Abstract
Information from exogenous donor DNA can be introduced into the genome via homology-directed repair (HDR) pathways. These pathways are stimulated by double strand breaks and by DNA damage such as interstrand cross-links. We have employed triple helix-forming oligonucleotides linked to psoralen (pso-TFO) to introduce a DNA interstrand cross-link at a specific site in the genome of living mammalian cells. Co-introduction of duplex DNA with target region homology resulted in precise knock in of the donor at frequencies 2–3 orders of magnitude greater than with donor alone. Knock-in was eliminated in cells deficient in ERCC1-XPF, which is involved in recombinational pathways as well as cross-link repair. Separately, single strand oligonucleotide donors (SSO) were co-introduced with the pso-TFO. These were 10-fold more active than the duplex knock-in donor. SSO efficacy was further elevated in cells deficient in ERCC1-XPF, in contrast to the duplex donor. Resected single strand ends have been implicated as critical intermediates in sequence modulation by SSO, as well as duplex donor knock in. We asked whether there would be a competition between the donor species for these ends if both were present with the pso-TFO. The frequency of duplex donor knock in was unaffected by a 100-fold molar excess of the SSO. The same result was obtained when the homing endonuclease I-SceI was used to initiate HDR at the target site. We conclude that the entry of double strand breaks into distinct HDR pathways is controlled by factors other than the nucleic acid partners in those pathways.
Double strand breaks (DSBs)2 are among the most dangerous forms of DNA damage and may result in deletion, rearrangement of chromosomal sequences, or, if unrepaired, chromosome loss and possibly cell death (1). There are multiple pathways for DSB repair that are distinguished by the identity of the proteins and enzymes involved and their potential for mutagenesis of the break site (2–8). Non-homologous end joining, the major pathway in mammalian cells, is homology-independent and often results in small deletions at the site of the break. There are two homology-directed repair (HDR) pathways in which resected single-stranded ends interact with homologous sequences. In single strand annealing (SSA), the single strand end anneals with a complementary single strand. SSA between two direct repeated sequences results in the retention of one copy of the repeat and deletion of the other copy and the intervening sequence. Homologous recombination repair involves invasion of a DNA duplex by a single strand end and can be error-free or mutagenic depending on the sequence of the invaded duplex. Because the sequences with which they interact need not be absolutely identical to the single strand end, the homology-directed pathways provide an opportunity to manipulate the sequence of the genome.
Gene conversion and recombination with exogenous double and single strand donor DNAs occur at impractically low frequencies in mammalian cells (9). However, DSBs in the genome markedly enhance the frequency of cis and trans recombination/gene conversion, as demonstrated in experiments with the homing endonuclease I-SceI (3, 10, 11). Exposure of cells to DNA-damaging agents such as ionizing radiation, UV light, or cross-linkers also stimulates HDR (12–15), presumably due to DSBs formed as the direct or indirect consequence of the damage (16). Thus a sequence-specific nuclease or targeted DNA damage would be the basis of a strategy for manipulation of the genome. Although considerable effort has been given to the development of chimeric nucleases for this purpose (17, 18), the alternate approach based on targeted DNA damage has received much less attention (9, 19).
One strategy for targeting DNA damage is based on triple helix-forming oligonucleotides (TFOs) coupled to DNA-reactive compounds. Triplexes can form when a third strand of nucleic acid lies in the major groove of an intact duplex target, typically on a polypurine:polypyrimidine element (20). The complex is sequence-specific and stabilized by hydrogen bonds between the third strand bases and the purines in the duplex. Although conventional deoxyoligonucleotides perform poorly under physiological conditions (21), we have identified a modification format that confers biological activity on a psoralen-TFO (pso-TFO) as measured in a site-specific gene mutation assay (22, 23). Mutagenesis of the targeted psoralen cross-link resulted in deletions that were consistent with repair by non-homologous end joining of DSBs. Base substitutions were also recovered and could be explained by error-prone bypass of a gapped intermediate formed after the initial incisions that released one cross-link strand from the other (24). Cross-link release is termed “unhooking” and is dependent on the activity of ERCC1-XPF, a component of nucleotide excision repair (25, 26).
Psoralen cross-links also stimulate recombination (27–29). Consequently, we were interested in examining the entry of the targeted cross-link site into HDR pathways. We have used two assays in which exogenous information was supplied by donor DNA introduced at the same time as the pso-TFO. In one case we used a standard double strand replacement vector, while in the other we employed single strand oligonucleotide (SSO) donors. We recovered stable cell lines with the targeted sequence changes with both donors and have measured the influence of nucleotide excision repair deficiencies on the activity of the two donors.
EXPERIMENTAL PROCEDURES
Cells, Plasmids, Oligonucleotides—The Chinese hamster ovary (CHO) wild type cell line AA8, the CHO repair-deficient cell lines UV5/XPD and UV41/XPF, and ERCC1-deficient CHO-727 (30) cells were grown in α-minimum essential medium supplemented with penicillin, streptomycin, and 10% fetal bovine serum. Prior to experiments, cells were cultured in medium containing HAT (10–4 m hypoxanthine, 5 × 10–6 m aminopterin, 10–5 m thymidine) to remove pre-existing Hprt-deficient cells. CHO were synchronized in G0/G1 by a variation of the method described by Sawai et al. (24, 31). Briefly, cells were plated at subconfluent levels and the next day the medium changed to α-minimal essential medium with 2% fetal bovine serum and 2% DMSO. After 48 h the cells were washed (85–88% G0/G1) and incubated with complete medium containing 100 μm mimosine for 16 h to block them in early S phase (∼90% early S phase cells). After 16 h the cells were released from the mimosine block by feeding with α-minimum essential medium/10% fetal bovine serum.
TFOs and Hprt Mutation Assay—The 17-nucleotide TFO, AE-07, against the hamster Hprt target, was synthesized and purified as described previously (22). Pso-TFOs were introduced by electroporation using an Amaxa nucleoporator. In experiments with the donor nucleic acids, 3 μg of the duplex donor AM200 and/or 3 μg of an SSO donor were cotransfected with the pso-TFO. Transfection was followed by incubation for 3 h and then exposure in the Rayonet chamber to UVA light for 3 min at 1.8 J/cm2. The cells were passaged twice over a 7-day period and then plated in culture medium containing 20 μm 6-thioguanine (6-TG). Cells were also plated in medium without 6-TG to determine plating efficiency. After 7–10 days, colonies were counted and the mutation frequencies calculated as the ratio of 6-TG-resistant colonies/total colony-forming cells. In experiments with double selection, 400 μg/ml Geneticin (Invitrogen) was added to medium in addition to 6-TG.
Gene Knock In and Sequence Conversion at an I-SceI Site—AMISI cells in random culture were electroporated with an I-SceI expression plasmid and variously an SSO and/or the AM200 duplex donor. Cells were maintained in culture for 7 days and then placed in 6-TG selection as above.
Analysis of Hprt Target Region in 6-TG-resistant Colonies—Individual colonies were picked and expanded in 96-well dishes. DNA was extracted and the exon 5 target region amplified using forward primer 5′-CTAGTTTGAGGCCAGCTTTGGC and reverse primer 5′-GGGATTCCAGGCATGCCTTACTG, which yield a 750-bp fragment with DNA from wild type cells. Digestion of the PCR product with the appropriate restriction enzyme was used to determine the frequency of sequence conversion by TGRC-1 or other SSO. Knock in of the AM200 duplex donor was examined by nested PCR with a first primer set of 5′-AATAAAACGCACGGTGTTGGG and 5′-GCAAGAAGGGAGGTAGATGATG and a second set of 5′-GTTTGTTCATAAACGCGGG and 5′-CCTGTAATGCCAGCACTTGAC. For Southern analysis, restriction enzyme-digested DNA from different clones was electrophoresed on 1.5% GTG-agarose gel, transferred to Hybond-N+ nylon membrane (Amersham Biosciences), and hybridized to a 32P-labeled 2-kb restriction fragment complementary to the region containing exon 5. The frequencies of all events are based on the number of cells in the population following treatment and passage.
RESULTS
The Hprt gene contains a polypurine:polypyrimidine element adjacent to exon 5 that can support the formation of a stable triplex (32). This sequence terminates in a 5′-TA step that is a preferred sequence for interstrand T-T cross-link formation by psoralen (Fig. 1). The pso-TFO, AE-07, used in these experiments contains a patch of four contiguous 2′-O-aminoethoxy ribose residues (33) with the remaining sugars as 2′-O-methyl ribose (22, 23). Treatment of cells in S phase resulted in the highest levels of targeted cross-linking (24). Levels of recombination functions are also greatest in S phase. Thus, unless otherwise indicated, experiments were performed with S phase cells.
FIGURE 1.
The sequences of the triplex target in the CHO Hprt gene and the triple helix-forming oligonucleotide linked to psoralen. The XbaI site is in italics, and the thymidine residues linked by the psoralen cross-link are in larger font.
Pso-TFO-mediated Gene Knock In—The double strand DNA fragment AM200 contained two 1.5-kb sequence elements identical to the regions on either side of the triplex target site. These flanked the gene for neomycin resistance (Fig. 2a). Targeted knock in of AM200 would delete 200 bp of the genomic target and inactivate the Hprt gene. Colonies carrying the targeted insertion were resistant to G418 and sensitive to 6-TG.
FIGURE 2.
Targeted knock in of a duplex donor by the pso-TFO. a, schematic of the chromosomal target with the TFO-targeted cross-link site and the replacement duplex donor. The regions of homology are not to scale. Targeted knock in of the donor produces a cell that is Hprt mutant and resistant to neomycin. b, Southern blot analysis of individual clones with targeted knock in of the duplex donor. The Hprt gene is single copy in CHO cells, and precise knock in results in the shift of the restriction fragment from the size denoted by the light arrow (the control sample, rightmost lane) to that indicated by the heavy arrow.
The pso-TFO and the AM200 donor were co-electroporated into CHO-AA8 cells. Following photoactivation and passage the cells were exposed to G418 in addition to 6-TG. Resistant colonies were expanded and DNA extracted and analyzed by PCR for the presence of targeted insertion of the donor vector. In experiments with the donor DNA but lacking the pso-TFO or with the pso-TFO but without photoactivation, we recovered colonies containing the targeted insertion at frequencies of 0.5 ± 0.1 × 10–6, consistent with previous work (34). However, when the pso-TFO was co-electroporated with the donor DNA followed by photoactivation, the frequency of clones containing the targeted insertion, determined by PCR analysis, was 0.19 ± 0.07 × 10–3. Positive clones were expanded and total genomic DNA extracted. Analysis by Southern blot hybridization demonstrated the shift of the target fragment to the size expected from a precise insertion/replacement event (Fig. 2b). The sequence of the regions corresponding to either end of the donor DNA in 12 clones was determined and found to be identical to that in the donor. Thus, the pso-TFO/UVA treatment of the cells could increase the frequency of targeted gene knock in by 300 to 400-fold.
Targeted Knock In in DNA Repair-deficient Cells—Previously we found that the frequency of deletion mutations rose in cells with deficiencies in nucleotide excision repair activities such as XPD or ERCC1-XPF (35). We interpreted this as a reflection of increased DSB formation at the targeted cross-link site as the result of the unavailability of repair pathways dependent on these genes. In light of the stimulatory effect of DSB on HDR pathways, we introduced the pso-TFO and the AM200 donor DNA into cells deficient in these functions. The knock-in frequency in XPD-deficient cells was ∼5-fold greater than in wild type cells (Fig. 3). In contrast, there was no knock-in activity in ERCC1-deficient cells. Identical results were obtained with cells deficient in XPF (not shown). These results were consistent with the interpretation that an increase in deletion mutations in the XPD-deficient cells was the result of an increase in DSBs, some of which could be captured by the AM200 donor. The absence of donor activity in the ERCC1-XPF-deficient cells most likely reflected the additional role of this complex in facilitating HDR pathways (36, 37). Knock in of the AM200 donor was at wild type levels in cells with repair deficiencies in DNAP-Kcs or MSH2 or MutSβ (not shown). In our previous work cells with these deficiencies had wild type deletion frequencies following pso-TFO treatment (35).
FIGURE 3.
Influence of repair deficiency on pso-TFO-targeted knock in. Knock in of the duplex replacement donor was measured in wild type cells or cells deficient in XPD or ERCC1. The results are presented as the mean and S.D. of three or more independent experiments.
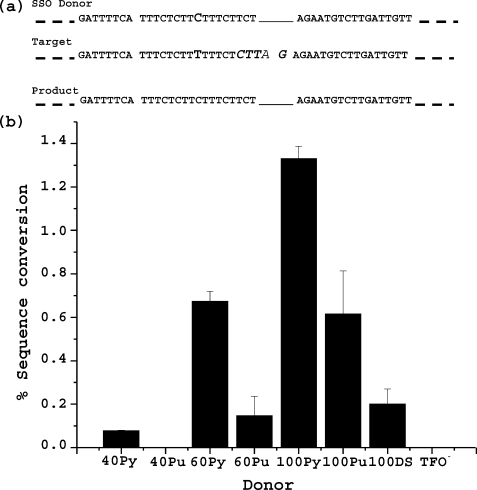
Targeted Sequence Manipulation by SSO Donors—Sequence modulation in mammalian cells following introduction of SSO donors has been reported, although frequencies have been variable and low (38–40). However, Resnick and co-workers (41, 42) have described high frequency repair by SSO donors of a DSB in yeast via an SSA pathway. Recently, repair of a DSB by SSO donors has also been shown in mammalian cells (43). We asked whether sequence information supplied by SSOs could be introduced at the site of the pso-TFO-targeted cross-link. For the initial experiments we took advantage of a cell line obtained in a previous study. This line, CHO-AM12, has a mutant Hprt gene as a result of changes in the sequence of the nucleotides adjacent to and within exon 5. These changes destroyed an XbaI site but created a new triplex target site adjacent to a TA step, appropriate for psoralen cross-linking (Fig. 4a). We synthesized a pso-TFO containing a patch of 2′-O-aminoethoxy residues according to guidelines described by us previously (23). This TFO (AE-AM12) was co-electroporated with SSOs of varying length and complementarity, designed to convert the target to wild type sequence (Fig. 4a). Following UVA treatment the cells were cultured for 4–5 days and then placed in HAT medium to select colonies with a functional Hprt gene. Individual HAT-resistant colonies were expanded, DNA extracted and amplified, and the introduction of the XbaI site (from the donor) verified by digestion of the appropriate PCR fragment. The results indicated that the frequency of sequence conversion varied as a function of donor oligonucleotide length and, to a certain extent, complementarity. The 100-mer donor containing the pyrimidine sequence in the triplex target was the most active, whereas the 40-mer in the opposite strand was inactive. The activity was completely dependent on the pso-TFO/UVA treatment (Fig. 4b). The sequence of the target region was determined in 25 HAT-resistant colonies and all had incorporated the entire donor sequence. However, it should be noted that the requirement for a functional Hprt gene would very likely restrict the diversity of sequences at the target site.
FIGURE 4.
Modification of the pso-TFO target site by SSO. a, sequence of the target and SSO. The target region in the AM12 cell line is shown with the sequence changes in italicized and enlarged font. The SSO donor restores the wild type sequence in the vicinity of the targeted cross-link and also introduces a C (in bold) in place of a T in the triplex target sequence. b, influence of SSO length and complementarity on donor activity at the pso-TFO target site. The designations Pu or Py reflect the strand containing either the purine or pyrimidine run in the triplex target. TFO– refers to the activity of the SSO in the absence of the pso-TFO. Error bars represent S.D.
To address this issue we asked whether the same strategy could be used to modulate the frequency of mutagenesis of the pso-TFO target site in wild type CHO-AA8 cells. We co-electroporated, with the pso-TFO, a 100-mer SSO donor, TGRC-1, designed to mutate the wild type target site (replacing the XbaI site with an XhoI site) (Fig. 5a). In a series of experiments the frequency of 6-TG-resistant colonies after treatment with the pso-TFO alone was ∼0.22%. However, when the TGRC-1 donor was co-electroporated with the pso-TFO, the frequency of 6-TG resistance was ∼2-fold greater (Fig. 5b). Analysis of the DNA extracted from individual colonies confirmed the replacement of the XbaI site by XhoI in 60–70% of the clones, such that the sequence conversion frequency was 0.25–0.3%. Determination of the sequence of the target region in 24 6-TG-resistant clones showed that 21 had the exact sequence expected from the TGRC-1 donor, while the other three had an additional single base variation from the donor sequence near the cross-link target site (not shown). These results suggested a relatively faithful incorporation of donor sequence despite the absence of functional constraints on the targeted sequence.
FIGURE 5.
Modulation of pso-TFO-targeted mutagenesis by wild type or mutant SSO donors. a, sequence of the mutant SSO donor TGRC-1 and the target region and product. The sequence of the wild type donor is shown in Fig. 4a. b, frequency of mutagenesis of the pso-TFO target with the TGRC-1 or wild type donor SSO. The open portion of the column for AE-07/TGRC-1 represents the frequency of TGRC-1-directed mutagenesis. Error bars represent the mean and S.D. of three or more independent experiments.
We then performed the counterpart experiment by co-introduction of the pso-TFO and an SSO carrying the wild type sequence into the wild type CHO cells. This resulted in a decline in 6-TG-resistant colonies to 0.12% (Fig. 5b). The implication of this result was that some DSB that would have become deletions were converted by the wild type SSO to wild type sequence.
Control Experiments—The assays for targeted sequence manipulation were based on the gain or loss of activity of the Hprt gene. Subsequent to the isolation of individual clones of drug-resistant cells, DNA was extracted, the target sequence region amplified, and the PCR products examined by restriction digestion. There are reports of artifacts due to donor oligonucleotides participating in, or interfering with, PCR analysis of genomic DNA (44–46). Although it seemed likely that the concentration of donor oligonucleotide would have been diluted to insignificance during the 30–40 doublings between treatment and PCR analysis, we performed three control experiments to address this issue. The TGRC-1 SSO was introduced into cells in the absence of the pso-TFO. The cells were cultured for a week and then diluted and plated so as to permit isolation of individual colonies (no selection). Individual colonies were expanded, DNA isolated, the target region amplified, and the products examined by restriction digestion. No positive clones were recovered. In a second experiment, DNA was extracted from 96 6-TG-resistant clones generated by treatment of AA8 cells with the pso-TFO/TGRC-1 donor combination. One aliquot of the DNA was examined by target region amplification and restriction digestion. Another aliquot was digested with Exonuclease I to remove single-stranded DNA, including oligonucleotides, prior to amplification and restriction digestion. No differences between the two samples were found in the frequency of restriction-sensitive products. Finally, we examined long term cultures (>90 days) of 6-TG-resistant/restriction-resistant clones and verified the stability of the phenotype. Consequently, we concluded that the conversion of the target site was stable.
Influence of DNA Repair Deficiency on SSO Donor Activity—The pso-TFO and TGRC-1 were co-electroporated into cells deficient in XPD and ERCC1-XPF, followed by UVA treatment. The sequence conversion results with the XPD-deficient cells were similar to those seen with the AM200 vector in that the frequency of targeted sequence modification was increased relative to wild type cells (Fig. 6, left). However, in marked contrast to the results with the AM200 donor, in ERCC1-deficient cells the relative frequency of sequence modification increased by 4- to 5-fold (Fig. 6, right). Thus, sequence conversion by the SSO did not require ERCC1-XPF-dependent events.
FIGURE 6.
Influence of repair deficiency on targeted mutagenesis by the TGRC-1 donor. Error bars represent the mean and S.D. of three or more independent experiments.
We also examined the activity of the pso-TFO/SSO in cells deficient in DNAPKCS, and the mismatch repair proteins MSH2 and MutSβ. As in the previous experiments with the AM200 donor, sequence conversion frequencies were unaffected by these deficiencies (not shown).
Competition between Single and Double Strand Donor DNA for Ends Derived from Targeted Cross-links—A key step in HDR of a DSB is 5′-3′-exonucleolytic digestion that produces single strand tails terminating in a 3′-OH. These could invade a duplex, as would occur during knock in of sequence information from the long duplex donor (Fig. 1). Alternatively, as proposed by the Resnick group (41, 42) based on experiments in Saccharomyces cerevisiae, the single strand tails might hybridize with an SSO. The central role of a single strand tail in both pathways raised the question of whether there would be a competition for this intermediate if both options were available. To address this question we introduced both donor species, along with the pso-TFO, into repair-proficient cells. The oligonucleotide donor was present at ∼100-fold molar excess relative to the duplex donor. Under these conditions, if the SSO were an effective competitor for ends at the target site, then the frequency of knock in of the AM200 duplex would be expected to decline. Experiments were performed with either the TGRC-1 or the wild type SSO. Analysis of 6-TG-resistant colonies indicated that frequency of AM200 knock in was similar in the presence or absence of the single strand donor (Fig. 7). Furthermore, the activity of the SSO was also unaffected by the AM200 donor (not shown). The experiment was repeated in XPD-deficient cells, and the same lack of competition between the two donors was observed (not shown). Thus, the presence of a considerable excess of an SSO donor did not influence the frequency of AM200 knock in.
FIGURE 7.
The SSO does not influence knock in of the duplex donor by the pso-TFO. Cells were electroporated with the pso-TFO and the AM200 duplex donor with or without the SSO. Error bars represent the mean and S.D. of three or more independent experiments.
Competition between Single and Double Strand Donor DNA for Ends Derived from I-SceI Cleavage—Our approach to introducing a targeted DSB was indirect, via a targeted cross-link. Given the novelty of this method, it was of interest to ask how the donor competition would play out when the target site was activated by direct cleavage by a well characterized reagent such as I-SceI (43, 47–49). To address this question directly we constructed a wild type variant of the AM12 cell line by pso-TFO-targeted introduction of an I-SceI site adjacent to the triplex target site. The SSO was designed to replace the mutant AM12 triplex target with a wild type sequence including the XbaI site. The donor also carried the 18-nt I-SceI recognition sequence positioned immediately upstream of the triplex target site (Fig. 8a). HAT-resistant clones were screened for the presence of the I-SceI site. Six clones with the I-SceI site were expanded and carried in culture for 3 months. At the end of this time the presence of the I-SceI site was verified in all the clones by sequence analysis of the appropriate PCR product, and one, AMIS1, was expanded. Cells in random culture were used in all the following experiments.
FIGURE 8.
Sequence modulation of an I-SceI site. a, targeted introduction of the I-SceI recognition sequence. The sequence of the donor SSO containing the I-SceI recognition sequence, the AM12 target sequence, and the sequence of the target region in the product cell line with the I-SceI recognition sequence adjacent to the triplex target. Also shown is the sequence of the competition donor SSO co-electroporated with the plasmid encoding I-SceI. b, sequence conversion by the SSO introduced into cells treated with the pso-TFO/UVA or the I-SceI expression plasmid. c, knock in of the AM200 donor in the presence or absence of the SSO in cells transfected with the I-SceI expression plasmid. Error bars represent the mean and S.D. of three or more independent experiments.
In an initial experiment we transfected AMIS1 cells with an expression plasmid that encoded I-SceI. After a week in culture the cells were plated at low density and 192 individual colonies picked and expanded without any selection. DNA was extracted, the target region amplified, and the PCR products digested with I-SceI. PCR fragments from 65% of the clones were resistant to digestion, an indication of the extensive cleavage of the site with concomitant loss of the recognition sequence (50, 51).
We synthesized a 100-mer SSO carrying sequence homology to either side of the I-SceI site (Fig. 8a). Transfer of sequence information from this donor to the I-SceI site would eliminate the I-SceI site, introduce an XhoI site, and inactivate the Hprt gene. This SSO was cotransfected with the I-SceI expression plasmid into AMIS1 cells. We found that the frequency of targeted sequence modification was ∼0.3%, the same as in the previous experiments with the pso-TFO (Fig. 8b).
AMIS1 cells were then cotransfected with the I-SceI expression plasmid and either the AM200 donor or both the AM200 and SSO donors. The knock-in frequency in cells treated with the I-SceI plasmid and the AM200 donor was 0.24%, ∼10-fold greater than in the experiments with the pso-TFO/UVA (Fig. 8c). When both donors were transfected, the activity of either donor was unaffected by the presence of the other. Thus, as in the experiment with the targeted cross-link, the frequency of targeted knock in of the AM200 duplex donor was not influenced by the presence of the SSO donor.
DISCUSSION
In previous work we described deletion mutagenesis at the site of the pso-TFO target and concluded that these were the consequence of DSBs formed during the cellular response to the cross-link (24, 32, 35). Here we show that following crosslinking the target sequence can enter HDR pathways and incorporate information supplied by exogenous duplex and single strand donors. Sequence modulation occurred at frequencies 2–3 orders of magnitude above those recovered in experiments with the donors alone. The use of the pso-TFO directed against an endogenous genomic target has the advantage in that every cell has the target. Thus, experiments can be done with host cells with different phenotypes (e.g. repair deficiencies) without the requirement of constructing a new cell line as is required for experiments with the I-SceI endonuclease. Of course, the pso-TFO-mediated sequence conversion strategies do have practical implications. Thus, we were able to readily construct a new cell line that allowed us to directly compare genomic modification initiated by nuclease cleavage or the targeted cross-link and address basic questions about the process.
Influence of DNA Repair Deficiency on Sequence Conversion Mediated by the Pso-TFO Cross-link—Our results with the pso-TFO and either donor indicated that the frequency of genome modification rose in XPD-deficient cells in which pso-TFO-mediated deletion mutation frequencies also increased. On the other hand, cells with repair deficiencies that did not result in increased deletion frequencies (shown in our previous work, Ref. 35) had wild type levels of sequence modulation by both donors. The exception to this straightforward correlation was the ERCC1-XPF deficiency. Here the interpretation is more complicated because this complex participates in repair of the cross-links as well as in HDR pathways. The ERCC1-XPF endonuclease is considered essential for the formation of the gapped intermediate (25) that is the substrate for error-prone bypass by pol ζ (35, 52, 53). Because the pso-TFO/SSO combination was active in the ERCC1-XPF-deficient cells, incision and gap formation were not required for sequence conversion by the SSO at the targeted cross-link site.
The ERCC1-XPF complex plays a role in the resolution of recombination intermediates that appear during gene knock in (36, 37), which explains the lack of duplex donor activity in the ERCC1-XPF-deficient cells. In the I-SceI experiments in yeast cells with mutations in the equivalent genes, SSO activity declined 5-fold (42). This contrasts with the increased SSO activity in ERCC1-deficient cells relative to wild type cells in our experiments. A requirement for the ERCC1-XPF complex has been described during SSA of direct repeat sequences, presumably to process single strand tails that appear as intermediates in the pathway (2, 54–56). However, our SSO donors were designed to generate mismatches or rather small tails upon hybridization to the target sequence. Additionally, Rad52 protein interacts with the ERCC1-XPF complex with an attendant loss of strand-annealing activity (57). Thus, the strand-annealing activity of Rad52 protein would be expected to rise in ERCC1-XPF-deficient cells, and, absent a concern for removal of long tails, this could increase the activity of the SSO.
Target Site Activation by the Pso-TFO Versus I-SceI—There is an important distinction between experiments with I-SceI (3, 58) and those with the pso-TFO. The cross-link is formed only in cells at the time of photoactivation. The frequency of the different end points reflects the different pathways taken during a single cycle of cross-link repair by those cells with targeted cross-links. Based on the data presented here and previously, some simple bookkeeping is possible. The targeted cross-link is formed in 25–30% of the pso-TFO/UVA-treated cells (24). Following repair and/or mutagenesis 5–6% of the cells have base substitutions and 0.2–0.4% have deletions at the target site. The data presented here indicate that sequence conversion by the SSO donor is in the 0.2–0.3% range, with a 10-fold lower frequency of duplex donor knock in.
In contrast, in the I-SceI experiments the encoding plasmid and enzyme are present for days and the frequency of end points reflects many rounds of cleavage/repair. Repair by non-homologous end joining could be either error-free, regenerating the I-SceI site, or error-prone, resulting in small deletions and loss of the recognition sequence (3). Multiple cycles of cleavage would drive the system toward deletion mutations as shown previously (50, 51) and in this report, in which 65% of the cells after treatment had lost the I-SceI site. The high frequency of cleavage explains the 10-fold increase in knock-in of the AM200 donor relative to the pso-TFO experiments. Consequently, it was somewhat unexpected that the frequency of SSO-mediated sequence conversion was similar in both the pso-TFO and I-SceI experiments. This does not appear to be unique to the experiments described here, as similar frequencies were reported in another study that employed I-SceI to initiate sequence conversion by an SSO (43). Because there are many more damage/repair cycles in the I-SceI experiments than with the pso-TFO, this suggests that some other factor(s) (perhaps a protein or enzyme) limits the activity of the SSO. Additional work will be required to elucidate the nature of this limitation. However, it would appear that sequence modulation by the SSO initiated by the pso-TFO is as efficient as that by I-SceI, with one-tenth the collateral mutagenesis.
Competition for Ends—The probability of entry of a DSB into a particular pathway can be influenced by manipulation of the levels of repair factors that are critical for the individual pathways (2, 4, 59, 60). The conclusion of many studies is that the pathways are competitive, although they have also been described as “compensatory” (61). While non-homologous end joining requires relatively little single strand resection of ends (62), long resected single strands, formed by the action of the MRE11-RAD50-NBS1 complex (63), are presumed to be the common precursor to the homologous recombination repair or SSA pathways (2). Entry into homologous recombination repair requires Rad51, while the frequency of SSA is dependent on Rad52. Long resected single strand ends have been proposed to be the key intermediate in SSO-mediated DSB repair in S. cerevisiae (41, 42). In that system a deficiency in Rad51 protein increased the frequency of SSO sequence conversion, while Rad52 protein was required for SSO activity. The authors concluded that SSO repair of a break required the annealing activity of Rad52 protein. They proposed a two-step model in which the SSO initiates repair by annealing to a single strand following resection of the 5′-strand, thus supplying a template for extension synthesis. The break would be sealed in the second step.
In our competition experiments we asked whether the SSO would compete for ends that would otherwise interact with the duplex knock-in donor. We found that the frequency of AM200 knock-in was unaffected by the presence of the SSO in experiments initiated either by the pso-TFO or I-SceI. Consequently, it would seem that the ends that engaged the duplex knock-in donor were not accessible to the SSO. This would suggest that commitment to a particular end-modification pathway is made by cellular functions rather than the availability of different nucleic acid partners. Assuming that the key intermediate for both homologous recombination repair and SSA/oligonucleotide pathway is a 5′-resected end (42), then there would have to be a subsequent fork in the processing pathway, with the SSO engagement restricted to one branch. If the arguments from the studies in S. cerevisiae extend to mammalian cells, then the formation of a Rad51-dependent complex on a resected end, competent for strand invasion, would preclude interaction of that end with the SSO. Consequently, the SSO would not compete for ends that engaged the AM200 knock-in donor (see supplemental Fig. S1 for a schematic and discussion of the interaction of the SSO with intermediates in DSB repair pathways). It will be of interest to determine which factors (including, presumably, Rad52) control the accessibility to ends by the SSO in mammalian cells.
This work was supported by the Intramural Research Program of the NIA, National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and supplemental references.
Footnotes
The abbreviations used are: DSB, double strand break; HDR, homology-directed repair; pso-TFO, psoralen-linked triple helix-forming oligonucleotide; SSO, single strand oligonucleotide; SSA, single strand annealing; Hprt, hypoxanthine phosphoribosyl transferase; 6-TG, 6 thioguanine; UVA, long wave ultraviolet light; CHO, Chinese hamster ovary.
References
- 1.van Gent, D. C., Hoeijmakers, J. H., and Kanaar, R. (2001) Nat. Rev. Genet. 2 196–206 [DOI] [PubMed] [Google Scholar]
- 2.Stark, J. M., Pierce, A. J., Oh, J., Pastink, A., and Jasin, M. (2004) Mol. Cell. Biol. 24 9305–9316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin, Y., Lukacsovich, T., and Waldman, A. S. (1999) Mol. Cell. Biol. 19 8353–8360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonoda, E., Hochegger, H., Saberi, A., Taniguchi, Y., and Takeda, S. (2006) DNA Repair (Amst.) 5 1021–1029 [DOI] [PubMed] [Google Scholar]
- 5.Couedel, C., Mills, K. D., Barchi, M., Shen, L., Olshen, A., Johnson, R. D., Nussenzweig, A., Essers, J., Kanaar, R., Li, G. C., Alt, F. W., and Jasin, M. (2004) Genes Dev. 18 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstock, D. M., Nakanishi, K., Helgadottir, H. R., and Jasin, M. (2006) Methods Enzymol. 409 524–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dronkert, M. L., Beverloo, H. B., Johnson, R. D., Hoeijmakers, J. H., Jasin, M., and Kanaar, R. (2000) Mol. Cell. Biol. 20 3147–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tutt, A., Bertwistle, D., Valentine, J., Gabriel, A., Swift, S., Ross, G., Griffin, C., Thacker, J., and Ashworth, A. (2001) EMBO J. 20 4704–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasquez, K. M., Marburger, K., Intody, Z., and Wilson, J. H. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 8403–8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang, F., Han, M., Romanienko, P. J., and Jasin, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 5172–5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott, B., Richardson, C., Winderbaum, J., Nickoloff, J. A., and Jasin, M. (1998) Mol. Cell. Biol. 18 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durant, S. T., Paffett, K. S., Shrivastav, M., Timmins, G. S., Morgan, W. F., and Nickoloff, J. A. (2006) Mol. Cell. Biol. 26 6047–6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujimura, T., Maher, V. M., Godwin, A. R., Liskay, R. M., and McCormick, J. J. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 1566–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, J., DePrimo, S. E., and Stringer, J. R. (1997) Mutat. Res. 374 233–243 [DOI] [PubMed] [Google Scholar]
- 15.Jonnalagadda, V. S., Matsuguchi, T., and Engelward, B. P. (2005) DNA Repair (Amst.) 4 594–605 [DOI] [PubMed] [Google Scholar]
- 16.Bessho, T. (2003) J. Biol. Chem. 278 5250–5254 [DOI] [PubMed] [Google Scholar]
- 17.Porteus, M. H. (2006) Mol. Ther. 13 438–446 [DOI] [PubMed] [Google Scholar]
- 18.Chevalier, B. S., Kortemme, T., Chadsey, M. S., Baker, D., Monnat, R. J., and Stoddard, B. L. (2002) Mol. Cell 10 895–905 [DOI] [PubMed] [Google Scholar]
- 19.Chin, J. Y., Schleifman, E. B., and Glazer, P. M. (2007) Front Biosci. 12 4288–4297 [DOI] [PubMed] [Google Scholar]
- 20.Felsenfeld, G., Davies, D. R., and Rich, A. (1957) J. Am. Chem. Soc. 79 2023–2024 [Google Scholar]
- 21.Seidman, M. M., and Glazer, P. M. (2003) J. Clin. Investig. 112 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri, N., Majumdar, A., Cuenoud, B., Natt, F., Martin, P., Boyd, A., Miller, P. S., and Seidman, M. M. (2002) Biochemistry 41 7716–7724 [DOI] [PubMed] [Google Scholar]
- 23.Puri, N., Majumdar, A., Cuenoud, B., Miller, P. S., and Seidman, M. M. (2004) Biochemistry 43 1343–1351 [DOI] [PubMed] [Google Scholar]
- 24.Majumdar, A., Puri, N., Cuenoud, B., Natt, F., Martin, P., Khorlin, A., Dyatkina, N., George, A. J., Miller, P. S., and Seidman, M. M. (2003) J. Biol. Chem. 278 11072–11077 [DOI] [PubMed] [Google Scholar]
- 25.Kuraoka, I., Kobertz, W. R., Ariza, R. R., Biggerstaff, M., Essigmann, J. M., and Wood, R. D. (2000) J. Biol. Chem. 275 26632–26636 [DOI] [PubMed] [Google Scholar]
- 26.Fisher, L. A., Bessho, M., and Bessho, T. (2008) J. Biol. Chem. 283 1275–1281 [DOI] [PubMed] [Google Scholar]
- 27.Saffran, W. A., Ahmed, S., Bellevue, S., Pereira, G., Patrick, T., Sanchez, W., Thomas, S., Alberti, M., and Hearst, J. E. (2004) J. Biol. Chem. 279 36462–36469 [DOI] [PubMed] [Google Scholar]
- 28.Cortes, F., Morgan, W. F., Varcarcel, E. R., Cleaver, J. E., and Wolff, S. (1991) Exp. Cell Res. 196 127–130 [DOI] [PubMed] [Google Scholar]
- 29.Faruqi, A. F., Seidman, M. M., Segal, D. J., Carroll, D., and Glazer, P. M. (1996) Mol. Cell. Biol. 16 6820–6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolig, R. L., Layher, S. K., Santi, B., Adair, G. M., Gu, F., Rainbow, A. J., and Nairn, R. S. (1997) Mutagenesis 12 277–283 [DOI] [PubMed] [Google Scholar]
- 31.Sawai, M., Takase, K., Teraoka, H., and Tsukada, K. (1990) Exp. Cell Res. 187 4–10 [DOI] [PubMed] [Google Scholar]
- 32.Majumdar, A., Khorlin, A., Dyatkina, N., Lin, F. L., Powell, J., Liu, J., Fei, Z., Khripine, Y., Watanabe, K. A., George, J., Glazer, P. M., and Seidman, M. M. (1998) Nat. Genet. 20 212–214 [DOI] [PubMed] [Google Scholar]
- 33.Cuenoud, B., Casset, F., Husken, D., Natt, F., Wolf, R. M., Altmann, K. H., Martin, P., and Moser, H. E. (1998) Angew. Chem. Intl. Ed. Eng. 37 1288–1291 [DOI] [PubMed] [Google Scholar]
- 34.Adair, G. M., Scheerer, J. B., Brotherman, A., McConville, S., Wilson, J. H., and Nairn, R. S. (1998) Somatic Cell Mol. Genet. 24 91–105 [DOI] [PubMed] [Google Scholar]
- 35.Richards, S., Liu, S. T., Majumdar, A., Liu, J. L., Nairn, R. S., Bernier, M., Maher, V., and Seidman, M. M. (2005) Nucleic Acids Res. 33 5382–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adair, G. M., Rolig, R. L., Moore-Faver, D., Zabelshansky, M., Wilson, J. H., and Nairn, R. S. (2000) EMBO J. 19 5552–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niedernhofer, L. J., Essers, J., Weeda, G., Beverloo, B., de Wit, J., Muijtjens, M., Odijk, H., Hoeijmakers, J. H., and Kanaar, R. (2001) EMBO J. 20 6540–6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aarts, M., Dekker, M., de Vries, S., van der Wal, A., and te Riele, H. (2006) Nucleic Acids Res. 34 e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen, P. A., McKeen, C., and Krauss, S. (2003) Gene Ther. 10 1830–1840 [DOI] [PubMed] [Google Scholar]
- 40.Olsen, P. A., Randol, M., Luna, L., Brown, T., and Krauss, S. (2005) J. Gene Med. 7 1534–1544 [DOI] [PubMed] [Google Scholar]
- 41.Storici, F., Durham, C. L., Gordenin, D. A., and Resnick, M. A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storici, F., Snipe, J. R., Chan, G. K., Gordenin, D. A., and Resnick, M. A. (2006) Mol. Cell. Biol. 26 7645–7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radecke, F., Peter, I., Radecke, S., Gellhaus, K., Schwarz, K., and Cathomen, T. (2006) Mol. Ther. 14 798–808 [DOI] [PubMed] [Google Scholar]
- 44.Becker, N. A., and Maher, L. J., III (1999) Antisense Nucleic Acid Drug Dev. 9 313–316 [DOI] [PubMed] [Google Scholar]
- 45.De Semir, D., and Aran, J. M. (2003) Oligonucleotides 13 261–269 [DOI] [PubMed] [Google Scholar]
- 46.Maurisse, R., Fichou, Y., De Semir, D., Cheung, J., Ferec, C., and Gruenert, D. C. (2006) Oligonucleotides 16 375–386 [DOI] [PubMed] [Google Scholar]
- 47.Richardson, C., Elliott, B., and Jasin, M. (1999) Methods Mol. Biol. 113 453–463 [DOI] [PubMed] [Google Scholar]
- 48.Richardson, C., and Jasin, M. (2000) Mol. Cell. Biol. 20 9068–9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin, Y., and Waldman, A. S. (2001) Genetics 158 1665–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varga, T., and Aplan, P. D. (2005) DNA Repair (Amst.) 4 1038–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honma, M., Sakuraba, M., Koizumi, T., Takashima, Y., Sakamoto, H., and Hayashi, M. (2007) DNA Repair (Amst.) 6 781–788 [DOI] [PubMed] [Google Scholar]
- 52.Shen, X., Jun, S., O'Neal, L. E., Sonoda, E., Bemark, M., Sale, J. E., and Li, L. (2006) J. Biol. Chem. 281 13869–13872 [DOI] [PubMed] [Google Scholar]
- 53.Sarkar, S., Davies, A. A., Ulrich, H. D., and McHugh, P. J. (2006) EMBO J. 25 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sargent, R. G., Rolig, R. L., Kilburn, A. E., Adair, G. M., Wilson, J. H., and Nairn, R. S. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 13122–13127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sargent, R. G., Meservy, J. L., Perkins, B. D., Kilburn, A. E., Intody, Z., Adair, G. M., Nairn, R. S., and Wilson, J. H. (2000) Nucleic Acids Res. 28 3771–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Minawi, A. Z., Saleh-Gohari, N., and Helleday, T. (2008) Nucleic Acids Res. 36 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Motycka, T. A., Bessho, T., Post, S. M., Sung, P., and Tomkinson, A. E. (2004) J. Biol. Chem. 279 13634–13639 [DOI] [PubMed] [Google Scholar]
- 58.Rouet, P., Smih, F., and Jasin, M. (1994) Mol. Cell. Biol. 14 8096–8106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierce, A. J., Hu, P., Han, M., Ellis, N., and Jasin, M. (2001) Genes Dev. 15 3237–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson, C., Stark, J. M., Ommundsen, M., and Jasin, M. (2004) Oncogene 23 546–553 [DOI] [PubMed] [Google Scholar]
- 61.Kim, J. S., Krasieva, T. B., Kurumizaka, H., Chen, D. J., Taylor, A. M., and Yokomori, K. (2005) J. Cell Biol. 170 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma, Y., Lu, H., Schwarz, K., and Lieber, M. R. (2005) Cell Cycle 4 1193–1200 [DOI] [PubMed] [Google Scholar]
- 63.Sartori, A. A., Lukas, C., Coates, J., Mistrik, M., Fu, S., Bartek, J., Baer, R., Lukas, J., and Jackson, S. P. (2007) Nature 450 509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]