Abstract
In vivo protein kinases A and G (PKA and PKG) coordinately phosphorylate a broad range of substrates to mediate their various physiological effects. The functions of many of these substrates have yet to be defined genetically. Herein we show a role for smoothelin-like protein 1 (SMTNL1), a novel in vivo target of PKG/PKA, in mediating vascular adaptations to exercise. Aortas from smtnl1-/- mice exhibited strikingly enhanced vasorelaxation before exercise, similar in extent to that achieved after endurance training of wild-type littermates. Additionally, contractile responses to α-adrenergic agonists were greatly attenuated. Immunological studies showed SMTNL1 is expressed in smooth muscle and type 2a striated muscle fibers. Consistent with a role in adaptations to exercise, smtnl1-/- mice also exhibited increased type 2a fibers before training and better performance after forced endurance training compared smtnl1+/+ mice. Furthermore, exercise was found to reduce expression of SMTNL1, particularly in female mice. In both muscle types, SMTNL1 is phosphorylated at Ser-301 in response to adrenergic signals. In vitro SMTNL1 suppresses myosin phosphatase activity through a substrate-directed effect, which is relieved by Ser-301 phosphorylation. Our findings suggest roles for SMTNL1 in cGMP/cAMP-mediated adaptations to exercise through mechanisms involving direct modulation of contractile activity.
The contractile state of smooth muscle (SM)2 is largely governed by phosphorylation of myosin regulatory light chain (LC20), which in turn is regulated by the opposing activities of myosin light chain kinase (MLCK) and myosin phosphatase, SMPP1M (1). In SM, both MLCK and SMPP1M are regulated, thereby enabling homeostatic control of contractile activity (2). Exquisite control is necessary because of the essential roles of SM in many physiological processes, such as maintenance of blood pressure. In most SMs, release of cyclic nucleotides (cGMP/cAMP), in response to hormonal or neuronal stimulation, promote relaxation either by lowering intracellular [Ca2+] or desensitizing the muscle to Ca2+ by inhibiting MLCK and/or activating SMPP1M (2).
Targeted deletions of both PKA and PKG in mice produce profound phenotypes, underscoring the importance of these kinases in many physiological processes (3, 4). In vivo, both kinases are known to selectively target a discrete number of substrates and current thinking suggests that selective targeting is the means by which these broadly acting enzymes bring about coordinated physiological responses (5). A few groups have begun to test this hypothesis by selectively deleting PKA/PKG targets in mice. Schlossmann et al. (6) demonstrated a major role for IRAG (inositol 1,4,5-trisphosphate receptor 1 IP3R1-associated protein, with exon 12 deleted by removing the 1,4,5-trisphosphate receptor binding domain) in PKG-mediated regulation of [Ca2+] in SM. Disruption of IRAG resulted in a selective loss of signaling response. Other PKG/PKA-mediated responses were largely intact, contrasting with deletion of PKG itself, which produced profound loss of most cGMP/PKG-dependent signaling.
Smoothelin-like protein 1 (SMTNL1, NP_077192) is an early target of PKG in SM and is phosphorylated in vitro by PKG and PKA at Ser-301 (7). The C terminus contains a highly conserved calponin homology (CH) domain (residues 342-459), also found in the smoothelin family of smooth muscle-specific proteins, whereas the N-terminal domain (residues 1-341) is composed of entirely unique sequence. Studies in permeabilized SM suggested a role in mediating Ca2+ desensitization in response to cGMP (7). To determine the physiological relevance of SMTNL1 in mediating cGMP/cAMP signaling in vivo, we generated smtnl1-/- mice. We show that smtnl1-/- mice have an exercised adapted phenotype, exhibiting better exercise performance and improved vasorelaxation/contractile responses compared with WT littermates. In addition we also show discrete expression of SMTNL1 to type 2a striated muscle fibers. In skeletal muscle (SKM), exercise activates a large set of muscle-specific genes leading to phenotypic changes such as 2b-to-2a fiber type switching, angiogenesis, and enhanced mitochondrial biogenesis, improving metabolic function, blood flow, and contractility (8, 9). PKA/PKG-dependent signaling pathways have been implicated as primary messengers in exercise adaptation in both SM and SKM (9-11). Our findings with smtnl1-/- mice suggest the physiological role of the protein is to mediate the actions of PKA/PKG in promoting adaptive responses to exercise in SM, and possibly SKM. In biochemical studies in vitro, purified SMTNL1 inhibits SMPP1M activity in its dephosphorylated state, and this effect is alleviated by phosphorylation at Ser-301 by PKA or PKG. In both intact isolated smooth and striated muscle Ser-301 is phosphorylated in response to agonists that elevate intracellular cAMP or cGMP. Collectively these findings suggest a potential mechanism by which adrenergic signals can promote exercise-induced adaptations through regulation of myosin phosphatase activity.
EXPERIMENTAL PROCEDURES
Targeted Deletion of the Mouse smtnl1 Gene—The smtnl1-/- mouse was created using standard protocols. Genomic DNA fragments were synthesized by high fidelity PCR from the smtnl1 locus and cloned into a gene targeting vector (OSfrtloxP) (Fig. S1A). The vector was electroporated into an ES cell line derived from strain 129P2/ola (E14Tg2a). Colonies were screened by PCR using a neomycin-specific primer (NeoPro: 5′-acgcgtcaccttaatatgc-3′) and a reverse primer outside the 3′ arm of homology (5′-gatgtgtgctccttccctgagctgtctg-3′). PCR-positive colonies were expanded and re-screened by Southern hybridization. The probe detects an 8.7-kb fragment in the WT locus and a 5.4-kb band in the targeted locus when genomic DNA is cut with Nde1 (supplemental Fig. S1B). Targeted clones were injected into blastocysts derived from strain C75BL/6(B6). Male chimeras demonstrating gene transmission were crossed with 129S6/SvEvTac(129) females, and heterozygous offspring from that cross were back-crossed over 9 generations to a 129 background. Mice were genotyped using a multiplex PCR reaction with three primers: NeoPro, acgcgtcaccttaatatgc; smtnlWT, ttcacctttgaccc; and smtnl1Rev, caaaagagacctggc (supplemental Fig. S1C).
Immunohistochemistry and Indirect Immunofluorescent Fiber Typing—Formalin (10%)-fixed, paraffin-embedded mouse tissues and timed embryos were analyzed by immunohistochemistry using anti-SMTNL1 (Proteintech Inc.). Fiber typing by immunofluorescent fiber typing was performed as described (12). Soleus (SOL) and plantaris (PL) muscles were harvested and frozen in liquid N2-cooled isopentane. Frozen cross-sections (8 μm) were immunostained against MHC I, 2a, and 2b, dystrophin, and SMTNL1. Longitudinal sections were immunostained with anti-myomesin (clone B4), and F-actin was visualized with phalloidin-Alexa488.
Treadmill Exercise Protocol—8-week-old 129 mice were maintained on a 12-h light/dark cycle. Mice exposed to the treadmill but not exercise comprised the sedentary (SED) group, those that ran on the treadmill were the exercise (EX) group. Mice were exercised 3 h into their dark cycle on a 2-lane enclosed treadmill. The treadmill stress and endurance protocols were performed as described (13). Mice were acclimated to the treadmill at 5 m/min at an 8° incline for 10 min for 2 days before testing. For baseline and final stress tests, an incremental protocol was used: 8.5 m/min, 0° incline, 9 min; then the speed increased by 2.5 m/min every 3 min to a maximum of 40 m/min and incline increase 5° every 9 min to a maximum of 15°. Time to fatigue was determined when mice could not maintain sufficient speed to remain off the shock grid for 15 s. This test was repeated twice with a 48-h rest between tests for each mouse before and after a 5-week endurance training protocol, and results were averaged. Performance was determined through calculation of time to fatigue (minutes), distance run (in meters, calculated from run time and speed of treadmill), and vertical work performed (kg·m). Vertical work was calculated as a product of body weight (kilograms) and vertical distance (meters). Mice were euthanized 24 h after the final stress test, and tissues and serum were collected.
Isometric Force Measurements in Aortic Rings—Descending thoracic aortas from SED and EX mice were placed in Krebs solution, continuously aerated with 95% O2/5% CO2, at 37 °C. Aortic rings (3 mm) were mounted in a wire myograph as described previously (14). Passive stretch was set to 15 millinewtons to simulate the wall tension generated by 100 mmHg of blood pressure. After 30 min of equilibration, 3 cycles of constriction to phenylephrine (PE) were completed, followed by a dose-response curve to PE. Aortic rings were preconstricted with PE, and dose-response curves to acetylcholine (ACh) were determined.
Western Blot of Ser-301 Phosphorylation in Vivo—The specificity of the pSMTNL1S301 antibody (Proteintech, Inc.) was tested against recombinant (rSMTLN1) and S301A SMTLN1 (rS301A) phosphorylated with purified PKA as described before (7). To test Ser-301 phosphorylation in vivo by Western, isolated aortas held under tension (15 millinewtons), with pinned bladder and SOL equilibrated in Krebs as described (15), were stimulated with vehicle, ISO, or 8-bromo-cAMP and snap frozen. Lysates were Western blotted with anti-pSMTNL1S301.
Assay of SMPP1M—SMPP1M and SM myosin were purified from pig bladder as described (16). The purified myosin was phosphorylated with rZIPK to ∼1.4 mol using the following conditions; 1.0 ml of 2 mg/ml myosin (SM or SKM), 10 μg of rZIPK (truncated at Ser-276), 200 μm ATP, 5 mm MgCl2, 50 mm Tris-HCl for 30 min (22 °C), followed by dialysis against 50 mm Tris-HCl, pH7.4, 1 mm dithiothreitol. Phosphatase assays were performed as described (16). For experiments examining the effects of SMTNL1 on SMPP1M activity in vitro, ∼2 mg of purified rSMTNL1 was phosphorylated to stoichiometry with purified PKA as described before (7). Direct phosphorylation site analysis confirmed exclusive phosphorylation of Ser-301.
RESULTS
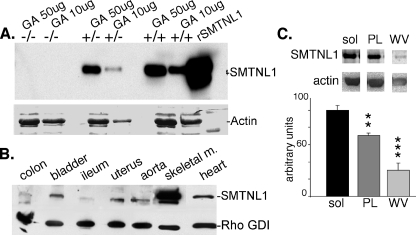
Disruption of the smtnl1 Gene—Genotyping of targeted mice by PCR and Southern hybridization confirmed gene deletion (supplemental Fig. S1, A-C). Deletion of SMTNL1 at the protein level was confirmed by Western analysis of SKM extracts (Fig. 1A). Basal activity, litter sizes, and embryonic development of the smtnl1-/- mice were normal. Extensive histological analysis of various blood vessels and general tissues showed these to be normal in smtnl1-/- mice. Additionally, electron microscopy of SKM sections showed the muscle ultrastructure was normal (supplemental Fig. S2). Western analysis of a battery of tissues showed SMTNL1 expression to be highest in SKM relative to SM and other tissues (Fig. 1B). Within individual striated muscles, SMTNL1 is significantly more expressed in SOL compared with PL or white vastus (WV), suggesting the protein is preferentially expressed in muscles containing Type I or Type 2a fibers (Fig. 1C).
FIGURE 1.
Analysis of SMTNL1 expression in smtnl1-/- and WT mice. In A, Western analysis of gastrocnemius muscle (GA) isolated from WT, smtnl1-/-, and smtnl1-/+ confirms deletion of SMTNL1 in null mice and an approximate 50% reduction in expression at the protein level in heterozygous (-/+) animals. B, representative Western analysis of levels of SMTNL1 expression between tissues. The indicated tissues were isolated from WT male mice and blotted for either SMTNL1 or Rho-GDI (loading control). C, a representative Western analysis of the expression of SMTNL1 in soleus (SOL), plantaris (PL), and white vastus (WV) muscles. Expression levels were quantified by densitometry, and levels in PL and WV were compared with SOL using α-actin as a loading control (n = 6 ± S.D.) (**, p < 0.01; ***, p < 0.001).
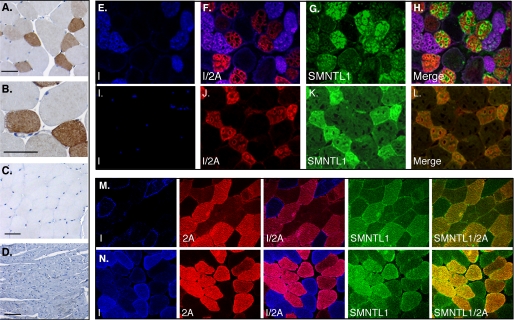
Histological Examination of WT, smtnl1-/- Mice and Human SKM Shows Exclusive Expression of SMTNL1 in Type 2a Fibers—Immunohistochemical analysis of SKM and heart showed strong staining in the mixed fiber muscle PL (Fig. 2, A and B) but was absent in sections of fast twitch type 2b muscle WV and the oxidative type I/b heart muscle (Fig. 2, C and D). To determine the specific fiber type expressing SMTNL1, sections from SOL and PL muscles were examined by immunofluorescent fiber typing confocal microscopy. Fig. 2 (E-L) shows SMTNL1 staining co-localized with MHC-2A fibers in SOL and PL, with no staining in MHC-1, MHC-2B, or MHC-2x/d fibers. Specificity of staining was confirmed by competition with rSMTNL1 and analysis of SKM from smtnl1-/- mice (supplemental Fig. S3). Longitudinal sections of SKM stained with phalloidin and anti-myomesin showed that SMTLN1 is specifically localized to the I-band and M-line, but absent in the Z-line (supplemental Fig. S4). Immunohistochemical analysis of timed embryos demonstrated SMTNL1 expression in axial SKM beginning at day E14.5 (supplemental Fig. S5). To verify relevance to human physiology, frozen sections of vastus lateralis biopsy from a study of young men pre and post 8 weeks of stationary bicycle training were evaluated by immunofluorescent fiber typing for SMTNL1 expression and fiber typing. Fig. 2 (M and N) shows expression of SMTNL1 is specific to type 2a fibers in humans. We are currently investigating whether exercise alters the SMTNL1 expression levels in human.
FIGURE 2.
SMTNL1 is expressed in type 2a fibers in mice and humans. In A and B the mixed fiber muscle plantaris (PL) was isolated from WT mice and prepared for immunohistochemical analysis using the SMTNL1 antibody. Discrete SMTNL1 expression was observed in some fibers and not others. The figure shows a muscle cross-section at low (A) and high (B) magnifications. In C and D, immunohistochemical analysis of white vastus and heart showed no SMTNL1 staining (scale = 100 μm). E-H, show the results of fiber typing experiments carried by immunofluorescence with antibodies to myosin heavy chains (MHC) I and 2a and SMTNL1 of cross-sections of soleus (SOL) and (I-L) plantaris muscle. The merged figures show specific localization of SMTNL1 in type 2A fibers only in both muscle types. In the 10 panels comprising M and N, SMTNL1 expression was measured in vastus lateralis from young men before (M) and after (N) training. The merged images show that in humans SMTNL1 is expressed in type 2a fibers also and induced following training. Color Key: In immunohistochemical experiments in A-D SMTNL1 is stained brown; in E-N MHC I is stained blue, MHC 2a is stained red, and SMTNL1 is green.
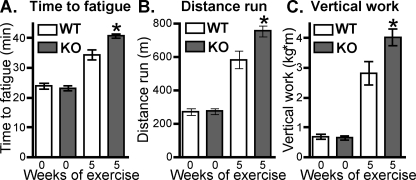
Male smtnl1-/- Mice Perform Better Than WT in Exercise Stress Test after Endurance Training—Given the role of type 2a fibers in endurance exercise adaptation and the finding that SMTNL1 is only expressed in those fibers within SKM of both mice and men, we investigated the exercise performance of smtnl1-/- mice. Fig. 3 shows all mice demonstrated similar performance at the beginning of the study. All EX mice ran the same time and distance during the endurance portion of the training, but subjectively, the smtnl1-/- mice demonstrated more willingness to run and required fewer motivational stimuli, such as air puffs, throughout the 5-week protocol. Forty-eight hours after the end of exercise training, the mice were subjected to two more graded stress tests. Results of the stress tests showed female EX smtnl1-/- mice did not differ significantly from the female EX WT mice: (time to fatigue: WT 24.6 ± 0.8 min baseline, 33.1 ± 1.0 min final; smtnl1-/- 24.2 ± 1.2 min baseline, 35.6 ± 1.1 min final). However, male EX smtnl1-/- mice showed significantly increased time to fatigue, distance run, and vertical work performed compared with male EX WT mice after training (Fig. 3, A-C).
FIGURE 3.
smtnl1-/- mice perform better in exercise stress tests. Mice were exercised, trained, and tested for endurance as described under “Experimental Procedures.” In A, mean time to fatigue was measured. In B the total mean distance run until fatigue was determined. In C, vertical work performed in a graded exercise test was determined by increasing the gradient of the treadmill. Data shown are from male WT and smtnl1-/- mice before (0 weeks) and after (5 weeks) forced treadmill endurance training; *, p < 0.05 (n = 5 ± S.D.). Results were similar in female mice, but less significant.
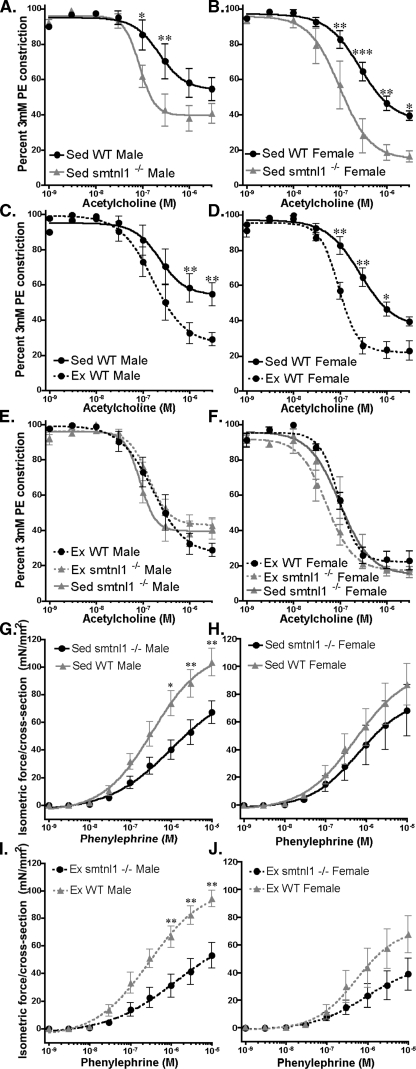
Endothelium-dependent Vasorelaxation of the Aorta Is Enhanced, and Responses to α-Adrenergic Constriction Are Reduced in smtnl1-/- Mice—Endurance training is known to improve vascular performance. To investigate the impact of SMTNL1 deletion on the contractile properties of SM, isolated aorta from SED and EX mice were mounted for isometric force measurement. To test NO-mediated, endothelium-dependent vasorelaxation, dose-response curves to ACh were generated. Aortas from SED smtnl1-/- mice showed significantly greater relaxation to ACh compared with WT littermates (Fig. 4, A-D). The extent of relaxation in smtnl1-/- was similar to that from EX WT mice. Therefore exercise did not improve relaxation in aortic rings from smtnl1-/- mice beyond that achieved in ACh-treated EX WT mice (Fig. 4, E and F). These findings show that deletion of SMTNL1 improves vascular responsiveness to ACh, suggesting a role in cGMP/PKG-dependent vascular adaptations to exercise. To determine if the improvements in vascular relaxation were due to alteration in endothelial nitricoxide synthase expression or phosphorylation, we also evaluated these parameters in WT and smtnl1-/- aorta lysates by Western blot. However, no differences in endothelial nitric-oxide synthase phosphorylation or expression were observed.
FIGURE 4.
Deletion of SMTNL1 mimics exercise induced adaptations in aorta. A and B show that deletion of SMTNL1 (smtnl1-/-) results in greater sensitivity to acetylcholine (ACh) in aortas isolated from sedentary (Sed) male (A) and female (B) mice, following precontraction with 3 μm phenylephrine (PE). C and D show that exercise (Ex) training improves sensitivity to ACh-induced relaxation in wild0type (WT) male (C) and female (D) mice. In E and F, the relaxant effects of ACh were compared in aortas isolated from Ex and Sed male (E) and female (F) smtnl1-/- mice and compared in the same experiment with aorta isolated from Ex WT littermates. The figure shows that aortas from Sed smtnl1-/- mice have the same sensitivity to ACh as aortas isolated from Ex trained animals. The figures also show that deletion of SMTNL1 does not enhance Ex-induced relaxation performance beyond that achieved by Ex alone. In G-J, the effects of the contractile agonist PE on force development were examined in aortas isolated WT and smtnl1-/- male and female mice Ex and Sed mice. The figure shows that Ex attenuates force in WT mice, but this effect is significantly enhanced in smtnl1-/- mice. In all cases forces were normalized to aorta cross-sectional area. (n = 5 ± S.D.) (*, p < 0.05; **, p < 0.01; and ***, p < 0.001).
To investigate the effects of SMTNL1 deletion on contractile activity in response to exercise, aortic rings were stimulated with increasing doses of PE. Fig. 4 (G and I) shows that WT SED and EX males generated greater isometric force per cross-section area at doses above 1 μm PE when compared with smtnl1-/- males in the same exercise category. In the case of SED or EX smtnl1-/- mice, the differences in force cannot be attributed to decreased sensitivity to PE. Comparison of EC50 in response to PE values with respect to isometric force development in both aortas from WT and smtnl1-/- did not show significant differences. These findings suggest that the absence of SMTNL1 does not result in receptor desensitization due to either reduced expression of α-adrenergic receptors or through compensatory signaling mechanisms that down-regulate receptor activity. In female mice, the differences in isometric force generated in response to PE between SED or EX WT and smtnl1-/- were less significant (Fig. 4, H and J). This may be reflective of expression levels of SMTNL1 between sexes. Generally, Western analysis of tissues expressing SMTNL1 showed 30-40% less expression in females relative to males (supplemental Fig. S6). As a result, SED WT female mice may already be closer to smtnl1-/- males with respect to their responsiveness to α-adrenergic agonists.
No effect on relaxation or force development was observed in tracheal rings isolated from SED or EX, WT or smtnl1-/- mice. These findings are consistent with immunoblot Western data showing that SMTNL1 is not expressed in airway SM (data not shown). Other vessels expressing SMTNL1 have yet to be evaluated.
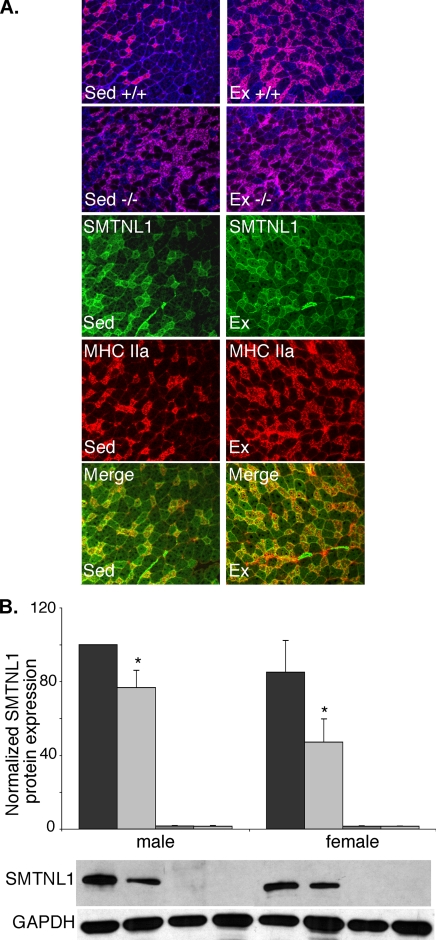
Deletion of SMTNL1 Induces Fiber Adaptations in SKM—To determine if deletion of SMTNL1 significantly altered the proportion type 2a fibers in a manner similar to that induced by exercise, we first evaluated SKM fiber adaptation by immunohistochemical approaches. As reported by others, in all examined fibers from WT mice, the percentage of 2a fibers increased, whereas, the percentage of 2b fibers decreased in response to endurance exercise (Fig. 5A). Interestingly, as determine by fiber typing, SED smtnl1-/- mice had the same percentage of 2a fibers as EX WT mice, suggesting SED smtnl1-/- mice have more type 2A fibers at baseline (Table 1). Although, the percentage of 2a fibers did not increase further in EX smtnl1-/- mice. Like Ex mice, SED smtnl1-/- mice showed significantly lower level of type1b fibers, implying an adaptive response had occurred. The numbers of type 1 and type 2x/d fibers were unaffected with respect to genotype or exercise status (Table 1).
FIGURE 5.
SMTNL1 deletion mimics exercise induced fiber adaptation in SKM. A, representative immunofluorescence confocal microscopy of fiber typing in plantarus of WT (+/+) and smtnl1-/- mice before and after exercise training. SMTNL1 is stained green, type2a fibers are red, type 1 fibers blue, and Type 2x/d fibers are unstained. B, Western blot analysis shows exercise reduces SMTNL1 expression in striated muscle isolated from male and female mice. Between SED and EX, the differences are significant in both males and females (n = 7 ± S.E. *, p < 0.029). For quantitative analysis of fiber types shown in A see Table 1.
TABLE 1.
SMTNL1 deletion induces type 2A fibers and reduces type 2B fibers to a similar extent to that observed in exercised mice
The table shows the results of fiber typing experiments measuring the effects of EX and SMTNL1 deletion on the expression of myosin heavy chains in plantaris muscle. Data show the percentage of type I, type 2A, type 2B, and type 2x/d fibers. 300 fibers were counted from 5 animals per group and compared with each other.
|
MHC-1
|
MHC-2A
|
MHC-2B
|
MHC-2x/d
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sed | Ex | Sed | Ex | Sed | Ex | Sed | Ex | ||
| % | |||||||||
| smtnl1+/+ | 1.9 ± 1.6 | 0.8 ± 0.2 | 26.5 ± 1.1 | 37.9 ± 2.0a | 54.4 ± 2.6 | 36.5 ± 2.6b | 17.3 ± 1.4 | 21.6 ± 1.1 | |
| smtnl1−/− | 1.4 ± 0.6 | 1.6 ± 0.4 | 34.3 ± 1.8c | 36.8 ± 1.5 | 44 ± 2.9d | 39.6 ± 3.0 | 22.0 ± 1.5 | 22.4 ± 1.1 | |
p < 0.001 versus WT Sec 2a.
p < 0.001 versus WT Sec 2b.
p < 0.01 versus WT Sed 2a.
p < 0.05 versus WT Sed.
The finding that SED smtnl1-/- mice exhibit fiber adaptations as well as perform better in exercise suggests that exercise may reduce SMTNL1 expression in vivo. Consistent with this hypothesis, Western analysis of SOL isolated from EX mice showed a significant reduction in expression of the protein in male and female mice (Fig. 5B). Similar trends were observed in SM, although the extent of the change was not statistically significant. These results indicate that improvements in exercise performance in smtnl1-/- mice are likely to be either related to improved blood flow due to adaptations within the vascular smooth muscle, and/or, alterations in pathways directly regulating contractile activity.
In addition to demonstrating changes in MHC2a and MH2b expression in fiber typing experiments, we also examined other known markers of exercise adaptation by Western blot, including the mitochondrial markers PGC-1α and COX1V by Western blot. Although, as shown by others, these markers were induced by exercise, no differences were observed between SED smtnl1-/- mice and SED WT littermates (supplemental Fig. S7A).
Body and Muscle Weight, Capillary Contacts, Glucose Tolerance, Serum Chemistry Values, PL Muscle Time to Fatigue in smtnl1-/- and WT Mice—Other potential compensatory mechanisms that could explain the smtnl1-/- phenotype were also evaluated, including overnight fasted glucose levels, resting blood pressure (supplemental Fig. S7, B and C) serum muscle enzyme activities (creatine kinase, aspartate aminotransferase, and lactate dehydrogenase), total Ca, Mg, P, Na+, K+, Cl-, and albumin levels. All values were within normal ranges with no differences between groups. Body and muscle weight were also not different (supplemental Table S1). We asked whether capillary density was altered in the smtnl1-/- mice. Capillary contacts increased in type 2A fibers with exercise as expected, but there was no difference between WT and smtnl1-/- mice (supplemental Fig. S8A). Evaluation of time to fatigue of PL muscle after tetanic stimulation in smtnl1-/- and WT mice showed similar times to fatigue of ∼20s (supplemental Fig. S8B). Because SKM consists of mixed fibers (e.g. PL, <30% type 2a; SOL, ∼40%) more complete assessment of the effects of SMTNL1 deletion on contractile activity in SKM will require studies on isolated 2a fibers.
SMTNL1 Phosphorylation Is Regulated in SM and SKM in Vivo—Previously, using a phosphoproteomic approach, we identified SMTNL1 as an early target of PKG activation following addition of 8-bromo-cGMP to permeabilized ileal smooth muscle (7). In vitro, using rSMTNL1, we showed that the protein is an excellent substrate for both PKG and PKA. Both protein kinases phosphorylate SMTNL1 at a single site, Ser-301. To determine if SMTNL1 is phosphorylated in intact smooth muscles, we generated phosphospecific antibodies to Ser-301. Specificity of the antibody was confirmed by Western blotting against WT rSMTNL1 and a recombinant S301A mutant of the protein (rSMTNL1S301A) following in vitro phosphorylation of the protein with purified PKA. Fig. 6A shows the antibody specifically recognizes only the phosphorylated form of the protein and not the non-phosphorylated form or rSMTNL1S301A. Having demonstrated specificity for the phospho-Ser-301 in vitro, we examined phosphorylation in vivo. Isolated intact aortas held under tension were treated with physiological concentrations of norepinephrine, isoproterenol (ISO), acetylcholine, 8-bromo-cGMP, and 8-bromo-cAMP for times observed to produced maximal relaxation as determined in Fig. 4. The treated vessels were immediately frozen in liquid nitrogen. Tissue extracts were blotted for Ser-301 phosphorylation. Fig. 6B shows that all five agonists produce robust phosphorylation of Ser-301, demonstrating that in aorta SMTNL1 is regulated by cyclic nucleotide-dependent pathways activated by both endothelial-dependent (ACh) and -independent pathways (norepinephrine and ISO). Significantly, maximal phosphorylation of Ser-301 was found to correlate with maximal relaxation of aorta in response to ACh as observed in Fig. 4. Similar results were also observed in studies with isolated bladder treated with 8-bromo-cGMP (data not shown). To test whether SMTNL1 is phosphorylated in striated muscle, isolated SOL held under tension were treated with ISO over the indicated time course and fixed time point then frozen in liquid nitrogen, and extracts were blotted for Ser-301 phosphorylation (Fig. 6, C and D). Fig. 6 (C and D) shows that SMTNL1 is robustly phosphorylated in striated muscle and therefore likely to be directly regulated by PKA in this tissue.
FIGURE 6.
SMTNL1 phosphorylation is regulated in vivo. In A, rSMTNL1 and rSMTNL1S301A were phosphorylated with purified PKA as described under “Experimental Procedures.” Reactions were stopped at the indicated times, and phosphorylation of Ser-301 was detected with the Ser-301 phosphospecific antibody. The figure illustrates the specificity of the antibody for the phosphorylated form of SMTNL1. In B, isolated aortas were held under tension and treated with the indicated agonists for 5 min. The vessels were snap frozen in liquid nitrogen, and extracts were blotted for Ser-301 phosphorylation. The extent of phosphorylation was determined by densitometry. Representative Western blots are shown WT = smtnl+/+ aorta; KO = smtnl1-/- aorta. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control (n = 5 ± S.E. *, p < 0.05). In C, soleus muscles were held under tension and treated for the indicated times with either vehicle (VEH) or 10 μm isoproterenol (ISO). The muscles were snap frozen with liquid nitrogen, and extracts were blotted for Ser-301 phosphorylation. In D, soleus muscles were treated for 5 min with 10 μm ISO, snap frozen with liquid nitrogen, and phosphorylation of Ser-301 was quantitated by Western blotting using total SNTNL1 (tSMTNL1) as the loading control (n = 6 ± S.E.; **, p < 0.01).
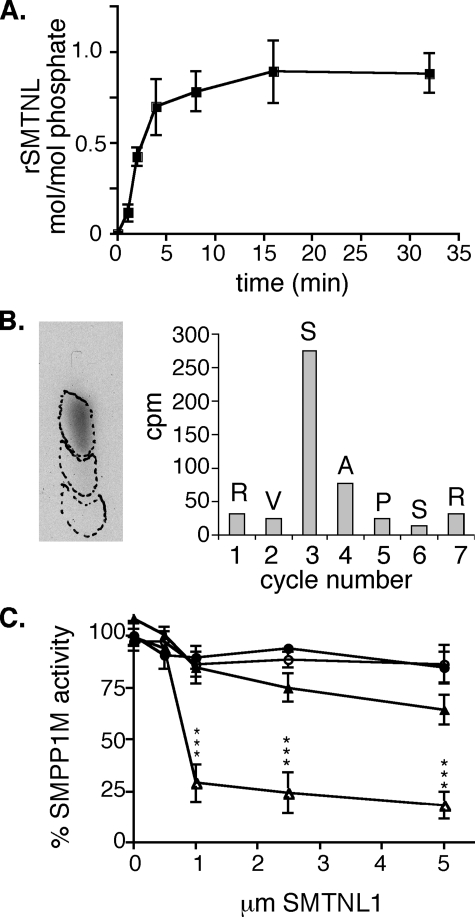
SMTNL1 Inhibits SMPP1M Activity toward Myosin through a Substrate-directed Effect That Is Alleviated by Ser-301 Phosphorylation—The finding that exercise reduces SMTNL1 expression in SM and SKM, as well as the finding that Ser-301 is phosphorylated in vivo in response to both endothelial dependent agonists as well β-adrenergic agonists, suggests a role in the regulation of contraction. This hypothesis is supported by earlier studies from our laboratory demonstrating an effect of rSMTNL1 on Ca2+-desensitization pathways when added to permeabilized SM (7). Myosin light chain kinase (MLCK), myosin, and myosin phosphatase (SMPP1M) have all been directly implicated in mediating Ca2+ desensitization in SM (1-3). The phosphorylated form of SMTNL1 was generated by phosphorylating rSMTNL1 to ∼1 mol/mol with purified PKA as described (7). Phosphopeptide sequence and phospho amino acid analysis confirmed that PKA phosphorylated SMTNL1 at a single site of Ser-301 (Fig. 7, A and B). To investigate whether phosphoS301rSMTNL1 or SMTNL1 directly regulate MLCK activity toward myosin, both forms of the protein were incubated with the kinase at increasing concentrations in the presence of MgATP. No effects on MLCK activity were observed even at the highest [SMTNL1] tested (30 μm) (data not shown). To examine the effect of SMTNL1 on myosin, we examined the effects of the recombinant phospho and dephospho forms on ATPase activity. Neither phospho nor dephospho-rSMTNL1 altered myosin ATPase activity under any conditions tested. Additionally, no effect was observed, with either form of rSMTNL1, on the rate at which SM myosin was phosphorylated by a non-Ca2+/calmodulin-dependent myosin kinase, such as Zipper-interacting protein kinase. Finally, as shown previously by our laboratory with dephospho-SMTNL1, the phosphorylated form of rSMTNL1 did not disrupt the ability of purified myosin to bind and release actin in the presence of ATP (7).
FIGURE 7.
SMTNL1 regulates SMPP1M activity toward myosin. In A, rSMTNL1 was phosphorylated with purified PKA (n = 3; ±S.E.). In B, phosphorylation of Ser-301 was confirmed by phospho amino acid analysis and cleaved radioactive peptide analysis as described (7). In C, SMPP1M activity was measured against 32P-labeled SM myosin (▵) and rLC20 (○) with or without phospho-SMTNL (filled) or dephospho-SMTNL1 (open). Purified SM myosin and recombinant SM LC20 were phosphorylated with purified SKM MLCK as described (16) (*, p < 0.001 n = 3; ±S.E.).
Because neither ATPase activity nor the ability of myosin to be phosphorylated were affected by the presence of rSMTNL1, next we examined the effects of the phospho and dephospho forms on SMPP1M activity toward myosin. Fig. 7C shows that addition of the dephospho-rSMTNL1 had an inhibitory effect of SMPP1M activity toward SM myosin. In contrast the phosphorylated form of SMTNL1 showed significantly reduced inhibition of SMPP1M activity. Full-length SMTNL1 was required to inhibit SMPP1M activity. Incubation with truncated forms of rSMTNL1 (SMTNL11-346 and rSMTNL1346-452) had no effect upon SMPP1M activity (data not shown). Importantly, 32P-labeled rSMTNL1 itself was poorly dephosphorylated by SMPP1M, in contrast to the catalytic subunits of PP1C or PP2A (data not shown).
Interestingly, neither phosphorylated form of rSMTNL1 had any effect on SMPP1M activity when LC20 was used as the substrate. The differences in SMPP1M activity toward whole myosin and LC20 suggest that SMTNL1 exerts a substrate directed effect on myosin itself, altering its accessibility to the phosphatase. These in vitro findings may in part explain the smtnl1-/- exercised adapted phenotype observed in aorta, in which greater relaxation is achieved in response to ACh, and contractile force is suppressed in response to PE. Loss of SMTNL1 relieves any inhibitory effect that the protein may have on dephosphorylation of myosin by SMPP1M. By these criteria, the exercise-induced improvements in vascular performance could also be attributed to reduced expression of SMTNL1 in this tissue and possibly SKM. The finding that Ser-301 phosphorylation also relieves the inhibitory effect of SMTNL1 on SMPP1M in vitro suggests one potential in vivo mechanism of action whereby PKG (and PKA) can regulate myosin phosphorylation through phosphorylation of SMTNL1, in addition to exercise-induced alterations in the level of its expression.
DISCUSSION
We have examined the physiological relevance of SMTNL1, a novel in vivo target of PKG/PKA previously identified in SM proteomically (7). The physiological role of the protein was not obvious from the primary sequence alone. Although the protein has a highly conserved calponin homology domain (CH domain) in its C terminus, two-thirds of the remaining N-terminal sequence is entirely unique within the mouse and human genomes. Preliminary CD analysis and NMR studies with N-terminal domain indicate the structure is disordered.3 Therefore, to determine SMTNL1 function, herein, we generated smtnl1-/- mice. Studies with smtnl1-/- mice in a 129 congenic background strongly suggest SMTLN1 plays a role in PKG/PKA-mediated adaptation to exercise. First, isolated SED smtnl1-/- aortas exhibit relaxation/contraction responses that are similar to those observed in aortal rings isolated from WT littermates subjected to 5 weeks of exercise training. Second, overall smtnl1-/- mice perform better in exercise compared with WT littermates. Third, in immunohistochemical studies we found SMTNL1 is exclusively expressed within type 2a SKM fibers in both mice and humans, and that SED smtnl1-/- mice have the same levels of type 2a fibers and MHC2a expression as EX WT mice. Fourth, exercise training reduces the expression of SMTNL1 in SM and SKM. Collectively, these findings suggest common roles for the protein in adaptations to exercise in SKM as well as SM.
Based on studies with purified SMPP1M and recombinant SMTNL1, a potential mechanism by which SMTNL1 may promote muscle adaptations is through regulation of contractile activity. In vitro, in its dephosphorylated state, SMTNL1 reduces SMPP1M activity, whereas phosphorylation at Ser-301 blocks this effect. These in vitro findings may provide a potential mechanism to explain the smtnl1-/- phenotype as well as suggest a function for the protein in vivo. If SMTNL1 suppress SMPP1M activity, its absence would result in higher rates of myosin dephosphorylation. This suggests that SMTNL1's normal role in SM is to act as a physiological break on contractile activity, which is alleviated through Ser-301 phosphorylation. Additionally, the finding that exercise itself reduces expression of SMTNL1 provides an additional longer term mechanism of regulation in which myosin dephosphorylation would be constantly favored over phosphorylation by increasing endogenous SMPP1M activity. Thus, receptor-mediated pathways can acutely regulate SMTNL1 function through Ser-301 phosphorylation, whereas exercise achieves the same effect by reducing its expression. As indicated in in vitro biochemical experiments, the molecular mechanism by which SMTNL1 alters SMPP1M activity appears to be through interactions with myosin itself rather than the phosphatase itself i.e. SMTNL1 alters myosins ability to be dephosphorylated by SMPP1M. This conclusion is supported by the finding that SMTNL1 had no apparent effect on SMPP1M activity when recombinant light chains were used as the substrate. The single CH domain on SMTNL1 is the most likely primary site of myosin binding. The sequence of the remaining two thirds of the protein predicts a disordered structure, suggesting it may fold around myosin. The Ser-301 site borders the CH domain, suggesting phosphorylation would interrupt interactions with myosin.
The changes observed in smtnl1-/- mice beg the question as to how a protein acting on myosin dephosphorylation can affect exercise induced adaptation in SM, and possibly SKM? In SM, multiple adaptations occur in response to physical exercise (10). The physical forces of increased shear stress, transmural pressure, and cyclic stretch are major stimuli. Various kinase signal transduction pathways are activated such as those mediated by PKA and PKG, Akt kinase, phosphatidylinositol 3-kinase, MLCK, and mitogen-activated protein kinase (10, 19). Therefore one explanation is that SMTNL1 provides a pharmaco-mechanical mechanism to initiate adaptations in response to elevations in cGMP/cAMP. Alterations in the sensitivity of SM muscles to Ca2+ through activation of myosin phosphatase and myosin dephosphorylation would have a profound effect on contraction/relaxation rates, and in SKM mechanical activity is itself a primary mediator of adaptive fiber switching (20). Alternatively, SMTNL1 could have additional roles as a direct transcriptional regulator of adaptive responses in muscle.
The interesting observation by immunohistochemistry and immunofluorescence that SKM from smtnl1-/- show increased type 2a fibers without exercise training suggests a direct role in SKM fiber adaptation process. The adaptive effect was not complete. Whereas type 2a fibers and MHC2a expression increase and type 2b fibers and MHC2b expression decrease by SMTNL1 deletion, other known exercise-induced proteins were unaffected. Other groups however, have demonstrated that individual proteins associated with exercise-induced adaptations can be divorced from one another in SKM (17). The mechanisms that promote MHC2a expression in smtnl1-/- mice could either be related to improved blood flow to the muscle as a result of the vascular adaptations mimicking the increased blood flow that occurs during exercise, or, as a result of a mechanical effect from alterations in contractile activity as a result loss of the effects on myosin phosphatase activity. Although phosphorylation of SKM myosin is not required for contractile activity, it does induce Ca2+ sensitization in SKM (21). Also, β-adrenergic signaling, which leads to activation of cAMP/PKA in SKM, is part of the contractile and adaptive responses to exercise and is essential for energy metabolism through its glycogenolytic effects (11).
Exercise-induced adaptations in both SM and SKM are generally recognized as having long term health benefits in humans. Our data suggest that suppression of SMTNL1 expression can promote these adaptations fully in SM, and at least in part in SKM, without the necessity of prolonged exercise training. The finding that exercise itself reduces SMTNL1 expression, clearly demonstrates that expression of the protein is highly regulated. As shown herein, Western analysis of various tissues shows different levels of expression of SMTNL1 between SM types. Therefore, different levels of SMTNL1 expression may contribute to differences in contractile responses between SM types. We also observed different levels of SMTNL1 expression between the genders. Generally, male mice have about twice the amount of SMTNL1 compared with females, suggesting expression is regulated by sex hormones. In humans and mice, the physical differences between male and female SKM are well documented, and generally premenopausal females exhibit lower blood pressure and are less prone to development of hypertension than males. Therefore, understanding the pathways that regulate SMTNL1 expression in SM and SKM may provide new therapeutic strategies to treat a variety of diseases associated with cardiovasculature.
Finally, although SMTNL1 shares homology with the smoothelin family of SM-specific proteins within its CH domain, the smtnl1-/- mouse has a very distinct phenotype from the smoothelin-deleted mouse. Targeted deletion of smoothelin A and B produced profound alterations in intestinal smooth muscle, including intestinal fragility, SM hypertrophy, and altered contractility (18). In contrast, smtnl1-/- mice have no alterations in SM architecture and appear normal on the surface. These findings clearly discriminate SMTNL1 functionally from the smoothelin family. Originally, our laboratories named SMTNL1 as CHASM, for calponin homology activated in smooth muscle. The protein was recently renamed Smoothelin-like protein 1 by the international committee governing gene ontology based on sequence alignments within the CH domain of SMTNL1. However we believe that, because the smtnl1-/- mice and smoothelin-/- mice have such distinct phenotypes, the SMTNL1 nomenclature may be misleading. Additionally, SMTNL1, unlike smoothelins, is also expressed in striated muscle and not exclusively in smooth muscles. Our original acronym, CHASM, may therefore be a more appropriate description of the protein.
Acknowledgments
We thank Cleo Proctor for technical assistance and Elizabeth Snyder for help in the preparation of the manuscript.
This work was supported by National Institutes of Health Grants DK065954-02 and 5 R01 HL078795-04. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S8 and Table S1.
Footnotes
The abbreviations used are: SM, smooth muscle; MLCK, myosin light chain kinase; PKA, protein kinase A; PKG, protein kinase G; CH, calponin homology; WT, wild type; SKM, skeletal muscle; SOL, soleus muscle; PL, plantaris muscle; SED, sedentary; EX, exercise; PE, phenylephrine; ACh, acetylcholine; ISO, isoproterenol; WV, white vastus; SMTNL1, smoothelin-like protein 1; LC20, myosin regulatory light chain.
T. Haystead, unpublished observations.
References
- 1.Somlyo, A. V., Khromov, A. S., Webb, M. R., Ferenczi, M. A., Trentham, D. R., He, Z. H., Sheng, S., Shao, Z., and Somlyo, A. P. (2004) Philos. Trans. R Soc. Lond. B Biol. Sci. 359 1921-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lincoln, T. M. (2007) Circ. Res. 100 10-12 [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer, A., Klatt, P., Massberg, S., Ny, L., Sausbier, M., Hirneiss, C., Wang, G. X., Korth, M., Aszodi, A., Andersson, K. E., Krombach, F., Mayerhofer, A., Ruth, P., Fassler, R., and Hofmann, F. (1998) EMBO J. 17 3045-3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings, D. E., Brandon, E. P., Planas, J. V., Motamed, K., Idzerda, R. L., and McKnight, G. S. (1996) Nature 382 622-626 [DOI] [PubMed] [Google Scholar]
- 5.Beene, D. L., and Scott, J. D. (2007) Curr. Opin. Cell Biol. 19 192-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiselhoringer, A., Werner, M., Sigl, K., Smital, P., Worner, R., Acheo, L., Stieber, J., Weinmeister, P., Feil, R., Feil, S., Wegener, J., Hofmann, F., and Schlossmann, J. (2004) EMBO J. 23 4222-4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borman, M. A., MacDonald, J. A., and Haystead, T. A. (2004) FEBS Lett. 573 207-213 [DOI] [PubMed] [Google Scholar]
- 8.Muoio, D. M., and Newgard, C. B. (2006) Annu. Rev. Biochem. 75 367-401 [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto, K., and Goodyear, L. J. (2002) J. Appl. Physiol. 93 369-383 [DOI] [PubMed] [Google Scholar]
- 10.Kojda, G., and Hambrecht, R. (2005) Cardiovasc. Res. 67 187-197 [DOI] [PubMed] [Google Scholar]
- 11.Plourde, G., Rousseau-Migneron, S., and Nadeau, A. (1993) J. Appl. Physiol. 74 1641-1646 [DOI] [PubMed] [Google Scholar]
- 12.Akimoto, T., Ribar, T. J., Williams, R. S., and Yan, Z. (2004) Am. J. Physiol. Cell Physiol. 287 C1311-C1319 [DOI] [PubMed] [Google Scholar]
- 13.Haubold, K. W., Allen, D. L., Capetanaki, Y., and Leinwand, L. A. (2003) J. Appl. Physiol. 95 1617-1622 [DOI] [PubMed] [Google Scholar]
- 14.Pannirselvam, M., Simon, V., Verma, S., Anderson, T., and Triggle, C. R. (2003) Br. J. Pharmacol. 140 701-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wooldridge, A. A., MacDonald, J. A., Erdodi, F., Ma, C., Borman, M. A., Hartshorne, D. J., and Haystead, T. A. (2004) J. Biol. Chem. 279 34496-34504 [DOI] [PubMed] [Google Scholar]
- 16.Shirazi, A., Iizuka, K., Fadden, P., Mosse, C., Somlyo, A. P., Somlyo, A. V., and Haystead, T. A. (1994) J. Biol. Chem. 269 31598-31606 [PubMed] [Google Scholar]
- 17.Rockl, K. S., Hirshman, M. F., Brandauer, J., Fujii, N., Witters, L. A., and Goodyear, L. J. (2007) Diabetes 56 2062-2069 [DOI] [PubMed] [Google Scholar]
- 18.Niessen, P., Rensen, S., van Deursen, J., De Man, J., De Laet, A., Vanderwinden, J. M., Wedel, T., Baker, D., Doevendans, P., Hofker, M., Gijbels, M., and van Eys, G. (2005) Gastroenterology 129 1592-1601 [DOI] [PubMed] [Google Scholar]
- 19.Boo, Y. C. (2006) Exp. Mol. Med. 38 63-71 [DOI] [PubMed] [Google Scholar]
- 20.Akimoto, T., Pohnert, S. C., Li, P., Zhang, M., Gumbs, C., Rosenberg, P. B., Williams, R. S., and Yan, Z. (2005) J. Biol. Chem. 280 19587-19593 [DOI] [PubMed] [Google Scholar]
- 21.Szczesna, D., Zhao, J., Jones, M., Zhi, G., Stull, J., and Potter, J. D. (2002) J. Appl. Physiol. 92 1661-1670 [DOI] [PubMed] [Google Scholar]