Abstract
The biosynthesis of DIMBOA, a pesticidal secondary metabolite of maize, branches off the tryptophan pathway. We have previously demonstrated that indole is the last intermediate common to both the tryptophan and hydroxamic acid pathways. The earliest discovered mutant in the DIMBOA pathway, bxbx (benzoxazineless), is deficient in the production of DIMBOA and related compounds. This paper presents evidence that a gene identified by Kramer and Koziel [Kramer, V. C. & Koziel, M. G. (1995) Plant Mol. Biol. 27, 1183–1188] as maize tryptophan synthase α (TSA) is the site of the genetic lesion in the DIMBOA-deficient mutant maize line bxbx. We demonstrate that the TSA gene has sustained a 924-bp deletion in bxbx compared with its counterpart in wild-type maize. We report that the TSA gene maps to the same location as the bxbx mutation, on the short arm of chromosome 4. We present evidence that the very early and very high level of expression of TSA corresponds to the timing and level of DIMBOA biosynthesis but is strikingly different from the expression of the maize tryptophan synthase β (TSB) genes. We show that feeding indole to bxbx seedlings restores their ability to synthesize DIMBOA. We conclude that the maize enzyme initially named tryptophan synthase α in fact is a DIMBOA biosynthetic enzyme, and we propose that it be renamed indole synthase. This work confirms and enlarges upon the findings of Frey et al. [Frey, M. Chomet, P., Glawischniq, E., Stettner, C., Grün, S., Winklmair, A., Eisenreich, W., Bacher, A., Meeley, R. B., Briggs, S. P., Simcox, K. & Gierl, A. (1997) Science 277, 696–699], which appeared while the present paper was in review.
Cyclic hydroxamic acids (Hx), which occur as glucosides in several grasses, including rye, wheat, and maize (1), and a few dicots (2), are of agricultural importance as chemical defense compounds. DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one), the aglycone liberated in damaged tissue by the action of a glucosidase, is toxic to a broad range of insects, bacteria, and fungi, including northern leaf blight (3, 4), Agrobacterium tumefaciens (5), the European, Asian, and southwestern corn borers (6–10), and aphids (11–16).
DIMBOA-glucoside is not present in the embryo or endosperm of cereals (17, 18) but can be isolated from both roots and shoots immediately after germination (17–19). In maize shoots, the concentration of DIMBOA-glucoside peaks at about 1–10 mmols⋅kg−1 fresh weight (f.w.) about 7 days after germination, and by approximately 15 days later levels have declined to around 0.3–3 mmols⋅kg−1 f.w. (19). It is not known whether the biosynthesis of DIMBOA-glucoside is shut down as the plant matures (and thus the reserve of DIMBOA-glucoside is diluted by growth) or whether biosynthesis continues at a rate insufficient to keep up with growth.
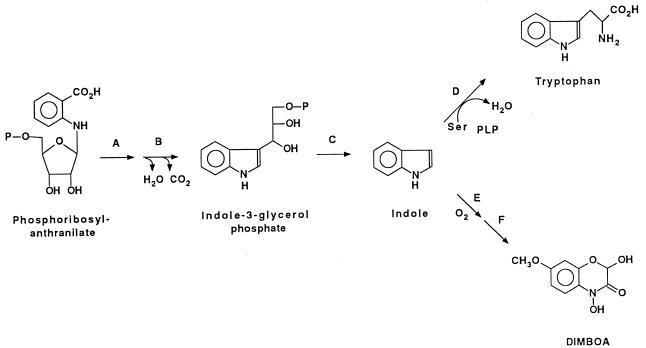
We are engaged in a collaborative effort to identify the intermediates, characterize the enzymes, and clone the genes involved in the biosynthetic pathway (Fig. 1) of DIMBOA. Our earlier work (20) has demonstrated that maize converts 2-14C-indole into 14C-DIMBOA, and that perdeuterated indole is converted by maize into DIMBOA that is deuterated both in the benzene ring and at the hemiacetal carbon of the heterocyclic ring. These results demonstrate that the DIMBOA biosynthetic pathway utilizes indole as a direct intermediate and raise the question of whether a specific indole synthase exists for this pathway or whether indole is diverted from the tryptophan biosynthetic pathway.
Figure 1.
Schematic of the biosynthetic pathway of DIMBOA and tryptophan. (A) Phosphoribosyl-anthranilate isomerase. (B) Indole-3-glycerol phosphate synthase. (C) Indole synthase (tryptophan synthase α). (D) Tryptophan synthase β. (E) A series of subsequent cytochrome P450-mediated oxygenation steps. (F) Methyl transferase.
Kramer and Koziel (21) have cloned a maize cDNA whose protein catalyzes indole synthesis. They identified it as a tryptophan synthase alpha (TSα) subunit gene. Evidence supporting this assignment appears indisputable: (i) its cDNA is homologous to trpA genes from bacteria and yeast and the TSA1 cDNA from Arabidopsis thaliana (22), and (ii) it complements a tryptophan synthase alpha mutant Escherichia coli. The function of tryptophan synthase alpha is the conversion of indole-glycerol-3-phosphate to indole; thus, a tryptophan synthase alpha is formally an indole synthase. The expression pattern of TSA (21) led us to investigate whether it might indeed be a gene specific for the DIMBOA pathway. We found that transcript levels of TSA are very high in young maize seedlings, long before they have exhausted endosperm tryptophan. Moreover, we found that the expression of TSA decreases as the plant grows. This expression pattern would be surprising for a tryptophan biosynthetic gene, but it correlates well with the levels of DIMBOA in the developing maize plant. Therefore, we investigated whether this gene might be dedicated to biosynthesis of DIMBOA and not of tryptophan. We present here evidence from several experimental approaches that demonstrates that it is indeed a part of the DIMBOA pathway.
MATERIALS AND METHODS
Plant Material.
As sources of material for DNA or RNA isolation, we germinated axenic seedlings from maize inbred line FRB73, from DIMBOA-deficient mutant lines, bxbx (23) and 14–75 (W.S.C., unpublished data), and from a crossed (W64 × bx) maize line. For DNA isolation, seedlings were harvested 2–4 days after germination, quick-frozen in liquid nitrogen, and stored at −80°C. For mRNA isolation, seedlings were harvested 10 days and 30 days after germination, quick-frozen, and stored as above. To test harvested seedlings for phenotype (Hx+ or Hx−), a 2- to 3-cm portion of the roots from each seedling was crushed and dipped into 5% methanolic ferric chloride. A blue to indigo color, indicative of the presence of hydroxamic acids and/or their glucosides, was taken as evidence that the seedling was Hx positive.
Total RNA Isolation.
RNA was isolated from shoots using a method first described by Kirby (24) and then modified by Parish and Kirby (25). The total RNA concentration and purity was quantified by UV spectrophotometric analysis.
DNA Isolation.
DNA was isolated from shoots using the CTAB (cetyltrimethylammonium bromide) protocol described by Murray and Thompson (26). DNA concentrations were determined fluorimetrically in a DyNA Quant 200 fluorometer (Hoefer) with Hoechst H 33258 dye.
32P-Labeling of DNA Probes.
Cloned DNA fragments were electrophoretically separated from the vector on preparative agarose gels and purified by the QIAquick gel extraction kit (Qiagen). The following probes were used in the course of this work: TSA, the maize tryptophan synthase alpha subunit cDNA (21); gen3–1, the corresponding genomic clone (both kindly provided by Vance Kramer, Novartis, Research Triangle Park, NC); and TSB1 and TSB2, the maize tryptophan synthase beta subunit cDNAs (kindly provided by Karen Cone, Univ. of Missouri, Columbia) (27). Probe fragments were labeled with [α-32P]dCTP (3,000 Ci/mmol; 1 Ci = 37 GBq) using an oligolabeling kit (Pharmacia). Unincorporated nucleotides were removed from labeled probes by the QIAquick nucleotide removal kit (Qiagen).
Northern Blot Analysis.
Total RNA was fractionated on a 1.2% agarose, 37% formaldehyde denaturing gel in 1× MSE (0.2 M Mops free acid/50 mM NaOAc/10 mM EDTA, pH 7.0). Because DNA controls often were included in Northern blots, gels were subjected to brief denaturation/neutralization as described in Sambrook et al. (28) before being blotted overnight on Hybond N+ nylon membranes according to the protocol suggested by the manufacturer (Amersham). Nucleic acids were fixed to the membrane by UV irradiation in a StrataLinker (Stratagene). Membranes were subjected to the hybridization procedure of Church and Gilbert (29) using labeled probe at a concentration of ≈0.2–1.0 × 106 cpm/ml of hybridization solution. Membranes were washed once for 15 min at 65°C in buffer I and twice for 15 min at 65°C in buffer II (29) before autoradiography on Kodak X-Omat film (2.5–24 hr at −70°C with two intensification screens).
PCR Analysis.
Oligonucleotides specific to the region of interest in the TSA genomic clone were designed using the software program primer (Scientific and Educational Software, Durham, NC). The sequences of the primers (TSA1180 and TSA2522c) are CAACGCTCCTCCATCCTGTA and GCCAAGCTGCGAGACATGAT, respectively. The GeneAmp PCR Kit and PCR System 9600 (Perkin–Elmer Cetus) were used to amplify products from genomic DNA from both the bxbx and FRB73 maize lines. The products were separated electrophoretically from the unincorporated primers and dNTPs, and the DNA was purified with the QIAquick gel extraction kit (Qiagen). The purified PCR products were then cloned into the PCR2.1 vector (Invitrogen) according to the manufacturer’s instructions. The resulting clones were sequenced using the primers described above and the Applied Biosystems Dye-Deoxy Terminator sequencing kit and Applied Biosystems Automated DNA Sequencer. The sequence was compared with the published sequence for the maize TSA genomic clone (accession number X76713) using align plus 2.0 (Scientific and Educational Software).
Indole Feeding Experiments.
Surface-sterilized seeds of bxbx and FRB73 maize lines were germinated on soft agar containing 2% sucrose (to facilitate detection of fungal contamination). When the emerging shoot reached a length of 3–5 mm and the root was 1–2 cm long, the endosperm was removed and plantlets were transferred to individual culture tubes containing 3 ml of sterile medium consisting of White’s salts (Sigma) supplemented with 20 mg/ml sucrose with or without addition of 0.1 mg/ml indole. Plantlets were grown under continuous light for 8 days.
Orange Pericarp (orp) Recessive Lethal Mutant.
Seed carrying the recessive lethal mutation orp1orp1/orp2orp2 produce maize plants auxotrophic for tryptophan that are mutated in both of the TSB genes but still produce indole (27). Orange pericarp (orp1orp1/orp2orp2) seeds and sibling yellow pericarp (orp1/orp1Orp2) seeds were coated with Captan and germinated on filter paper moistened with water containing White’s mineral salts (Sigma). Seedlings were harvested 2 weeks after planting and stored frozen (−20°C) until analyzed for DIMBOA content.
Extraction and Quantitation of DIMBOA.
Plants were harvested, blotted to remove adhering medium, and separated into roots and shoots before weighing. Pooled shoots or roots were frozen in liquid nitrogen and ground to a fine powder. After warming to room temperature, 5 ml water per gram of tissue was added and the samples were incubated for 1 hr at 22°C (to allow for hydrolysis of DIMBOA-glucoside by endogenous glucosidases). After centrifugation to clear the extract, the supernatant was filtered to remove any dislodged pellet particles and extracted four times with an equal volume of ethyl acetate. Pooled extracts were dried with anhydrous sodium sulfate, and the solvent was removed in a rotary evaporator. The solid residue was dissolved in 1 ml methanol per gram extracted tissue f.w. (one tissue equivalent concentration) for HPLC analysis. HPLC analysis was conducted on a 25-cm Bioadvantage (Thomson Instrument Co., Springfield, VA) RP C-18 column eluted isocratically with 10% tetrahydrofuran in water containing 0.5 ml formic acid per liter (DIMBOA retention time, 8.4 min.).
RESULTS
Involvement of TSA in Tryptophan Biosynthesis.
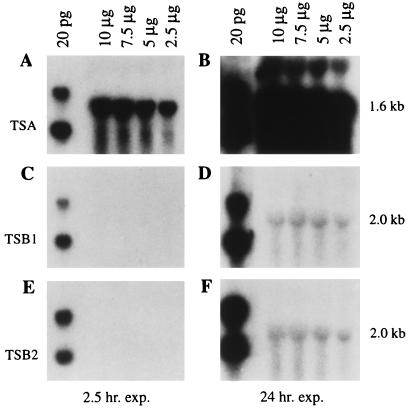
As part of a series of tests to determine whether Kramer’s gene is a part of the tryptophan biosynthetic pathway, we examined steady-state levels of mRNA for the two maize TSB genes vs. that of Kramer’s maize TSA gene by Northern blot analysis. To probe the Northern blots of total RNA from wild-type maize seedlings (FRB73) we used the TSA cDNA and each of the TSB cDNAs: TSB1 and TSB2. We found that the TSB gene transcripts are expressed at dramatically lower levels in wild-type maize than are TSA gene transcripts at 10 days after germination (Fig. 2).
Figure 2.
Analysis of TSA, TSB1, and TSB2 mRNAs in wild-type maize seedlings. Replicate blots contain 10, 7.5, 5, and 2.5 μg total RNA from the shoots of 10-day-old FRB73 maize seedlings and 20 pg of EcoRI-digested TSA (A and B), TSB1 (C and D), and TSB2 (E and F) cDNA clones. The blots were probed with the EcoRI insert from the TSA cDNA (A and B), TSB1 cDNA (C and D), and TSB2 cDNA (E and F), respectively.
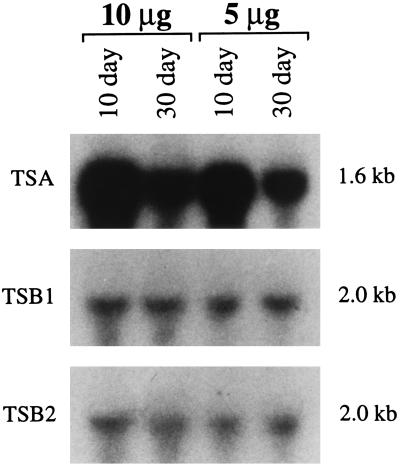
Time Course of Expression of TSA in Maize.
Confirming Kramer and Koziel’s earlier work (21) we found that the steady-state level of transcripts from the TSA gene is very high in 10-day-old seedlings, decreasing 2- to 3-fold in 30-day-old plants (Fig. 3). In contrast, the transcripts of the TSB genes are present at the same low steady-state level in 10- and 30-day-old maize.
Figure 3.
Developmental expression of the TSA, TSB1, and TSB2 transcripts in wild-type maize seedlings. Replicate blots contain 10 and 5 μg of total RNA from the shoots of 10- and 30-day-old FRB73 seedlings. The blots were probed with the EcoRI fragment from TSA, TSB1, and TSB2 cDNAs, respectively.
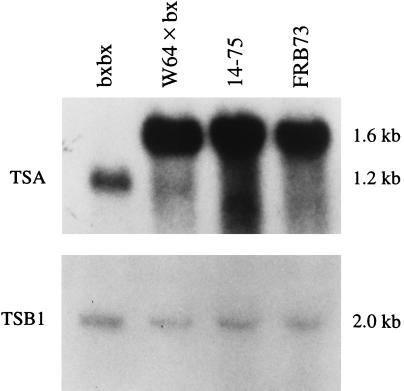
Aberrant Expression of TSA in a DIMBOA-Deficient Mutant Maize Line.
To test whether expression of the TSA gene might be disrupted in any of the known DIMBOA-deficient maize mutant lines, we used labeled-TSA cDNA to probe Northern blots of total RNA from two DIMBOA-deficient mutant maize lines whose genetic lesion was as yet unassigned (14–75 and bxbx) and from wild-type maize (FRB73). We found that there is very little TSA mRNA in 10-day-old bxbx seedlings, whereas TSA is highly expressed in both wild type and mutant 14–75 (Fig. 4). Moreover, the TSA transcript that does hybridize in bxbx seedlings is significantly smaller than the RNA from wild type or 14–75 seedlings: 1.2 kb rather than 1.6 kb. There is no difference between TSB transcript level in either of the mutant lines compared with wild type (Fig. 4).
Figure 4.
RNA gel blot analysis of TSA and TSB1 expression in mutant and wild-type maize lines. Each lane contains 5 μg total RNA from the shoots of 10-day-old bxbx seedlings, W64 × bx seedlings, 14–75 seedlings, and FRB73 seedlings, respectively. (Upper) Blot probed with the EcoRI insert from the TSA cDNA. (Lower) Blot probed with the EcoRI insert from the TSB1 cDNA.
Mapping of TSA in Maize.
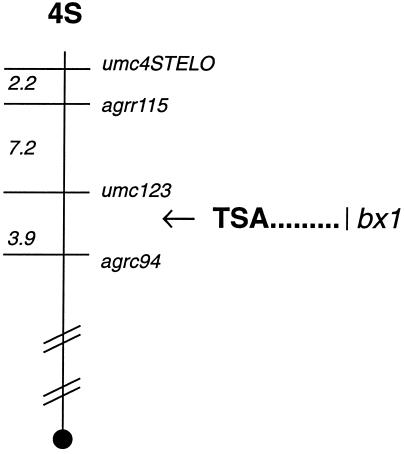
Due to the aberrant expression of TSA in the bxbx maize line, we suspected that the TSA gene was the site of the bx mutation. If so, then TSA must lie in the same position as the bx mutation on the maize genetic map. By probing blots of the CoTx recombinant inbred lines with the TSA gene, we found that it maps on the short arm of chromosome 4 between the umc123 and the Agrc94 markers, which is at the same location as the bxbx mutation (30) (Fig. 5).
Figure 5.
Map location of TSA and bxbx on the short arm of maize chromosome 4. The diagram shows a representation for the short arm of maize chromosome 4 (not drawn to scale). Morphological and molecular markers are indicated on the right of the chromosome. Numbers on the left represent distance in centimorgans for markers that have been mapped with molecular probes; the positions correspond to those compiled by the Maize Genetic Database. A solid circle indicates the centromere. The arrow indicates the position of the cloned TSA gene as determined by restriction fragment length polymorphism (RFLP) mapping.
TSA Gene Structure in Mutant Maize Lines.
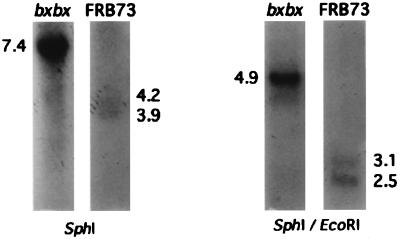
To determine whether the truncated message in the bxbx maize line reflected a genomic deletion, we conducted Southern blot analyses of genomic DNA from bxbx and wild-type FRB73 maize. To test for the presence of the two SphI sites near the first exon of TSA (21) we analyzed Southern blots of FRB73 and bxbx DNA digested with SphI. Because the flanking SphI sites are beyond the known map, the exact size of the hybridizing fragments could not be predicted, but the TSA probe, indicated in Fig. 6, should hybridize to two SphI fragments of molecular weight >2.5 kb. We observed bands of 4.2 and 3.9 kb, from which we deduced the approximate positions of two flanking SphI sites. SphI-digested bxbx DNA, in contrast, exhibited a single 7.4-kb hybridizing fragment (Fig. 6), indicating a deletion of ca. 0.7 kb of DNA encompassing the two central SphI sites and formation of a fusion fragment of 7.4 kb.
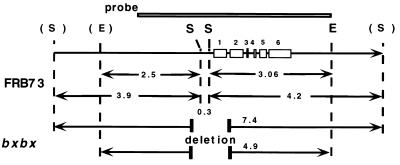
Figure 6.
Southern analysis of the TSA gene structure in bxbx and FRB73 maize lines. In the Southern blot lanes 1–4 contain 5 μg of SphI- or EcoRI/SphI-digested genomic DNA from bxbx or FRB73 maize seedlings. The blot was probed with the EcoRI fragment from the TSA genomic clone (as shown in the schematic). The cartoon is a schematic of the TSA gene structure and the location of the proposed deletion in the bxbx maize line. The open boxes indicate the TSA exons. The restriction sites shown are EcoRI (E) and SphI (S); the restriction sites in bold are known from the sequence of the TSA genomic clone, and the sites in parentheses are inferred from the Southern data above.
Southern analysis of the EcoRI/SphI digest of wild-type (FRB73) maize DNA revealed the expected 3.06-kb fragment and a 2.5-kb band indicative of the postulated EcoRI site on the left-hand side of the map. Again, bxbx DNA digested with EcoRI/SphI revealed a fusion fragment (4.9 kb), confirming that bxbx has a genomic deletion of ca. 0.7 kb including the central SphI sites.
Based on the results of the Southern analysis, we designed PCR primers to flank a 1350-bp sequence encompassing the proposed deletion. These primers amplified from the bxbx genomic DNA a 450-bp fragment, whereas from the wild-type (FRB73) DNA they produced the expected 1350-bp fragment. This suggested a deletion of ca. 900 bp.
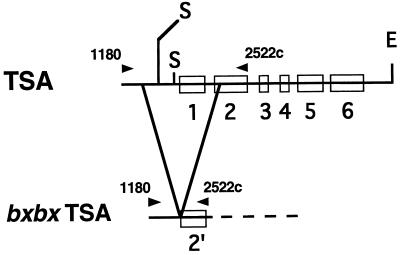
Comparison of the sequence of the product amplified from bxbx and the published sequence of the maize TSA gene (accession number X76713) revealed a deletion from base pair 1356 to base pair 2280 (924 bp), spanning 473 bp of the promoter/leader region, the entire first exon, first intron, and a portion of the second exon (Fig. 7). Back-extrapolation of the ORF that remains in the mutant reveals that there is no upstream ATG in frame that could allow leaky (partial) expression of enzyme activity. It appears that the deletion should totally inactivate this indole synthase gene.
Figure 7.
Schematic structure of the TSA gene and the location of the deletion in the bxbx maize line. The open boxes on the line represent the gene exons. The arrowheads above the line represent the location of the two PCR primers: TSA1108 and TSA2522c. The restriction sites shown are EcoRI (E) and SphI (S).
DIMBOA Production in bxbx Is Rescued by Feeding Indole.
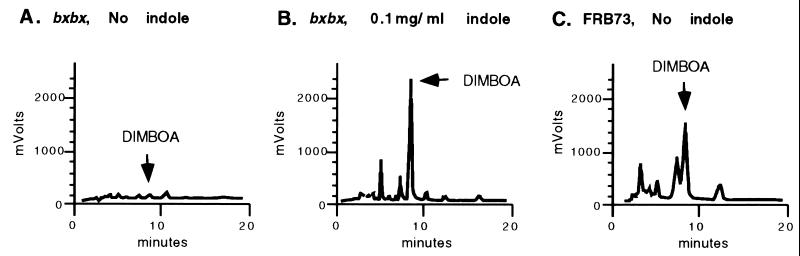
If the defect in DIMBOA production in bxbx is due to a block in indole synthase, feeding indole should reverse the deficiency. Indole was administered to seedlings as described in Materials and Methods. Ethyl acetate extracts from pools of eight shoots each were analyzed by HPLC. Shoots of FRB73 grown on 2% sucrose plus 0.1 mg/ml indole exhibited only a small increase in DIMBOA concentration as compared with shoots grown on sucrose alone. These concentrations are not substantially different from the concentration found in shoots of the same-age FRB73 plants grown from intact seeds in germination paper. In contrast, DIMBOA levels in shoots of the DIMBOA-deficient bxbx mutant, when fed indole, increased to 150% of the levels of FRB73 (Fig. 8).
Figure 8.
Reversed-phase HPLC of metabolites of bxbx mutant and FRB73 maize lines grown on salts and 2% sucrose without (A and C) and with (B) supplementation with 0.1 mg/ml indole.
Levels of DIMBOA in the Tryptophan Auxotroph orp Are in the Normal Range.
To confirm earlier evidence that tryptophan is not in the DIMBOA biosynthetic pathway, we exploited the tryptophan auxotrophic mutant orp, which has a homozygous recessive lethal mutation in both the TSB genes. Maize shoots of genotype tsb1/tsb1TSB2/TSB2 (yellow pericarp) contained 0.57 mmol DIMBOA/kg f.w., whereas those of genotype tsb1/tsb1/tsb2tsb2 (orange pericarp) contained 0.32 mmol DIMBOA/kg f.w.
DISCUSSION
Although the DIMBOA biosynthetic pathway formally shares several steps with that of tryptophan (Fig. 1), 14C-labeled tryptophan is not incorporated into DIMBOA (31). However, we have recently reported that the DIMBOA pathway does utilize indole as an intermediate (20). In bacteria and fungi, tryptophan synthase is an α2β2 tetrameric enzyme complex that catalyzes the conversion of indole-3-glycerol phosphate and serine to tryptophan via the channeling of the intermediate indole from the α subunit to the β subunit (32–37). In E. coli it has been demonstrated that the presence of serine (substrate for the TSβ subunit) activates the TSα subunit (38). The TSα subunit of Arabidopsis thaliana, when expressed in E. coli, was not active by itself, nor could it catalyze production of indole when coexpressed with the E. coli TSβ subunit (22). Only the A. thaliana TSβ subunit could activate it. In contrast, Kramer and Koziel (21) found that the maize TSα subunit did complement an E. coli trpA mutation without the need for any maize TSβ subunits. This suggests that the maize TSα subunit is capable of producing indole on its own. However, the possibility that the maize TSα subunit, unlike that of A. thaliana, is capable of forming a functional tetrameric complex with E. coli TSβ subunits is not ruled out.
Northern blot comparison of the steady-state levels of the TSA gene transcripts and those of the two TSB genes indicates that the TSA gene is expressed at dramatically higher levels than either of the two TSB genes. This supports the idea of functionality of the maize TSα subunit independent of TSβ subunits: transcripts of subunits of a multimeric enzyme complex would likely be expressed coordinately, i.e., at similar levels and at the same time in development. Although the bxbx homozygous line has a deletion encompassing the 5′ end of the TSA gene, the mutation is not lethal, whereas the orp homozygous mutation in both the TSB genes is lethal. We infer that there must be one or more additional genes encoding TSα subunits that are functional in the tryptophan pathway.
We conclude that the cDNA copied from mRNA of very young maize pith by Kramer and Koziel (21) and designated trpA (TSA) in fact encodes a DIMBOA biosynthetic enzyme that is TSβ independent, and we propose that TSA be renamed indole synthase. The α subunit of tryptophan synthase is formally an indole synthase. We hypothesize that at least one other TSA gene must exist and be expressed at a later developmental stage for the production of tryptophan after exhaustion of seed storage proteins from the endosperm.
There are several examples of duplication of tryptophan pathway genes (39–42) with substantial evidence that one acts in tryptophan production while the other affords intermediates for production of defensive secondary metabolites (43–45). In Ruta graveolens, one of the duplicate genes for anthranilate synthase alpha (ASα2) is constitutive and the corresponding enzyme is feedback-inhibited by l-tryptophan. The other gene is elicitor-induced, and its product ASα1 is not feedback-inhibited by tryptophan (44). It is plausible that the latter is responsible for the elicitable biosynthesis of anthranilate-derived acridone phytoalexins of Ruta.
In A. thaliana, defensive glucosinolates are produced from tryptophan constitutively, whereas biosynthesis of another defense compound, camalexin, from indole or indole-3-glycerol phosphate is elicitor-induced (46). Like Ruta, Arabidopsis also has duplicate genes for the tryptophan pathway-committing enzyme, anthranilate synthase α, one inducible by pathogens and the other not. In Arabidopsis, TSα and TSβ are also pathogen-induced; although TSβ and tryptophan are not required for synthesis of camalexin, increased tryptophan may be needed for synthesis of wound-inducible glucosinolates.
The biosynthetic pathway to DIMBOA formally shares the intermediate indole with the tryptophan pathway. However, our results show that indole destined for DIMBOA biosynthesis is the product of an indole synthase enzyme independent of the tryptophan pathway and under different regulation (different developmental time of gene expression and much higher level of expression than TSB genes). It is possible that genes dedicated to biosynthesis of DIMBOA extend back to anthranilate synthase, as is the case for the acridone alkaloids of Ruta graveolens and probably also for the phytoalexin of A. thaliana.
In a 1991 review (47), Poulsen and Verpoorte reported that an enzyme in the tryptophan biosynthesis pathway (anthranilate synthase) is feedback-inhibited by micromolar amounts of tryptophan. The maize tryptophan auxotroph orp is mutated in both of the TSB genes (27). Seedlings with this lethal mutation are able to germinate, but growth is arrested at the two-leaf stage upon depletion of tryptophan derived from seed storage proteins. Wright et al. (48) found that 10-day-old seedlings of normal maize contain ca. 0.3 mM free tryptophan, but the same-age orp mutant seedlings only contain 0.09 mM free tryptophan. However, the orp mutants at this stage contain an approximately normal concentration of DIMBOA. Thus, a 3-fold reduction in ambient levels of tryptophan has no effect on the levels of DIMBOA, arguing for independent regulation of the two. This suggests that DIMBOA is produced by an isozyme of the anthranilate synthase that is not regulated in the same manner. The presence of a second unregulated anthranilate synthase enzyme has been reported in some plants (44, 49). One can speculate that the entire tryptophan pathway may be duplicated for independent regulation of DIMBOA biosynthesis.
The mutant bxbx maize line has a deficiency in DIMBOA production, and here we present evidence that the mutation is in the TSA gene. The TSA transcript is not transcribed in bxbx at the levels seen in wild-type maize. Furthermore, the transcript expressed is of lower molecular weight than in wild-type maize. Although such truncation could conceivably be due to altered mRNA splicing or a greater instability of an altered mRNA, our Southern and PCR analyses provide evidence that there is indeed a deletion of 994 bp overlapping the 5′ end of the TSA coding region in bxbx. On examination of predicted ORFs in the deletion mutant, we found that no functional protein would be translated from the mutant mRNA. That TSA maps to the site of the bx mutation confirms that the deletion is indeed responsible for the DIMBOA deficiency in this maize line. Supporting this conclusion, we found that addition of 0.1 mg/ml indole to growth medium of bxbx mutant maize plantlets dramatically increases the DIMBOA concentration.
These findings, however, lead to an important question. If indole synthase is involved in DIMBOA biosynthesis and if there is a deletion in the bxbx indole synthase gene, then why does this line produce any DIMBOA, even a small amount? One idea is that a little indole might “leak” from the tryptophan pathway. It has been demonstrated that most of the indole generated by the TSα subunit is transferred directly to the active site of the TSβ subunit but these studies do not rule out the possible escape of very small amounts of indole from the enzyme complex (50). Perhaps the DIMBOA biosynthesis pathway in bxbx is able to scavenge small amounts of this free indole from the tryptophan biosynthesis pathway. A second possibility is that there is a second pathway leading to the production of DIMBOA that does not require this indole synthase.
Based on the data presented here we conclude that TSA is involved in the DIMBOA biosynthesis pathway and that the bxbx maize line is mutated in the TSA gene, and we propose that the bx gene is TSA. Directed alterations of the regulation of DIMBOA biosynthesis may afford an improvement of the crop-protection capability of this natural product.
Acknowledgments
We thank Vance Kramer and Karen Cone for providing cDNA clones. We thank Geneviève Hansen, Martha Wright, Laura Privalle, Dominique Robertson, and Brant Touchette for critical review of the manuscript. We gratefully acknowledge many scientific discussions with Geneviève Hansen. D.M.M thanks Novartis Seeds for providing facilities for a part of the work reported here. This research was supported by a grant from the National Science Foundation Molecular and Cellular Biosciences (9418155).
ABBREVIATIONS
- TSA
tryptophan synthase alpha gene
- TSB1 and TSB2
tryptophan synthase beta gene 1 and 2, respectively
- orp
orange pericarp mutant maize line
- bxbx
benzoxazineless mutant maize line
- FRB73
fungal resistant B73 maize line
- Hx
hydroxamic acids
- DIMBOA
2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one
- TSα and TSβ
tryptophan synthase alpha and beta protein subunit, respectively
- f.w.
fresh weight
References
- 1.Niemeyer H M. Phytochemistry. 1988;27:3349–3358. [Google Scholar]
- 2.Pratt K, Kumar P, Chilton W S. Biochem Syst Ecol. 1995;23:781–785. [Google Scholar]
- 3.Couture R M, Routley D G, Dunn G M. Physiol Plant Path. 1971;1:515–521. [Google Scholar]
- 4.Long B J, Dunn G M, Routley D G. Crop Sci. 1975;15:333–335. [Google Scholar]
- 5.Sahi S V, Chilton M, Chilton W S. Proc Natl Acad Sci USA. 1990;87:3879–3883. doi: 10.1073/pnas.87.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos F, Atkinson J, Arnason J T, Philogene B J R, Morand P, Werstiuk N H, Timmins G. J Chem Ecol. 1989;15:1989–2001. doi: 10.1007/BF01207432. [DOI] [PubMed] [Google Scholar]
- 7.Campos F, Donskov N, Arnason J T, Philogene B J R, Atkinson J, Morand P, Werstiuk N H. J Econ Entomol. 1990;83:357–360. [Google Scholar]
- 8.Xie Y S, Arnason J T, Philogene B J R, Lambert J D H, Atkinson J, Morand P. Can Entomol. 1990;122:1177–1186. [Google Scholar]
- 9.Reid L M, Arnason J T, Nozzolillo C, Hamilton R I. Crop Sci. 1991;31:1496–1502. [Google Scholar]
- 10.Xie Y S, Arnason J T, Philogene B J R, Atkinson J, Morand P. J Chem Ecol. 1992;18:945–957. doi: 10.1007/BF00980055. [DOI] [PubMed] [Google Scholar]
- 11.Cuevas L, Niemeyer H M. Phytochemistry. 1993;34:983–985. [Google Scholar]
- 12.Nicol D, Wratten S D, Eaton N, Copaja S V. Ann Appl Biol. 1993;122:427–433. [Google Scholar]
- 13.Argandona V H, Luza J G, Niemeyer H M, Corcuera L J. Phytochemistry. 1980;19:1665–1668. [Google Scholar]
- 14.Morse S, Wratten S D, Edwards P J, Niemeyer H M. Ann Appl Biol. 1991;119:239–249. [Google Scholar]
- 15.Morse S, Wratten S D, Edwards P J, Niemeyer H M. Ann Appl Biol. 1991;119:251–256. [Google Scholar]
- 16.Nicol D, Copaja S V, Wratten S D, Niemeyer H M. Ann Appl Biol. 1992;121:11–18. [Google Scholar]
- 17.Epstein W M, Rowsemitt C N, Berger P J, Negus N C. J Chem Ecol. 1986;12:2011–2020. doi: 10.1007/BF01041950. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa E, Amano T, Hirai N, Iwamura H. Phytochemistry. 1995;38:1349–1354. [Google Scholar]
- 19.Argandona V H, Corcuera L J. Phytochemistry. 1985;24:177–178. [Google Scholar]
- 20.Desai, S. R., Kumar, P. & Chilton, W. S. (1996) Chem. Commun. 1321.
- 21.Kramer V C, Koziel M G. Plant Mol Biol. 1995;27:1183–1188. doi: 10.1007/BF00020891. [DOI] [PubMed] [Google Scholar]
- 22.Radwanski E R, Zhao J, Last R L. Mol Gen Genet. 1995;248:657–667. doi: 10.1007/BF02191705. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton R H. Weeds. 1964;12:27–30. [Google Scholar]
- 24.Kirby K S. Biochem J. 1965;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parish J H, Kirby K S. Biochim Biophys Acta. 1966;129:554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- 26.Murray M G, Thompson W F. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright A D, Moehlenkamp C A, Perrot G H, Neuffer M G, Cone K C. Plant Cell. 1992;4:711–719. doi: 10.1105/tpc.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 7.37–7.52. [Google Scholar]
- 29.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1993. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simcox K D, Weber D F. Crop Sci. 1985;25:827–830. [Google Scholar]
- 31.Reimann J E, Byerrum R U. Biochemistry. 1964;3:847–851. doi: 10.1021/bi00894a021. [DOI] [PubMed] [Google Scholar]
- 32.Demoss J A. Biochim Biophys Acta. 1962;62:279–293. doi: 10.1016/0006-3002(62)90041-0. [DOI] [PubMed] [Google Scholar]
- 33.Creighton T E. Eur J Biochem. 1970;13:1–10. doi: 10.1111/j.1432-1033.1970.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 34.Matchett W H. J Biol Chem. 1974;249:4041–4049. [PubMed] [Google Scholar]
- 35.Kirschner K, Wiskocil R L, Foehn M, Rezeau L. Eur J Biochem. 1975;60:513–523. doi: 10.1111/j.1432-1033.1975.tb21030.x. [DOI] [PubMed] [Google Scholar]
- 36.Hyde C C, Ahmed S A, Padlan E A, Miles E W, Davies D R. J Biol Chem. 1988;263:17857–17871. [PubMed] [Google Scholar]
- 37.Dunn M F, Aguilar V, Brzovic B, Drewe W F J, Houben K F, Laja C A, Roy M. Biochemistry. 1990;29:8598–8607. doi: 10.1021/bi00489a015. [DOI] [PubMed] [Google Scholar]
- 38.Anderson K S, Miles E W, Johnson K A. J Biol Chem. 1991;266:8020–8033. [PubMed] [Google Scholar]
- 39.Last R L, Bissinger P H, Mahoney D J, Radwanski E R, Fink G R. Plant Cell. 1991;3:345–358. doi: 10.1105/tpc.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruitt K D, Last R L. Plant Physiol. 1993;102:1019–1027. doi: 10.1104/pp.102.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Zhao J, Rose A B, Schmidt R, Last R L. Plant Cell. 1995;7:447–461. doi: 10.1105/tpc.7.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radwanski E R, Last R L. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niyogi K K, Fink G R. Plant Cell. 1992;4:721–733. doi: 10.1105/tpc.4.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohlmann J, Lins T, Martin W, Eilert U. Plant Physiol. 1996;111:507–514. doi: 10.1104/pp.111.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J M, Last R L. Plant Cell. 1996;8:2235–2244. doi: 10.1105/tpc.8.12.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuji J, Zook M, Somerville S C, Last R L, Hammerschmidt R. Physiol Mol Plant Path. 1993;49:221–229. [Google Scholar]
- 47.Poulsen C, Verpoorte R. Phytochemistry. 1991;30:377–386. [Google Scholar]
- 48.Wright A D, Sampson M B, Neuffer M G, Michalczuk L, Slovin J P, Cohen J D. Science. 1991;254:998–1000. doi: 10.1126/science.254.5034.998. [DOI] [PubMed] [Google Scholar]
- 49.Brotherton J E, Hauptmann R M, Widholm J M. Planta. 1986;168:214–221. doi: 10.1007/BF00402966. [DOI] [PubMed] [Google Scholar]
- 50.Lane A N, Kirschner K. Biochemistry. 1991;30:479–484. doi: 10.1021/bi00216a025. [DOI] [PubMed] [Google Scholar]