Abstract
Sebaleic acid (5,8-octadecadienoic acid) is the major polyunsaturated fatty acid in human sebum and skin surface lipids. The objective of the present study was to investigate the metabolism of this fatty acid by human neutrophils and to determine whether its metabolites are biologically active. Neutrophils converted sebaleic acid to four major products, which were identified by their chromatographic properties, UV absorbance, and mass spectra as 5-hydroxy-(6E,8Z)-octadecadienoic acid (5-HODE), 5-oxo-(6E,8Z)-octadecadienoic acid (5-oxo-ODE), 5S,18-dihydroxy-(6E,8Z)-octadecadienoic acid, and 5-oxo-18-hydroxy-(6E,8Z)-octadecadienoic acid. The identities of these metabolites were confirmed by comparison of their properties with those of authentic chemically synthesized standards. Both neutrophils and human keratinocytes converted 5-HODE to 5-oxo-ODE. This reaction was stimulated in neutrophils by phorbol myristate acetate and in keratinocytes by oxidative stress (t-butyl-hydroperoxide). Both treatments dramatically elevated intracellular levels of NADP+, the cofactor required by 5-hydroxyeicosanoid dehydrogenase. In keratinocytes, this was accompanied by a rapid increase in intracellular GSSG levels, consistent with the involvement of glutathione peroxidase. 5-Oxo-ODE stimulated calcium mobilization in human neutrophils and induced desensitization to 5-oxo-6,8,11,14-eicosatetraenoic acid but not leukotriene B4, indicating that this effect was mediated by the OXE receptor. 5-Oxo-ODE and its 8-trans isomer were equipotent with 5-oxo-6,8,11,14-eicosatetraenoic acid in stimulating actin polymerization and chemotaxis in human neutrophils, whereas 5-HODE, 5-oxo-18-hydroxy-(6E,8Z)-octadecadienoic acid, and 5S,18-dihydroxy-(6E,8Z)-octadecadienoic acid were much less active. We conclude that neutrophil 5-lipoxygenase converts sebaleic acid to 5-HODE, which can be further metabolized to 5-oxo-ODE by 5-hydroxyeicosanoid dehydrogenase in neutrophils and keratinocytes. Because of its chemoattractant properties, sebum-derived 5-oxo-ODE could be involved in neutrophil infiltration in inflammatory skin diseases.
Human sebaceous glands and the sebum they produce have a unique fatty acid profile in that they contain high levels of the Δ6 C16 fatty acid sapienic acid along with its Δ5,8 elongation product sebaleic acid (1, 2). Sapienic acid is formed by the action of fatty acid desaturase-2 on palmitic acid (3). Although this enzyme is widely distributed, it does not normally act on palmitic acid but rather on linoleic acid or α-linolenic acid, which are much better substrates. However, only very small amounts of these polyunsaturated fatty acids (PUFA)3 are found in sebaceous glands, which instead accumulate high levels of palmitic acid. This is possibly due to the preferential removal of linoleic acid by β-oxidation in this tissue (4), leaving palmitic acid as the only substrate for fatty acid desaturase-2. Elongation of sapienic acid and insertion of a 5,6-double bond by Δ5-desaturase (fatty acid desaturase-1) results in the formation of sebaleic acid ((5Z,8Z)-octadecadienoic acid) (5), which, although present in relatively small amounts, is the major PUFA in human skin surface lipids, being present at levels over 2-fold higher than linoleic acid ((9Z,12Z)-octadecadienoic acid) (2). Although sapienic acid has been reported to have antibacterial activities (6), nothing is known about the possible role of sebaleic acid in the skin.
One possible route for the oxidative metabolism of sebaleic acid is the 5-lipoxygenase (5-LO) pathway. 5-LO converts the ω6 PUFA arachidonic acid (5,8,11,14-eicosatetraenoic acid) to 5-hydroperoxy-6,8,11,14-eicosatetraenoic acid and leukotrienes (7). In neutrophils, these intermediates are rapidly converted to leukotriene B4 (LTB4), a potent neutrophil chemoattractant, and 5-hydroxy-6,8,11,14-eicosatetraenoic acid (5-HETE), respectively (8). Since sebaleic acid is a Δ5,8-PUFA like arachidonic acid, we hypothesized that it might be a substrate for 5-LO and could possibly be converted to an analogous 5-hydroxy metabolite, although further conversion to leukotrienes would not be possible due to the lack of a Δ11-double bond.
Although 5-HETE has only weak biological activities on neutrophils and eosinophils, it is oxidized to the potent granulocyte chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) by the NADP+-dependent enzyme 5-hydroxyeicosanoid dehydrogenase (5-HEDH) (9). The biological actions of this compound are mediated by the highly selective OXE receptor (10, 11), which is a member of the G protein-coupled receptor family (12–14). The objective of the current study was to determine whether sebaleic acid is a substrate for the 5-LO pathway and whether it can be converted to biologically active proinflammatory products that could potentially be involved in attracting granulocytes to the skin.
EXPERIMENTAL PROCEDURES
Materials—5-Oxo-ETE (15) and LTB4 (16) were prepared by chemical synthesis as described previously. 13S-hydroxy-9Z,11E-octadecadienoic acid (13-HODE) was prepared by oxidation of linoleic acid with soybean lipoxygenase Type 1B (Sigma) (17). The following were purchased from Sigma: phorbol 12-myristate 13-acetate (PMA), tert-butyl-hydroperoxide (tBuOOH), GSH, GSSG, o-phthalaldehyde, acetophenone, formic acid, deamino-NAD+, tetrabutyl ammonium hydroxide, and 3-[(3-cholamidopropyl)dimethylammonio]-1-pro-panesulfonate (CHAPS). Me2SO was purchased from Fisher, whereas NADP+ and A23187 were from Roche Applied Science and Calbiochem, respectively. Ionomycin was purchased from Biomol (Plymouth Meeting, PA), and Indo-1 was obtained from Molecular Probes/Invitrogen.
Chemical Synthesis of Sebaleic Acid and Its 5-Hydroxy and 5-Oxo Derivatives—Sebaleic acid (Fig. 1A), 5S-hydroxy-(6E,8Z)-octadecadienoic acid (5S-HODE; Fig. 1B), 5S,18-dihydroxy-(6E,8Z)-octadecadienoic acid (5,18-diHODE; Fig. 1C), 5-oxo-18-hydroxy-(6E,8Z)-octadecadienoic acid (5-oxo-18-HODE; Fig. 1C), 5-oxo-(6E,8Z)-octadecadienoic acid (5-oxo-ODE; Fig. 1D), and 5-oxo-(6E,8E)-octadecadienoic acid (8-trans-5-oxo-ODE; Fig. 1E) were prepared by total chemical synthesis as illustrated in Fig. 1. 5R-HODE was prepared as described for 5S-HODE (Fig. 1B), except that the starting material was l-arabinose rather than d-arabinose.
FIGURE 1.
Chemical synthesis of sebaleic acid and its metabolites. Sebaleic acid (A), 5-HODE (B), the ω-oxidation products 5,18-diHODE and 5-oxo-18-HODE (C), 5-oxo-ODE (D), and 8-trans-5-oxo-ODE (E) were prepared by total chemical synthesis as shown. Full details of the conditions used for these syntheses will be published elsewhere.
Although Ludwig and Sprecher (18) previously reported the total synthesis of 14C-labeled sebaleic acid, no chemical syntheses have previously been reported for the other compounds listed above. The above products were stored at –80 °C until used. Sebaleic acid was repurified by RP-HPLC within 1 week of its use to remove any autoxidation products, whereas the oxygenated derivatives of sebaleic acid were repurified by RP-HPLC within 1 month of use. The detailed experimental conditions for these syntheses will be published separately.
Preparation of Neutrophils—Neutrophils were prepared by treatment of whole blood with Dextran T-500 (Amersham Biosciences) for 45 min, followed by centrifugation over Ficoll-Paque (Amersham Biosciences) and hypotonic lysis of any remaining red blood cells (9). The neutrophils were suspended in phosphate-buffered saline (PBS–).
Culture of Human Keratinocytes—Normal human keratinocytes were obtained from Cascade Biologics (Portland, OR) and cultured in serum-free EpiLife® medium containing EpiLife defined growth supplement (Cascade Biologics) according to the supplier's directions. Cells were seeded at a density of 2500 cells/cm2 and grown to 80% confluence, with the medium being changed every 2 days. The cells were used until passage 6 or 7.
Analysis of Metabolites of Sebaleic Acid and 5-HODE—Neutrophils (2 × 106 cells in 1 ml) were suspended in PBS– supplemented with 1.8 mm Ca2+ and 1 mm Mg2+ (PBS+) and incubated for various times with either sebaleic acid, 5-HODE, or 5-oxo-ODE in the presence or absence of A23187 (5 μm) or PMA (50 nm). Incubations were terminated by the addition of methanol (0.65 ml) containing 0.15% trifluoroacetic acid and cooling to 0 °C.
Keratinocytes were seeded in 6-well plates at a density of 2500 cells/cm2 as described above. When they reached 70–80% confluence, the medium was removed and replaced with PBS+ (1 ml), and the cells were incubated for different times with 5-HODE (4 μm) in the presence or absence of tBuOOH (100 μm). The medium was then removed, the internal standard (120 ng of 13-HODE) was added, and the wells were washed with 0.65 ml of MeOH, which was combined with the aqueous medium. The concentration of methanol in each sample was then adjusted to 30% by the addition of water. Eicosanoids were analyzed by precolumn extraction/RP-HPLC (19) using a Waters Alliance system (Waters Associates, Milford, MA). Products were quantitated by comparing the areas of their peaks of UV absorbance at their λmax with that of the internal standard. The extinction coefficients of 5-HODE and 5,18-di-HODE at 235 nm were assumed to be identical to that of 5-HETE (27,000), whereas those of 5-oxo-ODE and 5-oxo-18-HODE at 280 nm were assumed to be identical to that of 5-oxo-ETE (22,100). The extinction coefficients used for LTB4 and 13-HODE were 39,500 (280 nm) and 23,000 (235 nm).
Measurement of Glutathione in Keratinocytes—GSH and GSSG were measured by RP-HPLC as described previously (20) by modification of a postcolumn derivatization procedure in the literature (21). After incubation of keratinocytes with tBuOOH for various times in 6-well plates at 37 °C, the medium was removed, and 200 mm phosphoric acid (1 ml) containing 12 mm CHAPS at 0 °C was added. GSH and GSSG were separated by RP-HPLC, and each was converted to an identical isoindole adduct by in-line treatment of the column eluate with o-phthalaldehyde, followed by quantitation using a fluorescence detector. The amounts of GSH and GSSG were determined from a standard curve using the authentic compounds as external standards.
Measurement of NADP+ in Neutrophils and Keratinocytes—NADP+ was measured after conversion to a fluorescent naphthyridine derivative followed by quantitation by RP-HPLC, as previously described (20). Neutrophils (2 × 106 cells in 250 μl) were incubated for various times with PMA (50 nm). The incubations were terminated with acetophenone/KOH containing deamino-NAD+ (30 ng) as an internal standard. Alternatively, keratinocytes were cultured in 6-well plates and incubated with tBuOOH (100 μm). After various times, the medium was removed and added to acetophenone/KOH. After treatment with formic acid, extraction, and treatment with phenazine methosulfate, the samples were analyzed by RP-HPLC on an Ultracarb ODS column using a fluorescence detector as described previously (20).
Identification of Sebaleic Acid Metabolites by Fourier Transform Mass Spectrometry (FTMS)—Neutrophils (108 cells in 20 ml) were incubated for 60 min at 37 °C with sebaleic acid (50 μm) in the presence of PMA (50 nm) and A23187 (5 μm). The incubation was terminated by the addition of methanol to give a final concentration of 30%, followed by centrifugation. The supernatant was subjected to solid-phase extraction on a cartridge containing octadecylsilyl silica (C18 Sep-Pak; Waters), as previously described (22), except that the sample was loaded in 30% MeOH at neutral pH. The products were purified by RP-HPLC on a Spherisorb ODS-2 column (250 × 4.6 mm; 5-μm particle size; Phenomenex, Torrance, CA) using a mobile phase consisting of 51% acetonitrile in water containing 0.02% acetic acid at a flow rate of 1.3 ml/min. Metabolites I and II had tR values of 27 and 39 min, respectively, under these conditions.
To obtain metabolites III and IV, 5-HODE (4 μm) or 5-oxo-ODE (4 μm), respectively, were incubated with neutrophils (5 × 107 cells in 10 ml) for 40 min at 37 °C. The products were extracted by solid-phase extraction as described above and purified by RP-HPLC on a Spherisorb ODS-2 column using a mobile phase consisting of 40% acetonitrile containing 0.02% acetic acid and a flow rate of 1.3 ml/min The tR values for metabolites III and IV were 12 and 16 min, respectively.
Metabolites I–IV were identified by direct infusion electrospray ionization in negative ion mode, using an IonSpec 7.0-tesla Fourier transform mass spectrometer (Lake Forest, CA) calibrated with poly(ethylene glycol) diacid 600 (Fluka). A Z-spray source from Waters Corp. (Milford, MA) was used with a capillary voltage of 2800 V, a cone voltage of 30 V, and a sample flow rate of 1–3 μl/min. The solvent used was a 2:1 mixture of 90% MeOH containing 25 mm ammonium acetate and CH2Cl2. The elemental compositions of the four metabolites were determined by mass spectrometry, and the structures were determined through MS/MS. MS/MS experiments were performed after isolating the monoisotopic parent ion using 500 ms of sustained 1-kHz off-resonance irradiation collision-induced dissociation and a 90-ms nitrogen gas pulse. The resulting scans were internally calibrated using the exact mass of the parent ion.
Chiral Analysis of 5-HODE—5-HODE, prepared by incubation of sebaleic acid with neutrophils as described above, was converted to its methyl ester by treatment with diazomethane and purified by RP-HPLC. The neutrophil-derived 5-HODE was then analyzed by HPLC using a Chiralpak AD column (10-μm particle size, 4.6 × 250 mm; Chiral Technologies, Exton, PA). A mobile phase of 3% methanol in hexane was used at a flow rate of 1 ml/min. As previously reported (23), we confirmed that the performance of this column was vastly improved by using methanol instead of isopropyl alcohol as the polar modifying component of the mobile phase.
Measurement of Cytosolic Calcium Levels—Calcium levels were measured in neutrophils that were loaded with indo-1 by incubation with its acetoxymethyl ester for 30 min in PBS– (24). The cells were washed and resuspended in PBS containing Ca2+ and Mg2+. Following stabilization of the base line, fluorescence was measured using a Photon Technology International Deltascan 4000 spectrofluorometer with a temperature-controlled cuvette holder equipped with a magnetic stirrer.
Measurement of Actin Polymerization—Actin polymerization was measured in a mixed leukocyte fraction obtained as described above for neutrophils, except that centrifugation over Ficoll-Paque was omitted. Aliquots of the leukocyte suspension (90 μl, 5 × 106 cells/ml) were preincubated for 5 min at 37 °C, followed by the addition of either vehicle (10 μl of PBS containing 0.1% bovine serum albumin) or sebaleic acid metabolites. After 20 s, the incubations were terminated by the addition of formaldehyde, cytosolic F-actin was stained by incubation with NBD-phallacidin, and F-actin levels were measured by flow cytometry as previously described (25).
Measurement of Chemotactic Activity—Neutrophil migration was measured by the modified Boyden technique using 48-well microchemotaxis chambers (Neuro Probe Inc., Cabin John, MD) and Sartorius cellulose nitrate filters (8-μm pore size; 140-μm thickness; Neuro Probe Inc.) (24). Potential agonists were added to the bottom wells, whereas neutrophils (150,000 cells) were added to each of the top wells. After incubation for 2 h, the filters were stained, and the numbers of cells on the bottom surfaces were counted in five different fields at a magnification of ×400 for each incubation, each of which was performed in triplicate.
RESULTS
Metabolism of Sebaleic Acid by Neutrophils—To determine whether it can be metabolized by neutrophils, sebaleic acid (50 μm) was incubated with these cells (5 × 106 cells/ml) for 40 min in the presence of the calcium ionophore A23187 (5 μm) and PMA (50 nm). The products were analyzed by RP-HPLC as shown in Fig. 2A. Two major products were observed, one with a retention time (tR) of 32.9 min (metabolite I), which absorbed in the UV at 235 nm, and the other (metabolite II) with a tR of 34.3 min and UV absorbance at 280 nm. There were also smaller peaks with tR values at 13.2 min (235 nm; metabolite III) and 15.1 min (280 nm; metabolite IV). None of these peaks was present when sebaleic acid was omitted (data not shown). In addition, there were peaks corresponding to metabolites of endogenous arachidonic acid, which were also present when neutrophils were incubated with A23187 and PMA in the absence of sebaleic acid. These included 20-hydroxy-LTB4 (tR, 5.2 min), the two 6-trans isomers of LTB4 (tR, 17.0 and 17.6 min), LTB4 (tR, 18.5 min), 5-HETE (tR, 31.4 min), and 5-oxo-ETE (tR, 31.7 min).
FIGURE 2.
Chromatographic profile of sebaleic acid metabolites formed by neutrophils. A, human neutrophils (5 × 106 cells in 1 ml) were preincubated for 5 min with PMA (50 nm) and then incubated with sebaleic acid (50 μm) and A23187 (5 μm) for a further 40 min at 37 °C. The products were analyzed by automated precolumn extraction/RP-HPLC with 13-HODE (100 ng) as an internal standard (int std). The stationary phase was a Waters Nova-Pak C18 column (3.9 × 150 mm), whereas the mobile phase was a linear gradient over 40 min between water/acetonitrile/methanol/acetic acid (60:30:10:0.02) and water/acetonitrile/methanol/acetic acid (10:38:52:0.02) with a flow rate of 1 ml/min. The top chromatogram shows the profile for UV absorbance at 235 nm, whereas the bottom chromatogram shows absorbance at 280 nm. 5oETE, 5-oxo-ETE; 20h-LTB4, 20-hydroxy-LTB4. B, UV spectra of metabolites I (– – –), II (——), III (–··–), and IV (–·–).
The UV spectra of metabolites I–IV are shown in Fig. 2B. Metabolites I and III have virtually identical UV spectra, with λmax values at 233 nm, similar to that of 5-HETE, consistent with conjugated dienyl alcohols (i.e. monohydroxy octadecadienoic acids). Metabolites II and IV also have similar UV spectra with λmax values at 280 and 282 nm, respectively, similar to that of 5-oxo-ETE and consistent with conjugated dienones (i.e. oxo-octadecadienoic acids).
Identification of Sebaleic Acid Metabolites I and II—Because
of the presence of 5-LO and 5-HEDH in neutrophils, we suspected that sebaleic
acid was converted to 5-hydroxy and 5-oxo metabolites. To aid in the
identification of these products, 5-HODE and 5-oxo-ODE were prepared by total
chemical synthesis as shown in Fig.
1 and found to have chromatographic properties and UV spectra
identical with those of metabolites I and II, respectively. To obtain more
conclusive evidence for the identities of these metabolites, they were
prepared by incubation of sebaleic acid with neutrophils and purified by
RP-HPLC. Analysis by high resolution electrospray ionization-FTMS in MS mode
revealed that metabolites I and II had intense ions at m/z
295.2283
(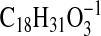 requires 295.2279) and 293.2122
(
requires 295.2279) and 293.2122
(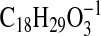 requires 293.2122), respectively, consistent with the carboxylate anions of
hydroxy and oxo derivatives of octadecadienoic acid.
requires 293.2122), respectively, consistent with the carboxylate anions of
hydroxy and oxo derivatives of octadecadienoic acid.
CID of the M-1 ion at m/z 295 for metabolite I resulted
in a product ion spectrum (Fig.
3A) with major ions at m/z 277.2173
(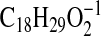 requires 277.2173; M-1-H2O), 259.2066
(
requires 277.2173; M-1-H2O), 259.2066
( requires 259.2067; M-1–2×H2O), 251.2381
(
requires 259.2067; M-1–2×H2O), 251.2381
(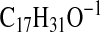 requires 251.2380; M-1-CO2), 235.2068
(
requires 251.2380; M-1-CO2), 235.2068
(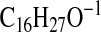 requires 235.2067; C3-C18-2), 233.2275
(
requires 235.2067; C3-C18-2), 233.2275
(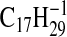 requires
233.2275; M-1-CO2-H2O), 223.2067
(
requires
233.2275; M-1-CO2-H2O), 223.2067
(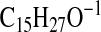 requires 223.2067; C4-C18), 179.1806
(C13H23– requires 179.1805;
C6-C18, formed by cleavage adjacent to the 5-hydroxyl
group), 115.0401
(
requires 223.2067; C4-C18), 179.1806
(C13H23– requires 179.1805;
C6-C18, formed by cleavage adjacent to the 5-hydroxyl
group), 115.0401
(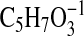 requires 115.0401; C1-C5-1, also formed by cleavage
adjacent to the 5-hydroxyl group), and 111.0453
(
requires 115.0401; C1-C5-1, also formed by cleavage
adjacent to the 5-hydroxyl group), and 111.0453
(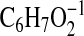 requires 111.0451; C1-C6-H2O). This mass
spectrum is virtually identical to that of authentic chemically synthesized
5-HODE (Fig. 3C). The
identical mass spectral, UV, and chromatographic properties of the
biologically and chemically derived compounds clearly indicate that metabolite
I is 5-HODE.
requires 111.0451; C1-C6-H2O). This mass
spectrum is virtually identical to that of authentic chemically synthesized
5-HODE (Fig. 3C). The
identical mass spectral, UV, and chromatographic properties of the
biologically and chemically derived compounds clearly indicate that metabolite
I is 5-HODE.
FIGURE 3.
Product ion mass spectra of sebaleic acid metabolites. Product ion spectra were obtained following collision-induced dissociation of the M-1 ion from neutrophil-derived metabolite I (5-HODE) (A) and metabolite II (5-oxo-ODE) (B), chemically synthesized 5-HODE (C) and 5-oxo-ODE (D), and neutrophil-derived metabolite III (5,18-diHODE) (E) and metabolite IV (5-oxo-18-HODE) (F). All of the above compounds were prepared as described under “Experimental Procedures” and analyzed by FTMS.
CID of the M-1 ion at m/z 293 for metabolite II gave a
product ion spectrum (Fig.
3B) with major ions at m/z 275.2016
(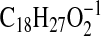 requires 275.2017; M-1-H2O), 257.1910
(
requires 275.2017; M-1-H2O), 257.1910
(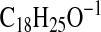 requires 257.1911; M-1–2×H2O), 249.2224
(
requires 257.1911; M-1–2×H2O), 249.2224
( requires 249.2224; M-1-CO2), 233.1911
(
requires 249.2224; M-1-CO2), 233.1911
(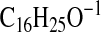 requires 233.1911; C3-C18-2), 231.2119
(
requires 233.1911; C3-C18-2), 231.2119
( requires
231.2118; M-1-CO2-H2O), 221.1911
(
requires
231.2118; M-1-CO2-H2O), 221.1911
(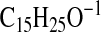 requires 221.1911; C4-C18), 179.1806
(
requires 221.1911; C4-C18), 179.1806
( requires
179.1805; C6-C18, formed by cleavage adjacent to the
5-oxo group), 129.0557
(
requires
179.1805; C6-C18, formed by cleavage adjacent to the
5-oxo group), 129.0557
(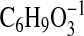 requires 129.0557; C1-C6+2), 113.0245
(
requires 129.0557; C1-C6+2), 113.0245
( requires 113.0244; C1-C5-1, formed by cleavage adjacent
to the 5-oxo group), and 111.0451
(
requires 113.0244; C1-C5-1, formed by cleavage adjacent
to the 5-oxo group), and 111.0451
(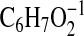 requires 111.0452). Except for some differences in the relative intensities of
some of the ions, probably because the spectra were run on different days,
this mass spectrum is virtually identical to that of authentic chemically
synthesized 5-oxo-ODE (Fig.
3D). The identity of the chromatographic properties, UV
spectrum, and mass spectrum of metabolite II with those of authentic 5-oxo-ODE
indicates that metabolite II is identical to this compound.
requires 111.0452). Except for some differences in the relative intensities of
some of the ions, probably because the spectra were run on different days,
this mass spectrum is virtually identical to that of authentic chemically
synthesized 5-oxo-ODE (Fig.
3D). The identity of the chromatographic properties, UV
spectrum, and mass spectrum of metabolite II with those of authentic 5-oxo-ODE
indicates that metabolite II is identical to this compound.
5-LO Is Responsible for the Formation of 5-HODE and 5-Oxo-ODE from Sebaleic Acid—To ensure that 5-HODE was synthesized enzymatically rather than by autoxidation of sebaleic acid, we investigated the chirality of the 5-hydroxyl group of 5-HODE using a Chiralpak AD column. All compounds were analyzed as their methyl ester (ME) derivatives. Chemically synthesized 5R-HODE-ME and 5S-HODE-ME were completely separated from one another, with tR values of 6.9 and 10.8 min, respectively (Fig. 4A). The identity of the first peak as the 5R isomer was confirmed by chromatographing synthetic 5R-HETE-ME (tR, 6.9 min) separately (Fig. 4B). 5S-HODE-ME, chromatographed separately, had a tR of 10.8 min (data not shown). Neutrophil-derived 5-HODE-ME had a tR of 10.8 min, identical to that of the chemically synthesized compound (Fig. 4C).
FIGURE 4.
Evidence for the formation of 5-HODE by 5-LO. A mixture of the methyl esters of chemically synthesized 5R-HODE and 5S-HODE (A), the methyl ester of chemically synthesized 5R-HODE (B), and 5-HODE methyl ester, isolated following incubation of neutrophils with sebaleic acid and A23187 as described under “Experimental Procedures” (C) were analyzed by HPLC on a column of Chiralpak AD. The brackets in each chromatogram show the retention times of 5R-HODE and 5S-HODE. D and E show the effects of different concentrations of MK-886 and zileuton, respectively, on the formation of 5-HODE (▴) and 5-oxo-ODE (○) by neutrophils incubated with sebaleic acid in the presence of A23187 and PMA as described under “Experimental Procedures.” The values are means ± S.E. of data from four independent experiments with different donors.
The involvement of 5-LO in the formation of sebaleic acid oxidation products by neutrophils was examined by using a FLAP antagonist (MK-886) and a 5-LO inhibitor (zileuton). MK-886 strongly inhibited the formation of both 5-HODE and 5-oxo-ODE from sebaleic acid with an IC50 of about 30 nm in both cases (Fig. 4D). Zileuton also had identical inhibitory effects on the formation of the two sebaleic acid metabolites but was less potent (IC50 ∼10 μm) (Fig. 4E).
Identification of Metabolites III and IV—To determine
whether the two polar metabolites III and IV were formed by further metabolism
of 5-HODE, chemically synthesized 5-HODE was incubated with neutrophils in the
presence of PMA, and the products were analyzed by RP-HPLC. 5-HODE was
converted to 5-oxo-ODE (metabolite II) along with metabolites III and IV
(Fig. 5A). Incubation
of 5-oxo-ODE with neutrophils (in the absence of PMA) gave rise almost
exclusively to metabolite IV (data not shown). Analysis of metabolites III and
IV by FTMS in MS mode revealed intense ions at m/z 311.2232
(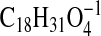 requires 311.2228) and 309.2071
(
requires 311.2228) and 309.2071
(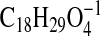 requires 309.2071), respectively, consistent with hydroxy metabolites of
5-HODE and 5-oxo-ODE.
requires 309.2071), respectively, consistent with hydroxy metabolites of
5-HODE and 5-oxo-ODE.
FIGURE 5.
Formation of 5-HODE metabolites and NADP+ by PMA-stimulated neutrophils. A, high performance liquid chromatogram of the products obtained after preincubation of neutrophils (2 × 106 cells in 1 ml) with PMA (50 nm) for 5 min, followed by incubation with 5-HODE (4 μm) for 20 min. The products were analyzed by precolumn extraction/RP-HPLC using a Waters Nova-Pak C18 column with a mobile phase consisting of a linear gradient between water/acetonitrile/acetic acid (65:35:0.02) and water/acetonitrile/acetic acid (25:75:0.02) at a flow rate of 1 ml/min. B and C, time courses for the formation of 5-oxo-ODE (5oODE)(•), 5-oxo-18-HODE (5o-18h; ○), and 5,18-diHODE (5,18-dh; ▵) by neutrophils preincubated for 5 min with either vehicle (B) or 50 nm PMA (C), followed by incubation with 5-HODE (4 μm) for various times. The products were analyzed by RP-HPLC as described above. The results are means ± S.E. (n = 4). D, time course for the effect of PMA (50 nm; added at 0 min) on intracellular NADP+ levels in neutrophils. NADP+ was measured as described under “Experimental Procedures.” Results are means ± S.E. (n = 5). int std, internal standard.
Further analysis of metabolite III by CID of the M-1 ion at
m/z 311 led to the formation of product ions
(Fig. 3E) at
m/z 293.2126
(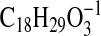 requires 293.2122; M-1-H2O), 275.2018
(
requires 293.2122; M-1-H2O), 275.2018
( requires 275.2017; M-1–2×H2O), 267.2333
(
requires 275.2017; M-1–2×H2O), 267.2333
( requires 267.2330; M-1-CO2), 249.2226
(
requires 267.2330; M-1-CO2), 249.2226
(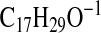 requires 249.2224; M-1-H2O-CO2), 239.2019
(
requires 249.2224; M-1-H2O-CO2), 239.2019
(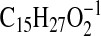 requires 239.2017; C4-C18), 221.1914
(
requires 239.2017; C4-C18), 221.1914
(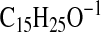 requires 221.1911; C4-C18-H2O), and 115.0399
(
requires 221.1911; C4-C18-H2O), and 115.0399
(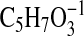 requires 115.0401; C1-C5-1). This mass spectrum is
similar to that of 5-HODE, except most of the ions occur at
m/z values 16 units higher, indicative of an additional
hydroxyl group. The diagnostic ions at m/z 115 and 239
derived from the carboxyl and ω-ends of the molecule, respectively,
indicate that the additional hydroxyl group is distal to the hydroxyl group at
C5, but from the mass spectral data alone it is not possible to
precisely define the position of this hydroxyl group.
requires 115.0401; C1-C5-1). This mass spectrum is
similar to that of 5-HODE, except most of the ions occur at
m/z values 16 units higher, indicative of an additional
hydroxyl group. The diagnostic ions at m/z 115 and 239
derived from the carboxyl and ω-ends of the molecule, respectively,
indicate that the additional hydroxyl group is distal to the hydroxyl group at
C5, but from the mass spectral data alone it is not possible to
precisely define the position of this hydroxyl group.
Fragmentation of the M-1 ion (m/z 309) for metabolite IV
by CID resulted in a product ion spectrum
(Fig. 3F) with major
ions at m/z 291.1966
(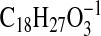 requires 291.1966; M-1-H2O), 273.1861
(
requires 291.1966; M-1-H2O), 273.1861
( requires 273.1860; M-1–2×H2O), 265.2174
(
requires 273.1860; M-1–2×H2O), 265.2174
( requires 265.2173; M-1-CO2), 249.1860
(
requires 265.2173; M-1-CO2), 249.1860
(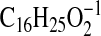 requires 249.1860; C3-C18-2), 247.2068
(
requires 249.1860; C3-C18-2), 247.2068
(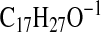 requires 247.2067; M-1-CO2-H2O), 237.1861
(
requires 247.2067; M-1-CO2-H2O), 237.1861
(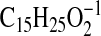 requires 237.1860; C4-C18), 177.1650
(
requires 237.1860; C4-C18), 177.1650
(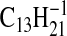 requires
177.1649; C6-C18-H2O), 129.0558
(
requires
177.1649; C6-C18-H2O), 129.0558
(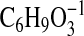 requires 129.0557; C1-C6+2), and 113.0245
(
requires 129.0557; C1-C6+2), and 113.0245
(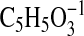 requires 113.0244; C1-C5-1, formed by cleavage adjacent
to the 5-oxo group). From the above mass spectrum, it can be concluded that
metabolite IV is a monohydroxy metabolite of 5-oxo-ODE in which the hydroxyl
group is distal to the oxo group at C5.
requires 113.0244; C1-C5-1, formed by cleavage adjacent
to the 5-oxo group). From the above mass spectrum, it can be concluded that
metabolite IV is a monohydroxy metabolite of 5-oxo-ODE in which the hydroxyl
group is distal to the oxo group at C5.
Although the exact position of the additional hydroxyl groups in metabolites III and IV could not be determined from the mass spectra described above, it seemed likely that they would be present in the ω-position at C18, since neutrophils contain an ω-hydroxylase (LTB4 20-hydroxylase; CYP4F3) that introduces a hydroxyl group at C20 in LTB4 (26), 5-HETE (27), and 5-oxo-ETE (24). To test this hypothesis, we prepared 5,18-diHODE and 5-oxo-18-HODE by total chemical synthesis. These compounds cochromatographed with metabolites III and IV, respectively, and had identical mass spectra (data not shown). Regioisomers in which the additional hydroxyl group was in another position should have had different retention times from the ω-hydroxy standards, as we have previously observed for ω, ω-1, and ω-2 metabolites of LTB4 (28, 29). We therefore conclude that metabolites III and IV are identical to 5,18-diHODE and 5-oxo-18-HODE, respectively.
Time Course for the Metabolism of 5-HODE by Neutrophils—When 5-HODE was incubated with neutrophils in the absence of PMA, it was rapidly converted to 5,18-diHODE, but only small amounts of 5-oxo-ODE and 5-oxo-18-HODE were detected (Fig. 5B). In contrast, when neutrophils were preincubated for 5 min with PMA prior to the addition of 5-HODE, there was a substantial reduction in the synthesis of 5,18-di-HODE coupled with dramatic increases in the synthesis of 5-oxo-ODE and 5-oxo-18-HODE (Fig. 5C).
PMA activates NADPH oxidase in neutrophils, resulting in the conversion of NADPH to NADP+, the cofactor required by 5-HEDH (9). To compare the time courses for the formation of 5-oxo-ODE and NADP+ in response to PMA, we measured NADP+ levels by RP-HPLC (Fig. 5D). This cofactor rose dramatically from a basal level of 0.8 ± 0.1 pmol/106 cells to 9.5 ± 1.8 pmol/106 cells by 2 min and continued to increase to a maximal level of 27.6 ± 4.1 pmol/106 cells by 12 min, after which time it slowly declined. In the absence of PMA, NADP+ remained at base-line levels (data not shown).
Metabolism of 5-HODE by Human Keratinocytes—To determine whether skin cells also have the ability to synthesize 5-oxo-ODE, keratinocytes were incubated with 5-HODE in the presence and absence of tBuOOH, which we recently found to increase 5-HEDH activity in epithelial cells by a mechanism dependent on the generation of GSSG and NADP+ (30). Keratinocytes were very active in synthesizing 5-oxo-ODE and even produced substantial amounts in the absence of tBuOOH (Fig. 6A). tBuOOH stimulated the formation of 5-oxo-ODE, the levels of which continued to increase up to at least 60 min, at which time they were 3-fold higher than in the presence of vehicle alone.
FIGURE 6.
Effects of tBuOOH on 5-oxo-ODE synthesis and GSSG and NADP+ levels in human keratinocytes. A, keratinocytes (3 × 105 cells/well) were incubated with 5-HODE (100 μm) and either vehicle (○) or tBuOOH (•) for various times at 37 °C. 5-Oxo-ODE was measured by RP-HPLC as described in the legend to Fig. 5. The results are means ± S.E. (n = 5). B, effects of tBuOOH, added at 0 min, on the intracellular levels of GSSG (•) and NADP+ (▵), measured as described under “Experimental Procedures,” in keratinocytes. The values are means ± S.E. (n = 4).
The addition of tBuOOH to keratinocytes induced a very rapid increase in GSSG levels, from 0.12 ± 0.03 nmol/106 cells to 24 ± 6 nmol/106 cells in only 1 min, after which time they remained fairly constant, diminishing by only about 25% after 60 min (Fig. 6B). This was mirrored by a dramatic decline in GSH levels, from 58 ± 16 to 6 ± 2 nmol/106 cells within 1 min of the addition of tBuOOH (data not shown). Basal levels of NADP+ in keratinocytes (25 ± 5 pmol/106 cells) were quite high compared with neutrophils (0.8 ± 0.1 pmol/106 cells; see above), and increased nearly linearly with time in response to tBuOOH, reaching levels of 1900 ± 590 pmol/106 cells by 60 min (Fig. 6B). In the absence of tBuOOH, both NADP+ and GSSG remained at base-line levels (data not shown).
5-Oxo-ODE Stimulates Calcium Mobilization in Neutrophils via the OXE Receptor—The addition of 5-oxo-ODE (100 nm) to neutrophils resulted in a strong calcium transient (Fig. 7A). 5-Oxo-ODE completely blocked the response to the subsequent addition of 5-oxo-ETE (10 nm) but not to LTB4 (10 nm). In contrast, when vehicle was added instead of 5-oxo-ODE, strong responses were observed to both 5-oxo-ETE and LTB4 (Fig. 7B). Both 5-oxo-ETE and LTB4 blocked the response to a subsequent addition of the same agonist (data not shown). These results indicate that 5-oxo-ODE selectively stimulates neutrophils via the OXE receptor.
FIGURE 7.
Effects of 5-oxo-ODE on intracellular calcium levels in neutrophils. Indo-1-loaded neutrophils were treated with either 5-oxo-ODE (5oODE) (10 nm)(A) or vehicle (B), followed by 5-oxo-ETE (5oETE) (10 nm), LTB4 (10 nm), and ionomycin (1 μm). Fluorescence was measured as described under “Experimental Procedures.” The results are representative of three similar experiments.
5-Oxo-ODE Is a Potent Stimulator of Actin Polymerization in Neutrophils—To further examine the biological properties of 5-oxo-ODE, we investigated its effects on actin polymerization in neutrophils (Fig. 8). Both 5-oxo-ODE and its 8-trans isomer (EC50 ∼ 10 nm) strongly stimulated actin polymerization in these cells with potencies nearly identical to that of 5-oxo-ETE. 5-HODE and 5-oxo-18-HODE were about 10 times less potent and exhibited lower maximal responses, whereas 5,18-diHODE was less active.
FIGURE 8.
Effects of sebaleic acid metabolites on actin polymerization in neutrophils. Neutrophils were treated for 20 s with various concentrations of 5-oxo-ODE (5oODE)(•), 8-trans-5-oxo-ODE (8t-5oODE)(▿), 5-oxo-ETE (5oETE) (□), 5-oxo-18-HODE (5o-18h)(○), 5-HODE (▴), and 5,18-diHODE (5,18-dh)(▵), and actin levels were measured by flow cytometry as described under “Experimental Procedures.” All values are means ± S.E. (n = 6).
5-Oxo-ODE Is a Potent Neutrophil Chemoattractant—The effects of 5-oxo-ODE on neutrophil migration were evaluated using microchemotaxis chambers. At a concentration of 10 nm, 5-oxo-ODE increased neutrophil migration by 3.8 ± 1.0-fold (p < 0.05) compared with the vehicle control (Fig. 9). The maximal response was observed at 1 μm, as was also the case with 5-oxo-ETE. The EC50 for 5-oxo-ODE (85 ± 37 nm) was a little higher than that for 5-oxo-ETE (59 ± 23 nm). The 8-trans isomer of 5-oxo-ODE also induced neutrophil migration but was slightly less potent (EC50 122 ± 31 nm). These differences were not statistically significant.
FIGURE 9.
Chemoattractant effects of 5-oxo-ODE. Chemotactic responses of neutrophils to 5-oxo-ODE (•), 8-trans-5-oxo-ODE (▿), and 5-oxo-ETE (□) were measured using microchemotaxis chambers as described under “Experimental Procedures.” 5oODE, 5-oxo-ODE; 8t-5oODE, 8-trans-5-oxo-ODE; 5oETE, 5-oxo-ETE. All values are means ± S.E. (n = 5) and are expressed as percentages of the maximal response to 5-oxo-ETE, which was 230 ± 32 cells/high power field, compared with 11 ± 2 cells/high power field for vehicle-treated controls.
DISCUSSION
Human sebaceous glands, unlike other tissues, contain only small amounts of arachidonic acid (1), the usual substrate for the formation of products of the 5-LO and cyclooxygenase pathways, and instead contain sebaleic acid, the major PUFA in skin surface lipids (2). The presence of a C7 methylene group activated by Δ5 and Δ8 double bonds in this fatty acid raised the possibility that it might be a substrate for 5-LO, although it could not be converted to leukotrienes, since it lacks a Δ11 double bond. Since sebaleic acid is not available commercially, we prepared it by total chemical synthesis. It proved to be an excellent substrate for 5-LO in neutrophils, being converted to two major products: 5-HODE and 5-oxo-ODE, along with two more polar ω-oxidation products, 5-oxo-18-HODE and 5,18-diHODE (Fig. 10).
FIGURE 10.
Metabolism of sebaleic acid by human neutrophils. Both 5-HODE and 5-oxo-ODE are assumed to be converted to 18-hydroxy products by the action of LTB4 20-hydroxylase (CYP4F3). Although 8-trans-5-oxo-ODE was not identified as a product of sebaleic acid metabolism by neutrophils, this substance has been reported to be a constituent of maca tubers.
To conclusively identify these novel metabolites and to investigate their biological properties, we prepared all of them by total chemical synthesis. The mass spectra, UV spectra, and chromatographic properties of the metabolites isolated from incubations with neutrophils were all identical to those obtained from the corresponding authentic chemically synthesized compounds. The 5-hydroxyl group of 5-HODE, synthesized from exogenous sebaleic acid by neutrophils, was exclusively in the S configuration, consistent with its formation by 5-LO. This was further supported by the strong inhibitory effects of the FLAP antagonist MK-886 and the 5-LO inhibitor zileuton on the formation of both 5-HODE and 5-oxo-ODE by neutrophils incubated with sebaleic acid. It would seem likely that the 18-hydroxy C18 metabolites we identified are formed by the action of LTB4 20-hydroxylase (CYP4F3), which is highly expressed in human neutrophils.
PMA strongly stimulates the conversion of exogenous 5-HODE to 5-oxo-ODE by neutrophils and inhibits the further metabolism of these two substances to ω-oxidation products. We previously found that the formation of 5-oxo-ETE is also increased by PMA and showed that this effect can be blocked by the NADPH oxidase inhibitor diphenylene iodonium (31), suggesting that NADPH oxidase-derived NADP+ was responsible. 5-HEDH is highly selective for NADP+ (32), which is normally present at only very low levels in cells (33). To provide further evidence for the involvement of NADP+, we measured its levels in neutrophils by RP-HPLC. The critical role of this cofactor in regulating 5-HEDH activity in these cells is supported by the rapid effect of PMA to induce and sustain high levels of NADP+, as shown in Fig. 5D.
Because of the presence of sebaleic acid in sebum, we wondered whether human keratinocytes also have the capacity to synthesize 5-oxo-ODE. We found that these cells can produce substantial amounts of 5-oxo-ODE from 5-HODE, even when not stimulated. This process is enhanced by oxidative stress, since tBuOOH increased 5-oxo-ODE formation while concomitantly inducing a dramatic 200-fold increase in GSSG levels in only 1 min, accompanied by a slower almost linear increase in NADP+. These effects are similar to those that we recently observed for the effects of oxidative stress on human airway epithelial cells (30). Hydroperoxides are reduced by cellular glutathione peroxidase, accompanied by the oxidation of GSH to GSSG. The latter is then recycled back to GSH by glutathione reductase, accompanied by oxidation of NADPH to NADP+, thus providing the cofactor required by 5-HEDH. The relatively high rate of conversion of 5-HODE to 5-oxo-ODE by unstimulated keratinocytes compared with unstimulated neutrophils may be due to the higher basal levels of NADP+ in these cells, which are about 30 times higher on a per cell basis. Keratinocytes are considerably more active than neutrophils in synthesizing 5-oxo-ODE from 5-HODE, producing about 7 times as much per cell following stimulation. These cells could therefore be an important source of this substance in the skin. Although they do not have appreciable 5-LO activity, they could potentially convert 5-HODE synthesized by 5-LO in sebaceous glands (34) and neutrophils to 5-oxo-ODE.
Although the present study is the first to identify 5-HODE, 5-oxo-ODE, 5,18-diHODE, and 5-oxo-18-HODE, the 8-trans isomer of 5-oxo-ODE along with its benzylamide derivative were previously identified by NMR spectroscopy as constituents of extracts from tubers of the South American maca plant (Lepidium meyenii) (35). This plant is found in the Peruvian Andes and is cultivated both for food and as a folk medicine (36, 37). Maca extracts are claimed to have fertility-enhancing and aphrodisiac effects along with other pharmacological properties (36, 38). Maca tubers contain a variety of constituents with potential biological activities, including various unsaturated fatty acids and oxo-fatty acids (macaenes) along with the corresponding benzylamides (macamides) (35, 36, 38). Whether or not 8-trans-5-oxo-ODE could account for some of the reputed biological activity of maca extracts is not known, but the present study shows that this substance is clearly a potent activator of human neutrophils.
Both of the double bonds of the macaene 8-trans-5-oxo-ODE are in the trans configuration, in contrast to the corresponding sebaleic acid-derived metabolite that we identified from neutrophils, in which the 8,9-double bond is in the cis configuration, as would be expected from a 5-LO-catalyzed reaction. Although we observed a small peak eluting shortly after 5-oxo-ODE (tR, 35.1 min; Fig. 2A), with a retention time similar to that of 8-trans-5-oxo-ODE, this material absorbed at both 280 and 235 nm and was also present in the absence of sebaleic acid. It therefore seems unlikely that neutrophils synthesize appreciable amounts of the 8-trans isomer. The biosynthetic route for the formation of maca-derived 8-trans-5-oxo-ODE may thus differ from that for the 8-cis isomer in neutrophils. Another possibility is that the 8-trans-5-oxo-ODE isolated from maca tubers could have been formed by isomerization of the 8-cis double bond of 5-oxo-ODE, since we have previously noted that 5-oxo fatty acids containing 8-cis double bonds are rather unstable when stored and are converted to the corresponding 8-trans isomers.
We have previously shown that 5-oxo-ETE is a potent chemoattractant for neutrophils and eosinophils and also stimulates actin polymerization and other responses in these cells (10). The present study clearly demonstrates that both neutrophil-derived 5-oxo-ODE and the macaene 8-trans-5-oxo-ODE have biological properties similar to those of 5-oxo-ETE with about the same potency and nearly the same efficacy. 5-Oxo-ODE completely desensitizes neutrophils to 5-oxo-ETE-induced Ca2+ mobilization but has no effect on the response to LTB4, indicating that its actions are mediated by the OXE receptor. The high potency of 5-oxo-ODE at this receptor is in marked contrast to the weak effects of a C18 analog of LTB4, which was reported to be about 50–100 times less potent than LTB4 in stimulating BLT1 receptor-mediated responses in neutrophils (39). The lesser ability of the 5-oxo-ETE receptor to distinguish differences in the ω-end of the molecule is understandable, in that the distinctive part of the molecule resides in the carboxylic acid end of the molecule (C1–C7), with the remainder (C8–C20) being identical to arachidonic acid. In the case of LTB4, the 12R-hydroxyl group, which is clearly very important for activation of the BLT1 receptor (40), is much closer to the ω-end of the molecule, which would be consistent with the greater ability of this receptor to sense changes in this portion of LTB4.
The biological activity of 5-oxo-ODE is markedly reduced by ω-oxidation to 5-oxo-18-HODE as well as by reduction back to its precursor 5-HODE, both of which result in about 10-fold reductions in potency. Metabolism of 5-oxo-ETE by these pathways has similar effects on its potency (24, 41), as does ω-oxidation of LTB4 (40). Although the OXE receptor is unable to distinguish shortening of the ω-end of the molecule by 2 carbons, it is clearly quite sensitive to the introduction of a polar ω-hydroxyl group. This suggests that an interaction between the ω hydrophobic portion of the ligand and the receptor is very important, although there is some flexibility with respect to the size of the hydrophobic group being recognized.
The potent chemoattractant effects of 5-oxo-ODE and the presence of its precursor fatty acid in sebaceous glands raise the possibility that it could be involved in inducing leukocyte infiltration in acne and seborrheic dermatitis. Although fatty acids in sebum are initially present in the form of triglycerides, they are subsequently freed by bacterial lipases so that in skin surface lipids they are present principally in the free form (42, 43). Furthermore, sebaceous glands have been reported to possess 5-LO activity (34), which, along with inflammatory cells, could convert sebaleic acid to 5-HODE. 5-HODE could then be oxidized to 5-oxo-ODE by 5-HEDH present in keratinocytes or inflammatory cells.
This work was supported by Canadian Institutes of Health Research Grant MOP-6254 (to W. S. P.), the J. T. Costello Memorial Research Fund, and National Institutes of Health Grant HL81873 (to J. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PUFA, polyunsaturated fatty acid(s); 13-HODE, 13S-hydroxy-(9Z,11E)-octadecadienoic acid; 5,18-diHODE, 5S,18-dihydroxy-(6E,8Z)-octadecadienoic acid; 5-HEDH, 5-hydroxyeicosanoid dehydrogenase; 5-HETE, 5S-hydroxy-(6E,8Z,11Z,14Z)-eicosatetraenoic acid; 5-, 5S-, and 5R-HODE, 5-, 5S-, and 5R-hydroxy-(6E,8Z)-octadecadienoic acid, respectively; 5-LO, 5-lipoxygenase; 5-oxo-18-HODE, 5-oxo-18-hydroxy-(6E,8Z)-octadecadienoic acid; 5-oxo-ETE, 5-oxo-(6E,8Z,11Z,14Z)-eicosatetraenoic acid; LTB4, leukotriene B4; 5-oxo-ODE, 5-oxo-(6E,8Z)-octadecadienoic acid; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; FTMS, Fourier transform mass spectrometry; ODS, octadecylsilyl; PBS, phosphate-buffered saline; PMA, phorbol 12-myristate 13-acetate; RP, reversed-phase; HPLC, high performance liquid chromatography; sebaleic acid, (5Z,8Z)-octadecadienoic acid; tBuOOH, tert-butyl-hydroperoxide; ME, methyl ester.
References
- 1.Stewart, M. E., Downing, D. T., Pochi, P. E., and Strauss, J. S. (1978) Biochim. Biophys. Acta 529 380–386 [DOI] [PubMed] [Google Scholar]
- 2.Nicolaides, N. (1974) Science 186 19–26 [DOI] [PubMed] [Google Scholar]
- 3.Ge, L., Gordon, J. S., Hsuan, C., Stenn, K., and Prouty, S. M. (2003) J. Invest. Dermatol. 120 707–714 [DOI] [PubMed] [Google Scholar]
- 4.Pappas, A., Anthonavage, M., and Gordon, J. S. (2002) J. Invest. Dermatol. 118 164–171 [DOI] [PubMed] [Google Scholar]
- 5.Thody, A. J., and Shuster, S. (1989) Physiol. Rev. 69 383–416 [DOI] [PubMed] [Google Scholar]
- 6.Wille, J. J., and Kydonieus, A. (2003) Skin Pharmacol. Appl. Skin Physiol. 16 176–187 [DOI] [PubMed] [Google Scholar]
- 7.Funk, C. D. (2001) Science 294 1871–1875 [DOI] [PubMed] [Google Scholar]
- 8.Borgeat, P., and Samuelsson, B. (1979) Proc. Natl. Acad. Sci. U. S. A. 76 3213–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell, W. S., Gravelle, F., and Gravel, S. (1992) J. Biol. Chem. 267 19233–19241 [PubMed] [Google Scholar]
- 10.Powell, W. S., and Rokach, J. (2005) Prog. Lipid Res. 44 154–183 [DOI] [PubMed] [Google Scholar]
- 11.Brink, C., Dahlen, S. E., Drazen, J., Evans, J. F., Hay, D. W., Rovati, G. E., Serhan, C. N., Shimizu, T., and Yokomizo, T. (2004) Pharmacol. Rev. 56 149–157 [DOI] [PubMed] [Google Scholar]
- 12.Hosoi, T., Sugikawa, E., Chikada, A., Koguchi, Y., and Ohnuki, T. (2005) Biochem. Biophys. Res. Commun. 334 987–995 [DOI] [PubMed] [Google Scholar]
- 13.Jones, C. E., Holden, S., Tenaillon, L., Bhatia, U., Seuwen, K., Tranter, P., Turner, J., Kettle, R., Bouhelal, R., Charlton, S., Nirmala, N. R., Jarai, G., and Finan, P. (2003) Mol. Pharmacol. 63 471–477 [DOI] [PubMed] [Google Scholar]
- 14.O'Flaherty, J. T., Taylor, J. S., and Kuroki, M. (2000) J. Immunol. 164 3345–3352 [DOI] [PubMed] [Google Scholar]
- 15.Khanapure, S. P., Shi, X. X., Powell, W. S., and Rokach, J. (1998) J. Org. Chem. 63 337–342 [Google Scholar]
- 16.Zamboni, R., and Rokach, J. (1982) Tetrahedron Lett. 23 2631–2634 [Google Scholar]
- 17.Hamberg, M., and Samuelsson, B. (1967) J. Biol. Chem. 242 5329–5335 [PubMed] [Google Scholar]
- 18.Ludwig, S. A., and Sprecher, H. (1979) Arch. Biochem. Biophys. 197 333–341 [DOI] [PubMed] [Google Scholar]
- 19.Powell, W. S. (1987) Anal. Biochem. 164 117–131 [DOI] [PubMed] [Google Scholar]
- 20.Erlemann, K. R., Cossette, C., Gravel, S., Stamatiou, P. B., Lee, G. J., Rokach, J., and Powell, W. S. (2006) Biochem. Biophys. Res. Commun. 350 151–156 [DOI] [PubMed] [Google Scholar]
- 21.Parmentier, C., Leroy, P., Wellman, M., and Nicolas, A. (1998) J. Chromatogr. B Biomed. Sci. Appl. 719 37–46 [DOI] [PubMed] [Google Scholar]
- 22.Powell, W. S. (1980) Prostaglandins 20 947–957 [DOI] [PubMed] [Google Scholar]
- 23.Schneider, C., Boeglin, W. E., and Brash, A. R. (2000) Anal. Biochem. 287 186–189 [DOI] [PubMed] [Google Scholar]
- 24.Powell, W. S., MacLeod, R. J., Gravel, S., Gravelle, F., and Bhakar, A. (1996) J. Immunol. 156 336–342 [PubMed] [Google Scholar]
- 25.Monneret, G., Li, H., Vasilescu, J., Rokach, J., and Powell, W. S. (2002) J. Immunol. 168 3563–3569 [DOI] [PubMed] [Google Scholar]
- 26.Hansson, G., Lindgren, J. Å., Dahlén, S. E., Hedqvist, P., and Samuelsson, B. (1981) FEBS Lett. 130 107–112 [DOI] [PubMed] [Google Scholar]
- 27.O'Flaherty, J. T., Wykle, R., Redman, J., Samuel, M., and Thomas, M. (1986) J. Immunol. 137 3277–3283 [PubMed] [Google Scholar]
- 28.Powell, W. S. (1987) Biochem. Biophys. Res. Commun. 145 991–998 [DOI] [PubMed] [Google Scholar]
- 29.Powell, W. S., and Gravelle, F. (1990) J. Biol. Chem. 265 9131–9139 [PubMed] [Google Scholar]
- 30.Erlemann, K. R., Cossette, C., Gravel, S., Lesimple, A., Lee, G. J., Saha, G., Rokach, J., and Powell, W. S. (2007) Free Radic. Biol. Med. 42 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell, W. S., Gravelle, F., and Gravel, S. (1994) J. Biol. Chem. 269 25373–25380 [PubMed] [Google Scholar]
- 32.Erlemann, K. R., Cossette, C., Grant, G. E., Lee, G. J., Patel, P., Rokach, J., and Powell, W. S. (2007) Biochem. J. 403 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer, F. Q., and Buettner, G. R. (2001) Free Radic. Biol. Med. 30 1191–1212 [DOI] [PubMed] [Google Scholar]
- 34.Alestas, T., Ganceviciene, R., Fimmel, S., Muller-Decker, K., and Zouboulis, C. C. (2006) J. Mol. Med. 84 75–87 [DOI] [PubMed] [Google Scholar]
- 35.Muhammad, I., Zhao, J., Dunbar, D. C., and Khan, I. A. (2002) Phytochemistry 59 105–110 [DOI] [PubMed] [Google Scholar]
- 36.Valentova, K., and Ulrichova, J. (2003) Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 147 119–130 [PubMed] [Google Scholar]
- 37.Bianchi, A. (2003) Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2 30–36 [Google Scholar]
- 38.Zheng, B. L., He, K., Kim, C. H., Rogers, L., Shao, Y., Huang, Z. Y., Lu, Y., Yan, S. J., Qien, L. C., and Zheng, Q. Y. (2000) Urology 55 598–602 [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi, Y., Shimazaki, T., Kawajiri, K., Shimizu, T., Seyama, Y., and Sato, F. (1994) Biochim. Biophys. Acta 1215 280–284 [DOI] [PubMed] [Google Scholar]
- 40.Powell, W. S., Rokach, J., Khanapure, S. P., Manna, S., Hashefi, M., Gravel, S., MacLeod, R. J., Falck, J. R., and Bhatt, R. K. (1996) J. Pharm. Exp. Ther. 276 728–736 [PubMed] [Google Scholar]
- 41.Powell, W. S., Gravel, S., MacLeod, R. J., Mills, E., and Hashefi, M. (1993) J. Biol. Chem. 268 9280–9286 [PubMed] [Google Scholar]
- 42.Toyoda, M., and Morohashi, M. (2001) Med. Electron Microsc. 34 29–40 [DOI] [PubMed] [Google Scholar]
- 43.Marples, R. R., Downing, D. T., and Kligman, A. M. (1971) J. Invest. Dermatol. 56 127–131 [DOI] [PubMed] [Google Scholar]












