Abstract
Many proteins involved in DNA replication and repair undergo post-translational modifications such as phosphorylation and ubiquitylation. Proliferating cell nuclear antigen (PCNA; a homotrimeric protein that encircles double-stranded DNA to function as a sliding clamp for DNA polymerases) is monoubiquitylated by the RAD6-RAD18 complex and further polyubiquitylated by the RAD5-MMS2-UBC13 complex in response to various DNA-damaging agents. PCNA mono- and polyubiquitylation activate an error-prone translesion synthesis pathway and an error-free pathway of damage avoidance, respectively. Here we show that replication factor C (RFC; a heteropentameric protein complex that loads PCNA onto DNA) was also ubiquitylated in a RAD18-dependent manner in cells treated with alkylating agents or H2O2. A mutant form of RFC2 with a D228A substitution (corresponding to a yeast Rfc4 mutation that reduces an interaction with replication protein A (RPA), a single-stranded DNA-binding protein) was heavily ubiquitylated in cells even in the absence of DNA damage. Furthermore RFC2 was ubiquitylated by the RAD6-RAD18 complex in vitro, and its modification was inhibited in the presence of RPA. The inhibitory effect of RPA on RFC2 ubiquitylation was relatively specific because RAD6-RAD18-mediated ubiquitylation of PCNA was RPA-insensitive. Our findings suggest that RPA plays a regulatory role in DNA damage responses via repression of RFC2 ubiquitylation in human cells.
Cellular DNA is continuously damaged by a vast variety of endogenous and exogenous genotoxicants. When genomic DNA is damaged, cells respond by activation of complex signaling networks that delay cell cycle progression, induce repair of lesions, activate damage tolerance pathways, and trigger apoptosis or senescence (1, 2). It is hypothesized that DNA damage-inducible signaling pathways serve important tumor-suppressive roles and prevent mutations that could lead to malignancy. Various genotoxins elicit different forms of DNA damage and result in distinct signal transduction pathways and biological outcomes. Distal steps of DNA damage-induced checkpoint signaling pathways that result in inhibition of the cell cycle are relatively well understood (3, 4). However, molecular details of proximal signaling events and lesion-specific DNA damage recognition events are less clear.
DNA replication and repair require the coordinated actions of multiple proteins on small regions of DNA. A limited number of proteins serve to coordinate multiple replication and repair events. Some proteins function commonly in DNA replication and repair and frequently have a crucial role in both processes. Three such examples are replication protein A (RPA),2 proliferating cell nuclear antigen (PCNA), and replication factor C (RFC). RPA was originally identified as a eukaryotic single-stranded DNA-binding protein essential for in vitro replication of SV40 DNA (5, 6). PCNA is a trimer of three identical subunits arranged head-to-tail to generate a ringlike structure with a large central cavity for encircling DNA. It is well established that PCNA provides a mobile platform to serve as anchor and processivity factor for DNA polymerases during chromosomal replication (7, 8). PCNA is loaded onto the primer-template junction in an ATP-dependent manner by a multiprotein clamp loader, RFC (9, 10). RFC binds preferentially to double-stranded/single-stranded junctions with a recessed 3′-end, which is the DNA target for PCNA loading.
RPA, PCNA, and RFC are key proteins that play central roles in DNA replication, participating in competitive polymerase switching during lagging strand synthesis. The DNA polymerase α-primase complex (Pol α) that synthesizes an RNA-DNA hybrid primer requires contact with RPA to remain stably attached to the primed site. For processive DNA synthesis to follow, Pol α must be replaced by DNA polymerase δ (Pol δ). Replacement of Pol α by Pol δ is initiated by interactions between RFC and RPA that disrupt Pol α-RPA interactions and result in removal of Pol α from DNA. After RFC loads PCNA onto the primed site, Pol δ associates with PCNA by displacing RFC. The switching process is indeed coordinated by RPA via cooperative interactions with PCNA and RFC (11, 12). RPA, RFC, and PCNA also play key roles in DNA repair by interacting with many DNA repair enzymes (13–15). Such interactions are believed to play roles in DNA damage recognition and in recruiting and positioning of DNA repair enzymes.
RFC consists of five different subunits, which are homologous to one another and are members of the AAA+ family of ATPases (16, 17). The RFC1(p140) subunit is sometimes referred to as the “large subunit” as it contains both N- and C-terminal extensions beyond its region of homology with the four “small” subunits. The four small RFC subunits are designated RFC2(p40), RFC3(p36), RFC4(p37), and RFC5(p38) in mammals. Three protein complexes with resemblance to RFC that are involved in maintaining genome stability have been described recently. These RFC-like complexes (RLCs) share four common small subunits (RFC2–5), and each carries a unique large subunit (RAD17, CTF18, or ELG1) replacing the RFC1. These RLCs are involved in the checkpoint response (RAD17-RFC), sister chromatid cohesion (CTF18-RFC), and maintenance of genome stability (ELG1-RFC) (18, 19).
DNA damage sensors and repair proteins must react in a rapid and efficient manner to execute their functions. Frequently the regulation of these proteins involves post-translational modifications, such as phosphorylation and ubiquitylation, to help modulate the assembly and disassembly of complexes and to assist targeting and the regulation of enzymatic activity in a timely manner. For example, RPA is hyperphosphorylated upon DNA damage or replication stress by several checkpoint kinases (20). Hyperphosphorylation alters RPA-DNA and RPA-protein interactions (15, 21). Recent studies in the DNA repair field have highlighted the expanding role of ubiquitylation in the regulation of diverse DNA repair processes and pathways. One of the most striking examples of how ubiquitylation can affect protein function is that of PCNA in the budding yeast Saccharomyces cerevisiae. Following DNA damage, PCNA can be monoubiquitylated or polyubiquitylated on the Lys-164 residue, and each modification results in a different outcome with respect to DNA synthesis and repair (22, 23). Monoubiquitylated PCNA directs translesion synthesis via error-prone DNA polymerases, whereas polyubiquitylated PCNA is associated with an error-free DNA repair pathway (22, 23). Mammalian PCNA also undergoes monoubiquitylation after UV irradiation, and monoubiquitylated PCNA preferentially binds to translesion synthesis polymerases that contain one or two copies of ubiquitin-binding domains (24–27).
In contrast to RPA and PCNA, damage-dependent modification of RFC has not been described. Recent studies have significantly broadened the scope of the role of ubiquitylation to include regulatory functions in DNA repair and damage response pathways. Therefore, in this study we investigated whether the clamp loader RFC is likewise subjected to regulated modification. We examined the modification of all subunits in RFC and RLCs. We demonstrated that RFC2 and RFC4 are ubiquitylated following treatment of cells with alkylating agents. The ubiquitylation was partially dependent on RAD18. Surprisingly RPA inhibited the RAD18-dependent ubiquitylation of RFC2. Our results suggest that RFC regulates the DNA damage response pathway via interaction with RPA and ubiquitylation.
EXPERIMENTAL PROCEDURES
Plasmid Constructs—To generate pCDNA·RFC2(p40)FLAG and pCDNA·RFC2(p40)HA, human p40 coding region was amplified by PCR as an EcoRI-XhoI fragment. The PCR product was inserted into the EcoRI-XhoI site either of pCDNA-C-FLAG or pCDNA-C-HA. To generate pCAGGS·RFC2(p40), the human p40 coding region was amplified by PCR as a SalI-XhoI fragment. The PCR product was inserted into the XhoI site of pCAGGS. pCDNA-C-FLAG and pCDNA-C-HA was constructed by inserting the FLAG or HA epitope into the XhoI-XbaI site of pCDNA3.1. Expression plasmids containing human RFC1-FLAG, human FLAG-RAD17, human FLAG-CTF18, human FLAG-p38, human FLAG-p37, and human FLAG-p36 were constructed by inserting their cDNA described previously (28) into pCDNA3. Although N-terminally and C-terminally tagged forms of each RFC2 subunit were used, the presence of the epitope tag did not affect RFC2 regulation at least in the context of experiments reported in this study. pCAGGS·FLAG-Ubiquitin and pCAGGS·hRAD18 were constructed as described previously (25). The expression plasmids for human RFC and PCNA were described earlier (29, 30), and that for human RPA, p11d-tRPA (31), was a generous gift of Dr. Marc S. Wold (University of Iowa College of Medicine, Iowa City, IA). Mouse E1 expression vector RLC (32, 33) was a generous gift of Dr. Hideyo Yasuda (School of Life Science, Tokyo University of Pharmacy and Life Science, Tokyo, Japan). Human cDNAs for RAD6A and RAD18 amplified from a HeLa cDNA library by PCR introducing a NdeI site at the start codon were cloned together into pET20b(+) (Novagen) as an artificial operon. After cloning the PCR fragments, the nucleotide sequences were verified. All the expression plasmids of PCNA, RPA, RFC, E1, RAD6A, and RAD18 were designed for production of intact proteins without any affinity tags.
Cell Culture and Transfection—293A and HCT116 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. HCT116 RAD18-/-cells were established as described previously (25). Cells were transfected with Lipofectamine Plus (Invitrogen) or Lipofectamine 2000 according to the manufacturer's protocol. 2.4 μg of plasmid DNA was used to transfect each 6-cm plate of cells. Transfected cells were treated with genotoxins 24 h post-transfection.
Genotoxin and Inhibitor Treatments—Asynchronous cell cultures were grown to approximately 80% confluency. For UV treatment, cells were washed with PBS, and exposed to UV light (254 nm) at a fluence rate of 43 J m-2/s. For genotoxin and inhibitor treatment, hydroxyurea (HU; 1 m in H2O), aphidicolin (dissolved in Me2SO), methyl methanesulfonate (MMS; dissolved in Me2SO), ethyl methanesulfonate (dissolved in Me2SO), N-methyl-N′-nitro-N-nitrosoguanidine (dissolved in Me2SO), H2O2 (diluted in PBS), mitomycin C, bleomycin (dissolved in H2O), or camptothecin (dissolved in Me2SO) was added to the culture media to give a final concentration of 2 mm, 0.025 mm, 0.1–1.7 mm, 20 mm, 0.7 mm, 0.5 mm, 0.01 mm, 0.05 mg/ml, or 20 nm, respectively, and cells were exposed for 8 h unless otherwise stated.
Antibodies—A mouse monoclonal antibody against Drosophila RFC40 (anti-dRFC40) was used for probing human RFC2(p40). A hybridoma cell line producing anti-dRFC40 antibody was a kind gift from Dr. Gerald M. Rubin (University of California, Berkeley), and monoclonal antibody was purified as described previously (34). To test whether anti-dRFC40 antibody cross-reacts with human RFC2(p40), an HA epitope-tagged form of hRFC2(p40) was overexpressed in 293A cells by transfection, and cell lysate was recovered 24 h post-transfection and then immunoblotted with either anti-dRFC40 or anti-HA antibody. An anti-dRFC40-reactive protein band migrating at 40 kDa was clearly observed only in extracts from HA-hRFC2(p40)-transfected cells and corresponded to the species detected with an anti-HA antibody (supplemental Fig. 1). Therefore, the anti-dRFC40 antibody recognizes human RFC2(p40). To avoid confusion we refer to the anti-dRFC40 antibody as “anti-RFC2” antibody in this study.
Other commercial antibodies used in this study were: anti-HA (Y-11, Santa Cruz Biotechnology), anti-FLAG (M2, Sigma), anti-RFC1 (H-300, Santa Cruz Biotechnology), anti-RAD17 (H-3, Santa Cruz Biotechnology), anti-tubulin (B-5-1-2, Sigma), anti-histone H3 (6.6.2, Upstate; and ab1791, Abcam), and anti-PCNA (PC10, Oncogene).
Preparation of Cell Lysate and Chromatin Fraction—293A cells in a 3.5- or 6-cm dish were washed twice with ice-cold PBS and then harvested into radioimmune precipitation assay buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, and protease inhibitor (Nacalai)). The cell suspensions were incubated for 30 min on ice, and then the Nonidet P-40, 0.1% SDS-insoluble fraction and -soluble fractions were separated by centrifugation. The soluble fraction was used as the supernatant (Sup) fraction. The resultant pellet was washed with radioimmune precipitation assay buffer four times and then sonicated after adding SDS-PAGE loading buffer (7% glycerol, 22% SDS, 50 mm Tris-HCl (pH 6.8), 5% β-mercaptoethanol). The resultant solution was used as the chromatin fraction. We confirmed that there were few contaminations in each Sup and chromatin fraction using anti-tubulin and anti-histone H3 antibodies (supplemental Fig. 2).
SDS-PAGE and Western Blotting—Cell extracts were resolved by electrophoresis by 7.5 or 10% SDS-PAGE. Following transfer onto polyvinylidene difluoride or nitrocellulose filters, the blots were incubated with antibodies, and immunoblots were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences; or DURA, Pierce) according to the manufacturers' instructions.
Immunoprecipitation—Cell extracts were incubated with monoclonal mouse anti-RFC2 (dRFC4(p40)) antibody for 1 h at 4 °C and then with 25 μl of protein A/G-agarose (Santa Cruz Biotechnology). After incubation for overnight at 4 °C, the beads were washed with PBS three times and boiled in Laemmli buffer for 5 min, and the bound proteins were analyzed by electrophoresis and immunoblotting.
Protein Purification—Human RFC, PCNA, and RPA were purified as described previously (29, 30). Mouse E1 was overproduced in insect cells and purified as described previously (35). Human RAD6A-RAD18 complex was overproduced in Escherichia coli cells and then purified by column chromatography (phosphocellulose, heparin-Sepharose, Mono Q, and gel filtration) from E. coli cell lysate. Protein concentrations were determined by Bio-Rad protein assay kit using bovine serum albumin as the standard. Bovine ubiquitin was purchased from Sigma.
In Vitro Ubiquitylation Assay—The reaction mixture (25 μl) contained 20 mm HEPES-NaOH (pH 7.5), 50 mm NaCl, 0.2 mg/ml bovine serum albumin, 1 mm dithiothreitol, 10 mm MgCl2, 1 mm ATP, 33 fmol of singly primed single-stranded M13 mp18 DNA (30), 1.0 μg (9.1 pmol) of RPA, 86 ng (1.0 pmol as a trimer) of PCNA, 75 ng (260 fmol) of RFC, 100 ng (850 fmol) of mouse E1, 175 ng (2.4 pmol) of RAD6A-RAD18 complex, and 12.5 μg (1460 pmol) of ubiquitin. After incubation at 30 °C for 60 min, reactions were terminated with 2 μl of 300 mm EDTA.
Structural Model Building—Homology modeling of the human clamp loader-clamp complex was performed using MODELLER version 7.7 (36). The homologous structures were defined using the fold recognition server FORTE (37). The atomic coordinate of the clamp-clamp loader complex (Protein Data Bank code 1SXJ) was selected as a template for model building. Before submission to MODELLER, the sequence-structure alignment obtained from FORTE was used. Due to the lack of the template structure, the N-terminal 582 residues of human RFC1 were not modeled. The figures were prepared using MOLMOL (38). Coloring of each RFC subunit and PCNA was according to Fig. 2 in the review by Bowman et al. (39).
FIGURE 2.
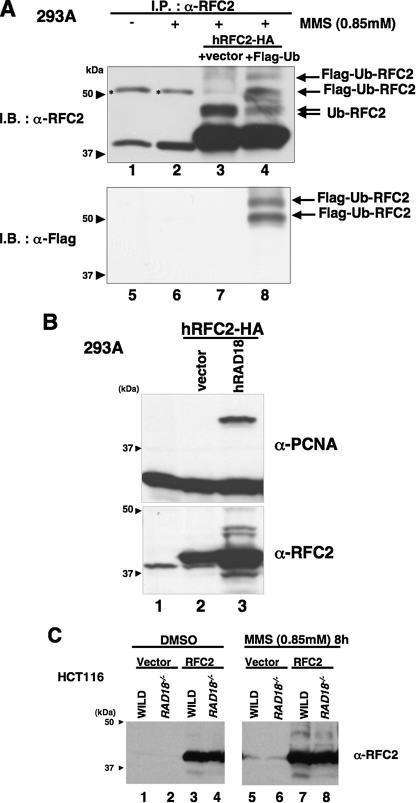
RFC2 monoubiquitylation in response to DNA-damaging agents is RAD18-dependent. A, lysates from RFC2-HA- and FLAG-ubiquitin-co-transfected 293A cells were analyzed by immunoprecipitation and Western blotting. pCDNA3·RFC2-HA was co-transfected either with pCAGGS-FLAG-Ubiquitin (lanes 4 and 8) or empty vector (lanes 3 and 7) in 293A cells. The following day, cells were treated with MMS for 8 h, and then cell extracts were recovered. Cell extracts were immunoprecipitated with anti-RFC2 antibody. The resulting immune complexes were recovered using protein A/G-agarose and detected by immunoblotting with anti-RFC2 antibody (lanes 1–4) or anti-FLAG antibody (lanes 5–8). Asterisks show nonspecific bands. B, Western blot of lysates from 293A cells overexpressing hRAD18. pCDNA3·RFC2-HA was co-transfected either with pCAGGS·hRAD18 (lane 3) or empty vector (lane 2) in 293A cells. Chromatin fractions were prepared and analyzed by Western blotting with anti-RFC2 (lower panel) or anti-PCNA (upper panel). The arrowheads indicate the position of molecular mass markers (kDa). C, Western blot of lysates from HCT116 cells (WILD) or RAD18-deficient HCT116 cells (RAD18-/-). HCT116 cells transfected either with empty vector or pCAGGS·hRFC2 were treated with 0.85 mm MMS for 8 h. Chromatin fractions from the resulting cells were analyzed by immunoblotting with anti-RFC2 antibody. The arrowheads indicate the position of molecular mass markers (kDa). I.P., immunoprecipitate; I.B., immunoblot; DMSO, Me2SO; Ub, ubiquitin.
RESULTS
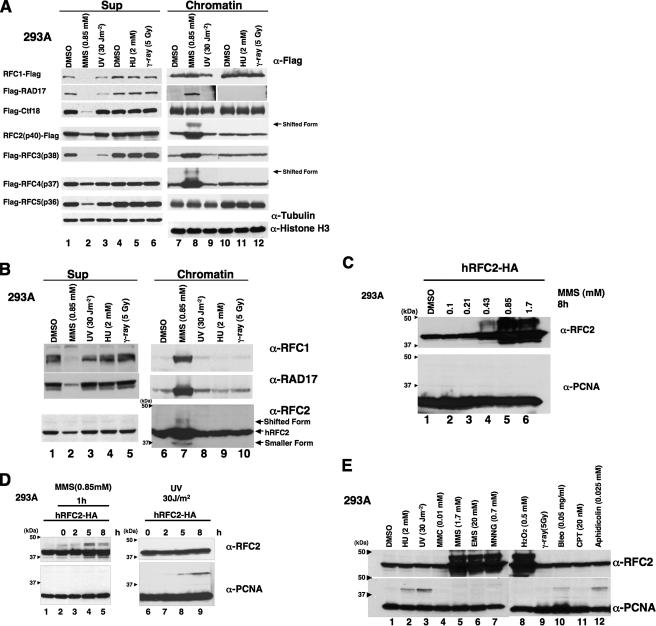
Specific DNA-damaging Agents Induce Modification of RFC2— To analyze the modification of each subunit of the RFC complex, a FLAG epitope-tagged form of each subunit of RFC and RLCs was expressed in human 293A cells. Transfected cells were treated with UV irradiation, γ-ray, HU, or MMS, and then cell extracts were prepared. The cell extracts were separated into Nonidet P-40-insoluble chromatin fractions and -soluble fractions (Sup). RFC and RLC subunits in each fraction were analyzed by SDS-PAGE and Western blotting (Fig. 1A). Following MMS treatment all of the subunits, except for CTF18 and RFC5, accumulated in the chromatin fraction, whereas no accumulation was observed following treatments with UV irradiation, γ-ray, or HU. Levels of soluble CTF18 and RFC5 decreased after MMS treatment, although we did not detect concomitant increases in the chromatin-bound levels of these subunits (Fig. 1A). Taken together, the results of Fig. 1A demonstrate that the levels and subcellular distribution of RFC and RLC subunits are regulated in response to MMS.
FIGURE 1.
Accumulation of RFC complex in chromatin fraction and modification of RFC2 following treatment of 293A cells with DNA-damaging agents. A, 293A cells transfected with a FLAG epitope-tagged form of each subunit of RFC and RLCs were irradiated with UV light (lanes 3 and 9) or γ-ray (lanes 6 and 12) or treated with Me2SO (DMSO; lanes 1, 4, 7, and 10), MMS (lanes 2 and 8), or HU (lanes 5 and 11) for 8 h. Cell extracts recovered from transfected cells were then separated into chromatin (lanes 7–12) and soluble (Sup; lanes 1–6) fractions and analyzed by Western blotting with anti-FLAG. Cell extracts recovered from RFC4-transfected cells were also analyzed by Western blotting with anti-tubulin or anti-histone H3 (lowest two blots). B, 293A cells were irradiated with UV light (lane 3) or γ-ray (lane 5) or treated with Me2SO (lane 1), MMS (lane 2), or HU (lane 4) for 8 h. Cell extracts recovered from transfected cells were then separated into chromatin and soluble (Sup) fractions and analyzed by Western blotting either with anti-RFC1, anti-RAD17, or anti-RFC2. The arrowheads indicate the position of molecular mass markers (kDa). C, 293A cells transfected with pCDNA3·RFC2-HA were treated with the indicated dose of MMS for 8 h. Chromatin fractions from the resulting cells were analyzed by immunoblotting with anti-RFC2 or anti-PCNA. The arrowheads indicate the position of molecular mass markers (kDa). D, 293A cells transfected with pCDNA3·RFC2-HA were treated with 0.85 mm MMS for 1 h (lanes 2–5) or UV light-irradiated at 254 nm with 30 J m-2 (lanes 6–9) and then incubated for the indicated times. Chromatin fractions were prepared and analyzed by Western blotting with anti-RFC2 and anti-PCNA. Cells treated with Me2SO (lane 1) are shown as control. The arrowheads indicate the position of molecular mass markers (kDa). E, 293A cells transfected with pCDNA3·RFC2-HA were treated with various genotoxic agents. Chromatin fractions were prepared and analyzed by Western blotting with anti-RFC2 or anti-PCNA. The arrowheads indicate the position of molecular mass markers (kDa). Gy, grays; MMC, mitomycin C; EMS, ethyl methanesulfonate; MNNG, N-methyl-N′-nitro-N-nitrosoguanidine; Bleo, bleomycin; CPT, camptothecin.
It was important to determine whether endogenous RFC and RLC subunits were also redistributed to chromatin in response to MMS. Therefore, we determined the effects of MMS on endogenous RFC1, RAD17, or RFC2 proteins for which good antibodies are available. As shown in Fig. 1B, endogenous RFC1, RAD17, and RFC2 accumulated in the chromatin fraction of MMS-treated 293A cells. Similar to ectopically expressed tagged proteins, endogenous RFC subunits were redistributed to chromatin in response to MMS treatment.
Interestingly we observed prominent forms of ectopically expressed RFC2 and RFC4 that migrated with reduced electrophoretic mobility on SDS-PAGE gels in chromatin fractions from MMS-treated 293A cells (Fig. 1A, lane 7). Electrophoretically retarded species of endogenous RFC2 were also evident in chromatin fractions of MMS-treated 293A cells (Fig. 1B, lane 7). The electrophoretically shifted form of RFC2 was more prominent than that of RFC4 (Fig. 1A). Therefore we focused on RFC2 and further analyzed its MMS-induced modification.
We performed quantitative analyses to determine the amount of chromatin-bound RFC2 relative to the soluble fraction in MMS-treated cells. In 293A cells ectopically expressing HA-tagged RFC2, more than 90% of the RFC2 accumulated in the chromatin fraction following 8 h of MMS treatment, whereas in untreated cells, less than 10% of RFC2 was present in the chromatin fraction (supplemental Fig. 3). Following MMS treatment, we consistently detected two electrophoretically retarded anti-RFC2-reactive proteins in the chromatin fraction. The apparent molecular mass of electrophoretically retarded RFC2 is consistent with ubiquitylation. The two putative ubiquitylated forms of RFC2 (shown in Fig. 1) might correspond to species that are monoubiquitylated on different residues. However, we cannot exclude the possibility that modifications other than ubiquitin are also present on the shifted RFC2. Furthermore smaller anti-RFC2-reactive proteins, possibly corresponding to degradation products, were detected in soluble and chromatin fractions from both control and MMS-treated cells (Fig. 1B and supplemental Fig. 3).
The electrophoretically retarded forms of RFC2 were induced by MMS in a dose-dependent manner (Fig. 1C). At lower concentrations of MMS (0.1 or 0.213 mm), no RFC2 band shift was detectable. However, treatment with higher concentrations of MMS (0.425, 0.85, or 1.7 mm) induced prominent electrophoretically retarded forms of RFC2 on chromatin (Fig. 1C).
In the experiments described above, the cells were treated with MMS for 8 h. We subsequently examined the kinetics of RFC2 modification by treating 293A cells with MMS (0.85 mm) for 1 h and preparing samples for immunoblotting at 0, 2, 5, and 8 h following MMS treatment. As shown in Fig. 1D, the shifted forms of RFC2 were detectable by 5 h after treatment of cells with MMS (lane 4). Similar to results of Fig. 1A, the genotoxin-induced RFC2 mobility shift was specific for MMS because UV irradiation (30 J/m2; lanes 7–9) did not induce RFC modification at any time point tested (although as expected, UV irradiation induced PCNA monoubiquitylation under these experimental conditions). Conversely little or no PCNA modification was detectable under the conditions used for the experiment shown in Fig. 1D (lanes 2–5), although low levels of PCNA ubiquitylation were observed when cells were treated with 0.85 mm MMS for longer times (data not shown).
The results of Fig. 1, A and D, indicated that MMS-induced RFC2 modification is not a general response to DNA damage. To gain insight into the significance of RFC2 modification, 293A cells ectopically expressing RFC2-HA were treated with a more extensive panel of DNA-damaging agents for 8 h, and proteins in resulting chromatin fractions were analyzed by immunoblotting with the anti-RFC2 antibody (Fig. 1E, upper panel). DNA-damaging agents we tested included alkylating agents (ethyl methanesulfonate and N-methyl-N′-nitro-N-nitrosoguanidine), an oxidizing agent (H2O2), a DNA cross-linking agent (mitomycin C), double strand break-inducing agents (bleomycin and ionizing radiation), and the topoisomerase I inhibitor camptothecin. Of the genotoxic agents tested, only ethyl methanesulfonate, N-methyl-N′-nitro-N-nitrosoguanidine, and H2O2 induced the shifted RFC2 band evident in MMS-treated cells (Fig. 1E, upper panel, lanes 7–10). Many of the agents failing to induce the RFC2 band shift nevertheless induced very robust PCNA monoubiquitylation (Fig. 1E, lower panel). Therefore, we conclude that RFC2 modification is a specific response to a subset of genotoxins.
RAD18-dependent Ubiquitylation of Human RFC2—To test whether the shifted RFC2-specific band in MMS-treated cells was due to ubiquitylation, RFC2-HA was co-expressed with FLAG-tagged ubiquitin in 293A cells. The transfected cells were treated with MMS. Endogenous and HA-tagged RFC2 proteins were immunoprecipitated with anti-RFC2 antibody from cell lysates, and the precipitated proteins were immunoblotted with either anti-RFC2 (Fig. 2A, upper panel) or anti-FLAG antibody to detect FLAG-ubiquitin-modified proteins (lower panel). Anti-RFC2-reactive bands migrating at the sizes expected for monoubiquitylated RFC2 (48 kDa) were observed in our anti-RFC2 immunoprecipitates (lanes 3 and 4, Ub-RFC2). In addition to the 48-kDa ubiquitin-RFC2 band, two extra, slowly migrating bands (51 and 62 kDa) were observed in the immunoprecipitates obtained from cells transfected with FLAG-tagged ubiquitin (lane 4, Flag-Ub-RFC2) that were also detectable by immunoblotting with anti-FLAG antibody (lane 8). From these results we conclude that the slow migrating forms of RFC2 in MMS-treated cells are ubiquitylated species.
In S. cerevisiae and human cells, monoubiquitylation of PCNA is dependent on the RAD18 E3 ubiquitin ligase (22, 24, 25). To determine whether ubiquitylation of RFC2 was similarly dependent on RAD18, RFC2 modification was tested in RAD18-overexpressing 293A cells (Fig. 2B) and RAD18-deficient HCT116 cells (Fig. 2C). As shown in Fig. 2B, overexpression of RAD18 induced the ubiquitylation of RFC2-HA and PCNA even in the absence of MMS treatment. Conversely MMS-induced ubiquitylated forms of RFC2 decreased considerably (by 50%) in HCT116 RAD18-/- cells compared with those in matched HCT116 RAD18+/+ cells (Fig. 2C). These results suggest that RFC2 monoubiquitylation in MMS-treated cells is mediated at least in large part by RAD18, most probably as a complex with RAD6. Interestingly RAD18 overexpression also induced chromatin accumulation of RFC2 (Fig. 2B). Ubiquitylation and chromatin accumulation of RFC2 (and also RFC4) was observed in response to MMS treatment and RAD18 overexpression. Because MMS treatment induced chromatin accumulation of each RFC subunit (Fig. 1A), it is most likely that increased chromatin loading of the entire RFC complex occurs in response to MMS.
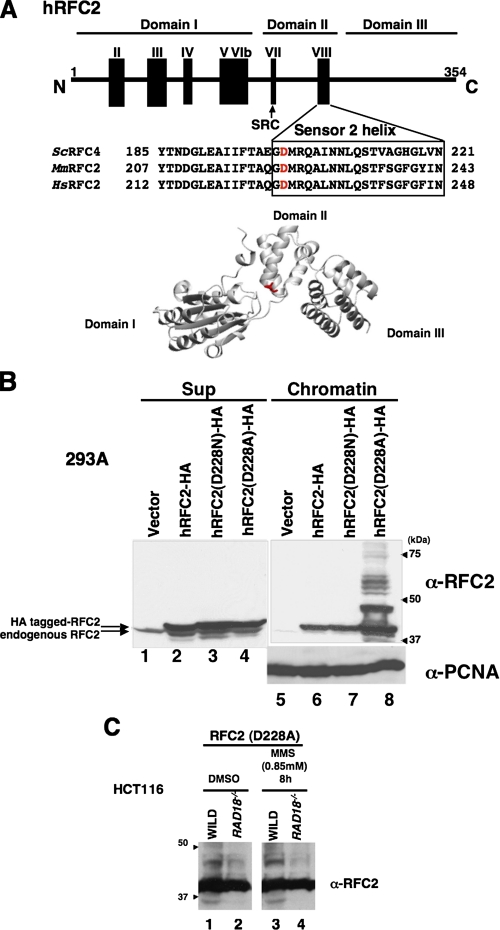
An RFC2 Mutant Is Ubiquitylated in the Absence of DNA Damage—It has been reported that the RFC2(p40) subunit of human RFC binds the large subunit of RPA (11). In S. cerevisiae, a mutation in rfc4 (yeast homolog of human RFC2(p40)) was found to display synthetic lethality with mutation in the gene encoding Rpa1 (the large subunit of S. cerevisiae RPA) (40). Interestingly this mutant Rfc4(p40) showed weaker physical interaction with RPA than did the wild type Rfc4(p40). This mutation, resulting in an amino acid change of aspartate to asparagine at residue 201, maps to the RFC box VIII, which is one of the conserved motifs found in all RFC subunits (16, 41). The Asp-201 residue of S. cerevisiae Rfc4 is conserved and found at an identical position in RFC2 from higher eukaryotes, including humans (Fig. 3A). We replaced Asp-228 of human RFC2 (which corresponds to S. cerevisiae Rfc4 Asp-201) with an asparagine residue (D228N) or an alanine (D228A). HA-tagged forms of mutant or wild-type RFC2 were expressed in 293A cells by transfection (Fig. 3B). The wild-type and mutant forms of RFC2-HA were expressed at similar levels; however, whereas the wild-type and D228N mutant RFC2 proteins showed no ubiquitylation of RFC2, the D228A mutant RFC2 protein underwent extensive modification without any genotoxin treatment (Fig. 3B, lane 8). The multiple shifted bands of RFC2 D228A decreased by 55% in HCT116 RAD18-/- cells compared with those in matched HCT116 RAD18+/+ cells (Fig. 3C). Therefore, we conclude that the multiple RAD18-dependent species we observed correspond to mono- and polyubiquitylated forms of RFC2. As described in the previous sections, we observed monoubiquitylated forms of the wild-type RFC2-HA in MMS-treated cells but did not observe high levels of its polyubiquitylated forms. The results of Fig. 3B indicate that the RFC2 D228A mutant is extensively ubiquitylated and accumulates as multiple polyubiquitylated species (even in the absence of genotoxin treatments) when ectopically expressed. Although the difference in susceptibility to spontaneous ubiquitylation between D228A and D228N is unexpected, by analogy with the S. cerevisiae Rfc4 D201N mutant protein, it is most likely that Asp-228 of human RFC2 is also involved in interaction with RPA. Although we have not formally verified the reduced interaction of human RFC D228A with RPA, we infer that RAD6-RAD18-mediated RFC2 ubiquitylation is regulated by interaction with RPA (see below).
FIGURE 3.
DNA damage-independent monoubiquitylation of hRFC2 D228A. A, schematic diagram and tertiary model of human (Hs) RFC2 showing the location of Asp-228 and the sequences of the surrounding regions. Corresponding sequences for S. cerevisiae (Sc) RFC2(p40) and mouse (Mm) RFC2 homologues are also shown. The conserved Sensor 2 helix is represented by a box, and the location of the conserved SRC motif is indicated by an arrow. Asp-228 of hRFC2, shown in red, corresponds to S. cerevisiae Asp-201, which shows synthetic lethality with mutation in Rpa-1(rfa1-Y29H). There are seven conserved RFC boxes numbered consecutively from the N terminus to C terminus. B, 293A cells were transfected with expression vectors encoding wild-type (lanes 2 and 6), D228N (lanes 3 and 7), or D228A (lanes 4 and 8) forms of hRFC2-HA. 24 h after transfection cells were harvested and separated into chromatin (lanes 5–8) and soluble fractions (lanes 1–4) and then immunoblotted with anti-RFC2 or anti-PCNA antibody. The arrowheads indicate the position of molecular mass markers (kDa). C, Western blot of lysates from HCT116 cells (WILD) or RAD18-deficient HCT116 cells (RAD18-/-). HCT116 cells transfected with pCAGGS·hRFC2(Asp-228) were treated with 0.85 mm MMS for 8 h. Chromatin fractions from the resulting cells were analyzed by Western blotting with anti-RFC2 antibody. The arrowheads indicate the position of molecular mass markers (kDa). DMSO, Me2SO.
RFC2 Is Modified by the RAD6-RAD18 Complex in Vitro— We subsequently examined whether RFC2 could be modified by the RAD6-RAD18 complex in vitro. Recombinant RFC complex (including RFC1–5 proteins of human origin) was expressed in E. coli and then purified. Monoubiquitylation of RFC2 in vitro was investigated by mixing the RFC1–5 complex with purified recombinant RAD6A (E2 ubiquitin-conjugating enzyme)-RAD18 (E3 ubiquitin ligase) complex. As shown in Fig. 4, RFC2 was monoubiquitylated in vitro when incubated in the presence of purified RAD6A and RAD18 plus ubiquitin and its activating enzyme (lane 2) although at a much lower efficiency when compared with PCNA. It should also be noted that the in vitro modification of RFC2 generated only a single monoubiquitylated species, whereas at least two monoubiquitylated forms of RFC2 (corresponding to monoubiquitylation at different residues) resulted from MMS treatment of intact cells. The reason for the differential patterns of RAD18-mediated RFC2 monoubiquitylation observed in vitro and in intact cells is not yet clear but could result from the existence of additional RFC2-directed E3 ligases in vivo. The difference also indicates that in vitro assay conditions do not fully recapitulate the complexity of events involved in RPA-sensitive RFC2 ubiquitylation at stalled replication forks in vivo. It should be noted that our in vitro assay uses primed M13 single-stranded DNA, which mimics the leading strand synthesis rather than the lagging strand synthesis that requires the RFC complex more frequently. PCNA did not affect RFC2 monoubiquitylation (lane 4), although the modification was dependent on the presence of DNA (data not shown). Interestingly, however, the addition of RPA inhibited RAD6-RAD18-dependent monoubiquitylation completely (lanes 3 and 5). In parallel reactions, RPA did not affect the monoubiquitylation of PCNA (lanes 4 and 5). Therefore, RPA specifically inhibits RAD18-dependent monoubiquitylation of RFC2. The inhibition of RAD18-mediated RFC2 ubiquitylation by RPA in vitro is consistent with our finding that the RFC2 D228A mutant is more extensively modified than wild-type RFC2 in intact cells.
FIGURE 4.
In vitro monoubiquitylation of RFC2. In vitro ubiquitylation was carried out by mixing RFC with mouse E1, RAD18-RAD6A complex, ubiquitin, and singly primed single stranded M13 mp18 DNA in the presence or absence of RPA or PCNA as indicated. The reaction products were analyzed by Western blotting with anti-RFC2 or anti-PCNA antibody.
DISCUSSION
Protein ubiquitylation is critical for numerous cellular functions, including the DNA damage response pathway. In this study we demonstrated that RFC2 is ubiquitylated in human cells via DNA damage-independent and genotoxin-inducible mechanisms. RFC2 ubiquitylation is partially dependent on RAD18 as demonstrated by the decreased MMS-induced RFC2 ubiquitylation in RAD18-/- cells compared with matched RAD18+/+ HCT116 cells (Fig. 2C). Conversely RFC2 undergoes genotoxin-independent monoubiquitylation in cells overexpressing RAD18. RAD18-dependent monoubiquitylation of RFC2 was also verified by in vitro reaction (Fig. 4). The RAD18-induced ubiquitylation of RFC2 in vitro and in RAD18-overexpressing cultured cells is similar to what we and others have observed for PCNA, a bona fide RAD18 substrate. These results are further indicative of a direct E3 ligase-substrate relationship between RAD18 and RFC2.
Our in vitro experiments clearly show an inhibitory effect of RPA on RFC2 monoubiquitylation (Fig. 4). The involvement of RPA in regulation of RFC2 ubiquitylation in vivo is also suggested by our experiments with the RFC2 D228A mutant (corresponding to an S. cerevisiae RPA interaction-deficient Rfc4 mutant). We have shown that RFC2 D228A underwent DNA damage-independent ubiquitylation, which was reduced substantially in RAD18-deficient cells (Fig. 3C). Our in vitro assay for RAD6-RAD18-dependent RFC2 ubiquitylation did not completely recapitulate all aspects of RFC2 modification in vivo, and the role of RFC2 D228 in mediating RPA associations is not yet clear. However, our results strongly suggest a key regulatory role of RPA in RFC2 ubiquitylation. We propose that RAD18-dependent RFC2 ubiquitylation is repressed by RPA in undamaged cells and that derepression of RFC2 ubiquitylation occurs following MMS-induced DNA damage.
Our experiments also indicate that the RFC2 D228A mutant is subject to extensive polyubiquitylation. It is likely that polyubiquitylated RFC2 is generated by linkage of additional ubiquitin molecules to lysine residues that are first monoubiquitylated by RAD18. By analogy, following genotoxin treatments PCNA is monoubiquitylated by RAD6-RAD18 on lysine 164, and subsequently the monoubiquitylated PCNA is polyubiquitylated in a reaction mediated by MMS2-UBC13 and RAD5 (22, 23, 42, 43). It will be interesting to determine whether RAD5 or alternative E3 ligases contribute to the RAD18-initiated polyubiquitylation of RFC2. Monoubiquitylated and polyubiquitylated species of PCNA promote different damage response pathways, error-prone and error-free postreplication repair, respectively. It will be interesting to determine whether the mono- and polyubiquitylated species of RFC2 similarly serve distinct effector functions. Several studies have demonstrated that a residual level of PCNA ubiquitylation is detectable in RAD18-deficient cells. Similarly we have shown that RAD18-deficiency did not completely ablate RFC2 ubiquitylation. Clearly further work is necessary to identify the E3 ligases involved in RAD18-independent ubiquitylation of PCNA and RFC2.
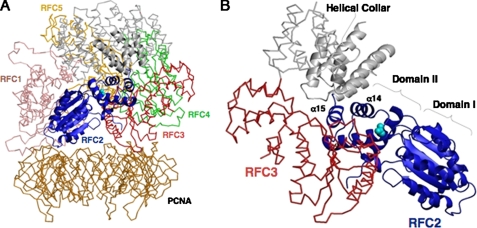
To obtain insight into the question of why the RFC2 D228A mutant is susceptible to ubiquitylation without DNA damage, we constructed tertiary structure models of human RFC2 (Fig. 3A) and RFC complex bound to PCNA (Fig. 5) by homology modeling using the reported yeast structure (41) as the template. Each RFC subunit contains three structurally conserved domains (Domains I, II, and III). Domains I and II comprise an ATPase module of the AAA+ family that is connected by a flexible linker to another helical domain (Domain III). Our structural model revealed that Asp-228 resides in the turn between helix14 and helix15 (Sensor 2 helix), which is located near the hinge region between Domains II and III (Fig. 5C). This implies that RFC2 Asp-228 is not exposed to the outer surface but instead is buried in the spiral structure. It is unlikely, therefore, that the Asp-228 residue directly associates with RPA as long as such a tight RFC-PCNA complex is maintained.
FIGURE 5.
A model for human clamp loader-clamp complex. Ribbon (RFC2) and wire (Cα trace, RFC1, RFC3–5, and PCNA) representations of the homology model for human RFC1–5-PCNA complex are shown. The five subunits of each clamp loader complex are denoted. The colors for each of the subunits are as follows with the helical collar domains (gray) at the top of the figure: pink, RFC1; navy, RFC2; red, RFC3; green, RFC4; orange, RFC5; gold, PCNA. The side chain atoms of Asp-228 of RFC2 are indicated as balls in cyan. A, a side view of the clamp loader-clamp complex in which RFC2 is in the front. B, views from the DNA-interacting pore of the clamp loader subunits. Domains I and II of AAA+ domain and α14 and α15 of RFC2 are indicated.
Whether the RFC complex remains around the primed end following PCNA loading is controversial (11, 30, 44–46). However, the RFC complex may stay associated with PCNA in a structure different from the tight complex as shown in Fig. 5A that allows RPA to associate with RFC2 around the Asp-228 residue. Another possibility is that the D228A mutation causes a conformational change in the RFC complex structure, possibly altering interactions with RPA and affecting susceptibility to ubiquitylation.
It is notable that RFC2 is ubiquitylated in human cells following treatment with alkylating agents but not in response to genotoxins that induce double strand breaks, bulky adducts, interstrand cross-links, or nucleotide depletion. Therefore, it appears that RFC2 monoubiquitylation is due to a specific alteration in DNA structure induced by alkylating agents or to a specific DNA repair intermediate. Identification of the DNA structure(s) responsible for RFC2 ubiquitylation may provide insight into the consequences of DNA damage due to particular genotoxins. Alkylating agents modify DNA by adding methyl or ethyl groups to a number of nucleophilic sites on the DNA bases (47). The predominant adduct in double strand DNA resulting from MMS or N-methyl-N′-nitro-N-nitrosoguanidine exposure is N7-methylguanine (N7-MeG) and N3-methyladenine (N3-MeA). N3-MeA blocks replication, whereas N7-MeG does not block replication or miscode. Another deleterious adduct is O6-methylguanine (O6-MeG). O6-MeG is produced at a relatively lower level compared with N7-MeG and N3-MeA but is highly mutagenic and toxic because O6-MeG-T mispairing not only results in G/C to A/T transition but also is recognized by mismatch repair in a process that is a potent signal of apoptosis (48). However, the human kidney cell line 293A cells, which were used in this study, are mismatch repair-deficient due to epigenetic silencing of the hMLH1 gene by promoter hypermethylation (49). Therefore, O6-MeG is not the lesion responsible for RFC2 monoubiquitylation, and instead N7-MeG and/or N3-MeA are the likely candidates. Treatment of 293A cells with an oxidative agent (H2O2) also induced RFC2 monoubiquitylation (Fig. 1E). Base excision repair is the common pathway for repairing N7-MeG, N3-MeA, and oxidative damage (47, 50, 51). Base excision repair is initiated with removal of altered bases by DNA glycosylase. The resulting apurinic/apyrimidinic (AP) sites are nicked, and repair is completed by resynthesis and ligation. Therefore, for proficient base excision repair, a proper balance of the individual steps involved in DNA repair is important. Imbalanced base excision repair may result in deleterious intermediates, such as AP sites. Furthermore methylation or oxidation of purines destabilizes the N-glycosyl bond, thus rendering the base more susceptible to hydrolysis to form an AP site. Therefore, AP sites are the lesions most likely to cause RFC2 monoubiquitylation, although precisely how RPA-RFC2 interaction is affected at AP sites is unclear.
Another possible role of RFC2 ubiquitylation is as the sensing signal for damage recognition. The RFC1–5 complex (containing RFC2) has several functions. During normal DNA replication RFC1–5 acts as clamp loader for PCNA, whereas in the DNA damage response RAD17-RFC2–5 loads the 9-1-1 complex. At present we do not know whether loading of PCNA, the 9-1-1 complex, or both is affected by RAD18-dependent RFC2 modification. Experiments to further address the significance of RFC2 modification and to identify relevant effectors of modified RFC are under way.
Supplementary Material
Acknowledgments
We greatly appreciate the gift of the expression plasmids for human RPA, p11d-tRPA, from Dr. Marc S. Wold (University of Iowa College of Medicine, Iowa City, IA) and mouse E1 expression vector RLC from Dr. Hideyo Yasuda (School of Life Science, Tokyo University of Pharmacy and Life Science, Tokyo, Japan).
This work was supported by grants-in-aid for Scientific Research A and B from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to T. T.) and by National Institutes of Health Grants ES09558 and ES12917 (to C. V.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
Footnotes
The abbreviations used are: RPA, replication protein A; PCNA, proliferating cell nuclear antigen; RFC, replication factor C; Pol, polymerase; RLC, RFC-like complex; HA, hemagglutinin; PBS, phosphate-buffered saline; HU, hydroxyurea; MMS, methyl methanesulfonate; Sup, supernatant; E1, ubiquitin-activating enzyme; E2, ubiquitin carrier protein; E3, ubiquitin-protein isopeptide ligase; AP, apurinic/apyrimidinic.
References
- 1.Hartwell, L. H., and Weinert, T. A. (1989) Science 246 629-634 [DOI] [PubMed] [Google Scholar]
- 2.Carr, A. M. (2002) DNA Repair (Amst.) 1 983-994 [DOI] [PubMed] [Google Scholar]
- 3.Kastan, M. B., and Bartek, J. (2004) Nature 432 316-323 [DOI] [PubMed] [Google Scholar]
- 4.Sancar, A., Lindsey-Boltz, L. A., Unsal-Kacmaz, K., and Linn, S. (2004) Annu. Rev. Biochem. 73 39-85 [DOI] [PubMed] [Google Scholar]
- 5.Bochkareva, E., Korolev, S., Lees-Miller, S. P., and Bochkarev, A. (2002) EMBO J. 21 1855-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binz, S. K., Sheehan, A. M., and Wold, M. S. (2004) DNA Repair (Amst.) 3 1015-1024 [DOI] [PubMed] [Google Scholar]
- 7.Kelman, Z., and O'Donnell, M. (1995) Nucleic Acids Res. 23 3613-3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyman, C., and Botchan, M. (1995) Curr. Biol. 5 334-337 [DOI] [PubMed] [Google Scholar]
- 9.Fien, K., and Stillman, B. (1992) Mol. Cell. Biol. 12 155-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna, T. S., Kong, X. P., Gary, S., Burgers, P. M., and Kuriyan, J. (1994) Cell 79 1233-1243 [DOI] [PubMed] [Google Scholar]
- 11.Yuzhakov, A., Kelman, Z., Hurwitz, J., and O'Donnell, M. (1999) EMBO J. 18 6189-6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsurimoto, T., and Stillman, B. (1991) J. Biol. Chem. 266 1961-1968 [PubMed] [Google Scholar]
- 13.Maga, G., and Hubscher, U. (2003) J. Cell Sci. 116 3051-3060 [DOI] [PubMed] [Google Scholar]
- 14.Warbrick, E. (2000) BioEssays 22 997-1006 [DOI] [PubMed] [Google Scholar]
- 15.Zou, Y., Liu, Y., Wu, X., and Shell, S. M. (2006) J. Cell. Physiol. 208 267-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullmann, G., Fien, K., Kobayashi, R., and Stillman, B. (1995) Mol. Cell. Biol. 15 4661-4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuwald, A. F., Aravind, L., Spouge, J. L., and Koonin, E. V. (1999) Genome Res. 9 27-43 [PubMed] [Google Scholar]
- 18.Kim, J., and MacNeill, S. A. (2003) Curr. Biol. 13 R873-R875 [DOI] [PubMed] [Google Scholar]
- 19.Majka, J., and Burgers, P. M. (2004) Prog. Nucleic Acids Res. Mol. Biol. 78 227-260 [DOI] [PubMed] [Google Scholar]
- 20.Zernik-Kobak, M., Vasunia, K., Connelly, M., Anderson, C. W., and Dixon, K. (1997) J. Biol. Chem. 272 23896-23904 [DOI] [PubMed] [Google Scholar]
- 21.Wu, X., Shell, S. M., and Zou, Y. (2005) Oncogene 24 4728-4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoege, C., Pfander, B., Moldovan, G. L., Pyrowolakis, G., and Jentsch, S. (2002) Nature 419 135-141 [DOI] [PubMed] [Google Scholar]
- 23.Stelter, P., and Ulrich, H. D. (2003) Nature 425 188-191 [DOI] [PubMed] [Google Scholar]
- 24.Kannouche, P. L., Wing, J., and Lehmann, A. R. (2004) Mol. Cell 14 491-500 [DOI] [PubMed] [Google Scholar]
- 25.Watanabe, K., Tateishi, S., Kawasuji, M., Tsurimoto, T., Inoue, H., and Yamaizumi, M. (2004) EMBO J. 23 3886-3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedberg, E. C., Lehmann, A. R., and Fuchs, R. P. (2005) Mol. Cell 18 499-505 [DOI] [PubMed] [Google Scholar]
- 27.Bienko, M., Green, C. M., Crosetto, N., Rudolf, F., Zapart, G., Coull, B., Kannouche, P., Wider, G., Peter, M., Lehmann, A. R., Hofmann, K., and Dikic, I. (2005) Science 310 1821-1824 [DOI] [PubMed] [Google Scholar]
- 28.Shiomi, Y., Shinozaki, A., Nakada, D., Sugimoto, K., Usukura, J., Obuse, C., and Tsurimoto, T. (2002) Genes Cells 7 861-868 [DOI] [PubMed] [Google Scholar]
- 29.Fukuda, K., Morioka, H., Imajou, S., Ikeda, S., Ohtsuka, E., and Tsurimoto, T. (1995) J. Biol. Chem. 270 22527-22534 [DOI] [PubMed] [Google Scholar]
- 30.Masuda, Y., Suzuki, M., Piao, J., Gu, Y., Tsurimoto, T., and Kamiya, K. (2007) Nucleic Acids Res. 35 6904-6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henricksen, L. A., Umbricht, C. B., and Wold, M. S. (1994) J. Biol. Chem. 269 11121-11132 [PubMed] [Google Scholar]
- 32.Honda, R., Tanaka, H., and Yasuda, H. (1997) FEBS Lett. 420 25-27 [DOI] [PubMed] [Google Scholar]
- 33.Imai, N., Kaneda, S., Nagai, Y., Seno, T., Ayusawa, D., Hanaoka, F., and Yamao, F. (1992) Gene (Amst.) 118 279-282 [DOI] [PubMed] [Google Scholar]
- 34.Harrison, S. D., Solomon, N., and Rubin, G. M. (1995) Genetics 139 1701-1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas, A. L., and Bright, P. M. (1988) J. Biol. Chem. 263 13258-13267 [PubMed] [Google Scholar]
- 36.Fiser, A., and Sali, A. (2003) Methods Enzymol. 374 461-491 [DOI] [PubMed] [Google Scholar]
- 37.Tomii, K., and Akiyama, Y. (2004) Bioinformatics (Oxf.) 20 594-595 [DOI] [PubMed] [Google Scholar]
- 38.Koradi, R., Billeter, M., and Wuthrich, K. (1996) J. Mol. Graph. 14 29-32 [DOI] [PubMed] [Google Scholar]
- 39.Bowman, G. D., Goedken, E. R., Kazmirski, S. L., O'Donnell, M., and Kuriyan, J. (2005) FEBS Lett. 579 863-867 [DOI] [PubMed] [Google Scholar]
- 40.Kim, H. S., and Brill, S. J. (2001) Mol. Cell. Biol. 21 3725-3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowman, G. D., O'Donnell, M., and Kuriyan, J. (2004) Nature 429 724-730 [DOI] [PubMed] [Google Scholar]
- 42.Motegi, A., Sood, R., Moinova, H., Markowitz, S. D., Liu, P. P., and Myung, K. (2006) J. Cell Biol. 175 703-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unk, I., Hajdu, I., Fatyol, K., Szakal, B., Blastyak, A., Bermudez, V., Hurwitz, J., Prakash, L., Prakash, S., and Haracska, L. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 18107-18112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes, X. V., and Burgers, P. M. (2001) J. Biol. Chem. 276 34768-34775 [DOI] [PubMed] [Google Scholar]
- 45.Podust, V. N., Tiwari, N., Stephan, S., and Fanning, E. (1998) J. Biol. Chem. 273 31992-31999 [DOI] [PubMed] [Google Scholar]
- 46.Moldovan, G. L., Pfander, B., and Jentsch, S. (2007) Cell 129 665-679 [DOI] [PubMed] [Google Scholar]
- 47.Wyatt, M. D., and Pittman, D. L. (2006) Chem. Res. Toxicol. 19 1580-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stojic, L., Brun, R., and Jiricny, J. (2004) DNA Repair (Amst.) 3 1091-1101 [DOI] [PubMed] [Google Scholar]
- 49.Trojan, J., Zeuzem, S., Randolph, A., Hemmerle, C., Brieger, A., Raedle, J., Plotz, G., Jiricny, J., and Marra, G. (2002) Gastroenterology 122 211-219 [DOI] [PubMed] [Google Scholar]
- 50.Hoeijmakers, J. H. (2001) Nature 411 366-374 [DOI] [PubMed] [Google Scholar]
- 51.Krokan, H. E., Nilsen, H., Skorpen, F., Otterlei, M., and Slupphaug, G. (2000) FEBS Lett. 476 73-77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.