Abstract
Hsp27 inhibits mitochondrial injury and apoptosis in both normal and cancer cells by an unknown mechanism. To test the hypothesis that Hsp27 decreases apoptosis by inhibiting Bax, Hsp27 expression was manipulated in renal epithelial cells before transient metabolic stress, an insult that activates Bax, induces mitochondrial injury, and causes apoptosis. Compared with control, enhanced Hsp27 expression inhibited conformational Bax activation, oligomerization, and translocation to mitochondria, reduced the leakage of both cytochrome c and apoptosis-inducing factor, and significantly improved cell survival by >50% after stress. In contrast, Hsp27 down-regulation using RNA-mediated interference promoted Bax activation, increased Bax translocation, and reduced cell survival after stress. Immunoprecipitation did not detect Hsp27-Bax interaction before, during, or after stress, suggesting that Hsp27 indirectly inhibits Bax. During stress, Hsp27 expression prevented the inactivation of Akt, a pro-survival kinase, and increased the interaction between Akt and Bax, an Akt substrate. In contrast, Hsp27 RNA-mediated interference promoted Akt inactivation during stress. Hsp27 up- or down-regulation markedly altered the activity of phosphatidylinositol 3-kinase (PI3-kinase), a major regulator of Akt. Furthermore, distinct PI3-kinase inhibitors completely abrogated the protective effect of Hsp27 expression on Akt activation, Bax inactivation, and cell survival. These data show that Hsp27 antagonizes Bax-mediated mitochondrial injury and apoptosis by promoting Akt activation via a PI3-kinase-dependent mechanism.
Hsp27, a member of the small heat shock protein family, is induced by stress and protects against heat shock, oxidative stress, hypertonic stress, and other forms of cellular injury in numerous cell types including neurons (1, 2), cardiac myocytes (3, 4), and endothelial cells (5) and mediates chemo-resistance in multiple cancer cell types (6, 7). In contrast, suppressing endogenous Hsp27 increases cellular susceptibility to apoptosis (8). In transgenic models of cerebral (1) and myocardial ischemia (9) Hsp27 expression also prevents tissue injury, suggesting that apoptotic cell death contributes to organ dysfunction (10).
Apoptotic signal transduction pathways converge at the mitochondrion to cause membrane permeabilization, an event regulated by mutually antagonistic members of BCL-2 protein family that includes Bcl-2 and Bax (11). In renal epithelial cells, as in other cell types, the balance between death and survival is determined by the ratio of these apoptosis-stimulating and suppressing BCL-2 proteins (12). Renal ischemia in vivo (13) as well as exposure to metabolic inhibitors in vitro causes mitochondrial membrane injury and Bax activation in epithelial cells (14, 15). In healthy cells, Bax exists as a 21-kDa cytosolic monomer. After a conformational change in both the carboxyl and amino termini, Bax forms toxic oligomers, translocates to the mitochondrial outer membrane (16), and either forms de novo pores or opens existing mitochondrial membrane channels that release pro-apoptotic proteins such as cytochrome c and apoptosis-inducing factor (16–19). Leakage of pro-apoptotic mediators normally sequestered in the intramembranous mitochondrial space results in activation of caspase-dependent and independent pathways that ultimately precipitate cell death (11, 20).
Recent evidence suggests that Bax activation is regulated by site-specific serine phosphorylation by kinases known to mediate apoptosis. Specifically, serine phosphorylation by Akt, a potent anti-apoptotic serine/threonine kinase, inactivates Bax (21), whereas serine phosphorylation at another site by glycogen synthase kinase 3β (GSK3β),2 an Akt substrate, promotes Bax activation and apoptosis (22). Taken together, these reports suggest that stressors that inactivate Akt and activate GSK3β promote Bax activation by a dual mechanism.
Several laboratories have investigated the mechanism of Hsp27-mediated cytoprotection. Specifically, Hsp27 inhibits caspase 3 and 9 activation and reduces apoptosome formation (8, 23, 24). However, each of these protective effects operates downstream of mitochondrial membrane injury and cannot explain the observation by multiple investigators that Hsp27 inhibits cytochrome c release after pro-apoptotic stress (8, 23–25). Despite these intriguing reports, the mechanism by which Hsp27 antagonizes mitochondrial injury and prevents apoptosis is not understood.
Hsp27 has been closely associated with Akt. However, most reports emphasize the effect of Akt on the phosphorylation and activation of Hsp27 rather than vice versa (26, 27). At least in neutrophils, Hsp27 and Akt co-exist in a large multiprotein complex, suggesting that Akt and Hsp27 regulate one another (28). Despite their apparent co-localization in these cells, direct evidence that Hsp27 modifies Akt activity has not been shown. This prompted us to speculate that Hsp27 inhibits Bax-mediated mitochondrial membrane injury by promoting the activation of phosphatidyl inositol 3 kinase (PI3-kinase), a major upstream regulator of Akt.
In the present study we report that Hsp27 expression reduces mitochondrial membrane injury and improves cell survival after stress, whereas Hsp27 down-regulation has the opposite effect on these parameters. Hsp27 expression enhances PI3-kinase activity, promotes Akt-Bax interaction, and inhibits Bax activation, oligomerization, and translocation to mitochondria. Importantly, each of the protective effects ascribed to Hsp27 is prevented by the addition of a PI3-kinase inhibitor. We propose that Hsp27-mediated regulation of PI3-kinase is responsible for the potent protective effects of Hsp27 on the outer mitochondrial membrane during stress.
EXPERIMENTAL PROCEDURES
Materials and Reagents—All reagents were purchased from Sigma-Aldrich unless otherwise specified.
Cell Culture—Previously characterized proximal tubular epithelial cell lines derived from the immortalized mouse; (29), from the normal human kidney (HK-2; ATCC, catalog #CRL-2190), or human embryonic kidney cells (293) were maintained as previously described (29).
Metabolic Stress—Cells were incubated for 15 min to 2 h at 37 °C in glucose-free medium (Dulbecco's modified Eagle's medium, Invitrogen 23800-014) that contained 5 mm sodium cyanide and 5 mm 2-deoxy-d-glucose as previously described by our laboratory (see Schwartz and co-workers (30) and Ref. 31). This maneuver results in apoptosis due to the rapid, persistent ATP depletion to <10% of base-line ATP content and is reversible with the removal of cyanide and the addition of exogenous glucose (32). In control, parallel medium changes were performed using Dulbecco's modified Eagle's medium.
Selective Hsp27 Expression—Wild type human Hsp27 expression was increased in renal cells using a previously characterized adenoviral construct (33). Control cells were infected with empty vector (AdTR5/GFP). Infection efficiency was >90–99% as determined by direct visualization of GFP in cells infected with 40–100 multiplicity of infection. Cells were infected with adenovirus for 16 h at 37 °C in Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum (FBS) followed by a 24-h washout period during which the medium was replaced with fresh Dulbecco's modified Eagle's medium containing 10% FBS. Increased Hsp27 expression was confirmed by immunoblot analysis and was titrated to approximate the level of expression detected after sublethal heat stress (43.0 °C × 45 min (34)) followed by 16 h of recovery (Fig. 1A).
FIGURE 1.
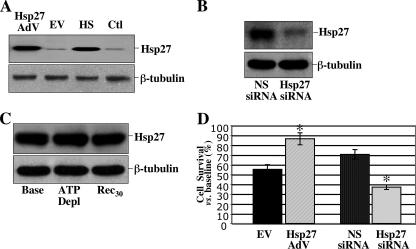
Effect of adenoviral infection, siRNA, or metabolic stress on Hsp27 expression and cell survival after metabolic stress. A, Hsp27 level in lysates harvested from non-infected cells (Ctl), cells subjected to transient heat stress (HS), or infected with adenovirus containing either empty vector (EV) or human Hsp27 (Hsp27). Lower panel, β-tubulin loading control. B, Hsp27 content after exposure to non-sense siRNA (NS siRNA) or specific Hsp27 siRNA (Hsp27 siRNA). Lower panel, β-tubulin loading control. C, Hsp27 content at base line (Base), after 60 min metabolic stress (ATP Depl), and after 30 min of recovery (Rec30). D, cell survival after 1 h of metabolic stress followed by 30 min of recovery in empty vector (EV), Hsp27 adenovirus (Hsp27 AdV), non-sense siRNA (NS siRNA), and Hsp27 siRNA (Hsp27 siRNA)-treated cells compared with base line. Data are the mean ± S.E. and were performed in triplicate; *, p < 0.05 versus base-line survival; immunoblots are representative of at least three independent experiments.
RNA Interference—HK-2 cells were transected with siRNA against Hsp27 (sc-29350) or control siRNA (sc-37007) from Santa Cruz Biotechnology (Santa Cruz, CA) as per the manufacturer's protocol. Briefly, subconfluent (50–60%) HK-2 cells grown in antibiotic free medium were transfected using siRNA Transfection Medium (sc-36868) and Transfection Reagent (sc-29528) at an siRNA concentration of 10 nm. After 8 h, cells were washed and cultured for 72 h in complete medium.
Mitochondrial Membrane Injury/Cytosolic Cytochrome c and Apoptosis-inducing Factor (AIF) Leakage—To assess mitochondrial membrane injury, the leakage of mitochondrial cytochrome c and AIF was measured by immunoblot analysis of cytosolic fractions using digitonin permeabilization as previously reported by us (30, 35, 36).
Co-localization of Bax and the Mitochondria with Confocal Microscopy—Subconfluent, live cells grown on glass coverslips were incubated with Mitotracker Green-FM (600 nm; Molecular Probes), a mitochondrial specific marker, for 30 min at 37 °C. Cells were then fixed in methanol (4 °C for 20 min), exposed to an anti-Bax (N-20) antibody (Santa Cruz, #sc-493), detected with a Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), and routine immunohistochemistry was performed (30). Confocal microscopy was used to localize Bax and Mitotracker Green using an Olympus confocal microscope (FV300-IX70) with a 60× UPlan apochromatic objective (Olympus, Melville, NY). Images were processed using Fluoview software.
Immunoblot Analysis and Immunoprecipitation—Immunoblot analysis was performed as described previously (31). Commercially available antibodies were used to detect Hsp27 (1:1000 dilution, StressGen Biotechnologies, Victoria BC, Canada, catalog #SPA-803 or Santa Cruz Biotechnology, catalog number sc-1048), apoptosis-inducing factor (1:250 dilution, Santa Cruz Biotechnology, catalog #sc-13116), cytochrome c (1:1000, Research Diagnostics, clone 6H2.B4), caspase-3 (1:1000 dilution, Cell Signaling, #9665), cleaved caspase-3 (1:1000 dilution, Cell Signaling, #9664), activated Bax (6A7; 1:1,000 dilution; Trevingen, Inc., Gaithersburg, MD, catalog #2281-MC-100), total Bax (5B7; 1:500 dilution; Pharmingen, catalog #556467), β-actin (1:750 dilution, Abcam, Inc., Cambridge, MA, catalog #ab6276-100), AKT/p-Ser473-AKT (1:1000 dilution, Cell Signaling, #9272/#9271), PI3-kinase p85 (1:1000 dilution, Santa Cruz, catalog #423), GSK3β/p-Ser9-GSK3β (1:1000 dilution, Cell Signaling, catalog #9315 and 9336), β-actin (Sigma catalog A5316), glyceraldehyde-3-phosphate dehydrogenase (N-14; Santa Cruz, catalog #sc-20356), and β-tubulin (1:1500 dilution, Sigma, catalog #A5441). Secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories) were used in combination with a chemiluminescence detection method (Amersham Biosciences) to visualize specific protein bands. For co-immunoprecipitation, 7.5 × 106 cells were harvested in buffer containing 150 mm NaCl, 10 mm Tris HCl, 5 mm EDTA, 1 mm EGTA, 1% Triton X-100, 0.5% Nonidet P-40, with protease inhibitors at pH 7.4 as previously described by our laboratory (30). Lysates were centrifuged at 20,000 × g for 10 min at 4 °C, and the supernatants were collected. Samples containing 400 μg of total protein were incubated with 5 μg of antibody directed against Hsp27 (Santa Cruz, anti-goat or StressGen, anti-rabbit), PI3-kinase p85a (Santa Cruz), active Bax (Trevingen, Gaithersburg, MD, clone YTH-6A7), or total Bax (Lab Vision/Neomarkers, Fremont, CA, clone 5B7) overnight at 4 °C. Immunoprecipitates were collected by the addition of protein G- or A-agarose (ImmunoPure Immobilized Protein G or A, Pierce) to each sample followed by incubation at 4 °C for 1 h. Complexes were harvested by centrifugation and washed 4× with lysis buffer or phosphate-buffered saline. Finally, the beads were resuspended in 1× SDS sample buffer (50 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% mercaptoethanol) and heated at 100 °C for 10 min before separation by SDS-PAGE and immunoblot analysis. In selected experiments cells were lysed in either Tris-buffered saline or 2% CHAPS lysis buffer, and the identical buffer was then used in all subsequent steps as described above.
Cell Viability—Viability was assessed with a modified colorimetric technique (3-(4,5 dimethylthiazol)-2,5-diphenyl tetrazolium bromide (MTT) assay) that is based on the ability of live cells to convert MTT, a tetrazolium compound, into purple formazan crystals as described by our laboratory (37).
Chemical Cross-linking—Cell extracts were prepared in 1× Tris-buffered saline containing protease and phosphatase inhibitors by subjecting 2.5 × 106 cells to lysis with one freeze-thaw cycle in a dry-ice-ethanol bath and 37 °C water bath, respectively. Lysates were then purified by centrifugation at 12,000 × g for 10 min at 4 °C. Samples containing 50 μg of protein were suspended for 30 min at room temperature in 50 μl of freshly dissolved disuccinimidyl tartrate (DST) with a 6.4-Å spacer arm (Pierce) in DMSO to achieve a final concentration of 2 mm. DMSO without DST was added to control. The reactions were then quenched by the addition of 15 mm Tris, pH 7.4. The samples were prepared for immunoblot analysis as described above and were probed with an anti-Bax antibody directed against total Bax (mouse monoclonal, Clone 5B7, Lab Vision/Neomarkers).
PI3-kinase Assay—Cell extracts harvested in the presence of Nonidet P-40 were obtained from human kidney (HK-2) cells infected with adenovirus containing either Hsp27 or empty adenovirus that were incubated overnight with an antibody directed against the non-catalytic PI3-kinase p85 subunit (Santa Cruz) followed by a 1-h incubation with protein A/G-agarose. The immunoprecipitates were washed 3 times with lysis buffer then twice in buffer that contained 10 mm Tris·HCl, 100 mm NaCl, and 1 mm EDTA, pH 7.5, then once in PI3-kinase assay buffer that contained 20 mm Tris·HCl, 100 mm NaCl 10 mm, MgCl2, 0.1 mm EGTA, 100 mm vanadate, 20 mm ATP, 200 mm adenosine, pH 7.5. After the last wash, 10 μl of sonicated, PI substrate (l-α-phosphatidylinositol, Sigma, 1 mg/ml in 10 mm HEPES, pH 7.5) was added to each sample, and the samples were incubated for 10 min on ice. The reaction was carried out at 25 °C for 20 min by adding 40 ml of PI3-kinase assay buffer containing 10 μCi of [γ32P]ATP and then quenched with 100 ml of 1 m HCl. Phospholipids were extracted once with 200 μl of CHCl3/MeOH (1:1) (8000 rpm × 3 min) using a bench-top centrifuge, and then the lower (organic) phase was transferred to a fresh Eppendorf tube and extracted twice with 160 μl of 1 m HCl/CH3OH (1:1 vol:vol). The organic phase was dried under 100% nitrogen gas and re-suspended in 20 μl of CHCl3/MeOH (1:1 vol:vol). Phosphorylated products were resolved on oxalate-impregnated silica 60 plates that were wetted with 1.2% potassium oxalate mixed (1:1 vol:vol) with CHCl3/MeOH and 4 m NH4OH (9:7:2) for 2 h, the gel was air-dried, and then autoradiography was performed. Radioactive bands representing PI3-kinase were separated and quantified using a liquid scintillation analyzer (Packard-PerkinElmer Life Sciences).
Densitometry and Statistical Analysis—After digitizing the immunoblot image (Hewlett-Packard, Desk Scan II), band densities were quantified using NIH ImageQuant Software. Data are expressed as the means ± S.E. Comparison of two groups was performed using a two-tailed Student's t test. Results involving more than one group were compared using 2-way analysis of variance and were then analyzed with the Fisher post hoc test. A result was considered significant if p < 0.05.
RESULTS
Hsp27 Expression Regulates Cell Survival After Metabolic Stress—In renal tubular epithelial cells, non-lethal heat stress (43 °C × 45 min with 16 h recovery; Fig. 1A, third lane)or infection with 40 multiplicity of infection adenovirus containing human Hsp27 (first lane) exhibited a similar, marked increase in Hsp27 content compared with either uninfected cells (fourth lane) or cells infected with adenovirus containing empty vector (second lane). To reproduce the physiologic cellular response to stress, Hsp27 expression was increased by adenovirus to the same level as that afforded by heat stress in all subsequent experiments. In contrast, siRNA directed against Hsp27 markedly decreased steady state Hsp27 content compared with non-sense siRNA (Fig. 1B). Importantly, metabolic stress did not itself induce Hsp27 within the time frame examined in our studies (Fig. 1C). Apparent differences in the amount of immunoreactive Hsp27 detected in control (first lane, Fig. 1A), non-sense siRNA (first lane, Fig. 1B), and in normal cells at base line (first lane, Fig. 1C) are due to differences in exposure time that were optimized for each study.
To assess its effect on survival, Hsp27 content was manipulated in epithelial cells after 1–1.5 h of metabolic stress, an insult that causes apoptosis (14, 32). Only 50% of control (empty vector) survived after stress (Fig. 1D). Hsp27 expression increased cell survival after stress to 87 ± 6%% (p < 0.05; n = 3). In contrast, Hsp27 knockdown with specific siRNA decreased survival by almost 50% compared with non-sense siRNA (38 ± 6 versus 71 ± 3%, p < .05; n = 4). Differences in relative cell survival between empty vector and non-sense siRNA are due to differences in the duration of stress (1 h empty vector versus 1.5 h non-sense siRNA) that were intentionally selected to optimize cytoprotection or injury caused by changes in Hsp27 expression.
Hsp27 Prevents Mitochondrial Membrane Injury and Caspase 3 Activation—Metabolic stress resulted in the progressive leakage of mitochondrial cytochrome c and AIF into the cytosol of cells exposed to digitonin (Fig. 2A) as previously reported by us (36). Compared with control, Hsp27 up-regulation markedly decreased mitochondrial cytochrome c and AIF leakage both during and after stress. In addition, enhanced Hsp27 expression decreased stress-induced activation of caspase 3 (Fig. 2B), a downstream consequence of cytochrome c leakage. In this study the decrease in pro-caspase 3 content parallels cytochrome c leakage and is a reliable estimate of caspase activity in these cells (30). Taken together, these results demonstrate that Hsp27 ameliorates stress-induced mitochondrial membrane injury and caspase activation, hallmarks of apoptosis.
FIGURE 2.
Effect of Hsp27 on stress-induced mitochondrial membrane injury and caspase 3 activation. A, leakage of mitochondrial AIF (upper panel) and cytochrome c (Cyto c; middle panel) into the cytosol of digitonin-permeabilized control (EV) versus cells with enhanced Hsp27 expression (Hsp27 AdV), at base line (Base), immediately after 1 h of injury (ATP depl), and after a 30-min recovery (R30). Bottom panel, β-actin loading control. AIF and cytochrome c were localized in 5 μg of cell lysate (WCL, lane 1). Other lanes contain 40 μg of total protein. B, content of pro-caspase 3, the inactive form of the enzyme (upper panel) in non-infected cells (Ctl, first lane) or after infection with AdV containing either empty vector or wild type Hsp27. Lower panel, β-tubulin loading control. Lanes contain 40 μg of total protein. Immunoblots are representative of at least two separate studies.
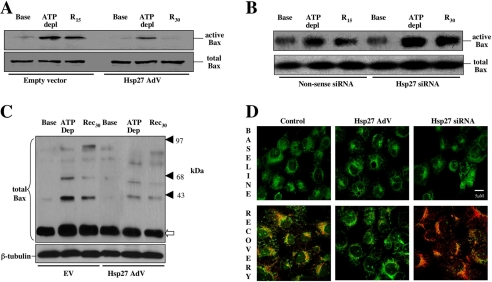
Hsp27 alters Bax activation, oligomerization, and translocation to mitochondria. Because Bax is a primary cause of stress-induced mitochondrial membrane injury after metabolic stress (14–16, 38), the effect of Hsp27 on Bax activation was examined. Compared with base line, metabolic stress markedly increased active Bax in cell lysates as assessed by a 6A7 epitope-specific antibody (Fig. 3A, upper panel) without changing total Bax content (bottom panel). Hsp27 expression markedly reduced conformational Bax activation during and after metabolic stress (Fig. 3A). In contrast, RNA interference-mediated Hsp27 knockdown resulted in a marked increase in activated Bax after stress (Fig. 3B, upper panel) without affecting total Bax (bottom panel). Hsp27 expression also reduced the formation of toxic Bax oligomers during and after stress (Fig. 3C). In the absence of stress, however, neither the detergent-free buffer nor the chemical cross-linker caused Bax oligomerization in normal or Hsp27-expressing cells. In intact cells, dual channel confocal microscopy was used to co-localize Bax with mitochondria, visualized as overlap between active Bax, stained in red with cy3, and mitochondria stained with Mitotracker™ green. Before stress, active Bax was not detected in cells exposed to empty adenovirus, Hsp27-containing adenovirus, non-sense siRNA (not shown), or specific Hsp27 siRNA (Fig. 3D, upper three panels). Compared with control (empty vector, lower left panel), Hsp27 expression markedly reduced co-localization of active Bax with mitochondria (lower row, middle panel). In contrast, suppressing Hsp27 further increased mitochondrial Bax accumulation in stressed cells. Thus, three distinct methodological approaches confirm that Hsp27 expression modifies Bax conformational activation, oligomerization, and translocation to mitochondria after metabolic stress.
FIGURE 3.
Effect of sp27 expression on Bax activation, oligomerization, and mitochondrial translocation after cell injury. Active Bax content assessed by a conformation-specific antibody directed against the 6A7 epitope and total Bax content before (Base), immediately after 1 h of metabolic stress (ATP depl), and after 15 min of recovery (R15) in cells infected with AdV containing Empty vector or Hsp27 AdV (A), cells exposed to non-sense siRNA or Hsp27 siRNA (B), Bax oligomerization (upper panel) in empty vector (EV), and Hsp27-expressing cells (C). The open arrow indicates monomeric Bax (21 kDa); solid arrowheads identify Bax oligomers that accumulate after cell injury. Lower panel, β-tubulin loading control, 40 μg of total protein per lane; each immunoblot represents 3–5 separate experiments. D, mitochondrial co-localization of active Bax and Mitotracker Green™ at base line and after metabolic stress followed by 30 min of recovery using confocal microscopy in normal cells (Control) and in cells with either increased (Hsp27 AdV) or decreased (Hsp27 siRNA) content. Bax (red) and MitoTracker™ (green) are shown as merged images; orange-yellow indicates co-localization; the bar indicates 5 μm.
Hsp27 Promotes Akt Activation and Akt-Bax Interaction—To examine the possibility that Hsp27 directly inhibits Bax, Bax and Hsp27 were alternatively immunoprecipitated, and the precipitates were probed for the other protein. In these bidirectional immunoprecipitation studies, no interaction between Hsp27 and Bax was detected at base line or after stress either in normal or Hsp27 expressing cells (data not shown). Furthermore, Hsp27-Bax failed to co-localize in intact cells by confocal microscopy after stress despite enhanced Hsp27 expression (data not shown). These data suggest that Hsp27 indirectly antagonizes Bax.
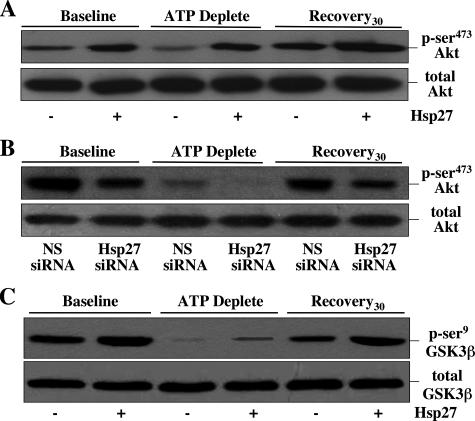
To test the hypothesis that Hsp27 inhibits Bax by activating Akt, a kinase that regulates Bax (21), p-Ser473 (active) and total Akt content were examined after metabolic stress. Compared with base line, stress resulted in marked Akt inactivation (Fig. 4A, upper panel) without altering total Akt content (lower panel). Compared with control, Hsp27 overexpression increased active Akt content before, during, and after injury (upper panel), whereas reduced Hsp27 expression (siRNA) markedly decreased Akt activation at all time points (Fig. 4B). Consistent with its effect on promoting Akt activation, Hsp27 expression also increased Ser9 phosphorylation (i.e. inactivation) of GSK3β, an Akt substrate, during and after metabolic stress (Fig. 4C). In contrast to Akt, GSK3β activates Bax (22).
FIGURE 4.
Effect of Hsp27 expression and cell stress on Akt and GSK3b activation. Active (p-Ser473) Akt content (upper panel of each pair) before (Baseline), immediately after 60 min ATP depletion (ATP Deplete), and after 30 min recovery (Recovery30) in cells infected with AdV containing either empty vector (Hsp27-) or wild type Hsp27 (Hsp27+)(A) and cells exposed to non-sense siRNA (NS siRNA) or Hsp27 siRNA (B). A and B, lower panels, total Akt loading control. C, inactive (p-Ser9) GSK3β content (upper panel), an Akt substrate. Lower panel, total GSK3β loading control; lanes contain 40 μg of total protein; immunoblots are representative of three separate studies.
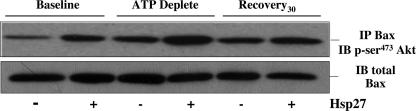
Minimal interaction between Hsp27 and Akt was detected at base line by immunoprecipitation, and neither metabolic stress nor Hsp27 expression increased this interaction (data not shown). These observations suggest that Akt-Hsp27 interaction is not required to promote Akt activation. Interestingly, stress modestly increased the interaction between Bax and GSK3β compared with base line in immunoprecipitates harvested from cell lysates. However, Hsp27 expression did not reduce this interaction (data not shown), suggesting that Hsp27 inhibits Bax upstream of GSK3β. In contrast to the minimal effects of Hsp27 expression on Akt-Hsp27 or GSK3β-Bax interaction, Hsp27 substantially increased the interaction between Akt and Bax, especially during metabolic stress (Fig. 5), indicating that Hsp27 inhibits Bax partly by promoting Akt-Bax interaction during cell stress.
FIGURE 5.
Effect of Hsp27 expression on stress-induced p-Ser473-Akt-Bax interaction. Total Bax content (using 5B7 antibody; upper panel) in immunoprecipitates (IP)harvested with an anti-p-Ser473-Akt antibody from lysates of cells infected with AdV containing either empty vector (Hsp27-) or wild type Hsp27 (Hsp27+) at base line (Baseline), after 1 h of metabolic stress (ATP Deplete), and after 30 min of recovery (Recovery30). Lower panel, total Bax content loading control. Immunoblots (IB) are representative of two separate studies; 400 μg of protein was immunoprecipitated in each sample.
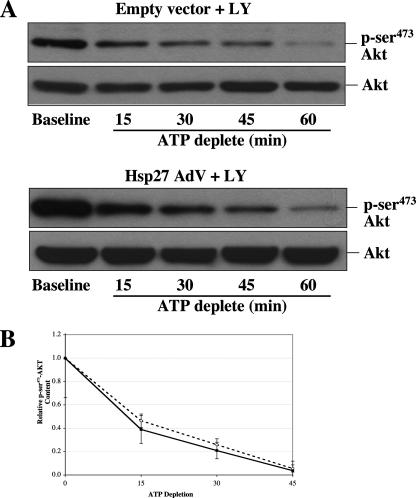
Hsp27 Does Not Inhibit p-Ser473-Akt Dephosphorylation—To evaluate the possibility that Hsp27-Akt interaction during stress reduces its susceptibility to dephosphorylation by PP2, an Akt phosphatase (Chiang et al. (44)), p-Ser473-Akt content was serially assessed in the presence of LY294002, a PI3-kinase inhibitor. By preventing de novo Akt phosphorylation, dephosphorylation can be clearly isolated from ongoing kinase activity. Densitometric evaluation of several independent studies confirmed that control and Hsp27-expressing cells exhibited a similar rate of decline in p-Ser473-Akt content during stress (Fig. 6B).
FIGURE 6.
Effect of Hsp27 expression on stress-induced p-Ser473-Akt dephosphorylation. A, p-Ser473-Akt content (upper panel of each pair) in lysates in the presence of 20 μm LY294002, a PI3-kinase inhibitor, at base line, and after 15–60 min of metabolic stress in control (Empty vector) or Hsp27-expressing cells (Hsp27 AdV). Lower panel of each pair; total Akt loading control. B, time course of changes in p-Ser473-Akt content in empty vector (open circles) and Hsp27-expressing (solid squares) cells assessed by densitometric analysis (n = 4 at each time point). Some standard error bars are too small to be seen. The slope of each curve is nearly identical.
Hsp27-mediated Cytoprotection Involves Akt/PI3-Kinase—To determine whether Hsp27 improves cell survival by activating Akt via PI3-kinase, distinct PI3-kinase inhibitors (LY and wortmannin) were used to block Akt activation. Importantly, 1 h of exposure to either of these agents did not significantly alter cell survival in the absence of stress (data not shown). Compared with empty vector, Hsp27 expression significantly improved cell survival after metabolic stress (p < 0.05; Fig. 7A). Like wortmannin, LY almost completely abrogated the pro-survival effect of enhanced Hsp27 expression. Although LY minimally altered Akt content at base line, it selectively inhibited Akt activation during recovery from stress, confirming its efficacy as an Akt inhibitor (Fig. 7B). Furthermore, inhibition of Akt by LY increased stress-induced Bax conformational activation (Fig. 7C). Compared with their respective base lines, LY exposure selectively increased stress-induced active Bax conformational activation in both groups without altering total Bax (Fig. 7, A and B, bottom panel). These studies show that Hsp27-mediated antagonism of Bax requires active PI3-kinase.
FIGURE 7.
Effect of PI3-kinase inhibition on Hsp27-mediated cytoprotection. A, relative survival compared with empty vector cells after 60 min of metabolic stress in Hsp27-expressing cells either with (HSP27 + LY)or without (HSP27)20 μm LY; *, p < 0.05 versus empty vector cells; NS, not significant. B, effect of LY on p-Ser473-Akt content at base line, immediately after ATP depletion, and after 30 min of recovery (upper panel). Lower panel, total Akt loading control. C, effect of LY on stress-induced Bax activation (6A7 antibody; upper panel of each pair) in cells infected with AdV containing either empty vector (upper pair) or wild type Hsp27 (lower pair). Bottom panel of each pair, total Bax loading control. Immunoblots are representative of three separate studies. Lanes contain 40 μg of total protein.
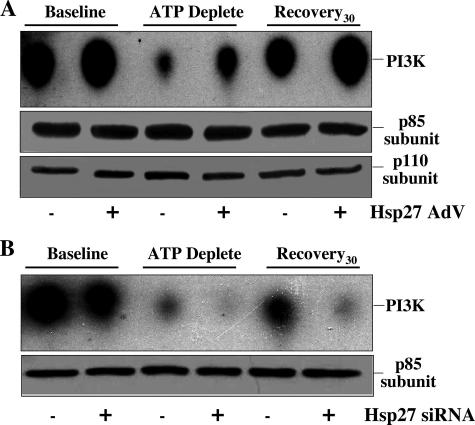
Hsp27 Regulates PI3-kinase Activity—To assess the hypothesis that Hsp27 promotes PI3-kinase activation, the effect of Hsp27 on PI3-kinase activity was directly assessed using a kinetic assay that detects the incorporation of 32P into a PI3-kinase-specific substrate. Compared with control, the stimulatory effect of Hsp27 expression on PI3-kinase activity was apparent before, during, and after metabolic stress (Fig. 8A). Similar amounts of the p85-kDa subunit were harvested in each immunoprecipitate (middle panel). Furthermore, similar amounts of the p100-kDa subunit were detected in each of the immunoprecipitates harvested using an antibody directed against the p85 subunit (bottom panel). In contrast to the effect of increased Hsp27 expression on PI3-kinase, Hsp27 knockdown exacerbated PI3-kinase inactivation during metabolic stress and recovery (Fig. 8B, upper panel). In this study Hsp27 expression had no effect on the amount of p85-kDa subunit of PI3-kinase detected in each immunoprecipitate (lower panel). This demonstrates that the effect of Hsp27 on PI3-kinase activity cannot be attributed to differences in loading or to Hsp27-mediated recruitment of p85 and p110 subunits into the active kinase complex. Bi-directional immunoprecipitation failed to detect interaction between Hsp27 and PI3-kinase even in cells with increased Hsp27 expression and despite preservation of kinase activity in these immunoprecipitates (Figs. 8, A and B).
FIGURE 8.
Effect of Hsp27 expression on PI3-kinase activity before and after metabolic stress. PI3-kinase activity in immunoprecipitates harvested at base line, immediately after 1 h metabolic stress, and after 30 min of recovery using an anti-p85 subunit antibody in cells infected with AdV containing Hsp27 (Hsp27+) or empty vector (Hsp27-)(A). Similar amounts of p85-kDa (middle panel) and p110kDa (lower panel) subunits were detected in immunoprecipitates harvested with an antibody directed against the p85 subunit. B, cells exposed to non-sense siRNA (Hsp27 siRNA-) or Hsp27 siRNA (Hsp27 siRNA+). p85-kDa subunit loading control (lower panel); results are representative of three separate studies. PI3K, PI3-kinase.
DISCUSSION
Bax is a major cause of organelle injury after ATP depletion in vitro (15) and organ dysfunction after renal (13), cardiac (39), or cerebral (40) ischemia. In the current study metabolic stress caused Bax activation, evidenced by the increased exposure of carboxyl-terminal amino acids 13–19 detected by a 6A7 epitope-specific antibody, promoted the accumulation of toxic Bax oligomers (Fig. 3B) in a pattern similar to that reported by others (18), and markedly increased Bax mitochondrial translocation (Fig. 3D), mitochondrial membrane injury (Fig. 2A), caspase 3 activation (Fig. 2B), and cell death (Fig. 1D).
When expressed to a level that replicates non-lethal heat stress, a physiologic stressor, Hsp27, markedly antagonized all measures of Bax activation (Fig. 3, A, C, and D), reduced the leakage of AIF and cytochrome c (Fig. 2A), inhibited caspase 3 activation (Fig. 2B), and significantly improved cell survival (Fig. 1D). Interestingly, Hsp27 expression reduced mitochondrial membrane injury (AIF and cytochrome leakage) and cell death by ≥50%, suggesting that the mitochondrion is a critical site for Hsp27-mediated cytoprotection. In contrast, suppression of endogenous Hsp27 expression promoted Bax conformational activation, translocation to mitochondria, and reduced cell survival after stress (Fig. 3B). The 30% change in cell survival caused by Hsp27 expression or suppression is important after renal ischemia when re-population of damaged epithelial cells is crucial for restoring organ function (41). These results clearly demonstrate that Hsp27 interferes with Bax activation, a proximal step in the apoptotic pathway and upstream of those previously reported for this cytoprotective protein (2, 8, 23–25).
Hsp27 could either directly or indirectly inhibit Bax by interfering with its conformational change, oligomerization, or translocation, key steps in the Bax activation pathway. A direct effect is unlikely, however, since Hsp27 and Bax did not interact as judged by bidirectional immunoprecipitation studies before or after stress despite increased Hsp27 expression. In addition, these two proteins failed to co-localize in intact cells analyzed by confocal microscopy before or after stress (data not shown). To our knowledge, interaction between Hsp27 and Bax has not been reported.
Alternatively, Hsp27 could indirectly regulate Bax by altering the activity of kinases such as Akt and/or GSK3β that phosphorylate it (21, 22). In fact, stress caused both transient Akt inactivation, resulting in the activation of GSK3β, an Akt substrate that is normally phosphorylated at Ser9, and inactivated by Akt (42). Hsp27 expression persistently activated Akt during metabolic stress (Fig. 4A) and inhibited Bax activation during stress (Fig. 3A). Gardai et al. (21), using site-specific Bax mutant proteins, showed that Akt phosphorylates Bax at Ser184, resulting in Bax inactivation and decreased apoptosis. Interestingly, Linseman et al. (22) recently showed that GSK3β activates Bax by phosphorylating Ser163. Thus, Bax activation during metabolic stress would be favored by the combined inactivation of Akt and the activation of GSK3β (21, 22), and Hsp27 inhibits Bax activation by opposing both untoward events.
The preponderance of evidence in the present study supports the notion that Hsp27-mediated changes in Akt and GSK3β regulate Bax. First, stress contemporaneously inactivated Akt (Fig. 4A) and activated both GSK3β (Fig. 4C) and Bax (Fig. 3A). Second, Hsp27 expression promoted Akt activation and inhibited both GSK3β and Bax activation. Third, Hsp27 expression promoted the interaction between Akt and Bax, especially during cell stress (Fig. 5). These interactions are specific, because neither stress nor Hsp27 expression altered Hsp27-GSK3β or GSK3β-Bax interaction. Fourth, inhibition of the Akt pathway by two distinct inhibitors completely abrogated the protective effect of Hsp27 expression on Akt (Fig. 7B), Bax (Fig. 7C), and cell survival (Fig. 7A), suggesting that the Akt-GSK3β-Bax pathway is crucial for Hsp27-mediated cytoprotection. By inhibiting initial conformational activation, Hsp27 (via Akt and GSK3β) also limits Bax oligomerization and translocation to mitochondria, membrane injury, caspase activation, and apoptosis.
The present study has some limitations. The lack of residue-specific phosphoserine antibodies and the presence of multiple Bax serine phosphorylation sites complicate direct assessment of the regulatory effects of Akt and GSK3β on Bax. It is also possible that in addition to its effect on GSK3β and Bax-GSK3β, Akt could exert anti-apoptotic effects by regulating forkhead transcription factor, Ika kinase-NFκB, Bad, a BH3 only protein that promotes Bax activation, and apoptosis signal-regulated kinase (Ask-1; for review, see Ref. 43). However, few of these pathways are likely to regulate Bax over the brief time course of cell injury examined in our study, and the role of Bad in stress-induced apoptosis is presently unclear.
How does Hsp27 activate Akt? Hsp27 could stimulate Akt by regulating its synthesis or degradation, by interfering with the phosphatase that inactivates Akt, and/or by directly or indirectly enhance kinase activation. However, neither metabolic stress nor Hsp27 expression changed total Akt content, indicating that Hsp27 alters Akt activity in a manner that is independent of its synthesis or degradation. Hsp27 expression also failed to slow the loss of phosphoserine473-Akt during stress (Fig. 6A), indicating that Hsp27 does not promotes Akt activation by regulating PP2, the phosphatase responsible for inactivating Akt (44). Alternatively, Hsp27 might directly regulate Akt activation. In fact, Rane and co-workers (28) recently suggested that Hsp27 inhibits apoptosis in human neutrophils by acting as a scaffolding protein to approximate Akt and mitogen-activated protein kinase 2 (MK2 or PDK2) in a manner that appeared to require Hsp27-Akt interaction. In epithelial cells, however, minimal interaction was detected between Hsp27 and Akt, and perhaps more importantly, neither metabolic stress nor increased Hsp27 expression significantly enhanced the interaction between Hsp27 and Akt in the present study. These findings show that interaction with Hsp27 is not required to promote Akt activation in epithelial cells and implicates a mechanism upstream of Akt itself.
In the present study Hsp27 expression dramatically increased, and knockdown of Hsp27 decreased PI3-kinase activity as demonstrated by a direct measure of its activity (Fig. 8). Because Hsp27 activates both PI3-kinase and Akt at base line (Figs. 4 and 8), it possible that Hsp27 “primes” the cell for survival during stress. In this scenario the relatively high activity of PI3-kinase and Akt at base line would be responsible for their persistent activation during stress and recovery (Figs. 4 and 8) as well as for the antagonism of Bax. Regardless of the mechanism, this is the most upstream anti-apoptotic effect demonstrated for Hsp27 and potentially explains prior observations that Hsp27 inhibits cytochrome c release (8, 23–25).
How Hsp27 increases PI3-kinase activity is presently unclear. Bidirectional co-immunoprecipitation did not detect interaction between PI3-kinase and Hsp27 at base line or after metabolic stress even in cells with increased Hsp27 expression (data not shown).
This could be due to the fact that it is technically difficult to harvest immunoprecipitates without disrupting the interactions between Hsp27 and membrane-bound proteins such as PI3-kinase or that the effect of Hsp27 does not require interaction with the kinase. However, because our technique for obtaining immunoprecipitates maintained the interaction between the p85 and p110 PI3-kinase subunits (a prerequisite for directly measuring kinase activity), it is tempting to speculate that Hsp27 promotes PI3-kinase activity by stabilizing cytoskeletal structures, an established function for this cytoprotective protein. Specifically, Hsp27 could enhance PI3-kinase by stabilizing E-cadherin, a key protein that mediates cell-cell interaction (45). E-cadherin is re-distributed into the cytosol and degraded in renal cells subjected to metabolic stress (46, 47) and has been shown to increase PI3-kinase activity by stabilizing its interaction with the cell membrane (48, 49). If true, then Hsp27 promotes cell survival by acting as a scaffolding protein that promotes Akt activation by maintaining the proximity of E-cadherin and PI3-kinase at the plasma membrane.
In summary, this study advances our mechanistic understanding of the anti-apoptotic effects of Hsp27, since it identifies a pre-mitochondrial site of protection and links this effect to Bax inactivation by the PI3-kinase-Akt pathway. Importantly, cytoprotection by Hsp27 effectively inhibits both caspase-dependent and independent cell death pathways by antagonizing early signal events that result in Bax-induced membrane injury. Dissecting the mechanism by which Hsp27 alters PI3-kinase activity deserves further study, since it may highlight potential targets for increasing cellular resistance to apoptogenic stress.
This work was supported by a National Kidney Foundation Fellowship grant (to A. H.) and by National Institutes of Health Grants DK-53387 (to S. C. B.) and DK-52898 (to J. H. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GSK3β, glycogen synthase kinase 3β; PI3-kinase, phosphatidylinositol 3-kinase; siRNA, small interfering RNA; p-Ser, phosphoserine; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; AIF, apoptosis-inducing factor.
References
- 1.Akbar, M. T., Lundberg, A. M., Liu, K., Vidyadaran, S., Wells, K. E., Dolatshad, H., Wynn, S., Wells, D. J., Latchman, D. S., and de Belleroche, J. (2003) J. Biol. Chem. 278 19956-19965 [DOI] [PubMed] [Google Scholar]
- 2.Benn, S. C., Perrelet, D., Kato, A. C., Scholz, J., Decosterd, I., Mannion, R. J., Bakowska, J. C., and Woolf, C. J. (2002) Neuron 36 45-56 [DOI] [PubMed] [Google Scholar]
- 3.Brar, B. K., Stephanou, A., Wagstaff, M. J., Coffin, R. S., Marber, M. S., Engelmann, G., and Latchman, D. S. (1999) J. Mol. Cell. Cardiol. 31 135-146 [DOI] [PubMed] [Google Scholar]
- 4.Martin, J. L., Mestril, R., Hilal-Dandan, R., Brunton, L. L., and Dillmann, W. H. (1997) Circulation 96 4343-4348 [DOI] [PubMed] [Google Scholar]
- 5.Ferns, G., Shams, S., and Shafi, S. (2006) Int. J. Exp. Pathol. 87 253-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oesterreich, S., Weng, C. N., Qiu, M., Hilsenbeck, S. G., Osborne, C. K., and Fuqua, S. A. (1993) Cancer Res. 53 4443-4448 [PubMed] [Google Scholar]
- 7.Garrido, C., Ottavi, P., Fromentin, A., Hammann, A., Arrigo, A. P., Chauffert, B., and Mehlen, P. (1997) Cancer Res. 57 2661-2667 [PubMed] [Google Scholar]
- 8.Paul, C., Manero, F., Gonin, S., Kretz-Remy, C., Virot, S., and Arrigo, A. P. (2002) Mol. Cell. Biol. 22 816-834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollander, J. M., Martin, J. L., Belke, D. D., Scott, B. T., Swanson, E., Krishnamoorthy, V., and Dillmann, W. H. (2004) Circulation 110 3544-3552 [DOI] [PubMed] [Google Scholar]
- 10.Ueda, N., Kaushal, G. P., and Shah, S. V. (2000) Am. J. Med. 108 403-415 [DOI] [PubMed] [Google Scholar]
- 11.Bernardi, P., Petronilli, V., Di Lisa, F., and Forte, M. (2001) Trends Biochem. Sci. 26 112-117 [DOI] [PubMed] [Google Scholar]
- 12.Korsmeyer, S. J., Shutter, J. R., Veis, D. J., Merry, D. E., and Oltvai, Z. N. (1993) Semin. Cancer Biol. 4 327-332 [PubMed] [Google Scholar]
- 13.Wolfs, T. G., de Vries, B., Walter, S. J., Peutz-Kootstra, C. J., van Heurn, L. W., Oosterhof, G. O., and Buurman, W. A. (2005) Am. J. Transplant. 5 68-75 [DOI] [PubMed] [Google Scholar]
- 14.Ruchalski, K., Mao, H., Li, Z., Wang, Z., Gillers, S., Wang, Y., Mosser, D. D., Gabai, V., Schwartz, J. H., and Borkan, S. C. (2006) J. Biol. Chem. 281 7873-7880 [DOI] [PubMed] [Google Scholar]
- 15.Saikumar, P., Dong, Z., Patel, Y., Hall, K., Hopfer, U., Weinberg, J. M., and Venkatachalam, M. A. (1998) Oncogene 17 3401-3415 [DOI] [PubMed] [Google Scholar]
- 16.Goping, I. S., Gross, A., Lavoie, J. N., Nguyen, M., Jemmerson, R., Roth, K., Korsmeyer, S. J., and Shore, G. C. (1998) J. Cell Biol. 143 207-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu, Y. T., and Youle, R. J. (1998) J. Biol. Chem. 273 10777-10783 [DOI] [PubMed] [Google Scholar]
- 18.De Giorgi, F., Lartigue, L., Bauer, M. K., Schubert, A., Grimm, S., Hanson, G. T., Remington, S. J., Youle, R. J., and Ichas, F. (2002) FASEB J. 16 607-609 [DOI] [PubMed] [Google Scholar]
- 19.Adachi, M., Higuchi, H., Miura, S., Azuma, T., Inokuchi, S., Saito, H., Kato, S., and Ishii, H. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287 695-705 [DOI] [PubMed] [Google Scholar]
- 20.Hengartner, M. O. (2000) Nature 407 770-776 [DOI] [PubMed] [Google Scholar]
- 21.Gardai, S. J., Hildeman, D. A., Frankel, S. K., Whitlock, B. B., Frasch, S. C., Borregaard, N., Marrack, P., Bratton, D. L., and Henson, P. M. (2004) J. Biol. Chem. 279 21085-21095 [DOI] [PubMed] [Google Scholar]
- 22.Linseman, D. A., Butts, B. D., Precht, T. A., Phelps, R. A., Le, S. S., Laessig, T. A., Bouchard, R. J., Florez-McClure, M. L., and Heidenreich, K. A. (2004) J. Neurosci. 24 9993-10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrido, C., Bruey, J. M., Fromentin, A., Hammann, A., Arrigo, A. P., and Solary, E. (1999) FASEB J. 13 2061-2070 [DOI] [PubMed] [Google Scholar]
- 24.Bruey, J. M., Ducasse, C., Bonniaud, P., Ravagnan, L., Susin, S. A., Diaz-Latoud, C., Gurbuxani, S., Arrigo, A. P., Kroemer, G., Solary, E., and Garrido, C. (2000) Nat. Cell Biol. 2 645-652 [DOI] [PubMed] [Google Scholar]
- 25.Concannon, C. G., Orrenius, S., and Samali, A. (2001) Gene Expr. 9 195-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi, H., Matsuzaki, H., Tanaka, M., Takemura, Y., Kuroda, S., Ono, Y., and Kikkawa, U. (1997) FEBS Lett. 410 493-498 [DOI] [PubMed] [Google Scholar]
- 27.Suga, H., Nakajima, K., Shu, E., Kanno, Y., Hirade, K., Ishisaki, A., Matsuno, H., Tanabe, K., Takai, S., Akamatsu, S., Kato, K., Oiso, Y., and Kozawa, O. (2005) Arch. Biochem. Biophys. 438 137-145 [DOI] [PubMed] [Google Scholar]
- 28.Wu, R., Kausar, H., Johnson, P., Montoya-Durango, D. E., Merchant, M., and Rane, M. J. (2007) J. Biol. Chem. 282 21598-21608 [DOI] [PubMed] [Google Scholar]
- 29.Sinha, D., Wang, Z., Price, V. R., Schwartz, J. H., and Lieberthal, W. (2003) Am. J. Physiol. Renal Physiol. 284 488-497 [DOI] [PubMed] [Google Scholar]
- 30.Li, F., Mao, H. P., Ruchalski, K. L., Wang, Y. H., Choy, W., Schwartz, J. H., and Borkan, S. C. (2002) Am. J. Physiol. Cell Physiol. 283 917-926 [DOI] [PubMed] [Google Scholar]
- 31.Schwartz, J. H., Shih, T., Menza, S. A., and Lieberthal, W. (1999) J. Am. Soc. Nephrol. 10 2297-2305 [DOI] [PubMed] [Google Scholar]
- 32.Wang, Y. H., and Borkan, S. C. (1996) Am. J. Physiol. 270 F1057-F1065 [DOI] [PubMed] [Google Scholar]
- 33.Martin, J. L., Hickey, E., Weber, L. A., Dillmann, W. H., and Mestril, R. (1999) Gene Expr. 7 349-355 [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, Y., Knowlton, A. A., Christensen, T. G., Shih, T., and Borkan, S. C. (1999) Kidney Int. 55 2224-2235 [DOI] [PubMed] [Google Scholar]
- 35.Borkan, S. C., Emami, A., and Schwartz, J. H. (1993) Am. J. Physiol. 265 F333-F341 [DOI] [PubMed] [Google Scholar]
- 36.Ruchalski, K., Mao, H., Singh, S. K., Wang, Y., Mosser, D. D., Li, F., Schwartz, J. H., and Borkan, S. C. (2003) Am. J. Physiol. Cell Physiol. 285 C1483-C1493 [DOI] [PubMed] [Google Scholar]
- 37.Lieberthal, W., Triaca, V., Koh, J. S., Pagano, P. J., and Levine, J. S. (1998) Am. J. Physiol. 275 F691-F702 [DOI] [PubMed] [Google Scholar]
- 38.Mikhailov, V., Mikhailova, M., Pulkrabek, D. J., Dong, Z., Venkatachalam, M. A., and Saikumar, P. (2001) J. Biol. Chem. 276 18361-18374 [DOI] [PubMed] [Google Scholar]
- 39.Huang, J., Nakamura, K., Ito, Y., Uzuka, T., Morikawa, M., Hirai, S., Tomihara, K., Tanaka, T., Masuta, Y., Ishii, K., Kato, K., and Hamada, H. (2005) Circulation 112 76-83 [DOI] [PubMed] [Google Scholar]
- 40.Hetz, C., Vitte, P. A., Bombrun, A., Rostovtseva, T. K., Montessuit, S., Hiver, A., Schwarz, M. K., Church, D. J., Korsmeyer, S. J., Martinou, J. C., and Antonsson, B. (2005) J. Biol. Chem. 280 42960-42970 [DOI] [PubMed] [Google Scholar]
- 41.Lieberthal, W., and Levine, J. S. (1996) Am. J. Physiol. 271 F477-F488 [DOI] [PubMed] [Google Scholar]
- 42.Grzesiak, J. J., Smith, K. C., Burton, D. W., Deftos, L. J., and Bouvet, M. (2005) Int. J. Cancer 114 522-530 [DOI] [PubMed] [Google Scholar]
- 43.Horbinski, C., and Chu, C. T. (2005) Free Radic. Biol. Med. 38 2-11 [DOI] [PubMed] [Google Scholar]
- 44.Chiang, G. J., Billmeyer, B. R., Canes, D., Stoffel, J., Moinzadeh, A., Austin, C. A., Kosakowski, M., Rieger-Christ, K. M., Libertino, J. A., and Summerhayes, I. C. (2005) BJU Int. 96 416-422 [DOI] [PubMed] [Google Scholar]
- 45.Aberle, H., Schwartz, H., and Kemler, R. (1996) J. Cell. Biochem. 61 514-523 [DOI] [PubMed] [Google Scholar]
- 46.Price, V. R., Reed, C. A., Lieberthal, W., and Schwartz, J. H. (2002) J. Am. Soc. Nephrol. 13 1152-1161 [DOI] [PubMed] [Google Scholar]
- 47.Bush, K. T., Tsukamoto, T., and Nigam, S. K. (2000) Am. J. Physiol. Renal Physiol. 278 847-852 [DOI] [PubMed] [Google Scholar]
- 48.Somasiri, A., Wu, C., Ellchuk, T., Turley, S., and Roskelley, C. D. (2000) Differentiation 66 116-125 [DOI] [PubMed] [Google Scholar]
- 49.Munshi, H. G., Ghosh, S., Mukhopadhyay, S., Wu, Y. I., Sen, R., Green, K. J., and Stack, M. S. (2002) J. Biol. Chem. 277 38159-38167 [DOI] [PubMed] [Google Scholar]