Abstract
There are several lines of evidence that the podocyte slit diaphragm (SD), which serves as a structural framework for the filtration barrier in kidney glomerulus, also plays an essential role as a signaling platform. Several SD components including nephrin and TRPC6 are known to be phosphorylated by a Src family tyrosine kinase (SFK), Fyn. Here we have characterized Neph1, another SD component, as a novel substrate of SFK. Fyn interacts with and phosphorylates the cytoplasmic domain of Neph1 in vitro and in intact cells. Peptide mass fingerprinting and site-directed mutagenesis identified several tyrosine phosphorylation sites. In pull-down assays using rat glomerular lysates, Neph1 but not nephrin specifically binds to adaptor protein Grb2 and tyrosine kinase Csk in a phosphorylation-dependent manner. Both tyrosine 637 and 638 of Neph1 are crucial for Neph1-Grb2 binding. Phosphorylation of tyrosine 637 is significantly up-regulated in in vivo models of podocyte injury. Furthermore, Neph1 attenuates ERK activation elicited by Fyn, and this inhibitory effect requires the intact binding motif for the Grb2 SH2 domain. Our results shown here demonstrate that Neph1 is a novel in vivo substrate of SFK and suggest that Neph1 modulates ERK signaling through phosphorylation-dependent interaction with Grb2. Thus, SFK orchestrates a wide spectrum of protein-protein interactions and intracellular signaling networks at SD through tyrosine phosphorylation.
The urinary side of the capillary loop in kidney glomerulus is covered by highly branched glomerular visceral epithelial cells, called podocytes (1). Podocytes form primary processes that further extend numerous foot processes. Foot processes from neighboring podocytes interdigitate with each other and surround the entire surface of capillary loops. These foot processes are bridged by a unique cell adhesion structure, the slit diaphragm (SD).2
Recent studies have identified several molecular components of SD. The first of these molecules to be identified was nephrin (2). Nephrin is a transmembrane protein encoded by the NPHS1 gene, and is a member of the immunoglobulin superfamily. Mutations in NPHS1 cause heavy proteinuria before birth and result in early death (congenital nephrotic syndrome of the Finnish type) (2, 3). Neph1 is structurally related to nephrin, and has five extracellular immunoglobulin-like motifs. Mice deficient in Neph1 develop proteinuria and die within the 8 weeks after birth (4). Several other molecules, including podocin (5), FAT1 (6), and CD2AP (7) are identified as components of SD, and gene disruption of these molecules in human diseases or in genetically manipulated mice results in similar phenotypic conditions; the flattening (effacement) of foot processes, loss of SD, and proteinuria. Nephrin interacts with Neph1 and podocin, forming a trimeric protein complex (8–11). These transmembrane proteins at SD further interact with the junctional scaffolding proteins, ZO-1 (12), CD2AP (13), calcium calmodulin-dependent serine protein kinase (14), and MAGI-1 (membrane-associated guanylate kinase inverted) (14, 15), which anchor the SD complex to the elaborate actin cytoskeleton network. Most of these proteins are crucial to both the development of the glomerulus and the filter function of SD.
In addition to its role as a structural framework of the filtration barrier, SD has been implicated as the signaling platform (16). Nephrin interacts with IQGAP (17), an effector protein of small GTPases Rac1 and Cdc42. Nephrin and CD2AP also interact with phosphatidylinositol 3-kinase p85, which leads to increased Akt activity and reduction in cell death induced by apoptotic stimuli (18).
There are also several lines of evidence that tyrosine phosphorylation may play a key role in the integrity of SD. The cytoplasmic domain of nephrin is tyrosine-phosphorylated by a Src family tyrosine kinase (SFK), Fyn (19). Tyrosine phosphorylation of nephrin results in the recruitment of the SH2-SH3 domain-containing adapter protein Nck to SD, and this nephrin-Nck interaction regulates actin polymerization (20, 21). Recently, the TRPC6 cation channel, whose activity is regulated by tyrosine phosphorylation by Fyn (22), was also identified as an SD-associated protein (23, 24). The crucial role of Fyn-mediated phosphorylation of SD in maintaining the integrity of renal glomerulus is also indicated by the effacement of foot processes and proteinuria in fyn-deficient mice (19, 25).
Neph1 interacts with nephrin at SD, and this hetero-oligomeric complex is considered to be an important determinant of glomerular permselectivity (26). Recently Neph1 is also considered as a signaling molecule. Neph1 has a longer cytoplasmic domain that contains a larger number of tyrosine residues than nephrin, and the carboxyl-terminal domain of Neph1 interacts with the PDZ domain of ZO-1, which not only connects Neph1 to actin cytoskeleton but also modulates the Neph1-mediated signal transduction by augmenting tyrosine phosphorylation of Neph1 (12). Neph1 is phosphorylated by Tec family tyrosine kinases in cotransfected cultured cells, and the phosphorylation leads to an increase of Neph1-mediated AP-1 activation (10).
Whereas tyrosine phosphorylation of ZO-1 (27) and nephrin (20) is dramatically increased in the rat protamine sulfate (PS) perfusion model, one of the in vivo models of podocyte injury, tyrosine phosphorylation of Neph1 in vivo, has not been reported. We hypothesize that Neph1 is tyrosine-phosphorylated in injured podocytes, and involved in intracellular signaling events.
In this study, we have demonstrated that Neph1 is tyrosine-phosphorylated in podocyte injury in vivo. Neph1 is phosphorylated by SFK in vitro and in intact cells. Tyrosine phosphorylation of Neph1 regulates its interaction with Grb2 and Csk, which may be involved in negative feedback control of tyrosine kinase signaling.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents—Mouse monoclonal anti-FLAG antibody (M2; Sigma), rabbit polyclonal anti-Grb2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-Csk antibody (BD Biosciences, San Jose, CA), mouse monoclonal anti-phosphotyrosine antibody (4G10; Upstate, Lake Placid, NY), mouse monoclonal anti-His antibody (Qiagen, Hilden, Germany), and rabbit polyclonal anti-Fyn antibody (Cell Signaling Technology, Danvers, MA) were obtained commercially. A rabbit polyclonal anti-Neph1 antibody was raised against a COOH-terminal peptide of 20 amino acids, CSYTSQHSDYGQRFQQRMQTH (the first cysteine is not part of the Neph1 sequence), coupled to keyhole limpet hemocyanin. The antiserum was affinity purified using the immunogen coupled to a SulfoLink column (Pierce). A rabbit polyclonal phosphospecific antibody (anti-pY637) was raised against the high pressure liquid chromatography-purified synthetic oligopeptide CDPTNGpYYNVRAH. The antiserum was affinity purified by the immunogen described above and absorbed with non-phosphorylated peptide CDPTGYYNVRAH. Western blotting was carried out with these antibodies diluted at 1/2000. PP2 was obtained from Biomol (Plymouth Meeting, PA). SU6656 was obtained from Calbiochem.
Cell Culture and Transfection—HEK293T cells were purchased from the ATCC (Manassas, VA). These cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Transfections were performed using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. A temperature-sensitive rat podocyte cell line, 2DNA1D7, was described previously (28). The cells were cultured in Dulbecco's modified Eagle's medium/F-12 medium supplemented with 10% fetal calf serum and insulin/transferrin/selenium A (Invitrogen).
Eukaryotic Expression Constructs—The following plasmids were prepared. For full-length Neph1-Flag, a cDNA fragment coding for full-length rat Neph1 was amplified by PCR using primers (5′-ccggaattcccgccatgactctggagaaccgtagcac-3′ and 5′-ccgctcgagcacgtgagtctgcatgcgctg-3′) and cloned into pCMV-Tag4A vector (Stratagene). For Flag-Neph1CD that includes the cytoplasmic domain of rat Neph1 (amino acids 585–789), a cDNA fragment amplified by PCR using primers (5′-ccggaattcgaccgggaggatgataccacc-3′ and 5′-ccgctcgagctacacgtgagtctgcatgcgc-3′) was cloned into pCMV-Tag2B. Mammalian expression plasmids encoding rat Neph1 phenylalanine substitution mutants, Y604F, Y637F, Y638F, Y654F, and Y657F, were prepared using standard PCR methods. For full-length Neph2-Flag and nephrin-Flag, cDNA fragments were amplified by PCR using primers (Neph2, 5′-cccgcggccgccgccatgagacctttccagctggatttgctc-3′ and 5′-ccgctcgaggacgtgagtctgcatccgccgctgcag-3′; nephrin, 5′-atttgcggccgcccgccatgggcgctaagagagtcactg-3′ and 5′-gcggtcgaccaccagatgtcccctcagctc-3′) and were cloned into pCMV-Tag4A vector. Mammalian expression plasmid encoding Fyn (29), Csk (gift from M. Okada and S. Nada, Department of Oncogene Research, Research Institute for Microbial Diseases, Osaka University) (30), and hemagglutinin-tagged ERK (gift from M. Maekawa and E. Nishida, Department of Cell and Developmental Biology, Graduate School of Biostudies, Kyoto University) (31) were previously described. Restriction digestion and DNA sequencing were performed to validate all constructs.
Bacterial Fusion Protein Expression—A rat Neph1 cDNA fragment encoding the cytoplasmic 171 amino acids (Neph1CD; amino acids 585–755) flanked with EcoRI (5′) and XhoI (3′) restriction sites was subcloned into pGEX-6P-1 (GE Healthcare). Bacterial pellets were resuspended and sonicated in a solution containing 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 2 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml antipain, and 10 μg/ml leupeptin, and insoluble material was removed by centrifugation. GST-tagged fusion protein was purified on a glutathione-Sepharose column followed by removal of GST with PreScission protease according to the manufacturer's instructions (GE Healthcare). We expressed Neph1CD-(585–755) because GST-Neph1-(552–789) encompassing the whole intracellular domain of Neph1 was insoluble in a buffer containing 3 m urea, 3 m guanidine, or 0.03% SDS. To express the His-tagged rat Csk-SH2 domain, a rat Csk cDNA fragment encoding the 91-amino acid residues (amino acids 81–171) flanked with EcoRI (5′) and NotI (3′) restriction sites was cloned into the pET28a(+) vector (Novagen, Madison, WI). His-tagged fusion protein was purified on a nickel-Sepharose column according to the manufacturer's instructions (GE Healthcare).
In Vitro Phosphorylation and Peptide Mass Analysis—Phosphorylation of recombinant Neph1CD-(585–755) was performed by incubation of 6.0 μg of Neph1CD with 30 ng of recombinant active Fyn (Upstate) in 10 μl of a kinase buffer (20 mm Tris-HCl, pH 7.5, 20 mm MgCl2, 1 mm ATP, and 1 mm sodium orthovanadate) for 60 min at 30 °C. Phosphorylated Neph1CD was digested with trypsin (Promega, Madison, WI). Acetonitrile was added to a final concentration of 90%, and the sample was desalted/concentrated using ZipTip HPL (Millipore, Bedford, MA). Peptides were eluted with 1 μl of matrix solution (0.1% acetic acid and 50% acetonitrile saturated with α-cyano-4-hydroxycinnamic acid) and applied onto a sample plate (Applied Biosystems, Foster City, CA). Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was performed using Voyager-DE PRO (Applied Biosystems). Part of the samples was subjected to liquid chromatography for separation of the peptides, and each peptide was analyzed by a MALDI-TOF/TOF tandem mass spectrometer (4700 Proteomics Analyzer; Applied Biosystems). Data were subjected to data base searches with the Mascot search engine (Matrix Science, London, UK).
Pull-down Assay—GST or GST-Neph1CD immobilized on glutathione-Sepharose beads (GE Healthcare) was incubated with Fyn in a kinase buffer for 60 min at 30 °C. 293T and glomerular lysates were incubated at 4 °C overnight with phosphorylated GST-Neph1CD or GST immobilized on beads. Beads were washed extensively with wash buffer (50 mm Tris-Cl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40). Neph1 and bound proteins were detached from the beads by digestion with PreScission protease and analyzed by Western blotting.
Immunoprecipitation—Cells were lysed with immunoprecipitation buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml antipain, 10 μg/ml leupeptin, 100 units/ml aprotinin, 50 mm NaF, 1 mm EDTA, and 1 mm orthovanadate) for 15 min on ice. Lysates were clarified by centrifugation and incubated with beads conjugated with M2 anti-FLAG antibody for 1 h at 4 °C. Beads were washed two times with immunoprecipitation buffer, and bound proteins were eluted with 100 mm glycine-HCl (pH 2.6).
Immunohistochemistry—Immunofluorescence studies were performed as previously described (32). Briefly, rat kidneys were perfused with 2% paraformaldehyde fixative buffered with 0.1 m phosphate buffer (pH 7.4). These samples were immersed in the same fixative for about 30 min. After washing with phosphate-buffered saline, the tissue was immersed successively in phosphate-buffered saline solution containing 10, 15, and 20% sucrose. After the tissue was embedded in OCT compound and frozen, cryosections (thickness 5–10 μm) were cut using a Jung Frigocut 2800E (Leica) and then mounted on silane-coated glass slides. The cryosections were rinsed with phosphate-buffered saline and blocked in blocking solution (0.1% bovine serum albumin in phosphate-buffered saline). The sections were incubated with first antibodies and visualized with rhodamine isothiocyanate-conjugated second antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Fluorescence specimens were viewed with confocal laser scanning microscope LSM510 (Carl Zeiss, Thornwood, NY).
Animals—All the experiments using animal models were carried out according to the guidelines set by the Animal Center of the Institute of Medical Science, the University of Tokyo. Perfusion of rat kidneys with PS was carried out essentially as previously described (32). Six-week-old male Wistar rats were purchased from Charles River Laboratories Japan, Inc. (Atsugi, Japan). The rats were anesthetized with pentobarbital. Kidneys were perfused through the aorta at 5 ml/min with Hanks' balanced salt solution for 20 min followed by PS solution (500 μg/ml in Hanks' balanced salt solution) for 20 min. The cryostat sections for immunohistological study and glomerular lysates were prepared as previously described (32). Induction of puromycin aminonucleoside (PAN) nephrosis was carried out as described previously (32). Young male rats (Wistar) were injected intraperitoneally with 10 mg/100 g body weight of PAN (Sigma). Animals were killed on days 2, 5, 7, and 11 for protein samples from glomeruli.
RESULTS
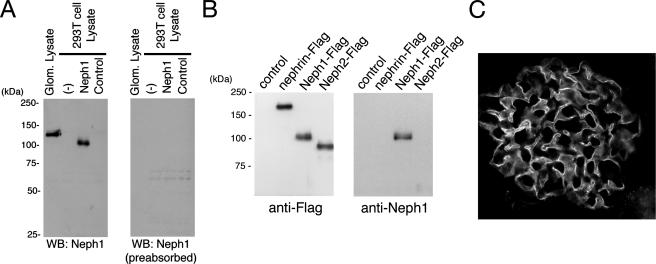
Characterization of Anti-Neph1C Antibody—We prepared a rabbit polyclonal antibody against rat Neph1 carboxyl-terminal 20 amino acid residues. To assess the specificity of the antibody, the full-length rat Neph1 cDNA was cloned and transiently expressed as Neph1-Flag in HEK (human embryonic kidney) 293T cells. A portion of the cell lysates was subjected to immunoblotting with the antibody. As shown in Fig. 1A, the anti-Neph1C antibody recognized a protein with an apparent molecular mass of ∼100 kDa in the Neph1-Flag-transfected cells but not in the empty vector transfectants. Rat glomeruli were isolated using a sieving protocol and their extracts were analyzed similarly. A protein with a slightly slower mobility than overexpressed Neph1-Flag in 293T cells, possibly due to some difference in post-translational modification, was identified.
FIGURE 1.
Detection of Neph1 with the anti-Neph1C antibody. A, lysates from isolated rat glomeruli, untransfected 293T cells, 293T cells transiently transfected with plasmid encoding Neph1, or a control vector were separated by SDS-PAGE (10%), transferred to nitrocellulose, and immunoblotted with anti-Neph1C (left panel) or anti-Neph1C preabsorbed with the peptide used for immunization (right panel). B, lysates from 293T cells transiently transfected with plasmid encoding FLAG-tagged Neph1, Neph2, or nephrin were immunoprecipitated by anti-FLAG antibody, and immunoprecipitates were immunoblotted with anti-FLAG or anti-Neph1C antibody. C, indirect immunofluorescence microscopy was performed to detect Neph1 in adult rat kidney cryosections with anti-Neph1C antibody. This antibody specifically labels glomerular podocytes.
Because COOH-terminal 20-amino acid sequences of Neph1 and Neph2 show 52.4% identity, we examined whether this antibody cross-reacts with Neph2 or nephrin using lysates from 293T cells expressing FLAG-tagged Neph1, Neph2, or nephrin. As shown in Fig. 1B, the anti-Neph1C antibody specifically reacted with Neph1, but not with Neph2 or nephrin. We immunostained kidney sections isolated from normal rats with this antibody (Fig. 1C). Neph1 was highly expressed in podocytes and also in surrounding cells at a relatively low level.
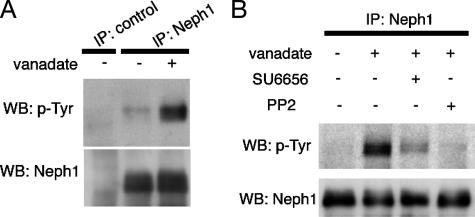
Neph1 Is Phosphorylated by Src Family Tyrosine Kinases in Cultured Rat Podocytes—As has been demonstrated in other cell-cell junctions, SD components are targets for tyrosine phosphorylation. Therefore, we expected that treatment with a tyrosine phosphatase inhibitor, pervanadate, may induce the phosphorylation of Neph1 in cultured rat podocytes (2DNA1D7). Neph1 was immunoprecipitated with anti-Neph1C from the cell lysates, and the immunoprecipitates were immunoblotted with anti-Neph1C or anti-phosphotyrosine (4G10). As shown in Fig. 2A, tyrosine phosphorylation of Neph1 was markedly increased in vanadate-treated podocytes.
FIGURE 2.
Tyrosine phosphorylation of endogenous Neph1 in pervanadate-treated cultured podocytes. A, lysates from cultured podocytes treated with or without 1 mm pervanadate for 15 min were immunoprecipitated with anti-Neph1C antibody, and the immunoprecipitates were immunoblotted with anti-Neph1C or anti-phosphotyrosine (p-Tyr) antibody. B, where indicated, cells were pretreated with 5 μm SU6656 for 60 min or 10 μm PP2 for 15 min prior to treatment with pervanadate. Anti-Neph1C immunoprecipitates (IP) were immunoblotted with anti-phosphotyrosine or anti-Neph1C antibody. WB, Western blot.
Previous studies have demonstrated that the SD protein nephrin is tyrosine-phosphorylated by SFKs (19). TRPC6, mutations of which cause familial focal segmental glomerular sclerosis, is also phosphorylated by Fyn (22). Moreover, gene targeting of fyn or yes results in partial effacement of podocyte foot processes (19, 25). These observations prompted us to further investigate the effect of SFK-specific inhibitors on the tyrosine phosphorylation of endogenous Neph1. As shown in Fig. 2B, PP2 completely abolished pervanadate-stimulated tyrosine phosphorylation of Neph1. SU6656, a more specific SFK inhibitor, also largely inhibit tyrosine phosphorylation. These data suggest that a Src family tyrosine kinase or kinases, at least in part, are responsible for the phosphorylation of Neph1 in podocytes.
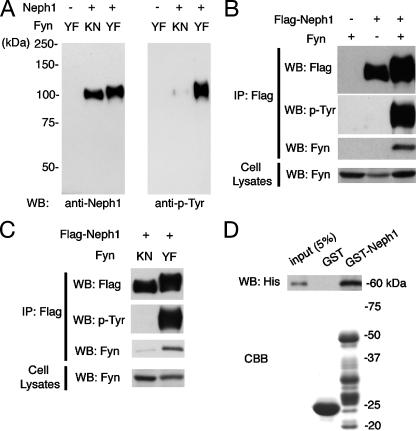
Fyn Binds to and Phosphorylates Neph1—Among the members of SFK, Fyn has been shown to be essential for SD integrity and podocyte structure (19, 25). Thus, we examined whether Fyn phosphorylates Neph1. We transiently cotransfected 293T cells with full-length Neph1 and either a kinase-active form (YF) or a kinase-dead form (KN) of Fyn. When coexpressed with active Fyn, some portion of Neph1 exhibited an upward mobility shift (Fig. 3A). Anti-phosphotyrosine antibody reacted with these upward-shifted bands, suggesting that Fyn phosphorylated Neph1 under these conditions. Under the same conditions, Fyn was coimmunoprecipitated with Neph1 (Fig. 3B). Kinase-dead Fyn neither induced phosphorylation of Neph1 (Fig. 3A) nor bound to Neph1 at all (Fig. 3C), indicating that the interaction between Neph1 and Fyn depends on Fyn kinase activity.
FIGURE 3.
Neph1 is bound to and phosphorylated by the Src family tyrosine kinase Fyn. A, 293T cells were transfected with the indicated plasmids encoding an active (YF) or inactive (KN) form of Fyn together with wild-type Neph1. Cell lysates were analyzed by immunoblotting with anti-Neph1C antibody or anti-phosphotyrosine (p-Tyr) antibody. B, 293T cells were transfected with the indicated plasmids, and anti-FLAG immunoprecipitates (IP) or cell lysates were analyzed by immunoblotting with the indicated antibodies. C, 293T cells were transfected with Neph1-Flag together with either the inactive or active form of Fyn. Anti-FLAG immunoprecipitates and cell lysates were analyzed by immunoblotting with antibodies as indicated. D, Fyn binds to Neph1 in vitro. GST or GST-tagged Neph1 cytoplasmic domain (GST-Neph1CD; amino acids 585–755) were immobilized on glutathione beads, and phosphorylated by His-tagged Fyn (active form) in vitro. GST pull-downs were immunoblotted with anti-His antibody. WB, Western blot.
To examine whether the binding of Fyn to Neph1 is direct or not, we performed pull-down assays using GST-Neph1CD (cytoplasmic domain, amino acids 585–755) and His-tagged Fyn. As shown in Fig. 3D, Fyn bound to GST-Neph1CD but not to GST, indicating the direct interaction between these two proteins.
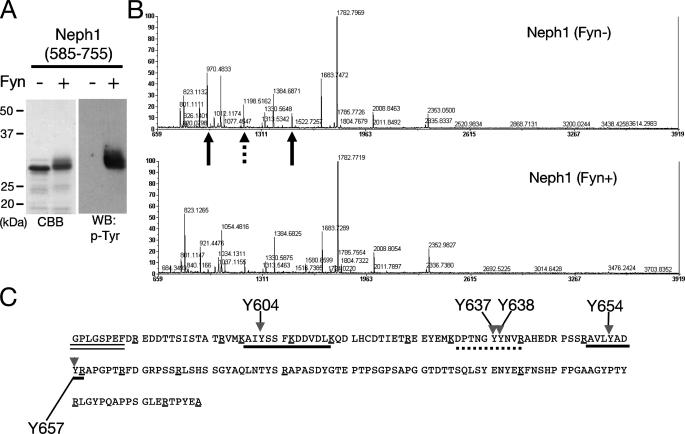
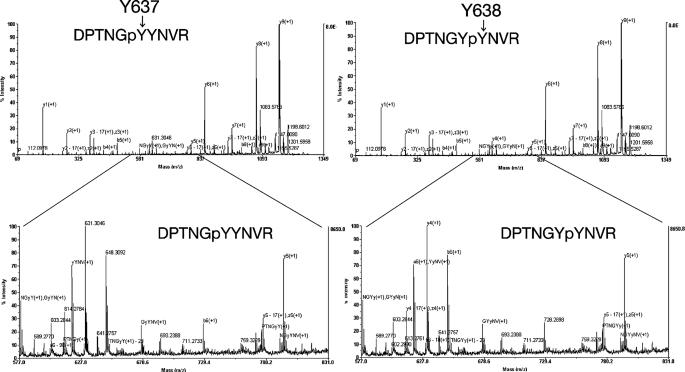
Identification of Tyrosine Residues of Neph1 Phosphorylated by Fyn—We next determined the tyrosine residues phosphorylated by Fyn. To identify these sites, we performed in vitro phosphorylation of Neph1 by recombinant active Fyn, and confirmed that Neph1CD was tyrosine-phosphorylated by Fyn in vitro (Fig. 4A). These samples (phosphorylated and non-phosphorylated Neph1CD) were digested with trypsin and their peptide mass fingerprints were compared. Fig. 4B shows the peptide mass spectra of non-phosphorylated (upper panel) and phosphorylated (lower panel) Neph1CD. When a peptide is phosphorylated, its peptide mass should increase by 80 Da. Occasionally, phosphorylated peptides cannot be detected due to its low efficiency of ionization. By this criterion, we could identify phosphorylated peptides by comparing these two spectra. Peptides of 970.5 Da (corresponding to amino acids 651–658) and 1500.7 Da (602–614) (indicated by arrows) were not detected in the phosphorylated sample, and a significant decrease in peak intensity was observed for a peptide of 1198.5 Da (632–641; an arrowhead). Because two of these three peptides contain two tyrosine residues (Fig. 4C), we further performed MS/MS analysis to identify the exact tyrosine residues that were phosphorylated by Fyn. Phosphorylation of Tyr637 and Tyr638 (Fig. 5) as well as Tyr604 and Tyr654 (data not shown) was confirmed unambiguously.
FIGURE 4.
Tyrosine phosphorylation of Neph1 by Fyn in vitro. A, the Neph1 cytoplasmic domain was incubated with or without Fyn in vitro, and the samples were immunoblotted with anti-phosphotyrosine antibody. B, identification of tyrosine residues of Neph1 phosphorylated by Fyn. The same samples as in A were digested with trypsin, and the peptides were analyzed by a MALDI-TOF mass spectrometer. A marked decrease of peak intensity was observed for two peptides (indicated by arrows; 970.5 and 1500.7 Da), and a moderate decrease for another peptide (1198.5 Da, dashed arrow). C, tyrosine phosphorylation sites in the Neph1 cytoplasmic domain. The amino acid sequence of the Neph1-cytoplasmic domain (585–755) with the amino-terminal linker sequence (indicated with a double underline). Peptides corresponding to 970.5 and 1500.7 Da are underlined. A peptide corresponding to 1198.5 Da is indicated by a dashed underline. Candidate phosphorylation sites are indicated by arrows with amino acid numbers. Lysine and arginine residues are marked with single underlines. CBB, Coomassie Brilliant Blue; WB, Western blot.
FIGURE 5.
Identification of tyrosine residues of Neph1 phosphorylated by Fyn by LC-MS/MS analysis. The Neph1 cytoplasmic domain phosphorylated by Fyn was digested with trypsin, and then the peptides were analyzed by offline nanoLC-MALDI-TOF/TOF analysis. The results shown in this figure reveals tyrosine phosphorylation of Neph1 at Tyr637 and Tyr638.
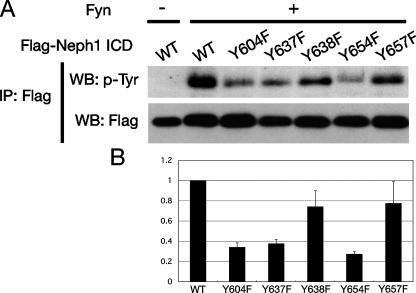
To evaluate the phosphorylation of these tyrosine residues in intact cells, we introduced a series of single phenylalanine substitutions for tyrosine, Y604F, Y637F, Y638F, Y654F, and Y657F into FLAG-tagged Neph1CD (amino acids 585–789), and expressed these proteins together with Fyn in 293T cells (Fig. 6). Tyrosine phosphorylation of Y604F, Y637F, and Y654F was clearly reduced compared with wild-type cells, suggesting that these tyrosine residues are phosphorylated by Fyn in intact cells.
FIGURE 6.
Several tyrosine residues of Neph1 are phosphorylated in intact cells. A, the FLAG-tagged wild-type (WT) or mutant Neph1 cytoplasmic region were transfected into 293T cells together with active Fyn. Cell lysates were immunoprecipitated (IP) with anti-FLAG antibody and immunoprecipitates were immunoblotted for phosphotyrosine. As a control, wild-type Neph1 was transfected without Fyn. B, the densitometry of A is shown. Values are normalized to wild-type Neph1.
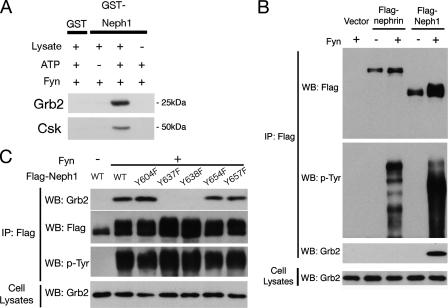
Identification of an SH2 Domain Containing Proteins That Associate with Phosphorylated Neph1—Having established that Neph1 is tyrosine-phosphorylated by Fyn, we searched for molecules that bind to Neph1 upon tyrosine phosphorylation. Because Fyn plays an essential role in the establishment of SD integrity, the binding partner(s) of tyrosine-phosphorylated Neph1 may also play important roles. To this end, we performed pull-down analysis. GST or GST-Neph1CD were immobilized on glutathione-Sepharose beads, and phosphorylated by Fyn in vitro. These samples were used to pull-down binding proteins from rat glomerular lysates. Proteins trapped on the beads were analyzed by immunoblot using antibodies against several SH2 domain containing proteins. Of these, an adapter protein Grb2 and a tyrosine kinase Csk specifically bound to Neph1 in a phosphorylation-dependent manner (Fig. 7A). Binding of phosphorylated Neph1 with Grb2 or Csk was also observed when 293T cell lysates were used (data not shown).
FIGURE 7.
Grb2 specifically binds to phosphorylated Neph1. A, recombinant GST-Neph1CD was bound to glutathione-Sepharose beads, and incubated with recombinant Fyn with or without ATP. After washing, the beads were incubated with glomerular lysates from normal rats, and bound proteins were analyzed by immunoblotting for Grb2 and Csk. B, Grb2 binds to phosphorylated Neph1 in 293T cells. 293T cells were transfected with the indicated vectors, and anti-FLAG immunoprecipitates (IP) and cell lysates were analyzed by Western blotting (WB) for FLAG tag, phosphotyrosine, and Grb2. C, both Tyr637 and Tyr638 are required for binding to Grb2. FLAG-tagged wild-type or the indicated mutant Neph1 were transfected into 293T cells together with Fyn, and anti-FLAG immunoprecipitates and cell lysates were analyzed by Western blotting for FLAG tag, phosphotyrosine, and Grb2.
Tyr637 and Tyr638 Are Critical for Grb2-Neph1 Interaction— Neph1 contains a putative Grb2 SH2 binding motif starting at Tyr637 (YYNV), and previously Sellin et al. (10) demonstrated that Neph1 interacts with Grb2 in transiently transfected 293T cells. We performed coimmunoprecipitation experiments to examine whether the interaction between Neph1 and Grb2 is phosphorylation-dependent. 293T cells were transfected with Neph1-Flag or nephrin-Flag together with or without Fyn (Fig. 7B). Grb2 was coimmunoprecipitated with phosphorylated Neph1, but not with non-phosphorylated Neph1 nor phosphorylated nephrin. Neph1 is phosphorylated by Fyn at Tyr637 and Tyr638 (Figs. 4, 5, 6) and these tyrosine residues of Neph1 are in the consensus binding motif for Grb2. Therefore, we examined the effect of phenylalanine substitution for these residues on the Neph1-Grb2 interaction. The substitution of either Tyr637 or Tyr638 with phenylalanine completely abolished the Neph1-Grb2 interaction (Fig. 7C), indicating that these two tyrosine residues are essential for this interaction.
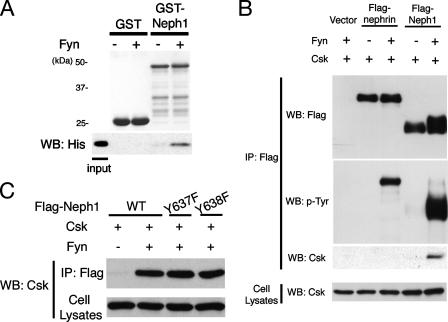
Csk Also Directly Binds to Phosphorylated Neph1—Next, the interaction between Neph1 and Csk was investigated. GST or GST-Neph1CD immobilized on glutathione beads was phosphorylated by Fyn in vitro and incubated with the His-tagged SH2 domain of Csk. Bound proteins were analyzed by SDS-PAGE and immunoblotted with anti-His antibody. As shown in Fig. 8A, the Csk SH2 domain bound directly to phosphorylated Neph1. We also confirmed this interaction in transiently transfected 293T cells. Csk was specifically coimmunoprecipitated with phosphorylated Neph1, but not with phosphorylated nephrin nor non-phosphorylated Neph1 (Fig. 8B). Csk still bound to Grb2 binding-deficient mutants (Y637F and Y638F) (Fig. 8C) and other phenylalanine substitution mutants (Y604F, Y654F, and Y657F) (data not shown) of Neph1, indicating that Csk binds to other tyrosine residue(s) of Neph1.
FIGURE 8.
The Csk SH2 domain directly binds to phosphorylated Neph1. A, recombinant GST or GST-Neph1CD were bound to glutathione-Sepharose beads, and incubated with or without Fyn. After washing, the beads were incubated with recombinant His-tagged SH2 domain of Csk, and bound proteins were immunoblotted with anti-His antibody. B, 293T cells were transfected with the indicated vectors, and anti-FLAG immunoprecipitates and cell lysates were analyzed by Western blotting (WB) for FLAG tag, phosphotyrosine (p-Tyr), and Csk. C, 293T cells were transfected with the indicated vectors, and anti-FLAG immunoprecipitates (IP) and cell lysates were analyzed by Western blotting for Csk.
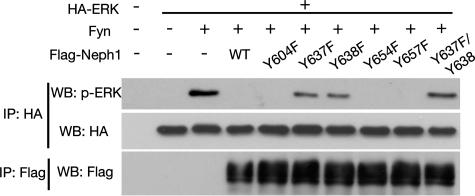
Neph1 Negatively Regulates ERK Signaling—Grb2 forms a stable complex with Sos, a guanine nucleotide exchange factor for Ras, and activates Ras upon recruitment to appropriate tyrosine-phosphorylated adaptors or autophosphorylated receptor tyrosine kinases via the SH2 domain (33, 34). Therefore, we examined whether the phosphorylation of Neph1 and recruitment of Grb2 to Neph1 may have some effects on the Ras-ERK pathway. To elucidate this, we evaluated the activation status of ERK in the presence of Neph1. The expression of active Fyn in 293T cells resulted in the activation of ERK (Fig. 9, third lane). Interestingly, the additional expression of wild-type Neph1 abolished this Fyn-induced ERK activation (lane 4). Furthermore, this suppression of ERK activation was partially prevented by the mutations of tyrosine residues that are critical for Grb2 binding (Y637F and Y638F), but not by other mutants, indicating that binding of Grb2 to phosphorylated Neph1 is required for the attenuation of ERK activation.
FIGURE 9.
Suppression of Fyn-induced activation of ERK by Neph1. 293T cells were co-transfected with hemagglutinin (HA)-tagged ERK expression vector and a Fyn expression vector together with an empty expression vector, a wild-type Neph1, or each of mutant Neph1 vectors. Anti-hemagglutinin or anti-FLAG immunoprecipitates (IP) were analyzed by Western blotting (WB) with the indicated antibodies.
Tyr637 Is Phosphorylated in Injured Podocytes in Vivo—Several tyrosine-phosphorylated proteins in the PS-induced podocyte injury model have been described by Kurihara and others (20, 32). Therefore, we expected that treatment with PS may induce the phosphorylation of Neph1 in vivo. Neph1 was immunoprecipitated with anti-Neph1C from glomerular lysates of normal or PS-treated rats, and the immunoprecipitates were immunoblotted with anti-Neph1C or anti-phosphotyrosine (4G10). As shown in Fig. 10A, Neph1 was weakly phosphorylated in normal rats, and this tyrosine phosphorylation of Neph1 was significantly increased in PS-treated glomeruli.
FIGURE 10.
Tyr637 is phosphorylated in injured podocytes in vivo. A, Neph1 is transiently tyrosine-phosphorylated during foot process effacement in the protamine sulfate-induced podocyte injury model. Rat kidneys were perfused with protamine sulfate for 20 min as described under “Experimental Procedures.” Anti-Neph1C immunoprecipitates (IP) from control or protamine sulfate-treated glomeruli were subjected to immunoblotting with anti-Neph1C or anti-phosphotyrosine antibody. B, recombinant wild-type, Y637F, or Y657F GST-Neph1CD was incubated in vitro with recombinant Fyn, followed by immunoblotting with affinity purified rabbit polyclonal anti-phospho-Neph1 antibody (anti-pY637). C, rat kidneys were perfused with protamine sulfate as in A. Glomerular lysate was immunoprecipitated by anti-Neph1C antibody and the immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-Neph1C or anti-phospho-Neph1. D, immunoblot analysis of glomerular lysates from normal and PAN-treated rat kidneys probed with anti-phospho-Neph1. WB, Western blot.
To develop a reagent that would be useful to investigate the phosphorylation of the critical residue of Neph1 in vivo, a rabbit polyclonal antibody against a phosphopeptide surrounding Tyr637 was raised as described under “Experimental Procedures” (anti-Tyr(P)637). Amino acid sequences surrounding Tyr637 are conserved among mouse, rat, and human species. After absorption with the non-phosphorylated peptide, this antibody specifically recognized phosphorylation of Tyr637 of Neph1 (Fig. 10B). Anti-Neph1C immunoprecipitates from glomerular lysates of normal or PS-treated rats were immunoblotted with anti-Tyr(P)637 or anti-Neph1C (Fig. 10C). Again, phosphorylation of Tyr637 was significantly up-regulated by protamine sulfate treatment. Furthermore, we investigated the phosphorylation status of Neph1 in PAN nephropathy, another podocyte injury model characterized by massive proteinuria and effacement of podocytes. As shown in Fig. 10D, phosphorylation of Neph1 was observed on days 7 and 11 in PAN nephropathy, when severe proteinuria is observed. These results clearly indicate that Tyr637, whose phosphorylation is critical for binding with Grb2, is phosphorylated in vivo in response to podocyte injury.
DISCUSSION
Podocytes are terminally differentiated epithelial cells. SD formed between adjacent interdigitating podocyte foot processes is a highly specialized cell adhesion structure, and functions as a critical size and charge barrier to prevent proteinuria (1). In most of the clinical settings characterized by proteinuria such as idiopathic nephrotic syndrome, focal segmental glomerulosclerosis, or diabetic nephropathy, the characteristic change in podocyte shape called effacement of foot processes is a common occurrence. This change is caused by damage to podocytes, including mechanical stress, high glucose concentration, reactive oxygen species, angiotensin II, transforming growth factor-β, and sometimes local infection of human immunodeficiency virus (35). But so far, the mechanism of how podocytes respond to injury or damage is largely unknown. Here, we describe evidence that (i) Neph1 is tyrosine-phosphorylated in podocyte injury models in vivo, (ii) Fyn tyrosine kinase physically interacts with and phosphorylates Neph1, (iii) Grb2 and Csk specifically bind to Neph1 in a phosphorylation-dependent manner, and (iv) binding of Grb2 to Neph1 contributes to negative regulation of tyrosine kinase signaling.
A growing number of reports suggest that protein complexes at SD serve as a signaling nexus. The components of SD such as nephrin, Neph1, and podocin are localized in lipid rafts, where they interact with each other. Nephrin interacts with CD2AP, and this interaction elicits phosphatidylinositol 3-kinase-dependent Akt signaling (18). Tyrosine residues of the nephrin cytoplasmic domain are phosphorylated in response to nephrin clustering (36). Recently, the Src family kinase was reported to be responsible for the phosphorylation of nephrin (19). This phosphorylation results in the recruitment of Nck via its SH2 domain, and promotes the assembly of actin filaments (20, 21).
There are two reports on tyrosine phosphorylation of Neph1. Sellin et al. (10) reported that Neph1 is phosphorylated by Tec family tyrosine kinases in transfected 293T cells, and the phosphorylation augment the Neph1-mediated AP-1 activation, indicating that Neph1 serve as a signaling molecule. Huber et al. (12) demonstrated that phosphorylation of Neph1 is augmented by cotransfected ZO-1. In the present study, we have demonstrated that Neph1 is tyrosine-phosphorylated in response to podocyte injury in vivo. In several podocyte injury models, tyrosine phosphorylation of ZO-1 is associated with the effacement of foot processes and glomerular integrity (27). Nephrin was also found to be phosphorylated in PS-treated rats (20). Our findings that Neph1 is tyrosine-phosphorylated in PS-treated or PAN-treated rats are intriguing because Neph1 can bind to both nephrin and ZO-1. Taken altogether, these observations indicate that global tyrosine phosphorylation events within the SD complex occur when podocytes are injured.
In cultured podocytes, phosphorylation of Neph1 is inhibited by PP2 and SU6656, indicating SFK-dependent phosphorylation of Neph1. There is a line of evidence suggesting that among the members of SFKs, Fyn plays a major role in the barrier function of podocyte. First, Src, Fyn, Yes, and Lyn are expressed in podocytes, and Fyn, but not Yes is coimmunoprecipitated with nephrin (36). Verma et al. (19) reported that Fyn and Yes, but not Src, fractionate with nephrin and podocin in Triton X-100-insoluble membrane fractions in glomerular lysate. They also found that phosphorylation of nephrin is abrogated in Fyn knock-out mice. Furthermore, fyn-/- mice represent proteinuria characterized by podocyte foot process effacement with B and T lymphocyte-independent mechanisms (19, 25), indicating that intrinsic Fyn in glomeruli, possibly in podocytes, might be crucial in the maintenance of podocyte structure. Therefore, we investigated the phosphorylation of Neph1 by Fyn. We further examined whether small interfering RNA of Fyn attenuated the phosphorylation of Neph1 in vanadate-treated cultured podocytes. However, we could not detect a significant decrease in tyrosine phosphorylation of Neph1 upon small interfering RNA for Fyn (data not shown). This result is probably attributed to phosphorylation by other SFKs because vanadate non-selectively inhibits protein-tyrosine phosphatases. During the revision of this manuscript, Garg et al. (37) reported the tyrosine phosphorylation of Neph1, which is greatly but not completely abolished in fyn-deficient mice. These observations altogether suggest that tyrosine phosphorylation of SD components by Fyn plays a physiologically important role in podocyte function.
We identified Grb2 as a binding partner of phosphorylated Neph1. Grb2 is composed exclusively of Src homology domains, one SH2 domain flanked by two SH3 domains. In fibroblasts, a substantial amount of Grb2 is constitutively associated via its SH3 domain with the COOH-terminal proline-rich region of the Ras guanine nucleotide exchange factor, Sos (33, 34). Many other effector molecules can bind to the SH3 domains of Grb2 as well (38). Because recruitment of the Grb2·Sos complex to the plasma membrane by epidermal growth factor receptor stimulation regulates the Ras-ERK pathway, we examined the effect of Grb2 binding to Neph1 on ERK activation. Unexpectedly, the activation of ERK by Fyn was inhibited by phosphorylation of wild-type Neph1, and this inhibitory effect was dependent on Tyr637 and Tyr638, two residues that are critical for Grb2 binding.
The spatiotemporal regulation of ERK activity is an emerging concept in the field of signal transduction (39, 40). Recently, several negative regulators for Ras/ERK signaling have been identified, and their detailed molecular mechanisms have been analyzed (41). Sprouty 1/2 (42, 43), Dok-3 (44), and DOC-2/DAB2 (45) inhibit the Ras-ERK pathway by sequestration of Grb2, whereas Dok-1/2 (46, 47) act by binding to Ras-GAP or by unidentified mechanisms. Unlike classical negative feedback factors that are transcriptionally induced by stimuli, these factors attenuate Ras activation in a tyrosine phosphorylation-dependent manner. Neph1 may also belong to this type of negative regulators for Ras/ERK signaling.
As a consequence of the high degree of differentiation of podocytes, it has been postulated that they, in analogy to neurons, are unable to proliferate (1). Recent studies revealed the role of ERK as a key regulator of neuronal apoptosis besides its role as a survival factor, because ERK activation promotes neuronal degeneration by causing plasma membrane damage (48). The roles of the MAP kinase family in injured podocytes are not well elucidated. Recent analysis by Koshikawa et al. (49) demonstrated that p38 MAP kinase and ERK are activated in podocyte injury models and complete inhibition of p38 MAP kinase and attenuation of ERK results in suppression of proteinuria, suggesting their deleterious effect in differentiated podocytes. In this regard, it may be an intriguing possibility that the in vivo phosphorylation of the Grb2 binding site of Neph1 (Tyr637/Tyr638) in podocyte injury protects podocytes from further damage by negatively regulating ERK signaling.
Garg et al. (37) also demonstrated that Fyn-dependent phosphorylation of Neph1, which is increased in the disease model, induces Neph1 binding to Grb2, and this complex reorganizes actin polymerization at the plasma membrane in cultured fibroblast. We have shown here using site-specific anti-phospho-Neph1 antibody that phosphorylation of the critical binding sites for Grb2 greatly increases in the disease models. These observations taken together suggest the essential role of Neph1 phosphorylation at Tyr637–Tyr638 to elicit diverse signaling pathways in living animals.
Neph1 is a member of a group of closely related proteins. Recently, other Neph family members, Neph2 and Neph3, have been reported to be involved in odorant receptor-specific segregation of axon termini, by their homophilic interaction (50). Phosphorylation of Neph2 or Neph3 is not well characterized, but the binding motif for Grb2 is not conserved in these two proteins, suggesting that negative feedback control for ERK by binding to Grb2 may be unique to Neph1.
Csk negatively regulates SFKs by phosphorylating a tyrosine residue in their carboxyl-terminal region (51). In brain and fibroblasts, Csk is translocated to the plasma membrane with the aid of a lipid-raft-associated membrane protein known as Csk-binding protein, and the membrane localization of Csk causes sustained inhibition of the SFK activity (30). Caveolin-1 is another adaptor protein that recruits Csk to the plasma membrane (52). Csk binds to caveolin-1 phosphorylated by SFK via its SH2 domain, and this binding is also known as a negative feedback control on SFK activity. We have shown here that Csk directly binds to phosphorylated Neph1. This may present another method of Csk regulation, because Neph1 is mainly localized in Triton X-100-insoluble lipid microdomain (8), where most SFK also localize. The spatiotemporal regulation of SFKs in SD may be very critical to maintain normal podocyte function in the kidney, integrating activating and inhibitory signals, but the mechanism of how tyrosine phosphorylation of SD proteins is regulated is still largely unknown. It is an intriguing hypothesis that membrane-targeted Csk modifies SFK activity at the slit diaphragm. Further studies are needed to determine the role of Csk in the regulation of tyrosine phosphorylation of SD components.
Neph1 and nephrin are essential for establishing selective glomerular filtration. The results presented here demonstrate that Neph1 as well as nephrin is tyrosine-phosphorylated at SD in vivo, which promotes the dynamic signaling complex formation depending on tyrosine phosphorylation. Interestingly, phosphorylated Neph1 binds to Grb2 and Csk, whereas phosphorylated nephrin binds to Nck. Therefore, tyrosine-phosphorylated Neph1 and nephrin at SD might regulate distinct signaling pathways through binding to different SH2 domain proteins. Thus, phosphorylation by SFK may orchestrate a wide spectrum of signaling pathways by various protein-protein interactions. Further studies of phosphorylation of SD will contribute to a better understanding of the molecular pathogenesis of proteinuria.
Acknowledgments
We thank Drs. M. Okada, S. Nada, M. Maekawa, and E. Nishida for providing plasmids and Dr. H. Fukuda for MS/MS analysis. We also thank Drs. N. Iida, M. Kobayashi, K. Shirakabe, M. Machida, and M.-Y. Han for valuable comments.
This work was supported by the program for the International Research and Educational Institute for Integrated Medical Sciences (IREIIMS). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SD, slit diaphragm; SFK, Src family tyrosine kinase; TRPC, transient receptor potential cation channel; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; ZO-1, zonula occludens; CD2AP, CD2-associated protein; SH, Src homology; HEK, human embryonic kidney; GST, glutathione S-transferase; PS, protamine sulfate; PAN, puromycin aminonucleoside; MS, mass spectrometry.
References
- 1.Pavenstadt, H., Kriz, W., and Kretzler, M. (2003) Physiol. Rev. 83 253-307 [DOI] [PubMed] [Google Scholar]
- 2.Kestila, M., Lenkkeri, U., Mannikko, M., Lamerdin, J., McCready, P., Putaala, H., Ruotsalainen, V., Morita, T., Nissinen, M., Herva, R., Kashtan, C. E., Peltonen, L., Holmberg, C., Olsen, A., and Tryggvason, K. (1998) Mol. Cell. 1 575-582 [DOI] [PubMed] [Google Scholar]
- 3.Putaala, H., Soininen, R., Kilpelainen, P., Wartiovaara, J., and Tryggvason, K. (2001) Hum. Mol. Genet. 10 1-8 [DOI] [PubMed] [Google Scholar]
- 4.Donoviel, D. B., Freed, D. D., Vogel, H., Potter, D. G., Hawkins, E., Barrish, J. P., Mathur, B. N., Turner, C. A., Geske, R., Montgomery, C. A., Starbuck, M., Brandt, M., Gupta, A., Ramirez-Solis, R., Zambrowicz, B. P., and Powell, D. R. (2001) Mol. Cell. Biol. 21 4829-4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roselli, S., Heidet, L., Sich, M., Henger, A., Kretzler, M., Gubler, M. C., and Antignac, C. (2004) Mol. Cell. Biol. 24 550-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciani, L., Patel, A., Allen, N. D., and ffrench-Constant, C. (2003) Mol. Cell. Biol. 23 3575-3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih, N. Y., Li, J., Karpitskii, V., Nguyen, A., Dustin, M. L., Kanagawa, O., Miner, J. H., and Shaw, A. S. (1999) Science 286 312-315 [DOI] [PubMed] [Google Scholar]
- 8.Barletta, G. M., Kovari, I. A., Verma, R. K., Kerjaschki, D., and Holzman, L. B. (2003) J. Biol. Chem. 278 19266-19271 [DOI] [PubMed] [Google Scholar]
- 9.Gerke, P., Huber, T. B., Sellin, L., Benzing, T., and Walz, G. (2003) J. Am. Soc. Nephrol. 14 918-926 [DOI] [PubMed] [Google Scholar]
- 10.Sellin, L., Huber, T. B., Gerke, P., Quack, I., Pavenstadt, H., and Walz, G. (2003) FASEB J. 17 115-117 [DOI] [PubMed] [Google Scholar]
- 11.Schwarz, K., Simons, M., Reiser, J., Saleem, M. A., Faul, C., Kriz, W., Shaw, A. S., Holzman, L. B., and Mundel, P. (2001) J. Clin. Investig. 108 1621-1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber, T. B., Schmidts, M., Gerke, P., Schermer, B., Zahn, A., Hartleben, B., Sellin, L., Walz, G., and Benzing, T. (2003) J. Biol. Chem. 278 13417-13421 [DOI] [PubMed] [Google Scholar]
- 13.Shih, N. Y., Li, J., Cotran, R., Mundel, P., Miner, J. H., and Shaw, A. S. (2001) Am. J. Pathol. 159 2303-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehtonen, S., Ryan, J. J., Kudlicka, K., Iino, N., Zhou, H., and Farquhar, M. G. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 9814-9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirabayashi, S., Tajima, M., Yao, I., Nishimura, W., Mori, H., and Hata, Y. (2003) Mol. Cell. Biol. 23 4267-4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benzing, T. (2004) J. Am. Soc. Nephrol. 15 1382-1391 [DOI] [PubMed] [Google Scholar]
- 17.Liu, X. L., Kilpelainen, P., Hellman, U., Sun, Y., Wartiovaara, J., Morgunova, E., Pikkarainen, T., Yan, K., Jonsson, A. P., and Tryggvason, K. (2005) FEBS J. 272 228-243 [DOI] [PubMed] [Google Scholar]
- 18.Huber, T. B., Hartleben, B., Kim, J., Schmidts, M., Schermer, B., Keil, A., Egger, L., Lecha, R. L., Borner, C., Pavenstadt, H., Shaw, A. S., Walz, G., and Benzing, T. (2003) Mol. Cell. Biol. 23 4917-4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma, R., Wharram, B., Kovari, I., Kunkel, R., Nihalani, D., Wary, K. K., Wiggins, R. C., Killen, P., and Holzman, L. B. (2003) J. Biol. Chem. 278 20716-20723 [DOI] [PubMed] [Google Scholar]
- 20.Verma, R., Kovari, I., Soofi, A., Nihalani, D., Patrie, K., and Holzman, L. B. (2006) J. Clin. Investig. 116 1346-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, N., Blasutig, I. M., Eremina, V., Ruston, J. M., Bladt, F., Li, H., Huang, H., Larose, L., Li, S. S., Takano, T., Quaggin, S. E., and Pawson, T. (2006) Nature 440 818-823 [DOI] [PubMed] [Google Scholar]
- 22.Hisatsune, C., Kuroda, Y., Nakamura, K., Inoue, T., Nakamura, T., Michikawa, T., Mizutani, A., and Mikoshiba, K. (2004) J. Biol. Chem. 279 18887-18894 [DOI] [PubMed] [Google Scholar]
- 23.Winn, M. P., Conlon, P. J., Lynn, K. L., Farrington, M. K., Creazzo, T., Hawkins, A. F., Daskalakis, N., Kwan, S. Y., Ebersviller, S., Burchette, J. L., Pericak-Vance, M. A., Howell, D. N., Vance, J. M., and Rosenberg, P. B. (2005) Science 308 1801-1804 [DOI] [PubMed] [Google Scholar]
- 24.Reiser, J., Polu, K. R., Moller, C. C., Kenlan, P., Altintas, M. M., Wei, C., Faul, C., Herbert, S., Villegas, I., Avila-Casado, C., McGee, M., Sugimoto, H., Brown, D., Kalluri, R., Mundel, P., Smith, P. L., Clapham, D. E., and Pollak, M. R. (2005) Nat. Genet. 37 739-744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, C. C., Yen, T. S., Lowell, C. A., and DeFranco, A. L. (2001) Curr. Biol. 11 34-38 [DOI] [PubMed] [Google Scholar]
- 26.Liu, G., Kaw, B., Kurfis, J., Rahmanuddin, S., Kanwar, Y. S., and Chugh, S. S. (2003) J. Clin. Investig. 112 209-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurihara, H., Anderson, J. M., and Farquhar, M. G. (1995) Am. J. Physiol. 268 R514-R524 [DOI] [PubMed] [Google Scholar]
- 28.Matsui, I., Ito, T., Kurihara, H., Imai, E., Ogihara, T., and Hori, M. (2007) Lab. Investig. 87 273-283 [DOI] [PubMed] [Google Scholar]
- 29.Tezuka, T., Umemori, H., Akiyama, T., Nakanishi, S., and Yamamoto, T. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 435-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabuchi, M., Satomi, Y., Takao, T., Shimonishi, Y., Nada, S., Nagai, K., Tarakhovsky, A., and Okada, M. (2000) Nature 404 999-1003 [DOI] [PubMed] [Google Scholar]
- 31.Maekawa, M., Nishida, E., and Tanoue, T. (2002) J. Biol. Chem. 277 7783-7787 [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi, S., Mori, H., Kansaku, A., Kurihara, H., Sakai, T., Shimizu, F., Kawachi, H., and Hata, Y. (2005) Lab. Investig. 85 1528-1543 [DOI] [PubMed] [Google Scholar]
- 33.Lowenstein, E. J., Daly, R. J., Batzer, A. G., Li, W., Margolis, B., Lammers, R., Ullrich, A., Skolnik, E. Y., Bar-Sagi, D., and Schlessinger, J. (1992) Cell 70 431-442 [DOI] [PubMed] [Google Scholar]
- 34.Buday, L., and Downward, J. (1993) Cell 73 611-620 [DOI] [PubMed] [Google Scholar]
- 35.Durvasula, R. V., and Shankland, S. J. (2006) Curr. Opin. Nephrol. Hypertens. 15 1-7 [DOI] [PubMed] [Google Scholar]
- 36.Lahdenpera, J., Kilpelainen, P., Liu, X. L., Pikkarainen, T., Reponen, P., Ruotsalainen, V., and Tryggvason, K. (2003) Kidney Int. 64 404-413 [DOI] [PubMed] [Google Scholar]
- 37.Garg, P., Verma, R., Nihalani, D., Johnstone, D. B., and Holzman, L. B. (2007) Mol. Cell. Biol. 27 8698-8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buday, L. (1999) Biochim. Biophys. Acta 1422 187-204 [DOI] [PubMed] [Google Scholar]
- 39.Shilo, B. Z. (2005) Development 132 4017-4027 [DOI] [PubMed] [Google Scholar]
- 40.Freeman, M. (2000) Nature 408 313-319 [DOI] [PubMed] [Google Scholar]
- 41.Torii, S., Nakayama, K., Yamamoto, T., and Nishida, E. (2004) J. Biochem. (Tokyo) 136 557-561 [DOI] [PubMed] [Google Scholar]
- 42.Hanafusa, H., Torii, S., Yasunaga, T., and Nishida, E. (2002) Nat. Cell. Biol. 4 850-858 [DOI] [PubMed] [Google Scholar]
- 43.Guy, G. R., Wong, E. S., Yusoff, P., Chandramouli, S., Lo, T. L., Lim, J., and Fong, C. W. (2003) J. Cell Sci. 116 3061-3068 [DOI] [PubMed] [Google Scholar]
- 44.Honma, M., Higuchi, O., Shirakata, M., Yasuda, T., Shibuya, H., Iemura, S., Natsume, T., and Yamanashi, Y. (2006) Genes Cells 11 143-151 [DOI] [PubMed] [Google Scholar]
- 45.Zhou, J., and Hsieh, J. T. (2000) J. Biol. Chem. 276 27793-27798 [DOI] [PubMed] [Google Scholar]
- 46.Jones, N., and Dumont, D. J. (1999) Curr. Biol. 9 1057-1060 [DOI] [PubMed] [Google Scholar]
- 47.Gerard, A., Favre, C., Garcon, F., Nemorin, J. G., Duplay, P., Pastor, S., Collette, Y., Olive, D., and Nunes, J. A. (2004) Oncogene 23 1594-1598 [DOI] [PubMed] [Google Scholar]
- 48.Subramaniam, S., Zirrgiebel, U., von Bohlen Und Halbach, O., Strelau, J., Laliberte, C., Kaplan, D. R., and Unsicker, K. (2004) J. Cell Biol. 165 357-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koshikawa, M., Mukoyama, M., Mori, K., Suganami, T., Sawai, K., Yoshioka, T., Nagae, T., Yokoi, H., Kawachi, H., Shimizu, F., Sugawara, A., and Nakao, K. (2005) J. Am. Soc. Nephrol. 16 2690-2701 [DOI] [PubMed] [Google Scholar]
- 50.Serizawa, S., Miyamichi, K., Takeuchi, H., Yamagishi, Y., Suzuki, M., and Sakano, H. (2006) Cell 127 1057-1069 [DOI] [PubMed] [Google Scholar]
- 51.Chong, Y. P., Mulhern, T. D., and Cheng, H. C. (2005) Growth Factors 23 233-244 [DOI] [PubMed] [Google Scholar]
- 52.Cao, H., Courchesne, W. E., and Mastick, C. C. (2002) J. Biol. Chem. 277 8771-8774 [DOI] [PubMed] [Google Scholar]