Abstract
α-Synuclein, a protein implicated in the pathogenesis of Parkinson disease (PD), is thought to affect mitochondrial functions, although the mechanisms of its action remain unclear. In this study we show that the N-terminal 32 amino acids of human α-synuclein contain cryptic mitochondrial targeting signal, which is important for mitochondrial targeting of α-synuclein. Mitochondrial imported α-synuclein is predominantly associated with the inner membrane. Accumulation of wild-type α-synuclein in the mitochondria of human dopaminergic neurons caused reduced mitochondrial complex I activity and increased production of reactive oxygen species. However, these defects occurred at an early time point in dopaminergic neurons expressing familial α-synuclein with A53T mutation as compared with wild-type α-synuclein. Importantly, α-synuclein that lacks mitochondrial targeting signal failed to target to the mitochondria and showed no detectable effect on complex I function. The PD relevance of these results was investigated using mitochondria of substantia nigra, striatum, and cerebellum of postmortem late-onset PD and normal human brains. Results showed the constitutive presence of ∼14-kDa α-synuclein in the mitochondria of all three brain regions of normal subjects. Mitochondria of PD-vulnerable substantia nigra and striatum but not cerebellum from PD subjects showed significant accumulation of α-synuclein and decreased complex I activity. Analysis of mitochondria from PD brain and α-synuclein expressing dopaminergic neuronal cultures using blue native gel electrophoresis and immunocapture technique showed the association of α-synuclein with complex I. These results provide evidence that mitochondrial accumulated α-synuclein may interact with complex I and interfere with its functions.
Parkinson disease (PD)2 is associated with the degeneration of dopaminergic neurons in the substantia nigra pars compacta. One of the pathological hallmarks of PD and related synucleinopathies is the presence of intracellular inclusions called Lewy bodies that consist of aggregates of the presynaptic soluble protein called α-synuclein.(1–3). In addition, it is well known that mitochondrial dysfunction and oxidative stress are associated with the pathogenesis of PD (4–7). Triplication of α-synuclein gene and point mutations in the coding region has been associated with early onset of familial forms of PD. The familial PD represents only a minor proportion of all PD cases, whereas the majority of cases belong to sporadic form of the disease. However, some forms of familial PD have also been linked to mutations in PINK1 and DJ1 (2, 3, 8–10). Studies have shown that these proteins localize to mitochondria and gain toxic functions due to the mutations, which may lead to mitochondrial dysfunction (11–13). Furthermore, studies on overexpression of wild-type and familial mutant forms of α-synuclein in cell culture models and transgenic mouse models have reported cellular changes, including mitochondrial abnormalities (14, 15). However, the key issues on either mitochondrial localization of α-synuclein or precise mechanisms affecting mitochondrial functions in the normal and in PD human brains have not been addressed in detail.
Studies from our laboratory demonstrated the mitochondrial targeting of several endoplasmic reticulum-destined cytochrome P450 isoforms, cytosolic glutathione S-transferase, and Alzheimer amyloid precursor protein with a noncanonical, cryptic mitochondrial targeting signal (16–20). These atypical signals require activation either by proteolytic processing or protein modifications. Recently, using postmortem human AD brains and human cortical neuronal cell cultures, we showed that the progressive accumulation of amyloid precursor protein into mitochondrial import channels was associated with the impairment of mitochondrial function (21). In the present study, we identified the cryptic mitochondrial targeting sequence at the N terminus of α-synuclein. Mitochondrial targeting of α-synuclein under in vivo and in vitro conditions and also mitochondrial electron transport chain defects in α-synuclein accumulating neuronal cell systems led us to hypothesize that mitochondrial accumulation of α-synuclein may be an important cause of mitochondrial dysfunction as seen in the pathogenesis of PD. This possibility was further investigated in human brains affected by PD. Our results show that mitochondria are one of the direct targets of α-synuclein during the pathogenesis of PD.
MATERIALS AND METHODS
Cloning of α-Synuclein cDNAs—Human α-synuclein cDNA containing a 5′ HindIII site and a 3′ XbaI site was generated by reverse transcriptase-based PCR using total RNA from human brain tissue. Various N-terminal deletions were generated by PCR amplification of the parent cDNA using the appropriate sense primers containing an ATG codon and upstream Kozak consensus (22) for translation initiation. A53T/synuclein construct was generated by overlap PCR using wild-type synuclein as a template. All constructs were engineered to contain 5′ HindIII site and a 3′ Xbal site, and these cDNAs were cloned into pCR TOPO vectors. Simultaneously, α-synuclein constructs containing a 5′ HindIII site and a 3′ XhoI site were generated, and these constructs were cloned into the mammalian 3 FLAG pcDNA3 vector (gift from Dr. Serge Fuchs). The sequence properties of all the plasmid constructs were verified prior to use.
Cell Culture, Transfection, and Subcellular Localization of Proteins—Human fetal dopaminergic primary neuronal (DAN) cultures (Clonexpress, Inc., Gaithersburg, MD) were grown as according to the supplier's instructions. Cells at 60% confluence were transfected with wild-type and N-terminal truncated α-synuclein FLAG-tagged cDNA constructs (5 μg/plate) using FuGENE. Cells were harvested and homogenized (25–30 strokes) in four volumes of H medium using a Teflon-fitted glass hand homogenizer. The homogenates were centrifuged at 2,000 × g for 10 min, and the 2000 × g supernatant fraction was centrifuged at 15,000 × g for 15 min to obtain mitochondrial pellet. The resultant post mitochondrial supernatant was centrifuged at 100,000 × g to obtain cytosol and microsomes. The pellet (mitochondrial fraction) was resuspended in H medium and layered onto 1 m sucrose and centrifuged at 18,000 × g for 20 min. The resultant pellet was resuspended in suspension buffer and used for biochemical analysis without further delay. Cross-contamination of mitochondrial fractions with other subcellular organelles was investigated by Western blotting with antibodies to mitochondrial, cytosol, and endoplasmic reticulum markers.
Knockdown of α-Synuclein in DAN Cells—Knockdown of α-synuclein in DAN cells was carried out by using α-synuclein siRNA (h) kit from Santa Cruz Biotechnology Inc. Transfection of human α-synuclein-specific siRNA (consists of three target-specific 20- to 25-nucleotide siRNAs), and control siRNA (contains scrambled sequence) was carried out according to the manufacturer's protocol. Following the 48 h of transfection, cells were subjected to mitochondrial isolation to assay complex activities.
In Vitro Mitochondrial Import—Fresh mitochondria from rat liver were isolated essentially as described (18). Using cDNAs encoding the wild-type, familial, and N-terminal truncated α-synuclein, 35S-labeled translation products were generated in a transcription-coupled translation system (Promega, WI) according to the manufacturer's protocol. Import of the in vitro-synthesized proteins was carried out in a 200-μl reaction volume as described previously (17, 18). The incubation mixture was subjected to limited trypsin treatment (150 μg/ml reaction), and mitochondria were re-isolated by layering through 0.8 m sucrose and subsequent centrifugation at 15,000 × g for 15 min. Mitochondrial proteins were separated by SDS-PAGE and subjected to fluorography. The mitochondrially imported radioactive bands were visualized using the STORM (Molecular Dynamics) system. In another set of experiments, α-synuclein translation product was generated in the presence of unlabeled methionine. Mitochondrial import of unlabeled α-synuclein was carried out as described above, and the resulting mitochondrial pellet was subjected to immunoelectron microscopy using antibodies to α-synuclein.
Double Immunofluorescence Microscopy—DAN cells grown on coverslips were transfected with WT/syn FLAG and +33/syn FLAG cDNA constructs for 40 h and processed for antibody staining as previously described (20). Cells permeabilized with 0.1% Triton X-100 were stained with mouse monoclonal antibody to the FLAG epitope (Sigma) and rabbit polyclonal antibody to TOM20. The cells then were stained with appropriate fluorescence-tagged secondary antibodies and subjected to fluorescence microscopy.
Postmortem Human Subjects—Twenty-two autopsy brain hemispheres belonging to both genders were procured from the National Disease Resource Interchange (NDRI, Philadelphia, PA) of which eleven were clinically diagnosed with PD (PD subjects, n = 11) and eleven were normal brains with no neurological disorders (normal subjects, n = 11). These subjects had a postmortem time and ages ranging from 6 to 13 h and 52 to 93 years, respectively (supplemental Table S1). All procedures used to collect postmortem human brains from NDRI were approved by the institutional review board committee. The PD cases used in the study were late-onset. All studies were carried out on the fresh specimen without any further delay. Brains were dissected into various regions, and the subcellular fractions were isolated for biochemical analysis. For immunoelectron microscopy and immunohistochemistry analysis a portion of tissue from each region was fixed for 48 h in 20 mm phosphate buffer containing 4% formaldehyde and 2% glutaraldehyde immediately. PD subjects used in the study were positive for the presence of Lewy bodies, whereas controls showed no Lewy body staining.
Isolation of Subcellular Fractions from Fresh Postmortem Brain Tissue—Approximately 1 g of brain tissue was homogenized in four volumes of homogenization medium containing 70 mm sucrose, 210 mm mannitol, 2 mm HEPES, and 0.1 mm EDTA, pH 7.4 (H medium). Homogenates were centrifuged at 2000 × g for 10 min. Mitochondrial, microsomal and cytosolic fractions from the resulting supernatant were isolated as described (21). Isolated subcellular fractions were subjected to biochemical analysis without further delay. Protein concentration was measured by the Lowry assay (23). Cross-contamination of mitochondrial fractions with other subcellular organelles was investigated by Western blotting with antibodies to mitochondrial anti-rabbit TOM20 (1:1500, Santa Cruz Biotechnology), anti-mouse nuclear P97 (1:2000, Santa Cruz Biotechnology), anti-goat cytosolic marker actin (1:1500, Santa Cruz Biotechnology), anti-rabbit endoplasmic reticulum marker Sec 61(1:1000, Upstate), and anti-mouse Golgi marker TGN 38 (1:1500, Affinity Bioreagents).
Treatments, Immunoblots, and Quantitative ELISA—Mitochondria (50 μg) were suspended in H medium and were subjected to trypsin, digitonin, and sodium carbonate treatments separately as described (24). Mitochondrial proteins were separated by 14% SDS-PAGE and subjected to immunoblot analysis with ant rabbit α-synuclein (Santa Cruz Biotechnology), anti-mouse mitochondrial mtHSP70 (1:1000, Ab cam), anti-mouse cytochrome c oxidase subunit I (1;1500, Mitosciences), and anti-rabbit TOM20 (1;1500, Santa Cruz Biotechnology). The blots were immunostained with appropriate primary and secondary antibodies and the bands were developed using the Pierce West Femto Super Signal ULTRA chemiluminescent substrate kit and a Versa Doc (Bio-Rad) imaging system with Quantity One software. Quantitative ELISA of α-synuclein in cytosol and mitochondria (100 μg each in triplicates) from PD subjects, and controls was carried out using the “Human α-Synuclein ELISA kit” (BioSource) as per the manufacturer's protocol.
Immunoelectron Microscopy—Immunoelectron microscopy was carried out in the “Biomedical Imaging Core Laboratory” at the University of Pennsylvania as described (20, 21). Ultrathin sections from freshly fixed tissue were incubated with primary antibody to α-synuclein at 4 °C for 24 h. The grids were rinsed with TBST for 15 min and incubated with anti-rabbit colloidal gold-conjugated IgG. The sections were examined and imaged using an electron microscope (JEOL JEM-1010).
Measurement of Mitochondrial Complex Activities—Mitochondrial complex I, II, and IV activities were measured essentially as described (25). The activity of Rotenone sensitive complex I (NADH:ubiquinone oxidoreductase), in the mitochondria was assayed by following the decrease in absorbance due to the oxidation of NADH at 340 nm (ε = 6.81 mm-1 cm-1) using 50 μg of mitochondria. Complex II activity was measured by following the reduction of 2,6-dichlorophenolindophenol at 600 nm (e = 19.1 mm-1 cm-1) in the presence of succinate. Complex IV activity was assayed spectrophotometrically by measuring the decrease in absorbance of ferrocytochrome c at 550 nm. Activities of ubiquinol cytochrome c reductase (complex III) and NADH cytochrome c reductase (complex I–III link) was measured essentially as described (26). In another set of experiments, mitoplast (50 μg) isolated from substantia nigra of normal subject #9 was suspended in phosphate buffer, pH 7.4, containing 0.02% lauryl maltoside and incubated with varying amounts of purified human α-synuclein (Calbiochem) at 37 °C for 30 min simultaneously. Control reactions containing mitoplast and varying concentration of α-synuclein dilution buffer (100 mm NaCl, 20 mm, Tris-HCl (pH 7.5) and 1 mm MgCl2) were incubated at 37 °C for 30 min. Following the preincubation, the samples were assayed for mitochondrial complex activities as described (25, 26).
Measurement of ROS in Isolated Mitochondria—ROS was measured in the freshly isolated mitochondrial fractions from DAN cells transfected with wild-type and mutant α-synuclein constructs using hydroxyphenyl fluorescein. Mitochondria (50 μg) were incubated with hydroxyphenyl fluorescein (1 μm) in 1 ml of a medium containing 70 mm sucrose, 210 mm mannitol, 2 mm HEPES (pH 7.4), and 0.1 mm EDTA, 10 mm glutamate, 2 mm malate, and 2 mm ADP at 37 °C for 60 min in the dark. Fluorescence was measured using a spectrofluorometer utilizing excitation at 488 nm and emission at 515 nm.
Blue Native Gel Electrophoresis-coupled Western Blot— Freshly isolated mitochondrial samples (striatum) were solubilized in 0.2% lauryl maltoside and were subjected to blue native gel electrophoresis coupled Western blot as described (21). The blots were immunostained with antibodies to 39-kDa subunit of complex I (NDUFA9, Mitosciences) and α-synuclein.
Immunocapture of Complex I—Immunocapture of complex I was carried out using “Complex I Immunocapture Kit” from Mitosciences according to the manufacturer's protocol from 5 mg of mitochondrial fraction. The final elution of complex I was carried out using glycine buffer (0.2 m glycine-HCl, pH 2.5, containing 0.05% lauryl maltoside). Eluted complex I was subjected to 14% SDS-PAGE followed by Western immunoblot analysis using antibodies to α-synuclein and NDUFA9.
Statistical Analysis—Statistical analyses were performed using Origin 7.5 software (MicroCal Software, Northampton, MA). The correlation among mitochondrial α-synuclein content and complex I activity was statistically analyzed using scatter plots. Statistical analysis and the level of significance were assessed by one-way analysis of variance, followed by Tukey-Kramer post hoc test. In some instances statistical significance was tested by Student's t test.
RESULTS
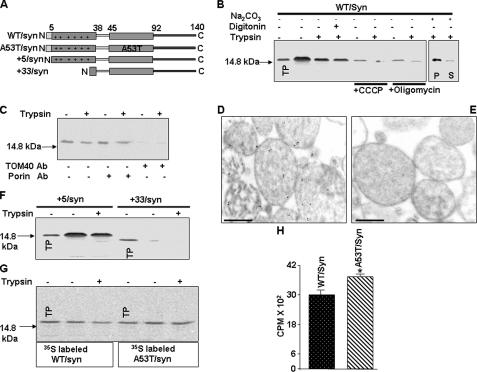
Mitochondrial Import Signals of Human α-Synuclein—As seen from Fig. 1A the N terminus of α-synuclein is rich in positively charged amino acids (lysine). Furthermore, this region can also form an α-helical structure (27) thus elucidating the physico-chemical characteristics of mitochondrial targeting signals (28, 29). We also used the Bioinformatics program, WoLFPSORT, which predicts the signal targeting activities of proteins. The analysis suggests a multi subcellular localization of α-synuclein, including mitochondria (Table 1). The percent distribution of α-synuclein to mitochondria was 16.5% when the whole protein sequence was used for analysis. Interestingly, the mitochondrial distribution of α-synuclein increased to 25.5% when the N-terminal 40-amino acid sequence was used for analysis, suggesting that the N terminus of human α-synuclein may function as mitochondrial targeting signal. These results prompted a detailed investigation on the mitochondrial transport of human α-synuclein using in vitro mitochondrial import and in vivo expression in neuronal cell system.
FIGURE 1.
Characterization of mitochondrial targeting properties of α-synuclein by mitochondrial import assay. A, description of wild-type (WT), familial, and N-terminal deletion constructs of α-synuclein. B, in vitro mitochondrial import of 35S-labeled WT/synuclein, in isolated rat liver mitochondria as described under “Materials and Methods” using 200 μg of trypsin/ml of reaction (+). Mitochondria were preincubated with or without added inhibitors (50 μm carbonyl cyanide-m-lorophenylhydrazone (CCCP) or 50 μm oligomycin) at 25 °C for 10 min before initiating the in vitro transport of WT/synuclein. C, effects of antibodies to porin and TOM40 on mitochondrial import of α-synuclein. Mitochondria were preincubated with antibodies (5 μg/ml) to porin and TOM40 at room temperature for 30 min prior to the initiation of import. Immunoelectron microscopy of mitochondrial imported unlabeledα-synuclein using rabbit polyclonal antibodies toα-synuclein (D) and the preabsorbed α-synuclein antibody (E). Sections were stained with secondary antibody conjugated to 10 nm gold. Bar = 500 nm. F, in vitro mitochondrial import of +5/synuclein and +33/synuclein. G and H, effects of A53T mutation on mitochondrial import of α-synuclein (G, autoradiogram and H, quantitation of trypsin-protected α-synuclein bands). Values are mean ± S.D. from three separate experiments. *, p < 0.05 as compared with WT/synuclein (Student's t test). 250 μg of mitochondrial protein were used for electrophoresis. TP = translation product (20% of total input), S = supernatant, P = pellet.
TABLE 1.
Prediction of distribution of α-synuclein into various subcellular organelles
|
Subcellular organelle
|
% distribution of α synuclein
|
|
|---|---|---|
| Complete protein sequence used for prediction | N-terminal 40 amino acids sequence used for prediction | |
| Mitochondria | 16.2 | 25.5 |
| Cytosol | 32.2 | 22 |
| Nucleus | 16.2 | 12.5 |
| Secretory vesicles | 35.4 | 40 |
Mitochondrial Import of α-Synuclein Is Energy-dependent and Requires Import Channel—We investigated whether the α-synuclein protein localizes to the mitochondria using an in vitro mitochondrial import assay, in which protection against limited proteolytic digestion was used as a criterion for the import of 35S-labeled synuclein protein. The cDNA constructs (Fig. 1A) were translated in the presence of [35S]methionine using the Quick Gold TNT system and incubated with freshly isolated rat liver mitochondria. Trypsin treatment of in vitro 35S-labeled wild-type α-synuclein (WT/syn) incubated with mitochondria resulted in the protection of the full-length ∼14-kDa 35S-labeled protein. This result suggests a complete translocation of this protein to mitochondria. In contrast, α-synuclein in reaction without added mitochondria was completely degraded by trypsin (data not shown).
Further characterization of the mitochondrial import of α-synuclein is shown in Fig. 1B. Results showed that digitonin treatment (75 μg/mg of protein), which strips off the mitochondrial outer membrane, had no effect on mitochondrial import of α-synuclein protein suggesting the intramitochondrial localization of α-synuclein. The import of 35S-labeled α-synuclein into the mitochondria was markedly inhibited by carbonyl cyanide-m-lorophenylhydrazone, which disrupts the mitochondrial membrane potential (Fig. 1B), and also by oligomycin, which inhibits mitochondrial ATP synthase leading to the depletion of the ATP pool (Fig. 1B). Consistent with the established views on energy requirements for mitochondrial protein transport, our results show that the transport of α-synuclein requires membrane potential and that it is dependent on the mitochondrial ATP pool. Furthermore, mitochondrially imported α-synuclein was mostly associated with pellet (P) fraction following the sodium carbonate extraction of digitonin treated mitochondria (mitoplast) (Fig. 1B, right panel). We further investigated the requirement of mitochondrial outer membrane receptor system for the import of α-synuclein in the presence of antibodies to TOM40 an import channel forming protein. As shown in the Fig. 1C, mitochondria preincubated with antibodies to porin, an outer membrane poreforming protein, were able to import 35S-labeled α-synuclein. However, antibodies to TOM40 inhibited the mitochondrial import of 35S-labeled α-synuclein. Collectively, these results suggest that the mitochondrial import of α-synuclein is physiological and requires outer membrane protein import channel.
Next, we investigated the mitochondrial localization of imported α-synuclein by immunoelectron microscopy. In vitro translated α-synuclein in the presence of unlabeled methionine was incubated with mitochondria in the presence of an energy-generating system as described under “Materials and Methods.” Following import mitochondria were pelleted through 1 m sucrose and subjected to immunoelectron microscopy using antibodies to α-synuclein. In support of results from carbonate extraction (Fig. 1B), immunoelectron micrograph presented in the Fig. 1D showed that most of α-synuclein-reactive gold particles were localized inside the mitochondrial compartment mostly in close proximity to the inner membrane suggesting the association of imported α-synuclein predominantly with inner membrane of mitochondria. Antibodies pre-adsorbed to α-synuclein antigenic peptide failed to show any gold labeling in the mitochondria indicating the specificity of α-synuclein antibody used in the present study (Fig. 1E).
Positively Charged Residues at N Terminus Are Required for Mitochondrial Targeting of α-Synuclein—Next we evaluated the sequence requirements for the targeting of α-synuclein to the mitochondria by generating N-terminal progressive deletion constructs. Deletion of first four amino acids (+5/syn) did not reduce the mitochondrial import efficiency of α-synuclein (Fig. 1F). However, the deletion of first 32 amino acids (+33/syn) markedly reduced the mitochondrial import of α-synuclein as no detectable 35S-labeled protein was protected in trypsin treated mitochondria (Fig. 1F). In support of our signal sequence prediction in Table 1, these results provide evidence that the N-terminal 32-amino acid function is a putative mitochondrial-targeting signal.
We also tested the mitochondrial import efficiency of familial mutation of α-synuclein. We chose the A53T mutation on α-synuclein, because this mutation has been shown to generate cellular abnormalities faster than wild-type α-synuclein (30). Autoradiogram presented in the Fig. 1G showed that 35S-labeled A53T/Syn was imported in to mitochondria. Quantitation of trypsin-protected 35S-labeled α-synuclein showed that A53T/syn was imported in to mitochondria with significantly higher efficiency (∼20%) as compared with wild type (Fig. 1H).
Time-dependent Accumulation of α-Synuclein in the Mitochondria of Human Dopaminergic Neuronal Cultures—The in vivo bimodal targeting of α-synuclein to mitochondria was studied in human fetal DANs, which are primarily affected in the pathogenesis of PD. The DAN culture system has been reported to provide a good model to study human dopamine-related disorders (31). We therefore studied the mitochondrial functions in DAN cells transfected with wild-type and mutant α-synuclein cDNA constructs. DAN cells express low but detectable levels of intracellular α-synuclein. To avoid the interference from endogenous α-synuclein, we cloned α-synuclein constructs in a mammalian cell expression vector (3-FLAG pcDNA3 vector) with a FLAG epitope at the 3′-end. DAN cells were transfected with WT/syn-FLAG and +33/syn-FLAG cDNAs. Twenty hours post transfection mitochondria and cytosol fractions were isolated, and the levels of FLAG antibody-reactive proteins were determined by immunoblot analysis. The results showed that, over time, WT/syn-FLAG protein (Fig. 2A) accumulated in the mitochondrial compartment. In support of in vitro mitochondrial import results, the +33/syn-FLAG protein showed vastly reduced mitochondrial localization (Fig. 2D) suggesting that the first 32 amino acids of α-synuclein are important for mitochondrial targeting under in vivo and in vitro conditions. The levels of mitochondrial marker TOM20 and cytosolic marker actin remained constant in DAN cells transfected with WT/syn-FLAG (Fig. 2, B and C, respectively) and +33/syn-FLAG (Fig. 2, E and F, respectively). Notably, actin immunoreactivity was detectable only in cytosol, whereas TOM20 was detected in mitochondria. These results suggest that mitochondrial and cytosolic fractions used in this study did not contain significant cross contamination and were of high purity. Results from quantitative ELISA showed that the mitochondrial accumulation of WT/syn, which varied from 3 to 10 ng of mitochondrial protein at various time points (Fig. 2G). Interestingly, the corresponding cytosol-associated WT/syn levels declined with time (Fig. 2G). However, the total cellular expression of WT/syn, and +33/syn remained the same at all three time points tested (Fig. 2H).
FIGURE 2.
Mitochondrial targeting of WT/α-synuclein and +33/α-synuclein in DAN neuronal cultures. DAN cells were transfected with WT/synuclein-FLAG (A) and +33/synuclein-FLAG (D). Cytosol (cyto.) and mitochondrial (mito.) fractions (100 μg) were probed with FLAG antibodies. B and C show the levels of loading controls: TOM20 and actin, respectively, for A. E and F show the levels of loading controls: TOM20 and actin, respectively, for D. G, quantitation of WT/synuclein and +33/synuclein in the mitochondrial and cytosolic fractions by ELISA at different time points of transfection. Values are mean ± S.D. from three separate experiments. *, p < 0.05 compared with 20-h transfection. H, following the transfection, the whole cell lysates (homogenate) from DAN cells were tested for the levels of α-synuclein at different time points using quantitative ELISA. Values are mean ± S.D. from three separate experiments. No significant variation was observed (one-way analysis of variance with Tukey-Kramer post test).
The localization of α-synuclein in mitochondria was also investigated by immuno-colocalization of the protein in DAN cells transfected with WT/syn-FLAG and +33/syn-FLAG cDNA constructs. Triton-permeabilized cells were subjected to double immunostaining with FLAG antibody and antibody to the mitochondrial outer membrane receptor, TOM20. Cells transfected with WT/syn-FLAG showed a robust intracellular α-synuclein staining pattern that included extranuclear granulate structures (Fig. 3A) and mitochondria specific marker TOM20 staining (Fig. 3B). Fig. 3C shows that most of the α-synuclein staining colocalized with TOM20 staining. However, the expression of +33/syn-FLAG cDNA yielded predominantly extramitochondrial α-synuclein staining (Fig. 3D) and mitochondrial TOM20 staining (Fig. 3E), which did not overlap with each other (Fig. 3F). These results confirm that ectopically expressed full-length α-synuclein is targeted to mitochondria and that the N-terminal 32 residues are important for mitochondrial targeting.
FIGURE 3.
Mitochondrial localization of WT/α-synuclein and +33/α-synuclein by immunofluorescence microscopy. DAN cells were transfected with WT/synuclein-FLAG (A–C) or +33/synuclein-FLAG (D–F). Cells (A and B, and D and E) were double immunostained with antibodies to FLAG and TOM20, respectively. C and F are the merged image of A and B, and D and E, respectively. Bar = 10 mm.
Mitochondrial Accumulation of Wild-type α-Synuclein Induces Oxidative Stress and Impaired Complex I Activity—Overexpression of α-synuclein in cell cultures is associated with mitochondrial dysfunction and oxidative stress (14). In addition, impaired complex I activity is one of the prominent mitochondrial lesions in PD (32, 33). We, therefore, investigated the effect of mitochondrial accumulation of α-synuclein on mitochondrial complex I activity. The mitochondrial fraction of DAN cells transfected with wild-type, A53T/syn, and +33/syn FLAG cDNA constructs were assessed for complex I activity. Time-dependent accumulation of wild-type α-synuclein in DAN cell mitochondria (Fig. 4A) was accompanied by a significant decline in rotenone sensitive complex I activity (Fig. 4C), and an increase in reactive oxygen species (ROS) (Fig. 4D) as compared with control cells transfected with vector DNA alone. Furthermore, the familial A53T/syn FLAG showed significant mitochondrial accumulation by as early as 20 h as compared with WT/syn (Fig. 4A). Interestingly, A53T/syn FLAG-expressing cells showed a significant reduction in complex I activity (Fig. 4C) and elevated ROS (Fig. 4D) at an earlier time point than the WT/syn FLAG. However, the levels of mitochondrial TOM20, which served as a loading control, were unchanged (Fig. 4B). Mitochondrial accumulation of α-synuclein showed no significant reduction in complex II, III, and IV activities (data not shown). Transfection with +33/syn FLAG, which showed no detectable mitochondrial localization (Fig. 2F), did not result in significant effect on either mitochondrial complex I activity (Fig. 4C) or the production of ROS (Fig. 4D). Similar results were obtained when DAN cells were transfected with α-synuclein constructs without FLAG tag (data not shown). These results show for the first time that progressive accumulation of α-synuclein in the mitochondrial compartment may impair vital mitochondrial functions and induce oxidative stress.
FIGURE 4.
Mitochondrial complex activities in DAN cells expressing various α-synuclein constructs. A, Western immunoblot analysis of mitochondrial (100 μg) localization of WT/synuclein-FLAG and A53T/synuclein-FLAG with time using antibodies to FLAG. B, levels of TOM20. Mitochondrial complex I activity (C) and the production of ROS (D) were estimated at various time points following the expression of WT/synuclein-FLAG, A53T/α-synuclein-FLAG, and +33/synuclein-FLAG cDNA constructs. *, p < 0.05 compared with vector control. **, p < 0.05 compared with vector control, and +33/α-synuclein-FLAG constructs. #, p < 0.05 compared with WT/synuclein-FLAG (one-way analysis of variance with Tukey-Kramer post test). Western immunoblot analysis of endogenous α-synuclein (E), actin (F), and TOM20 (G) in total homogenate (100 μg) and mitochondria (100 μg) following 48-h transfection of α-synuclein-specific siRNA in DAN cells. Mitochondrial NADH cytochrome c reductase (H) and complex I (I) in control and α-synuclein-specific siRNA transfected DAN cells. *, p < 0.05 (Student t test).
Role of Endogenous α-Synuclein on Mitochondrial Functions—A recent study showed the impairment of connection between mitochondrial complex I and III in α-synuclein knock-out mice (26). We therefore investigated the role of the constitutive presence of α-synuclein on complex I activity in human DAN cells by siRNA-mediated knockdown of α-synuclein. Results showed low but detectable levels of α-synuclein in the mitochondrial fraction of control siRNA-transfected cells (Fig. 4E). Following the post 48-h transfection of DAN cells with α-synuclein-specific siRNA, the protein levels of α-synuclein were undetectable in the total homogenate as well as in mitochondrial fraction as compared with control siRNA-transfected DAN cells suggesting there was efficient knockdown of endogenous α-synuclein (Fig. 4E). However, the transfection of neither control siRNA nor α-synuclein-specific siRNA had no effect on the levels of actin (Fig. 4F) and mitochondrial TOM20 (Fig. 4G). In agreement with Ellis et al. (26) there was a significant reduction in the NADH cytochrome c reductase, which reflects the connection between complex I and complex III, in the mitochondria from α-synuclein knockdown DAN cells (Fig. 4H). Complex I activity in these cells showed a marginal but statistically insignificant decrease (Fig. 4I). These results suggest an important role for the constitutively expressed α-synuclein in maintaining the integrity of mitochondrial complexes.
α-Synuclein Accumulates in the Mitochondria of Striatum and Substantia Nigra of PD—We investigated the PD relevance of mitochondrial targeting and accumulation of α-synuclein in in vitro import and DAN neuronal culture system using human fresh postmortem PD and normal brains. The purity of mitochondria and cytosolic fractions isolated from human PD and normal brains by Western immunoblot analysis using antibodies to cytosol-specific marker actin, mitochondrial specific marker TOM20, nuclear marker p97 (Fig. 5A), endoplasmic reticulum marker Sec 61, and Golgi marker TGN 38 (Fig. 5B). The results showed that none of the marker proteins except mitochondrial TOM20 was detected in the mitochondrial fractions (Fig. 5, A and B) suggesting that the mitochondrial fractions used in this study were free from cytosolic, nuclear, endoplasmic reticulum, and Golgi contamination.
FIGURE 5.
Subcellular purity and mitochondrial localization of α-synuclein in human PD and normal brains. Western immunoblot analysis of marker proteins for different subcellular fractions from substantia nigra (100 μg of protein each) was carried out using antibodies to p97, TOM20, actin (A), and Sec 61 and TGN 38 (B). C, amounts of α-synuclein present in the mitochondrial fractions from substantia nigra of normal and PD subjects as quantitated by ELISA. Values are mean ± S.D. from three separate experiments. Western immunoblot analysis of mitochondria (50 μg) using antibodies to α-synuclein of substantia nigra (SN, D), striatum (ST, G), and cerebellum (CE, J) and. F, I, and L are Western immunoblot analysis of TOM20 levels (as a loading control) in D, G, and J blots, respectively. E, H, and K represent the quantification of α-synuclein immunoreactivity in Western blots D, G, and J, respectively. Values are mean ± S.D. from three separate experiments. *, p < 0.05 compared with corresponding normal levels (one-way analysis of variance with Tukey-Kramer post test). NS = normal subjects, N = nucleus, M = mitochondria, MC = microsomes, and C = cytosol.
Using quantitative ELISA technique we determined the levels of α-synuclein in the mitochondria isolated from substantia nigra of PD and normal subjects, because this brain region is the part of basal ganglia structure, which is known to be vulnerable during the pathogenesis of PD. Results showed low levels of α-synuclein (2–6 ng/mg of mitochondrial protein) in the mitochondria from normal subjects while mitochondria from PD subjects contained significantly higher levels (∼10–100 ng/mg of mitochondria) α-synuclein (Fig. 5C). We further investigated the levels and molecular weight of mitochondria-localized α-synuclein in substantia nigra as well as in another basal ganglia structure such as striatum of randomly chosen PD and control subjects by employing Western immunoblot techniques. Results were compared with respective non-basal ganglia structure such as cerebellum. Immunoblots of mitochondrial α-synuclein and their quantitation showed low and almost equal levels of ∼14-kDa α-synuclein in mitochondria from substantia nigra (Fig. 5, D and E), striatum (Fig. 5, G and H), and cerebellum (Fig. 5, J and K) of control subjects suggesting the constitutive presence of α-synuclein in the mitochondria of these brain regions. In support of results from quantitative ELISA (5C), mitochondria from substantia nigra (Fig. 5, D and E) and striatum (Fig. 5, G and H) but not cerebellum from PD subjects showed a significant accumulation of α-synuclein as compared with normal subjects. However, the levels of α-synuclein in the mitochondria of cerebellum from PD and normal subjects were similar (Fig. 5, J and K). Importantly, mitochondrial loading control TOM20 levels were similar in all cases of PD and normal subjects (Fig. 5, I and L). These results suggest the preferential accumulation of α-synuclein in the mitochondria of substantia nigra and striatum of basal ganglia in PD subjects. Furthermore, mitochondrial accumulation of α-synuclein in substantia nigra and striatum of PD subjects was not associated with either age or postmortem intervals (data not shown).
α-Synuclein Is Predominantly Associated with Inner Membrane of Mitochondria in PD Brain—Mitochondrial localization and membrane topology of α-synuclein in PD brain were investigated by treatment with trypsin or digitonin and also by sodium carbonate (Na2CO3) extraction. The representative blot showed that the limited trypsin treatment, which removes the cytosol-exposed outer mitochondrial membrane proteins as well as contaminating proteins sticking to the mitochondria outer membrane, protected ∼14-kDa α-synuclein (Fig. 6A), matrix-associated mitochondrial HSP70 (Fig. 6B), and inner membrane cytochrome c oxidase subunit 1 (Fig. 6C), but not the cytosolic-exposed outer membrane TOM20 (Fig. 6D). However, the similar trypsin treatment completely digested α-synuclein in the cytosolic fraction (Fig. 6E). Results also showed that digitonin treatment of mitochondria, which strips off the outer membrane, significantly reduced the levels of TOM20 (Fig. 6D) but not of ∼14-kDa α-synuclein (Fig. 6A), HSP 70 (Fig. 6B), or cytochrome c oxidase subunit 1 (Fig. 6C) suggesting the association of α-synuclein with the inner membrane or matrix compartments. Furthermore, extraction of digitonin-treated mitochondria (mitoplast) with alkaline Na2CO3 resulted in near complete recovery of mitochondrial matrix HSP70 in the soluble fraction (Fig. 6B). However, α-synuclein (Fig. 6A) and cytochrome c oxidase subunit 1, an inner membrane protein (Fig. 6C), both partitioned in the insoluble fraction. In support of results from in vitro import data (Fig. 1, B and D), these data suggest a membrane-intrinsic association of α-synuclein with the inner membrane of mitochondria of PD brain.
FIGURE 6.
Biochemical and immunoelectron microscopy characterization of mitochondrial α-synuclein in human PD and normal brains. Western blot analysis of 50 μg of mitochondrial fraction from substantia nigra of PD #1 for α-synuclein (A), mitochondrial (mt)HSP70 (B), CcO (C), and TOM20 (D) following the treatment with trypsin, digitonin, and sodium carbonate. Sup = supernatant, Dig = digitonin. E, Western blot analysis of 50 μg of cytosol from substantia nigra of PD #1 treated with or without trypsin (30 μg/mg of protein) using antibodies to α-synuclein. Immunoelectron microscopy analysis of α-synuclein in the mitochondria of substantia nigra of PD subject (PDS #1) (F) and normal subject (NS #11) (G) using anti rabbit antibodies toα-synuclein. The sections were stained with secondary antibody conjugated to 20 nm (E) and 10 nm (F), respectively. M = mitochondrion, V = vesicle, C = cytosol. Bar = 100 nm. Arrows indicate association of gold particle to the outer membrane of mitochondria.
To substantiate the above biochemical observation, we carried out immunoelectron microscopy of substantia nigra of multiple PD and normal subjects using α-synuclein antibody, and co-stained with colloidal gold-conjugated secondary antibodies. In support of in vitro import of α-synuclein (Fig. 1, B and D), Western blot (Fig. 6, D, G, and J), and results of carbonate extraction (Fig. 6A), the representative electron micrograph of PD subject (#1) sections showed the α-synuclein-specific electron dense particles mostly associated with the mitochondrial (M) inner membrane (average of ∼10 particles/mitochondria) (Fig. 6F), whereas normal subjects showed one or two foci of α-synuclein labeling in the mitochondria (Fig. 6G). However, occasionally we observed the association of one or more gold particles (one or two) on the outer membrane of a few mitochondria (arrows) of both PD (Fig. 6F) and normal subjects (Fig. 6G). As expected, the extramitochondrial α-synuclein immunolabeling in both PD and normal subjects was associated with vesicles (V) and cytosol (C) (Fig. 6, F and G).
Mitochondrial Functional Parameters in PD Brain—Progressive accumulation of α-synuclein in mitochondria was associated with impaired complex I activity and oxidative stress in the DAN culture system. Hence, we examined whether the accumulation of α-synuclein in the mitochondria of basal ganglia structures of PD subjects was correlated with functional parameters, namely rotenone sensitive complex I activities. Results showed ∼3-fold decrease in the mitochondrial complex I activities in the substantia nigra of PD subjects as compared with the activities of normal subjects (Fig. 7A). Striatum from PD subjects also showed similar results (data not shown). On the contrary, mitochondrial complex I activities in the cerebellum of PD were similar to the activities of normal subjects (Fig. 7B). Next, we analyzed the correlation between the levels of α-synuclein in the mitochondria of substantia nigra from PD subjects and corresponding mitochondrial complex I activities by using linear regression plots, which showed highly significant negative correlation (Fig. 7C). On the contrary, the cerebellum of PD, which did not show the accumulation of α-synuclein in the mitochondrial fraction, yielded no significant correlation between mitochondrial α-synuclein levels and complex I activities (Fig. 7D).
FIGURE 7.
Correlation between mitochondrial α-synuclein and complex I activity in PD subjects. Distribution of complex I activities in the mitochondria of substantia nigra (A) and cerebellum (B) from PD and control subjects. Scatter plots of mitochondrial α-synuclein levels versus complex I activity in substantia nigra of PD subjects (C) and scatter plots of mitochondrial α-synuclein levels versus complex I activity in cerebellum (D). Regression analysis was carried out using Origin 7.5 software.
Association of Mitochondrial Accumulated α-Synuclein with Complex I—We studied the possible role of α-synuclein on complex I inhibition by two approaches, because α-synuclein was predominantly associated with the mitochondrial inner membrane compartment. In the first set of experiments, we isolated mitoplast from substantia nigra of normal brain and incubated with varying concentrations of purified α-synuclein and analyzed the complex I activity as described under “Materials and Methods.” Results presented in Fig. 8A showed that complex I activity was inhibited by α-synuclein in a concentration-dependent fashion. Next, we tested the specificity of α-synuclein in disrupting other complexes such as complexes II, III, and IV. Incubation with the highest concentration of α-synuclein (1.5 μg/mg of mitochondrial protein, which impaired complex I activity very significantly) with mitoplast didn't result in the significant reduction of activities of complexes II (Fig. 8B), III (Fig. 8C), and IV (Fig. 8D). These results suggest the possibility of preferential interaction of α-synuclein with complex I. To substantiate this in vitro observation, we carried out the blue native electrophoresis of solubilized mitochondrial complexes (200 μg of protein) from striatum of PD subjects and normal subjects as well as DAN cells expressing wild-type and truncated α-synuclein mutants. The complexes were transferred to polyvinylidene difluoride membranes, and companion blots were probed with either antibodies to α-synuclein or the 39-kDa subunit of complex I (NDUFA9), which detects the subcomplexes of 250, 600, and the holo complex of 950 kDa (34). A representative blot (Fig. 8E) showed that antibodies to NDUFA9 recognized three bands migrating around 250, 600, and 950 kDa in mitochondria from PD brain and DAN cells transfected with wild-type for 60 h. Interestingly, antibodies to α-synuclein recognized ∼950 and ∼600 kDa complexes (Fig. 8F). However, antibodies to NDUFA9 recognized the major 950-kDa complex I from mitochondria of normal striatum, 20-h WT, and 60-h +33/syn transfected (both 20 and 40 h) cells (Fig. 8E), although these complexes did not cross-react with antibodies to α-synuclein (Fig. 8F). To further document the association of mitochondrial accumulated α-synuclein, we carried out the immunocapture of complex I from mitochondria from striatum of PD and normal as well as DAN cells expressing α-synuclein. Immunoprecipitated complex I was subjected to SDS-PAGE coupled Western immunoblot analysis with antibodies to α-synuclein (Fig. 8G) and NDUFA9 (Fig. 8H). Results showed the presence of high amounts of α-synuclein in the immunocaptured complex I from PD brain and wild-type α-synuclein expressing DAN cell mitochondria (Fig. 8G). As expected the immunocaptured complexes I from mitochondria isolated from PD, normal, and DAN cells expressing wild-type and +33/syn contained 39-kDa subunit of complex I, NDUFA9 protein (Fig. 8H). These results provide compelling evidence that mitochondrially accumulated α-synuclein in human dopaminergic neuronal cultures and PD brains may interact with complex I, which is likely causing mitochondrial dysfunction.
FIGURE 8.
Interaction of α-synuclein with mitochondrial complex I. A, effect of in vitro incubation of mitoplast from substantia nigra of control subject #9 with dilution buffers (a) and varying concentrations of α-synuclein (b). Effect of α-synuclein (1.5 μg/mg of mitochondrial protein) on the activities of complex II (B), complex III (C), and complex IV (D). Mitochondria from striatum of a normal subject (NS) and a PD subject (PDS), as well as DAN cells expressing α-synuclein constructs were solubilized and run on blue native PAGE. Following the separation, complexes were transferred on polyvinylidene difluoride membranes and stained with antibodies to NDUFA9 (E) and α-synuclein (F). Co-immunoprecipitation of α-synuclein using complex I immunocapture kit (Mitosciences). Immunocaptured mitochondrial complexes I (4 μg) from striatum of PD and normal subjects as well as DAN cells expressing α-synuclein constructs were resolved on 14% SDS-PAGE and subjected to Western immunoblot using antibodies to α-synuclein (G) and NDUFA9 (H).
DISCUSSION
In this study we show for the first time that human α-synuclein is imported into mitochondria under both in vitro and in vivo conditions in human DAN cells. Mitochondrial import of α-synuclein is dependent on mitochondrial transmembrane potential (ΔΨm) and mitochondrial ATP suggesting that the mitochondrial entry of α-synuclein is physiological and follows established mitochondrial import paradigms. Furthermore, we also show that α-synuclein accumulates in the mitochondrial compartment of basal ganglia structures (striatum and substantia nigra) from brains from PD patients.
Using multiple approaches we have characterized the putative mitochondrial targeting signal of α-synuclein. The N-terminal progressive deletion analysis shows that the N-terminal 32-amino acid region of α-synuclein, which contains evenly spaced positive residues, is critical for mitochondrial targeting. Furthermore, the mitochondrial targeting signal of α-synuclein is noncleavable. In support of results from in vitro import, we show that α-synuclein is constitutively localized to mitochondria of dopaminergic neurons and fresh postmortem human brains. Interestingly, the biochemical and immunoelectron microscopy data presented in this study provide compelling evidence that mitochondria-localized α-synuclein is predominantly associated with inner membrane in the human system. A recent report showed the constitutive presence of low levels of α-synuclein on the outer mitochondrial membrane of mouse brain (35). In support of this, we have also observed a few specific labeling of α-synuclein on the outer mitochondrial membranes of human brain. However, the reason for the predominant association of α-synuclein with mitochondrial inner membrane in the human system versus predominant outer membrane localization of in the mouse system is not clear.
The physiological functions of constitutively present α-synuclein in the mitochondrial and extramitochondrial compartments are not clear. Results from knockdown of α-synuclein in the human system (Fig. 4, H and I) and α-synuclein knock-out mice (26) showed impaired connectivity between complexes I and III. Furthermore, using α-synuclein knock-out mice (26) a possible role for extramitochondrial α-synuclein in the synthesis of precursors of cardiolipin, a mitochondrial inner membrane-associated lipid, has been suggested. Cardiolipin is thought to be responsible for maintaining the physical connectivity of mitochondrial electron transport chain complexes involved in oxidative phosphorylation (36). Several lines of evidence suggest that constitutive α-synuclein may be a chaperone protein and can also bind to cardiolipin (3, 37–39). The present study suggests that mitochondria localized α-synuclein is in association with complex I and lowering the endogenous mitochondrial levels by siRNA may impair connectivity between complex I and III in human DAN cell culture. This may also be related to a possible chaperone role for mitochondrial localized constitutive α-synuclein in maintaining the physiological functions of mitochondrial electron transport chain. Collectively, observations by us and Ellis et al. (26) suggest an important role for endogenous α-synuclein in maintaining the normal functions of mitochondrial complexes involved in the oxidative phosphorylation.
Interestingly, our in vivo data using human DAN cell culture and PD brains suggests that progressive accumulation of α-synuclein in the mitochondria impairs complex I function and causes increased oxidative stress. This conclusion is further supported by experiments showing that overexpression of +33/syn that lacks the mitochondrial targeting signal not only failed to localize to mitochondria but also did not cause mitochondrial abnormalities. Accumulation of α-synuclein in the mitochondria from striatum and substantia nigra of PD subjects varied from ∼2- to 15-fold as compared with normal subjects. However, the reason for mitochondrial accumulation of α-synuclein in the basal ganglia structures in the PD subjects is not clear. One possibility is that there is an increased mitochondrial targeting of α-synuclein protein during the pathogenesis of PD. In a series of previous studies we have reported an important role for post translation modifications in the mitochondrial targeting of a number of physiologically important proteins (16, 17, 19). We showed that mitochondrial targeting of CYP 2B1 and 2E1 requires protein kinase A-mediated phosphorylation of Ser residues at positions 128 or 129, respectively (16, 17). However, in the case of GSTA4–4, phosphorylation of a tandem protein kinase A/protein kinase C target site close to the C terminus is required for the activation of the mitochondrial targeting signal (19). It has been shown that α-synuclein undergoes a number of post translational modifications, including phosphorylation and nitration under normal and disease status (40, 41). It is likely that these modifications contribute to increased mitochondrial accumulation by altering the rate of mitochondrial targeting. The second possibility is that mitochondrially translocated α-synuclein is turned over at slower rate during the pathogenesis of PD. However, at present, the nature of mitochondrial proteases involved in the turnover of mitochondria-associated α-synuclein under normal and disease conditions is not known. Currently, work is in progress to investigate the role of post translational modification on mitochondrial targeting of α-synuclein as well as the proteases involved in the turnover of α-synuclein under normal and disease conditions.
Our results also showed a significant correlation between the accumulation of α-synuclein levels and decreased complex I activities in human PD basal ganglia. Decrease in complex I activity in PD brain is in agreement with other reports that showed 30 and 39% reductions in complex I and complex I/III activities, respectively, in the substantia nigra (42) and 32% reduction in the cortex of PD patients (32). Besides these, several groups have also emphasized the importance of impaired mitochondrial complex I activity as the cause of mitochondrial dysfunction in PD models (4, 43, 44). Another line of direct evidence for the involvement of decreased complex I activity in the pathogenesis of PD comes from studies administering complex I inhibitors such as rotenone and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to rodents, which showed mitochondrial dysfunction as well as the loss of dopaminergic neurons (45, 46). Interestingly, our results also show the concentration-dependent inhibition of complex I activity by externally added purified α-synuclein under in vitro conditions. An important observation of this study is that α-synuclein was found in direct association with ∼950-kDa holo complex I both in mitochondria of PD basal ganglia and dopaminergic neurons transfected with WT α-synuclein, where higher mitochondrial levels of α-synuclein was found. These results suggest that α-synuclein may directly target the holo complex I.
Mechanistically our results suggest that direct association of mitochondrial accumulated α-synuclein with complex I may be a critical factor leading to complex I dysfunction and increased oxidative stress. In particular, association of α-synuclein with the 600-kDa subcomplex of complex I suggest two possibilities: It is likely that α-synuclein binds to subcomplexes that are in the process of becoming a stepwise assembly of holo complex I (34, 47, 48). Alternatively, α-synuclein binding may cause the disassociation of holo complex I to subcomplexes. In contrast, mitochondria from normal striatum and DAN cells transfected with α-synuclein cDNA for 20 h, which showed low levels of mitochondrial α-synuclein did not show any detectable association of α-synuclein with complex I. Furthermore, extramitochondrially accumulated N-terminal truncated α-synuclein protein neither associated with complex I nor affected its activity significantly. Taken together our results suggest that higher levels of α-synuclein-mediated mitochondrial dysfunction primarily may stem from its action on mitochondria, and more preferentially with complex I.
Results from in vivo and in vitro studies suggest that the mitochondrial accumulation of familial A53T/syn occurred at faster rates than wild type and showed the mitochondrial abnormalities at an earlier time point. Although in vitro import data suggest an increase in the mitochondrial targeting of A53T/syn, it is likely that in vivo this mutation may also decrease the turnover of α-synuclein in the mitochondrial compartment. Besides A53T, other mutations such as A30P and E46K are also known to occur in the familial PD (49, 50). However, the precise effects of these familial mutations on mitochondrial transport and its functions in the pathogenesis of PD are a subject of future investigation.
In summary, using multiple human model systems, we show for the first time that constitutive low levels of mitochondrial α-synuclein may be important for the normal functions of mitochondrial complexes, whereas mitochondrial accumulation of α-synuclein may contribute to the impairment of complex I function. Thus the balance between low and high levels of mitochondrial α-synuclein may be an important factor in the outcome of mitochondrial dysfunction as seen in PD.
Acknowledgments
We thank Dr. Serge Fuchs (University of Pennsylvania, Philadelphia, PA) for providing 3 FLAG pcDNA3 vector and NDRI, Philadelphia for providing fresh postmortem human brains.
This work was supported by NIA, National Institutes of Health Grant AG 021920. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
Footnotes
The abbreviations used are: PD, Parkinson disease; DAN, dopaminergic primary neuronal; NDRI, National Disease Resource Interchange; ELISA, enzyme-linked immunosorbent assay; ROS, reactive oxygen species; siRNA, small interference RNA.
References
- 1.Bennett, M. C. (2005) Pharmacol. Ther. 105 311-331 [DOI] [PubMed] [Google Scholar]
- 2.Dawson, T. M., and Dawson, V. L. (2003) Science 302 819-822 [DOI] [PubMed] [Google Scholar]
- 3.Shults, C. W. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 1661-1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou-Sleiman, P. M., Muqit, M. M., McDonald, N. Q., Yang, Y. X., Gandhi, S., Healy, D. G., Harvey, K., Harvey, R. J., Deas, E., Bhatia, K., Quinn, N., Lees, A., Latchman, D. S., and Wood, N. W. (2006) Ann. Neurol. 60 414-419 [DOI] [PubMed] [Google Scholar]
- 5.Fiskum, G., Starkov, A., Polster, B. M., and Chinopoulos, C. (2003) Ann. N. Y. Acad. Sci. 991 111-119 [DOI] [PubMed] [Google Scholar]
- 6.Greenamyre, J. T., Betarbet, R., and Sherer, T. B. (2003) Parkinsonism. Relat. Disord. 9 Suppl. 2, S59-S64 [DOI] [PubMed] [Google Scholar]
- 7.Lin, M. T., and Beal, M. F. (2006) Nature 443 787-795 [DOI] [PubMed] [Google Scholar]
- 8.Bonifati, V., Rizzu, P., Squitieri, F., Krieger, E., Vanacore, N., van Swieten, J. C., Brice, A., van Duijn, C. M., Oostra, B., Meco, G., and Heutink, P. (2003) Neurol. Sci. 24 159-160 [DOI] [PubMed] [Google Scholar]
- 9.Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., Dekker, M. C., Squitieri, F., Ibanez, P., Joosse, M., van Dongen, J. W., Vanacore, N., van Swieten, J. C., Brice, A., Meco, G., van Duijn, C. M., Oostra, B. A., and Heutink, P. (2003) Science 299 256-259 [DOI] [PubMed] [Google Scholar]
- 10.Valente, E. M., bou-Sleiman, P. M., Caputo, V., Muqit, M. M., Harvey, K., Gispert, S., Ali, Z., Del, T. D., Bentivoglio, A. R., Healy, D. G., Albanese, A., Nussbaum, R., Gonzalez-Maldonado, R., Deller, T., Salvi, S., Cortelli, P., Gilks, W. P., Latchman, D. S., Harvey, R. J., Dallapiccola, B., Auburger, G., and Wood, N. W. (2004) Science 304 1158-1160 [DOI] [PubMed] [Google Scholar]
- 11.Canet-Aviles, R. M., Wilson, M. A., Miller, D. W., Ahmad, R., McLendon, C., Bandyopadhyay, S., Baptista, M. J., Ringe, D., Petsko, G. A., and Cookson, M. R. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 9103-9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore, D. J., Zhang, L., Troncoso, J., Lee, M. K., Hattori, N., Mizuno, Y., Dawson, T. M., and Dawson, V. L. (2005) Hum. Mol. Genet. 14 71-84 [DOI] [PubMed] [Google Scholar]
- 13.Silvestri, L., Caputo, V., Bellacchio, E., Atorino, L., Dallapiccola, B., Valente, E. M., and Casari, G. (2005) Hum. Mol. Genet. 14 3477-3492 [DOI] [PubMed] [Google Scholar]
- 14.Hsu, L. J., Sagara, Y., Arroyo, A., Rockenstein, E., Sisk, A., Mallory, M., Wong, J., Takenouchi, T., Hashimoto, M., and Masliah, E. (2000) Am. J. Pathol. 157 401-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, L. J., Pan, Y., Price, A. C., Sterling, W., Copeland, N. G., Jenkins, N. A., Price, D. L., and Lee, M. K. (2006) J. Neurosci. 26 41-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anandatheerthavarada, H. K., Biswas, G., Mullick. J., Sepuri, N. B., Otvos, L., Pain, D., and Avadhani, N. G. (1999) EMBO J. 18 5494-5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robin, M.-A., Anandatheerthavarada, H. K., Biswas, G., Naresh Babu V. Sepuri., Gordon, D. M., Pain, D and Avadhani, N. G. (2002) J. Biol. Chem. 277 40583-40593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addya, S., Anandatheerthavarada, H. K., Biswas, G., Bhagwat, S. V., Mullick, J., and Avadhani, N. G. (1997) J. Cell Biol. 139 589-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robin, M. A., Prabu, S. K., Raza, H., Anandatheerthavarada, H. K., and Avadhani, N. G. (2003) J. Biol. Chem. 278 18960-18970 [DOI] [PubMed] [Google Scholar]
- 20.Anandatheerthavarada, H. K., Biswas, G., Robin, M. A., and Avadhani, N. G. (2003) J. Cell Biol. 161 41-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devi, L., Prabhu, B. M., Galati, D. F., Avadhani, N. G., and Anandatheerthavarada, H. K. (2006) J. Neurosci. 26 9057-9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak, M. (1986) Cell 44 283-292 [DOI] [PubMed] [Google Scholar]
- 23.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265-275 [PubMed] [Google Scholar]
- 24.Anandatheerthavarada, H. K., Addya, S., Dwivedi, R. S., Biswas, G., Mullick, J., and Avadhani, N. G. (1997) Arch. Biochem. Biophys. 339 136-150 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Biswas G, Prabu, S. K., and Avadhani N. G. (2006) Biochem. Pharmacol. 72 881-892 [DOI] [PubMed] [Google Scholar]
- 26.Ellis, C. E., Murphy, E, J., Mitchell, D. C., Golovko, M. Y., Scaglia, F., Barcelo′-Coblijn, G. C., and Nussbaum, R. L. (2005) Mol. Cell. Biol. 25 10190-10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulmer, T. S., and Bax, A. (2005) J. Biol. Chem. 280 43179-43187 [DOI] [PubMed] [Google Scholar]
- 28.Von Heijne, G. (1986) EMBO J. 5 1335-1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehlin, P., Katrin Brandner, K., and Pfanner, N. (2004) Nat. Rev. Mol. Cell Biol. 5 519-530 [DOI] [PubMed] [Google Scholar]
- 30.Smith, W. W., Jiang, H., Pei, Z., Tanaka, Y., Morita, H., Sawa, A., Dawson, V. L., Dawson, T. M., and Ross, C. A. (2005) Hum. Mol. Gen. 14 3801-3811 [DOI] [PubMed] [Google Scholar]
- 31.Xu, J., Kao, S. Y., Lee, F. J., Song, W., Jin, L. W., and Yankner, B. A. (2002) Nat. Med. 8 600-606 [DOI] [PubMed] [Google Scholar]
- 32.Keeney, P. M., Xie, J., Capaldi, R. A., and Bennett, J. P., Jr. (2006) J. Neurosci. 26 5256-5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krige, D., Carroll, M. T., Cooper, J. M., Marsden, C. D., and Schapira, A. H. (1992) Ann. Neurol. 32 782-788 [DOI] [PubMed] [Google Scholar]
- 34.Ugalde, C., Vogel, R., Huijbens, R., Van Den, H. B., Smeitink, J., and Nijtmans, L. (2004) Hum. Mol. Genet. 13 2461-2472 [DOI] [PubMed] [Google Scholar]
- 35.Li, W. W., Yang, R., Guo, J. C., Ren, H. M., Zha, X. L., Cheng, J. S., and Cai, D. F. (2007) Neuroreport 18 1543-1546 [DOI] [PubMed] [Google Scholar]
- 36.Chicco, A. J., and Sparagna, G. C. (2007) Am. J. Physiol. Cell Physiol. 292 C33-C44 [DOI] [PubMed] [Google Scholar]
- 37.Kim, T. D., Paik, S. R, Yang, C. H., and Kim, J. (2000) Protein Sci. 9 2489-2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn, M., Kim, S., Kang, M., Ryu, Y., and Kim, T. D. (2006) Biochem. Biophys. Res. Commun. 346 1142-1149 [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan, M., Jensen, P. H., and Marsh, D. (2003) Biochemistry 42 12919-12926 [DOI] [PubMed] [Google Scholar]
- 40.Recchia, A., Debetto, P., Negro, A., Guidolin, D., Skaper, S. D., and Giusti, P. (2004) FASEB J. 18 617-626 [DOI] [PubMed] [Google Scholar]
- 41.Okochi, M., Walter, J., Koyama, A., Nakajo, S., Baba, M., Iwatsubo, T., Meijer, L., Kahle, P. J., and Haass, C. (2000) J. Biol. Chem. 275 390-397 [DOI] [PubMed] [Google Scholar]
- 42.Schapira, A. H., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P., and Marsden, C. D. (1990) J. Neurochem. 54 823-827 [DOI] [PubMed] [Google Scholar]
- 43.Gu, M., Gash, M. T., Cooper, J. M., Wenning, G. K., Daniel, S. E., Quinn, N. P., Marsden, C. D., and Schapira, A. H. (1997) Mov. Disord. 12 418-422 [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow, R. H., Parks, J. K., Davis, J. N., 2nd, Cassarino, D. S., Trimmer, P. A., Currie, L. J., Dougherty, J., Bridges, W. S., Bennett, J. P., Jr., Wooten, G. F., and Parker, W. D. (1998) Ann. Neurol. 44 873-881 [DOI] [PubMed] [Google Scholar]
- 45.Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., and Greenamyre, J. T. (2000) Nat. Neurosci. 3 1301-1306 [DOI] [PubMed] [Google Scholar]
- 46.Ali, S. F., David, S. N., Newport, G. D., Cadet, J. L., and Slikker, W., Jr. (1994) Synapse. 18 27-34 [DOI] [PubMed] [Google Scholar]
- 47.Vogel, R. O., Janssen, R. J., Ugalde, C., Grovenstein, M., Huijbens, R. J., Visch, H. J., van den Heuvel, L. P, Willems, P. H., Zeviani, M., Smeitink, J. A., and Nijtmans, L. G. (2005) FEBS J. 272 5317-5326 [DOI] [PubMed] [Google Scholar]
- 48.Antonicka, H., Ogilvie, I., Taivassalo, T., Anitori, R. P., Haller, R. G., Vissing, J., Kennaway, N. G., and Shoubridge, E. A. (2003) J. Biol. Chem. 278 43081-43088 [DOI] [PubMed] [Google Scholar]
- 49.Cookson, M. R. (2005) Annu. Rev. Biochem. 74 29-52 [DOI] [PubMed] [Google Scholar]
- 50.Gasser, T. (2001) J. Neurol. 248 833-840 [DOI] [PubMed] [Google Scholar]