Abstract
The Ewing's sarcoma family of tumors (ESFT) includes Ewing's sarcoma (ES), Askin's tumor of the chest wall, and peripheral primitive neuroectodermal tumor. Basic fibroblast growth factor (FGF2) suppresses the growth of ESFT cells and causes their apoptosis. The underlying mechanism is unclear. Using a human peripheral primitive neuroectodermal tumor cell line, SK-N-MC, we demonstrated FGF2 stimulated phosphorylation of ERK1 and ERK2 (pERK1/2) and GSK3β (pGSK3β(Tyr-216)), all of which were primarily retained in the cytoplasm. FGF2 promoted the association between ERK and pGSK3β(Tyr-216). Inhibitors for GSK3β (TDZD and LiCl) and ERK (PD98059) protected cells from FGF2-induced apoptosis. On the other hand, inhibitors of GSK3β, but not PD98059 decreased ERK/pGSK3β(Tyr-216) association and caused a nuclear translocation of pERK1/2. Similarly, expression of a kinase-deficient (K85R) GSK3β or GSK3β-small interfering RNA inhibited FGF2-regulated ERK/pGSK3β(Tyr-216) association and translocated pERK to the nucleus. Both K85R GSK3β and small interfering RNA offered protection against FGF2-induced cell death. In contrast, overexpression of wild-type GSK3β sensitized cells to FGF2 cytotoxicity. Hydrogen peroxide and ethanol enhanced FGF2-stimulated pGSK3β(Tyr-216), ERK/pGSK3β(Tyr-216) association, and cytoplasmic retention of pERK1/2. As a result, they potentiated FGF2-induced cell death. Taken together, our results suggested that FGF2-induced accumulation of pERK1/2 in the cytoplasm is toxic for SK-N-MC cells. The formation of an ERK·GSK3β complex retained pERK1/2 in the cytoplasm. In contrast, disruption of the ERK·GSK3β complex resulted in nuclear translocation of pERK1/2 and offered protection.
The Ewing's sarcoma family of tumors (ESFT)3 including Ewing's sarcoma, Askin's tumor of the chest wall, and peripheral primitive neuroectodermal tumor are common bone and soft tissue tumors among children and young adults. It is generally believed that ESFT are derived from pluripotent neural crest cells (1). They are malignant tumors of childhood and adolescence (1). The outcomes of treatment of these tumors are poor; less than 20% of patients with metastatic disease are long-term survival patients (2). Therefore, development of new treatment strategies for these tumors is important.
Basic fibroblast growth factor (bFGF or FGF2) belongs to the FGF family, which consists of up to 23 members (3, 4). FGFs and their cell surface receptors (FGFR) make up a large and complex family of signaling molecules that play an important role in a variety of processes of embryonic development and tissue homeostasis, as well as pathogenesis of some morphogenetic disorders and cancers. FGF2 is ubiquitously expressed, but is most abundant in the nervous system (5). In embryonic tissues, FGF2 plays a critical role in morphogenesis by regulating cell proliferation, differentiation, and cell migration. In adult organisms, FGF2 plays an important role in the function of the nervous system, tissue repair, wound healing, and tumor angiogenesis (3, 4).
FGF2 is generally viewed as a mitogen or pro-survival factor. Dysregulation of FGF signaling has been implicated in tumorigenesis and malignant progression (6). However, the response to FGF2 depends on cell type and developmental status (7, 8). For example, FGF2 causes apoptosis in chondrocytes and breast cancer cells (9, 10). FGF2 suppresses the growth of ESFT cells by inducing apoptosis of tumor cells in vivo (2). FGF2-induced death of ESFT cells is confirmed in vitro using various ESFT cell lines (2, 11–13). However, the cellular and molecular mechanisms underlying FGF2-mediated death of ESFT cells remain unclear.
Key components of FGF2 signaling are mitogen-activated protein kinases (MAPKs) (3). In mammals, three major groups of MAPKs have been identified: extracellular signal-regulated kinases (ERKs), p38 MAPK, and c-Jun N-terminal kinase (JNK). The ERKs are stimulated by receptor tyrosine kinases and G protein-coupled receptors, and their activation generally leads to mitogenic or growth response. JNK and p38 MAPK are stimulated by cellular stresses, such as free radicals and inflammatory agents, leading to apoptotic cell death. Although ERKs have been generally known for their mitogenic and survival promoting functions, many studies indicate that ERK activation may lead to cell death (14). It appears that the subcellular localization of ERK plays an important role in determining the function of ERKs (15).
In the present study, we used human SK-N-MC cells, which were derived from soft tissue peripheral primitive neuroectodermal tumors, to investigate the mechanism of FGF2-induced apoptosis. SK-N-MC cells endogenously express FGF2 and FGF receptor (FGFR-1) (2). We demonstrate here that FGF2 induces a sustained phosphorylation of ERK1 and ERK2 (pERK1/2), whereas it has a modest effect on JNK and p38 MAPK. The FGF2-induced pERK1/2 is predominantly retained in the cytoplasm and forms a complex with GSK3β. The cytoplasmic accumulation of pERK is accountable for FGF2-induced death of SK-N-MC cells. Our findings underscore a novel mechanism by which FGF2 induces cell death.
EXPERIMENTAL PROCEDURES
Materials—Antibodies against GSK3β (catalog number 9332), Histone 3 (catalog number 9715), pERK1/2 (catalog number 9101), pJNK (catalog number 9251), poly(ADP-ribose) polymerase (catalog number 9542), and cyclin D1 (catalog number 2926) were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Anti-tubulin antibody (catalog number T6199) was purchased from Sigma. Anti-pGSK3β(Ser-9) (catalog number 44-600G) and pGSK3β(Tyr-216) antibodies (catalog number 44-604G) were obtained from Invitrogen. Anti-p-p38 antibody (catalog number 7973) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). GSK3β inhibitors (TDZD-8 and LiCl) and MEK1 inhibitor (PD98059) were purchased from Calbiochem (La Jolla, CA). Annexin-V FITC Apoptosis Detection Kit was obtained from Biovision Inc. (Mountain View, CA). Other chemicals were obtained from Sigma.
Cell Culture—Human SK-N-MC cells obtained from ATCC were grown in Eagle's minimal essential medium containing 10% fetal bovine serum, 2 mm l-glutamine and 25 μg/ml gentamicin, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C with 5% CO2.
Determination of Cell Viability and Apoptosis—Cell viability was determined by MTT assay as previously described (16). Apoptosis was quantified by the expression of Annexin-V using an Annexin-V FITC Apoptosis Detection Kit as previously described (17). Briefly, cells (1 × 106) were suspended in 500 μl of binding buffer (10 mm HEPES/NaOH, pH 7.4, 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, and 1.8 mm CaCl2) and treated with 10 μl of FITC-conjugated anti-Annexin-V antibody and 10 μl of propidium iodide for 10 min in the dark at room temperature. The samples were analyzed by flow cytometry (excitation = 488 nm; emission = 530 nm).
Preparation of Cell Lysates of Whole Cell and Cytoplasmic and Nuclear Fractions—For the lysates of whole cells, cells were washed with phosphate-buffered saline (pH 7.4) and lysed with RIPA buffer (150 mm NaCl, 50 mm Tris (pH 8.0), 1% Nonidet P-40 (Nonidet P-40), 0.1% SDS, 0.5% deoxycholic acid sodium, 0.1 mg/ml phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate and 3% aprotinin) on ice for 10 min, solubilized cells were centrifuged, the supernatant was collected and designated as the whole cell lysates. For extraction of cytoplasmic and nuclear fractions, cells were lysed in phosphate-buffered saline that contained 5 mm EDTA, 1% Nonidet P-40, 1 mm dithiothreitol (Sigma), 10 μg/ml leupeptin, and 1 mm Pephabloc SC. After 5 min incubation on ice, the lysate was centrifuged at 12,000 × g for 5 min. The supernatant was designated the cytoplasmic fraction. The pelleted nuclei were sonicated in nuclear extraction buffer (20 mm Tris-HCl, pH 7.5, 1% SDS, 5 mm EGTA, 0.5% Triton X-100, 150 mm NaCl, 1 mm dithiothreitol, 10 mg/ml leupeptin, and 1 mm Pephabloc SC) and centrifuged at 12,000 × g for 5 min. The supernatant was collected and designated the nuclear fraction.
Immunoblotting—The immunoblotting procedure has been previously described (16). Briefly, after the protein concentrations were determined, aliquots of the protein samples (30 μg) were loaded into the lanes of a SDS-polyacrylamide gel. The protein samples were separated by electrophoresis, and the separated proteins were transferred to nitrocellulose membranes. The membranes were blocked with either 5% bovine serum albumin or 5% nonfat milk in 0.01 m phosphate-buffered saline (pH 7.4) and 0.05% Tween 20 (TPBS) at room temperature for 1 h. Subsequently, the membranes were probed with primary antibodies directed against target proteins for 2 h at room temperature or overnight at 4 °C. After three quick washes in TPBS, the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences) diluted at 1:2000 in TPBS for 1 h. The immune complexes were detected by the enhanced chemiluminescence method (Amersham Biosciences). In some cases, the blots were stripped and re-probed with either an anti-tubulin or an anti-Histone H3 antibody. The density of immunoblotting was quantified with the software of Quantity One (Bio-Rad). The expression of target proteins was normalized to the levels of either tubulin or Histone H3.
Cell Transfection and Establishing Stable Transfectants—Stable transfectants expressing various GSK3β constructs were established as previously described (18). V5-tagged GSK3β constructs (wild-type and K85R) carried by vector pcDNA3 were generous gifts from Dr. Thilo Hagen (University Hospital Nottingham, Nottingham, UK). Constitutively active MEK1 plasmid (CA-MEK1) was obtained from Stratagene (pFC-MEK1, catalog number 219065). Cell transfection was carried out with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Stable cell clones expressing exogenous GSK3β were selected by treatment of G418 (600 μg/ml) for 3–4 weeks. Positive clones were verified by the expression of the V5 tag protein, as well as the evidence of GSK3β overexpression. The clones expressing the highest levels of exogenous GSK3β were selected for subsequent experiments.
GSK3β Small Interfering RNA (siRNA)—GSK3β siRNA (catalog number 6301; Cell Signaling Technology, Inc.) or Silencer Negative Control siRNA (catalog number 4611; Ambion, Inc., Austin, TX) were transfected into SK-N-MC cells with either oligofectamine reagent (Invitrogen) or Nucleofector™ (Amaxa Inc., Gaithersburg, MD) according to the manufacturer's protocol. At 48 h after transfection, the cells were subjected to immunoblotting analysis for GSK3β expression.
Statistical Analysis—Differences among treatment groups were tested using analysis of variance. Differences in which p was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests.
RESULTS
ERK1/2 Mediate FGF2-induced Death of SK-N-MC Cells— We examined the effect of FGF2, forskolin, and heregulin β1 on the viability of SK-N-MC cells. Forskolin and heregulin β1 were selected for comparison because forskolin-induced pERK1/2 was mainly localized in the nucleus (15), whereas heregulin β1 did not affect pERK1/2 (data not shown). As determined by MTT assay, FGF2 decreased the viability of SK-N-MC cells, whereas forskolin enhanced the survival of SK-N-MC cells; heregulin β1 had little effect on cell viability (Fig. 1A). FGF2-induced apoptosis of SK-N-MC cells was confirmed by Annexin-V staining and the cleavage of poly(ADP-ribose) polymerase (Fig. 1, B and C).
FIGURE 1.
FGF2-induced death of SK-N-MC cells. A, SK-N-MC cells were treated with FGF2 (10 ng/ml), heregulin β1 (30 ng/ml), or forskolin (10 μm) for 48 h. Cell viability was determined by MTT as described under “Experimental Procedures.” Each data point (±S.E.; bar) is the mean of three experiments. Asterisk denotes significant difference from controls. B, SK-N-MC cells were treated with FGF2 (10 ng/ml) for 12 h. Annexin-V-positive cells were analyzed via flow cytometry as described under “Experimental Procedures.” C, SK-N-MC cells were treated with FGF2 (10 ng/ml) for 12 h. The expression of poly(ADP-ribose) polymerase (PARP) was determined with immunoblotting.
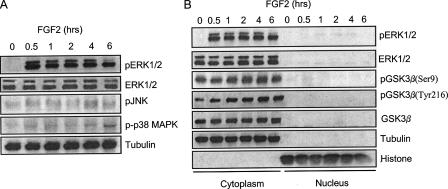
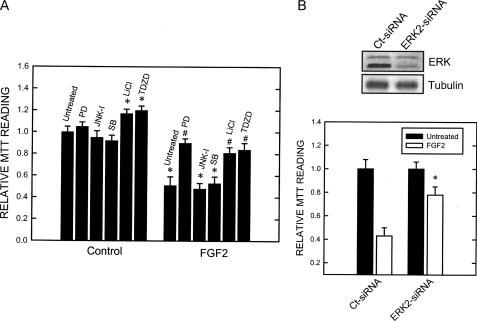
FGF2 caused a prolonged phosphorylation of ERK1 and ERK2, whereas it had a modest effect on the phosphorylation of JNK and p38 MAPK (Fig. 2A). FGF2-induced pERK1/2 persisted for at least 6 h. The majority of FGF2-induced pERK1/2 was retained in the cytoplasm (Fig. 2B). GSK3β is a substrate of ERK, and ERK phosphorylates GSK3β at serine 9 (19). Full activity of GSK3β generally requires phosphorylation at tyrosine 216, and conversely, phosphorylation at serine 9 inhibits GSK3β activity (19). We thus sought to determine whether FGF2 affected GSK3β phosphorylation. As shown in Fig. 2B, FGF2 had little effect on the phosphorylation of GSK3β at serine 9 (pGSK3β(Ser-9)), but enhanced its phosphorylation at tyrosine 216 (pGSK3β(Tyr-216)); both phosphorylated and non-phosphorylated forms of GSK3β were localized to the cytoplasm (Fig. 2B). ERK1/2 are substrates of MEK1 (14). PD98059, a selective inhibitor of MEK1, effectively blocked FGF2-induced ERK phophorylation (Fig. 5A). PD98059, but not inhibitors for p38 MAPK and JNK, protected SK-N-MC cells against FGF2-induced cell death (Fig. 3A), suggesting pERK1/2 were required for FGF2 cytotoxicity. To confirm that ERK-mediated FGF2 induced cell death, we knocked down the expression of ERK2 by an ERK2 siRNA. As shown in Fig. 3B, ERK2 siRNA significantly protected cells against FGF2 cytotoxicity. Inhibitors of GSK3β (LiCl and TDZD) also offered significant protection against FGF2-induced cell death (Fig. 3A), suggesting that GSK3β is also involved in FGF2-induced cell death. It was noted that GSK3β inhibitors by themselves promoted cell survival (Fig. 3A).
FIGURE 2.
Effect of FGF2 on the phosphorylation of MAPKs and GSK3β. A, SK-N-MC cells were maintained in serum-free medium overnight and then exposed to FGF2 (10 ng/ml) for the indicated times. Total cell lysates were collected and examined for the expression of phosphorylated ERK1/2, JNK, and p38 MAPK. B, SK-N-MC cells were maintained in serum-free medium overnight and then exposed to FGF2 (10 ng/ml) for the indicated times. The cytoplamic and nuclear proteins were collected. The expression of ERK1/2 and GSK3β as well as phosphorylated ERK1/2 (pERK1/2) and pGSK3β(Ser-9 and Tyr-216) was examined with immunoblotting. The purity of cytoplamic and nuclear protein was verified by the expression of tubulin and histone H3, respectively. The experiment was replicated three times.
FIGURE 5.
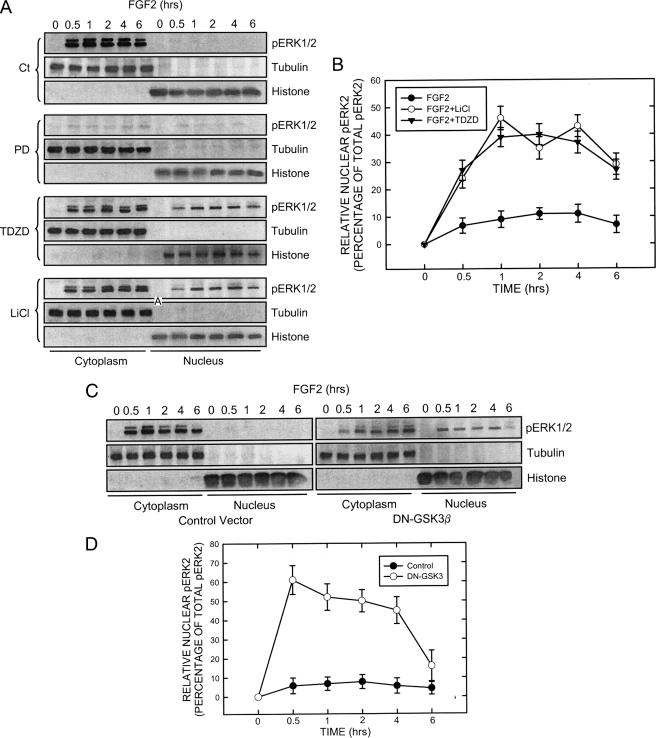
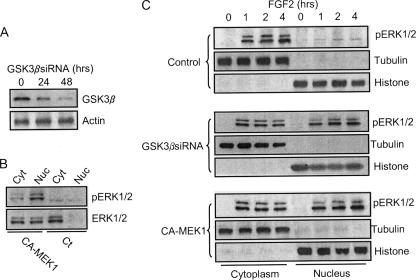
Effect of GSK3β inhibitors and dominant negative (DN) GSK3β on the distribution of pERK1/2. A, SK-N-MC cells were maintained in serum-free medium overnight and pretreated with inhibitors of MEK1 (PD98059, 50 μm) and GSK3β (LiCl, 20 mm; TDZD, 10 μm) for 30 min. After that, cells were exposed to FGF2 (10 ng/ml) for the indicated times. The expression of phosphorylated ERK (pERK1/2) in both the cytoplasm and nucleus were examined with immunoblotting. B, FGF2-induced up-regulation of pERK2 (42 kDa) was much larger than pERK1 (44 kDa). The relative amounts of pERK2 in the nucleus were measured microdensitometrically, normalized to the level of total cellular pERK2, and expressed as the percentage of total pERK. Each data point (±S.E.; bar) is the mean of three replications. C, SK-N-MC cells were transfected with either an empty vector or a dominant negative (DN) GSK3β construct (kinase-deficient mutant) for 48 h. After that, cells were treated with FGF2 (10 ng/ml) for the indicated times. Cytoplasmic and nuclear protein were collected. The expression of pERK1/2, tubulin, and histone H3 was determined by immunoblotting. D, the relative amounts of pERK2 in the nucleus were measured microdensitometrically as described above. Each data point (±S.E.; bar) is the mean of three replications.
FIGURE 3.
Effect of inhibitors of MAPKs and GSK3β as well as ERK2 siRNA on FGF2-induced death of SK-N-MC cells. A, SK-N-MC cells were pretreated with inhibitors of MEK1 (PD98059, 50 μm), JNK (JNK-I, 1 μm), p38 MAPK (SB203580, 10 μm), and GSK3β (LiCl, 20 mm; TDZD, 10 μm) for 30 min. After that, cells were exposed to FGF2 (10 ng/ml) for 48 h. Cell viability was determined by MTT as described under “Experimental Procedures.” The experiment was replicated three times. Asterisk denotes significant difference from untreated controls. Number sign denotes significant difference from the group treated with FGF2 but without inhibitors. B, SK-N-MC cells were treated with an ERK2 siRNA for 48 h. The expression of ERK1/2 was determined with immunoblotting (top panel). After transfection with ERK2 siRNA or control siRNA for 48 h, cells were treated with FGF2 (0 or 10 ng/ml) for 48 h. Cell viability was determined by MTT (bottom panel). Each data point (±S.E.; bar) is the mean of three replications. Asterisk, ERK2 siRNA-mediated protection; denotes significant difference from the control siRNA-treated group (open bar in ERK2 siRNA-treated group versus open bar in control siRNA-treated group).
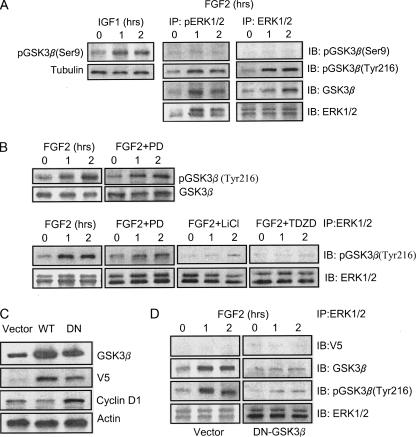
FGF2 Modulates the Interaction between ERK1/2 and GSK3β— It has been shown that pERK was retained in the cytoplasm by forming a heterotrimeric complex with other cytosolic proteins, and cytoplamic retention of pERK1/2 is cytotoxic to SK-N-MC cells (15). We demonstrated here that FGF2 induced an accumulation of pERK1/2 in the cytoplasm. We therefore sought to determine the mechanisms underlying the cytoplasmic retention of pERK1/2. Our results indicated that FGF2 promoted the association between ERK and GSK3β; the association mostly occurred between ERK and pGSK3β(Tyr-216) (Fig. 4A), suggesting that ERK1/2 preferably associated with active GSK3β. To test this hypothesis, we examined the effect of GSK3β inhibitors on ERK/pGSK3β(Tyr-216) association. As shown in Fig. 4B, LiCl and TDZD disrupted FGF2-induced ERK/GSK3β association. A similar result was obtained using another specific GSK3β inhibitor (L803) (data not shown). On the other hand, the phosphorylation status of ERK did not affect the ERK/GSK3β association because both ERK and pERK associated with pGSK3β(Tyr-216) to a similar extent; PD98059 had little effect on pGSK3β(Tyr-216) as well as ERK/pGSK3β(Tyr-216) association (Fig. 4B). To further verify the active form of GSK3β is required for the interaction, we transfected SK-N-MC cells with a kinase-deficient GSK3β (K85R) mutant. The mutant functions as a dominant negative protein and inhibits the activation of endogenous GSK3β (20). Overexpression of the K85R mutant in SK-N-MC cells was verified by the expression of V5 tag protein and high levels of GSK3β, as well as up-regulation of cyclin D1 (Fig. 4C) because GSK3β phosphorylates cyclin D1 and induces its degradation (21). In contrast, transfection of wild-type GSK3β decreased cyclin D1 expression. As shown in Fig. 4D, overexpression of the K85R GSK3β mutant inhibited FGF2-mediated ERK/GSK3β association.
FIGURE 4.
FGF2-mediated association between ERK1/2 and GSK3β. A, SK-N-MC cells were treated with FGF2 (10 ng/ml) for the indicated times. Total protein lysates were collected and immunoprecipitated (IP) with either anti-ERK1/2 or anti-pERK1/2 antibody. The immunoprecipitates were then immunoblotted (IB) for the expression of GSK3β, pGSK3β(Ser-9 and Tyr-216), and ERK. IGF1-induced pGSK3β(Ser-9) served as a positive control. The experiment was replicated three times. B, SK-N-MC cells were pretreated with inhibitors of MEK1 (PD98059, 50 μm), JNK (JNK-I, 1 μm), p38 MAPK (SB20358, 10 μm), and GSK3β (LiCl, 20 mm; TDZD, 10 μm) for 30 min. After that, cells were exposed to FGF2 (10 ng/ml) for the indicated times. Cell lysates were immunoprecipitated with an anti-ERK1/2 antibody and immunoblotted using an anti-pGSK3β(Tyr-216) antibody. The experiment was replicated three times. C, SK-N-MC cells were transfected with either an empty vector or GSK3β constructs (wild type, WT; dominant negative, DN) for 48 h. The expression of GSK3β, V5 tag protein, cyclin D1, and actin was examined with immunoblotting. D, SK-N-MC cells were transfected with either an empty vector or a dominant negative GSK3β construct for 48 h. After that, cells were treated with FGF2 (10 ng/ml) for the indicated times. Cell lysates were immunoprecipitated with an anti-ERK1/2 antibody and immunoblotted for the expression of V5 tag protein, GSK3β, pGSK3β(Tyr-216), and ERK1/2. The expression was replicated three times.
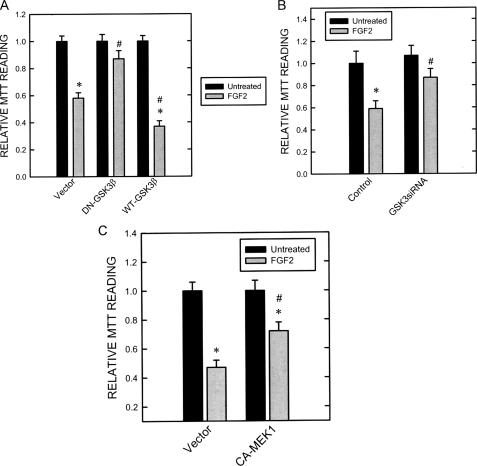
Because inhibitors of GSK3β disrupted ERK/pGSK3β(Tyr-216) association, we sought to determine their effect on the distribution of pERK1/2. As shown in Fig. 5, A and B, inhibitors of GSK3β significantly enhanced the nuclear localization of pERK1/2. The K85R GSK3β mutant failed to associate with ERK. Like GSK3β inhibitors, overexpression of this mutant greatly enhanced pERK1/2 translocation to the nucleus (Fig. 5, C and D). The role of GSK3β in the regulation of pERK1/2 distribution was further confirmed by knocking down GSK3β using a specific GSK3β siRNA. As shown in Fig. 6A, GSK3β siRNA effectively knocked down the expression of GSK3β. Like GSK3β inhibitors and the K85R GSK3β mutant, GSK3β siRNA enhanced pERK1/2 translocation to the nucleus (Fig. 6C). Consistent with the protective effect of GSK3β inhibitors (Fig. 3), overexpression of the K85R GSK3β mutant (Fig. 7A) and GSK3β siRNA (Fig. 7B) also significantly ameliorated FGF2-mediated cell death. Contrarily, overexpression of wild-type GSK3β sensitized SK-N-MC cells to FGF2 cytotoxicity (Fig. 7A). To further demonstrate that cytoplasmic pERK1/2 were cytotoxic for SK-N-MC cells, we transfected cells with a CA-MEK1. Because MEK1 had little effect on pGSK3β(Tyr-216) and bypassed FGF2-initiated signaling, CA-MEK1 expression resulted in pERK localization in the nucleus (Fig. 6, B and C). It was noted that CA-MEK1 only induced weak pERK expression (Fig. 6B). On the other hand, CA-MEK1 did increase pGSK3β(Ser-9), which resulted in reduced association between GSK and ERK (data not shown); this likely caused pERK nuclear translocation. As shown in Fig. 7C, FGF2 cytotoxicity was significantly diminished in cells expressing CA-MEK1. Taken together, FGF2-induced formation of the ERK·GSK3β complex caused a cytoplasmic retention of pERK, which was cytotoxic to SK-N-MC cells.
FIGURE 6.
Effect of GSK3β siRNA on the distribution of pERK. A, SK-N-MC cells were treated with a GSK3β siRNA for 24 or 48 h as described under “Experimental Procedures.” The expression of GSK3β was determined with immunoblotting. B, SK-N-MC cells were transfected with CA-MEK1 for 48 h. The expression of cytoplasmic (Cyt) and nuclear (Nuc) pERK1/2 and ERK1/2 was determined with immunoblotting. C, after being treated with GSK3β siRNA, control siRNA, or CA-MEK1 for 48 h, SK-N-MC cells were exposed to FGF2 (10 ng/ml) for the indicated times. The expression of cytoplasmic and nuclear pERK1/2 was determined as described above. The experiment was replicated three times.
FIGURE 7.
Effect of dominant negative (DN), wild-type (WT) GSK3β, GSK3β siRNA, and CA-MEK1 on FGF2-induced cell death. A, SK-N-MC cells stably expressing dominant negative or wild-type GSK3β constructs were established as described under “Experimental Procedures.” These cells were treated with FGF2 (0 or 10 ng/ml) for 48 h. Cell viability was determined by MTT as described under “Experimental Procedures.” Each data point (±S.E.; bar) is the mean of three experiments. Asterisk, FGF2-induced death; denotes significant difference from untreated controls (black bar versus gray bar within group). #, DN-GSK3β-mediated protection; denotes significant difference from FGF2-exposed cultures in the empty vector-treated group (gray bar in DN-GSK3β-treated group versus gray bar in empty vector-treated group). B, after transfection with GSK3β siRNA or control siRNA for 48 h, cells were treated with FGF2 (0 or 10 ng/ml) for 48 h. Cell viability was determined by MTT. C, after transfection with CA-MEK1 or empty vector for 48 h, cells were treated with FGF2 (0 or 10 ng/ml) for 48 h. Cell viability was determined by MTT. Each data point (±S.E.; bar)is the mean of three experiments. Asterisk, FGF2-induced cell death (black bar versus gray bar within group). #, GSK3β siRNA or CA-MEK1 mediate protection (gray bars in GSK3β siRNA- or CA-MEK1-treated group versus gray bars in empty vector-treated group).
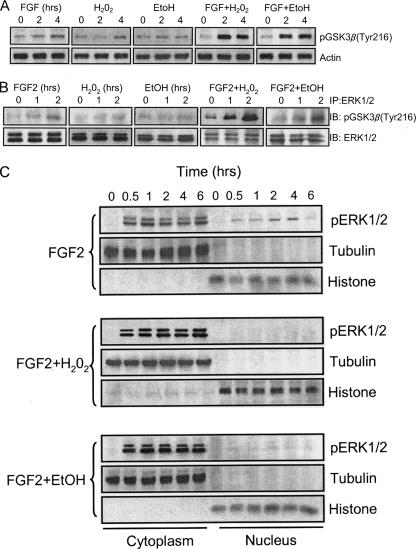
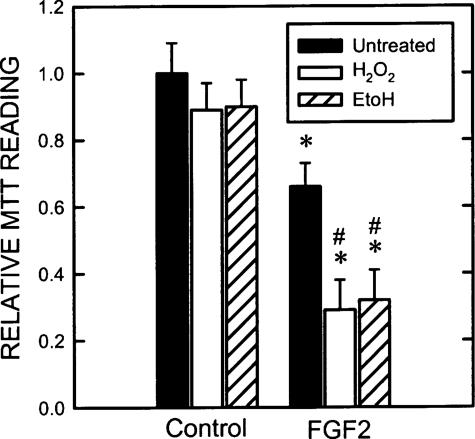
Hydrogen Peroxide and Ethanol Potentiate FGF2-induced Cell Death by Modulating ERK/GSK3β Interaction—To further establish the role of cytoplamic pERK1/2 in the regulation of cell death, we investigated the effect of hydrogen peroxide on ERK/GSK3β association and subcellular distribution of pERK because oxidative stress-induced cell death may be mediated by ERK activation (14). We sought to determine whether oxidative stress may promote the death of SK-N-MC cells by retaining pERK in the cytoplasm. Both hydrogen peroxide and ethanol cause cellular oxidative stress. We treated SK-N-MC cells with FGF2 with/without hydrogen peroxide or ethanol and examined the expression of pGSK3β(Tyr-216), formation of ERK/pGSK3β(Tyr-216) complex, and subcellular distribution of pERK1/2. As shown in Fig. 8A, although hydrogen peroxide or ethanol alone had a modest or little effect on pGSK3β(Tyr-216), they greatly enhanced FGF2-stimulated pGSK3β(Tyr-216). Hydrogen peroxide and ethanol also drastically promoted FGF2-induced ERK/pGSK3β(Tyr-216) association (Fig. 8B). Hydrogen peroxide and ethanol alone did not cause ERK1/2 phosphorylation and alteration in their localization (data not shown). Consistent with our prediction, hydrogen peroxide and ethanol increased FGF2-mediated pERK1/2 in the cytoplasm while decreasing their amount in the nucleus, indicating a promotion of cytosolic retention of pERK1/2 (Fig. 8C). As a result, hydrogen peroxide and ethanol potentiated FGF2 cytotoxicity (Fig. 9). Taken together, these results suggested that promotion of the ERK/pGSK3β(Tyr-216) complex by hydrogen peroxide and ethanol enhanced cytoplamic retention of pERK1/2 and subsequently potentiated FGF2-induced cell death.
FIGURE 8.
Effect of hydrogen peroxide and ethanol on FGF2-mediated ERK/GSK3β interaction and pERK1/2 distribution. A, SK-N-MC cells were treated with FGF2 (10 ng/ml) with/without hydrogen peroxide (50 μm) or ethanol (400 mg/dl) for the indicated times. The expression of pGSK3β(Tyr-216) was examined with immunoblotting as described above. B, SK-N-MC cells were treated with FGF2 (10 ng/ml) with/without hydrogen peroxide (50 μm) or ethanol (400 mg/dl) for the indicated times. Total protein lysates were immuoprecipitated (IP) with anti-ERK1/2 antibody and immunoblotted (IB) for the expression of pGSK3β(Tyr-216) and ERK1/2. The experiment was replicated three times. C, SK-N-MC cells were treated with FGF2 (10 ng/ml) with/without hydrogen peroxide (50 μm) or ethanol (400 mg/dl) for the indicated times. The distribution of pERK1/2 was determined as described above. The experiment was replicated three times.
FIGURE 9.
Effect of hydrogen peroxide and ethanol on FGF2-mediated death. SK-N-MC cells were treated with FGF2 (10 ng/ml) with/without hydrogen peroxide (50 μm) or ethanol (400 mg/dl) for 48 h. Cell viability was determined by MTT as described under “Experimental Procedures.” The experiment was replicated three times. Asterisk, FGF2-induced cell death; denotes significant difference from untreated control (black bar versus black bar). #, the potentiation of hydrogen peroxide or ethanol on cell death; denotes significant difference from the group treated with FGF2 but without hydrogen peroxide and ethanol.
DISCUSSION
This is the first report to show that ERK mediates FGF2-induced death of SK-N-MC cells. We demonstrate that FGF2 causes phosphorylation of ERK1/2 and the phosphorylated ERK1/2 are primarily retained in the cytoplasm. Accumulation of cytosolic pERK1/2 is cytotoxic for SK-N-MC cells.
ERK1/2 belong to a family of MAPKs that is comprised of ERKs, c-Jun N-terminal kinase/stress-activated protein kinases, and p38 kinases. ERK1/2 are the most extensively studied ERKs and are regulated by the dual-specificity kinase MEK1 and MEK2. ERK1/2 are generally stimulated by receptor tyrosine kinases and G protein-coupled receptors; they are also activated in response to various stress stimuli through divergent mechanisms involving the Ras-Raf-MEK pathway (14). Depending upon the cell type, the stimulus and the duration of activation, a variety of biological responses, i.e. cell proliferation, survival, migration, differentiation or cell death, are associated with ERK1/2 activation (14).
In resting cells, ERK1/2 are mainly kept in the cytoplasm by the microtubule cytoskeleton, which serves as a major docking matrix (22). Other putative cytosol-anchoring proteins of ERKs include MAP kinase phosphatases (23, 24). ERKs are also retained in the cytosol by their association with MEK1/2 (25). After phosphorylation by MEK1/2, pERK1/2 are detached from the cytosolic anchors and rapidly translocated into the nucleus (26). Once in the nucleus, pERK1/2 phosphorylate various substrates, such as transcription factors, thereby transmitting signals received by cell surface receptors to the nucleus. The translocation of pERK1/2 from the cytoplasm to the nucleus is believed to be mandatory for efficient regulation of cell survival and proliferation (23, 27). However, pERK1/2 may be abnormally retained in the cytoplasm, resulting in low transcription activity, lack of a mitogenic response, or cytotoxicity (15, 28–31). The cytoplamic retention of pERK1/2 may be caused by forming a complex with other cytosolic proteins. For example, it is demonstrated that pERK1/2 form stable heterotrimetric complexes with the G protein-coupled receptors and β-arrestin2, which are retained in the cytoplasm (15, 28–31).
GSK3β is a multifunctional serine/threonine kinase. The activation of GSK3β is regulated by site-specific phosphorylation. Full activity of GSK3β generally requires phosphorylation at Tyr-216, and conversely, phosphorylation at Ser-9 inhibits GSK3β activity (19). GSK3β directly interacts with ERK1/2 (32). In the hepatoma cell line, ERK/GSK3β association “primes” GSK3β for its subsequent phosphorylation at Ser-9 by p90RSK (33). In human colon cancer cell lines, ERK/GSK3β association inhibits ERK activity in a protein kinase C-dependent manner (34). We demonstrate here that ERK1/2 preferably associate with pGSK3β(Tyr-216) in SK-N-MC cells and inhibitors of GSK3β disrupt the association, suggesting that an active GSK3β is required for the association. This argument is further supported by the result showing that a kinase-deficient GSK3β fails to associate with ERK1/2. FGF2-stimulated ERK/GSK3β association in SK-N-MC cells is likely mediated by its effect on pGSK3β(Tyr-216) because FGF2 does not stimulate GSK3β phosphorylation at Ser-9, but causes its phosphorylation at Tyr-216 (Fig. 2B). Compared with pGSK3β(Ser-9), regulation of GSK3β activity by pGSK3β(Tyr-216) is less common. Stimulation of pGSK3β(Tyr-216) can be mediated by alterations in intracellular calcium levels or by Fyn, a member of the Src tyrosine family (35, 36). Recent evidence indicates that pGSK3β(Tyr-216) is regulated by MEK1/2 (37). FGF2 is shown to regulate intracellular calcium levels and activate Src family kinases including Fyn (38). FGF2 likely activates MEK1/2 in SK-N-MC cells because FGF2-induced pERK1/2 is blocked by PD98059, a MEK1 inhibitor. However, MEK1/2 seem minimally involved in FGF2 regulation of pGSK3β(Tyr-216) in SK-N-MC cells because PD98059 has little effect on FGF2-stimulated pGSK3β-(Tyr-216) expression.
It appears that ERK1/2·GSK3β association is important for cytoplasmic retention of pERK1/2 because disruption of the ERK1/2·GSK3β complex by specific GSK3β inhibitors, dominant negative protein, or down-regulation of GSK3β by siRNA promotes nuclear translocation of pERK1/2. In contrast, agents that promote ERK/GSK3β association, such as hydrogen peroxide and ethanol, retain pERK1/2 in the cytoplasm. Cytoplasmic accumulation of pERK1/2 is toxic for SK-N-MC cells. This is supported by the results showing that forced nuclear localization of pERK1/2 by inhibition of GSK3β or expression of a CA-MEK1 offer protection against FGF2 cytotoxicity. ERK1/2 are believed to mediate oxidative stress-induced apoptosis (14). Oxidative stress can cause Fyn activation, phosphorylation of GSK3β at Tyr-216, and disruption of intracellular calcium levels (39–41). Hydrogen peroxide and ethanol are oxidative stress inducers, and they enhance both FGF2-stimulated pGSK3β(Tyr-216) and ERK/GSK3β association. As predicted, they potentiate FGF2-induced cytoplamic retention of pERK1/2 and subsequent cell death. The result further supports the theory that association of ERK1/2 with active GSK3β results in cytosolic retention of ERK1/2 and promotes cell death. A recent study shows that hydrogen peroxide alone can induce pGSK3β(Tyr-216) in human Hepa-1 cells (40). Our results indicate that hydrogen peroxide only produces a modest increase in the pGSK3β(Tyr-216) level, but drastically potentiated FGF2-induced pGSK3β(Tyr-216) in SK-N-MC cells (Fig. 8). The discrepancy may be due to the use of different model systems.
FGF2 and oxidative stress initiate different signal pathways. pGSK3β(Tyr-216) appears to be a converging point for FGF2 and oxidative stress-mediated signaling, and therefore mediates the interaction between FGF2 and oxidative stress. Oxidative stress potentiates the effect of FGF2 by enhancing pGSK3β(Tyr-216) expression. It is currently unclear why active GSK3β is required for ERK/GSK3β interaction. It cannot be excluded that other cytoplasmic proteins may be part of the complex; an active GSK3β may be necessary for recruiting these proteins for the complex formation. The investigation of this mechanism is currently underway. Our finding that ERK/GSK3β association causes cytosolic retention of pERK1/2 provides additional insight into the regulation of the function of ERK. Similar to FGF2, dopamine or agonists for dopamine receptor cause ERK1/2-dependent apoptosis of SK-N-MC cells (15). The majority of pERK1/2 stimulated by dopamine or dopamine receptor agonist is retained in the cytoplasm and forms a stable heterotrimeric complex with D1 dopamine receptor and β-arrestin2. In contrast, forskolin, which induces pERK1/2 localization in the nucleus, enhances survival of SK-N-MC cells (15) (Fig. 1).
The mechanisms of cytosolic pERK1/2-induced cell death are unclear. If abnormally retained in the cytoplasm, pERKs may phosphorylate multiple proteins in plasma membranes as well as cytoplasmic and cytoskeletal substrates (42), resulting in inappropriate cellular events. For example, cytosolic ERK may directly target mitochondria and interfere with mitochondial functions that cause the release of cytochrome c and subsequent caspase activation and apoptosis (43–45). Cytosolic pERK1/2 may suppress survival signaling, such as the phosphatidylinositol 3-kinase/AKT pathway, and promote cell death (46). The significance of cytosolic retention of pERKs is underscored by findings in postmortem tissues of neurodegenerative diseases where neuronal inclusion bodies were found to contain substantially high levels of aggregated pERK1/2. pERK1/2 were found to be localized in the cytoplasm and not in the nucleus of degenerating neurons of Parkinson disease, Alzheimer disease, and Pick disease (47–49). GSK3β is believed to play an important role during neurodegeneration (32). Our study, which shows that ERK/GSK3β association causes cytosolic retention of pERK, provides new insight into mechanisms of GSK3β-mediated neurotoxicity.
This work was supported in part by National Institutes of Health Grant AA015407, National Natural Science Foundation of China Grants 30470544, 30471452, and 30570580, and Ministry of Science and Technology of China Grant 2007CB947100. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ESFT, Ewing's sarcoma family of tumors; ERK1/2, extracellular signal-regulated kinases 1 and 2; bFGF2, basic fibroblast growth factor; GSK3β, glycogen synthase kinase 3β; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK1/2, MAPK kinase 1 and 2; p38 MAPK, 38-kDa mitogen-activated protein kinase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; FITC, fluorescein isothiocyanate; CA-MEK1, constitutively active MEK1; siRNA, small interfering RNA.
References
- 1.Scurr, M., and Judson, I. (2006) Oncologist 11 65-72 [DOI] [PubMed] [Google Scholar]
- 2.Sturla, L. M., Westwood, G., Selby, P. J., Lewis, I. J., and Burchill, S. A. (2000) Cancer Res. 60 6160-6170 [PubMed] [Google Scholar]
- 3.Eswarakumar, V. P., Lax, I., and Schlessinger, J. (2005) Cytokine Growth Factor Rev. 16 139-149 [DOI] [PubMed] [Google Scholar]
- 4.Grothe, C., and Nikkhah, G. (2001) Anat. Embryol. (Berl.) 204 171-177 [DOI] [PubMed] [Google Scholar]
- 5.Ledoux, D., Gannoun-Zaki, L., and Barritault, D. (1992) Prog. Growth Factor Res. 4 107-120 [DOI] [PubMed] [Google Scholar]
- 6.Grose, R., and Dickson, C. (2005) Cytokine Growth Factor Rev. 16 179-186 [DOI] [PubMed] [Google Scholar]
- 7.Burchill, S. A., and Westwood, G. (2002) Apoptosis 7 5-12 [DOI] [PubMed] [Google Scholar]
- 8.Dailey, L., Ambrosetti, D., Mansukhani, A., and Basilico, C. (2005) Cytokine Growth Factor Rev. 16 233-247 [DOI] [PubMed] [Google Scholar]
- 9.Maloof, P., Wang, Q., Wang, H., Stein, D., Denny, T. N., Yahalom, J., Fenig, E., and Wieder, R. (1999) Breast Cancer Res. Treat. 56 153-167 [DOI] [PubMed] [Google Scholar]
- 10.Sahni, M., Raz, R., Coffin, J. D., Levy, D., and Basilico, C. (2001) Development 128 2119-2129 [DOI] [PubMed] [Google Scholar]
- 11.Kim, M. S., Kim, C. J., Jung, H. S., Seo, M. R., Juhnn, Y. S., Shin, H. Y., Ahn, H. S., Thiele, C. J., and Chi, J. G. (2004) J. Pathol. 202 103-112 [DOI] [PubMed] [Google Scholar]
- 12.Russo, V. C., Andaloro, E., Fornaro, S. A., Najdovska, S., Newgreen, D. F., Bach, L. A., and Werther, G. A. (2004) J. Cell. Physiol. 199 371-380 [DOI] [PubMed] [Google Scholar]
- 13.Williamson, A. J., Dibling, B. C., Boyne, J. R., Selby, P., and Burchill, S. A. (2001) J. Biol. Chem. 279 47912-47928 [DOI] [PubMed] [Google Scholar]
- 14.Zhuang, S., and Schnellmann, R. G. (2006) J. Pharmacol. Exp. Ther. 319 991-997 [DOI] [PubMed] [Google Scholar]
- 15.Chen, J., Rusnak, M., Luedtke, R. R., and Sidhu, A. (2004) J. Biol. Chem. 279 39317-39330 [DOI] [PubMed] [Google Scholar]
- 16.Chen, G., Bower, K. A., Ma, C., Fang, S., Thiele, C. J., and Luo, J. (2004) FASEB J. 18 1162-1164 [DOI] [PubMed] [Google Scholar]
- 17.Cai, G., Wang, J., Xin, X., Ke, Z., and Luo, J. (2007) Int. J. Oncol. 31 657-662 [PubMed] [Google Scholar]
- 18.Chen, G., Ma, C., Bower, K. A., Ke, Z., and Luo, J. (2006) J. Biol. Chem. 281 15909-15915 [DOI] [PubMed] [Google Scholar]
- 19.Doble, B. W., and Woodgett, J. R. (2003) J. Cell Sci. 116 1175-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma, C., Wang, J., Gao, Y., Gao, T., Chen, G., Bower, K., Odetallah, M., Ding, M., Ke, Z. J., and Luo, J. (2007) Cancer Res. 67 7756-7764 [DOI] [PubMed] [Google Scholar]
- 21.Germain, D., Russell, A., Thompson, A., and Hendley, J. (2000) J. Biol. Chem. 275 12074-12079 [DOI] [PubMed] [Google Scholar]
- 22.Reszka, A. A., Seger, R., Diltz, C. D., Krebs, E. G., and Fischer, E. H. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 8881-8885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet, A., Roux, D., Lenormand, P., Dowd, S., Keyse, S., and Pouysségur, J. (1999) EMBO J. 18 664-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camps, M., Nichols, A., Gillieron, C., Antonsson, B., Muda, M., Chabert, C., Boschert, U., and Arkinstall, S. (1998) Science 280 1262-1265 [DOI] [PubMed] [Google Scholar]
- 25.Fukuda, M., Gotoh, Y., and Nishida, E. (1997) EMBO J. 16 1901-1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, R. H., Sarnecki, C., and Blenis, J. (1992) Mol. Cell. Biol. 12 915-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochholdinger, F., Baier, G., Nogalo, A., Bauer, B., Grunicke, H. H., and Uberall, F. (1999) Mol. Cell. Biol. 19 8052-8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFea, K. A., Zalevsky, J., Thoma, M. S., Déry, O., Mullins, R. D., and Bunnett, N. W. (2000) J. Cell Biol. 148 1267-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, W. E., and Lefkowitz, R. J. (2001) Curr. Opin. Cell Biol. 13 139-145 [DOI] [PubMed] [Google Scholar]
- 30.Shenoy, S. K., and Lefkowitz, R. J. (2003) J. Biol. Chem. 278 14498-14506 [DOI] [PubMed] [Google Scholar]
- 31.Tohgo, A., Choy, E. W., Gesty-Palmer, D., Pierce, K. L., Laporte, S., Oakley, R. H., Caron, M. G., Lefkowitz, R. J., and Luttrell, L. M. (2003) J. Biol. Chem. 278 6258-6267 [DOI] [PubMed] [Google Scholar]
- 32.Jope, R. S., and Johnson, G. V. (2004) Trends Biochem. Sci. 29 95-102 [DOI] [PubMed] [Google Scholar]
- 33.Ding, Q., Xia, W., Liu, J. C., Yang, J. Y., Lee, D. F., Xia, J., Bartholomeusz, G., Li, Y., Pan, Y., Li, Z., Bargou, R. C., Qin, J., Lai, C. C., Tsai, F. J., Tsai, C. H., and Hung, M. C. (2005) Mol. Cell. 19 159-170 [DOI] [PubMed] [Google Scholar]
- 34.Wang, Q., Zhou, Y., Wang, X., and Evers, B. M. (2006) Oncogene 25 43-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartigan, J. A., and Johnson, G. V. (1999) J. Biol. Chem. 274 21395-21401 [DOI] [PubMed] [Google Scholar]
- 36.Lesort, M., Jope, R. S., and Johnson, G. V. (1999) J. Neurochem. 72 576-584 [DOI] [PubMed] [Google Scholar]
- 37.Takahashi-Yanaga, F., Shiraishi, F., Hirata, M., Miwa, Y., Morimoto, S., and Sasaguri, T. (2004) Biochem. Biophys. Res. Commun. 316 411-415 [DOI] [PubMed] [Google Scholar]
- 38.Landgren, E., Blume-Jensen, P., Courtneidge, S. A., and Claesson-Welsh, L. (1995) Oncogene 10 2027-2035 [PubMed] [Google Scholar]
- 39.Abe, J., Okuda, M., Huang, Q., Yoshizumi, M., and Berk, B. C. (2000) J. Biol. Chem. 275 1739-1748 [DOI] [PubMed] [Google Scholar]
- 40.Jain, A. K., and Jaiswal, A. K. (2007) J. Biol. Chem. 282 16502-16510 [DOI] [PubMed] [Google Scholar]
- 41.Valencia, A., and Moran, J. (2004) Free Radic. Biol. Med. 36 1112-1125 [DOI] [PubMed] [Google Scholar]
- 42.Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., and Cobb, M. H. (2001) Endocr. Rev. 22 153-183 [DOI] [PubMed] [Google Scholar]
- 43.Kim, Y. K., Kim, H. J., Kwon, C. H., Kim, J. H., Woo, J. S., Jung, J. S., and Kim, J. M. (2005) J. Appl. Toxicol. 25 374-382 [DOI] [PubMed] [Google Scholar]
- 44.Nowak, G. (2002) J. Biol. Chem. 277 43377-43388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X., Martindale, J. L., and Holbrook, N. J. (2000) J. Biol. Chem. 275 39435-39443 [DOI] [PubMed] [Google Scholar]
- 46.Sinha, D., Bannergee, S., Schwartz, J. H., Lieberthal, W., and Levine, J. S. (2004) J. Biol. Chem. 279 10962-10972 [DOI] [PubMed] [Google Scholar]
- 47.Ferrer, I., Blanco, R., Carmona, M., and Puig, B. (2001) J. Neural Transm. 108 1397-1415 [DOI] [PubMed] [Google Scholar]
- 48.Ferrer, I., Blanco, R., Carmona, M., Ribera, R., Goutan, E., Puig, B., Rey, M. J., Cardozo, A., Vinals, F., and Ribalta, T. (2001) Brain Pathol. 11 144-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, J. H., Kulich, S. M., Oury, T. D., and Chu, C. T. (2002) Am. J. Pathol. 161 2087-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]