Abstract
Many candidates have been proposed as zona pellucida-binding proteins. Without precluding a role for any of those candidates, we focused on mouse sperm protein ZP3R/sp56, which is localized in the acrosomal matrix. The objective of this study was to analyze the role of ZP3R/sp56 in mouse fertilization. We expressed recombinant ZP3R/sp56 as a secreted protein in HEK293 cells and purified it from serum-free, conditioned medium. In the presence of reducing agents, the recombinant ZP3R/sp56 exhibited a molecular weight similar to that observed for the native ZP3R/sp56. Reminiscent of the native protein, recombinant ZP3R/sp56 formed a high molecular weight, disulfide cross-linked oligomer consisting of six or more monomers under non-reducing conditions. Recombinant ZP3R/sp56 bound to the zona pellucida of unfertilized eggs but not to 2-cell embryos, indicating that the changes that take place in the zona pellucida at fertilization affected the interaction of this protein with the zona pellucida. The extent of in vitro fertilization was reduced in a dose-dependent manner when unfertilized eggs were preincubated with recombinant ZP3R/sp56 (74% drop at the maximum concentrations assayed). Eggs incubated with the recombinant protein showed an absence of or very few sperm in the perivitelline space, suggesting that the reduction in the fertilization rate is caused by the inhibition of sperm binding and/or penetration through the zona pellucida. These results indicate that sperm ZP3R/sp56 is important for sperm-zona interactions during fertilization and support the concept that the acrosomal matrix plays an essential role in mediating the binding of sperm to the zona pellucida.
Sperm-zona pellucida (ZP)3 binding is an important step of gamete interaction in many species. The actual mechanism of binding is still unresolved. For mammals, some researchers favor a two-step process whereby primary binding with the ZP occurs through a plasma membrane receptor(s) of sperm with intact acrosomes, and secondary binding utilizes intraacrosomal proteins that are exposed during the course of or following acrosomal exocytosis (“Acrosome Reaction Model”) (1, 2). We and others feel that is difficult to ascertain the definitive acrosomal status at the time of binding to the zona pellucida. It is possible that during sperm capacitation subtle changes occur to the acrosomal region that initiate the acrosomal exocytotic process, thereby exposing acrosomal proteins involved in binding to the ZP (Acrosomal Exocytosis Model) (1, 3).
While there are only a few ZP sulfoglycoproteins involved in these binding events, a large number of sperm molecules have been shown to possess ZP binding activity (2). The list includes β-1,4 galactosyltransferase (4), zonadhesin (5), SED-1 (6), spermadhesins (7), and ZP3R/sp56 (formerly sp56) (8). Without precluding a role for any of these candidates in sperm-ZP interactions, we focused on mouse sperm protein ZP3R/sp56, because of our interest in defining the function of the acrosomal matrix.
ZP3R/sp56 was initially discovered by photoaffinity cross-linking with ZP3 (9) and later characterized as having specific affinity for mouse ZP3 oligosaccharides involved in sperm binding (10). The cloning of ZP3R/sp56 demonstrated that it is a member of the superfamily of C3/C4 binding proteins, possessing multiple short consensus repeats (so-called “sushi” domains) characteristic of complement regulatory proteins; however, the protein lacked an obvious carbohydrate recognition domain (8). Because fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans, the specificity of carbohydrate-mediated binding is still a matter of controversy (4, 11–13). ZP3R/sp56 is first detectable during late meiosis although during the course of spermatid differentiation, the acrosomal glycoprotein undergoes post-translational modifications, which may include the modification of carbohydrate side chains (14). Initial reports suggested that ZP3R/sp56 is a plasma membrane protein (10, 15). However, our laboratory later demonstrated by conventional immunoelectron microscopy that ZP3R/sp56 is an intra-acrosomal protein and is, in fact, part of a stable acrosomal matrix (14, 16).
In view of the model of primary/secondary binding of sperm to the ZP, the finding that ZP3R/sp56 is not a plasma membrane protein would discount it as a protein important in the initial adhesion of spermatozoa to the ZP. However, from the perspective of the Acrosomal Exocytosis Model, ZP3R/sp56 can have an important functional role as a ZP-binding protein (3). In this model, the outer acrosomal membrane and the plasma membrane of capacitated spermatozoa partially fuse in limited areas, exposing the acrosomal contents at the sperm surface. Some of the exposed components on the outer perimeter of the acrosomal matrix come in contact with the ZP and mediate gamete adhesion. Acrosomal components assist the penetration of spermatozoa through the ZP by restricted disassembly of this structure either enzymatically or stoichiometrically. We hypothesized that the particulate nature of ZP3R/sp56 in the acrosomal matrix may allow it to remain transiently associated with the sperm surface after being exposed to the external environment during the course of acrosomal exocytosis (3, 17).
In this study, we examined the role of ZP3R/sp56 in the fertilization process using recombinant protein. Here, we show that ZP3R/sp56 is important in the process of binding and/or penetration of the zona pellucida. The recombinant protein had an affinity for the ZP of unfertilized eggs but not the ZP from 2-cell embryos. When unfertilized eggs were incubated in the presence of the recombinant ZP3R/sp56, sperm binding and/or penetration through the zona pellucida was reduced, leading to decreased fertilization rates. These findings provide further evidence for the Acrosomal Exocytosis Model for sperm binding to the ZP and support a role for intermediates of acrosomal exocytosis during capacitation and sperm-ZP interactions.
EXPERIMENTAL PROCEDURES
Cloning of Mouse Zp3r—A cDNA region encoding murine ZP3R/sp56 was amplified by PCR from the first-strand cDNA prepared by reverse transcription of mouse mixed germ cell RNA (8). The following primers were used to amplify the sequence: Forward: 5′-GGA TCC GGC AAC ACT AGA GTC TTC CTA CG-3′; Reverse: 5′-AGC CAA GTA GAC AAA TTG AAC CA-3′. A full-length cDNA encoding mouse ZP3R/sp56 was cloned between the EcoRI and BamH1 restriction sites of the eukaryotic expression vector pcDNA3.1 (Invitrogen). The resulting plasmid was sequenced to verify that the insert was in-frame, and that the nucleotide sequence was correct. Restriction enzyme digests, ligations, transformations, and selection of transformants were performed according to standard protocols (18).
Expression and Purification of Recombinant Proteins—Human embryonic kidney 293 cells (HEK293: ATCC number 1573-CRL) were transfected with the ZP3R/sp56 expression vector using the Superfect transfection reagent according to the manufacturer's instructions (Qiagen, Inc.). Zeocin, at a concentration of 25 μg/ml was used for selection of transfected cells. Colonies of cells showing the highest expression levels, as judged by immunoblotting of the expression medium, were expanded in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 3.4 mm glutamine, 100 units/ml of penicillin and streptomycin, and 25 μg/ml Zeocin. Medium from transfected cells was collected at 2-day intervals, during which time, the cells were cultured in serum-free Dulbecco's modified Eagle's medium. The conditioned medium was then stored at -20 °C. For purifying ZP3R/sp56, the medium was thawed, centrifuged for 30 min at 5000 × g to remove cell debris, and applied at a flow of 1 ml/min on a HiTrap® QFF anion-exchange column, attached to an Akta FPLC system (GE Healthcare Inc.). The absorbance at 280 nm was continuously monitored to detect the protein. The column was washed with 20 mm Tris-buffered saline, pH 8.2, and the recombinant protein was subsequently eluted with 1 m NaCl, 20 mm Tris-HCl, pH 8.2, followed by dialysis against PBS. All preparative work was done at 4 °C. The purity of the sample was evaluated by Coomassie Blue staining after SDS gel electrophoresis and surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) analysis.
Surface-enhanced Laser Desorption/Ionization Time-of-Flight Mass Spectrometry—The purity and molecular weight of the purified recombinant mouse ZP3R/sp56 protein were determined by SELDI-TOF-MS (19). Briefly, 2 μl of protein solution was applied to a WCX2 protein chip array (Ciphergen Biosystems), which was then incubated at room temperature for 1 h. Following washes with 50 mm sodium acetate buffer (pH 4.0) and a final wash with water, the array was allowed to dry, and a saturated solution of sinapinic acid in 50% (v/v) acetonitrile, and 0.5% (v/v) trifluoroacetic acid was added to each spot and dried. The protein chip array was analyzed using a SELDI ProteinChip Biomarker System (PBS-II, Ciphergen Biosystems). Spectra were collected by the accumulation of 192 shots at a laser intensity of 220 in a positive mode. The protein masses were calibrated externally using protein molecular mass standards (Ciphergen Biosystems).
Fluorescent Bead (FluoSphere) Binding Assay—Red fluorescent FluoSphere® sulfate microspheres, diameter 1.0 μm, were obtained from Molecular Probes, Inc. Recombinant mouse ZP3R/sp56 was dissolved in 1 ml of 50 mm phosphate buffer, pH 7.4, containing 0.9% NaCl. This solution was then mixed with 2 ml of 2% aqueous suspension of microspheres and incubated at room temperature for 12 h. The mixtures were centrifuged to separate the ZP3R/sp56-labeled microsphere particles from unreacted protein (3000–5000 × g for 20 min). The pellet was resuspended in 50 mm PBS by gentle vortexing. The washing step was repeated twice more (total of three washes). The protein-conjugated microspheres were resuspended in 3 ml of 50 mm PBS buffer, pH 7.4, containing 1% BSA. Sodium azide was added to a final concentration of 2 mm, and the FluoSpheres were stored at 4 °C. For the binding assay, ∼25 mouse metaphase II-arrested eggs and ∼15 2-cell embryos were mixed and combined in 40-μl droplets with 100 μl of freshly washed, protein-conjugated FluoSpheres for 30 min at 37 °C, 5% CO2. Then, the suspension of eggs and embryos was washed three times in PBS containing 0.1% BSA and 0.01% polyvinyl alcohol (PVA) with a microbore pipette to remove unbound FluoSpheres and subsequently examined under phase contrast and fluorescence microscopy.
In Vitro Fertilization—In vitro fertilization of metaphase II-arrested mouse eggs was performed as described previously (20) with modifications. Briefly, cumulus cell-enclosed, metaphase-arrested eggs were harvested from 6-week-old CF1 females (Charles River) at ∼14 h post-human chorionic gonadotropin injection. The collection medium was Whitten's/Hepes containing 0.01% PVA(21). PVA was used prior to insemination in culture media devoid of BSA to keep the oocytes from sticking to the culture dish. Sperm were obtained from (C57BL/6J X SJL/J) F1 males (The Jackson Laboratory). Sperm from the vasa deferentia and caudae epididymides were removed and placed in a 900-μl drop of TYH medium (22) supplemented with 4 mg/ml BSA (Invitrogen) under mineral oil. The tissue was cut into sections, and the sperm were allowed to swim out into the medium for 10–20 min. Eggs and sperm (105/ml) were mixed in a 30-μl drop of TYH medium containing 4 mg/ml BSA and then cultured at 37 °C in an atmosphere of 5% CO2, 5% O2, 90% N2 for 3 h. Eggs were removed from the drop, washed three times in Whitten's/HEPES, and then cultured in KSOM medium (Specialty Media) (23). Phase contrast microscopy was used to evaluate fertilization. The success of fertilization was determined by morphological assessment of pronucleus formation after 6 h and cleavage to the 2-cell stage after 27 h. In some cases, embryos were cultured to the blastocyst stage.
Preparation of Culture Media and Spermatozoa—The basic medium used throughout these studies for the preparation and culture of mouse sperm was a modified Krebs-Ringer bicarbonate medium (HMB-HEPES-buffered), as described by Lee and Storey (24). This medium was first prepared in the absence of calcium, bovine serum albumin, NaHCO3, and pyruvate, sterilized by filtration through a 0.22-μm tissue culture filter unit (Nalgene) and frozen at -20 °C in aliquots for single use. The working “complete” medium was prepared by adding CaCl2 (1.7 mm), pyruvate (1 mm), NaHCO3 (25 mm), and BSA (3 mg/ml), followed by adjusting the pH to 7.3 and gassing with 5% CO2, 95% air. Uncapacitated cauda epididymal sperm were collected from mature (8-week-old) mice by placing minced caudae epididymides in 1 ml of medium HM without Ca2+, BSA, and NaHCO3. After 5 min, the sperm were washed in 1 ml of the same medium by centrifugation at 800 × g for 10 min at room temperature. The sperm were resuspended to a final concentration of 2 × 107 sperm/ml.
Acid Extraction of Sperm Proteins—Cauda epididymal mouse sperm were washed twice by centrifugation for 5 min at 300 × g and resuspended in PBS (5 × 108 sperm/ml). After the final wash, the sperm pellet was resuspended in two volumes of acid extraction buffer (1% glacial acetic acid with protease inhibitor mixture containing 1 mg/ml leupeptin, 1 mg/ml aprotinin, and 100 μm p-aminobenzamidine, pH 3.0) to 1 volume of sperm pellet. The sperm suspension was placed on a rocking platform for 1 h at 4 °C and then centrifuged at 10,000 × g for 30 min at 4 °C. The supernatant was then dialyzed with several changes against 1 mm HCl, pH 3.0 at 4 °C for 12–24 h. The protein concentration of extract was determined by the BCA assay (Pierce).
Sperm Egg Binding and Penetration Assay—Approximately 10 eggs and sperm (105/ml) were mixed in a 30-μl drop of TYH medium containing 4 mg/ml BSA with or without recombinant ZP3R/sp56 (100 μg/ml). The eggs were preincubated with the protein for 45 min before adding the spermatozoa. For the penetration assay, the mixture was then cultured at 37 °C in an atmosphere of 5% CO2, 5% O2, 90% N2.for 3 h. Eggs were removed from the drop, washed three times in Whitten's/HEPES, fixed in 3.8% paraformaldehyde, and stained with the DNA dye DAPI at a final concentration of 2 μg/ml. Eggs were viewed by epifluorescence microscopy, and the number of sperm in the perivitelline space were counted visually. For sperm egg binding assays, gamete interactions were allowed to proceed for 30 min at 37 °C in an atmosphere of 5% CO2, 5%O2, 90% N2. Afterward, sperm egg complexes were washed free of unbound or loosely bound sperm in Whitten's/HEPES medium. Two to three 2-cell embryos from superovulated females were included in every drop as negative controls. The washing was stopped when less than 3 sperm remained bound to 2-cell embryos. Sperm-egg complexes were fixed in 3.8% paraformaldehyde, and the number of sperm attached to each zona pellucida was determined. The results were expressed as a percentage of the control (0 μg/ml ZP3R/sp56).
Immunoblotting—Serum-free conditioned medium was collected from the transfected HEK293 cells, and proteins were mixed with Laemmli SDS-PAGE sample buffer containing a protease inhibitor mixture (Roche Applied Sciences). The proteins were separated by SDS-PAGE in a 10% polyacrylamide gel according to the method of Laemmli (25) and transferred to polyvinylidene difluoride membranes by the method of Towbin et al. (26). Blots were blocked in 5% nonfat dry milk in TBS (20 mm Tris-HCl, 150 mm NaCl, pH 7.5) and incubated for 2 h at room temperature with anti-CPT, an anti-peptide antibody against a central region of ZP3R/sp56, (14) diluted in blocking buffer (1:1000). After washing to remove the primary antibody, the blots were incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibody diluted 1:2500 in blocking buffer. Blots were developed using enhanced chemifluorescence and analyzed on a STORM 860 system (Molecular Dynamics).
Statistical Analysis—The statistical analysis of the IVF experiments was done by chi-square using Prism 4.0 software (GraphPad Software). Results were expressed as mean ± S.D. of the mean. All tests were two-tailed with a statistical significance assessed at the p < 0.05 level.
RESULTS
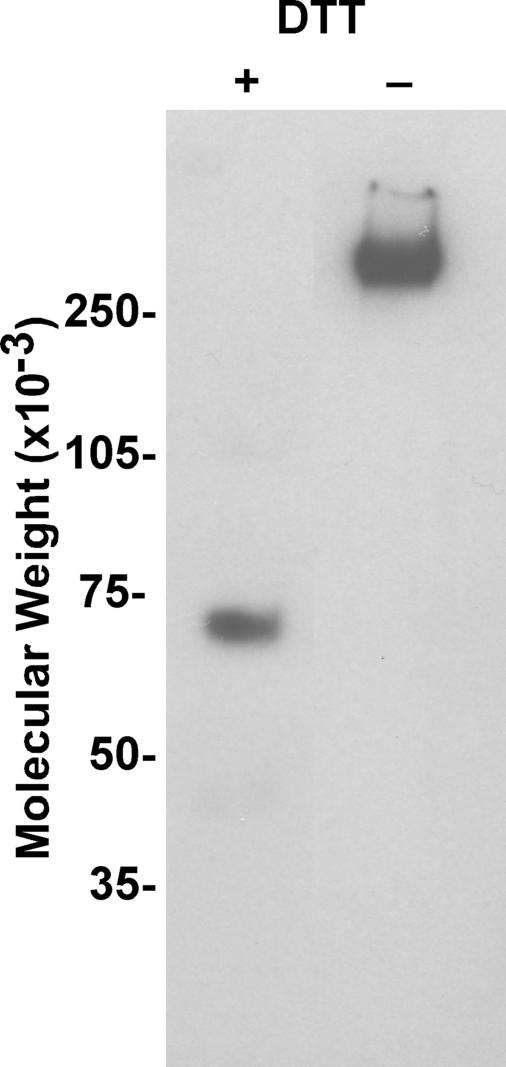
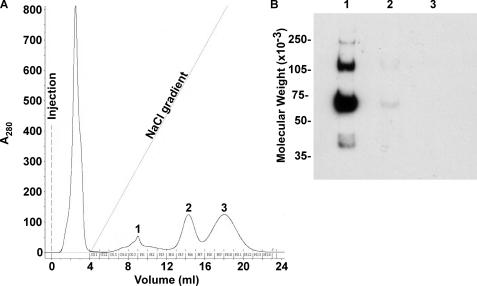
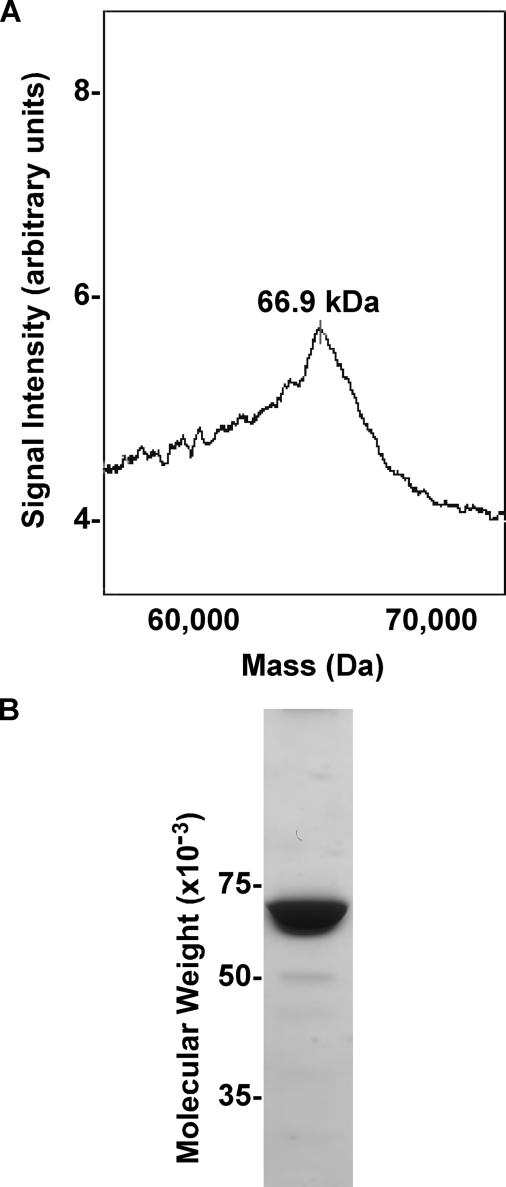
Production and Analysis of Recombinant Mouse ZP3R/sp56—To study the biological function of mouse ZP3R/sp56 during fertilization, recombinant protein was produced (Fig. 1). Analysis of the conditioned culture medium by immunoblotting revealed that recombinant ZP3R/sp56 exhibited molecular weights similar to that observed for native ZP3R/sp56 under reducing and non-reducing conditions (Fig. 1). The protein existed as a large complex under non-reducing conditions and had a molecular weight greater than 250,000 due to the formation of intra- and intermolecular disulfide bonds. The disruption of disulfide bonds by reduction with dithiothreitol (DTT) produced a major immunoreactive protein with a molecular weight of 67,000. Occasionally, minor species with molecular weights of 43,000 and 31,000 were also detected; immunoreactive species of similar sizes have previously been detected in extracts of mouse sperm and may represent slightly degraded ZP3R/sp56 monomers (14). The recombinant ZP3R/sp56 was purified from serum-free conditioned medium using FPLC chromatography (Fig. 2). Analysis by SELDI-TOF-MS after protein reduction confirmed the monomeric subunit size of ∼66,900 (Fig. 3A). The purity was assessed by SDS-PAGE and Coomassie Blue staining (Fig. 3B), showing that the purified preparation consisted primarily of a single protein of 67,000 Mr.
FIGURE 1.
Cloning and expression of recombinant ZP3R/sp56. Immunoblot analysis of recombinant ZP3R/sp56 under non-reducing and reducing conditions. The protein was diluted in sample buffer with or without DTT, and 1 μg of protein was analyzed per lane by immunoblotting with the anti-peptide antibody anti-CPT (14).
FIGURE 2.
Purification of recombinant ZP3R/sp56 using FPLC. A, chromatogram from anion exchange purification of recombinant ZP3R/sp56 from serum-free conditioned media. The linear salt gradient ranged from 0 to 1 m NaCl. Values under the curve (... 613, H1,...) represent the numbers of the collected fractions. B, immunoblotting analysis under reducing conditions of different chromatogram peaks to detect recombinant ZP3R/sp56 with anti-CPT antibody.
FIGURE 3.
Analysis of recombinant ZP3R/sp56. A, SELDI-MS spectrum analysis of purified recombinant ZP3R/sp56 (after reduction with DTT) showing the correct molecular mass. B, Coomassie Blue staining of the purified protein by anion exchange FPLC.
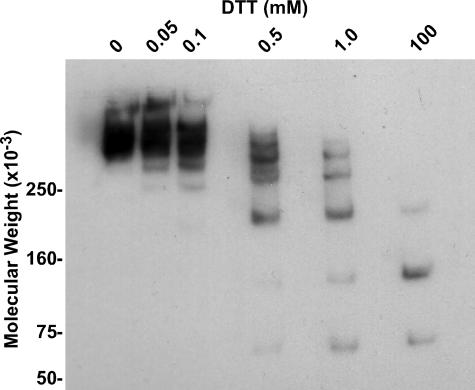
Native ZP3R/sp56 forms high molecular weight (>>250,000) oligomers cross-linked by disulfide bonds (14). Under non-reducing conditions, the same is true for recombinant ZP3R/sp56 (Fig. 4, lane 1). When the recombinant ZP3R/sp56 was processed with increasing amounts of DTT in the sample buffer prior to electrophoresis and immunoblotting, the high molecular weight oligomer was broken down into a series of smaller oligomers as a function of the concentration of DTT. At the highest concentration of DTT used (100 mm), ZP3R/sp56 monomers were produced (Fig. 4, last lane). This technique has occasionally been used to determine the number of monomers present in a given high molecular weight oligomer. Although it is apparent that there are at least 6 monomers in the ZP3R/sp56 complex, we were unable to form an accurate estimate of the number of monomers in the ZP3R/sp56 oligomer due to the complexity of the electrophoretic pattern in the higher molecular weight region of the gel. In addition, we cannot clearly estimate of the size of the ZP3R/sp56 oligomer because its relative mobility is slower than our largest molecular weight marker.
FIGURE 4.
Titration of ZP3R/sp56 disulfides by dithiothreitol. Recombinant ZP3R/sp56 was prepared for SDS-PAGE with increasing amounts of DTT in the sample buffer. DTT concentrations ranged from 0 to 100 mm. Each lane contained 1 μg of recombinant protein, and detection was achieved by immunoblotting with the anti-CPT antibody.
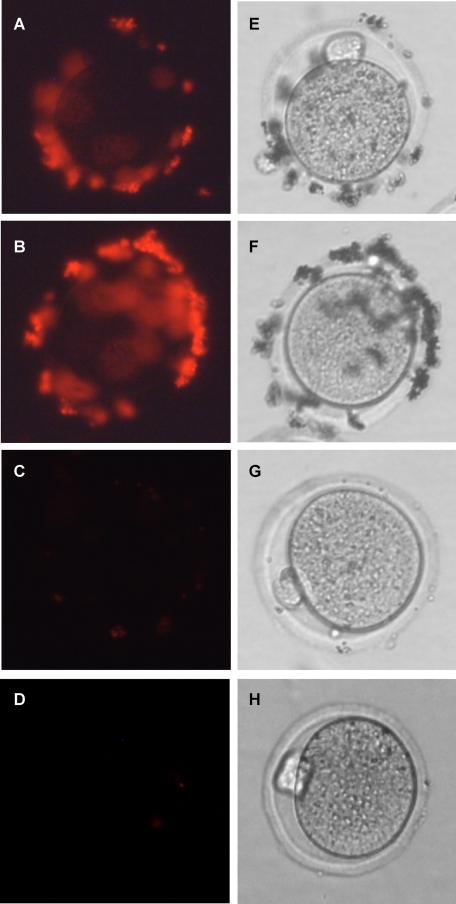
Recombinant ZP3R/sp56 Binds to the ZP of Unfertilized Eggs but Not to 2-Cell Embryos—The biological activity of ZP3R/sp56 was initially assessed by evaluating its ability to bind to unfertilized eggs. Fluorescent beads conjugated to recombinant ZP3R/sp56 were added to droplets containing unfertilized metaphase II-arrested eggs or 2-cell embryos. Beads coated with recombinant ZP3R/sp56 bound to the ZP of unfertilized eggs but not to fertilized 2-cell embryos, indicating that the changes that take place in the ZP upon fertilization (27) affected the interaction of this protein with the ZP (Figs. 5 and 6, respectively). As a positive control, we used beads coated with proteins from an acid extract of uncapacitated sperm, which contains ZP3R/sp56 and, possibly, other proteins that can bind to the ZP. As a negative control, we coated beads with a cleaved form of ZP3R/sp56 (an N-terminal 43,000 Mr fragment released from sperm that had undergone acrosomal exocytosis), which did not show binding activity.4 The binding of ZP3R/sp56-coated beads was also reduced by preincubating the egg with recombinant ZP3R/sp56 for 45 min (Fig. 5D).
FIGURE 5.
Mouse sperm protein ZP3R/sp56 binds to unfertilized eggs. Protein-conjugated fluorescent beads (1 μm) were added to droplets containing ovulated eggs and incubated for 30 min. Unbound beads were removed by washing. Beads coated with ZP3R/sp56 bound to the zonae of unfertilized oocytes (A). The binding of beads coated with acid-extracted sperm protein (containing acrosomal proteins) served as a positive control (B). As a negative control, BSA-coated FluorSpheres were used (C). The binding of ZP3R/sp56-coated beads was reduced by preincubating the egg with recombinant ZP3R/sp56 for 45 min (D). E–H are the phase contrast images of A–D, respectively. The experiment was repeated five times with similar results.
FIGURE 6.
Mouse sperm protein ZP3R/sp56 does not bind to 2-cell embryos. Fluorescent beads (1 μm) coated with ZP3R/sp56 were added to droplets containing 2-cell embryos and incubated for 30 min. Unbound beads were removed by washing. Beads coated with ZP3R/sp56 did not bind to the zona of 2-cell embryos (D). The binding of beads coated with acid-extracted sperm protein (containing acrosomal proteins) served as a positive control (E). As a negative control, BSA-coated FluoSpheres were used (F). A–C are the phase contrast images of D–F, respectively. The experiment was repeated five times with similar results.
Normally, the bead binding assay was performed as soon as possible after egg collection (within an hour). However, when we performed these experiments 6 h after egg collection, we did not see a clear binding of ZP3R/sp56 beads relative to BSA-coated beads (negative control). It is possible that the lack of binding is related to zona pellucida hardening, an aging-related phenomenon seen in mouse and rat oocytes cultured in serum-free medium (28, 29). The hardened ZP is refractory to sperm binding and penetration.
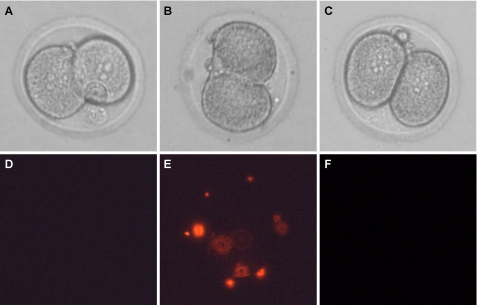
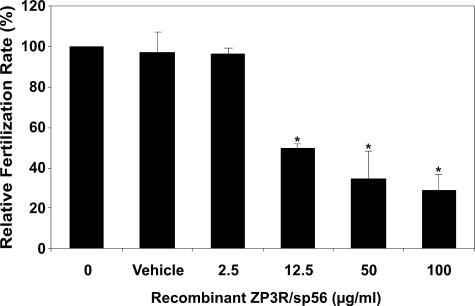
Incubation of Eggs with Recombinant ZP3R/sp56 Reduces the in Vitro Fertilization Rate—To evaluate a potential role of ZP3R/sp56 in sperm-gamete interactions, we preincubated unfertilized eggs in varying concentrations of recombinant ZP3R/sp56 and then performed in vitro fertilization in the presence of the recombinant protein. Successful sperm fusion was assessed by the formation of pronuclei and subsequent progression to the 2-cell embryo stage (Fig. 7). The extent of in vitro fertilization was reduced in a dose-dependent manner when oocytes were preincubated in recombinant ZP3R/sp56 (74% drop at the maximal concentration assayed). To discount any possible effect of compounds present in the conditioned media, in one set of experiments, we preincubated the eggs in the presence of conditioned medium from cells transfected with an empty plasmid. The fertilization rate observed in this situation was similar to the controls (not shown). The 2-cell embryos generated from eggs treated with ZP3R/sp56 generally progressed to the blastocyst stage without displaying any abnormalities, indicating the incubation of eggs in ZP3R/sp56 did not have toxic consequences. In addition, sperm motility appeared to be unaffected after 3 h of incubation, even at concentrations as high as 100 μg/ml (not shown).
FIGURE 7.
Incubation of eggs in recombinant ZP3R/sp56 reduces the relative rate of in vitro fertilization. Recombinant ZP3R/sp56 reduced the fertilization rates in a dose-dependent manner. The eggs were preincubated with the protein for 45 min before adding the spermatozoa. After 27 h of incubation, eggs were classified as fertilized if they had undergone pronuclear formation or cleaved to the 2-cell stage. The results were normalized against the fertilization rates of 0 μg/ml of ZP3R/sp56. The recombinant ZP3R/sp56 was diluted in PBS (vehicle). Each bar represents the mean ± S.D. of five independent experiments.
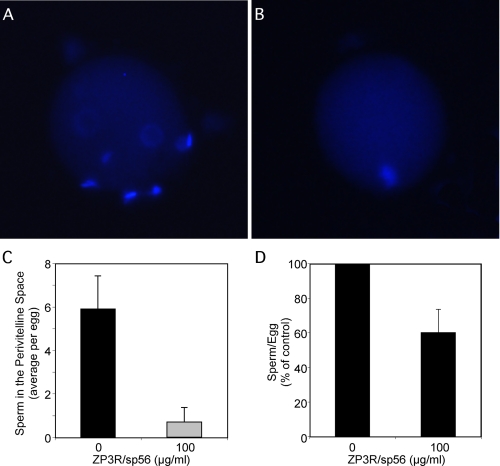
Reduced Sperm Penetration in Eggs Incubated with Recombinant ZP3R/sp56—Eggs incubated for 3 h in the presence of mouse sperm with or without recombinant ZP3R/sp56 (100 μg/ml) were washed and stained with the DNA dye DAPI (Fig. 8). Those eggs incubated with the recombinant protein showed an absence of or very few sperm in the perivitelline space compared with those incubated without the protein, suggesting that the reduction in the fertilization rate is due to the inhibition of sperm penetration through the zona pellucida. On average, the eggs showed from 5.9 ± 1.5 sperm per egg, which is in accordance with previous reports (30). On the other hand, the eggs incubated with ZP3R/sp56 displayed 0.7 ± 0.7 sperm per egg.
FIGURE 8.
Incubation of eggs with recombinant ZP3R/sp56 reduces the sperm penetration of the zona pellucida as assessed by the number of sperm in the perivitelline space. The eggs were preincubated with the protein for 45 min before adding the spermatozoa. After 3 h of incubation, the eggs were washed and stained with DAPI. Representative pictures of eggs incubated without ZP3R/sp56 (A) or with ZP3R/sp56 (B). The average number of sperm per egg in the perivitelline space and the number of sperm attached to the zona pellucida are shown in C and D, respectively. The experiment was repeated three times (∼10 eggs for each condition).
DISCUSSION
The acrosome is a critical exocytotic organelle overlying the anterior aspect of sperm from many species. Although its role during fertilization is not completely understood, a mammalian spermatozoon must have a properly formed acrosome to be fully functional in the process of binding and penetrating the zona pellucida, the extracellular matrix surrounding the egg. Men or mice carrying mutations affecting the formation of the sperm acrosome are infertile (31–34). Our studies showing that the mouse sperm zona pellucida-binding protein ZP3R/sp56 is intra-acrosomal, and not on the plasma membrane, have led us to reexamine the events of acrosomal exocytosis and the role of the sperm acrosomal matrix in the fertilization process (14, 16).
To expand these investigations, ZP3R/sp56 was expressed in HEK293 cells because there is no spermatogenic cell system for the expression of recombinant proteins. HEK293 cells secrete proteins that undergo glycosylation and oligomerization, two post-translational modifications that are not possible in a bacterial expression system. Furthermore, we were able to adapt methodology used for studies on the closely related protein C4BP (35). Recombinant ZP3R/sp56 exhibited the expected monomer molecular weight under reducing conditions and formed higher molecular weight oligomers similar to the native mouse sperm protein, properties shared by C4BP. The oligomerization of ZP3R/sp56 is dependent on the formation of intermolecular disulfide bonds between cysteines in the C-terminal region of each monomer. As a result of amino acid sequence similarities between the two proteins, structural features can be inferred for ZP3R/sp56 from the wealth of data concerning human C4BP. This latter protein consists of seven identical α-subunits and a unique β-chain linked together by disulfide bridges (36). Synchroton x-ray scattering and hydrodynamic analysis indicates that C4BP in solution is a bundle of seven extended arms held together at their C termini (37). As far as we know, there is not a β-subunit associated with the ZP3R/sp56 oligomer although we frequently observed a band with a molecular weight of ∼43,000. This could be an unidentified ZP3R/sp56 β-subunit or a fragment of the 67,000 Mr monomer.
Using the FluoSphere binding assay, we demonstrated that beads coated with ZP3R/sp56 bound to the zona pellucida of unfertilized eggs, confirming its role as a ZP-binding protein. After fertilization, the zona pellucida undergoes critical modifications to prevent the binding or penetration of additional sperm (38, 39). Egg cortical granules exocytose their contents, which modify the zona matrix. Although other biochemical changes have been inferred, only the proteolytic cleavage of ZP2 has been experimentally observed (13, 27). As expected for ZP modified as a consequence of cortical granule exocytosis, recombinant ZP3R/sp56 did not bind to fertilized 2-cell embryos.
If ZP3R/sp56 binds to specific recognition sites of ZP glycoproteins, one would predict that preincubation of unfertilized eggs with purified recombinant ZP3R/sp56 would greatly reduce sperm binding to the ZP, and that fertilization rates would be adversely affected. We performed in vitro fertilization after preincubating the eggs with increasing concentrations of recombinant ZP3R/sp56. The binding of ZP3R/sp56 to the ZP decreased the ability of sperm to fertilize the eggs in a dose-dependent manner. The interference with fertilization was at the level of the zona pellucida, because the total or partial saturation of the ZP3R/sp56-binding sites reduced the number of sperm in the perivitelline space, as shown in Fig. 8. We did expect to see a reduction in the fertilization rate but not a complete inhibition, because the binding to the zona pellucida seems to be a very redundant process, i.e. sperm proteins other than ZP3R/sp56 are also involved in sperm-ZP binding (2). For example, the targeted deletion of three proteins involved in the binding to the ZP such as SED-1 (6), galactosyltransferase (40), and proacrosin (41) generated some level of subfertility or disregulation of acrosomal exocytosis. Few of these studies have looked at details concerning acrosomal architecture or composition in the mutant mice.
With several proteins identified as having a role in sperm-ZP binding, it is difficult to define the actual mechanism of sperm binding and penetration of the ZP because of the challenges in definitively assessing the state of acrosomal integrity at any given moment. The Acrosomal Exocytosis Model proposed by our laboratory acknowledges a role for the acrosomal matrix in the ZP binding and penetration steps (3). This model does not require that the acrosome be absolutely intact for sperm binding to the ZP. Because this alternative paradigm predicts that a hallmark of capacitated sperm is that they have initiated but not completed exocytosis, the ZP is proposed to accelerate the exocytotic process rather than induce it. In a recent report, Baibakov et al. (13) used a filter penetration assay and suggested that the passage of sperm through a matrix such as the zona initiates a mechanosensory signal transduction process necessary to trigger the acrosome reaction. However, this model ignores the rather unambiguous observations that acid-solubilized ZP or purified ZP3 can stimulate acrosomal exocytosis (42–47). In our view, any model for acrosomal exocytosis must take into account the microenvironment at the site of spermzona interaction. For example, several studies have noted that green fluorescent protein (GFP) loss from the acrosomes of transgenic sperm on the zona is slow (13, 48) and interpret this to mean that acrosomal exocytosis has not occurred. However, we imagine that the biophysical nature of this site is not fully appreciated in these studies and envision that the glycoprotein nature of the ZP components may form a gel-like microenvironment (13, 48). The diffusion of GFP away from the acrosomes of sperm embedded in the surface of the ZP may be impeded relative to sperm swimming free in solution.
In summary, these results indicate that sperm ZP3R/sp56 is important for sperm-zona interactions during fertilization, consistent with a role in adhesion to the zona pellucida, and support the concept that the acrosomal matrix plays an essential role in mediating the binding of the sperm to the ZP.
Acknowledgments
We thank Drs. Paula Stein, Jose Conejo-Garcia, and Xue-Jun Fan for help with technical issues. We thank Dr. Bayard Storey for his insightful comments on the manuscript.
This work was supported by Grant HD-41552 (to G. L. G.) and the Fogarty International Center Fellowship Program 5D43TW000671 (to M. G. B.) from the National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ZP, zona pellucida; DTT, dithiothreitol; FPLC, fast protein liquid chromatography; SELDI-MS, surface-enhanced laser desorption ionization mass spectrometry; ZP3R/sp56, ZP3 receptor; PBS, phosphate-buffered saline; BSA, bovine serum albumin; PVA, polyvinyl alcohol; DAPI, 4′,6-diamidino-2-phenylindole; HEK, human embryonic kidney cells.
M. G., Buffone and G. L. Gerton, unpublished results.
References
- 1.Gerton, G. L. (2002) in Function of the Sperm Acrosome, Fertilization (Hardy, D. M., ed), pp. 265-302, Academic Press, San Diego, CA
- 2.Tanphaichitr, N., Carmona, E., Bou Khalil, M., Xu, H., Berger, T., and Gerton, G. L. (2007) Front. Biosci. 12 1748-1766 [DOI] [PubMed] [Google Scholar]
- 3.Kim, K. S., and Gerton, G. L. (2003) Dev. Biol. 264 141-152 [DOI] [PubMed] [Google Scholar]
- 4.Miller, D. J., Macek, M. B., and Shur, B. D. (1992) Nature 357 589-593 [DOI] [PubMed] [Google Scholar]
- 5.Gao, Z., and Garbers, D. L. (1998) J. Biol. Chem. 273 3415-3421 [DOI] [PubMed] [Google Scholar]
- 6.Ensslin, M. A., and Shur, B. D. (2003) Cell 114 405-417 [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Martinez, H., Iborra, A., Martinez, P., and Calvete, J. J. (1998) Reprod. Fertil. Dev. 10 491-497 [DOI] [PubMed] [Google Scholar]
- 8.Bookbinder, L. H., Cheng, A., and Bleil, J. D. (1995) Science 269 86-89 [DOI] [PubMed] [Google Scholar]
- 9.Bleil, J. D., and Wassarman, P. M. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 5563-5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, A., Le, T., Palacios, M., Bookbinder, L. H., Wassarman, P. M., Suzuki, F., and Bleil, J. D. (1994) J. Cell Biol. 125 867-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleil, J. D., and Wassarman, P. M. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 6778-6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams, S. A., Xia, L., Cummings, R. D., McEver, R. P., and Stanley, P. (2007) J. Cell Sci. 120 1341-1349 [DOI] [PubMed] [Google Scholar]
- 13.Baibakov, B., Gauthier, L., Talbot, P., Rankin, T. L., and Dean, J. (2007) Development 134 933-943 [DOI] [PubMed] [Google Scholar]
- 14.Kim, K. S., Cha, M. C., and Gerton, G. L. (2001) Biol. Reprod. 64 36-43 [DOI] [PubMed] [Google Scholar]
- 15.Cohen, N., and Wassarman, P. M. (2001) Int. J. Dev. Biol. 45 569-576 [PubMed] [Google Scholar]
- 16.Foster, J. A., Friday, B. B., Maulit, M. T., Blobel, C., Winfrey, V. P., Olson, G. E., Kim, K. S., and Gerton, G. L. (1997) J. Biol. Chem. 272 12714-12722 [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. S., Foster, J. A., and Gerton, G. L. (2001) Biol. Reprod. 64 148-156 [DOI] [PubMed] [Google Scholar]
- 18.Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual., 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 19.Tang, N., Tornatore, P., and Weinberger, S. R. (2004) Mass Spectrom. Rev. 23 34-44 [DOI] [PubMed] [Google Scholar]
- 20.Visconti, P. E., Bailey, J. L., Moore, G. D., Pan, D., Olds-Clarke, P., and Kopf, G. S. (1995) Development 121 1129-1137 [DOI] [PubMed] [Google Scholar]
- 21.Whitten, W. K., and Biggers, J. D. (1968) J. Reprod. Fertil. 17 399-401 [DOI] [PubMed] [Google Scholar]
- 22.Toyoda, Y., Yokoyama, M., and Hoshi, T. (1971) Jpn. J. Anim. Reprod. 16 147-151 [Google Scholar]
- 23.Summers, M. C., McGinnis, L. K., Lawitts, J. A., Raffin, M., and Biggers, J. D. (2000) Hum. Reprod. 15 1791-1801 [DOI] [PubMed] [Google Scholar]
- 24.Lee, M. A., and Storey, B. T. (1986) Biol. Reprod. 34 349-356 [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. (1970) Nature 227 680-685 [DOI] [PubMed] [Google Scholar]
- 26.Towbin, H., Staehelin, T., and Gordon, J. (1979) Proc. Natl. Acad. Sci. U. S. A. 76 4350-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleil, J. D., Beall, C. F., and Wassarman, P. M. (1981) Dev. Biol. 86 189-197 [DOI] [PubMed] [Google Scholar]
- 28.Schroeder, A. C., Schultz, R. M., Kopf, G. S., Taylor, F. R., Becker, R. B., and Eppig, J. J. (1990) Biol. Reprod. 43 891-897 [DOI] [PubMed] [Google Scholar]
- 29.Murayama, Y., Mizuno, J., Kamakura, H., Fueta, Y., Nakamura, H., Akaishi, K., Anzai, K., Watanabe, A., Inui, H., and Omata, S. (2006) Hum. Cell 19 119-125 [DOI] [PubMed] [Google Scholar]
- 30.Yanagimachi, R., Lopata, A., Odom, C. B., Bronson, R. A., Mahi, C. A., and Nicolson, G. L. (1979) Fertil. Steril. 31 562-574 [DOI] [PubMed] [Google Scholar]
- 31.Schill, W. B. (1991) Hum. Reprod. 6 969-978 [DOI] [PubMed] [Google Scholar]
- 32.Sotomayor, R. E., and Handel, M. A. (1986) Biol. Reprod. 34 171-182 [DOI] [PubMed] [Google Scholar]
- 33.Chemes, E. H., and Rawe, Y. V. (2003) Hum. Reprod. Update 9 405-428 [DOI] [PubMed] [Google Scholar]
- 34.Dam, A. H., Feenstra, I., Westphal, J. R., Ramos, L., van Golde, R. J., and Kremer, J. A. (2007) Hum. Reprod. Update 13 63-75 [DOI] [PubMed] [Google Scholar]
- 35.Blom, A. M., Webb, J., Villoutreix, B. O., and Dahlback, B. (1999) J. Biol. Chem. 274 19237-19245 [DOI] [PubMed] [Google Scholar]
- 36.Blom, A. M. (2002) Biochem. Soc. Trans. 30 978-982 [DOI] [PubMed] [Google Scholar]
- 37.Perkins, S. J., Chung, L. P., and Reid, K. B. (1986) Biochem. J. 233 799-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braden, A. W., Austin, C. R., and David, H. A. (1954) Aust. J. Biol. Sci. 7 391-409 [DOI] [PubMed] [Google Scholar]
- 39.Ducibella, T. (1996) Hum. Reprod. Update 2 29-42 [DOI] [PubMed] [Google Scholar]
- 40.Lu, Q., and Shur, B. D. (1997) Development 124 4121-4131 [DOI] [PubMed] [Google Scholar]
- 41.Baba, T., Azuma, S., Kashiwabara, S.-I., and Toyoda, Y. (1994) J. Biol. Chem. 269 31845-31849 [PubMed] [Google Scholar]
- 42.Bleil, J. D., and Wassarman, P. M. (1983) Dev. Biol. 95 317-324 [DOI] [PubMed] [Google Scholar]
- 43.Miller, D. J., Gong, X., and Shur, B. D. (1993) Development 118 1279-1289 [DOI] [PubMed] [Google Scholar]
- 44.Nixon, B., Lu, Q., Wassler, M. J., Foote, C. I., Ensslin, M. A., and Shur, B. D. (2001) Cells Tissues Organs 168 46-57 [DOI] [PubMed] [Google Scholar]
- 45.Rockwell, P. L., and Storey, B. T. (2000) Mol. Reprod. Dev. 55 335-349 [DOI] [PubMed] [Google Scholar]
- 46.Ward, C. R., Storey, B. T., and Kopf, G. S. (1992) J. Biol. Chem. 267 14061-14067 [PubMed] [Google Scholar]
- 47.Ward, C. R., Storey, B. T., and Kopf, G. S. (1994) J. Biol. Chem. 269 13254-13258 [PubMed] [Google Scholar]
- 48.Nakanishi, T., Ikawa, M., Yamada, S., Parvinen, M., Baba, T., Nishimune, Y., and Okabe, M. (1999) FEBS Lett. 449 277-283 [DOI] [PubMed] [Google Scholar]