Abstract
Histone deacetylases (HDACs) are protein deacetylases that play a role in repression of gene transcription and are emerging targets in cancer therapy. Here, we characterize the structure and enzymatic activity of the catalytic domain of human HDAC7 (cdHDAC7). Although HDAC7 normally exists as part of a multiprotein complex, we show that cdHDAC7 has a low level of deacetylase activity which can be inhibited by known HDAC inhibitors. The crystal structures of human cdHDAC7 and its complexes with two hydroxamate inhibitors are the first structures of the catalytic domain of class IIa HDACs and demonstrate significant differences with previously reported class I and class IIb-like HDAC structures. We show that cdHDAC7 has an additional class IIa HDAC-specific zinc binding motif adjacent to the active site which is likely to participate in substrate recognition and protein-protein interaction and may provide a site for modulation of activity. Furthermore, a different active site topology results in modified catalytic properties and in an enlarged active site pocket. Our studies provide mechanistic insights into class IIa HDACs and facilitate the design of specific modulators.
The level of histone acetylation is regulated by the action of two classes of enzymes, histone acetyltransferases and histone deacetylases (HDACs).3 Histone acetyltransferases and HDACs are found in large multiprotein complexes, and recruitment of histone acetylase or deacetylase complexes by coactivators or corepressors is thought to cause a local change in the chromatin structure, resulting in either activation or repression of gene transcription (1). Humans have 18 HDACs and, based on their sequence similarity to yeast factors, they are grouped into four classes (class I–IV). Class II HDACs are homologous to yeast histone deacetylase HDA1 and have been implicated as global regulators of gene expression during cell differentiation and development (2). In humans, class II HDACs are subdivided into classes IIa (HDAC4, HDAC5, HDAC7, and HDAC9) and IIb (HDAC6 and HDAC10). Class IIa HDACs contain two functionally important regions, a highly conserved C-terminal catalytic domain and an N-terminal extension that has no similarity with other proteins, mediates the signal-dependent shuttling between the nucleus and the cytoplasm, and harbors binding sites for transcriptional regulators (2, 3). Class IIa HDACs interact with corepressors such as N-CoR (nuclear receptor corepressor) and the MEF2 (myocyte enhancer factor 2) family of transcription factors that is not only important for controlling gene expression in normal cellular programs like muscle differentiation, T-cell apoptosis, neuronal survival, and synaptic differentiation but has also been linked to cardiac hypertrophy, asthma, atherosclerosis, hypertension, and other pathological conditions (3–5). To date all four class IIa HDACs have been knocked out in mice, and the resulting abnormal phenotypes have been extensively characterized (6–9). HDAC7 for example, plays an important role in cardiovascular development and disease (7).
HDACs catalyze the deacetylation of lysine residues in the N-terminal tails of core histones. In addition to histones, nonhistone proteins may also serve as substrates (10, 11). The currently accepted catalytic mechanism of HDACs has features of both metallo- and serine proteases. In a first step a tetrahedral oxyanion intermediate is formed after the nucleophilic attack of a zinc-activated water molecule on the carbonyl carbon of the substrate acetyl group. The negative charge of the tetrahedral oxyanion intermediate is stabilized by interactions with the zinc and the side chain of an active site tyrosine. The reaction is completed by the transfer of a proton to the scissile nitrogen, yielding the acetate and lysine products (12–15). The activity of the class IIa HDACs is regulated at several levels, including tissue-specific gene expression, nucleocytoplasmic shuttling that is dependent on their phosphorylation state and subsequent binding of 14-3-3 proteins, and recruitment of distinct cofactors (2, 3). In vivo, the enzymatic activity of class IIa HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR (silencing mediator for retinoid and thyroid receptor) (16, 17).
HDACs are considered to be among the most promising targets in drug development for cancer therapy (18, 19), and some compounds like suberoylanilide hydroxamic acid (SAHA) are in late stage clinical trials (20). Most of the HDAC inhibitors developed so far display their effects in nano- and micromolar concentrations (21). However, only a few compounds are emerging as preferential inhibitors of class I versus class II HDACs, and even fewer are able to discriminate efficiently among HDACs that belong to the same class (19). HDAC structures in complex with various inhibitors revealed a pharmacophore model for HDAC inhibition. The pharmacophore consists of (i) a metal binding domain, which chelates the active site zinc and blocks the enzymatic activity, (ii) a linker domain, which mimics the substrate and occupies the enzymatic channel, and (iii) a surface domain, which makes contacts with the rim of the binding pocket (19, 22).
To date the crystal structures of human HDAC8 and a bacterial HDAC homolog from Aquifex aeolicus, both representing class I HDACs, and the bacterial class IIb HDAC-like amidohydrolase from Bordetella/Alcaligenes strain FB188 have been reported (12–15, 23–25). However, there is no structural information available for eukaryotic class II and class IIa HDACs. To provide insight into the structure and function of this subclass, we report here the crystal structure of the catalytic domain of human HDAC7 (cdHDAC7) and two cdHDAC7-hydroxamate inhibitor complex structures. The crystal structure of cdHDAC7 is the first structure of the catalytic domain of a eukaryotic class IIa HDAC, revealing significant structural differences compared with previously reported human and bacterial class I and bacterial class IIb-like HDAC structures. cdHDAC7 has a novel zinc binding motif adjacent to the active site, which is conserved in class IIa HDACs and likely participates in substrate recognition and protein-protein interaction. cdHDAC7 also has a variant active site topology resulting in altered catalytic properties and in an enlarged active site pocket. Our cdHDAC7 studies provide mechanistic insights into eukaryotic class IIa HDACs and may enable the design of class-specific HDAC modulators.
EXPERIMENTAL PROCEDURES
Reagents—Thrombin was obtained from Sigma. HDAC inhibitors were purchased from commercial suppliers except those synthesized as described in supplemental Fig. S1. All other chemicals were of the highest purity available.
Protein Cloning, Expression, and Purification—Residues 483–903 of isoform 1 of cdHDAC7 were amplified by PCR from the Mammalian Gene Collection clone and subcloned into a modified pET28a vector using ligation-independent cloning. cdHDAC7 (both wild type and variants) was expressed in Escherichia coli BL21 (DE3) Codon Plus RIL (Stratagene) in Terrific Broth medium in the presence of 50 μg/ml kanamycin. Cells were grown at 37 °C to an A600 of about 2, cooled down to 12 °C, and induced by 1 mm isopropyl-1-thio-d-galactopyranoside overnight. For purification, cells were suspended in lysis buffer (1× phosphate-buffered saline, pH 7.4, 0.5 m NaCl, 5% glycerol, 0.1% CHAPS, 1 mm DTT, and 0.1 mm phenylmethylsulfonyl fluoride) and lysed by passing through a Microfluidizer (Microfluidics Corp.) at 18,000 p.s.i. The purification procedure comprised two chromatographic steps, an affinity chromatography on a 5-ml HiTrap Chelating column (GE Healthcare) charged with Ni2+ and anion exchange chromatography on a Source 30Q column (10 × 10, GE Healthcare). The affinity column was washed with 10 column volumes of 20 mm Tris-HCl buffer, pH 8.0, containing 250 mm NaCl, 0.1% CHAPS, and 20 mm imidazole, and the protein was eluted with elution buffer (equilibration buffer plus 250 mm imidazole). Thrombin was added to pooled cdHDAC7 fractions and incubated overnight at 4 °C while dialyzing against 20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5% glycerol, and 1 mm DTT. The protein was further purified to homogeneity by anion-exchange chromatography after diluting the dialyzed sample 10 times with equilibration buffer (20 mm Tris-HCl, pH 8.0). cdHDAC7 was eluted with a linear gradient of NaCl up to 400 mm (15 column volumes). The purified protein was concentrated to the desired concentration in presence of 2.5% 1,2-propandiol and 5 mm DTT.
Ligand Binding Assay—Ligand binding was detected by monitoring the increase in thermostability of proteins in the presence of ligands using differential scanning light scattering as previously described (26). Fifty microliters of protein (0.4 mg/ml) was heated from 27 to 80 °C at a rate of 1 °C/min in each well of a clear-bottom 384-well plate (Nunc, Rochester, NY) under a variety of solution conditions. Protein aggregation was monitored by measuring the intensity of the scattered light every 30 s with a CCD camera. The pixel intensities in a preselected region of each well were integrated to generate a value representative of the total amount of scattered light in that region. These total intensities were then plotted against temperature for each sample well and fitted to the Boltzmann equation by nonlinear regression. The resulting point of inflection of each resulting curve was defined as the Tagg.
Enzyme Activity Assays—Histone deacetylase activity was assayed using the Fluor de Lys™ Kit from Biomol (AK-500, Biomol International LP) according to the manufacturer's protocol. The increase in fluorescence intensity at 460 nm (emission) after excitation at 360 nm was monitored using a multiplate reader (Spectromax Gemini, Molecular Devices). To determine Km and kcat values, cdHDAC7 at 2.5 μm was assayed using different concentrations of Fluor de Lys substrate (1 μm to 1 mm), and reactions were prolonged to 150 min to be able to detect the low level of activity of the wild-type enzyme. For the cdHDAC7 variants, enzyme concentrations of 1, 1, and 0.02 μm of H843F, H843A, and H843Y were used, respectively. Each reaction was performed in 7 min. HDAC activity was also measured in the presence of potential inhibitors (LAQ824, LBH589, oxamflatin, PDX101, SAHA, TSA, and tubacin) at concentrations ranging from 1.9 μm to 2 mm using 10 μm of Fluor de Lys substrate. IC50 values were calculated using SigmaPlot 9.0 software.
Protein Crystallization—Purified cdHDAC7 was crystallized using the hanging drop vapor diffusion method at 20 °C by mixing 3 μl of the protein solution (in 20 mm Tris-HCl, pH 8.0, 0.1 m NaCl, 5 mm DTT, and 2.5% 1,2-propandiol) with 3 μl of the reservoir solution. First, cdHDAC7 was co-crystallized with a 7-fold molar excess of the hydroxamate inhibitor LAQ824. However, no ligand was observed in the cdHDAC7 model. Moreover, a water molecule coordinates the catalytic zinc ion as seen in the apo structure of the HDAC-like protein from A. aeolicus (12, 27) (Fig. 2A). To obtain the cdHDAC7-hydroxamate inhibitor complex structures, co-crystallization with the inhibitor was insufficient, and 2 mm compound needed to be included in the cryosolution during crystal freezing (soaking). The SAHA complex was prepared by directly adding 2 mm SAHA to concentrated cdHDAC7 (10–13 mg/ml). TSA complex crystals were obtained by adding 2 mm TSA to diluted protein solution followed by co-concentration up to about 10 mg/ml. All crystals were grown in a condition containing 10–15% polyethylene glycol 3350, 0.1 m HEPES-NaOH, pH 7.5, 10% isopropanol, and 10 mm DTT. Before flash-freezing the crystal in liquid nitrogen, the crystal was soaked in a cryoprotectant consisting of 80% reservoir solution and 20% ethylene glycol.
FIGURE 2.
Stereo view of the cdHDAC7 active site. Shown are the interactions in the structure of apo-cdHDAC7 (A) and in the cdHDAC7·TSA complex structure (B). Water molecules are shown as blue spheres and potential hydrogen bonds as blue dashed lines.
Data Collection and Processing—X-ray diffraction data for apo- and complexed cdHDAC7 were collected at beamlines X12C and X26C of National Synchrotron Light Source at Brookhaven National Laboratory. X-ray data were processed using the Hkl2000 program package (28). Data statistics are reported in supplemental Table 1.
Structure Determination and Refinement—All cdHDAC7 structures were solved by molecular replacement using the program Phaser (29). For the structure of apo-cdHDAC7, the model of Bordetella class IIb HDAC-like protein served as a template (1ZZ0) (14), and for cdHDAC7-inhibitor complex structures the model of apo-cdHDAC7 was used as a search model (3C0Y). Refinement of the model was performed with Refmac (30). Graphics program Coot was used for model building and visualization (31). Statistics on structure refinement are summarized in supplemental Table 1.
Structural Analysis—Sequence alignments were performed with ClustalW (32). Structure comparisons were carried out using the program Pymol. Protein interface prediction and pocket mapping were carried out with ICM (Molsoft LLC) (34). Docking of an acetylated p53 peptide (PPGSTK(Ac)R) was performed by Monte Carlo energy minimization (35). The rotameric conformational space available to His-843 was sampled by a Monte Carlo energy minimization procedure in the absence of the active site water. The lowest energy level was observed with His-843 pointing toward the catalytic site. The water molecule was positioned manually a posteriori. Figures were created with Pymol.
Accession Numbers—The x-ray data and the atomic coordinates have been deposited into the Protein Data Bank as 3C0Y, 3C0Z, and 3C10.
RESULTS
Deacetylase Activity and Inhibition of Wild-type cdHDAC7—Here we describe the bacterial expression, purification, and catalytic activity of a human class IIa HDAC enzyme. Using a standard fluorimetric activity assay that has been successfully applied for other class II HDACs (36–38), we determined the Michaelis-Menten constants for cdHDAC7 (Table 1). The Km for the Fluor de Lys substrate (17 μm) is in the same range as those of other fluorogenic substrates tested for purified full-length HDACs (39, 40). cdHDAC7 showed the same level of catalytic activity as rat liver HDAC (39), which is 34 times less than that of the class IIb-like HDAC from Bordetella (39).
TABLE 1.
Kinetic parameters for human cdHDAC7 Deacetylase activity of wild-type cdHDAC7 and its variants was assayed using the Fluor de Lys™ kit from Biomol. Wild-type cdHDAC7 was assayed at 2.5 μm in the HDAC assay buffer (50 mm Tris/Cl, pH 8.0, 137 mm NaCl, 2.7 mm KCl, 1 mm MgCl2). For the cdHDAC7 variants, enzyme concentrations of 1, 1, and 0.02 μm of H843F, H843A, and H843Y were used, respectively.
| cdHDAC7 | Km | kcat |
|---|---|---|
| μm | min–1 | |
| Wild type | 17 ± 7 | 0.012 ± 0.004 |
| H843Y | 13 ± 4 | 66 ± 9 |
| H843F | 16 ± 1 | 1.0 ± 0.02 |
| H843A | 16 ± 2 | 1.1 ± 0.06 |
To identify potential inhibitors of human HDAC7, we first determined the relative binding affinity of a series of known HDAC inhibitors by monitoring the increase in thermostability of cdHDAC7 in the presence of each compound (Table 2). cdHDAC7 showed significant binding to hydroxamate inhibitors such as TSA, LAQ824, PXD-101, LBH-589, and SAHA. No binding was found for benzamide inhibitors (MS-275, CI-994, and MCGD-0103), cyclic tetrapeptides (apicidin), and short chain fatty acids (butyrate and valproic acid). The results presented here are comparable with the inhibitor specificities described for the class II-like HDAC from Bordetella strain FB188 (39).
TABLE 2.
Effect of known HDAC inhibitors on stability of wild-type cdHDAC7 Stabilizing effect of 23 different HDAC inhibitors on cdHDAC7 was assayed by monitoring the increase in thermostability of proteins (ΔTagg) in the presence of inhibitors using DSLS. Any effect exceeding 2 °C is considered above experimental error.
| Compounds | ΔTagg |
|---|---|
| °C | |
| Hydroxamic acid | |
| TSA | 3.4 |
| PXD101 | 5.5 |
| LBH589 | 3.3 |
| Oxamflatin | 2.3 |
| SAHA | 3.3 |
| Tubacin | 6.5 |
| LAQ824 | 3.3 |
| MAZ1523-V-24a | 4.5 |
| NK170-II-4-1a | 4.3 |
| NK50-I-290a | 3.9 |
| Scriptaid | 3.9 |
| Suberic bishydroxamic acid | 2.8 |
| Pyroxamide | 2.3 |
| MAZ1514-V-15a | 0 |
| CRA-024781 | 0 |
| Cyclic tetrapeptide | |
| Apicidin | 0 |
| Short-chain fatty acid | |
| Butyrate | 0 |
| Valproic acid | 0 |
| Benzamide | |
| CI-994 (tacedinaline) | 0 |
| MS-275 | 0 |
| MCGD-0103 | 0 |
| Miscellaneous | |
| Depudecin | 0 |
| Nicotinamide | 0 |
These names are conventional, and the structures and related chemical information for these compounds are presented in supplemental Fig. S1
To test the effect and potency of hydroxamate inhibitors on human class IIa HDACs, we first measured enzyme activity in the presence of 1 mm concentrations of each inhibitor (data not shown). Again, hydroxamic acids were the most potent inhibitors of deacetylase activity. IC50 values were then determined for the seven most potent inhibitors (Table 3, supplemental Fig. S2). The most effective inhibitor for cdHDAC7 deacetylase activity was the nonspecific, general HDAC inhibitor TSA with an IC50 value of 0.3 μm (Table 3). Similar IC50 values have been reported for the class IIb-like HDAC from Bordetella strain FB188 (41) (1.2 μm), full-length human class I HDACs (HDAC1, HDAC3, and HDAC8), and the A. aeolicus enzyme (IC50 values of 0.1–0.3 μm) (12, 27).
TABLE 3.
Effect of known HDAC inhibitors on activity of cdHDAC7 The IC50 values were determined for selected hydroxamate inhibitors.
|
Compounds
|
IC50
|
||
|---|---|---|---|
| Wild type | H843Y | ||
| μm | |||
| TSA | 0.3 ± 0.1a | 0.04 ± 0.01b | |
| PXD101 | 4.3 ± 0.1 | 0.02 ± 0.002 | |
| LBH589 | 15.4 ± 1.1 | 0.13 ± 0.03 | |
| Oxamflatin | 98 ± 16 | 2.6 ± 1.1 | |
| SAHA | 113 ± 16 | 0.3 ± 0.05 | |
| Tubacin | 225 ± 30 | 0.8 ± 0.3 | |
| LAQ824 | 553 ± 38 | 3.6 ± 0.3 | |
Standard fitting errors
Deviation from three different experiments
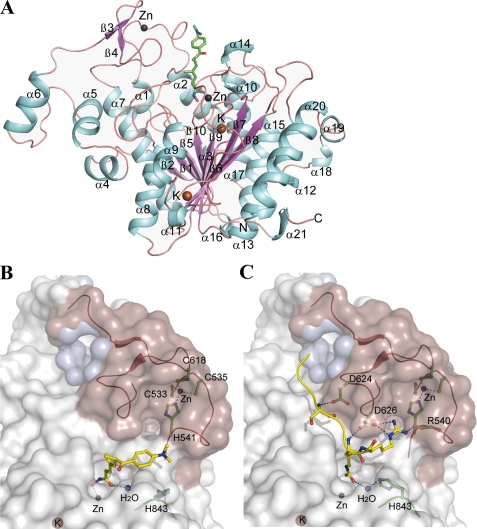
cdHDAC7 Contains a Class IIa HDAC-specific Zinc Binding Domain—To better understand the mechanism of catalysis and inhibition of HDAC7, we solved the crystal structure of its isolated catalytic domain as well as two complexes with the hydroxamate inhibitors TSA and SAHA. In all three cdHDAC7 crystal structures there are three independent protein molecules in the asymmetric unit. Each monomer consists of 21 α-helices and 10 β-strands organized in a single domain with an open α/β-fold (Fig. 1A). The final model of cdHDAC7 comprises amino acid residues 515–900. In the apo-cdHDAC7 structure the catalytic zinc tetrahedrally coordinates to the side chains of amino acids Asp-707, His-709, and Asp-801 and one water molecule (Fig. 2A). Amino acid residues that are involved in binding the catalytic zinc are highly conserved in the class I and II HDACs (supplemental Fig. S3). Two potassium ions are bound per cdHDAC7 monomer and are also structurally conserved in human HDAC8 and in the HDAC-like protein from Bordetella strain FB188 (14, 23).
FIGURE 1.
Structure of human cdHDAC7. A, overall structure of human cdHDAC7 bound to TSA. α-Helices and β-strands are labeled. The zinc and potassium ions are shown as gray and brown spheres, respectively. TSA is shown as stick model colored as per atom type: carbon in green, oxygen in red, sulfur in yellow, and nitrogen in blue. B, a surface representation of cdHDAC7 bound to TSA (carbon in yellow). The class IIa-specific insertion (ribbon in red, surface in salmon) forms a hydrophobic cavity (inner cavity surface in blue, see also supplemental Fig. 4) that is predicted to constitute a protein recruitment site. Amino acids coordinating the second zinc and forming the CCHC-motif are shown as sticks (carbon in green). The CCHC motif delineates an extended groove where substrate can bind. C, a surface representation of cdHDAC7 docked to the acetyl-lysine-containing peptide. The active site histidine was modeled in its inward orientation to illustrate potential function in catalysis after stimulation, possibly by binding of another factor in the class IIa-specific groove. Some cdHDAC7 residues interacting with the docked peptide are shown as stick models (carbon in green). Zinc and potassium ions are shown as gray and brown and water as blue spheres, respectively.
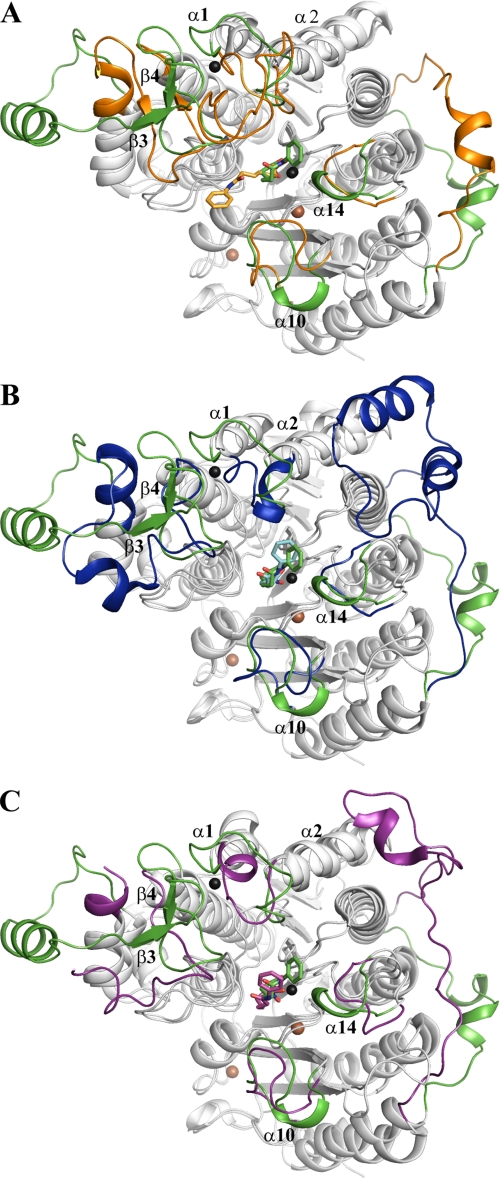
The overall fold of cdHDAC7 is similar to other reported HDAC structures. The Cα atoms of the structures of human cdHDAC7 and class II HDAC-like amidohydrolase from Bordetella strain FB188 (1ZZ1) superimpose with a root mean square deviation of 1.8 Å. The Cα atoms of the structures of human HDAC8 (1T69) and HDAC-like protein from A. aeolicus (1C3S), both representing class I HDACs, superimpose with a root mean square deviation of 3.8 and 2.9 Å on human cdHDAC7, respectively. The greatest structural diversity is found in loop regions around the active site entrance (Fig. 3, A–C). In cdHDAC7, the positioning of helix α14 and its preceding loop results in a more closed conformation of the active site. Structural differences are seen in the helix α10 and its following loop, in the loop connecting helices α1 and α2, and residues between helices α6 and α7, comprising β3 and β4. Interestingly, these regions harbor residues that differ most in class I and class II HDACs (supplemental Fig. S3) and, thus, are likely to be involved in regulation of substrate binding and/or specificity.
FIGURE 3.
Superimposition of cdHDAC7 structure with other HDAC crystal structures. The crystal structure of human cdHDAC7 (green) bound to SAHA was superimposed on the SAHA-complexed structures of the class II HDAC-like amidohydrolase from Bordetella/Alcaligenes (orange, 1ZZ1) (A), HDAC-like protein from A. aeolicus (blue, 1C3S) (B), and on human HDAC8 (purple, 1T69) (C). Structural differences are highlighted. Helices α1, α2, α10, and α14 and the unique insertion (β3 and β4) that is involved in binding the second zinc ion in cdHDAC7 are labeled. The SAHA molecules are represented as stick models. The zinc and potassium atoms are shown as gray and brown spheres, respectively.
The most striking structural feature of cdHDAC7 is a novel zinc binding motif arising from sequences unique to class IIa HDACs (HDAC4, HDAC5, HDAC7, and HDAC9). This motif is formed by a class IIa HDAC-specific insertion comprising a β-hairpin positioned by two antiparallel β-strands (β3 and β4) and the loop between helices α1 and α2 (Fig. 1A). The zinc ion tetrahedrally coordinates the side chains of Cys-533, Cys-535, His-541, and Cys-618, residues that are exclusively conserved in class IIa HDACs with a consensus pattern of CXCX5HX75–76C (Fig. 1B and supplemental Fig. S3). The α1-α2 loop, which is located near the active site entrance, is in close proximity to the hydrophobic capping group of the inhibitor seen in our cdHDAC7-hydroxamate inhibitor structures (Fig. 1A and Fig. 3A). The structural consequences of the second zinc binding region, referred to here as the CCHC motif, are 2-fold. First, this motif delineates a distinct groove contiguous to the opening of the active site channel. Second, the CCHC motif stabilizes a deep hydrophobic pocket, which may represent a site for docking of additional factors and/or regulatory proteins (Fig. 1, B and C, and supplemental Fig. S4).
To envision how a substrate peptide could interact with both the active site and the CCHC motif, we used Monte Carlo energy minimization to dock an acetyl-lysine-containing peptide, analogous to that of the recently published HDAC8-p53 peptide-bound structure (15). In the lowest energy conformation, the peptide lies in the groove delineated by the class IIa-specific insertion and CCHC motif, and its acetyl-lysine occupies the active site (Fig. 1C). The peptide makes several hydrogen bonds with cdHDAC7, including a hydrogen bond between the backbone nitrogen of the acetylated lysine to the side chain of Asp-626, a feature similar to the recently published HDAC8 substrate structure (15). The arginine C terminus of the acetylated lysine lies in a small cavity and makes three hydrogen bonds to cdHDAC7, suggesting that Lys-Arg is a favorable motif for HDAC7 substrates. However, we note that there is currently no evidence that p53 is a substrate for HDAC7, and further studies in search of native HDAC7 substrates are necessary.
Taken together, a model is suggested whereby the class IIa HDAC-specific CCHC-motif generates two separate structural features, a groove and a cavity, which mediate substrate and possibly binding of partner proteins. Thus, the class IIa HDAC-specific CCHC motif may be involved in substrate specificity and/or regulation of activity.
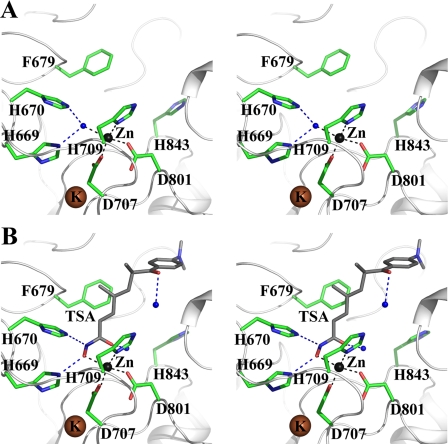
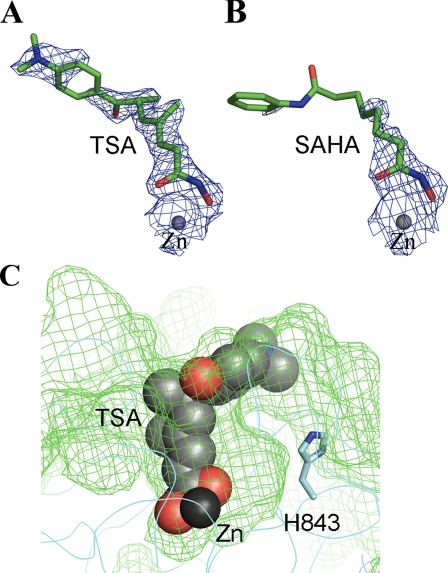
Mode of Hydroxamate Inhibitor Binding—The electron density map of both the TSA-cdHDAC7 and the SAHA-cdHDAC7 complex structures reveal poor electron density for the capping group but strong and clear electron density for the hydroxamate moiety of the inhibitors (Fig. 4, A and B). The hydroxamate moiety forms hydrogen bonds with the side chains of two active site histidines (His-669 and His-670), mimicking the interactions of the water molecule in the active site of apo-cdHDAC7 (Fig. 2, A and B). The hydroxyl group of the inhibitor replaces the water molecule, which was found in the active site of apo-cdHDAC7. Surprisingly, the hydroxamate group of the inhibitor forms a monodentate complex with the catalytic zinc ion involving only the hydroxyl oxygen rather than the expected bidentate complex seen in the hydroxamate-complexed HDAC structures reported so far (both the hydroxyl and the carbonyl oxygen of the inhibitor coordinate the zinc ion) (Fig. 2B). The aberrant monodentate coordination of TSA and SAHA in the cdHDAC7-complex structures originates from the different topology of the active site. While in the other HDAC structures a tyrosine residue (Tyr-312 in HDAC-like protein from Bordetella, Tyr-297 in A. aeolicus, and Tyr-306 in human HDAC8) orients the carbonyl oxygen of the hydroxamate group, cdHDAC7 lacks this active site tyrosine. In cdHDAC7, the histidine residue His-843 is found at this position, pointing away from the active site (Fig. 2). Whereas the active site tyrosine is conserved among the bacterial and class I and IIb HDACs, the histidine residue is found exclusively in class IIa HDACs (supplemental Fig. S3). As a result of the different topology of the active site at the histidine position, the hydroxamate carbonyl oxygen is turned away from the zinc and forms a hydrogen bond with a water molecule, which is absent in the other HDAC structures but found in all three cdHDAC7 structures (Fig. 2). The water molecule is 2.5 Å away from the carbonyl oxygen of the hydroxamide group, as is the tyrosine hydroxyl in human HDAC8.
FIGURE 4.
Hydroxamate inhibitor binding of cdHDAC7. A and B, the 2|F0 - Fc| electron density maps for the bound zinc and inhibitor molecules in the active site of cdHDAC7 are contoured at 0.9σ (blue). TSA (A) and SAHA (B) are shown as stick models colored as per atom type: carbon in green, oxygen in red, and nitrogen in blue. C, the TSA molecule bound in the cdHDAC7 active site is shown as a sphere model. The histidine residue His-843 points away from the active site, resulting in an enlarged active site pocket.
Upon inhibitor binding, the side chain of some amino acids, which are at the periphery of the pocket and mostly solvent-exposed, change conformation to accommodate the inhibitor (e.g. Phe-679, Thr-625, Asp-626, Leu-810). Otherwise, there are no significant structural changes upon inhibitor binding (see also Fig. 2).
Mutational Analysis of cdHDAC7—It was shown for the HDAC-like protein from Bordetella strain FB188 that mutation of the active site tyrosine residue into phenylalanine (Y312F) completely abolished deacetylase activity with an acetylated lysine peptide (42). To assess the role of the corresponding residue His-843 in cdHDAC7 catalysis, we generated three variants (H843Y, H843F, and H843A) and assayed for deacetylase activity. All three cdHDAC7 variants were more active than the wild-type enzyme, with the mutation of histidine to tyrosine (H843Y) showing the greatest effect (Table 1). cdHDAC7 variant H843Y is about 6000 times more active than wild-type cdHDAC7 and also significantly more active than any class I HDAC tested with a fluorogenic substrate (39, 40, 42). Thus, the lack of the tyrosine in cdHDAC7 may partially explain the relatively low level of deacetylase activity found for wild-type cdHDAC7. Our data further substantiate the important role of residue 843 in catalysis. We assume that Tyr at this position acts by stabilizing the tetrahedral oxyanion intermediate as described for other HDACs. This strongly suggests that the Tyr-843 side chain is oriented toward the active site, unlike the native His-843 side chain seen in our structures.
As seen in Table 1, deacetylase activity is also modestly increased for the H843F and H843A variants. Because the Km values are similar for wild-type cdHDAC7 and all cdHDAC7 variants, mutation of the active site histidine (His-843) seems to affect the turnover rate rather than substrate binding. Our IC50 determinations of the tyrosine variant, however, clearly show that IC50 values of the inhibitors are significantly decreased for the tyrosine variant compared with wild-type cdHDAC7 (Table 3), reinforcing the role of the side chain of the tyrosine in inhibitor binding and stabilizing the oxyanion intermediate during catalysis.
DISCUSSION
HDAC7 was previously reported to have enzymatic activity only when bound to the SMRT/N-CoR·HDAC3 complex (16, 17). Because this finding was derived from coimmunoprecipitation experiments, it was not clear if the observed HDAC activity was due to HDAC7 or the complex partner HDAC3. Two models were suggested. First, HDAC7 might not be a functional HDAC in the context of the SMRT/N-CoR complex but might serve to recruit pre-existing, enzymatically active SMRT/N-CoR complexes that contain HDAC3. In this model, the SMRT/N-CoR co-repressors provide a structural link between active HDAC3 and inactive HDAC7. In the second model, HDAC7 might become activated only in the presence of HDAC3 (2, 16).
Another class IIa HDAC, HDAC4, was shown to have intrinsic deacetylase activity, but its activity levels were inversely proportional to the expression level, suggesting that an elusive factor is required for deacetylase activity (43). More recently, HDAC4 and other class IIa HDACs were reported to only regulate transcription by bridging the enzymatically active SMRT/N-CoR·HDAC3 complex and transcription factors independent of any intrinsic HDAC activity (16, 17). This finding was derived from experiments using coimmunoprecipitated protein and chemically acetylated H4 peptide as substrate.
The kinetic data presented in this study show that the isolated and purified catalytic domain of the human class IIa HDAC, cdHDAC7, has an intrinsic low level of deacetylase activity in the absence of any complex partner which can be inhibited by known HDAC inhibitors such as the hydroxamic acid TSA. In parallel to our studies, Lahm et al. (38) also showed that the isolated catalytic domain of class IIa HDACs have weak but measurable intrinsic catalytic activity on chemically acetylated core histones. Interestingly, they also demonstrated a gain of function for class IIa HDACs upon mutation of the active site histidine into tyrosine, stressing the importance of this residue in catalysis (38).
Whereas the substrate affinity (Km) and inhibitory constants (IC50) of wild-type cdHDAC7 are comparable with other HDACs (39), the observed catalytic rate constant of wild-type cdHDAC7 is somewhat lower. This might be due to the general difficulty in defining catalytic properties of HDAC7 and other class IIa HDACs, since their biological substrates are unknown, and it has been shown that HDACs show different catalytic efficiencies with different substrate peptides (38, 40, 41, 44). On the other hand, the fact that the side chain of His-843 points away from the cdHDAC7 active site could also explain the lower activity of cdHDAC7 since in this position His-843 is unlikely to contribute to the orientation of the substrate acetyl oxygen and the stabilization of the tetrahedral oxyanion intermediate during catalysis as proposed for the tyrosine of other HDACs. However, it is conceivable that upon binding of a protein partner and/or substrate binding, the side chain of His-843 could rotate inward, thereby orienting the histidine ring toward the catalytic site and acetyl group of the substrate (Fig. 1C). Our mutational analysis supports the ability of cdHDAC7 to accommodate an inward conformation of the side chain of residue 843, because a tyrosine at this position significantly increases the catalytic rate as well as decreases IC50 values for inhibitors compared with wild-type cdHDAC7. Thus, criteria for the residue at position 843 to contribute to catalysis are (i) an inward orientation relative to the active site and (ii) bearing a side chain capable of hydrogen bonding to the reaction intermediate or able to accommodate one or more water molecules that can hydrogen-bond to the intermediate. In the case of the Phe and Ala variants of cdHDAC7 as well as the native apo structure with His-843 oriented outward, stabilization likely occurs via water.
Verdin et al. (2) proposed that class II HDAC enzymatic activity may be modulated by means of its interactions with other proteins, ensuring that the enzymes only become enzymatically active after incorporation in an appropriate multiprotein complex. Clemente et al. (45) proposed that substrate peptide specificity mainly depends on cofactors that form complexes with HDACs, and recently it was shown that corepressor binding can modulate the selectivity of enzymatic substrate conversion (40). Our structural analysis of cdHDAC7 reveals a potential molecular mechanism for modulation of specificity and activity of class IIa HDACs within a multiprotein complex. The class IIa HDAC-specific CCHC motif delineates a groove in the vicinity of the catalytic site (Fig. 1, B and C) and forms a putative protein binding cavity (Fig. 1, B and C, and supplemental Fig. 4). This hydrophobic protein binding cavity is close to but distinct from the active site and may represent a substrate recruitment platform or a docking site for protein-protein interactions. Homology models for the class IIa HDACs HDAC4 and HDAC5 (46) as well as amino acid sequence analysis confirm that residues lining the cavity are well conserved within the class IIa HDAC family (see also supplemental Fig. S3). Thus, it is likely that the cavity could represent the recruitment interface for these complex partners. Moreover, due to its proximity to the active site and peptide binding groove, binding of partner proteins may modulate HDAC activity and/or substrate specificity. Significantly, His-843 in cdHDAC7 is oriented away from the active site and toward the class IIa HDAC-specific peptide binding groove. Therefore, it is possible that conformational changes within a multiprotein complex could re-orient His-843 toward the active site, providing improved stabilization of the oxyanion intermediate, thereby stimulating the cryptic HDAC activity (Fig. 1C). The fact that mutations of His-843 have a greater HDAC activity supports this concept.
Alternatively, the cavity may also regulate class IIa HDAC activity upon nuclear export. Class IIa HDACs show nucleocytoplasmic shuttling, and phosphorylation-dependent intracellular trafficking has been shown for HDAC4, HDAC5, and HDAC7 (33, 47). In the nucleus, class IIa HDACs function as transcriptional repressors. After T-cell receptor activation, phosphorylation occurs, and HDACs shuttle into the cytoplasm. Because biological substrates for class IIa HDACs are not known, it is possible that deacetylation of substrates other than nuclear histones could be regulated via this potential binding pocket. Taken together, we suggest that the class IIa HDACs likely evolved the CCHC motif to control activity and substrate specificity and to adapt to various regulatory processes.
It has been suggested that inhibitors specific to class I HDACs may be more clinically efficacious, and activators of class II HDACs were proposed to be of potential therapeutic value (3). The crystal structure of human cdHDAC7 described in this work represents a framework for the development of such HDAC modulators. As a result of the tyrosine-histidine exchange, the active site pocket of cdHDAC7 is enlarged, providing avenues for the development of highly specific class IIa HDAC modulators (Fig. 4C). More specifically, the hydrophobic bed defined between Phe-679 and Arg-547 may accommodate binding of compounds with aromatic rings that can be directed in this region. Another strategy for the development of more specific HDAC modulators might be to modify the hydrophobic capping group of the inhibitor for preferential interaction with residues 531–544 of the CCHC motif as well as targeting the putative protein binding pocket adjacent to the active site. The fact that cdHDAC7 activity can be modulated by changes of the chemical environment within the catalytic site is substantiated by our analysis of three cdHDAC7 variants that, interestingly, all displayed increased catalytic rate constants compared with wild-type cdHDAC7.
In conclusion, our studies provide the first structural view of the catalytic domain of a class IIa HDAC and reveal for this subclass specific features (i) a novel zinc binding motif that is likely to be involved in substrate binding and/or protein-protein interactions and may provide a site for modulation of activity and (ii) a unique active site topology resulting in a cryptic catalytic activity and in an enlarged active site pocket. Finally, our structures may enable the design of specific class IIa HDAC modulators. Further analysis of class II HDACs will be necessary to yield insights into the molecular basis of related human diseases and to develop HDAC modulators with potential therapeutic value.
Acknowledgments
We thank J. Bradner for fruitful discussions.
The atomic coordinates and structure factors (codes 3C0Y, 3C0Z, and 3C10) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported in part by federal funds from the NCI, National Institutes of Health Initiative for Chemical Genetics under Contract N01-CO-12400. The Structural Genomics Consortium is a registered charity (no. 1097737) that receives funds from the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, Genome Canada through the Ontario Genomics Institute, GlaxoSmithKline, Karolinska Institutet, the Knut and Alice Wallenberg Foundation, the Ontario Innovation Trust, the Ontario Ministry for Research and Innovation, Merck, the Novartis Research Foundation, the Swedish Agency for Innovation Systems, the Swedish Foundation for Strategic Research, and the Wellcome Trust. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Table 1.
Footnotes
The abbreviations used are: HDAC, histone deacetylase; cdHDAC7, catalytic domain of human HDAC7; TSA, trichostatin A; SAHA, suberoylanilide hydroxamic acid; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; DTT, dithiothreitol.
References
- 1.Kao, H. Y., Downes, M., Ordentlich, P., and Evans, R. M. (2000) Genes Dev. 14 55-66 [PMC free article] [PubMed] [Google Scholar]
- 2.Verdin, E., Dequiedt, F., and Kasler, H. G. (2003) Trends Genet. 19 286-293 [DOI] [PubMed] [Google Scholar]
- 3.Yang, X. J., and Gregoire, S. (2005) Mol. Cell. Biol. 25 2873-2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregoire, S., Xiao, L., Nie, J., Zhang, X., Xu, M., Li, J., Wong, J., Seto, E., and Yang, X. J. (2007) Mol. Cell. Biol. 27 1280-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertos, N. R., Wang, A. H., and Yang, X. J. (2001) Biochem. Cell Biol. 79 243-252 [PubMed] [Google Scholar]
- 6.Vega, R. B., Matsuda, K., Oh, J., Barbosa, A. C., Yang, X., Meadows, E., McAnally, J., Pomajzl, C., Shelton, J. M., Richardson, J. A., Karsenty, G., and Olson, E. N. (2004) Cell 119 555-566 [DOI] [PubMed] [Google Scholar]
- 7.Chang, S., Young, B. D., Li, S., Qi, X., Richardson, J. A., and Olson, E. N. (2006) Cell 126 321-334 [DOI] [PubMed] [Google Scholar]
- 8.Chang, S., McKinsey, T. A., Zhang, C. L., Richardson, J. A., Hill, J. A., and Olson, E. N. (2004) Mol. Cell. Biol. 24 8467-8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, C. L., McKinsey, T. A., Chang, S., Antos, C. L., Hill, J. A., and Olson, E. N. (2002) Cell 110 479-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, Y., Li, N., Caron, C., Matthias, G., Hess, D., Khochbin, S., and Matthias, P. (2003) EMBO J. 22 1168-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbert, C., Guardiola, A., Shao, R., Kawaguchi, Y., Ito, A., Nixon, A., Yoshida, M., Wang, X. F., and Yao, T. P. (2002) Nature 417 455-458 [DOI] [PubMed] [Google Scholar]
- 12.Finnin, M. S., Donigian, J. R., Cohen, A., Richon, V. M., Rifkind, R. A., Marks, P. A., Breslow, R., and Pavletich, N. P. (1999) Nature 401 188-193 [DOI] [PubMed] [Google Scholar]
- 13.Somoza, J. R., Skene, R. J., Katz, B. A., Mol, C., Ho, J. D., Jennings, A. J., Luong, C., Arvai, A., Buggy, J. J., Chi, E., Tang, J., Sang, B. C., Verner, E., Wynands, R., Leahy, E. M., Dougan, D. R., Snell, G., Navre, M., Knuth, M. W., Swanson, R. V., McRee, D. E., and Tari, L. W. (2004) Structure 12 1325-1334 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen, T. K., Hildmann, C., Dickmanns, A., Schwienhorst, A., and Ficner, R. (2005) J. Mol. Biol. 354 107-120 [DOI] [PubMed] [Google Scholar]
- 15.Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfi, A., De Francesco, R., Steinkühler, C., and Di Marco, S. (2007) EMBO Rep. 8 879-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischle, W., Dequiedt, F., Hendzel, M. J., Guenther, M. G., Lazar, M. A., Voelter, W., and Verdin, E. (2002) Mol. Cell 9 45-57 [DOI] [PubMed] [Google Scholar]
- 17.Fischle, W., Dequiedt, F., Fillion, M., Hendzel, M. J., Voelter, W., and Verdin, E. (2001) J. Biol. Chem. 276 35826-35835 [DOI] [PubMed] [Google Scholar]
- 18.Drummond, D. C., Noble, C. O., Kirpotin, D. B., Guo, Z., Scott, G. K., and Benz, C. C. (2005) Annu. Rev. Pharmacol. Toxicol. 45 495-528 [DOI] [PubMed] [Google Scholar]
- 19.Minucci, S., and Pelicci, P. G. (2006) Nat. Rev. Cancer 6 38-51 [DOI] [PubMed] [Google Scholar]
- 20.Marks, P. A. (2007) Oncogene 26 1351-1356 [DOI] [PubMed] [Google Scholar]
- 21.Bolden, J. E., Peart, M. J., and Johnstone, R. W. (2006) Nat. Rev. Drug Discov. 5 769-784 [DOI] [PubMed] [Google Scholar]
- 22.Miller, T. A., Witter, D. J., and Belvedere, S. (2003) J. Med. Chem. 46 5097-5116 [DOI] [PubMed] [Google Scholar]
- 23.Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., Chakravarty, P., Paolini, C., De Francesco, R., Gallinari, P., Steinkuhler, C., and Di Marco, S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 15064-15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parra, M., Kasler, H., McKinsey, T. A., Olson, E. N., and Verdin, E. (2005) J. Biol. Chem. 280 13762-13770 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen, T. K., Hildmann, C., Riester, D., Wegener, D., Schwienhorst, A., and Ficner, R. (2007) Acta Crystallogr. F Struct. Biol. Crystalliz. Comm. 63 270-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vedadi, M., Niesen, F. H., Allali-Hassani, A., Fedorov, O. Y., Finerty, P. J., Jr., Wasney, G. A., Yeung, R., Arrowsmith, C., Ball, L. J., Berglund, H., Hui, R., Marsden, B. D., Nordlund, P., Sundstrom, M., Weigelt, J., and Edwards, A. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 15835-15840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu, E., Dul, E., Sung, C. M., Chen, Z., Kirkpatrick, R., Zhang, G. F., Johanson, K., Liu, R., Lago, A., Hofmann, G., Macarron, R., de los Frailes, M., Perez, P., Krawiec, J., Winkler, J., and Jaye, M. (2003) J. Pharmacol. Exp. Ther. 307 720-728 [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 472-494 [Google Scholar]
- 29.McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C., and Read, R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61 458-464 [DOI] [PubMed] [Google Scholar]
- 30.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. D. Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 31.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. D. Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 32.Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) Nucleic Acids Res. 22 4673-4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao, H. Y., Verdel, A., Tsai, C. C., Simon, C., Juguilon, H., and Khochbin, S. (2001) J. Biol. Chem. 276 47496-47507 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Recio, J., Totrov, M., and Abagyan, R. (2003) Proteins 52 113-117 [DOI] [PubMed] [Google Scholar]
- 35.Abagyan, R., and Totrov, M. (1994) J. Mol. Biol. 235 983-1002 [DOI] [PubMed] [Google Scholar]
- 36.Zhou, X., Marks, P. A., Rifkind, R. A., and Richon, V. M. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 10572-10577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurvich, N., Tsygankova, O. M., Meinkoth, J. L., and Klein, P. S. (2004) Cancer Res. 64 1079-1086 [DOI] [PubMed] [Google Scholar]
- 38.Lahm, A., Paolini, C., Pallaoro, M., Nardi, M. C., Jones, P., Neddermann, P., Sambucini, S., Bottomley, M. J., Lo Surdo, P., Carfi, A., Koch, U., De Francesco, R., Steinkuhler, C., and Gallinari, P. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 17335-17340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hildmann, C., Wegener, D., Riester, D., Hempel, R., Schober, A., Merana, J., Giurato, L., Guccione, S., Nielsen, T. K., Ficner, R., and Schwienhorst, A. (2006) J. Biotechnol. 124 258-270 [DOI] [PubMed] [Google Scholar]
- 40.Riester, D., Hildmann, C., Grunewald, S., Beckers, T., and Schwienhorst, A. (2007) Biochem. Biophys. Res. Commun. 357 439-445 [DOI] [PubMed] [Google Scholar]
- 41.Hildmann, C., Ninkovic, M., Dietrich, R., Wegener, D., Riester, D., Zimmermann, T., Birch, O. M., Bernegger, C., Loidl, P., and Schwienhorst, A. (2004) J. Bacteriol. 186 2328-2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreth, K., Riester, D., Hildmann, C., Hempel, R., Wegener, D., Schober, A., and Schwienhorst, A. (2007) Biochem. J. 401 659-665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, A. H., Bertos, N. R., Vezmar, M., Pelletier, N., Crosato, M., Heng, H. H., Th'ng, J., Han, J., and Yang, X. J. (1999) Mol. Cell. Biol. 19 7816-7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riester, D., Wegener, D., Hildmann, C., and Schwienhorst, A. (2004) Biochem. Biophys. Res. Commun. 324 1116-1123 [DOI] [PubMed] [Google Scholar]
- 45.Clemente, S., Franco, L., and Lopez-Rodas, G. (2001) Biochemistry 40 10671-10676 [DOI] [PubMed] [Google Scholar]
- 46.Cardozo, T., Totrov, M., and Abagyan, R. (1995) Proteins 23 403-414 [DOI] [PubMed] [Google Scholar]
- 47.McKinsey, T. A., Zhang, C. L., Lu, J., and Olson, E. N. (2000) Nature 408 106-111 [DOI] [PMC free article] [PubMed] [Google Scholar]