Abstract
Insulin increases glucose transport by stimulating the trafficking of intracellular GLUT4 to the cell surface, a process known as GLUT4 translocation. A key protein in signaling this process is AS160, a Rab GTPase-activating protein (GAP) whose activity appears to be suppressed by Akt phosphorylation. Tbc1d1 is a Rab GAP with a sequence highly similar to that of AS160 and with the same Rab specificity as that of AS160. The role of Tbc1d1 in regulating GLUT4 trafficking has been unclear. Our previous study showed that overexpressed Tbc1d1 inhibited insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes, even though insulin caused phosphorylation on its single canonical Akt motif. In the present study, we show in 3T3-L1 adipocytes that Tbc1d1 is only 1/20 as abundant as AS160, that knockdown of Tbc1d1 has no effect on insulin-stimulated GLUT4 translocation, and that overexpressed Tbc1d1 also inhibits GLUT4 translocation elicited by activated Akt expression. These results indicate that endogenous Tbc1d1 does not participate in insulin-regulated GLUT4 translocation in adipocytes and suggest that the GAP activity of Tbc1d1 is not suppressed by Akt phosphorylation. In addition, we discovered that Tbc1d1 is much more highly expressed in skeletal muscle than fat and that the AMP-activated protein kinase (AMPK) activator 5′-aminoimidazole-4-carboxamide ribonucleoside partially reversed the inhibition of insulin-stimulated GLUT4 translocation by overexpressed Tbc1d1 in 3T3-L1 adipocytes. 5′-Aminoimidazole-4-carboxamide ribonucleoside activation of the kinase AMPK is known to cause GLUT4 translocation in muscle. The above findings strongly suggest that Tbc1d1 is a component in the signal transduction pathway leading to AMPK-stimulated GLUT4 translocation in muscle.
Insulin rapidly stimulates glucose transport into adipose tissue, skeletal muscle, and heart. The basis for this effect is a rapid increase in the amount of the glucose transporter GLUT4 in the plasma membrane. These cell types contain specialized intracellular vesicles enriched in GLUT4. Insulin stimulates the movement of the GLUT4 vesicles to the plasma membrane and their fusion with it (1). This process is known as insulin-stimulated GLUT4 translocation. A key signal transduction pathway for GLUT4 translocation proceeds from the insulin receptor to activation of the protein kinase Akt (1). One way in which Akt controls GLUT4 translocation is through phosphorylation of the Rab GAP3 AS160 (also known as Tbc1d4). Current evidence supports the following scheme (2–6). In the absence of insulin AS160 keeps Rab10 and possibly other Rabs in their inactive GDP form; insulin treatment causes Akt phosphorylation of AS160; phosphorylation suppresses its GAP activity and thereby results in an elevation of the active GTP form of Rab10 and possibly other Rabs; the GTP form of the Rab(s) triggers the machinery for the docking of GLUT4 vesicles to the plasma membrane, which then leads to the fusion with the membrane.
We recently partially characterized a Rab GAP known as Tbc1d1 that is highly related to AS160 (7). Tbc1d1 and AS160 are 47% identical over their entire length and 79% identical in their GAP domains. We showed that Tbc1d1 and AS160 have the same Rab substrate specificities; notably Tbc1d1 also acts on Rab10. We found that similarly to AS160, Tbc1d1 can regulate insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. However, there is a major difference between the two GAPs. Overexpression of Tbc1d1 markedly inhibits GLUT4 translocation, whereas overexpression of AS160 does not. This finding suggested that unlike AS160, the Tbc1d1 GAP activity might not be suppressed by Akt phosphorylation of Tbc1d1. Tbc1d1 contains one canonical Akt phosphorylation motif (RXRXX(S/T)), which we established is phosphorylated in response to insulin (7). In contrast, AS160 contains five canonical Akt motifs that increase in phosphorylation in response to insulin (2).
The present study was undertaken to characterize Tbc1d1 further. We present evidence that although Tbc1d1 is expressed in 3T3-L1 adipocytes, the endogenous protein is unlikely to participate significantly in insulin-stimulated GLUT4 translocation and that Akt phosphorylation is unlikely to regulate ectopic Tbc1d1. In the course of this study we discovered that Tbc1d1 is highly expressed in skeletal muscle, with little or no expression in adipose tissue and heart. In skeletal muscle, contraction and activation of AMPK, as well as insulin, stimulate GLUT4 translocation (1, 8, 9). Hence, this discovery raised the possibility that Tbc1d1 participates in the regulation of GLUT4 translocation in response to contraction and/or AMPK activation. In support of this possibility we found that the inhibitory effect of overexpressed Tbc1d1 on insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes is partially relieved by AICAR, an agent that activates AMPK.
EXPERIMENTAL PROCEDURES
Plasmids—The cDNA encoding the large splice variant of mouse Tbc1d1 (gi37538012) was obtained from the Kazusa Foundation. The cDNA sequence was amplified by PCR and cloned into the NotI site of 3× FLAG-CMV-7.1 expression vector, which introduces a 3× FLAG tag at the amino terminus. Plasmids for expression of two GST fusion proteins with portions of mouse Tbc1d1 were generated by PCR amplification of regions encoding amino acids 2–269 (designated NT) and amino acids 443–627 (designated PG) and ligation into the BamHI/NotI sites of pGEX-5X-3. The plasmids for expression of 3× FLAG-tagged short splice variant of human Tbc1d1 (gi 54887445) and the T642A mutant were those described in Ref. 7; the S237A mutant was generated from the wild-type form as described in Ref. 7. The plasmids for expression of human 3× FLAG-tagged AS160 (gi 114688046) and HA-GLUT4-GFP were those described previously (2). The plasmid for expression of myrAkt1 with a carboxyl-terminal Myc tag (catalog number 17-253) was purchased from Millipore (Temecula, CA).
Antibodies—Affinity-purified antibodies against the NT and PG portions of the NT and PG GST fusion proteins of mouse Tbc1d1 were prepared as follows. The GST fusion proteins were expressed in Escherichia coli and purified on glutathione-agarose. Rabbits were immunized with the fusion proteins. The antibodies were affinity-purified from the immune serum on the immobilized GST fusion protein, as described in Ref. 10. Antibodies against the GST portion were then removed by adsorption on immobilized GST. Unless stated otherwise, the PG antibody was used for immunoprecipitation and immunoblotting of Tbc1d1. Affinity-purified rabbit polyclonal antibody against a peptide of amino acids 876–891 of mouse AS160, which sequence is identical in human AS160, was prepared as in Ref. 11. Affinity-purified rabbit antibody against a GST fusion protein with a portion of mouse AS160 (gi 67462068) encompassing amino acids 600–841, but without amino acids 685–747 from the alternately spliced exon within this region, was the one described previously (4). Unless stated otherwise, this antibody was used for immunoblotting AS160. An affinity-purified antibody against phosphoserine 237 of Tbc1d1 was prepared by 21st Century Biochemicals (Marlboro, MA) through immunization of rabbits with the phosphopeptide N-acetyl-PMRKSF-pSQPGLRSLC-amide. Antibodies were purchased from the following sources (catalog numbers in parentheses): horseradish peroxidase-conjugated goat α-rabbit immunoglobulin (170-6515) from Bio-Rad; Cy3-conjugated goat α-mouse immunoglobulin (5-165-146) from Jackson ImmunoResearch (West Grove, PA); anti-HA tag (MMS-101P) from Covance (Berkeley, CA); horseradish peroxidase-conjugated mouse anti-FLAG (A8592) from Sigma; anti-Myc tag (06-549) from Millipore (Temecula, CA); anti-phospho-Akt substrate motif (PAS antibody) (9611) and antibodies against AMPK (2532), phospho-Thr172 on AMPK (2531), eEF2 (2332), and phospho-Thr56 on eEF2 (2331) from Cell Signaling Technology (Danvers, MA).
Cell Culture and Assay of Cell Surface GLUT4—3T3-L1 fibroblasts were maintained in culture and differentiated into adipocytes as described in Ref. 12. The relative amount of GLUT4 at the cell surface was measured by the quantitative single-cell fluorescence assay that employs the reporter construct of GLUT4 with an HA tag in the amino-terminal extracellular loop and GFP fused to the carboxyl terminus (HA-GLUT4-GFP). This method is described in detail in Ref. 2. A brief description is as follows. Adipocytes on day 4 of differentiation were transfected by electroporation with the plasmid for HA-GLUT4-GFP together with the plasmids for Tbc1d1, myrAkt, and/or vector controls. For all the transfection experiments herein, the 3× FLAG-tagged human Tbc1d1 was used. After 24 h the cells were treated with insulin, AICAR, and/or both as described in the figure legends. Insulin treatment was always for 30 min with 160 nm insulin. Subsequently cells were fixed with formaldehyde or made into SDS samples. The fixed cells were labeled with anti-HA and then with Cy3-conjugated secondary antibody. The Cy3 and GFP fluorescence intensities of individual cells were quantitated. After correction for background fluorescence, the Cy3 to GFP ratio was calculated. This ratio is a measure of the relative amount of HA-GLUT4-GFP at the cell surface normalized to the level of GLUT4 expression for that cell. For each condition the ratio in ∼50 cells was measured. In our earlier experiments (see Figs. 2 and 3) insulin caused approximately a 5-fold increase in GLUT4 at the cell surface, whereas in our later ones (see Fig. 5) the effect was ∼10-fold. The difference was traced to the use of different lots of 3T3-L1 cells from the American Type Culture Collection.
FIGURE 2.
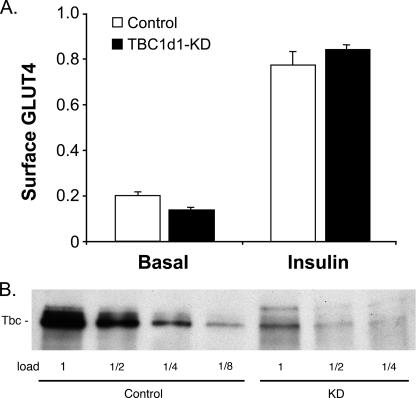
Effect of Tbc1d1 knockdown on cell surface GLUT4. A, the relative amount of HA-GLUT4-GFP at the cell surface in basal and insulin-stimulated adipocytes at day 5 of differentiation expressing control shRNA or Tbc1d1 shRNA (KD) was measured as described under “Experimental Procedures.” The results are the averages ± S.E. from three measurements. B, Tbc1d1 was immunoprecipitated from an SDS/C12E9 lysates of day 5 adipocytes expressing control shRNA or Tbc1d1 shRNA, and equal portions of the immunoprecipitates were immunoblotted for Tbc1d1. A repetition of the experiments in A and B starting with the generation of the cells expressing shRNAs gave similar results.
FIGURE 3.
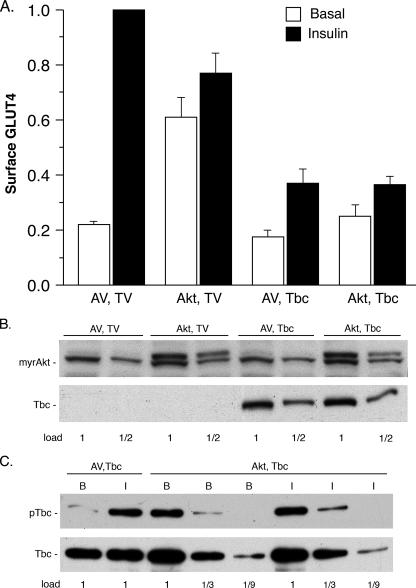
Effect of myrAkt on cell surface GLUT4 and Tbc1d1 phosphorylation. A, 3T3-L1 adipocytes were co-transfected with the plasmid for HA-GLUT4-GFP and the plasmids for myrAkt1 with a carboxyl-terminal Myc tag (Akt) and 3× FLAG-tagged human Tbc1d1 (Tbc) or for the corresponding empty vectors (AV, TV). Cell surface HA-GLUT4-GFP was measured as described under “Experimental Procedures.” The values are the averages ± S.E. for four experiments. The values in each experiment were normalized to 1.0 for the AV, TV control in the insulin state. B, immunoblots of the SDS samples of the transfected cells in A for myrAkt with anti-Myc tag and for Tbc1d1 with anti-FLAG tag. The 1× load contained 20 μg of protein. The anti-Myc also cross-reacted with a protein that migrated just below myrAkt, which serves as a loading control. C, SDS/C12E9 lysates of the transfected cells in A were immunoprecipitated with anti-FLAG agarose. The immunoadsorbates were immunoblotted for phosphorylation of Tbc1d1 with the PAS antibody (upper panel) and for Tbc1d1 with anti-FLAG (lower panel). A repetition of B and C with a second set of samples gave similar results.
FIGURE 5.
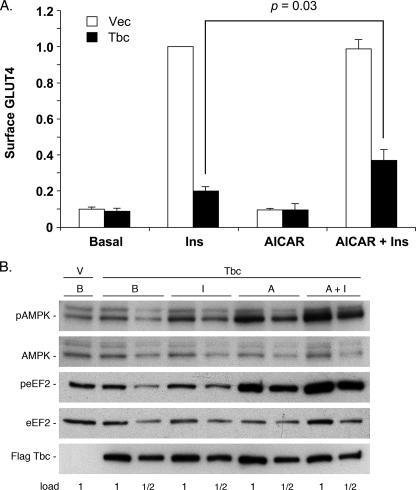
Effect of AICAR on Tbc1d1 inhibition of GLUT4 translocation. 3T3-L1 adipocytes were electroporated with HA-GLUT4-GFP and either control vector plasmid (V) or the plasmid for 3× FLAG-tagged human Tbc1d1. The cells were untreated (basal, B) or treated with insulin (I), 1 mm AICAR (A), or 1 mm AICAR plus insulin (Ins). Treatment with AICAR was 70 min. Insulin was added during the final 30 min. A, cell surface HA-GLUT4-GFP was measured as described under “Experimental Procedures.” The values are the averages ± S.E. for four independent experiments. They have been normalized to a value of 1.0 for the vector (Vec) control in the insulin state. The significance of the difference between surface GLUT4 in the presence of insulin versus AICAR plus insulin for cells overexpressing Tbc1d1 was calculated by the Student's two-tailed, paired t test. B, SDS samples of the cells in A were immunoblotted for pThr172 AMPK, AMPK, pThr56 eEF2, eEF2, and 3× FLAG-tagged Tbc1d1. The 1× load contained 30 μg of protein. A repetition of these blots with the samples from a second experiment gave similar results.
For shRNA-mediated knockdown of Tbc1d1, the pSiren-RetroQ system (Clontech, CA) was employed, as described previously (4). In this method 3T3-L1 fibroblasts were infected with a retrovirus containing the shRNA and the puromycin resistance gene. Fibroblasts stably expressing the shRNA were selected with puromycin, differentiated into adipocytes, and assayed for HA-GLUT4-GFP at the cell surface as described above. The sequences of the shRNA for knockdown of Tbc1d1 and for the control (13) were GCAGCGAGAGAATGAATTA and CAGTCGCGTTTGCGACTGG, respectively.
Immunoprecipitation, Mass Spectrometry, and Immunoblotting—For the isolation of endogenous Tbc1d1 for mass spectrometry identification, we followed the immunoprecipitation procedure described in Ref. 14. Ten 10-cm plates of 3T3-L1 adipocytes at day 7 of differentiation were solubilized in SDS/dithiothreitol, and then the sulfhydryl groups were capped with N-ethylmaleimide. An excess of the nonionic detergent C12E9 over SDS (3.5-fold by weight) was added to the lysate, followed by the PG antibody against Tbc1d1 and protein A-Sepharose. The immunoadsorbate was washed, and the adsorbed proteins were released with SDS sample buffer, separated by SDS-PAGE, and stained with Coomassie Blue. The expected Tbc1d1 band was excised from the gel and digested in gel with trypsin. The tryptic peptides were subjected to microcapillary liquid chromatography tandem mass spectrometry on an LTQ-Orbitrap mass spectrometer (ThermoFisher, San Jose, CA). Using SEQUEST v. 27 and the NCBI nr protein data base, Tbc1d1 peptides were sequenced and qualified in Sequest Summary of Proteomics Brower suite v3.3.22 requiring mass accuracy of 2.5 ppm or less, a minimum of five peptides with score final > 0.80, and the sum of score final > 9.9. For isolation of endogenous Tbc1d1 to assess its knockdown by immunoblotting (see Fig. 2B), we used the same immunoprecipitation procedure with single 10-cm plates on day 5 of differentiation. For isolation of 3× FLAG-tagged Tbc1d1 to measure its phosphorylation by immunoblotting (see Fig. 3C), we also used this immunoprecipitation procedure with anti-FLAG agarose (Sigma), as described previously (7). For immunoblotting, SDS samples were separated by SDS-PAGE, and the proteins were transferred to ImmobilonP membrane (Millipore, Temecula, CA) and detected with primary antibody followed by horseradish peroxidase-conjugated secondary antibody and SuperSignal chemiluminescence reagent (Pierce).
Recombinant Proteins and Tissue and Cell Samples for Immunoblotting—Recombinant FLAG-tagged mouse Tbc1d1 and human AS160 were expressed by transfecting 10-cm plates of HEK293F cells with 10 μg of the plasmids for these proteins using the Lipofectamine 2000 reagent (Invitrogen). SDS/C12E9 lysates of the cells were prepared as described above, and each protein was isolated by immunoadsorption with anti-FLAG conjugated to agarose and then released with SDS sample buffer. This method yielded relatively pure preparations of each protein contaminated with only smaller amounts of the lower molecular weight heavy and light chains of anti-FLAG, as assessed by SDS-PAGE and Coomassie Blue staining. The concentration of each protein in the SDS sample was estimated by visual comparison of the intensity of its Coomassie Blue staining with that of known nanogram amounts of molecular weight markers (Bio-Rad) whose staining intensities bracketed that of the Tbc1d1 and AS160 samples.
Mouse tissues were dissected from mice euthanized with carbon dioxide and immediately frozen in liquid nitrogen. The tissues were obtained littermate wild-type and AS160 knock-out mice that were on a mixed 129, C57BL/6 background. The characterization of the AS160 knock-out mouse, which was purchased from Lexicon Genetics Inc., will be reported elsewhere. The frozen tissues were disrupted with a polytron in cold 40 mm Hepes, 150 mm NaCl, 2 mm EDTA, pH 7.5, containing a mixture of protease inhibitors (1 μm pepstatin A, 10 μm leupeptin, 10 μm EP475, and 1 μg/ml aprotinin). The homogenates, in which the protein concentration was about 4 mg/ml, were made 1.5% in C12E9, and microcentrifuged for 10 min. The supernatants were removed and made into SDS samples by the addition of one-third volume of concentrated sample buffer. The protein concentration of these samples, as well as that of other SDS samples herein, was determined by the precipitating Lowry assay (15).
L6 myoblasts expressing Myc-tagged GLUT4 were a generous gift from Dr. Amira Klip (Hospital for Sick Children, Toronto, Canada). These were cultured in 10% fetal bovine serum for carrying as myoblasts, and confluent plates were cultured in 2% serum for differentiation into myotubes. Fused cells were first visible on day 4 of differentiation into myotubes. At various days in the differentiation process, a 10-cm plate was washed with phosphate-buffered saline and then dissolved in 1 ml of SDS sample buffer with 10 mm dithiothreitol and protease inhibitors (see above) and held at 100 °C for 5 min. This same procedure was used to generate SDS samples of 3T3-L1 cells during differentiation.
RESULTS
Expression of Tbc1d1 in 3T3-L1 Adipocytes—Although it has been reported that Tbc1d1 mRNA is expressed in 3T3-L1 adipocytes (16), expression of the protein has not been established. To determine whether Tbc1d1 protein is expressed, we immunoprecipitated Tbc1d1 from a SDS/C12E9 lysate of 3T3-L1 adipocytes. The precipitated proteins were then separated by SDS-PAGE and stained with Coomassie Blue. A band in the region of 140 kDa was observed. It was digested with trypsin, and the tryptic peptides were analyzed by liquid chromatography tandem mass spectrometry. Fourteen unique tryptic peptides from Tbc1d1, covering 12.4% of the sequence, were identified (data not shown).
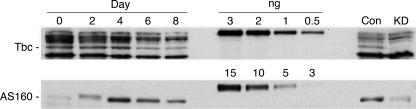
We next examined Tbc1d1 expression during the course of differentiation of 3T3-L1 adipocytes and compared its expression to that of AS160 (Fig. 1). Mouse Tbc1d1 and human AS160 recombinant proteins were included to allow quantitation of the amounts of each protein. In addition, samples of 3T3-L1 adipocytes in which Tbc1d1 or AS160 were knocked down were included to verify the identification of each protein. In the case of Tbc1d1, a protein at ∼140 kDa, as well as some other proteins of higher and lower size, were detected (Fig. 1, upper panel, left). The 140-kDa protein is Tbc1d1, because it was markedly reduced in the sample from adipocytes expressing Tbc1d1 shRNA (Fig. 1, upper panel, Con versus KD lanes). The Tbc1d1 in the adipocytes migrated more rapidly than the recombinant mouse Tbc1d1, which migrated at ∼170 kDa (Fig. 1, upper panel, middle). Most likely the reason is that adipocytes express the short splice variant of Tbc1d1, whereas the recombinant protein is the long splice variant. The long and short splice variants of Tbc1d1 differ by 94 amino acids (7). In addition, the recombinant protein has a 3× FLAG tag of 27 amino acids at the amino terminus. Expression of Tbc1d1 was constant between day 0 of differentiation and day 4 and then decreased at days 6 and 8. The full adipocyte phenotype is reached at about day 6 (17). Hence, Tbc1d1 decreases somewhat upon differentiation. This finding contrasts with the report that its mRNA increases upon 3T3-L1 adipocyte differentiation (16). From comparison with the known amounts of recombinant protein, cells at day 4 contained ∼5 ng of Tbc1d1/mg of protein.
FIGURE 1.
Expression of Tbc1d1 and AS160 during 3T3-L1 adipocyte differentiation. SDS samples of 3T3-L1 adipocytes as confluent fibroblasts (day 0) and on days 2, 4, 6, and 8 following the initiation of differentiation were immunoblotted for Tbc1d1 and AS160. The loads/lane were 100 μg for the Tbc1d1 blot and 50 μg for the AS160 blot. Known amounts (ng) of recombinant 3× FLAG-tagged mouse Tbc1d1 and human AS160 were included as standards. SDS samples of adipocytes on day 5 of differentiation expressing control (Con) shRNA or shRNA for Tbc1d1 or AS160 (KD) were also included (see Fig. 2 and (4), respectively, for the generation of these samples). Immunoblotting for AS160 was done with an affinity-purified antibody against amino acids 876–891 of mouse AS160, which has the same sequence in human and mouse AS160. A repetition of this experiment gave similar results.
In contrast to Tbc1d1 the expression of AS160 was very low in fibroblasts (day 0) and increased to a maximum at day 4 and then decreased somewhat (Fig. 1, lower panel, left). The mobility of AS160 on day 2 is slightly less than on the other days because insulin present in the differentiation medium on day 2 leads to phosphorylation on multiple sites. Because the complete cDNA for mouse AS160 is not available, we used recombinant human AS160 as the standard and immunoblotted for AS160 with an antibody against a peptide that is identical in human and mouse AS160, so that reactivity with the mouse and human protein should be the similar. Based upon comparison with the standards (Fig. 1, lower panel, middle), cells at day 4 expressed ∼100 ng of AS160/mg of protein. Thus, AS160 is 20 times more abundant than Tbc1d1 in 3T3-L1 adipocytes. The adipocyte AS160 exhibited a higher mobility than the recombinant human AS160 (∼160 and 180 kDa, respectively). Similarly to Tbc1d1, there is a long and short splice variant of AS160 (7). In mouse AS160 these differ by a single exon of 63 amino acids. Through immunoblotting with an antibody against a peptide in this exon, we have found that 3T3-L1 adipocytes express mainly the short variant of AS160 (data not shown). Because the recombinant human AS160 was the long variant and also contained the 3× FLAG tag at its amino terminus, the adipocyte AS160 migrated more rapidly than the recombinant AS160.
Effect of Knockdown of Tbc1d1 on Cell Surface GLUT4— Knockdown of AS160 in 3T3-L1 adipocytes elevates GLUT4 at the cell surface in the absence of insulin, to a level that is ∼30% of that seen with insulin (4). Here we examined the effect of knockdown of Tbc1d1 on the relative amount of GLUT4 at the cell surface. Approximately 75% knockdown of Tbc1d1 protein was achieved through stable expression of shRNA by retroviral infection of 3T3-L1 fibroblasts (Fig. 2B). Knockdown of Tbc1d1 had little or no effect on GLUT4 at the cell surface in either the basal or insulin state (Fig. 2A). Insulin treatment increased the amount of GLUT4 at the cell surface by ∼4-fold. The lack of an effect of Tbc1d1 knockdown suggests that endogenous Tbc1d1 does not play a significant role in keeping the Rab(s) required for GLUT4 translocation in the inactive GTP form, most probably because of its low expression in comparison with that of AS160.
Effect of Akt Phosphorylation of Tbc1d1—Overexpression of Tbc1d1 in 3T3-L1 adipocytes markedly inhibits insulin-stimulated GLUT4 translocation, whereas overexpression of AS160 does not inhibit (2, 7). Both proteins are phosphorylated by Akt (2, 7). One possible explanation for this difference is that although the GAP activity of both proteins is suppressed by Akt phosphorylation, the Akt phosphorylation of ectopic Tbc1d1 is incomplete. Possibly the endogenous Akt activity in insulin-treated 3T3-L1 adipocytes is insufficient to phosphorylate the overexpressed Tbc1d1 effectively. To test this possibility, we examined the effect of ectopic activated Akt on Tbc1d1 inhibition of GLUT4 translocation. This approach also allowed determination of whether the Tbc1d1 inhibition was, as expected, downstream of Akt activation. Ectopic activated Akt was achieved through expression of a truncated Akt1 construct containing a myristoylation sequence at its amino terminus (myrAkt) (18).
Fig. 3A presents the effect of myrAkt on HA-GLUT4-GFP at the cell surface in the presence and absence of insulin and of the myrAkt on inhibition of GLUT4 translocation by Tbc1d1. Insulin treatment caused a 5-fold increase of HA-GLUT4-GFP at the cell surface in cells co-transfected with the vector controls (Fig. 3A, AV, TV). Expression of myrAkt increased GLUT4 at the cell surface in the absence of insulin by 3-fold, and there was a further slight increase in the presence of insulin (Fig. 3A, Akt, TV). This effect of myrAkt qualitatively agrees with a previous study, although in that study the increase in cell surface HA-GLUT4-GFP caused by myrAkt was as large as that caused by insulin (3). As expected from our previous study, overexpression of Tbc1d1 inhibited GLUT4 translocation, such that the amount of HA-GLUT4-GFP at the cell surface in the presence of insulin was reduced by 60% (Fig. 3A, AV, Tbc). Overexpression of Tbc1d1 also inhibited the increase in HA-GLUT4-GFP at the cell surface elicited by myrAkt, by ∼50% in both the absence and the presence of insulin (Fig. 3A, Akt, Tbc).
Thus, activated Akt did not substantially reduce the inhibitory effect of Tbc1d1 on cell surface HA-GLUT4-GFP. To establish that the effects in Fig. 3A were not due to differences in expression of myrAkt and Tbc1d1, we immunoblotted SDS lysates of the transfected cells for myrAkt and Tbc1d1 (Fig. 3B). The myrAkt and Tbc1d1 were equally expressed under the two conditions where they were introduced.
We previously showed that insulin treatment of 3T3-L1 adipocytes causes phosphorylation of Tbc1d1 on Thr596 in the Akt phosphorylation motif RRRANTL (7), which can be detected with the PAS antibody. We examined the phosphorylation of the overexpressed Tbc1d1 in basal and insulin-treated adipocytes that coexpressed myrAkt or not (Fig. 3C, upper panel). As was expected, in cells without myrAkt, insulin stimulated phosphorylation of Tbc1d1. In cells with myrAkt, Tbc1d1 was phosphorylated in the absence or presence of insulin. The extent of Tbc1d1 phosphorylation was the same in insulin-treated cells without myrAkt, in basal cells with myrAkt, and in insulin-treated cells with myrAkt. A control immunoblot for Tbc1d1 showed that its recovery was approximately the same under all conditions (Fig. 3C, lower panel). Because activated Akt did not increase the phosphorylation of Tbc1d1 over that with insulin alone, most likely phosphorylation of Tbc1d1 on Thr596 in the presence of insulin is complete. The results in this section thus indicate that incomplete phosphorylation of Tbc1d1 is unlikely to account for its inhibition of insulin-stimulated GLUT4 translocation. In addition, they show that the inhibition by Tbc1d1 occurs at a step that is beyond Akt activation.
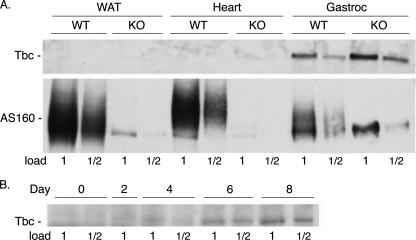
Tissue Expression of Tbc1d1—To determine where Tbc1d1 might be functioning in GLUT4 translocation in mouse tissues, we examined its expression, as well as that of AS160, in white fat, heart, and skeletal muscle, the main tissues of GLUT4 expression. SDS samples of these tissues from wild-type and AS160 knock-out mice were immunoblotted for the two proteins. Tbc1d1 was detected only in skeletal muscle (Fig. 4A, upper panel). With the samples shown in Fig. 4A, the amount of Tbc1d1 in quadriceps of the knock-out mouse was about twice that in its littermate wild-type mouse. However, this difference was not a consistent finding. We have immunoblotted the quadriceps muscle from three other littermate pairs of wild-type and AS160 knock-out mice for Tbc1d1; in two sets the wild-type and knock-out mice had approximately equal amounts of Tbc1d1, and in the third set there was more Tbc1d1 in the wild type (data not shown). Thus, these differences are due to mouse-to-mouse variation rather than to the AS160 knock-out.
FIGURE 4.
Tbc1d1 expression in tissues and L6 cells. A, SDS samples of tissues from white adipose tissue (WAT), heart, and gastrocnemius (Gastroc) muscle from wild-type (WT) and AS160 knock-out (KO) mice were immunoblotted for Tbc1d1 and AS160. The 1× load was 100 μg of protein. Two replicates of these blots with tissue samples from two other littermate sets of wild-type and AS160 knock-out mice gave similar results. B, SDS samples of L6 cells on the stated days after initiation of differentiation were immunoblotted for Tbc1d1. The 1× load was 100 μg of protein. The blot shown was probed with the NT antibody against Tbc1d1. A similar pattern was obtained when the samples were immunoblotted with the PG antibody.
In contrast to Tbc1d1, AS160 was expressed in white fat, heart, and skeletal muscle (Fig. 4A, lower panel). As expected, the tissues from the knock-out mouse showed no AS160. In muscle, and to a lesser extent in fat, the antibody against AS160 cross-reacted with an unknown protein that co-migrated with the lower portion of the AS160 band. To obtain better separation from this cross-reacting band, we ran the AS160 to the bottom of the gel, and as a consequence the AS160 band in the immunoblot is spread out. This cross-reacting band is not Tbc1d1, because our antibody against AS160 did not immunoblot recombinant mouse Tbc1d1 (data not shown). On the basis of the relative intensity of the AS160 band, fat and heart contain about equal amounts of AS160 per mg protein, whereas the amount in muscle was about half that in fat and heart.
We also examined the expression of Tbc1d1 during differentiation of cultured muscle cells. Rat L6 myoblasts were differentiated into myotubes, and SDS samples of the cells were prepared at various times during the differentiation. Tbc1d1 was not detectable in the myoblasts. It became detectable on day 4 of differentiation, when myotube formation was first evident, and increased to day 8 (Fig. 4B). Unfortunately, our antibodies against AS160 did not give sufficiently clean immunoblots with the L6 cell SDS samples to allow reliable identification of AS160. These experiments thus suggest that the major site of Tbc1d1 action is skeletal muscle, whereas AS160 acts in fat, heart, and muscle.
It is notable that in these blots the mouse and rat muscle Tbc1d1 migrated at about 160 kDa, in contrast to the 3T3-L1 adipocyte Tbc1d1, which migrated at about 140 kDa (see above). An immunoblot for Tbc1d1 that contained samples of mouse muscle and 3T3-L1 adipocytes side-by-side confirmed this difference in mobility (data not shown). Most likely the explanation is that the longer splice variant of Tbc1d1 (see above) is expressed in muscle. The AS160 in mouse muscle also migrated at about 160 kDa. Thus, Tbc1d1 and AS160 in muscle are not easily separated by SDS-PAGE.
Effect of AMPK on Tbc1d1 Inhibition of GLUT4 Translocation—Activation of AMPK in muscle by the compound AICAR is known to stimulate GLUT4 translocation and glucose transport (9). Hence, by analogy with the mode of action of AS160 in insulin-stimulated GLUT4 translocation, it seemed possible that AMPK phosphorylation of Tbc1d1 might inhibit its Rab GAP activity and thereby allow GLUT4 translocation.
To test this possibility, we examined the effect of AICAR on cell surface GLUT4 in 3T3-L1 adipocytes in the basal and insulin-stimulated states, without or with Tbc1d1 overexpressed. AICAR treatment of adipocytes without ectopic Tbc1d1 had no effect on the cell surface GLUT4 in the basal or insulin state (Fig. 5A, open bars). With or without AICAR, insulin caused a 10-fold increase in cell surface GLUT4. As was seen previously (Ref. 7 and Fig. 3), overexpression of Tbc1d1 markedly decreased GLUT4 at the cell surface in the insulin state, without any effect on the amount in the basal state (Fig. 5A, solid bars). In this set of experiments cell surface GLUT4 was reduced to 20% of the level in the absence of Tbc1d1 overexpression. When the adipocytes overexpressing Tbc1d1 were treated with AICAR as well as insulin, the inhibition caused by Tbc1d1 was less. The amount of GLUT4 at the cell surface in the presence of both agents was 40% of that in the absence of Tbc1d1 overexpression. Thus, AICAR treatment partially reversed the inhibition by Tbc1d1 overexpression, such that twice as much GLUT4 was at the cell surface in cells overexpressing Tbc1d1 in the presence of both AICAR and insulin. This result suggests that AMPK phosphorylation of Tbc1d1 reduces its inhibitory action on GLUT4 translocation.
To show that this effect was due to activation of AMPK and not to different levels of Tbc1d1 expression, SDS lysates of the transfected cells were immunoblotted for activation of AMPK and for Tbc1d1. Phosphorylation of AMPK on Thr172 results in its activation and can be detected with an antibody against the Thr172 phosphopeptide (19). AICAR-treated adipocytes with ectopic Tbc1d1 showed approximately a 2-fold increase in pThr172 (Fig. 5B, top panel, compare lane B with lane A and lane A+I with lane I). The amount of AMPK protein was the same under all conditions (Fig. 5B, second panel). As a second measure of AMPK activation, we immunoblotted for phosphorylation on Thr56 of eEF2, which is a known site of AMPK phosphorylation (20). AICAR increased phosphorylation on this site by more than 2-fold, in the absence or presence of insulin, with the amount of eEF2 the same under all conditions (Fig. 5B, third and fourth panels). In the experiments shown here, the cells were treated with 1 mm AICAR for 70 min. This concentration of AICAR gave maximal activation of AMPK, because SDS samples of cells treated with 1 and 3 mm AICAR for 70 min gave similar signals for pThr172 AMPK (data not shown). Expression of ectopic Tbc1d1 was the same under all the conditions (Fig. 5B, bottom panel).
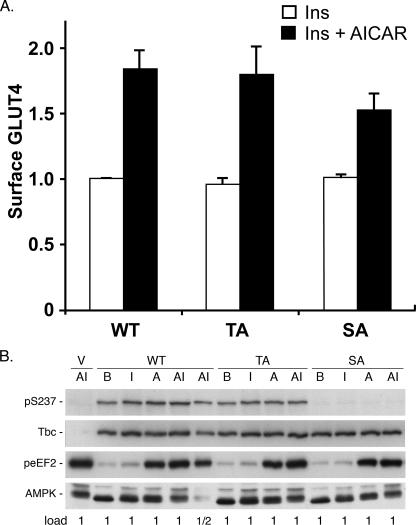
Human Tbc1d1 contains a predicted site for AMPK phosphorylation at Ser237 (7, 19). In mass spectrometry analysis of human Tbc1d1 isolated from HEK 293 cells overexpressing the protein, we have found tryptic phosphopeptide corresponding to phosphorylation on Ser237 (data not shown). Moreover, while this manuscript was in revision, a study by Chen et al. (21) showed that activation of AMPK leads to increased phosphorylation on Ser237 of Tbc1d1 in HEK 293 cells and in L6 myotubes. Consequently, we investigated whether phosphorylation on Ser237 might underlie the effect of AICAR to relieve in part Tbc1d1 inhibition of the insulin-stimulated increase in cell surface GLUT4. To do so, we examined whether mutation of Ser237 to Ala in Tbc1d1 blunted the AICAR effect. In addition, as a control, we also examined the effect of mutation of Thr596, the site of insulin-stimulated phosphorylation by Akt. The S237A mutant reduced the increase in cell surface GLUT4 in response to AICAR slightly, from 1.8-fold with the wild-type Tbc1d1 to 1.6-fold with this mutant (Fig. 6A). However, the difference was not statistically significant. The T596A mutant had no effect. In combination with this analysis of cell surface GLUT4, we also examined phosphorylation on Ser237 through the use of a phosphopeptide-specific antibody (Fig. 6B). Ser237 was phosphorylated in the basal state, and there was little or no increase in its phosphorylation in response to insulin. The specificity of the antibody was verified by the fact that there was virtually no signal for the S237A mutant. Immunoblotting for Tbc1d1 with anti-FLAG showed that the wild type, S237A, and T596A were equally well expressed, and immunoblotting for peEF2 showed that the AICAR was effective in activating AMPK. These results thus indicate that AMPK phosphorylation of Ser237 is unlikely to account for the AICAR effect.
FIGURE 6.
Effect of phosphorylation of Ser237 of Tbc1d1. A, the relative amount of HA-GLUT4-GFP at the cell surface in adipocytes expressing FLAG-tagged wild-type Tbc1d1 (WT), T596A Tbc1d1 (TA), or S237A Tbc1d1 (SA) in the presence of insulin (Ins) or AICAR plus insulin was determined as described in the legend to Fig. 5. The values are the averages ± S.E. for four independent experiments. They have been normalized to 1.0 for the wild type in the insulin state. For each Tbc1d1 construct the difference between insulin and AICAR plus insulin was significant at p < 0.05, whereas the difference between the AICAR plus insulin for SA compared with AICAR plus insulin for WT and TA was not significant at p < 0.05. B, 3T3-L1 adipocytes were electroporated with vector (V) or the plasmids for FLAG-tagged wild-type, T596A, or S237A Tbc1d1 and treated with insulin, AICAR, or AICAR plus insulin as described in the legend to Fig. 5. The samples were immunoblotted for phospho-Ser237, FLAG-tagged Tbc1d1 (anti-FLAG), peEF2, and, as a loading control, AMPK. The 1× load was 50 μg of protein. A replicate of this experiment gave similar results.
DISCUSSION
This study shows that Tbc1d1 was expressed in 3T3-L1 adipocytes, but at one-twentieth the level of AS160. Moreover, Tbc1d1 was not detected by immunoblotting of white adipose tissue, whereas AS160 was readily detectable. Tbc1d1 and AS160 have the same Rab specificity and approximately the same GAP activity toward these Rab substrates (7). Thus, it seems likely that in adipocytes endogenous Tbc1d1 does not contribute significantly to the total GAP activity toward the Rab(s) involved in GLUT4 translocation. In support of this conclusion, knockdown of Tbc1d1 did not increase the amount of GLUT4 at the cell surface in the absence of insulin, whereas knockdown of AS160 does (4). Our data thus indicate that AS160 is the primary Rab GAP involved in the regulation of GLUT4 translocation in adipocytes. The same situation is likely to apply in heart, because heart also contains AS160 but no detectable Tbc1d1.
Despite the absence of a role for endogenous Tbc1d1 in adipocytes, the robust insulin-stimulated GLUT4 translocation in these cells provided a system in which to examine the regulation of ectopic Tbc1d1. Previously we showed that insulin treatment led to phosphorylation of ectopic Tbc1d1 on Thr596 within an Akt substrate motif (7). Tbc1d1 has a number of other possible, albeit relatively poor, sites for Akt phosphorylation as predicted by the Scansite program, and insulin-activated Akt may also phosphorylate one or more of these. Nevertheless, phosphorylation by Akt did not seem to be regulatory, because ectopic Tbc1d1 markedly inhibited insulin-stimulated GLUT4 translocation (7). A potential alternative explanation for the absence of regulation was that the endogenous Akt was insufficient to phosphorylate ectopic Tbc1d1 to the extent required. Our present results make this explanation unlikely. Expression of constitutively active myrAkt did not increase the phosphorylation of ectopic Tbc1d1 on Thr596 over that elicited by insulin. Moreover, in the presence of myrAkt, ectopic Tbc1d1 still markedly reduced the level of GLUT4 at the cell surface. Thus, the activity of Tbc1d1 is almost certainly not regulated by Akt phosphorylation alone. Tbc1d1 differs in this way from AS160, where Akt phosphorylation alone is sufficient to block its inhibitory action on insulin-stimulated GLUT4 translocation (2). In addition our previous results did not determine whether ectopic Tbc1d1 inhibited Akt activation or inhibited downstream of Akt. Because Tbc1d1 inhibited GLUT4 translocation to the cell surface in response to myrAkt, the site of inhibition must be downstream of Akt. This site agrees with the site of action of AS160 (3). Based upon the current model for AS160 action (see the Introduction), most likely ectopic Tbc1d1 inhibits insulin and myrAkt-stimulated GLUT4 translocation by maintaining Rab10 and possibly other Rabs in their GDP form even in the presence of insulin.
A major finding of this study is that the main tissue for expression of Tbc1d1 is skeletal muscle. The appearance of Tbc1d1 during differentiation of L6 myoblasts into myotubes supports this finding. AS160 is also present in muscle. Hence, muscle contains two Rab GAPs that regulate GLUT4 translocation.
The relative abundance of Tbc1d1 in muscle compared with fat led us to examine the possibility that an alternative stimulus might regulate the inhibitory effect of Tbc1d1 on GLUT4 translocation. In muscle, activation of AMPK by AICAR is known to stimulate GLUT4 translocation (9). In contrast, we found that AICAR treatment of 3T3-L1 adipocytes did not alter the amount of GLUT4 at the cell surface in either the basal or insulin state. This situation thus allowed us to examine the effect of AICAR on inhibition of insulin-stimulated GLUT4 translocation by ectopic Tbc1d1. We found that AICAR treatment partially reversed the inhibition. This result strongly indicates that the GAP activity of Tbc1d1 is suppressed by AMPK. It seems likely that the suppression is through direct phosphorylation of Tbc1d1 by AMPK. To test this possibility, we examined the effect of mutation of the AMPK site Ser237 of Tbc1d1 on cell surface GLUT4 and also the extent of phosphorylation of this site. However, the S237A mutant did not significantly reduce the AICAR effect. This result is in agreement with our finding that AICAR caused only a minimal increase in phosphorylation of Ser237. An approximate consensus motif for AMPK phosphorylation has been deduced (19). In addition to Ser237, human Tbc1d1 contains three other Ser/Thr residues that conform to this consensus at amino acid residues 69, 372, and 565. In the future, it will be important to determine whether AMPK treatment leads to phosphorylation on one or more of these sites or other sites, and if so, whether the effect of AICAR is through phosphorylation on these sites.
In regard to the above, we note that our finding that AICAR treatment of 3T3-L1 adipocytes had no effect on the amount of GLUT4 at the cell surface in either the basal or insulin-stimulated states does not entirely agree with two studies of 3T3-L1 adipocytes in the literature, which are themselves in disagreement. One reports that by assay of plasma membrane lawns, 0.5 mm AICAR had no effect on plasma membrane GLUT4 in the absence of insulin and reduced it by one-half in the presence of insulin (22). The other reports that by a quantitative fluorescence assay with GLUT4-GFP 1 mm AICAR elevated GLUT4 at the cell surface by 1.5-fold in the absence of insulin but did not examine the effect of AICAR in the presence of insulin (23). We do not have an explanation for these differences.
In skeletal muscle, both insulin and contraction stimulate glucose transport through GLUT4 translocation to the plasma membrane (1, 8). Considerable evidence indicates that insulin signals translocation through the protein kinase Akt (1, 8). In contrast, the signaling pathway(s) from contraction to GLUT4 translocation remains to be established. Even though contraction activates Akt, this pathway appears not to participate in contraction-elicited translocation (8). Contraction also activates AMPK as a result of elevated AMP and calmodulin-dependent protein kinases and protein C kinases as a result of elevated calcium (8). Current evidence indicates that activation of AMPK accounts for only part of contraction-stimulated GLUT4 translocation, and the roles of the calmodulin-dependent and C kinases in this process have not been clearly established (1, 8, 9).
In this regard the interpretations of the data in recent studies on the role of AS160 in muscle, performed before knowledge of the presence of Tbc1d1, will need to be reevaluated. In the studies where phosphorylation of endogenous AS160 was examined by immunoblotting of muscle lysates with the PAS antibody (24–27), it may be that a significant part of the phosphorylation is on Tbc1d1, which migrates at about the same mobility as AS160 in muscle. In other studies (28, 29) overexpression of wild-type AS160 partially inhibited contraction-stimulated glucose transport (by about 20%), and overexpression of a nonphosphorylatable form of AS160 or a form that cannot bind calcium-calmodulin was somewhat more inhibitory (by about 40%). The inhibition by the wild-type AS160 cannot be taken as evidence of a role for AS160 in contraction, because overexpression of AS160 may act on that portion of Rab10 and other Rabs normally controlled by Tbc1d1. The somewhat greater inhibition by the nonphosphorylatable form of AS160 and the form that cannot bind calcium-calmodulin does indicate a role for AS160 in contraction. But its participation may be limited, because the inhibition of glucose transport by these forms beyond that of the wild-type AS160 is only 20%.
On the basis of the results here and those of other studies (2–4, 7), during insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes Akt phosphorylation suppresses the GAP activity of AS160 but not that of ectopic Tbc1d1. Hence, in adipocytes insulin signals GLUT4 translocation through AS160 and not Tbc1d1. Because insulin-stimulated Akt phosphorylation does not regulate ectopic Tbc1d1 in adipocytes, it may be that in muscle insulin-stimulated GLUT4 translocation also proceeds primarily through Akt phosphorylation of AS160 and not that of Tbc1d1. The relative contributions of AS160 and Tbc1d1 to the regulation of AICAR and contraction-stimulated GLUT4 translocation remain to be determined. Our results suggest that Tbc1d1 will prove to have a major role in AICAR and contraction-stimulated GLUT4 translocation. AMPK, a kinase that may participate in contraction-stimulated GLUT4 translocation, partially relieved the inhibitory effect of ectopic Tbc1d1 on GLUT4 translocation. In addition, Tbc1d1 may be regulated through phosphorylation by calmodulin-dependent kinases and protein C kinases, as well as by the direct binding of calcium-calmodulin to its calmodulin-binding motif (7). Hence, Tbc1d1 has the potential to integrate a number of signaling events activated by contraction. By contrast, AS160 may primarily regulate insulin-stimulated GLUT4 translocation and play a lesser role in AICAR and contraction-stimulated GLUT4 translocation. A recent publication, which appeared while this manuscript was in revision, provides suggestive support for this hypothesis. The binding of 14-3-3 protein to phosphorylated AS160 appears to be required for the insulin-stimulated increase in GLUT4 at the cell surface (30). Chen et al. (21) have now reported that insulin treatment of L6 myotubes results in enhanced binding of 14-3-3 to AS160 but not to Tbc1d1, whereas activation of AMPK in L6 myotubes with the agent A-769662 results in enhanced binding of 14-3-3 to Tbc1d1 but not to AS160. In the future it will be important to elucidate the roles of Tbc1d1 and AS160 in insulin and contraction-stimulated GLUT4 translocation in muscle.
Acknowledgments
We thank Dr. Lee Witters for advice about AMPK.
This work was supported by National Institutes of Health Grants DK25336 and DK42816. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GAP, GTPase-activating protein; AICAR, 5′-aminoimidazole-4-carboxamide ribonucleoside; AMPK, AMP-activated protein kinase; C12E9, nonaethyleneglycol dodecyl ether; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, hemagglutin epitope tag; shRNA, small hairpin RNA; eEF, eukaryotic elongation factor.
References
- 1.Huang, S., and Czech, M. P. (2007) Cell Metab. 5 237-252 [DOI] [PubMed] [Google Scholar]
- 2.Sano, H., Kane, S., Sano, E., Miinea, C. P., Asara, J. M., Lane, W. S., Garner, C. W., and Lienhard, G. E. (2003) J. Biol. Chem. 278 14599-14602 [DOI] [PubMed] [Google Scholar]
- 3.Zeigerer, A., McBrayer, M. K., and McGraw, T. E. (2004) Mol. Biol. Cell 15 4406-4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguez, L., Lee, A., Chavez, J. A., Miinea, C. P., Kane, S., Lienhard, G. E., and McGraw, T. E. (2005) Cell Metab. 2 263-272 [DOI] [PubMed] [Google Scholar]
- 5.Sano, H., Eguez, L., Teruel, M. N., Fukuda, M., Chuang, T. D., Chavez, J. A., Lienhard, G. E., and McGraw, T. E. (2007) Cell Metab. 5 293-303 [DOI] [PubMed] [Google Scholar]
- 6.Jiang, L., Fan, J., Bai, L., Wang, Y., Chen Y., Yang, L., Chen, L., and Xu, T. (2008) J. Biol. Chem. 283 8508-8516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roach, W. G., Chavez, J. A., Miinea, C. P., and Lienhard, G. E. (2007) Biochem. J. 403 353-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessen, N., and Goodyear, L. J. (2005) J. Appl. Physiol. 99 330-337 [DOI] [PubMed] [Google Scholar]
- 9.Fujii, N., Jessen, N., and Goodyear, L. J. (2006) Am. J. Physiol. 291 E867-E877 [DOI] [PubMed] [Google Scholar]
- 10.Keller, S. R., Scott, H. M., Mastick, C. C., Aebersold, R., and Lienhard, G. E. (1995) J. Biol. Chem. 270 23612-23618 [DOI] [PubMed] [Google Scholar]
- 11.Lamphere, L., and Lienhard, G. E. (1992) Endocrinology 131 2196-2202 [DOI] [PubMed] [Google Scholar]
- 12.Frost, S. C., and Lane, M. D. (1985) J. Biol. Chem. 260 2646-2652 [PubMed] [Google Scholar]
- 13.Mitra, P., Zheng, X., and Czech, M. P. (2004) J. Biol. Chem. 279 37431-37435 [DOI] [PubMed] [Google Scholar]
- 14.Kane, S., Sano, H., Liu, S. C., Asara, J. M., Lane, W. S., Garner, C. C., and Lienhard, G. E. (2002) J. Biol. Chem. 277 22115-22118 [DOI] [PubMed] [Google Scholar]
- 15.Peterson, G. L. (1977) Anal. Biochem. 83 346-356 [DOI] [PubMed] [Google Scholar]
- 16.Guo, X., and Liao, K. (2000) Gene (Amst.) 251 45-53 [DOI] [PubMed] [Google Scholar]
- 17.El-Jack, A. K., Kandror, K. V., and Pilch, P. F. (1999) Mol. Biol. Cell 10 1581-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohn, A. D., Summers, S. A., Birnbaum, M. J., and Roth, R. A. (1996) J. Biol. Chem. 271 31372-32378 [DOI] [PubMed] [Google Scholar]
- 19.Towler, M. C., and Hardie, D. G. (2007) Circ. Res. 100 328-341 [DOI] [PubMed] [Google Scholar]
- 20.Horman, S., Browne, G. J., Krause, U., Patel, J. V., Vertommen, D., Bertrand, L., Lavoinne, A., Hue, L., Proud, C. G., and Rider, M. H. (2002) Curr. Biol. 12 1419-1423 [DOI] [PubMed] [Google Scholar]
- 21.Chen, S., Murphy, J., Toth, R., Campbell, D. G., Morrice, N. A., and Mackintosh, C. (2008) Biochem. J. 409 449-459 [DOI] [PubMed] [Google Scholar]
- 22.Salt, I. P., Connell, J. M. C., and Gould, G. W. (2000) Diabetes 49 1649-1656 [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi, S., Katahira, H., Ozawa, S., Nakamichi, Y., Tanaka, T., Shimoyama, T., Takahashi, K., Yoshimoto, K., Imaizumi, M. O., Nagamatsu, S., and Ishida, H. (2005) Am. J. Physiol. 289 E643-E649 [DOI] [PubMed] [Google Scholar]
- 24.Bruss, M. D., Arias, E. B., Lienhard, G. E., and Cartee, G. D. (2005) Diabetes 54 41-50 [DOI] [PubMed] [Google Scholar]
- 25.Kramer, H. F., Witczak, C. A., Fujii, N., Jessen, N., Taylor, E. B., Arnolds, D. E., Sakamoto, K., Hirshman, M. F., and Goodyear, L. J. (2006) Diabetes 55 2067-2076 [DOI] [PubMed] [Google Scholar]
- 26.Karlsson, H. K. R., Zierath, J. R., Kane, S., Krook, A., Lienhard, G. E., and Wallberg-Henriksson, H. (2005) Diabetes 54 1692-1697 [DOI] [PubMed] [Google Scholar]
- 27.Treebak, J. T., Glund, S., Deshmukh, A., Klein, D. K., Long, Y. C., Jensen, T. E., Jorgensen, S. B., Viollet, B., Andersson, L., Neumann, D., Wallimann, T., Richter, E. A., Chibalin, A. V., Zierath, J. R., and Wojtaszewski, J. F. P. (2006) Diabetes 55 2051-2058 [DOI] [PubMed] [Google Scholar]
- 28.Kramer, H. F., Witczak, C. A., Taylor, E. B., Fujii, N., Hirshman, M. F., and Goodyear, L. J. (2006) J. Biol. Chem. 281 31478-31485 [DOI] [PubMed] [Google Scholar]
- 29.Kramer, H. F., Taylor, E. B., Witczak, C. A., Fujii, N., Hirshman, M. F., and Goodyear, L. J. (2007) Diabetes 56 2854-2862 [DOI] [PubMed] [Google Scholar]
- 30.Ramm, G., Larance, M., Guilhaus, M., and James, D. E. (2006) J. Biol. Chem. 281 29174-29180 [DOI] [PubMed] [Google Scholar]