Abstract
The objective of this study was to establish a new lipoprotein lipase (LPL) and hepatic lipase (HL) activity assay method. Seventy normal volunteers were recruited. Lipase activities were assayed by measuring the increase in absorbance at 546 nm due to the quinoneine dye. Reaction mixture-1 (R-1) contained dioleoylglycerol solubilized with lauryldimethylaminobetaine, monoacylglycerol-specific lipase, glycerolkinase, glycerol-3-phosphate oxidase, peroxidase, ascorbic acid oxidase, and apolipoprotein C-II (apoC-II). R-2 contained Tris-HCl (pH 8.7) and 4-aminoantipyrine. Automated assay of lipase activities was performed with an automatic clinical analyzer. In the assay for HL + LPL activity, 160 μl R-1 was incubated at 37°C with 2 μl of sample for 5 min, and 80 μl R-2 was added. HL activities were measured under the same conditions without apoC-II. HL and LPL activities were also measured by the conventional isotope method and for HL mass by ELISA. Lipase activity detected in a 1.6 M NaCl-eluted fraction from a heparin-Sepharose column was enhanced by adding purified apoC-II in a dose-dependent manner, whereas that eluted by 0.8 M NaCl was not. Postheparin plasma-LPL and HL activities measured in the present automated method had high correlations with those measured by conventional activity and mass methods. This automated assay method for LPL and HL activities is simple and reliable and can be applied to an automatic clinical analyzer.
Keywords: dioleylglycerol, quinonediimine dye, automatic clinical analyzer
Lipoprotein lipase (LPL) plays a central role in lipoprotein metabolism by catalyzing hydrolysis of triglycerides (TGs) in chylomicrons and VLDL particles (1). Hepatic lipase (HL) is synthesized by hepatocytes and bound to heparin sulfate proteoglycans at the surface of liver sinusoidal capillaries, which hydrolyzes TGs and phospholipids in chylomicron remnants, intermediate density lipoproteins (IDLs), and HDLs (1).
Patients with LPL deficiency present with marked hypertriglyceridemia with accumulation of chylomicrons (1–3). Patients with HL deficiency present with hypercholesterolemia and hypertriglyceridemia and accumulation of β-VLDLs, chylomicron remnants, IDLs, and TG-rich LDLs and HDLs (4–9).
The diagnosis of LPL and HL deficiency is based on the lack of their respective enzyme activities in postheparin plasma (PHP) from affected individuals (1). The conventionally available method for measuring LPL and HL activity has been the use of 3H- or 14C-labeled trioleoyl glycerol as substrate in the presence of 1 M NaCl or the anti-LPL monoclonal antibody, 5D2 (10, 11); remaining activity is considered to be HL activity under these conditions. However, this assay procedure is complicated and is not suitable for routine clinical or research work. Methods for measuring LPL or HL protein mass by ELISA in PHP have been developed by several groups, including ours, in the last two decades (10–15). These methods have provided considerable evidence for understanding how the amount of LPL or HL protein changes in variable metabolic disorders. To diagnose LPL or HL deficiency, it is essential to determine the absence of the respective lipase activity in PHP, because there are patients with normal mass and lipase activity for each (1). Very recently, we reported a new and simple assay method for measuring HL activities in PHP using 1,2-dioleylglycerol as a substrate and a specific monoglyceride lipase, producing a violet quinonediimine dye, which does not necessitate isotope-labeled substrate (16).
In consideration of the above, we have developed and established a novel and simple assay system for measuring both LPL and HL activities in PHP using dioleylglycerol as a substrate, which is applicable to an automatic clinical analyzer.
MATERIALS AND METHODS
Materials
Seventy normal volunteers [44 African American (M/F 20/24), 26 Japanese American (M/F 9/17)] were recruited (Table 1).
TABLE 1.
Clinical profile of study subjects
| Total (n = 70)
|
AA (n = 44)
|
JA (n = 26)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age, yr | 41.6 | 12.0 | 3.93 | 11.1 | 45.5 | 12.6 |
| BMI, kg/m2 | 27.0 | 5.3 | 28.3 | 5.6 | 24.7 | 3.8 |
| Total cholesterol, mg/dl | 181 | 37.7 | 176 | 36.4 | 191 | 38.6 |
| Triglycerides, mg/dl | 69.0 | 52–109 | 56.5 | 48–86 | 87.5 | 69–143 |
| RLP-triglycerides, mg/dl | 5.6 | 3.9–9.8 | 5.0 | 3.7–7.5 | 7.5 | 5.1–17.3 |
| HDL-cholesterol, mg/dl | 55.5 | 14.9 | 53.7 | 13.3 | 58.5 | 17.2 |
AA, African American; BMI, body mass index; JA, Japanese American; RLP, remnant-like particle. Data are shown as mean ± SD except for triglycerides. Triglycerides are shown as median (interquartile range).
A statement of institutional approval of the study in accordance with the Declaration of Helsinki was provided, and informed consent was obtained from all of the participants in this study. Exclusion of subjects included those diagnosed with diabetes mellitus, abnormal liver or muscle enzymes, creatinemia, use of lipid-lowering drugs, habitual alcohol intake of more than three standard drinks per day, or endocrinological disorder. Blood samples were obtained following at least 12 h fasting. PHP was obtained 10 min after 60 U/kg intravenous heparin injection. Purified human apolipoprotein C-II (apoC-II) was purchased from Sigma Co. Ltd (St. Louis, MO). All of the assays were done in a blinded fashion.
Method for measuring lipase activities in partially purified HL and LPL fractions
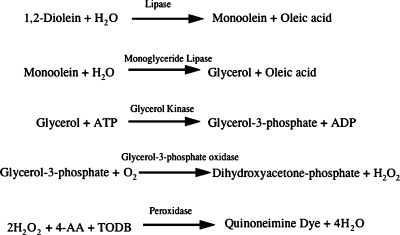
The reaction sequence is shown in Fig. 1.
Fig. 1.
The reaction sequence of the automated method. Hepatic lipase (HL) or lipoprotein lipase hydrolyzes the clear substrate solution to produce a 2-monoglyceride, which in turn releases glycerol by the action of a 2-monoglyceride lipase. Produced glycerol is then assayed by a sequence of enzymatic actions (glycerol kinase, glycerol phosphate oxidase, and peroxidase) that produce a violet quinone monoimine dye. AA, aminoantipyrine; TODB, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-m-toluidone.
HL and LPL were prepared from PHP from a Japanese normal volunteer using heparin-Sepharose column chromatography (14). HL and LPL were eluted with 0.8 M NaCl and 1.6 M NaCl, respectively. Lipase activities were assayed measuring the increase in absorbance at 546 nm (subwave length; 660 nm) due to the production of quinonediimine dye based on the previously described assay procedure (17, 18). Reaction mixture-1 (R-1) contained 0.7 mM dioleylglycerol solubilized with 0.03% lauryldimethylaminobetaine (ampholitic detergent), 3 mM ATP, 3 mM MgCl2, 1.5 mM CaCl2, monoacylglycerol-specific lipase (0.75 U/ml), glycerolkinase (0.75 U/ml), glycerol-3-phosphate oxidase (37.5 U/ml), 0.05% N, N-bis-(4-sulfobutyl)-3-methylaniline-2 Na, peroxidase (3 U/ml), ascorbic acid oxidase (10 U/ml), and partially purified apoC-II fraction obtained from porcine plasma. R-2 contained 200 mM Tris-HCl (pH 9.5), 0.15% 4-aminoantypirine. Automated assay of lipase activity was performed with a Model 7080 Hitachi automatic clinical analyzer. In the lipase assay, 160 μl of R-1 with apoC-II was incubated at 37°C with 2 μl of sample for 5 min, then 80 μl of R-2 was added. The increasing absorbance at 546 nm after 3 min lag time was measured (HL + LPL activity). Simultaneously, HL activities were measured at another channel of the analyzer using R-1 without apoC-II, and then R-2 was added. LPL activities were calculated by deducting HL activity from HL + LPL activity.
Lipase activities generating 1 μmol monoglyceride from diglyceride per min were defined as 1 unit.
Within-run coefficient of variation was 2.2% (n = 10).
Separation of HL and LPL by heparin-Sepharose CL6B
PHP (5 ml) was dialyzed against 1 liter of 1.6 M NaCl containing 10 mM PIPES-NaOH (pH 7.5) at 4°C for 18 h. The solution was dialyzed against 500 ml of 0.3 M NaCl containing 10 mM PIPES-NaOH (pH 7.5) at 4°C for 18 h, and then applied to a heparin-Sepharose CL6B column (1.6 × 2.5 cm). The column was washed with 30 ml of 0.3 M NaCl containing 0.1 mM EDTA, 10% sucrose, 0.025% BSA, and 10 mM PIPES-NaOH (pH 7.5). HL and LPL were eluted with 100 ml of 0.8 M NaCl containing 0.1 mM EDTA, 10% sucrose, 0.025% BSA, 10 mM PIPES-NaOH (pH 7.5), and 50 ml of 1.6 M NaCl containing 0.1 mM EDTA, 10% sucrose, 0.025% BSA, and 10 mM PIPES-NaOH (pH 7.5), respectively. These HL and LPL fractions were concentrated with an Amicone Ultra (molecular cut; 30 kDa), reconstituted with 2 ml of 10 mM PIPES-NaOH (pH 7.5) containing 10% sucrose and 0.1 mM EDTA. The concentrated HL and LPL were stored at −20°C until used.
Method for measuring LPL and HL activities and their mass in PHP by conventional methods
Total lipase activities in PHP were measured using [14C]triolein, based on the previously reported method (10, 11). The remaining activity in the presence of monoclonal antibody 5D2 was defined as HL activity. LPL activities were calculated as the differences between total lipase and HL activities. LPL and HL mass were measured using a sandwich ELISA following the previously described methods (13, 15), with the exception that PHP samples were diluted 1:10 prior to assay for HL mass.
Statistical analysis
Statistical evaluation was performed using Stat View-J 5.0 software (SAS Institute, Cary, NC, on a Macintosh Computer). Pearson's correlation coefficients analysis was carried out. Results were expressed as mean ± SD, and the significance levels were set at P < 0.05.
RESULTS
Lipase activity detected in the fractions eluted by 0.8 M and 1.6 M NaCl using reaction mixtures containing 0.5 mM dioleylglycerol
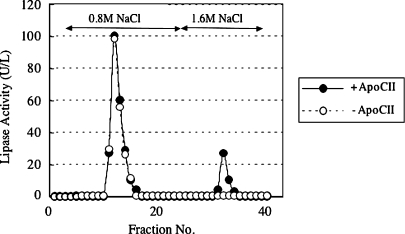
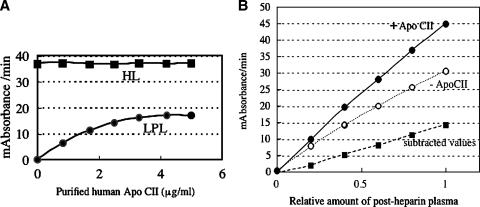
Lipase activities were detected in the fractions of heparin-Sepharose chromatography eluted in 0.8 M NaCl and 1.6 M NaCl (Fig. 2). The lipase activities in the fraction eluted in 0.8 M NaCl were not enhanced at all in the presence of apoC-II, indicating that the activities in this fraction derived from HL, whereas those eluted 1.6 M NaCl were enhanced in a dose-dependent manner, reaching a maximum at an apoC-II concentration of 5 μg/ml (Fig. 3A).
Fig. 2.
Separation of HL and lipoprotein lipase (LPL) by heparin-Sepharose CL6B. Postheparin plasma (PHP) sample (5 ml) was dialyzed against 1 liter of 1.6 M NaCl containing 10 mM PIPES-NaOH (pH 7.5) at 4°C for 18 h. The solution was dialyzed against 500 ml of 0.3 M NaCl containing 10 mM PIPES-NaOH (pH 7.5) at 4°C for 18 h, and then applied to a heparin-Sepharose CL6B column (1.6 × 2.5 cm). The column was washed with 30 ml of 0.3 M NaCl containing 0.1 mM EDTA, 10% sucrose, 0.025% BSA, and 10 mM PIPES-NaOH (pH 7.5). HL and LPL were eluted with 100 ml of 0.8 M NaCl containing 0.1 mM EDTA, 10% sucrose, 0.025% BSA, 10 mM PIPES-NaOH (pH 7.5), and 50 ml of 1.6 M NaCl containing 0.1 mM EDTA, 10% sucrose, 0.025% BSA, and 10 mM PIPES-NaOH (pH 7.5), respectively. These HL and LPL fractions were concentrated with an Amicone Ultra (molecular cut; 30 kDa), reconstituted with 2 ml of 10 mM PIPES-NaOH (pH 7.5) containing 10% sucrose and 0.1 mM EDTA. The concentrated HL and LPL were stored at −20°C until used. Lipase activities were assayed in either the presence or the absence of 5 μg purified human apolipoprotein C-II (apoC-II) as described in Materials and Methods.
Fig. 3.
Effect of addition of purified human apoC-II on HL or LPL activities. A: The lipase activities in the fraction eluted in 0.8 M NaCl (HL) were not enhanced with apoC-II, whereas those eluted in 1.6 M NaCl (LPL) were enhanced in a dose-dependent manner until apoC-II concentration in the reaction mixture was 5 μg/ml. B: Measurement of lipase activities in serially diluted PHP from a volunteer in the presence or absence of purified human apoC-II. Each point represents the average of duplicate values and the duplicate values differed by less than 5%. A 30 milliabsorbance/min at 550nm on the vertical line corresponds to 213 U/l of activities.
Measurement of lipase activities in serially diluted PHP from a volunteer in the presence or absence of purified human apoC-II was conducted (Fig. 3B). Those curves corresponding to total (apoC-II+), HL (apoC-II−), and LPL (subtracted values between total and HL) activities showed linearity.
Correlation between LPL and HL activity by the automated method and those determined by the conventional method in PHP
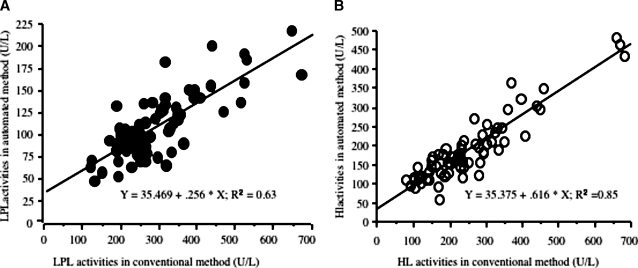
LPL activity in PHP was highly correlated with that by the conventional isotope method (r2 = 0.63) (Fig. 4A) and was similar in both ethnic groups.
Fig. 4.
A: Correlation graph of automated LPL activities in PHP and those in the conventional isotope method in whole subjects (n = 70). B: Correlation graph of automated HL activities in PHP and those in the conventional isotope method in whole subjects (n = 70).
HL activity in PHP also was highly correlated with that by the conventional isotope method (r2 = 0.85) (Fig. 4B).
Correlation of HL activity by the automated method and those determined by the conventional method with HL mass
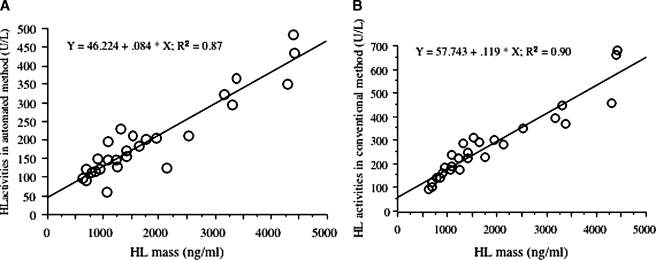
Both the automated and isotope HL activities correlated with HL mass by ELISA [r2 = 0.87 (n = 27), r2 = 0.90 (n = 27), respectively] (Fig. 5).
Fig. 5.
Correlation of automated (A) and isotope (B) HL activity with HL mass by ELISA. N = 27 subjects.
Correlation of LPL activity by the automated method and those determined by the conventional method with LPL mass
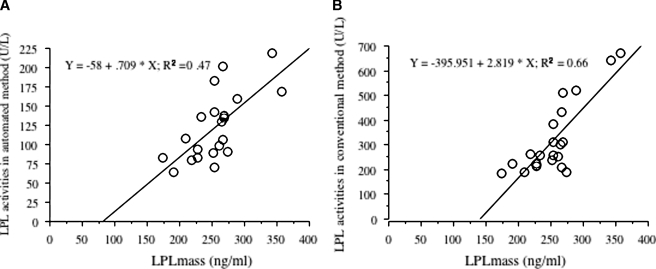
Both the automated and isotope LPL activities correlated with LPL mass by ELISA [r2 = 0.47 (n = 21), r2 = 0.66 (n = 21), respectively] (Fig. 6).
Fig. 6.
Correlation of automated (A) and isotope (B) LPL activity with LPL mass by ELISA. N = 21 subjects.
The automated LPL activity and the HL activity correlated with HDL-cholesterol levels in all subjects (r = 0.20, P = 0.08; r = −0.343, P = 0.004, respectively). The automated HL activity also correlated with plasma TG (r = 0.500, P < 0.0001) and remnant-like particle TG (r = 0.511, P < 0.0001) in all subjects.
DISCUSSION
In the present study, we have described an automated kinetic colorimetric method for assaying LPL and HL activities in PHP by using a natural long-chain fatty acid, 1,2-diglyceride. In the presence of apoC-II, LPL hydrolyzes the clear substrate solution to produce a 2-monoglyceride, which in turn releases glycerol by the action of a 2-monoglyceride lipase. The glycerol produced is then assayed by a sequence of enzymatic actions (glycerol kinase, glycerol phosphate oxidase, and peroxidase) that produce a violet quinone monoimine dye (Fig. 1). One of the key elements of expressing LPL and HL activities in this method was the property of the detergent in the reaction mixture. In a previous study, we reported a new and simple assay method of measuring HL activities in PHP using 1,2-dioleylglycerol as a substrate and a specific monoglyceride lipase, producing a violet quinonediimine dye (16). In that method, we discovered that the existence of polyoxyethylene-nonylphenylether, a nonionic detergent, in the reaction mixture could be a critical factor contributing to expressing the enzyme activities of HL. We assumed that this nonionic detergent would be useful for the determination of LPL activities as well. However, unexpectedly, this detergent was found to strongly inhibit LPL activity (data not shown), prompting us to look for other detergents to obtain optimal conditions for LPL activities. In the present study, we found that an ampholitic detergent, lauryldimethylaminobetaine, at certain concentrations increased both LPL and HL activities.
The result seen in Fig. 2, showing that lipase activities in the 1.6 M NaCl-eluted fraction of the heparin-Sepharose column were detected only in the presence of apoC-II, is consistent with the biochemical property of LPL. The result seen in Fig. 3A confirmed that the 1.6 M NaCl-eluted fraction contained LPL, whereas the 0.8 M NaCl-eluted fraction contained HL. These results indicate that our current method using the ampholytic detergent is useful for measuring both LPL and HL activities, which was not the case with the method using the above-mentioned nonionic detergent.
To further validate the automated LPL and HL activity assays, we compared them with an established isotope method for each and to LPL and HL ELISAs. The automated LPL activity assay correlated highly with both the isotope LPL activity assay and the LPL ELISA, which indicates that each of these three assays are measuring properties of the same protein. The LPL ELISA identifies nonhomodimeric mass as well as homodimer, which is consistent with the intersect of the regression line shown in Fig. 6. The data are remarkable when one considers that both the automated and isotope LPL activities are calculated from the total activity from which is subtracted the HL activity. Similarly, the automated HL activity correlated even better with both the isotope HL activity and mass by ELISA. The isotope LPL and HL activity assays were considered the “gold standard” for the automated assays. This is further validated by their relationships to the ELISA for LPL and HL mass.
PHP LPL activity is derived from both adipose tissue and muscle tissue, which are regulated in opposite directions. To obviate the problem with multiple tissue sources, adipose tissue LPL activity has been studied in relation to plasma TG metabolism (1). By the isotope method, human adipose tissue LPL activity per cell has been shown to be highly correlated with VLDL fractional catabolic rates (r = 0.80, P < 0.005) (19). Further, the change of plasma TG following a carbohydrate meal is inversely correlated with the change in adipose tissue LPL activity in humans (r = 0.76, P < 0.01) (20). In addition, LPL activity is zero in patients with functional mutations in both alleles, and at half-normal in the obligate heterozygote parents of these children with LPL deficiency (11).
HL activity by the isotope method is highly correlated with the amount of visceral adipose tissue (r = 0.70, P < 0.001) measured by abdominal computed tomography (CT) scan (21). Further, the change in HL activity with intensive lipid-lowering therapy in the Familial Atherosclerosis Trial correlated with the change in LDL peak density (P < 0.001); and both peak density and HL activity correlated (P < 0.001) with regression of coronary artery disease as assessed by repeat quantitative angiography (22). Genetic variants in the HL gene promoter produce consistent changes in HL activity among different ethnic groups (23).
Thus, both the LPL and HL isotope activity assays have been extensively related to physiological measures of lipid metabolism. The automated method reflects the activities of the isotope methods, but is more convenient to use.
HL automated lipase activity correlated with HDL-cholesterol, further validating the new methodologies. In addition, HL activity was associated with TG levels. The correlation of plasma TG level with automated HL activity is puzzling. We have noted that TG (r = 0.70, P < 0.001) and isotope HL activity (r = 0.46, P < 0.001) are both related to intra-abdominal fat by CT scan in patients with type 1 diabetes, but more weakly with each other (r = 0.32, P = 0.02) (unpublished data). Perhaps the same phenomenon is occurring in this study. Also, we previously reported that in Japanese subjects, HL activity in PHP showed positive correlation with plasma TG levels, which is not inconsistent with the present observation (24).
In summary, we have developed a novel and simple automated method for the assay of LPL and HL activities in PHP, which is suitable for application to an automatic clinical analyzer. The ability to perform hundreds of assays per run, rather than six to ten, provides an opportunity for further understanding the functions of these two critical enzymes in lipid and lipoprotein metabolism.
Acknowledgments
The authors wish to thank Alegria Albers for the performance of the isotope assays and Steve Hashimoto for the performance of the hepatic lipase ELISA.
Published, JLR Papers in Press, March 14, 2008.
Footnotes
This study was supported in part by National Institutes of Health Grants HL-64332 and HL30086. Part of this study was performed at the General Clinical Research Center of the University of Washington (National Institutes of Health Grant MO-1 RR37).
References
- 1.Brunzell, J. D., and S. S. Deeb. 2001. Familial lipoprotein lipase deficiency, apoC-II deficiency, and hepatic lipase deficiency. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, D. Valle, B. Childs, K. W. Kinzler, and B. Vogelstein, editors. McGraw-Hill, New York. 2789–2816.
- 2.Havel R. J., and R. S. Gordon, Jr. 1960. Idiopathic hyperlipemia: metabolic studies in an affected family. J. Clin. Invest. 39 1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkel M., R. H. Eckel, and I. J. Goldberg. 2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43 1997–2006. [DOI] [PubMed] [Google Scholar]
- 4.Breckenridge W. C., J. A. Little, P. Alaupovic, C. S. Wang, A. Kuksis, G. Kakis, F. Lindgren, and G. Gardiner. 1982. Lipoprotein abnormalities associated with a familial deficiency of hepatic lipase. Atherosclerosis. 45 161–179. [DOI] [PubMed] [Google Scholar]
- 5.Hegele R. A., J. A. Little, C. Vezina, G. F. Maguire, L. Tu, T. S. Wolever, D. J. A. Jenkins, and P. W. Connelly. 1993. Hepatic lipase deficiency. Clinical, biochemical, and molecular genetic characteristics. Arterioscler. Thromb. 13 720–728. [DOI] [PubMed] [Google Scholar]
- 6.Carlson L. A., L. Holmquist, and P. Nilsson-Ehle. 1986. Deficiency of hepatic lipase activity in post-heparin plasma in familial hyper-[alpha]-triglyceridemia. Acta Med. Scand. 219 435–447. [DOI] [PubMed] [Google Scholar]
- 7.Brand K., K. A. Dugi, J. D. Brunzell, D. N. Nevin, H. B. Brewer, Jr., and S. Santamarina-Fojo. 1996. A novel A-G mutation in intron I of the hepatic lipase gene leads to alternative splicing resulting in enzyme deficiency. J. Lipid Res. 37 1213–1223. [PubMed] [Google Scholar]
- 8.Knudsen P., M. Antikainen, S. Ehnholm, M. Uusi-Oukari, H. Tenkanen, S. Kahri, J. Lahdenpera, M. Tilly-Kiesi, A. Bensadoun, M. R. Taskinen, et al. 1996. A compound heterozygote for hepatic lipase gene mutations Leu334→Phe and Thr383→Met: correlation between hepatic lipase activity and phenotypic expression. J. Lipid Res. 37 825–834. [PubMed] [Google Scholar]
- 9.Zambon A., S. S. Deeb, A. Bensadoun, K. E. Foster, and J. D. Brunzell. 2000. In vivo evidence of a role for hepatic lipase in human apoB-containing lipoprotein metabolism, independent of its lipolytic activity. J. Lipid Res. 41 2094–2099. [PubMed] [Google Scholar]
- 10.Babirak S. P., B. G. Brown, and J. D. Brunzell. 1992. Familial combined hyperlipidemia and abnormal lipoprotein lipase. Arterioscler. Thromb. 12 1176–1183. [DOI] [PubMed] [Google Scholar]
- 11.Babirak S. P., P. H. Iverius, W. Y. Fujimoto, and J. D. Brunzell. 1989. Detection and characterization of the heterozygote state for lipoprotein lipase deficiency. Arteriosclerosis. 9 326–334. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi J., N. Sasaki, J. Tashiro, H. Inadera, Y. Saito, and S. Yoshida. 1993. A missense mutation (Ala334→Thr) in exon 7 of the lipoprotein lipase gene in a case with type I hyperlipidemia. Biochem. Biophys. Res. Commun. 191 1046–1054. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi J., H. Hashimoto, I. Fukamachi, J. Tashiro, K. Shirai, Y. Saito, and S. Yoshida. 1993. Lipoprotein lipase mass and activity in severe hypertriglyceridemia. Clin. Chim. Acta. 216 113–123. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda Y., A. Takagi, and A. Yamamoto. 1989. Purification and characterization of lipoprotein lipase and hepatic triglyceride lipase from human postheparin plasma: production of monospecific antibody to the individual lipase. Biochim. Biophys. Acta. 1003 254–269. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura M., Y. Ohkaru, H. Ishii, N. Sunahara, A. Takagi, and Y. Ikeda. 2000. Development and evaluation of a direct sandwich-enzyme-linked immunosorbent assay for the quantification of human hepatic triglyceride lipase mass in human plasma. J. Immunol. Methods. 235 41–51. [DOI] [PubMed] [Google Scholar]
- 16.Imamura S., J. Kobayashi, S. Sakasegawa, A. Nohara, K. Nakajima, M. Kawashiri, A. Inazu, M. Yamagishi, J. Koizumi, and H. Mabuchi. 2007. A novel method for measuring human hepatic lipase activity in postheparin plasma. J. Lipid Res. 48 453–457. [DOI] [PubMed] [Google Scholar]
- 17.Fossati P., M. Ponti, P. Paris, G. Berti, and G. Tarenghi. 1992. Kinetic colorimetric assay of lipase in serum. Clin. Chem. 38 211–215. [PubMed] [Google Scholar]
- 18.Panteghini M., F. Pagani, and R. Bonara. 1993. Clinical and analytical evaluation of a continuous enzymatic method for measuring pancreatic lipase activity. Clin. Chem. 39 304–308. [PubMed] [Google Scholar]
- 19.Magill P., S. N. Rao, N. E. Miller, A. Nicoll, J. D. Brunzell, J. St. Hilaire, and B. Lewis. 1982. Relationships between the metabolism of high-density and very-low-density lipoproteins in man: studies of apolipoprotein kinetics and adipose tissue lipoprotein lipase activity. Eur. J. Clin. Invest. 12 113–120. [DOI] [PubMed] [Google Scholar]
- 20.Brunzell J. D., R. S. Schwartz, R. H. Eckel, and A. P. Goldberg. 1981. Insulin and adipose tissue lipoprotein lipase activity in humans. Int. J. Obes. 5 685–694. [PubMed] [Google Scholar]
- 21.Carr M. C., J. E. Hokanson, S. S. Deeb, J. Q. Purnell, E. S. Mitchell, and J. D. Brunzell. 1999. A hepatic lipase gene promoter polymorphism attenuates the increase in hepatic lipase activity with increasing intra-abdominal fat in women. Arterioscler. Thromb. Vasc. Biol. 19 2701–2707. [DOI] [PubMed] [Google Scholar]
- 22.Zambon A., J. E. Hokanson, B. G. Brown, and J. D. Brunzell. 1999. Evidence for a new pathophysiological mechanism for coronary artery disease regression: hepatic lipase-mediated changes in LDL density. Circulation. 99 1959–1964. [DOI] [PubMed] [Google Scholar]
- 23.Carr M. C., J. D. Brunzell, and S. S. Deeb. 2004. Ethnic differences in hepatic lipase and HDL in Japanese, black, and white Americans: role of central obesity and LIPC polymorphisms. J. Lipid Res. 45 466–473. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi J., M. Kusunoki, Y. Murase, M. Kawashiri, T. Higashikata, K. Miwa, S. Katsuda, M. Takata, A. Asano, A. Nohara, et al. 2005. Relationship of lipoprotein lipase and hepatic triacylglycerol lipase activity to serum adiponectin levels in Japanese hyperlipidemic men. Horm. Metab. Res. 37 505–509. [DOI] [PubMed] [Google Scholar]