell Biol. 1999;19:4819–24. doi: 10.1128/mcb.19.7.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, et al. Transgenic RNAi-mediated reduction of MSY2 in mouse oocytes results in reduced fertility. Dev Biol. 2004;268:195–206. doi: 10.1016/j.ydbio.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Zhao H, et al. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A. 2002;99:14795–800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.