Abstract
Apolipoprotein B (apoB) is required for the hepatic assembly and secretion of very low density lipoprotein (VLDL). The LDL receptor (LDLR) promotes post-translational degradation of apoB and thereby reduces VLDL particle secretion. We investigated the trafficking pathways and ligand requirements for the LDLR to promote degradation of apoB. We first tested whether the LDLR drives apoB degradation in an endoplasmic reticulum (ER)-associated pathway. Primary mouse hepatocytes harboring an ethyl-nitrosourea-induced, ER-retained mutant LDLR secreted comparable levels of apoB with LDLR-null hepatocytes, despite reduced secretion from cells expressing the wild-type LDLR. Additionally, treatment of cells with brefeldin A inhibited LDLR-dependent degradation. However, this rescue was reversible, and degradation of apoB occurred upon removal of brefeldin A. To characterize the lipoprotein reuptake pathway of degradation, we employed an LDLR mutant defective in constitutive endocytosis and internalization of apoB. This mutant was as effective in reducing apoB secretion as the wild-type LDLR. However, the effect was dependent on apolipoprotein E (apoE) as only the wild-type LDLR, and not the endocytic mutant, reduced apoB secretion in apoE-null cells. Treatment with heparin rescued a pool of apoB in cells expressing the endocytic mutant, indicating that reuptake of VLDL via apoE still occurs with this mutant. Finally, an LDLR mutant defective in binding apoB but not apoE reduced apoB secretion in an apoE-dependent manner. Together, these data suggest that the LDLR directs apoB to degradation in a post-ER compartment. Furthermore, the reuptake mechanism of degradation occurs via internalization of apoB through a constitutive endocytic pathway and apoE through a ligand-dependent pathway.
Apolipoprotein B (apoB)3 is the major protein component of very low density lipoprotein (VLDL), the triglyceride-enriched lipoprotein particle produced by the liver. apoB is essential for the assembly and secretion of nascent VLDL particles (1–3). apoB is constitutively expressed, and its stability is regulated through co- and post-translational degradation (4). Therefore, the number of VLDL particles secreted is a function of the proportion of apoB that escapes degradation.
The LDL receptor (LDLR) is a ubiquitously expressed protein responsible for the clearance of cholesterol-rich lipoproteins from the bloodstream through its ligands, apoB and apolipoprotein E (apoE). A loss in LDLR activity, as occurs in humans with familial hypercholesterolemia (FH), results in a defect in LDL clearance (5). In addition to its role in mediating lipoprotein clearance, the LDLR also regulates VLDL secretion. Studies in humans (6–8), mice (9–11), primary hepatocytes (12–15), and human hepatoma cells (16) have revealed that the loss of LDLR activity leads to increased secretion of VLDL particles, due to a decrease in the degradation of apoB. The particles secreted from livers lacking LDLR activity are small (6, 10, 14) and have decreased triglyceride content (6, 11), suggesting that the LDLR preferentially targets underlipidated particles for degradation. Initial mechanistic studies have suggested that the LDLR operates through rapid reuptake of nascent VLDL particles (12, 16) in a process similar to the LDLR-mediated internalization of LDL, as well as through a direct intracellular targeting of nascent VLDL particles to degradation (12, 15).
In the current study, we addressed two questions. First, we asked whether the LDLR acts on apoB in the ER or at a post-ER step in the secretory pathway. Second, we studied the ligand requirements for LDLR-mediated reuptake of VLDL particles. We show that LDLR-dependent apoB degradation occurs after exit from the ER and that it involves both of the LDLR ligands on the VLDL particle, apoB and apoE. We also show that an FH LDLR mutant defective in constitutive endocytosis is still capable of mediating the reuptake of VLDL via a recently described, non-canonical endocytic mechanism (17, 18). This study sheds light on general mechanisms of LDLR-mediated degradation of apoB and specifically predicts pathways by which an FH LDLR mutant may regulate VLDL secretion.
EXPERIMENTAL PROCEDURES
Mice and Hepatocyte Isolation—Wild-type (C57BL/6J), C678Y (HLB301), Ldlr-/- (Ldlrtm1Her), and apoE-/- (apoEtm1Unc) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in our animal care facility. Mice were weaned at 3–4 weeks of age, fed standard chow and water ad libitum, and housed on a 12-h dark/light cycle. Mice were sacrificed between 8 and 18 weeks of age for hepatocyte isolation. Hepatocytes were isolated by liver perfusion as described previously (11). For confocal microscopy, cells were plated in low glucose DMEM supplemented with 10% fetal bovine serum, penicillin-streptomycin, l-glutamine, and sodium pyruvate (Invitrogen) at a density of 3.6 × 105 cells/well on collagen-coated coverslips (BD Biosciences) in 6-well plates. For all other applications, cells were plated at a density of 0.8 × 106 cells/dish in collagen-coated 60-mm dishes (BD Biosciences). Cells not infected with virus were used within 16 h.
Generation of Tetracycline-inducible Adenoviral Vectors—A reverse tetracycline-controlled transactivator driven by the cytomegalovirus promoter was cut from pWHE146 (19) (a generous gift from Wolfgang Hillen, Universitat Erlangen) with MluI and BamHI and was inserted into the XbaI site of pVQ E3 3.1 Δ XbaI (ViraQuest, North Liberty, IA). A minimal cytomegalovirus promoter with seven repeats of the tetracycline operator (tet-on promoter) was cut from pUHC13-3 (20) (also kindly supplied by Wolfgang Hillen) with XhoI and BamHI and inserted into the XhoI/BamHI site of pVQ Ad5 K-NpA (ViraQuest). LacZ was cut from pVQ AdRSV ntLacZ (ViraQuest) with HindIII and SpeI and inserted into the HindIII and SpeI site of pVQ Ad5 K-NpA downstream of the tet-on promoter. LDLR mutants were created by site-directed mutagenesis of the wild-type (WT) LDLR (12) in the pCI vector (Promega, Madison, WI). The LDLR cDNAs were cut from pCI with NheI and XbaI and inserted into the XbaI site of pVQ Ad5 K-NpA downstream of the tet-on promoter. The pVQ E3 3.1 Δ XbaI shuttle containing the reverse tetracycline-controlled transactivator and the pVQ Ad5 K-NpA shuttles containing the operators and genes of interest were combined by Viraquest to generate tetracycline-inducible adenoviral vectors.
Adenoviral Infection—Four hours after isolation, hepatocytes were washed once in supplemented DMEM without fetal bovine serum and then infected in the same medium containing virus at the indicated concentrations for 2 h. Infected cells were incubated overnight in supplemented DMEM with 10% tetracycline-free fetal bovine serum (Clontech) and re-fed the following day with the same medium. All viral expression experiments were performed ∼40 h after isolation and 36 h after infection.
Western Blots and Confocal Microscopy—Western blots were performed as described (12) except that proteins were transferred to Westran S polyvinylidene difluoride membrane (Whatman, Florham Park, NJ) and probed with a polyclonal rabbit anti-LDLR antibody (1:500). The anti-LDLR antibody was generated with a truncated LDLR containing the first 354 amino acids of the N terminus (21). To detect protein localization by immunofluorescence, hepatocytes were fixed, permeabilized, stained, and mounted as described previously (22). The antibodies and stains used were polyclonal anti-LDLR (1:300), Alexa Fluor 488-conjugated concanavalin A (50 μg/ml, Molecular Probes, Carlsbad, CA), 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA), and donkey anti-rabbit IgG-Rhodamine Red-X (1:200, Jackson Immuno-Research, Bar Harbor, ME). For antibody internalization experiments, cells were incubated with cold supplemented DMEM containing polyclonal anti-LDLR antibody (1:300) on ice for 1 h. The cells were either washed once with cold medium and fixed immediately with 2% paraformaldehyde or re-fed with warm medium and incubated at 37 °C for 10 min before fixation. All cells were then stained with donkey anti-rabbit IgG-fluorescein isothiocyanate (1:200, Jackson Immuno-Research, Bar Harbor, ME) and mounted according to the standard protocol (above). Confocal microscopy was performed with a Nikon Eclipse TE2000-U laser-scanning microscope with 408-, 488-, and 543-nm laser lines. Images were processed with Adobe PhotoShop CS2, version 9.0 (Adobe, San Jose, CA).
Protein Secretion Assays—Hepatocytes were starved for 1 h in supplemented methionine and cysteine-free DMEM (Invitrogen) without serum. For continuous labeling experiments, cells were incubated in starve medium supplemented with l-[35S]methionine/cysteine (100 μCi/dish, PerkinElmer Life Sciences) for 4 h before harvesting cells and media. For pulse-chase experiments, cells were incubated with 200 μCi/dish [35S]methionine/cysteine for 1 h, washed with DMEM supplemented with 10 mm unlabeled cysteine and methionine, and chased for the indicated times in the same medium. Brefeldin A (BFA, Epicenter, Madison, WI) was dissolved in water and added to the chase medium at a final concentration of 10 μg/ml. Heparin (Sigma) was dissolved in water and added to the labeling medium at a final concentration of 6 mg/ml. Cells were lysed, and immunoprecipitations for apoB and albumin were performed from media and cell lysates as described (12). Antibodies used for apoB and albumin immunoprecipitations were polyclonal rabbit anti-pig LDL (12) and polyclonal rabbit anti-human albumin (Sigma), respectively. Immunoprecipitated, radiolabeled proteins were resolved by SDS-PAGE, detected by autoradiography with a PhosphorImager screen followed by scanning with a Typhoon 9410 variable mode imager, and quantitated with ImageQuant TL v2002.01 (GE Healthcare, Uppsala, Sweden). In experiments testing endogenous genetic effects, apoB values were normalized by albumin values to correct for differences in hepatocyte isolation efficiencies and specific activity of incorporated 35S among cell types. In experiments testing effects of virally expressed proteins, values were normalized by cell protein (23). The data were expressed in mean arbitrary units and show the standard error. Student's t test was performed to determine differences between two treatment conditions. One-way analysis of variance followed by Fisher's protected test of least significant difference (LSD) were performed to determine differences among multiple treatment conditions.
RESULTS
The LDLR Regulates apoB Secretion through a Post-ER Mechanism—Our laboratory previously reported that transfection of cDNAs encoding ER-retained LDLR mutants reduced apoB secretion to the same extent as those expressing the WT LDLR (13). This suggested that the LDLR directs apoB to degradation through an ER-associated pathway. To test this result in a more physiological system, we used a newly generated hypercholesterolemic mouse obtained from an ethyl-nitrosourea mutagenesis screen with high throughput phenotyping (24). The hypercholesterolemia in this mouse results from a mutation in the Ldlr gene leading to a C678Y amino acid substitution (numbering excludes the 21-amino-acid signal peptide).4
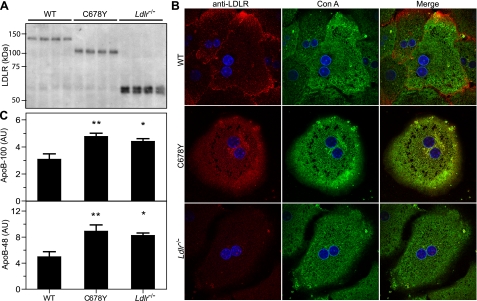
To determine whether the mouse C678Y LDLR mutation confers ER retention, we blotted for the LDLR with lysates from primary hepatocytes derived from WT, mutant, and Ldlr-/- mice. The WT LDLR migrated at ∼150 kDa (Fig. 1A), which is characteristic of the mature, fully glycosylated receptor. By contrast, the C678Y mutant migrated at ∼100 kDa (Fig. 1A), which is characteristic of the newly synthesized, immature form of the receptor. Glycosylation of the LDLR occurs after exit from the ER, in the Golgi (26). Therefore, the increased mobility of the mutant LDLR suggests that it is retained in the ER. To verify ER retention, we immunostained the LDLR and determined its subcellular localization with confocal microscopy. The LDLR in the WT cells showed punctate staining, largely in the periphery but also in the interior of the cells, which did not overlap with the ER marker, concanavalin A (Con A) (Fig. 1B). In contrast, the C678Y mutant showed a reticular staining pattern, which completely overlapped with the ER marker.
FIGURE 1.
An endogenous ER-retained LDLR mutant does not reduce apoB secretion. Hepatocytes were isolated from age-matched WT, C678Y, and Ldlr-/- mice. A, a Western blot with cellular lysate (7 μg of protein) was performed with a polyclonal anti-LDLR antibody. B, hepatocytes were fixed, permeabilized, and stained with a polyclonal anti-LDLR antibody, an ER marker (concanavalin A (Con A)), and a nuclear marker (4′,6-diamidino-2-phenylindole, blue). Stained cells were visualized at ×100 by confocal microscopy. C, hepatocytes were continuously radiolabeled with [35S]Met/Cys for 4 h, and secreted levels of apoB-100, apoB-48, and albumin were detected as described under “Experimental Procedures.” The secreted levels of apoB were normalized to secreted albumin levels. Each bar represents the mean from three independent experiments in which all genotypes were compared. AU, arbitrary units. **, p < 0.01 and *, p < 0.05 versus WT, Fisher's protected LSD.
To assess whether the ER-retained LDLR mutant reduces apoB secretion, we determined the secreted protein levels from primary hepatocytes isolated from WT, C678Y, and Ldlr-/- mice. Hepatocytes from WT, but not the C678Y mice, secreted less apoB-100 and apoB-48 than Ldlr-/- hepatocytes (Fig. 1C). Since the ER-retained LDLR was unable to decrease apoB secretion, this suggests that the LDLR-dependent regulation of apoB secretion requires exit of the LDLR from the ER.
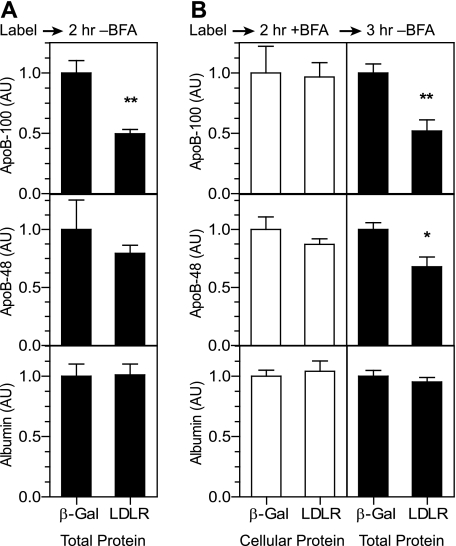
To determine whether retention of both the LDLR and apoB in the ER affects degradation of apoB, we investigated the effect of BFA on LDLR-dependent apoB degradation. BFA is a fungal metabolite that reversibly blocks anterograde transport of secretory cargo from the ER (27). Notably, BFA does not inhibit the ER-associated degradation of apoB observed in some cell types (28, 29). We employed adenoviral vectors expressing genes of interest in a tetracycline-inducible manner. This viral system allowed for a low basal level of LDLR expression in uninduced conditions, indicated by the ratio of the 100-kDa band (ER pool) relative to the 150-kDa band (post-ER pool, supplemental Fig. S1A), and enabled 90–95% infection efficiency (supplemental Fig. S1B). To establish a baseline of LDLR activity with this system in the absence of BFA, we infected Ldlr-/- mouse hepatocytes with adenoviral vectors to express β-galactosidase (Ad-β-Gal) or the wild-type human LDLR (Ad-LDLR-WT) in uninduced conditions. We radiolabeled the infected cells for 1 h and chased with cold medium for 2 h. The presence of the LDLR resulted in a reduction in total recovered apoB-100 (cellular and secreted) and a trend toward a reduction in apoB-48 (Fig. 2A), indicating that degradation of apoB occurred in the presence of the LDLR.
FIGURE 2.
Anterograde transport from the ER is required for LDLR-dependent degradation of apoB. Ldlr-/- primary hepatocytes were infected with Ad-β-Gal (β-Gal) or Ad-LDLR-WT (WT) at a titer of 0.8 × 108 pfu/dish. In A, cells were radiolabeled with [35S]Met/Cys for 1 h and chased in cold media for 2 h. AU, arbitrary units. In B, cells were chased in cold medium with 10 μg/ml BFA for 2 h and then further chased in the absence of BFA for 3 h. In both A and B, total levels of apoB-100, apoB-48, and albumin were calculated as the sum of protein-normalized cellular and secreted levels. The data are expressed as the mean fractional decrease in LDLR-expressing cells relative to β-Gal-expressing cells (n = 5). Black bars indicate total protein levels; white bars indicate cellular protein levels. Similar results were obtained in three independent experiments. *, p < 0.05, **, p < 0.01, Student's t test.
To determine the effect of BFA, we chased the cells in the presence of BFA for 2 h after labeling and then further chased for an additional 3 h in the absence of BFA to reverse the effects. Treatment with BFA increased cellular levels of apoB-100, apoB-48, and albumin (not shown), indicating that BFA indeed blocked transport from the ER. Importantly, the cellular levels of apoB did not differ between the β-Gal- and LDLR-expressing cells treated with BFA (Fig. 2B, left panels). However, removal of BFA during the second chase revealed an LDLR-dependent decrease in total (cellular and secreted) apoB-100 and apoB-48 (Fig. 2B, right panels). These results support a lack of an LDLR-dependent effect on apoB in the ER and suggest that exit from the ER is required for LDLR-mediated apoB degradation.
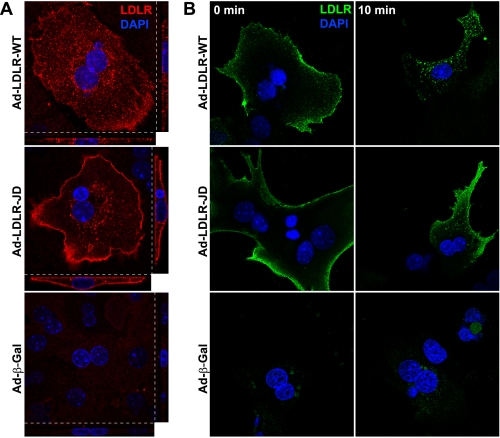
An LDLR Mutant Defective in Constitutive Endocytosis Reduces apoB Secretion as Effectively as the Wild Type—We next investigated mechanisms of LDLR-mediated apoB degradation downstream of ER exit, specifically those involving reuptake through endocytosis (12, 16). We employed an LDLR mutant, the “JD” LDLR (30), which has a substitution of cysteine for the critical tyrosine (Tyr-807) in the FDNPVY sequence in the C-terminal tail. This peptide signal is required for the constitutive endocytic activity responsible for internalization and degradation of apoB-containing lipoproteins in immortalized cells (30, 31). To verify that the JD LDLR is also defective in endocytosis in our primary hepatocyte system, we expressed the WT and JD LDLR with adenoviral vectors in Ldlr-/- hepatocytes and stained the LDLR to determine its steady-state localization. As with the endogenous mouse LDLR (Fig. 1B), the virally expressed WT LDLR showed punctate localization in both the periphery and the interior of the cell (Fig. 3A). In contrast, the JD LDLR was highly enriched at the cell surface and showed relatively little intracellular localization (Fig. 3A).
FIGURE 3.
The JD LDLR is defective in constitutive endocytosis. A, Ldlr-/- primary hepatocytes were infected with Ad-LDLR-WT, Ad-LDLR-JD, or Ad-β-Gal (1.35 × 108 pfu/well). Cells were fixed, permeabilized, and stained with a polyclonal anti-LDLR antibody (red) and 4′,6-diamidino-2-phenylindole (DAPI, blue). Confocal serial XY sections (×100, 0.25 μm/section) were imaged and reconstructed into XZ and YZ planes. XY planes (large panels) and the XZ (small panels, bottom) and YZ (small panels, right) reconstructions are shown. B, Ldlr-/- primary hepatocytes were prepared as above but infected with 2.25 × 108 pfu/well of virus. After treating overnight with doxycycline (100 ng/ml) to induce expression, cells were chilled on ice and incubated with a polyclonal anti-LDLR antibody for 1 h. Cells were washed with chilled medium (4 °C) and either fixed immediately or warmed to 37 °C for 10 min before fixation. Cells were then permeabilized, incubated with fluorescein isothiocyanate-conjugated anti-rabbit IgG, and imaged at ×100 by confocal microscopy.
To obtain a functional readout of the endocytic activity of the JD LDLR, Ldlr-/- hepatocytes expressing the WT or JD LDLR were incubated with a polyclonal anti-LDLR antibody on ice to allow for binding at the cell surface. The cells were then either immediately fixed or warmed to 37 °C for 10 min to allow for internalization of the bound antibody. Cells expressing the WT and JD LDLR both showed the presence of the antibody at the cell surface prior to warming (Fig. 3B, 0 min). However, only cells expressing the WT LDLR showed significant internalization of the antibody from the cell surface (Fig. 3B, 10 min). Identical results were observed with the C7 monoclonal anti-LDLR antibody (not shown). These data show that the JD LDLR is defective in constitutive endocytosis in primary hepatocytes.
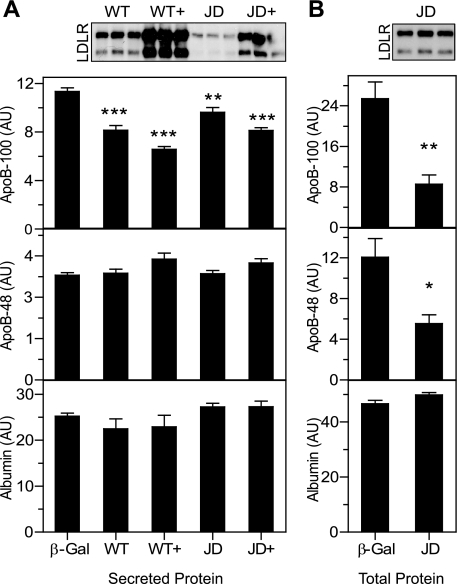
We hypothesized that the JD LDLR would be at least partially defective in regulating apoB secretion due to its defect in endocytosis. To test this hypothesis, we compared the secretion of apoB among Ldlr-/- hepatocytes expressing various levels of WT LDLR, JD LDLR, and β-Gal. Surprisingly, both the WT and the JD LDLR induced dose-dependent reductions in apoB-100 secretion (Fig. 4A). Importantly, similar levels of LDLR expression of the variants (Fig. 4A, inset, WT versus JD+) led to comparable reductions in apoB100 secretion. To verify that the JD LDLR is functional in promoting degradation of apoB, we performed a pulse-chase analysis. As with the WT LDLR (Fig. 2, A and B), cells expressing the JD LDLR showed a reduction in the recovery of total apoB-100 and apoB-48 (Fig. 4B). These data reveal that despite the defect in constitutive endocytic activity, the JD LDLR is effective in regulating apoB secretion.
FIGURE 4.
The JD LDLR is not defective in regulating apoB secretion. A, Ldlr-/- primary hepatocytes were infected at a dose of either 3 × 108 or 6 × 108 pfu/dish with Ad-β-Gal, Ad-LDLR-WT (WT, WT+), or Ad-LDLR-JD (JD, JD+). Cells were radiolabeled with [35S]Met/Cys for 4 h. Secreted levels of apoB-100, apoB-48, and albumin were determined and normalized to cell protein. The two Ad-β-Gal infection conditions were averaged (shown as β-Gal) for comparison with the varying Ad-LDLR infection conditions. n = 5 for each condition. Similar results were obtained from three independent experiments. B, Ldlr-/- primary hepatocytes were infected with Ad-β-Gal (β-Gal) or Ad-LDLR-JD (JD) at a titer of 4 × 108 pfu/dish (n = 6). Cells were labeled with [35S]Met/Cys for 1 h and chased for 4 h in cold medium. Total levels of apoB-100, apoB-48, and albumin were calculated as the sum of protein-adjusted cellular and secreted levels. Insets show Western blots for the LDLR with 23 μg (A) and 12 μg (B) of cellular protein lysate. *, p < 0.05, **, p < 0.01, ***, p < 0.001 versus Ad-β-Gal, Fisher's protected LSD. AU, arbitrary units.
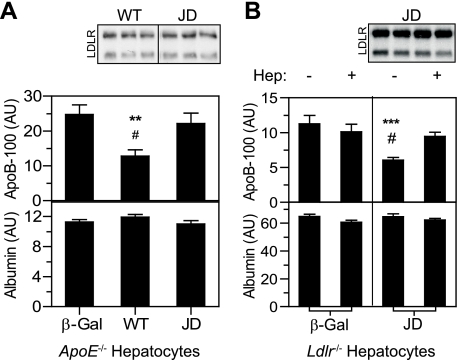
apoE Mediates the JD LDLR-dependent Reduction in apoB Secretion—The ability of the JD LDLR to regulate apoB secretion suggests that the process is independent of endocytosis. However, recently published data provide another potential explanation. ARH is an LDLR-specific clathrin adaptor that binds to the LDLR at the FDNPVY peptide sequence to mediate its endocytosis (32–34). The Y807C substitution in the JD LDLR disrupts this interaction (32), which accounts for its loss in constitutive endocytic activity in hepatocytes. However, recent studies show that despite a defect in the internalization of apoB-containing lipoproteins, Arh-/- hepatocytes and JD LDLR fibroblasts both maintain the ability to internalize apoE (17, 18). We therefore hypothesized that the JD LDLR may regulate apoB secretion through reuptake of VLDL via apoE. To test this hypothesis, we compared the secretion of apoB from apoE-/- hepatocytes ectopically expressing the WT or JD LDLR. No receptor-dependent effects were observed with apoB-48 (not shown). However, expression of the WT LDLR lowered apoB-100 secretion (Fig. 5A), suggesting that at least a portion of apoB degradation occurs through a direct interaction with the LDLR. By contrast, expression of the JD LDLR was completely ineffective in reducing apoB-100 secretion (Fig. 5A). These data indicate that the ability of the JD LDLR to reduce apoB secretion from hepatocytes with an intact apoE gene results from binding to apoE on the VLDL particle.
FIGURE 5.
The JD LDLR mediates LDLR-dependent regulation of apoB secretion through reuptake via apoE. A, apoE-/- primary hepatocytes were titrated with Ad-β-Gal (β-Gal), Ad-LDLR-WT (WT), or Ad-LDLR-JD (JD) at a titer of 3 × 108-12 × 108 pfu/dish. Secreted levels of apoB-100, apoB-48, and albumin were determined and normalized to cell protein. Comparisons shown were from conditions showing similar levels of WT LDLR and JD LDLR expression. n = 6 for each condition. Insets show two sections of a single Western blot for the LDLR with 13 μg of cellular protein lysate. Similar results were obtained from three independent experiments. **, p < 0.01 versus β-Gal; #, p < 0.05 versus JD LDLR, Fisher's protected LSD. AU, arbitrary units. B, Ldlr-/- primary hepatocytes infected with Ad-LDLR-JD or Ad-β-Gal (12 × 108 pfu/cell) were radiolabeled with [35S]Met/Cys for 4 h in the absence or presence of 6 mg/ml heparin. Secreted levels of apoB-100, apoB-48, and albumin were determined and normalized to cell protein. n = 9 for each condition. Similar results were obtained from three independent experiments. The inset shows a Western blot for the LDLR with 7 μg of protein lysate from cells incubated without and with heparin. ***, p < 0.001 versus Ad-β-Gal; #, p < 0.05 versus heparin, Fisher's protected LSD. For both A and B, no LDLR-dependent reductions in secreted apoB-48 were observed.
The previous experiment suggests that apoE is the ligand responsible for the ability of the JD LDLR to regulate apoB secretion. To determine whether this regulation occurs through lipoprotein reuptake, as our hypothesis predicts, we tested the effect of heparin on the ability of the JD LDLR to regulate apoB secretion. Heparin binds apoB and apoE in the extracellular space and disrupts their interaction with the LDLR (35). Thus, heparin inhibits any LDLR activity occurring at the cell surface (12). Treatment with heparin had no effect in β-Gal-expressing cells but rescued a pool of apoB in JD LDLR-expressing cells (Fig. 5B). This confirms that the JD LDLR is indeed capable of regulating apoB secretion through selective internalization of apoE.
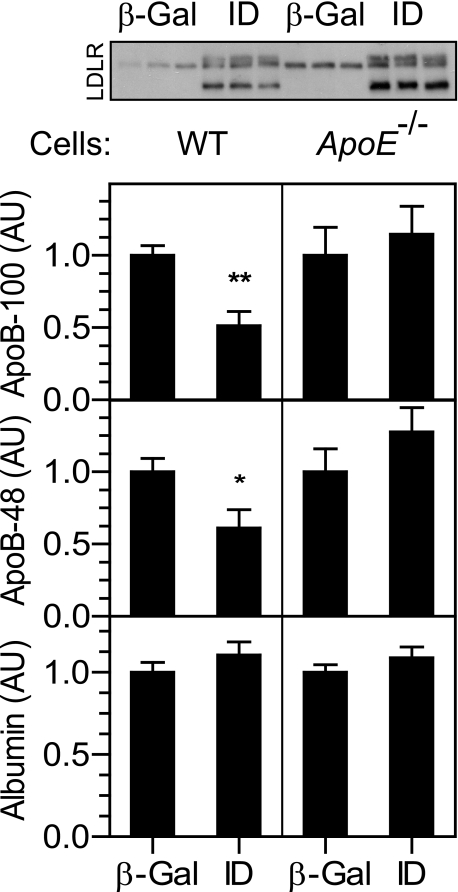
To determine whether the apoE-dependent internalization of apoB observed with the JD LDLR occurs with normal endocytic trafficking or results from a loss in the constitutive endocytic pathway, we employed an LDLR mutant (I140D; “ID” LDLR) that is defective in binding apoB but binds and internalizes apoE (36). Expression of this construct reduced apoB secretion in wild-type hepatocytes but failed to do so in apoE-/- hepatocytes (Fig. 6). This indicates that the LDLR-mediated internalization of apoE occurs under normal endocytic trafficking and is not a result of a defect in the constitutive endocytic pathway.
FIGURE 6.
An LDLR mutant defective in binding apoB reduces apoB secretion in an apoE-dependent manner. Wild-type (left panels) and apoE-/- (right panels) hepatocytes were infected with Ad-β-Gal (β-Gal) or Ad-LDLR-ID (ID) at a titer of 3 × 108 pfu/dish. Cells were radiolabeled with [35S]Met/Cys for 4 h. Secreted levels of apoB-100, apoB-48, and albumin were determined and normalized to cell protein. n = 5 for each condition. Similar results were obtained from at least two independent experiments. The inset shows a Western blot for the LDLR with 7 μg of protein lysate from wild-type (left) and apoE-/- (right) cells. *, p < 0.05, **, p < 0.01 versus Ad-β-Gal, Student's t test. AU, arbitrary units.
DISCUSSION
In this investigation, we studied mechanisms by which the LDLR regulates the secretion of apoB-containing lipoproteins. We investigated two possible sites of LDLR activity: in the ER, where the nascent VLDL particle is assembled, and at the plasma membrane, where VLDL particle reuptake occurs. We provided evidence that the LDLR does not induce the degradation of apoB within the ER (Fig. 1) and that anterograde transport from the ER is required for LDLR activity (Fig. 2). In investigating mechanisms downstream of the ER, we verified that the LDLR indeed controls apoB secretion through reuptake of nascent VLDL and showed that both apoB and apoE participate in this process (Figs. 5 and 6). In so doing, we revealed that the LDLR regulates apoB secretion through a non-canonical endocytic mechanism via internalization of apoE (Figs. 3, 4, 5).
Much of the post-translational apoB degradation that controls VLDL secretion occurs in the ER during the early steps of VLDL assembly (1, 2). Our previous study showed that expression of two independent ER-retained LDLR mutants induced apoB degradation (13), suggesting that the LDLR was intervening at this early step. In the current study, we took advantage of a mouse mutant with an endogenous mutation in the LDLR that results in its retention in the ER. Mice with this mutation have hypercholesterolemia and develop atherosclerosis, cholesterol gallstones, and skin and brain xanthomas when fed an atherogenic diet,4 thus mimicking humans with “class 2” FH mutations (5). This mouse therefore provided a physiological model to study the effects of an LDLR retained in the ER on apoB secretion. In contrast to our previous results, the endogenously expressed, ER-retained (C678Y) LDLR mutant failed to reduce apoB secretion (Fig. 1). Additionally, the transient blockage of apoB and LDLR transport from the ER with BFA prevented the ability of the LDLR to induce apoB degradation (Fig. 2). Together, these results suggest that the LDLR controls VLDL secretion in post-ER compartments. This conclusion is supported by the fact that very little of the ER pool of the LDLR (i.e. the 100-kDa form) is visible at steady state with endogenous expression (Fig. 1A).
Since the LDLR likely exerts its effects in post-ER compartments, it appears that LDLR-dependent degradation occurs after completion of assembly and lipidation of the VLDL particle. As mentioned previously, much evidence indicates that the LDLR preferentially inhibits secretion of small, poorly lipidated particles (6, 10, 11, 14). The downstream LDLR activity may therefore function as a quality control “filter” to prevent the secretion of such particles.
We believe that the discrepancy between our previous and most recent data concerning the ER-retained mutants lies in the expression level of the LDLR. The cDNA constructs in the original study were driven by the cytomegalovirus promoter in an unregulated manner. We suspect that the high expression of the ER-retained proteins may have stimulated degradation of apoB in a compartment where it does not occur under physiological conditions. By contrast, the tetracycline-induced adenoviral system employed in this study allowed for relatively low expression levels in uninduced conditions (Fig. 6 and supplemental Fig. S1A) with high infection efficiency (supplemental Fig. S1B). Two lines of evidence suggest that the LDLR-induced reduction in apoB secretion observed in this study, particularly that observed with the JD LDLR, did not artifactually result from viral overexpression. First, viral expression of the LDLR did not induce degradation of apoB in the ER (Fig. 2), where apoB would be most susceptible to perturbations resulting from overexpression. Second, the inability of the JD LDLR to reduce apoB secretion in apoE-/- hepatocytes relative to the WT LDLR (Fig. 5A) resulted strictly from a change in the LDLR variant rather than its expression level.
Although unanticipated, the ability of the JD LDLR to regulate apoB secretion through reuptake of apoE supports recent data characterizing a novel, non-canonical endocytic mechanism employed by the LDLR. Unlike many signaling receptors, which internalize upon ligand binding (37), the LDLR was traditionally thought to primarily undergo constitutive endocytosis (38–40). However, recent work suggests that two independent mechanisms drive endocytosis of the LDLR, a constitutive pathway mediated by binding of the clathrin adaptor protein, ARH, to the FDNPVY sequence and an FDNPVY-independent, ligand-regulated pathway (17, 18). Importantly, binding of apoE, but not apoB, induces the ligand-regulated pathway. Thus, disruption of FDNPVY-mediated endocytosis, either through loss of ARH or through the Y807C mutation in the JD LDLR mutant, confers a defect in constitutive endocytosis and internalization of apoB (30, 31, 34). This results in hypercholesterolemia in both humans (31) and mice (34), due to a defect in LDL clearance. However, a loss of the FDNPVY-dependent pathway still allows for the selective internalization of apoE-enriched lipoproteins, such as VLDL remnants (17, 18). The current study supports these findings; the ability of the JD LDLR to reduce apoB secretion through reuptake was dependent on the expression of apoE (Figs. 4 and 5), suggesting that the JD LDLR operates through the ligand-dependent endocytic pathway. This result uncovers a physiological function of this novel pathway. In addition, it predicts that the overproduction of VLDL, as occurs with individuals with many forms of FH (6–8), would not occur in individuals with the Y807C mutation and perhaps other mutations disrupting endocytosis.
This is the first study to directly implicate apoE as a ligand that mediates the effect of the LDLR on apoB secretion. Our previous studies have shown an LDLR-dependent lowering of apoB-48 secretion in addition to apoB-100 secretion (12, 13). Since apoB-48 lacks the LDLR binding site, which resides in the C terminus of the protein (41), the previous data indirectly implicated apoE in mediating the LDLR effect on VLDL secretion. By contrast, the apoE-dependent ability of the JD LDLR and the ID LDLR to reduce apoB secretion (Figs. 5 and 6) directly implicates apoE in the process. In the current study, we also observed LDLR-dependent reductions in apoB-48 secretion (Fig. 1C), although the effect was inconsistent in our viral expression experiments. A severe shift in the relative production of apoB-100 and apoB-48 occurs between 20 and 40 h after isolation, such that by the latter time point, apoB-100 constitutes ∼70–75% of the total apoB secreted (compare Fig. 1C with Fig. 4A). As our viral infection protocol requires such a time period to allow for protein expression, we attribute these inconsistent results with apo-B48 secretion to the limiting amount of substrate for the LDLR.
The participation of apoE in the LDLR-dependent regulation of VLDL secretion introduces a new role of apoE in lipoprotein assembly and secretion. It is well established that expression levels of apoE positively correlate with the secretion of VLDL particles (42–44), a phenomenon that is independent of the LDLR (11). By contrast, this study reveals that apoE is required for a reduction in VLDL secretion in a process that is dependent on the LDLR. The apparent paradox is resolved by the fact that expression of apoE appears to facilitate the lipoprotein assembly process within the ER (45), whereas LDLR activity occurs after exit from the ER. This indicates that apoE operates in two independent but opposing mechanisms to regulate in the assembly and secretion of VLDL. Since apoE-/- mice secrete less VLDL than wild-type mice (44, 46), its role in promoting lipoprotein assembly appears to be dominant, perhaps because of its priority in the sequence of events.
Interestingly, several of the conclusions reached in this investigation support a recent study of VLDL secretion in human FH patients (8). A comparison between FH patients with a varied assortment of LDLR defects and controls showed only a trend toward an increase in VLDL secretion in the FH individuals. However, removal of a subset of the FH cases from the comparison revealed a significant increase in VLDL secretion in the FH patients. Notably, among the cases removed from the comparison were a mutant that is defective in binding apoB but still binds apoE (S156L) and a mutant that reaches the cell surface but does not recycle after internalization (E387K). Cases included in the comparison were truncated, ER-retained mutants (C660X, where X indicates a nonsense mutation). These data support a lack of LDLR activity in the ER as well as the internalization of apoE in the regulation of apoB secretion by the LDLR.
In addition to the reuptake pathway characterized in this study, several lines of evidence indicate that the LDLR also operates through an intracellular mechanism (12, 14, 15). Our initial study of the regulation of apoB secretion estimated that approximately half of LDLR activity occurs through a reuptake mechanism and half occurs through an intracellular mechanism (12). We believe that the LDLR may act intracellularly in a manner analogous to the cation-independent mannose-6-phosphate receptor. The cation-independent mannose-6-phosphate receptor binds lysosomal hydrolases in the late Golgi and can bypass the plasma membrane to transport them directly to late endosomes (47). It is possible that the LDLR diverts nascent VLDL from the late secretory pathway to the endocytic pathway in the same manner. Indeed, several lines of evidence suggest that such a direct route may exist for the LDLR. For example, PCSK9 is a secretory protein that binds to the LDLR and induces its degradation in lysosomes (48–50). The addition of PCSK9-conditioned medium in trans is sufficient for degradation of the LDLR in wild-type but not Arh-/- hepatocytes (25), indicating a mechanism dependent on endocytosis. However, PCSK9 induces LDLR degradation in Arh-/- hepatocytes when expressed in cis (49), suggesting that the LDLR can also directly access endocytic and lysosomal compartments from the secretory pathway. Indeed, we have observed a pool of LDLR in the Golgi at steady state that co-localizes with PCSK9 (22), which may represent a rate-limiting transport step in this pathway.
Data from this study and others cumulatively support a model in which synthesis of apoB and lipidation of the nascent VLDL particle occur in the ER. After exit from the ER, the VLDL particle is susceptible to binding the LDLR via apoB or apoE, either within the cell or at the cell surface. This is followed by a targeting of the particle to the endocytic pathway for degradation. LDLR activity in this model thus serves as a quality control mechanism that operates late in the secretory and endocytic pathways, likely to inhibit the secretion of small, underlipidated VLDL particles.
Acknowledgments
We thank Karen L. Svenson for providing the HLB301 mice, Wolfgang Hillen for providing the reverse tetracycline-controlled transactivator and tet operator vectors, Roger Davis and Mark Keller for critical readings of the manuscript, Kathryn L. Schueler for breeding and maintaining the mice used in this study, and Brian Yandell for statistical advice.
This project was funded by NHLBI, National Institutes of Health Grant HL56593. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains a supplemental figure.
Footnotes
The abbreviations used are: apoB, apolipoprotein B; apoE, apolipoprotein E; ARH, autosomal hypercholesterolemia; LDL, low density lipoprotein; VLDL, very low density lipoprotein; LDLR, LDL receptor; ER, endoplasmic reticulum; β-Gal, β-galactosidase; BFA, brefeldin A; FH, familial hypercholesterolemia; LSD, least significant difference; WT, wild type; DMEM, Dulbecco's modified Eagle's medium; Ad, adenoviral; pfu, plaque-forming unit.
The Jackson Laboratory Stock Number 005061.
References
- 1.Davis, R. A. (1999) Biochim. Biophys. Acta 1440 1-31 [DOI] [PubMed] [Google Scholar]
- 2.Fisher, E. A., and Ginsberg, H. N. (2002) J. Biol. Chem. 277 17377-17380 [DOI] [PubMed] [Google Scholar]
- 3.Blasiole, D. A., Davis, R. A., and Attie, A. D. (2007) Mol. Biosyst. 3 608-619 [DOI] [PubMed] [Google Scholar]
- 4.Borchardt, R. A., and Davis, R. A. (1987) J. Biol. Chem. 262 16394-16402 [PubMed] [Google Scholar]
- 5.Brown, M. S., and Goldstein, J. L. (1986) Science 232 34-47 [DOI] [PubMed] [Google Scholar]
- 6.James, R. W., Martin, B., Pometta, D., Fruchart, J. C., Duriez, P., Puchois, P., Farriaux, J. P., Tacquet, A., Demant, T., Clegg, R. J., Munro, A., Oliver, M. F., Packard, C. J., and Shepherd, J. (1989) J. Lipid Res. 30 159-169 [PubMed] [Google Scholar]
- 7.Tremblay, A. J., Lamarche, B., Ruel, I. L., Hogue, J. C., Bergeron, J., Gagne, C., and Couture, P. (2004) J. Lipid Res. 45 866-872 [DOI] [PubMed] [Google Scholar]
- 8.Millar, J. S., Maugeais, C., Ikewaki, K., Kolansky, D. M., Barrett, P. H., Budreck, E. C., Boston, R. C., Tada, N., Mochizuki, S., Defesche, J. C., Wilson, J. M., and Rader, D. J. (2005) Arterioscler. Thromb. Vasc. Biol. 25 560-565 [DOI] [PubMed] [Google Scholar]
- 9.Horton, J. D., Shimano, H., Hamilton, R. L., Brown, M. S., and Goldstein, J. L. (1999) J. Clin. Investig. 103 1067-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassir, F., Xie, Y., Patterson, B. W., Luo, J., and Davidson, N. O. (2004) J. Lipid Res. 45 1649-1659 [DOI] [PubMed] [Google Scholar]
- 11.Teusink, B., Mensenkamp, A. R., van der Boom, H., Kuipers, F., van Dijk, K. W., and Havekes, L. M. (2001) J. Biol. Chem. 276 40693-40697 [DOI] [PubMed] [Google Scholar]
- 12.Twisk, J., Gillian-Daniel, D. L., Tebon, A., Wang, L., Barrett, P. H., and Attie, A. D. (2000) J. Clin. Investig. 105 521-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillian-Daniel, D. L., Bates, P. W., Tebon, A., and Attie, A. D. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 4337-4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson, S. L., Skogsberg, J., and Bjorkegren, J. (2004) J. Biol. Chem. 279 831-836 [DOI] [PubMed] [Google Scholar]
- 15.Jiang, X. C., Qin, S., Qiao, C., Kawano, K., Lin, M., Skold, A., Xiao, X., and Tall, A. R. (2001) Nat. Med. 7 847-852 [DOI] [PubMed] [Google Scholar]
- 16.Williams, K. J., Brocia, R. W., and Fisher, E. A. (1990) J. Biol. Chem. 265 16741-16744 [PubMed] [Google Scholar]
- 17.Jones, C., Garuti, R., Michaely, P., Li, W. P., Maeda, N., Cohen, J. C., Herz, J., and Hobbs, H. H. (2007) J. Clin. Investig. 117 165-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaely, P., Zhao, Z., Li, W. P., Garuti, R., Huang, L. J., Hobbs, H. H., and Cohen, J. C. (2007) EMBO J. 26 3273-3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urlinger, S., Baron, U., Thellmann, M., Hasan, M. T., Bujard, H., and Hillen, W. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 7963-7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gossen, M., and Bujard, H. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 5547-5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dirlam, K. A., Gretch, D. G., LaCount, D. J., Sturley, S. L., and Attie, A. D. (1996) Protein Expression Purif. 8 489-500 [DOI] [PubMed] [Google Scholar]
- 22.Nassoury, N., Blasiole, D. A., Tebon Oler, A., Benjannet, S., Hamelin, J., Poupon, V., McPherson, P. S., Attie, A. D., Prat, A., and Seidah, N. G. (2007) Traffic 8 718-732 [DOI] [PubMed] [Google Scholar]
- 23.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265-275 [PubMed] [Google Scholar]
- 24.Svenson, K. L., Von Smith, R., Magnani, P. A., Suetin, H. R., Paigen, B., Naggert, J. K., Li, R., Churchill, G. A., and Peters, L. L. (2007) J. Appl. Physiol. 102 2369-2378 [DOI] [PubMed] [Google Scholar]
- 25.Lagace, T. A., Curtis, D. E., Garuti, R., McNutt, M. C., Park, S. W., Prather, H. B., Anderson, N. N., Ho, Y. K., Hammer, R. E., and Horton, J. D. (2006) J. Clin. Investig. 116 2995-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings, R. D., Kornfeld, S., Schneider, W. J., Hobgood, K. K., Tolleshaug, H., Brown, M. S., and Goldstein, J. L. (1983) J. Biol. Chem. 258 15261-15273 [PubMed] [Google Scholar]
- 27.Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S., and Klausner, R. D. (1989) Cell 56 801-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato, R., Imanaka, T., Takatsuki, A., and Takano, T. (1990) J. Biol. Chem. 265 11880-11884 [PubMed] [Google Scholar]
- 29.Wu, X., Sakata, N., Lele, K. M., Zhou, M., Jiang, H., and Ginsberg, H. N. (1997) J. Biol. Chem. 272 11575-11580 [DOI] [PubMed] [Google Scholar]
- 30.Davis, C. G., Lehrman, M. A., Russell, D. W., Anderson, R. G., Brown, M. S., and Goldstein, J. L. (1986) Cell 45 15-24 [DOI] [PubMed] [Google Scholar]
- 31.Brown, M. S., and Goldstein, J. L. (1976) Cell 9 663-674 [DOI] [PubMed] [Google Scholar]
- 32.He, G., Gupta, S., Yi, M., Michaely, P., Hobbs, H. H., and Cohen, J. C. (2002) J. Biol. Chem. 277 44044-44049 [DOI] [PubMed] [Google Scholar]
- 33.Mishra, S. K., Watkins, S. C., and Traub, L. M. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 16099-16104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, C., Hammer, R. E., Li, W. P., Cohen, J. C., Hobbs, H. H., and Herz, J. (2003) J. Biol. Chem. 278 29024-29030 [DOI] [PubMed] [Google Scholar]
- 35.Goldstein, J. L., Basu, S. K., Brunschede, G. Y., and Brown, M. S. (1976) Cell 7 85-95 [DOI] [PubMed] [Google Scholar]
- 36.Russell, D. W., Brown, M. S., and Goldstein, J. L. (1989) J. Biol. Chem. 264 21682-21688 [PubMed] [Google Scholar]
- 37.Hopkins, C. R., Miller, K., and Beardmore, J. M. (1985) J. Cell Sci. 3 173-186 [DOI] [PubMed] [Google Scholar]
- 38.Anderson, R. G., Brown, M. S., Beisiegel, U., and Goldstein, J. L. (1982) J. Cell Biol. 93 523-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu, S. K., Goldstein, J. L., Anderson, R. G., and Brown, M. S. (1981) Cell 24 493-502 [DOI] [PubMed] [Google Scholar]
- 40.Goldstein, J. L., Brown, M. S., Anderson, R. G., Russell, D. W., and Schneider, W. J. (1985) Annu. Rev. Cell Biol. 1 1-39 [DOI] [PubMed] [Google Scholar]
- 41.Boren, J., Lee, I., Zhu, W., Arnold, K., Taylor, S., and Innerarity, T. L. (1998) J. Clin. Investig. 101 1084-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang, Y., Ji, Z. S., Brecht, W. J., Rall, S. C., Jr., Taylor, J. M., and Mahley, R. W. (1999) Arterioscler. Thromb. Vasc. Biol. 19 2952-2959 [DOI] [PubMed] [Google Scholar]
- 43.Mensenkamp, A. R., Teusink, B., Baller, J. F., Wolters, H., Havinga, R., van Dijk, K. W., Havekes, L. M., and Kuipers, F. (2001) Arterioscler. Thromb. Vasc. Biol. 21 1366-1372 [DOI] [PubMed] [Google Scholar]
- 44.Maugeais, C., Tietge, U. J., Tsukamoto, K., Glick, J. M., and Rader, D. J. (2000) J. Lipid Res. 41 1673-1679 [PubMed] [Google Scholar]
- 45.Mensenkamp, A. R., Van Luyn, M. J., Havinga, R., Teusink, B., Waterman, I. J., Mann, C. J., Elzinga, B. M., Verkade, H. J., Zammit, V. A., Havekes, L. M., Shoulders, C. C., and Kuipers, F. (2004) J. Hepatol. 40 599-606 [DOI] [PubMed] [Google Scholar]
- 46.Kuipers, F., Jong, M. C., Lin, Y., Eck, M., Havinga, R., Bloks, V., Verkade, H. J., Hofker, M. H., Moshage, H., Berkel, T. J., Vonk, R. J., and Havekes, L. M. (1997) J. Clin. Investig. 100 2915-2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maxfield, F. R., and McGraw, T. E. (2004) Nat. Rev. 5 121-132 [DOI] [PubMed] [Google Scholar]
- 48.Benjannet, S., Rhainds, D., Essalmani, R., Mayne, J., Wickham, L., Jin, W., Asselin, M. C., Hamelin, J., Varret, M., Allard, D., Trillard, M., Abifadel, M., Tebon, A., Attie, A. D., Rader, D. J., Boileau, C., Brissette, L., Chretien, M., Prat, A., and Seidah, N. G. (2004) J. Biol. Chem. 279 48865-48875 [DOI] [PubMed] [Google Scholar]
- 49.Park, S. W., Moon, Y. A., and Horton, J. D. (2004) J. Biol. Chem. 279 50630-50638 [DOI] [PubMed] [Google Scholar]
- 50.Maxwell, K. N., Fisher, E. A., and Breslow, J. L. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2069-2074 [DOI] [PMC free article] [PubMed] [Google Scholar]