Abstract
Met30 is the substrate recognition subunit of the essential ubiquitin ligase SCFMet30. The essential function of Met30 is the inactivation of the Saccharomyces cerevisiae transcription factor Met4, because fully activated Met4 induces a cell cycle arrest. Met4 regulates expression of genes involved in the sulfur assimilation pathway and coordinates the transcriptional program and cell cycle progression in response to cadmium and arsenic stress. Met4 lacks DNA binding activity and requires either Cbf1 or one of the two homologous proteins Met31 and Met32 for promoter association. Accordingly, met4 mutants, cbf1 mutants, and met31 met32 double mutants are methionine auxotroph. We isolated a truncated version of Met32 (Met32Δ145-192) as a dominant suppressor of the cell cycle defect of met30 mutants. Expression of Met32Δ145-192 significantly reduced induction of Met4-regulated genes. Interestingly, both Cbf1- and Met31/32-dependent genes were affected by Met32Δ145-192. Mechanistically, Met32Δ145-192 prevented recruitment of Met4 to both Cbf1 and Met31/32-dependent promoters. We further demonstrated that Met32 is part of the Cbf1-Met4 complex bound to Cbf1-recruiting promoter elements and that Met31/32 are required for formation of a stable Met4-Cbf1 transcription complex. These results suggest a regulatory role of Met32 as part of the Cbf1-Met4 complex and provide molecular insight into coordination of cell cycle response and modulation of gene expression programs.
The cellular response to changing environmental conditions is frequently orchestrated by induction of specific transcription programs that affect multiple pathways. For example, nutrient availability or stress situations often require coordinated modulation of metabolic pathways, induction of protective measures, and a response of the cell division cycle. One well studied regulatory network is the budding yeast sulfur amino acid synthesis pathway (1). Central to this pathway is the regulation of transcription factor complexes containing the transactivating factor Met4 (2). Met4 regulation is also critical for the cellular response to cadmium and arsenic stress (3-7). Active Met4 induces expression of a group of genes, commonly referred to as MET genes, that are involved in sulfur assimilation and synthesis of sulfur-containing amino acids (1). In addition, Met4 promotes synthesis of the tripeptide glutathione for detoxification under cadmium and arsenic stress conditions by inducing GSH1 expression, a gene that encodes for γ-glutamyl cysteine synthase, the rate-limiting enzyme in glutathione synthesis (4-6, 8). Met4 regulation links these metabolic responses to regulation of cell proliferation, because activation of Met4 can induce a complex cell cycle arrest that involves down-regulation of G1 and S phase cyclin expression, destabilization of pre-replication complexes, a block of metaphase to anaphase transition, and reduction in translation (3, 5, 9, 10).
Met4 is a basic leucine zipper protein that can associate with at least four other transcription factors, the basic helix-loop-helix protein Cbf1, the basic leucine zipper protein Met28, and the two homologous zinc finger factors Met31 and Met32 (1, 2, 11-13). Met4 is the sole factor with transactivating activity in these complexes but depends on the DNA binding activity of Cbf1 and Met31/32 for promoter recruitment. The basic leucine zipper protein Met28 does not directly bind DNA but has been shown to enhance promoter binding of the Cbf1-Met4 complex by an unknown mechanism (11). Cbf1 recognizes the sequence TCACGTG, which is also present at centromeres (CDE1 element) where Cbf1 is important for high fidelity chromosome segregation (13, 14). The cis-element for Met31 and Met32 binding was defined as AAACTGTG (12). Although some MET genes contain only one type of cis-element, frequently both binding elements are found in the promoter regions of Met4-controlled genes (2). MET gene expression is therefore thought to be coordinated by two types of Met4-containing transcription complexes, namely a Cbf1-Met28-Met4 and a Met31/Met32-Met28-Met4 complex, which are tethered to the two promoter elements by Cbf1 and Met31/Met32, respectively (2). Activation of these transcription complexes occurs under conditions where the sulfur-containing compounds cysteine, methionine, or S-adenosylmethionine are limiting (1, 15). In addition, cadmium and arsenic stress will lead to Met4 activation (5-8). In response to these nutritional and heavy metal stress conditions, Met4-dependent programs coordinate modulation of metabolic and detoxification pathways with cell cycle arrest (3). Interestingly, although all of the Met4-associated proteins are required for methionine biosynthesis, some show pathway-specific roles. For example, Met28 appears to be largely dispensable for the response to cadmium stress, because in contrast to met4, cbf1, and met31 met32 double mutants, met28 mutants are not cadmium-sensitive (5, 6). Even more surprising, despite the overlapping function of Met31 and Met32 in sulfur amino acid synthesis and cadmium detoxification (5, 6, 12), Met32 is the primary mediator of cell cycle arrest, because only deletion of MET32, and not deletion of MET31, can suppress the cell division block that is induced by fully activated Met4 (10).
Because fully activated Met4 inhibits cell division, cells need to repress Met4 activity to multiply, and components involved in Met4 inactivation are thus essential. Met4 is inactivated by ubiquitination (16, 17). The ubiquitin ligase SCFMet30 together with the ubiquitin-conjugating enzyme Cdc34 catalyze polyubiquitination of Met4 at a single, invariant lysine residue (18). Remarkably, polyubiquitination can inactivate Met4 without inducing its degradation by the 26 S proteasome, because a ubiquitin-interacting motif present in Met4 effectively protects polyubiquitinated Met4 from recognition by the 26 S proteasome (19). This proteolysis-independent inactivation by ubiquitination allows for a rapid response to environmental conditions, because the existing pool of inactive Met4 can be rapidly activated by deubiquitination without the need for Met4 transcription and translation (19). It is important to mention that under certain growth conditions, which remain to be defined but appear to be related to cell growth phase, ubiquitinated Met4 can be degraded by the 26 S proteasome for inactivation (15, 18, 20, 21). In either case, the ubiquitin ligase SCFMet30 is the key regulator of Met4 activity and cell cycle arrest. SCF-type ubiquitin ligases are multisubunit complexes with usually a single subunit directing substrate-specific ubiquitination (22). The F-box protein Met30 is the substrate specificity factor in SCFMet30 and links this ubiquitin ligase to Met4 regulation (16, 17). Consistent with its role in Met4 inactivation, deletion of MET30 is lethal (23), but lethality can be suppressed by deletion of MET4 or MET32 (10, 17). Met30 coordinates cell proliferation with availability of sulfur-containing compounds or cadmium and arsenic stress and is thus a key regulator important for maintenance of cellular integrity during nutritional and heavy metal stress conditions (3).
Here we report the results of a genetic approach to identify regulators of the Met30-controlled events and describe the characterization of a dominant allele of MET32. The results demonstrate that the Met4 transcription complex contains both DNA binding factors Met31/32 and Cbf1 and that Met31/32 are required for formation of a stable Met4-Cbf1 transcription complex. Together, these findings provide molecular insight into coordination of cell cycle regulation and transcriptional responses to environmental changes.
EXPERIMENTAL PROCEDURES
Yeast Strains, Growth Conditions, and Genetic Selection—All strains used in this study are isogenic to 15DaubΔ, a bar1Δ ura3Δns, a derivative of BF264-15D (24). Yeast strains were grown at 30 °C (unless otherwise stated) in standard culture media, and standard yeast genetic methods were used (25). Synthetic complete medium (SC)3 was prepared as described (18).
Genetic Selection of met30-6 Suppressor Mutants—A temperature-sensitive met30-6 mutant containing a GAL1-RGS6H-MET4 allele integrated at the LEU2 locus was used as the screening strain. Approximately 3 × 108 cells were plated on YEPD agar plates and irradiated with a half-lethal dose of UV light (254 nm, 30 J/m2). Plates were incubated at 25 °C for 1 day and then shifted to 35 °C for 2 days. Plates were then replicaplated to YEP-galactose plates to induce expression of the GAL1-RGS6H-MET4 allele to eliminate suppressor mutants caused by loss of function of the endogenous MET4. Plates were incubated for an additional 2 days at 35 °C, and the remaining suppressor mutants were retested and then analyzed for Met4 ubiquitination as described in the legend to Fig. 1A.
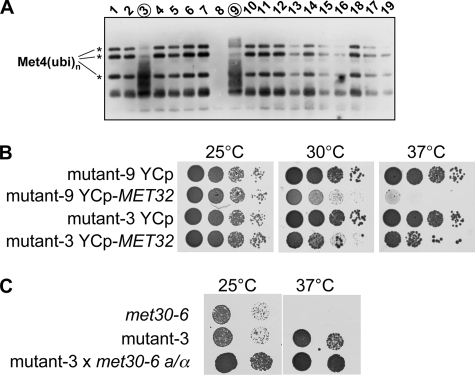
FIGURE 1.
Suppressors of met30 mutants. A, analysis of mutants that allowed suppression of the growth defect of temperature-sensitive met30-6 cells. Cells containing a GAL1-RGS6H-MET4 allele were grown in YEP-Gal medium to induce expression of the RGS6H-tagged Met4 first at 23 °C and then shifted to 37 °C for 2 h to inactivate Met30-6 function. The Met4 ubiquitination status was analyzed by immunoblotting using antibodies to the RGS6H epitope. Ubiquitinated forms of Met4 are indicated by asterisks. B, mutant-9 and -3 were transformed with a centromeric plasmid (URA3 marker) containing the MET32 gene (YCp-MET32) or the plasmid backbone (YCp) as a control. Cells were grown in dextrose-containing media lacking uracil to select for the plasmids, and serial dilutions were spotted on dextrose-containing synthetic medium plates lacking uracil (SC-Ura). Plates were incubated at the temperatures indicated. C, mutant-3 was mated with met30-6 cells to form a diploid homozygous for the met30-6 allele but heterozygous for the mutation causing suppression. Two dilutions of the cell suspensions indicated were spotted on YEPD plates, and plates were incubated at 25 and 37 °C. Growth of the diploid (mut-3 × met30-6) indicated the dominant character of the identified suppressor mutation.
Cell Spotting—Cells were collected at midlog phase, diluted to the same optical density (based on A600 reading) before spotting. 10-Fold serial dilutions starting with ∼5000 cells (assuming 1 A600 = 107 cells/ml) using sterile H2O were spotted onto agar plates.
Protein Analyses—Total cell lysates for immunoblot analysis were prepared under denaturing conditions as described (19). For protein complex purification experiments, between 40 and 60 A600 units of midlog phase cells were collected and lysed as reported (19). For immunoprecipitation with anti-Met4 or anti-Myc antibodies (SC-789-G, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), cell lysates were mixed with 2 μl of polyclonal antibody at 4 °C for at least 3 h. Protein G beads were incubated with 1 mg/ml bovine serum albumin before adding to the IP mixtures. The IP mixtures with resins were rotated at 4 °C for 3 h (or overnight). Immunocomplexes were washed three times with 1 ml of lysis buffer, and proteins were eluted by boiling in SDS-PAGE loading buffer. Purification of TAP-tagged proteins was similar, but lysates were incubated with IgG-Sepharose (Amersham Biosciences), washed, and eluted as above. RGS6H-tagged proteins were purified on Ni2+-Sepharose (Amersham Biosciences). Purified protein complexes were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and analyzed by immunoblotting. Antibodies were used at the following dilutions: anti-Met4 (1:10,000; gift from M. Tyers), anti-HA and anti-Myc (1:2000; Covance, Princeton, NJ), anti-RGS4H (1:2000; Qiagen, Germantown, MD). Peroxidase-conjugated anti-peroxidase (1:1000; Pierce).
RNA Analyses—RNA was isolated using the RNeasy kit (Qiagen, Germantown, MD) following the manufacturer's protocol with previously described modifications (5). First strand cDNA synthesis and real time quantitative PCRs (qPCRs) were performed as reported (5). The amount of cDNAs was calculated relative to the standard curve and normalized to the control (ACT1) samples. Primer sequences are available upon request.
Chromatin Immunoprecipitation (ChIP)—ChIP assays were performed as described (9). Briefly, cross-linked cell lysates were sonicated using a Misonix 3000 sonicator with a cup horn device (5.5 inches) (Misonix, Farmingdale, NY). After clarification, proteins of interest were purified. For Myc-tagged Met4, the lysates were incubated with 3 μl of antibody (c-Myc (A-14)-G; Santa Cruz Biotechnology) and protein G beads (Pierce) for at least 6 h at 4 °C. TAP-tagged proteins were purified with 20 μl of IgG-Sepharose suspension (Amersham Biosciences). Protein-DNA complexes were eluted with 125 μl of elution buffer (50 mm Tris, pH 8.0, 10 mm EDTA, 1% SDS), and DNA-protein cross-linking was reversed at 65 °C overnight. DNA was further purified on QIAquick PCR columns (Qiagen, Germantown, MD) according to the manufacturer's instructions. qPCR and primer selection was as described above and in Ref. 9. 1 μl of the eluted DNA (IP and input DNA) was used in a 20-μl PCR. All reactions were run in triplicates. For each experiment, a standard curve was generated using 5-fold dilutions of input DNA. ChIPs were normalized to the input DNA, and background obtained from samples expressing untagged proteins (normalized to input DNA) was subtracted from the ChIPs.
RESULTS
A Genetic Screen for Suppressor Mutations of Temperature-sensitive met30 Mutants—The F-box protein Met30 is essential for cell proliferation. In order to better understand the cell cycle function of Met30, we conducted a genetic screen for extragenic suppressor mutations of Met30 deficiency. We reasoned that loss of Met30 function activates pathways that inhibit cell cycle progression and that mutations in components of these pathways should, at least partially, suppress the cell cycle defect of temperature-sensitive met30 mutants. In support of this concept, it has previously been demonstrated that deletion of either MET4 or MET32 can rescue the lethality of met30 mutants (10, 17).
We used UV radiation to mutagenize a yeast strain carrying the temperature-sensitive met30-6 allele. To reduce the fraction of suppressor mutants carrying mutations in MET4 and thus to increase the chance of identifying other genes involved in the Met30-controlled cell cycle steps, we integrated a second allele of MET4 into the screening strain. The additional MET4 gene was expressed under control of the regulated GAL1 promoter with an RGS6H epitope tag fused to its amino terminus to facilitate detection by immunoblotting. Approximately 3 × 108 cells were mutagenized with UV and grown on dextrose-containing plates at the restrictive temperature. To eliminate mutants that have mutations in MET4, we replica-plated the colonies onto plates containing galactose to induce expression of the GAL1-RGS6HMET4 allele and incubated plates at the restrictive temperature. All but 31 of the initially more than 300 recovered suppressor mutants failed to grow at restrictive temperature when expression of GAL1-RGS6HMET4 was induced. The 31 suppressor mutants were also tested for growth on plates lacking methionine. All mutants were methionine prototroph, suggesting that suppression of these mutants was probably due to mutations in genes other than MET4.
Met4 is kept in an inactive state by the attachment of a regulatory ubiquitin chain that prevents Met4 activity (17-19, 21). Failure to ubiquitinate Met4, such as in met30 mutants, leads to full Met4 activation and induces a cell cycle arrest. Suppression of met30-6 mutants could either be a result of restored Met4 ubiquitination or due to mutations in genes that are required for induction of a cell cycle arrest by active Met4. To separate the isolated suppressor mutants by these criteria, we analyzed the ubiquitination status of Met4 in the suppressor mutants (Fig. 1A) (data not shown). Ubiquitinated Met4 can be distinguished from the active phosphorylated form of Met4 by immunoblotting due to the distinct migration patterns (17, 18). Met4 ubiquitination was restored in all but two suppressor mutants (Fig. 1A) (data not shown). Mutants indicated with number 3 and 9 in Fig. 1A showed a dramatic reduction in the ubiquitinated species of Met4 and the appearance of a “smear,” which we have shown previously by phosphatase treatment to be indicative of phosphorylated, hyperactive Met4 (17, 18). Met4 ubiquitination in met30-6 mutants suggests that these strains acquired intragenic mutations that either reversed the mutation in met30-6 or restored activity of the mutated Met30-6 protein. An alternative, albeit unlikely, explanation would be that negative regulators of Met4 ubiquitination were inactivated in these mutants. Our interest was in identification of factors involved in the Met30-controlled cell cycle arrest that function downstream of Met4. We therefore focused on the two suppressor mutants that did not show ubiquitinated Met4 (mutant-3 and mutant-9 in Fig. 1A).
We used a genomic library on a centromeric plasmid to identify the mutated gene in mutant-9 by complementation cloning. Suppression of mutant-9 was reversed by plasmids containing MET32 (Fig. 1B). These results are consistent with a previous report, which demonstrated that deletion of MET32 can suppress the lethality of met30 mutants (10). Mutant-3 could not be complemented by MET32 (Fig. 1B). However, we noticed that mutant-3 had a dominant character (Fig. 1C), which was evident when we analyzed diploid cells generated by mating of mutant-3 with a met30-6 strain of the opposite mating type. The resulting diploid was homozygous for the met30-6 allele but heterozygous for the mutated gene. Nevertheless, the mutated gene was able to suppress the cell cycle defect of met30 mutants although a wild-type copy was present (Fig. 1C).
Carboxyl-terminal Truncated Met32 Is a Dominant Suppressor of the Cell Cycle Arrest Caused by Met30 Deficiency—To identify the mutated gene that confers dominant suppression in mutant-3, we constructed a genomic library with DNA isolated from this mutant in a centromeric plasmid. The library was used to transform a met30-6 temperature-sensitive mutant, and plasmids were recovered from colonies that grew at 37 °C. The genomic fragments harbored in the recovered plasmids contained the region surrounding MET32. Subcloning and retransformation of different parts of the genomic fragments showed that MET32 isolated from mutant-3 was a dominant suppressor of met30-6 mutants. We refer to the dominant MET32 allele isolated from mutant-3 as MET32-1. Sequence analyses of MET32-1 revealed a point mutation that changed a tyrosine residue at position 145 to a termination codon (TAT changed to TAG). MET32-1 results therefore in expression of a truncated version of Met32 (Fig. 2A). Expression of a truncated version of Met32 in mutant-3 was confirmed by integrating a GAL1-TAP fragment in front of the first codon of MET32-1 and the wild-type MET32 gene in a control strain. Integration of the GAL1-TAP construct resulted in the expression of an amino-terminal TAP-tagged Met32 in both strains, and immunoblotting showed that Met32-1 was significantly shorter than wild-type Met32 (Fig. 2B).
FIGURE 2.
A carboxyl-terminal truncation of Met32 acts as a dominant bypass mutation of met30 mutants. A, schematic representation of the Met32-1 mutant. The COOH-terminal C2HC-type zinc finger is indicated with black filled circles representing the zinc coordinating residues. The first zinc finger of Met32 is not shown. The relative position of the mutation changing the codon encoding tyrosine to a termination codon (TAG) in MET32-1 is indicated. B, a PCR-based strategy was used to integrate the GAL1 promoter fused to the TAP tag at the MET31 and MET32 loci in wild-type cells or the MET32 locus in mutant-3. Cells were grown in medium containing galactose to express the TAP-tagged proteins, and total cell lysates were analyzed by immunoblotting using a peroxidase-conjugated anti-peroxidase antibody to detect the TAP tag. Two dilutions of the cell lysates were loaded on the gel. C, cells expressing MET30 under control of the GAL1 promoter as the only source of Met30 were transformed either with a control plasmid or with a centromeric plasmid containing the MET32-1 allele expressed under control of the MET32 promoter. Cells were grown in galactose-containing medium for expression of Met30 and washed in dextrose medium, and serial dilutions were spotted on dextrose-containing plates. Met30 expression was completely repressed under these growth conditions, as indicated by the lack of growth of met30 cells.
We next tested whether MET32-1 can bypass deletion of MET30. To this end, we used a met30Δ GAL1-MET30 strain, which when grown on dextrose-containing media does not express Met30 and thus arrests. We transformed this strain with a centromeric plasmid carrying the MET32-1 allele expressed under control of its own promoter and tested growth on dextrose-containing plates. MET32-1 could bypass loss of Met30 function (Fig. 2C). Furthermore, the bypass was dominant, since this strain expressed wild-type Met32.
Met32-1 Is a Defective Transcription Factor—Met31 and Met32 are two homologous zinc finger-containing transcription factors with redundant function in MET gene transcription (2, 12). Despite their redundant function in MET gene transcription, it is thought that only Met32 is involved in induction of the SCFMet30-controlled cell cycle arrest, because met30 met32 but not met30 met31 double mutants are viable (10). Identification of a COOH-terminal truncated Met32 (Met32-1) as a suppressor of met30 mutants could suggest that the COOH-terminal region of Met32 specifies the cell cycle function. To test this idea, we generated hybrid proteins with the NH2-terminal Met32 region and the COOH-terminal Met31 region (Met-n32c31) or the reverse (Met-n31c32). In addition, we created a mutant version of Met32 that contained the entire second zinc finger but is missing the COOH-terminal 34 residues (Met321-157) (Fig. 3A).
FIGURE 3.
Domain requirement for methionine auxotrophy and cell cycle arrest. A, schematic representation of the proteins expressed. The COOH-terminal C2HC zinc finger is represented by the two filled ovals connected with a line. Each of the ovals represents two zinc-coordinating residues (oval 1, Cys125/Cys128 in Met31 and Cys128/Cys131 in Met32; oval 2, His141/Cys147 in Met31 and His144/Cys150 in Met32). Only the second zinc finger is represented. B, met31 met32 double mutants were transformed with centromeric plasmids expressing the indicated proteins under control of the GAL1 promoter. Serial dilutions of the cells were spotted onto SC plates containing either dextrose or galactose as the carbon source to repress or induce expression from the GAL1 promoter. Plates lacking methionine served to score the ability of the indicated constructs to complement loss of Met31/32 function. C, temperature-sensitive met30-6 mutants carrying a deletion of MET32 to suppress cell cycle arrest induced by Met30 inactivation were transformed with the plasmids as indicated and described for B. Serial dilutions were spotted on SC plates lacking uracil to select for the plasmids, and plates were incubated at the temperatures indicated. Induction of cell cycle arrest is evident by growth inhibition upon induction of expression on galactose-containing plates.
We first tested whether the hybrid proteins and the Met32 truncations formed functional transcription factors by complementation of the methionine auxotrophy of met31 met32 double mutants (Fig. 3B). Deletion of MET32 or MET31 results in methionine prototroph mutants due to the redundant function of these two genes. However, met31 met32 double mutants are methionine auxotroph (12). Both hybrid proteins, but not Met32-1, complemented the methionine auxotrophy of met31 met32 double mutants (Fig. 3B). However, by moving the truncation point of Met32-1 twelve residues toward the COOH terminus to retain the zinc-coordinating cysteine residue of the second zinc finger in Met321-157, Met32 function was restored (Fig. 3B).
We next tested the potential of the hybrid proteins and Met32 truncations to mediate cell cycle arrest in response to loss of Met30 function. To this end, we expressed the proteins in met30-6 met32Δ cells and monitored growth at different temperatures (Fig. 3C). As expected, restoring Met32 function by induction of Met32 expression from the GAL1 promoter induced a cell cycle arrest at the restrictive temperature, but expression of Met32-1 allowed growth (Fig. 3C). Remarkably, overexpression of Met31 could also prevent cell proliferation at the restrictive temperature (Fig. 3C). This was unexpected, because unlike met32 mutants, deletion of MET31 cannot suppress the cell cycle arrest of met30 mutants (10). These results suggest that either the intrinsic activity of Met31 to induce the cell cycle arrest pathway is less potent or that the Met31 expression level is lower as compared with Met32.
Both hybrid proteins could induce cell cycle arrest in met30-6 mutants (Fig. 3C). Interestingly, expression of the hybrid protein formed by exchanging the COOH-terminal region of Met32 with that of Met31 (Met-n32c31) resulted in a more potent cell cycle inhibition, since growth of met30-6 mutants was already significantly inhibited at the permissive temperature (Fig. 3C, 23 °C).
To more directly address the function of Met32-1 in transcription, we compared expression levels of several Met4-regulated genes in met31 met32 double mutants expressing either wild-type Met32 or Met32-1 (Fig. 4A). To induce expression of MET genes, cells were grown in medium supplemented with repressing amounts of methionine and then shifted to methionine-free medium (1). Induction of MET16, MET17/MET25 (MET17 is the official name for YLR303W, but it is frequently called MET25), and MET3 was significantly induced by expression of wild-type Met32 but not Met32-1 (Fig. 4A), confirming the genetic results (Fig. 3B). Furthermore, consistent with its ability to complement the methionine auxotrophy of met31 met32 double mutants (Fig. 3B), the hybrid protein Met-n32c31 and the COOH-terminal truncated Met321-157 supported induction of these MET genes (Fig. 4A). Similar results were obtained when MET gene expression was induced by inactivation of the temperature-sensitive met30-6 allele instead of methionine depletion (data not shown).
FIGURE 4.
Met32-1 is inactive as a transcription factor but does not affect Met4 deubiquitination. A, met31 met32 double mutants expressing the proteins indicated under control of the GAL1 promoter were grown at 30 °C in galactose-containing media with repressive amounts of methionine (1 mm). Cells were collected by filtration, washed with prewarmed galactose medium lacking methionine (SC-Gal/-Met), and resuspended in SC-Gal/-Met medium to induce expression of MET genes. Cells were collected 15, 30, and 60 min after induction and expression levels of the three MET genes indicated were analyzed by RT-qPCR. Expression levels are represented normalized to ACT1 expression. The highest expression level was arbitrarily set to 100%. B, cells as described for A were grown in SC-Gal/+Met medium, collected by filtration, washed, and resuspended in SD-Gal/-Met medium to activate Met4. Cell lysates were prepared after incubation in SD-Gal/-Met for the time indicated. Total cell lysates were separated by SDS-PAGE and analyzed by immunoblotting using polyclonal antibodies directed against Met4. The proteasome subunit Rpt1 was detected as a loading control. Met4 activation is evident by loss of ubiquitinated forms and the appearance of phosphorylated Met4.
It is worth mentioning that under our experimental conditions, MET17 expression was repressed in met31 met32 double mutants in the presence of methionine and was not induced upon methionine depletion (Fig. 4A). It has been previously reported that met31 met32 double mutants fail to repress MET17 expression in the presence of methionine (12). These differences are probably due to different growth medium formulations and/or strain backgrounds.
Despite the defect of Met32-1 in induction of MET gene expression in met32 met31 double mutants, Met32-1 had no obvious effect on activation of Met4 deubiquitination (Fig. 4B). Met4 deubiquitination can be visualized by immunoblotting, based on the conversion of ubiquitinated forms of Met4 into phosphorylated Met4 species. This change in Met4 modification correlates well with Met4 activation by diverse stimuli and seems to be a reliable indicator for Met4 activation (5, 17, 18, 26). met31 met32 double mutants expressing either Met31, Met32, or Met32-1 were grown under repressive conditions and shifted to medium lacking methionine to activate Met4. All three strains show conversion of the ubiquitinated forms of Met4 into phosphorylated species indicative of Met4 activation (Fig. 4B). Thus, although Met32-1 did not block Met4 deubiquitination, it was unable to support efficient MET gene transcription.
Overexpression of Met32-1 Inhibits MET Gene Transcription—Analyses of MET gene expression indicate that Met32-1 is inactive as a transcription factor (Figs. 3B and 4A). However, we isolated the MET32-1 mutation as a dominant suppressor of met30 mutants, which cannot be readily explained by MET32-1 being simply a loss of function allele. We therefore asked if Met32-1 might have a dominant inhibitory effect on MET gene expression. MET gene expression can be induced by either methionine depletion or inactivation of SCFMet30 (1, 16, 17). We used the temperature-sensitive met30-6 strain to induce MET gene expression upon shifting cells to the restrictive temperature. Consistent with previous results (17), met30-6 mutants expressing wild-type Met32 induced expression of MET genes when shifted to the restrictive temperature (Fig. 5A). Endogenous levels of Met32-1 in addition to wild-type Met32 only modestly reduced expression of the three tested MET genes, MET16, MET17, and MET5 (Fig. 5A). Despite this subtle effect on MET gene expression, Met32-1 suppressed the cell cycle defect of met30-6 mutants (Fig. 1) (data not shown), indicating that the Met30-controlled cell cycle function is particularly sensitive to the Met32-1 defect. This explains why MET32-1 mutants are methionine prototroph. We next asked whether overexpression of Met32-1 can block MET gene expression in the presence of wild-type Met32 and Met31. Expression of all MET genes analyzed was severely reduced under these conditions (Fig. 5B).
FIGURE 5.
Inhibition of MET gene expression by Met32-1. A, temperature-sensitive met30-6 mutants carrying either a control plasmid or a centromeric plasmid harboring the MET32-1 allele under control of its own promoter were used to assess the effect of Met32-1 on MET gene expression. Cells expressed the endogenous MET32 gene in addition to MET32-1. MET gene expression was induced by inactivation of the temperature-sensitive met30-6 allele. Note that MET gene expression is not completely repressed in met30-6 mutants grown at permissive temperature (25 °C). Total RNA was prepared from cells after the time intervals indicated, expression of the three MET genes shown was analyzed by RT-qPCR, and expression levels are presented normalized to ACT1 expression. B, the experiment was as described in A, but cells expressed Met32-1 under control of the GAL1 promoter, and cells were grown in galactose-containing media to induce expression of Met32-1. C, Met32 and Met32-1 binding to Met4 was compared in met31 met32 double mutants carrying two centromeric plasmids expressing RGS6H-tagged Met32 and RGS6H-tagged Met32-1 under control of the GAL1 promoter. Cells were grown in SD-Gal/+Met medium, and Met4 was activated by shifting the cells to medium lacking methionine as described for Fig. 4B. Met4 was immunopurified with anti-Met4 antibodies, and the immunocomplexes were separated by SDS-PAGE. The relative amount of associated Met32 and Met32-1 was analyzed by immunoblotting using anti-RGS6H antibodies. Met4 was detected with anti-Met4 antibodies and Rpt1 was detected as loading control. WCL, whole cell lysates. Control IP was performed in parallel without the addition of anti-Met4 antibodies. Met4 activation is evident by the loss of ubiquitinated forms and the appearance of phosphorylated Met4. D, binding of Met31, Met32, and truncation mutants to Met4 was assessed in met31 met32 double mutants expressing the proteins indicated under control of the GAL1 promoter. Cells were collected after growth in SC-Gal/+Met (+Met samples) or 30 min after they were shifted to SD-Gal/-Met (-Met samples). RGS6H-tagged proteins were purified on Ni2+-chelate beads, and the purified protein complexes were separated by SDS-PAGE and analyzed by immunoblotting using anti-Met4 antibodies or antibodies directed against the RGS6H epitope. Cells expressing no RGS6H-tagged protein were processed in parallel as control.
Mechanistically, the dominant effect of Met32-1 on cell cycle progression could be explained if Met32-1, which is inactive as a transcription factor (Figs. 3B and 4A), binds Met4 with higher affinity than wild-type Met32 and thus displaces the active factor to lock most of Met4 in an inactive Met4-Met32-1 complex. To test this hypothesis, we expressed both Met32 and Met32-1 in the same cell, immunopurified Met4, and analyzed the relative amounts of Met32 and Met32-1 bound to Met4 (Fig. 5C). Both Met32 and Met32-1 were expressed as an NH2-terminal fusion with the RGS6H epitope so that the intensities of the immunoblot signals were directly comparable. Roughly the same amount of Met32 and Met32-1 was bound to Met4 (Fig. 5C). We therefore conclude that Met32-1 cannot displace wild-type Met32 from Met4 complexes. Consistent with these results, affinity purification of His6-tagged Met31, Met32, Met321-157, or Met32-1 demonstrated that about equal amounts of Met4 were associated with the four different proteins under both repressive and activating growth conditions, indicating that the truncation in Met32-1 does not significantly change its binding to Met4 (Fig. 5D). These results also demonstrated that the interaction of the zinc finger proteins Met31 and Met32 with Met4 is constitutive and not regulated by methionine or the Met4 activity/modification status.
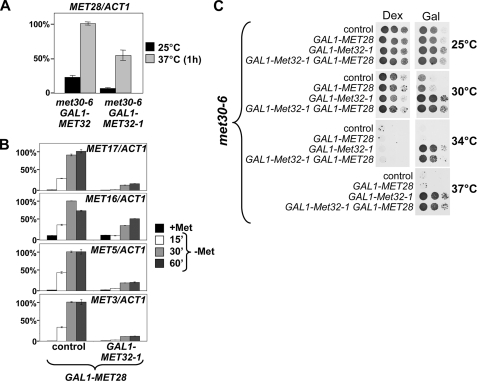
Met32-1 overexpression blocked expression of several MET genes (Fig. 5B). The effect on MET16 and MET5 expression was very surprising, because these genes lack canonical Met31/Met32 promoter elements and are thought to associate with Met4 exclusively via their Cbf1 binding elements (1). We further explored this effect to obtain some insight into the role of Met32/31 in Met4 regulation. It was possible that Met32-1 could have a direct effect on transactivation by the Cbf1-Met4 transcription complex. A more trivial interpretation would be an indirect mechanism. Indeed, Met28 has been shown to be an important component of the Cbf1-Met4 complex. Although lacking a DNA binding domain, Met28 has been shown to stabilize interaction of Cbf1 with target promoters (11). Since MET28 expression depends on Met31/32, one possible explanation was that Met32-1 blocked expression of the transcription factor Met28 and so affected Cbf1-dependent genes. To test this idea, we first looked at MET28 expression levels. We activated MET gene expression by inactivation of the met30-6 allele by a temperature shift and compared MET28 mRNA levels in cells containing wild-type Met32 or Met32-1 (Fig. 6A). Overexpression of Met32-1 resulted in a 50% reduction in MET28 expression. This was a significant but relatively modest decrease in MET28 transcript levels. Met32-1 had even less effect on MET28 expression when MET gene expression was induced by methionine depletion (data not shown).
FIGURE 6.
Overexpression of Met28 cannot overcome the effect of Met32-1 on MET gene expression or the cell cycle. A, temperature-sensitive met30-6 mutants expressing either Met32 or Met32-1 under the control of the GAL1 promoter were grown at 25 °C, shifted to 37 °C for 1 h to activate MET gene expression. MET28 expression levels were analyzed by RT-qPCR and are represented normalized to ACT1 expression. B, Met28 was overexpressed from the GAL1 promoter in wild-type cells and cells expressing GAL1-MET32-1. Cells were grown in SD-Gal/+Met and shifted to SD-Gal/-Met medium to induce MET gene expression, and samples were collected at the time points indicated. Expression levels of the genes indicated were analyzed by RT-qPCR and are represented normalized to ACT1 expression. C, the effect of Met28 overexpression on cell cycle arrest suppression by Met32-1 was analyzed by comparing growth of temperature-sensitive met30-6 mutants overexpressing either Met28, Met32-1, or both under control of the GAL1 promoter. Serial dilutions of cells were spotted on plates containing dextrose (Dex) or plates containing galactose (Gal), and plates were incubated at the temperatures indicated. Induction of Met32-1 expression on galactose plates suppressed the met30-6 cell cycle defect, and Met28 overexpression could not reverse suppression.
It was unlikely that a 50% reduction in MET28 expression could have such a dramatic effect on Cbf1-dependent genes. In addition, Met32-1 affected MET17 expression, which has been shown to be independent of Met28 (27). Nevertheless, we asked whether overexpression of Met28 from the strong GAL1 promoter could overcome the inhibitory effect of Met32-1 on MET gene expression (Fig. 6B). Cells were grown in galactose medium to constitutively express high levels of Met28 and then shifted to methionine-depleted medium to activate MET genes (Fig. 6B). Consistent with previous results overexpression of Met28 was not sufficient to induce MET genes in the presence of methionine (2). As expected, once shifted to medium lacking methionine, MET gene expression was rapidly induced. However, with the exception of MET16, overexpression of Met28 did not significantly induce expression of MET genes in cells containing Met32-1 (Fig. 6B).
To further address the role of Met28 expression in connection to Met32-1, we used a phenotypic readout (Fig. 6C). We have initially isolated MET32-1 as a suppressor of the cell cycle arrest phenotype associated with loss of Met30 function. We therefore asked whether overexpression of Met28 could reverse Met32-1-dependent suppression of the met30-6 cell cycle arrest (Fig. 6C). If Met32-1 acted indirectly via repression of MET28, overexpression of Met28 should restore cell cycle arrest in met30-6 MET32-1 mutants. Overexpression of Met28 did not result in cell cycle arrest in met30-6 MET32-1 double mutants. These results are also consistent with genetic studies reported previously that demonstrated that Met28 function is not important for the Met30-controlled cell cycle checkpoint, because deletion of MET28 could not overcome cell cycle arrest in met30 mutants (10).
Together, these results argue against an indirect, Met28-dependent mechanism by which Met32-1 represses MET gene expression and suppresses cell cycle arrest in met30 mutants.
Met32-1 Prevents Association of Met4 Transcription Complexes with MET Gene Promoter—The mutation in Met32-1 disrupts the second zinc finger motif (Fig. 2A), which could interfere with its DNA binding activity. Therefore, we asked whether Met32-1 associates with target promoters in vivo.To this end, we performed ChIP experiments. met30-6 met31 met32 mutants expressing either TAP-tagged Met31, Met32, or Met32-1 were shifted to the restrictive temperature for 1 h to activate MET gene expression. Cells expressed TAP-tagged Met31, Met32, or Met32-1 under control of the GAL1 promoter to ensure equal expression levels for the different proteins. met30-6 mutants expressing untagged Met31 and Met32 were processed in parallel as a control. Met31 and Met32 were bound to all three MET gene promoters we analyzed. Surprisingly, we found Met31 and Met32 binding to MET5 and MET16, which lack canonical Met31/Met32 binding elements (1), suggesting that association with the promoter of these genes might be indirect and mediated by Cbf1 (Fig. 7A). Met32-1 could not be detected at any of the tested promoters (Fig. 7A). These results are consistent with the experiments showing that Met32-1 is inactive as a transcription factor (Figs. 3B and 4A). The DNA binding defect of Met32-1 cannot explain the dominant phenotype of this mutation, considering that Met32-1 cannot displace wild-type Met32 or the functionally redundant Met31 from Met4 (Fig. 5C). Furthermore, it was puzzling how Met32-1 can affect transcription of genes that are dependent on the Cbf1-Met4 complex and do not have promoter binding sites for Met31/32, such as MET16 and MET5. We reasoned that perhaps Met31, Met32, and/or Met32-1 bind to the Cbf1-Met4 transcription complex and can have a regulatory function in this transcription complex. Formally, there is no reason to exclude Met31 or Met32 from the Cbf1-Met4 complex, because the binding sites for Cbf1 and Met31/32 have been mapped to different domains in Met4 (2). To test this idea, we first asked whether HA-tagged Cbf1 and TAP-tagged Met32 could be co-purified on IgG-beads. Met32 co-purified with Cbf1 (Fig. 7B). Importantly, co-purification was dependent on Met4, suggesting that Cbf1 and Met32 can simultaneously bind to the same Met4 molecule. Interestingly, the interaction of Cbf1 with the Met4-Met32 complex was induced by methionine depletion. This is consistent with previous results that indicated that Cbf1 interacts preferentially with the deubiquitinated, active form of Met4 (17).
FIGURE 7.
Met32-1 prevents recruitment of Met4 to MET gene promoters. A, met30-6 met31 met32 mutants expressing TAP-tagged versions of either Met31, Met32, or Met32-1 under control of the GAL1 promoter were analyzed by chromatin immunoprecipitation. Binding of TAPMet31, TAPMet32, and TAPMet32-1 to the promoter regions indicated was analyzed in cells grown at 25 °C and after MET gene expression was induced by inactivation of Met30-6 for 1 h at 37 °C. Cells were grown in YEP-galactose medium to continuously express the TAP-tagged proteins. TAP-tagged proteins were purified from formaldehyde cross-linked samples on IgG beads, and promoter fragments were detected by qPCR. Promoter binding is represented on the y axis as the relative signal obtained in the ChIP compared with the input from whole cell lysates. The highest value was arbitrarily set to 100%. The dotted line represents the background-binding signal obtained by analysis of binding to the ADH1 promoter region. B, to test whether Met32 and Cbf1 are found in the same Met4-containing transcription complex, met31 met32 double and met31 met32 met4 triple mutants expressing endogenous HA3-tagged Cbf1 and TAP-tagged Met32 under control of the GAL1 promoter were analyzed. Cells were grown in SD-Gal/+Met, and MET gene expression was induced for 30 min by shifting cells to SD-Gal/-Met medium. TAPMet32 was purified on IgG beads, and the purified protein complexes were separated by SDS-PAGE and analyzed by immunoblotting using antibodies directed against the HA tag or peroxidase-conjugated anti-peroxidase antibody to detect the TAP tag. Cbf1 binding was induced by methionine depletion and depended on Met4. C, promoter association of Met31, Met32, and Met32-1 was analyzed by ChIP. met31 met32 double and met31 met32 cbf1 triple mutants expressing TAP-tagged Met31, Met32, or Met32-1 under the control of the GAL1 promoter were grown in SD-Gal/+Met, and MET gene expression was induced by shifting cells to SD-Gal/-Met for 30 min. ChIP and data representation are as described for A. D, to test the effect of Met32-1 on recruitment of Met4 to MET gene promoters, cells expressing endogenous 18-Myc-tagged Met4 and either Met31, Met32, or Met32-1 under control of the GAL1 promoter were analyzed by ChIP. Cell growth and MET gene induction were as described for C. Met4 was immunopurified with anti-Myc antibodies and ChIP analyses, and presentation of binding data is as described for A. Note that cells express endogenous levels of Met31 and Met32 in addition to the proteins expressed under GAL1 control. WCL, whole cell lysates.
We next asked whether Met31 and Met32 could be detected at the MET gene promoter, including genes lacking canonical Met31/32 binding elements, upon activation by methionine depletion (Fig. 7C). To this end, we conducted ChIP experiments under repressive as well as activating conditions. Met31 and Met32, but not Met32-1, were bound to MET promoters. Promoter association of Met31 and, albeit to a lesser extent, Met32 was induced by methionine depletion. Importantly, Met31 and Met32 binding to the promoter regions of MET17, MET16, and MET5 was strictly dependent on Cbf1, suggesting that Cbf1 recruits the Met4-Met32 complex to these promoters. These results together with the co-purification experiments shown in Fig. 7B support the idea of a Cbf1-Met4-Met32 (and Cbf1-Met4-Met31) complex and are consistent with the concept that Met32 could have regulatory functions in this complex. Accordingly, Met32-1 could lead to inactivation of Cbf1-Met4-Met32-1 complexes and thus affect genes lacking canonical Met31/32 binding elements. To this end, we asked whether expression of Met32-1 could prevent recruitment of Met4 to the promoter regions of MET genes (Fig. 7D). Consistent with the report by Kuras et al. (21), Met4 promoter recruitment was induced by methionine depletion. Importantly, ChIP experiments demonstrated that Met32-1 blocked recruitment of Met4 to promoter regions of MET genes.
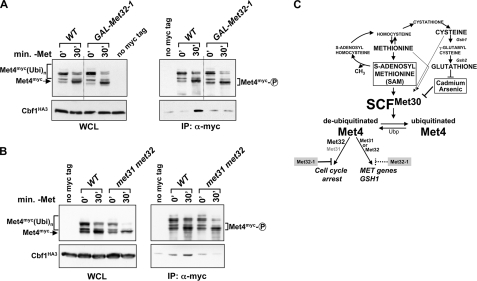
To more directly address the mechanism by which Met32-1 prevents binding of Met4 to target promoters, we analyzed the effect of Met32-1 on composition of the Met4 transcription complex (Fig. 8). We overexpressed Met32-1 in cells and analyzed binding of Cbf1 to Met4 in response to methionine depletion by co-immunopurification (Fig. 8A). Met32-1 significantly reduced interaction of Met4 with Cbf1, providing a rationale for the lack of Met4 recruitment to Cbf1-dependent promoter elements in the presence of Met32-1 (Fig. 7D). These results suggested that perhaps the two redundant zinc finger proteins Met31 and Met32 are involved in formation of a stable Met4-Cbf1 complex. Indeed, immunopurification experiments demonstrated that Met4 and Cbf1 cannot form a stable complex in the absence of Met31/32 (Fig. 8B). Together, these experiments show that the active Met4 complex contains the two DNA-binding factors Cbf1 and Met31/32 and suggest a regulatory function of Met31/32 in formation of a stable Met4-Cbf1 complex.
FIGURE 8.
Met31/32 are important for formation of a stable Met4-Cbf1 complex. A, cells expressing endogenous HA3-tagged Cbf1 and Myc-tagged Met4 were grown in SD-Gal/+Met to overexpress Met32-1 as indicated, and MET gene expression was induced by shifting cells to SD-Gal/-Met for 30 min. Met4 complexes were immunopurified and analyzed by immunoblotting. A yeast strain expressing untagged Met4 was processed in parallel. Please note that wild-type cells (WT) carried 18 copies of the Myc tag fused to Met4, and cells expressing GAL-MET32-1 had a 13-Myc-tagged Met4. For the sake of clarity, we aligned the figure to eliminate the observed differences in mobility. This is indicated by the dotted line. B, experiment as in A, but cells were grown in media containing dextrose. All cells expressed 13-Myc-tagged Met4. C, diagram illustrating regulation of the Met4 transcription complex. WCL, whole cell lysates.
DISCUSSION
In an effort to identify second site suppressor mutations of the cell cycle defect associated with loss of Met30 function, we isolated the dominant MET32-1 allele. MET32-1 expresses a COOH-terminally truncated protein that lacks DNA binding activity but interestingly could prevent expression of a variety of MET genes, including genes lacking promoter binding elements for Met32 or the closely related Met31 (Figs. 5B and 6B). Cells expressing Met32-1 no longer responded with cell cycle arrest to inactivation of the ubiquitin ligase SCFMet30. Experiments addressing cell cycle arrest induction of mutant versions of Met32 as well as that of hybrid proteins formed by fusion of parts of Met32 with Met31 (Fig. 3, B and C) showed that overexpression of Met31 from the GAL1 promoter could induce cell cycle arrest (Fig. 3C). This was unexpected, because deletion of MET32, but not deletion of MET31, can bypass the cell cycle block associated with loss of Met30 function (10). Thus, cell cycle regulation has been considered a specific function of Met32 that is lacking in Met31. Our results suggest that both Met32 and Met31 have the potential to induce cell cycle arrest but that Met31 expression levels are either too low or that the cell cycle inhibitory function of Met31 is significantly weaker than that of Met32. We did find that endogenous Met31 levels were considerably lower than Met32 levels under activating conditions, supporting the former explanation (data not shown). On the other hand, analyses of the cell cycle arrest activity of hybrid proteins formed with Met31 and Met32 suggested that the NH2-terminal region of Met32 contains the more potent cell cycle-inhibitory activity (Fig. 3C). These results suggest that the combination of both a higher expression level and higher cell cycle inhibitory activity of Met32 as compared with Met31 makes Met32 the primary mediator of the Met30-controlled cell cycle checkpoint (Fig. 8C).
The transactivating factor Met4 lacks DNA binding activity and requires association with DNA-binding factors for promoter recruitment. The DNA-binding factors Cbf1, and Met31/32 associate with Met4 and mediate sequence-specific promoter binding. Accordingly, two distinct Met4 complexes that are distinguished by their DNA-binding components, namely a Cbf1 and a Met31/32-containing complex, are thought to regulate MET gene expression (1). In addition, it was hypothesized that Met4 might be anchored simultaneously by both Cbf1 and Met31/32 to promoter regions containing both binding elements (2). The results reported here demonstrated that Met4 is simultaneously bound to both DNA-binding factors in vivo. Met4-dependent co-immunopurification of Met32 and Cbf1 provided direct evidence for a Cbf1-Met4-Met32 complex (Fig. 7B). Further confirmation comes from ChIP experiments in which Met4 complexes bound to MET gene promoter were analyzed. Two of the promoter regions we assayed, namely MET16 and MET5, contained only Cbf1-dependent binding sites and were devoid of Met31/32 binding elements. We found Met31 and Met32 associated with all MET gene promoters analyzed (Fig. 7C). Importantly, association was strictly dependent on Cbf1, not only supporting the idea of a Cbf1-Met4-Met32 (and Cbf1-Met4-Met31) complex but also demonstrating that such complexes are associated with target gene promoters. The presence of Met32-1 in these complexes could efficiently prevent recruitment of Met4 to MET gene promoter, including promoter regions containing Cbf1 but not Met31/32-binding elements (MET16 and MET5) (Fig. 7D). This indicates a general role for Met31/32 in the Met4 transcription complex. Experiments probing the interaction between Met4 and Cbf1 support this notion. Cbf1 did not interact stably with Met4 in cells lacking Met31/32 or in cells expressing the dominant negative Met32-1 protein (Fig. 8). We were unable to form a Met4-Cbf1-Met32 complex in vitro using recombinant, purified proteins. Met32 associated with Met4, but Cbf1 could not be bound to this complex efficiently. It is unlikely that the recombinant Cbf1 was defective, because Met4 provided in total yeast lysates bound to recombinant Cbf1 (17). These in vitro results suggest that posttranslational modifications and/or additional protein factors are involved in regulation of the Met4 transcription complex. There might also be additional roles for Met31/32 that are independent of their sequence-specific DNA binding activity. For example, Met31/32 could be crucial to stabilize interaction of the Cbf1-Met4-transcription complex and DNA through sequence-independent or less sequence-dependent DNA binding. In addition, Met31/32 could be involved in the recruitment of other proteins that are important for MET gene expression. For example, Met4-dependent recruitment of the mediator and SAGA complexes independent from each other has been reported recently (28). Met31/Met32 could play a direct role in one of these recruitment events. Together, our results demonstrate that formation of the active Met4-Cbf1 transcription complex depends on the cell cycle regulatory function of Met32 and thus provide insight into how cell cycle and transcriptional responses are synchronized. The precise mechanism of Met31/32 function in the context of the Cbf1-Met4 transcription complex remains to be elucidated, but further work in this direction will be important to understand how sulfur amino acid metabolism, response to cadmium and arsenic stress, and cell proliferation are coordinated.
Acknowledgments
We are grateful to Karin Flick for helpful suggestions and comments on the manuscript. We thank the members of the Kaiser and the Nomura laboratories for helpful discussions.
This work was supported by National Institutes of Health Grant GM66164 (to P. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SC, synthetic complete medium; IP, immunoprecipitation; ChIP, chromatin immunoprecipitation; qPCR, quantitative PCR; RT, reverse transcription; HA, hemagglutinin; TAP, tandem affinity purification.
References
- 1.Thomas, D., and Surdin-Kerjan, Y. (1997) Microbiol. Mol. Biol. Rev. 61 503-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaiseau, P. L., and Thomas, D. (1998) EMBO J. 17 6327-6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser, P., Su, N. Y., Yen, J. L., Ouni, I., and Flick, K. (2006) Cell Div. 1 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudouin-Cornu, P., and Labarre, J. (2006) Biochimie (Paris) 88 1673-1685 [DOI] [PubMed] [Google Scholar]
- 5.Yen, J. L., Su, N. Y., and Kaiser, P. (2005) Mol. Biol. Cell 16 1872-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbey, R., Baudouin-Cornu, P., Lee, T. A., Rouillon, A., Zarzov, P., Tyers, M., and Thomas, D. (2005) EMBO J. 24 521-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauchon, M., Lagniel, G., Aude, J. C., Lombardia, L., Soularue, P., Petat, C., Marguerie, G., Sentenac, A., Werner, M., and Labarre, J. (2002) Mol. Cell 9 713-723 [DOI] [PubMed] [Google Scholar]
- 8.Dormer, U. H., Westwater, J., McLaren, N. F., Kent, N. A., Mellor, J., and Jamieson, D. J. (2000) J. Biol. Chem. 275 32611-32616 [DOI] [PubMed] [Google Scholar]
- 9.Su, N. Y., Flick, K., and Kaiser, P. (2005) Mol. Cell. Biol. 25 3875-3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton, E. E., Peyraud, C., Rouillon, A., Surdin, K. Y., Tyers, M., and Thomas, D. (2000) EMBO J. 19 1613-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuras, L., Barbey, R., and Thomas, D. (1997) EMBO J. 16 2441-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaiseau, P. L., Isnard, A. D., Surdin-Kerjan, Y., and Thomas, D. (1997) Mol. Cell. Biol. 17 3640-3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas, D., Jacquemin, I., and Surdin-Kerjan, Y. (1992) Mol. Cell. Biol. 12 1719-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai, M., and Davis, R. W. (1990) Cell 61 437-446 [DOI] [PubMed] [Google Scholar]
- 15.Menant, A., Baudouin-Cornu, P., Peyraud, C., Tyers, M., and Thomas, D. (2006) J. Biol. Chem. 281 11744-11754 [DOI] [PubMed] [Google Scholar]
- 16.Rouillon, A., Barbey, R., Patton, E. E., Tyers, M., and Thomas, D. (2000) EMBO J. 19 282-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser, P., Flick, K., Wittenberg, C., and Reed, S. I. (2000) Cell 102 303-314 [DOI] [PubMed] [Google Scholar]
- 18.Flick, K., Ouni, I., Wohlschlegel, J. A., Capati, C., McDonald, W. H., Yates, J. R., and Kaiser, P. (2004) Nat. Cell Biol. 6 634-641 [DOI] [PubMed] [Google Scholar]
- 19.Flick, K., Raasi, S., Zhang, H., Yen, J. L., and Kaiser, P. (2006) Nat. Cell Biol. 8 509-515 [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekaran, S., Deffenbaugh, A. E., Ford, D. A., Bailly, E., Mathias, N., and Skowyra, D. (2006) Mol. Cell 24 689-699 [DOI] [PubMed] [Google Scholar]
- 21.Kuras, L., Rouillon, A., Lee, T., Barbey, R., Tyers, M., and Thomas, D. (2002) Mol. Cell 10 69-80 [DOI] [PubMed] [Google Scholar]
- 22.Petroski, M. D., and Deshaies, R. J. (2005) Nat. Rev. Mol. Cell. Biol. 6 9-20 [DOI] [PubMed] [Google Scholar]
- 23.Thomas, D., Kuras, L., Barbey, R., Cherest, H., Blaiseau, P. L., and Surdin-Kerjan, Y. (1995) Mol. Cell. Biol. 15 6526-6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, S. I., Hadwiger, J. A., and Lorincz, A. T. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 4055-4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthrie, C., and Fink, G. R. (1991) Guide to Yeast Genetics and Molecular Biology, Academic Press, Inc., San Diego
- 26.Barbey, R., Baudouin-Cornu, P., Lee, T. A., Rouillon, A., Zarzov, P., Tyers, M., and Thomas, D. (2005) EMBO J. 3 521-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuras, L., Cherest, H., Surdin-Kerjan, Y., and Thomas, D. (1996) EMBO J. 15 2519-2529 [PMC free article] [PubMed] [Google Scholar]
- 28.Leroy, C., Cormier, L., and Kuras, L. (2006) Mol. Cell. Biol. 26 3149-3163 [DOI] [PMC free article] [PubMed] [Google Scholar]