Abstract
Although synthesized in the same pituitary gonadotropes, the secretion profiles of lutropin (LH) and follitropin (FSH) differ. LH is secreted through a regulated pathway and associated with a bolus release at mid-estrous cycle. In contrast, the majority of FSH is secreted constitutively with an incremental increase until ovulation. Both share an identicalα subunit, and thus theβ subunit contains determinants for sorting into the regulated pathway. Previously, we demonstrated that a hydrophobic carboxyl-terminal heptapeptide of the LHβ subunit (Leu-Ser-Gly-Leu-Leu-Phe-Leu), not found in the FSHβ subunit, influences the intracellular behavior of the LH dimer. To test the hypothesis that the peptide contributes to differential sorting, we monitored the fates of LH and LHΔT (LHβ subunit lacking the carboxyl-terminal seven amino acids) dimers in the rat somatotrope-derived GH3 cell line in which both the regulated and constitutive secretory pathways operate. Pulse-chase labeling demonstrated that the LHΔT dimer was diverted to the constitutive pathway, resulting in a significant decrease in the corresponding intracellular pool. Forskolin stimulated LH dimer release 3-fold, which was accompanied by a parallel decrease of intracellular LH; only marginal forskolin stimulation of LHΔT was seen. Immunofluorescence after cycloheximide treatment demonstrated decreased retention of LHΔT compared with LH, consistent with increased constitutive secretion of LHΔT. We also demonstrated that fusing the heptapeptide to the carboxyl terminus of the FSHβ subunit resulted in an increased regulated secretion of this FSH analog compared with wild-type FSH. These data are the first to identify a novel structural determinant responsible for the sorting of a member of the glycoprotein hormone family into the regulated secretory pathway.
Lutropin (LH)4 and follitropin (FSH) are synthesized and secreted by pituitary gonadotropes and are members of the glycoprotein hormone family, which also includes thyrotropin (TSH) and the placental hormone chorionic gonadotropin (CG). They are heterodimers that share a common α subunit but differ in their hormone-specific β subunits (1, 2). Both subunits are glycosylated, containing asparagine (N)-linked oligosaccharides (3, 4). The mature carbohydrate structures are hormone-specific in that the terminal oligosaccharide is sulfate for LH (3, 5), whereas FSH contains sialic acid (3).
LH and FSH play key roles in regulating reproductive function. In females, FSH stimulates follicular growth, maintaining a steady concentration during the early follicular stage, and is required to facilitate selection of follicles to the preovulatory phase. At this time, low levels of LH stimulate steroidogenesis in thecal cells by enhancing androgen synthesis, which in turn is converted to estradiol in the presence of FSH. The gradual increase in estradiol is essential to initiate the LH surge. The reciprocal relationship between FSH and estrogen concentrations during the follicular phase of the menstrual cycle is an exquisitely sensitive feedback pathway that governs the selection of the preovulatory follicle (6, 7). The modes of secretion for LH and FSH are linked to their function; LH is released in pulses via a regulated pathway (i.e. LH is stored in secretory granules) and associated with a bolus release at midcycle to rupture the follicle and form the corpus luteum (6-9). By contrast, FSH is primarily constitutively secreted, tightly coupled to its synthesis rate with gradual incremental increases until ovulation (9, 10).
The N-linked oligosaccharides play a critical role in the extracellular stability of both hormones. Sulfated oligosaccharides lead to a rapid clearance of LH in vivo, regulating its pulsatile release (3, 10, 11). Sialylation results in greater extracellular longevity of FSH as compared with LH. Although the structural motifs that govern the differential sorting of LH and FSH have yet to be identified, critical structural cues exist that might account for these intracellular events. LH and FSH are synthesized in the same cell and share an identical α subunit, and thus the β subunit must represent the key determinant for the specificity of carbohydrate processing and for the intracellular segregation of one or both hormones. Of all the human glycoprotein hormone β subunits, the LHβ subunit is the most hydrophobic, particularly in the region between residues 75 and 121 (12, 13). DNA sequences for the LHβ (12) and thyrotropin β (14) subunits encode hydrophobic stretches of seven and six amino acids, respectively, at their carboxyl termini, but a similar heptapeptide is not observed at the carboxyl terminus of the FSHβ subunit. This difference points to the carboxyl end of the LHβ subunit as a potential candidate for a sorting determinant.
Some important observations that might explain the unique secretion patterns discussed above have been obtained from transfected animal cell lines. Earlier studies from our laboratory and others demonstrated that the unassembled pituitary β subunits do not efficiently exit the ER in the absence of the α subunit (15-17). Although co-expression with the α subunit rescues the β subunits, differences exist in the extent of assembly of the α/β subunit pairs. For example, in the case of LHβ, the amount of dimer formed in transfected CHO cells is less than 10% (12, 15, 18), whereas more than 80% of the steady-state FSHβ subunit is secreted as a component of the heterodimer (16). The terminal LHβ heptapeptide (Leu-Ser-Gly-Leu-Leu-Phe-Leu) accounts, in part, for this inefficient assembly (13, 18). Based on these observations, we proposed that this sequence serves as a signal capable of governing the intracellular sorting and trafficking of LH (13).
The experiments described above were performed with CHO cells, which secrete proteins only by the constitutive route (13, 16-19) without intracellular accumulation of mature hormone dimers, thus precluding studies of glycoprotein hormone secretion by the regulated pathway. To investigate sorting of the LH/FSH dimers and free subunits, we have used the GH3 cell line (20, 21), which is derived from pituitary somatotropes and contain storage vesicles responsive to secretagogues (20-22). Importantly, we previously demonstrated that transfected GH3 cells secrete LH and FSH primarily through regulated and constitutive secretory pathways, respectively (21), and that the LH N-linked carbohydrates were sulfated (20). The above observations were in marked contrast with data obtained from transfected CHO cells in which no detectable responses to secretagogues or LH sulfation were observed (20, 21). Here we tested the requirement of the LHβ heptapeptide to direct LH to the regulated pathway of GH3 cells. The data indicate that the carboxyl-terminal heptapeptide contributes to the trafficking of LH dimer to the regulated secretory pathway.
EXPERIMENTAL PROCEDURES
Cell Culture and Stable Transfection—GH3 cells were a gift from Dr. Dennis Shields (Albert Einstein College of Medicine, New York). The cells were grown (no more than 30 passages) at 37 °C in Ham's F-12 medium (Mediatech Inc., Herndon, VA) supplemented with 12.5% horse serum (Invitrogen), 2.5% fetal bovine serum (Harlan Bioproducts for Science, Inc., Indianapolis, IN), 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified 5% CO2 incubator. Transfections were performed using Lipofectamine 2000 (Invitrogen) on semiconfluent cells in 6-well plates. Cells were transfected with 4 μg of the α, LHβ, LHβ114 (designated LHβΔT), FSHβ, or FSHβ-LHβ chimera (designated FSHβ-L) subunit genes (Fig. 1) contained in the vector pM2HA (19) to obtain clones expressing the individual subunits and corresponding dimers. Stable clones were selected ∼16 days later with 0.25 mg/ml G418 (Research Product International, Mt. Prospect, IL). Single colonies were isolated and subsequently screened by immunoprecipitating the media and lysates of metabolically labeled cells (see below). Several clones (n = 5 per dimer) expressing the dimers LH, LHΔT, FSH, or FSH-L were maintained in culture in the presence of 0.125 mg/ml G418 and used for the experiments described below.
FIGURE 1.
Schematic diagram of the gonadotropin subunits. These include: α subunit; LHβ, luteinizing hormone β subunit (the cross-hatched area of region 115-121 denotes the hydrophobic heptapeptide); LHβΔT, carboxyl-terminal mutant of LHβ subunit truncated at amino acid 114; FSHβ, follicle-stimulating hormone β subunit; FSHβ-L, the cross-hatched area of region 112-118 denotes the hydrophobic heptapeptide of LHβ. N, Asn-linked oligosaccharides.
The mutant LHβΔT described previously lacks a seven-amino acid extension (Leu-Ser-Gly-Leu-Leu-Phe-Leu) at the carboxyl terminus of the LHβ subunit (13). To construct FSHβ-L, the heptapeptide sequence of LHβ subunit (plus the stop codon) was inserted in-frame at the 3′-end of the FSHβ subunit by using overlapping PCR mutagenesis. The PCR was performed using KlenTag DNA polymerase (Sigma) and GenAmp PCR system 2400 (PerkinElmer Life Sciences). The following primers were used in the construction of the FSHβ-L chimera: oligo 1 (universal primer for pM2HA); 5′-TTC TCC CCC GCA GCC CTA GAA GAC GTT CCA-3′; oligo 2; 5′-GAG GAG GCC TGA GAG TTC TTT CAT TTC ACC-3′; oligo 3; 5′-GGT GAA ATG AAA GAA CTC TCA GGC CTC CTC-3′; oligo 4 (universal primer for pM2HA); 5′-TTT TCA CTG CAT TCT AGT TGT GGT TTG TCC-3′.
The universal primers (oligos 1 and 4) corresponded to the sequences in pM2HA vector located upstream and downstream of multiple cloning sites. In the first PCR reaction, the FSHβ-CTP-α gene was used as a template with primers 1 and 2 to amplify product A containing the entire FSHβ sequence and the beginning of the heptapeptide of LHβ subunit. A parallel reaction containing primers 3 and 4 and the LHβ subunit as a template generated PCR product B comprising part of FSHβ exon 3 and the heptapeptide sequence of LHβ with its stop codon. The overlapping PCR was performed using fragments A and B with primers 1 and 4, resulting in the final product FSHβ-L (FSHβ with the seven amino acids of LHβ subunit), which was sequenced to ensure no errors occurred during the PCR reactions. The FSHβ-L chimera was enzymatically digested by BamHI and inserted into the pM2HA vector. The pM2HA vector is a pSV2 neo derivative, which contains the ampicillin resistance and neomycin resistance genes and the Harvey murine sarcoma virus long terminal repeat.
Metabolic Labeling and Immunoprecipitation—For continuous labeling experiments, cells were plated into 6- or 12-well dishes and grown for ∼4 days to near confluency. Cells were labeled for 16 h with 20 μCi/ml [35S]cysteine (specific activity > 1000 Ci/mmol, MP Biomedicals Inc., Irvine, CA) in Ham's F-12 medium minus cysteine and G418 but supplemented with 7.5% dialyzed fetal bovine serum, glutamine, and antibiotics. Labeling with inorganic sulfate was performed for 16 h in Ham's F-12 sulfate-free medium with 0.7 mCi/ml carrier-free sulfate (Na2[35S]O4; MP Biomedicals Inc.) supplemented with 7.5% dialyzed fetal bovine serum, glutamine, and antibiotics (20).
For pulse-chase experiments, confluent cells grown on 6-well plates were preincubated for 1.5 h with cysteine-free medium followed by a 20 min pulse in this medium containing 80 μCi/ml [35S]cysteine. At the end of the pulse, the medium was aspirated, and the cells were washed twice with prewarmed chase medium comprising Ham's F-12, 1 mm unlabeled l-cysteine (Sigma), 7.5% dialyzed fetal bovine serum, glutamine, and antibiotics and incubated in this medium for up to 24 h.
All collected media and cell lysates were treated with iodoacetamide and phenylmethanesulfonyl fluoride to inhibit proteases. After centrifugation to remove cell debris, samples (2 ml) were precleared with 7.5 μl/ml normal rabbit serum and Pansorbin (EMD Biosciences Inc., La Jolla, CA). The supernates were divided into two aliquots and immunoprecipitated for 2 h at room temperature by anti-α or anti-CGβ (which cross-reacts with the LHβ subunit) rabbit polyclonal sera. The immune complexes were precipitated with Pansorbin and subjected to SDS-PAGE on 12.5 or 15% gels. For quantitative comparisons of LH and LHΔT, equal volumes of samples were always loaded on the gel. The gels were soaked in 1 m sodium salicylate for 15 min, dried, and exposed to x-ray film (18).
Forskolin-stimulated Secretion—To examine gonadotropin storage and regulated secretion of dimers, five wells of LH and LHΔT cells were labeled for 16 h with [35S]sulfate or [35S]cysteine. Medium and lysate from one well were collected and served as the 0 time controls for the subsequent experiments. Cells in the remaining four wells were preincubated in chase medium (containing 1 mm unlabeled l-cysteine, 7.5% dialyzed fetal bovine serum, glutamine, and antibiotics) for 2 h to reduce the background of the constitutive pool. After the 2-h preincubation, media were collected and frozen for immunoprecipitation. During a second 2-h period, two wells received fresh chase medium with forskolin (25 μm final concentration; Sigma) (20), and the remaining two wells were incubated without secretagogue. Cells expressing LHΔT were also incubated in the presence or absence of forskolin immediately after a 16-h labeling without the 2-h preincubation period. All labeled culture media and cell lysates were then immunoprecipitated with α antiserum and analyzed by 12.5% SDS-PAGE.
To examine the storage and secretion of FSH and FSH-L dimers, cells were labeled for 4 h with [35S]cysteine instead of sulfate, because the N-linked oligosaccharides of FSH are not sulfated as they are in LH. Medium and lysate from one well were collected and served as the 0 time controls for the subsequent experiment. Cells in one well received chase medium for 2 h with forskolin (25 μm final concentration) (20), and the remaining well was incubated without secretagogue. All labeled culture media and cell lysates were then immunoprecipitated with FSH dimer-specific antibody (FSH 54B) and analyzed by 15% SDS-PAGE.
Immunofluorescence—GH3 cells expressing LH or LHΔT were grown on Fisherbrand Superfrost-Plus microscope slides (Fisher Scientific) in Petri dishes as described above. The cells were incubated at 37 °C for 4 h in the presence or absence of cycloheximide (CHX) (15 μg/ml; Sigma). Immediately following CHX treatment, all cells were fixed in 4% paraformaldehyde for 20 min and permeabilized with 0.2% Tween for 10 min. Cells were then incubated in 20% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 h to block nonspecific binding and washed three times for 10 min in 2% bovine serum albumin (Sigma). After washing, cells were incubated in rabbit anti-CGβ antiserum (1:250; also used for immunoprecipitation experiments) for 30 min at room temperature. The cells were washed three times in 2% bovine serum albumin and incubated in goat anti-rabbit Alexa Fluor 488 (1:250; Invitrogen) for 20 min. Cells were then washed three times in 2% bovine serum albumin and once in phosphate-buffered saline and counterstained with TOPRO-iodide (Invitrogen) for 15 min. Cells were washed three times in phosphate-buffered saline for 10 min; coverslips were added using VectaShield mounting medium (Vector Laboratories), and cells were examined by confocal microscopy using an Olympus FV-500 microscope with a ×60 water objective. The experiment was repeated three times. Cells from at least two fields from each slide were counted by two individuals (one blind to treatment) and were scored as either negative or positive; ∼200 cells per slide were counted. Cells were considered positive if multiple discrete puncta were observed. Negative cells were devoid of puncta, similar to cells incubated with normal rabbit serum.
Analysis of Data—The labeled bands from autoradiography were scanned using a GS-710 calibrated imaging densitometer, and the intensity of bands was quantitated by densitometric analysis using Quantity One software (Bio-Rad Laboratories). Equal exposure times for the autoradiograms were used when comparing the results of synthesis and protein secretion for LH, LHΔT, FSH, and FSH-L dimers. The secretion t½ for the dimers corresponds to the time when 50% of the labeled dimer, as determined from the presence of α subunit, accumulated in the medium when immunoprecipitating with CGβ antisera. Recovery of subunit (%) was determined as the amount of labeled subunit in medium as a fraction of the total. The sorting index of LH, LHΔT, FSH, and FSH-L dimer corresponds to the ratio of the band intensity on autoradiograms obtained with forskolin (+F) to the band intensity obtained without (-F) forskolin. For immunofluorescence, results are expressed as the percentage of positive cells. Each experiment was repeated three to eight times, analyzed using a paired t test, and the results are expressed as mean ± S.E., with p < 0.05 considered significantly different.
RESULTS
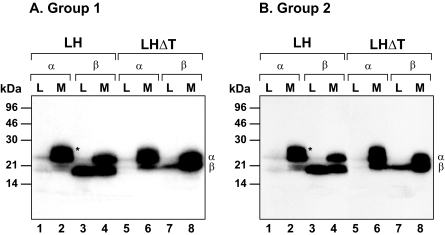
Secretion of LH and LHΔT After Steady-state Labeling—Previously we showed in CHO cells that deleting the seven-amino acid hydrophobic carboxyl-terminal extension from the LHβ subunit enhances secretion and alters processing of the N-linked oligosaccharides of this truncated subunit (designated LHβΔT, Fig. 1) compared with the wild-type LHβ subunit (19). Thus, we suspected that, because this sequence affects the intracellular disposition of the subunit, it contributes to LH sorting in the regulated pathway. Because CHO cells lack a regulated secretory pathway, we examined the secretion of LH and the mutant LHΔT in GH3 cells, which contain both constitutive and regulated routes (20, 21). Fig. 2 shows the secretion of two sets of representative clones (Groups 1 and 2) expressing either LH or LHΔT heterodimers after 16 h of labeling with [35S]cysteine.
FIGURE 2.
Secretion of representative clones (Group 1 (A) and Group 2 (B)) expressing either LH (n = 2 clones) or LHΔT (n = 2 clones) dimers after being labeled for 16 h with 20 μCi/ml [35S]cysteine. Media (M) and lysates (L) were immunoprecipitated with anti-α and CGβ sera. The immunoprecipitates were resolved by SDS-PAGE and subjected to autoradiography. The migration of α and β subunits and the molecular mass markers are indicated. The data shown are representative of five independent experiments. Asterisks represent free α subunit.
Immunoprecipitation of LH dimer secreting cells with α antiserum co-precipitates the β subunit, reflecting the extent of heterodimer formation (Fig. 2, A and B, lane 2), and the combined and free (asterisks) forms of α subunit. The unassembled α subunit is unique in that it undergoes a post-translational modification resulting in a more heterogeneous form, which clearly distinguishes it from the dimer species (Fig. 2, A and B, lane 2). The free α subunit is rapidly secreted with no detectable intracellular accumulation (Fig. 2, A and B, lane 1). As reported previously (18, 20), after overnight labeling the secreted heterodimer precipitated with α antiserum contains labeled α subunit associated with only a fraction of the total labeled β subunit seen in the lysate (Fig. 2, A and B, compare lanes 2 and 3). This is presumably due to the presence of a stable, nonlabeled intracellular pool of assembly-competent LHβ subunit (see “Discussion”). Precipitation of LH dimer by β subunit antiserum co-precipitates α subunit from the lysate and medium (Fig. 2, A and B, lanes 3 and 4). Note that the α subunit is only weakly observed in the lysate (Fig. 2, A and B, lane 3), reflecting the extent of heterodimer accumulating after steady state labeling. Note also the absence of the heterogeneous free α subunit in the media (Fig. 2, A and B, compare lanes 2 and 4). In contrast to the free α subunit, a significant fraction of the unassembled β subunit accumulates intracellularly (Fig. 2, A and B, lane 3), despite the presence of excess α subunit, and is confined to the ER (12, 14). This result agrees with earlier studies showing that in transfected cell lines the LHβ subunit inefficiently heterodimerizes with the α subunit (18, 19) and the unassembled β subunit is not secreted efficiently.
As expected from our previous work in CHO cells (18), the extent of heterodimer formation is significantly increased when the heptapeptide is deleted (Fig. 2, A and B, lanes 5-8). This is reflected in the 3.1 ± 0.4-fold reduction (p < 0.05) in the lysate fraction of the unassembled LHβΔT subunit immunoprecipitated with β antiserum compared with the LHβ subunit (Fig. 2, A and B, lanes 3 and 7). In addition, immunoprecipitation with α antiserum demonstrated a 2.6 ± 0.1-fold decrease (p < 0.05) in the amount of intracellular LHΔT (Fig. 2, A and B, lane 5) and 2.3 ± 0.2-fold increase (p < 0.05) in the secretion of LHΔT compared with LH dimer (lanes 2 and 6). This is associated with an increase in the incorporation of the labeled LHβΔT subunit in heterodimer compared with the wild-type LHβ subunit. It is curious that the truncated LHβΔT subunit migrates slower in the gel compared with the wild-type LHβ subunit. It is unclear whether this is due to the unmasking of a site for post-translational change or an effect on the mobility of the subunit on the gel. These data show that the total LHΔT secreted from GH3 cells is greater than LH dimer.
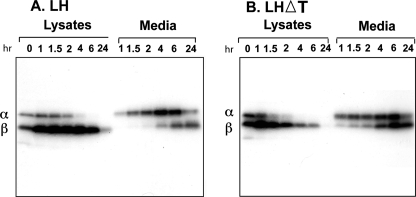
Pulse-Chase Kinetics—The steady-state labeling suggested that less of the LHΔT dimer is retained in GH3 cells consistent with an increased constitutive secretion. To assess this point more directly, we examined the kinetics of LH and LHΔT secretion by pulse-chase labeling (Fig. 3). GH3 cells were pulse-labeled for 20 min with [35S]cysteine and chased for the indicated times (Fig. 3). As expected in the case of wild-type LH (Fig. 3A), there is an excess accumulation of intracellular β subunit compared with the α subunit. The t½ for secretion of LH was 3.5 ± 0.06 h based on the appearance of dimer (determined from co-precipitated α subunit) in the media. It is also evident that labeled α subunit was chased into the secreted dimer more quickly than the labeled β subunit. Because β subunit antiserum was used, the labeled α subunit detected at the earliest time point is likely combining with predominantly unlabeled assembly-competent LHβ subunit as described previously (15) (see also “Discussion”). In the case of LHΔT (Fig. 3B), similar to earlier reports in which CHO cells were used, the efficiency of assembly was doubled as compared with LH (only 17% of the LHβ subunit assembled with the α subunit versus 32% of LHβΔT), and its secretion into the media was faster (t½ = 1.8 ± 0.21 h) compared with LH. The recovery of both proteins in the media was greater than 90%, and thus differences in the secretion of LH and LHΔT are not likely related to degradation. The labeled LHβΔT subunit appeared earlier in the secreted dimer as compared with wild-type β subunit in the LH dimer. Taken together, the data suggest that removal of the heptapeptide increased secretion through the constitutive pathway and that this sequence is associated with directing LH to the regulated pathway.
FIGURE 3.
Pulse-chase kinetics of LH (A) and LHΔT(B) dimers. GH3 cells were pulse-labeled with 80 μCi/ml [35S]cysteine for 20 min and then chased for the indicated times. Chase at 0 h indicates the lysate sample prepared immediately after the pulse. Lysates and media were immunoprecipitated with CGβ antiserum and subjected to SDS-PAGE followed by autoradiography. The migration of α and β subunits is indicated. The t½ was calculated from the appearance of the α subunit in the media, which indicates the extent of dimer formation. The data shown are representative of three independent experiments.
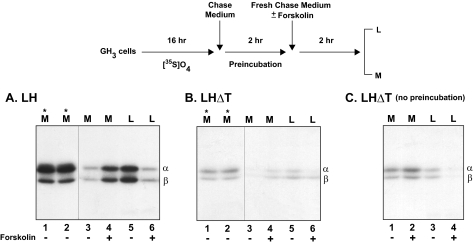
Secretagogue-stimulated Secretion of LH/LHΔT—Earlier we showed that LH accumulates in a secretagogue-sensitive pool, reflecting segregation to the regulated pathway (20, 21). If deleting the heptapeptide leads to increased constitutive secretion, as proposed above, the amount of forskolin-releasable LHΔT dimer should be decreased compared with LH. To investigate this issue we labeled the cells with [35S]sulfate (Fig. 4). Because the LH N-linked oligosaccharides are sulfated in GH3 cells (20), we reasoned that, given that this modification occurs in the trans-Golgi and is a final step in the maturation of the oligosaccharides just prior to secretion, only those mature forms of LH/LHΔT accumulating in the late stages of the secretion pathway will contain [35S]sulfate. Presumably this enriched population is in a secretagogue-releasable pool, and as a result the background would be reduced compared with [35S]cysteine labeling. After a 16-h labeling, media and lysates collected from one well of each dimer served as the 0 time controls for the subsequent experiments. The distribution of the intracellular and secreted proteins is comparable with that seen for the cysteine labeled products (see Fig. 2). To reduce the background of constitutive secretion after labeling, LH and LHΔT cells were preincubated for 2 h in chase medium. At the end of the initial 2 h of preincubation, prior to forskolin addition, cells from the control well and the well to receive forskolin secreted comparable amounts of either LH or LHΔT into the medium (Fig. 4, A and B, lanes 1 and 2, asterisks). The addition of forskolin during the second 2-h period stimulated release of sulfate-labeled LH dimer (2.9 ± 0.4-fold, p < 0.05) compared with cells incubated without forskolin (Fig. 4A, lanes 3 and 4; Table 1). Importantly, the increased secretion was accompanied by a decrease of intracellular LH (3.2 ± 0.2-fold, p < 0.05) compared with un-stimulated cells (Fig. 4A, lanes 5 and 6; Table 1). It is also noteworthy that no shift in electrophoretic migration between the intracellular and secreted subunits was detected, indicating that both intracellular forms were fully processed to complex oligosaccharides, and thus the mature proteins accumulated intracellularly after traversing the late Golgi compartment (20).
FIGURE 4.
Secretagogue-stimulated secretion of LH and LHΔT dimers from GH3 cells. The top part of the figure illustrates the experimental protocol. Cells were incubated for 16 h with [35S]sulfate (0.7 mCi/ml) followed by a 2-h incubation in chase media. Media (M, asterisks) of LH (A) and LHΔT (B) collected at the end of the 2-h preincubation period demonstrate that cells in each pair of wells synthesized the same amount of LH/LHΔT prior to forskolin addition. Subsequently, these media were replaced with fresh chase media, and the incubation was extended for an additional 2 h in the presence (+, lanes 4 and 6) or absence (-, lanes 3 and 5) of 25 μm forskolin. Panel C represents LHΔT incubated in the presence (+, lanes 2 and 4) or absence (-, lanes 1 and 2) of forskolin for 2 h without preincubation. All cell lysates (L) and media (M) were immunoprecipitated with α antiserum. The data shown are representative of four independent experiments.
TABLE 1.
Forskolin (F)-stimulated secretion of dimers from transfected GH3 cells 35S-Sulfated (LH, LHΔT) or cysteine-labeled (FSH, FSH-L) and immunoprecipitated proteins from x-rays similar to those shown in Figs. 5, A and B, and 8B, respectively, were quantitated by densitometry. The sorting index (+F/-F) values for medium correspond to the ratio of the band intensity (volume OD/mm2) obtained with forskolin-stimulated release (+F; 25 μm) to the band intensity obtained without forskolin (-F). Values for lysates (-F/+F) represent the decrease of accumulation of protein after forskolin stimulation. The data represent the mean values of four independent experiments ± S.E. *, p < 0.05 for LH vs. LHΔT and FSH vs. FSH-L.
|
Protein
|
Sorting index
|
|
|---|---|---|
| Medium (+F/–F) | Lysate (–F/+F) | |
| LH | 2.90 ± 0.40* | 3.20 ± 0.23* |
| LHΔT | 1.20 ± 0.07 | 1.15 ± 0.06 |
| FSH | 1.05 ± 0.04 | 1.24 ± 0.05 |
| FSH-L | 2.24 ± 0.13* | 2.09 ± 0.12* |
In contrast to LH dimer, there was minimal stimulation of LHΔT secretion by forskolin (1.25 ± 0.07-fold versus no forskolin; Fig. 4B, lanes 3 and 4; Table 1). In addition, the unstimulated pool for LHΔT during the forskolin stimulation period was reduced by 4.4 ± 0.5-fold (p < 0.05) compared with LH dimer (Fig. 4, B, lane 5, versus A, lane 5). Although it is clear that the forskolin-stimulated release of LHΔT was reduced, it was difficult to accurately quantitate the density of bands (even after the prolonged exposure) because of its decreased intracellular accumulation during the entire 4-h incubation. To address this issue, an additional experiment was performed where the initial 2-h preincubation period was eliminated. Cells expressing LHΔT were incubated for only 2 h in the presence or absence of forskolin immediately after a 16-h labeling (Fig. 4C). In this case, there was a larger pool of intracellular LHΔT, compared with the 4-h chase, as reflected by the increase in the nonstimulated level of LHΔT (Fig. 4C, lane 3). However, in the presence of forskolin, the secretion of LHΔT was stimulated only 1.39 ± 0.11-fold compared with nonstimulated cells (Fig. 4C, lanes 1 and 2) and accompanied by a 1.38 ± 0.14-fold decrease in the lysate pool (lanes 3 and 4). These results confirm the 4-h chase data for LHΔT showing that the intracellular stores of LHΔT are significantly reduced relative to LH dimer. Similar differences were observed after forskolin-stimulated secretion with cysteine-labeled LH and LHΔT dimers (data not shown). Overall, these experiments indicate that the absence of the heptapeptide in the LHβ subunit leads to less regulated secretion of LHΔT.
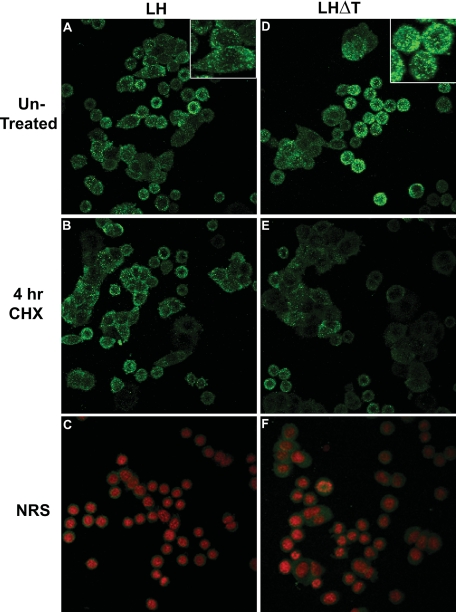
Immunofluorescence—We next examined whether the constitutive nature of LHΔT described above could be observed using immunofluorescence. Before fixation, cells were incubated for 4 h in the presence or absence of CHX to inhibit protein synthesis. This experiment predicts that in the absence of newly synthesized protein, the extent of LHΔT secretion will be greater than LH, thus having less intracellular retention. Untreated GH3 cells expressing LH or LHΔT have a similar fluorescence pattern with randomly dispersed puncta (Fig. 5, A and D). These puncta presumably represent post-Golgi LH- or LHΔT-containing vesicles and are not observed in cells incubated with normal rabbit serum instead of primary antiserum (Fig. 5, C and F). Note that for Fig. 5, A, B, D, and E, the counterstaining of the nucleus is not shown to better visualize the green puncta. The percentage of positive cells is similar between untreated and 4-h CHX-treated cells (82.4 ± 4.5 versus 77.3 ± 2.1%). In contrast, the percentage of LHΔT positive cells decreases from 80.4 ± 5.2% in untreated cells to 57.3 ± 4.5% after 4 h of CHX treatment (p < 0.05; Fig. 6), reflecting less regulated secretion of LHΔT.
FIGURE 5.
Immunofluorescence of untreated and 4-h CHX (15 μg/ml)-treated LH and LHΔTGH3 cells. Untreated LH (A) and LHΔT(D) cells have a similar fluorescence pattern with randomly dispersed puncta (green) around the nucleus. Insets show a higher magnification of LH and LHT cells for greater definition of the puncta. Counterstaining of the nucleus (red; C and F) is not shown in A, B, D, and E to enhance the contrast of the puncta. These puncta were not observed in cells incubated with normal rabbit serum (NRS; C and F) instead of primary antibody.
FIGURE 6.
Percentage of LH- or LHΔT-positive cells after incubation in the presence or absence (Untreated) of CHX. Results are the mean ± S.E. *, p < 0.05.
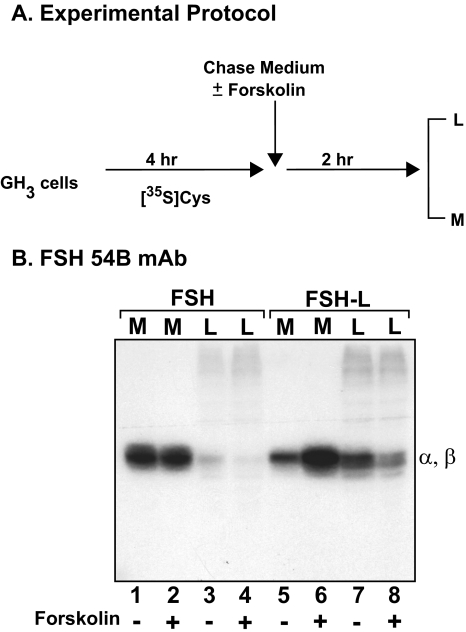
Forskolin-stimulated Secretion of FSH/FSH-L—We next asked whether the LHβ heptapeptide was sufficient to reroute a constitutively secreted protein to the regulated pathway. To examine this question, we fused the LHβ heptapeptide to the carboxyl terminus of the FSHβ subunit (FSHβ-L; Fig. 1). In this experiment, two parameters were compared between FSH-L and wild-type FSH: the extent of intracellular accumulation and the sensitivity to forskolin stimulation. Previously we showed that after stimulation with forskolin, a small fraction of newly synthesized FSH was secretagogue-sensitive and thus entered the regulated pathway (21). Those analyses used polyclonal antisera to FSH/FSHβ. Here we used a high affinity FSH dimer-specific monoclonal antibody (23, 24) to identify the proteins because it does not cross-react with the uncombined FSHβ subunit, thus reducing the background. As shown previously, the α and FSHβ subunits migrate at similar rates on SDS gels, and their resolution is not clear (21). It is evident that after 4 h of labeling and subsequent chase, more FSH-L accumulates in the lysate compared with FSH (Fig. 7B, lanes 3 and 7); this is accompanied by a corresponding decrease in the secretion of FSH-L (lane 5). Incubation with forskolin stimulated FSH-L 2.24 ± 0.13-fold (Fig. 7B, lanes 5 and 6) and FSH 1.05 ± 0.04-fold (lanes 1 and 2; Table 1). Moreover, there was a greater depletion of the intracellular pool (lysate) of FSH-L (2.09 ± 0.12-fold; Fig. 7B, lanes 7 and 8) than FSH (1.24 ± 0.05-fold; lanes 3 and 4) following forskolin stimulation (Table 1). Similar results were observed using different clones for FSH and FSH-L. These results indicate that the carboxyl-terminal seven amino acids of LHβ can reroute a fraction of FSH to the regulated pathway.
FIGURE 7.
Secretagogue-stimulated secretion of FSH and FSH-L dimers from GH3 cells. A, experimental protocol. B, represents FSH and FSH-L incubated in the presence (+, lanes 2, 4, 6, and 8) or absence (-, lanes 1, 3, 5, and 7) of forskolin. All cell lysates (L) and media (M) were immunoprecipitated with FSH 54B monoclonal antibody (mAb). Note that the α and FSHβ subunits co-migrate on SDS gels at ∼23 kDa. The data shown are representative of four independent experiments.
DISCUSSION
An important functional feature of the gonadotropins is their characteristic secretion patterns. LH is released in pulses via the regulated pathway in response to the secretagogue GnRH, whereas FSH is secreted constitutively. Clearly, this routing is essential for the coordinated roles of LH and FSH in the reproductive tract. Defining the sorting signals governing this trafficking is critical for understanding the link between their secretion and reproductive function. Although no common linear amino acid sequences capable of diverting secretory proteins into the regulated secretory pathway have been identified, signaling information has been observed in numerous domains of several secretory proteins (25-32). Here we describe a novel sorting signal (comprising a carboxyl-terminal seven-amino acid peptide in the LHβ subunit) that contributes to the diversion of newly synthesized LH to the regulated pathway of GH3 cells.
How the heptapeptide functions as a sorting signal is not clear. It could act directly as a sorting signal or indirectly through changes in the conformation of the molecule. Although the latter option cannot be excluded, the ability of the heptapeptide to reroute a portion of FSH to the regulated pathway suggests that the sequence per se is a sorting determinant. Recently, it was shown that α-helical sequences bearing stretches of hydrophobic amino acids at carboxyl-end regions can direct a variety of secretory proteins into the regulated secretion pathway (33). We examined the predicted secondary structure for the LHβ subunit with PSIPRED (protein structure prediction server) (34, 35). According to this analysis, the last 40 amino acids prefer a coil configuration. As such, it appears that the proposed sorting activity of the heptapeptide does not require a helical structure, although analogous to the above sorting sequence, it contains a hydrophobic cluster. Inspection of the heptapeptide sequence reveals a similarity to the sorting sequence XXXLL found in the cytoplasmic domain of numerous transmembrane proteins (36). Although recognition of the LH heptapeptide occurs in the luminal compartment rather than the cytoplasm, it is intriguing to consider that the heptapeptide has analogous recognition properties. For example, the heptapeptide might represent a granule-targeting domain that associates with a receptor in the vesicle membrane. It is likely that the heptapeptide works in concert with other signals in the LH molecule, because a portion of LHΔT still enters the regulated pathway. Importantly, the sequence studied here is well conserved in mammals (37), consistent with a proposed sorting function.
Further supporting the sorting feature associated with the heptapeptide are the observations that although the DNA sequence of the LHβ subunit gene encodes the heptapeptide, this hydrophobic stretch is not detected in the intact dimer isolated from pituitary tissue or urine (38, 39). Moreover, in the latter study (39), heterogeneity among carboxyl-terminal residues 114-121 of the LHβ subunit has been observed. That absence of the heptapeptide leads to less regulated secretion, with a concomitant enhanced constitutive secretion, supports its role in governing the trafficking of the LHβ subunit in the gonadotrope.
One critical finding regarding the expression of the gonadotropin family from transfected cell lines is that, although LHβ and FSHβ are retained intracellularly as monomers, only the LHβ subunit is slow to assemble with the α subunit (13, 15). Also, like the LHβ subunit, the presence of the heptapeptide on FSHβ decreases the extent of assembly with the α subunit (data not shown). The other β subunits assemble with the α subunit at an efficiency of more than 90%. Moreover, in transfected GH3 cells expressing LH dimer, a significant fraction of the pulse-labeled α subunit is secreted in association with unlabeled LHβ subunit, whereas the labeled β subunit remains unassociated. One explanation for the delay is the under-representation of labeled subunits by a large pool of nonlabeled species. Such pools of unassembled subunits have been observed in other systems (40-42). However, what is unusual in cells that contain LH dimers is that the population of free LHβ subunit persists despite the presence of excess α subunits. Thus, one explanation is that the LHβ subunit accumulates in at least two different luminal pools. Consistent with this view, several reports suggest that the ER is functionally mosaic and comprises specialized subdomains (43-48). These subcompartments may have distinct functions and a distinct distribution of resident and transient proteins. Thus, one model for how cells segregate LH/FSH heterodimers in the gonadotrope is assembly in distinct sub-ER compartments as proposed previously (23). This predicts that LH devoid of the heptapeptide will lose specificity in these domains. Alternatively, the selective routing of LH/FSH to the regulated/constitutive secretory pathway may be determined at the level of the trans-Golgi (49).
One issue that arises is whether in vivo LH and FSH dimers are routed via monohormone-containing vesicles or co-localize with the same carriers that bifurcate into monohormonal units. Given that our present experiments were performed with clonal GH3 cells, gonadotrope heterogeneity is not a variable, implying that the existence of only monohormonal LH and FSH secretion is governed primarily by general regulated and constitutive release mechanisms, respectively. Therefore, if a substantial population of vesicles initially contain both hormones, then encoded motifs are likely responsible for dictating the recognition to their respective pathways. Further characterization of the cognate recognition molecules for sorting determinants will be essential for understanding the underlying mechanisms by which LH and FSH are differentially sorted. Such investigations will also provide more general insight into how the entry of proteins into regulated secretory compartments is controlled.
Acknowledgments
We thank Drs. Phyllis Hanson, T. Rajendra Kumar, and Rick Sifers for helpful comments. We are also grateful to Dennis Oakley for help with confocal microscopy and to Laura Kyro, Barbara Machalek, Amanda Fisher, and Linda Lobos for preparation of the manuscript.
This manuscript was supported in part by National Institutes of Health (NIH) Grant DR065155, NIH Neuroscience Blueprint Core Grant NS057105 to Washington University, and the Bakewell Family Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LH, lutropin (luteinizing hormone); FSH, follitropin (follicle-stimulating hormone); CG, chorionic gonadotropin; CHX, cycloheximide; ER, endoplasmic reticulum; CHO, Chinese hamster ovary.
References
- 1.Pierce, J. G., and Parsons, T. F. (1981) Annu. Rev. Biochem. 50 465-495 [DOI] [PubMed] [Google Scholar]
- 2.Sairam, M. R. (1983) in Hormonal Proteins and Peptides: Gonadotropic Hormones (Li, C. H., ed) pp. 1-79, Academic Press, New York
- 3.Green, E. D., Boime, I., and Baenziger, J. U. (1986) Mol. Cell. Biochem. 72 81-100 [DOI] [PubMed] [Google Scholar]
- 4.Kessler, M. J., Mise, T., Ghai, R. D., and Bahl, O. P. (1979) J. Biol. Chem. 254 7909-7914 [PubMed] [Google Scholar]
- 5.Parsons, T. F., and Pierce, J. G. (1980) Proc. Natl. Acad. Sci. U. S. A. 77 7089-7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luderer, U., and Schwartz, N. B. (1992) in Serono Symposium on FSH: Regulation of Secretion and Molecular Mechanisms of Action (Hunzicker-Dunn, M., and Schwartz, N. B., eds) pp. 1-25, Springer-Verlag, New York
- 7.Farnworth, P. G. (1995) J. Endocrinol. 145 387-395 [DOI] [PubMed] [Google Scholar]
- 8.Currie, R. J., and McNeilly, A. S. (1995) J. Endocrinol. 147 259-270 [DOI] [PubMed] [Google Scholar]
- 9.Thomas, S. G., and Clarke, I. J. (1997) Endocrinology 138 1347-1350 [DOI] [PubMed] [Google Scholar]
- 10.Baenziger, J. U., Kumar, S., Brodbeck, R. M., Smith, P. L., and Beranek, M. C. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 334-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baenziger, J. U. (1994) Faseb. J. 8 1019-1025 [DOI] [PubMed] [Google Scholar]
- 12.Talmadge, K., Vamvakopoulos, N. C., and Fiddes, J. C. (1984) Nature 307 37-40 [DOI] [PubMed] [Google Scholar]
- 13.Matzuk, M. M., Spangler, M. M., Camel, M., Suganuma, N., and Boime, I. (1989) J. Cell Biol. 109 1429-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashizaki, Y., Miyai, K., Kato, K., and Matsubara, K. (1985) FEBS Lett. 188 394-400 [DOI] [PubMed] [Google Scholar]
- 15.Corless, C. L., Matzuk, M. M., Ramabhadran, T. V., Krichevsky, A., and Boime, I. (1987) J. Cell Biol. 104 1173-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keene, J. L., Matzuk, M. M., Otani, T., Fauser, B. C., Galway, A. B., Hsueh, A. J., and Boime, I. (1989) J. Biol. Chem. 264 4769-4775 [PubMed] [Google Scholar]
- 17.Kaetzel, D. M., Virgin, J. B., Clay, C. M., and Nilson, J. H. (1989) Mol. Endocrinol. 3 1765-1774 [DOI] [PubMed] [Google Scholar]
- 18.Muyan, M., Furuhashi, M., Sugahara, T., and Boime, I. (1996) Mol. Endocrinol. 10 1678-1687 [DOI] [PubMed] [Google Scholar]
- 19.Matzuk, M. M., Krieger, M., Corless, C. L., and Boime, I. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 6354-6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielinska, M., Rzymkiewicz, D., and Boime, I. (1994) Mol. Endocrinol. 8 919-928 [DOI] [PubMed] [Google Scholar]
- 21.Muyan, M., Rzymkiewicz, D. M., and Boime, I. (1994) Mol. Endocrinol. 8 1789-1797 [DOI] [PubMed] [Google Scholar]
- 22.Dannies, P. S. (1999) Endocr. Rev. 20 3-21 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Campayo, V., Jablonka-Shariff, A., and Boime, I. (2004) J. Biol. Chem. 279 44286-44293 [DOI] [PubMed] [Google Scholar]
- 24.Jablonka-Shariff, A., Kumar, T. R., Eklund, J., Comstock, A., and Boime, I. (2006) Mol. Endocrinol. 20 1437-1446 [DOI] [PubMed] [Google Scholar]
- 25.Childs, G. R. (1985) in Endocrinology (Labrie, F., and Proulx, L., eds) pp. 499-503, Elsevier, Amsterdam
- 26.Arvan, P., Zhang, B. Y., Feng, L., Liu, M., and Kuliawat, R. (2002) Curr. Opin. Cell Biol. 14 448-453 [DOI] [PubMed] [Google Scholar]
- 27.Tooze, S. A., Martens, G. J., and Huttner, W. B. (2001) Trends Cell Biol. 11 116-122 [DOI] [PubMed] [Google Scholar]
- 28.Dannies, P. S. (2001) Mol. Cell. Endocrinol. 177 87-93 [DOI] [PubMed] [Google Scholar]
- 29.Cool, D. R., Normant, E., Shen, F., Chen, H. C., Pannell, L., Zhang, Y., and Loh, Y. P. (1997) Cell 88 73-83 [DOI] [PubMed] [Google Scholar]
- 30.Brechler, V., Chu, W. N., Baxter, J. D., Thibault, G., and Reudelhuber, T. L. (1996) J. Biol. Chem. 271 20636-20640 [DOI] [PubMed] [Google Scholar]
- 31.Bundgaard, J. R., Birkedal, H., and Rehfeld, J. F. (2004) J. Biol. Chem. 279 5488-5493 [DOI] [PubMed] [Google Scholar]
- 32.Assadi, M., Sharpe, J. C., Snell, C., and Loh, Y. P. (2004) Biochemistry 43 7798-7807 [DOI] [PubMed] [Google Scholar]
- 33.Dikeakos, J. D., Lacombe, M. J., Mercure, C., Mireuta, M., and Reudelhuber, T. L. (2007) J. Biol. Chem. 282 1136-1143 [DOI] [PubMed] [Google Scholar]
- 34.McGuffin, L. J., Bryson, K., and Jones, D. T. (2000) Bioinformatics (Oxf.) 16 404-405 [DOI] [PubMed] [Google Scholar]
- 35.Jones, D. T. (1999) J. Mol. Biol. 292 195-202 [DOI] [PubMed] [Google Scholar]
- 36.Bonifacino, J. S., and Traub, L. M. (2003) Annu. Rev. Biochem. 72 395-447 [DOI] [PubMed] [Google Scholar]
- 37.Watanabe, N., Hatano, J., Asahina, K., Iwasaki, T., and Hayakawa, S. (2007) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146 105-118 [DOI] [PubMed] [Google Scholar]
- 38.Keutmann, H. T., Williams, R. M., and Ryan, R. J. (1979) Biochem. Biophys. Res. Commun. 90 842-848 [DOI] [PubMed] [Google Scholar]
- 39.Pantel, J., Robert, P., Troalen, F., Kujas, M., Bellet, D., and Bidart, J. M. (1998) Endocrinology 139 527-533 [DOI] [PubMed] [Google Scholar]
- 40.Tartakoff, A., and Vassalli, P. (1979) J. Cell Biol. 83 284-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho, M. K., and Springer, T. A. (1983) J. Biol. Chem. 258 2766-2769 [PubMed] [Google Scholar]
- 42.Kvist, S., Wiman, K., Claesson, L., Peterson, P. A., and Dobberstein, B. (1982) Cell 29 61-69 [DOI] [PubMed] [Google Scholar]
- 43.Sitia, R., and Meldolesi, J. (1992) Mol. Biol. Cell 3 1067-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths, G. (1996) Protoplasma 195 37-58 [Google Scholar]
- 45.Cabral, C. M., Choudhury, P., Liu, Y., and Sifers, R. N. (2000) J. Biol. Chem. 275 25015-25022 [DOI] [PubMed] [Google Scholar]
- 46.Kamhi-Nesher, S., Shenkman, M., Tolchinsky, S., Fromm, S. V., Ehrlich, R., and Lederkremer, G. Z. (2001) Mol. Biol. Cell 12 1711-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu, Y., and Hendershot, L. M. (2007) Adv. Exp. Med. Biol. 594 37-46 [DOI] [PubMed] [Google Scholar]
- 48.Avezov, E., Frenkel, Z., Ehrlich, M., Herscovics, A., and Lederkremer, G. Z. (2008) Mol. Biol. Cell 19 216-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakatsu, F., and Ohno, H. (2003) Cell Struct. Funct. 28 419-429 [DOI] [PubMed] [Google Scholar]